Abstract
Background
Antineutrophil cytoplasmic antibodies (ANCAs) have been reported to occur in 7% to 10% of patients with idiopathic pulmonary fibrosis (IPF), but their clinical relevance remains unclear. The aim of this study was to estimate the prevalence of ANCAs in a North American population with IPF and evaluate their clinical significance.
Methods
This was a retrospective study of two independent cohorts of patients diagnosed with IPF at the University of California San Francisco (discovery cohort) and the University of Chicago (replication cohort). Myeloperoxidase (MPO) and proteinase 3 (PR3) ANCAs were measured in all patients. Prevalence and associations of ANCAs with clinical characteristics and transplant-free survival were evaluated.
Results
A total of 14 of 353 (4.0%; 95% CI, 2.2-6.5) and 20 of 392 (5.1%; 95% CI, 3.1-7.8) patients with IPF were positive for ANCAs at the time of diagnosis in the discovery and replication cohorts, respectively. Among those positive for MPO antibodies, two of six (33%) in the discovery cohort and three of 12 (25%) in the replication cohort developed vasculitis. None of the patients who were PR3-positive developed vasculitis. Patients who were ANCA-positive were more likely to be women than patients who were ANCA-negative, and were more likely to have some ground-glass opacities on CT scan. In the combined cohort of 745 patients, median transplant-free survival was not significantly different in patients who were ANCA-positive vs ANCA-negative (P = .57).
Conclusions
ANCA positivity is uncommon in North American patients with IPF and not associated with baseline disease severity or transplant-free survival; however, a significant proportion of patients who are MPO-positive with IPF develop clinical vasculitis.
Key Words: antibodies, antineutrophil cytoplasmic, idiopathic pulmonary fibrosis, interstitial lung disease, interstitial pneumonia with autoimmune features, vasculitis
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; CTD, connective tissue disease; ILD, interstitial lung disease; IPAF, interstitial pneumonia with autoimmune features; IPF, idiopathic pulmonary fibrosis; MPA, microscopic polyangiitis; MPO, myeloperoxidase; PR3, proteinase 3; UC, University of Chicago; UCSF, University of California San Francisco; UIP, usual interstitial pneumonia
As more is understood about the differences in treatment response and outcomes between idiopathic pulmonary fibrosis (IPF), interstitial pneumonia with autoimmune features (IPAF), and connective tissue disease (CTD)-associated interstitial lung disease (ILD), there is an increasing need to understand the significance of various autoantibodies in patients with ILD. The proposal of the term interstitial pneumonia with autoimmune features by the European Respiratory Society/American Thoracic Society task force,1 along with specific criteria to define it, has enabled the study of patients with idiopathic interstitial pneumonia and features suggestive of, but not diagnostic for, CTD. However, IPAF criteria does not include antineutrophil cytoplasmic antibodies (ANCAs) in its serologic domain and does not include typical features of vasculitis in its clinical domain. Similar to ongoing research into whether a designation of IPAF has prognostic significance, it is important to study whether ANCA positivity in patients with IPF has prognostic significance.
Although ANCAs are most often associated with systemic vasculidities rather than CTD, ILD is reported to be a manifestation of ANCA-associated vasculidities, particularly microscopic polyangiitis (MPA).2 Despite this finding, testing for ANCAs, specifically myeloperoxidase (MPO) and proteinase 3 (PR3) antibodies, is not part of the current major society recommendations for serologic evaluation in patients with suspected IPF, CTD-associated ILD, or IPAF.1, 3, 4, 5
Autoantibodies are found in up to 22% of patients with IPF.6 ANCAs are estimated to be present in 7% to 10% of patients with IPF without other symptoms of systemic vasculitis at the time of IPF diagnosis and are estimated to develop in another 10% of patients during follow-up.7, 8, 9 This is significantly higher than the prevalence noted in a study of an unselected Greek population of > 10,000 persons with a mean age of 60 years, which found the total prevalence of either MPO or PR3 antibodies to be 1.35%.10 After exclusion of persons diagnosed with small-vessel vasculitis, the prevalence was only 0.37%, suggesting that false-positives for MPO and PR3 antibodies are uncommon.10
Several small single-center studies have examined the clinical significance of MPO and PR3 antibodies with inconsistent findings in how these patients may differ from patients with ANCA-negative IPF with respect to clinical, radiographic, or pathologic findings.7, 8, 9, 11, 12 Differences in survival between patients with ANCA-positive and ANCA-negative IPF have also been inconsistent across studies to date.7, 8, 9, 12
Almost all of the studies exploring the prevalence and significance of ANCAs in IPF have occurred in Japanese populations, where MPO antibodies and MPA are more common in the general population.2 This is notable because MPA is the vasculitis mostly commonly associated with ILD. The clinical significance of ANCAs in North American patients with IPF remains unclear. Therefore, we aimed to estimate the prevalence of ANCAs in two North American populations diagnosed with IPF and explore any associated differences in survival and clinical, radiographic, and pathologic features between patients with ANCA-positive and ANCA-negative IPF.
Methods
Study Population
This was a retrospective cohort study of consecutive patients diagnosed with IPF prospectively enrolled in longitudinal registries and biorepositories at the University of California San Francisco (UCSF) (discovery cohort) and the University of Chicago (UC) (replication cohort). All new patients evaluated at the UCSF ILD clinic from 2002 to 2017 and UC ILD clinic from 2006 to 2017 were invited to enroll in these ongoing registries. To be included in this study, patients were required to have a diagnosis of IPF, determined by in-person multidisciplinary discussion using clinical data available at the time, including demographics, exposures, laboratory values, radiographic and pathologic findings, and supported by clinical guidelines.3, 4 Included patients at UCSF were also required to have at least 50 μL of stored serum (stored at −80°C) obtained at diagnosis. All included patients from UC had ANCA testing as part of their ILD clinic evaluation. This study was conducted in accordance with the amended Declaration of Helsinki and was approved by the UCSF committee on human research (10-01592) and UC institutional review board (14163-A). All patients provided written informed consent at the time of enrollment.
Data Collection
Baseline clinical information, including demographics, pulmonary function tests, radiologic and histopathologic studies, and blood samples, was collected at the time of enrollment. The date of death was recorded clinically and confirmed using the US Social Security Death Index at 6-month intervals. Lung transplants and date of lung transplants were also recorded clinically and cross-referenced with transplant records. For patients found to have ANCAs, a more in-depth, retrospective chart review was performed using a standardized data collection form for the following information: any diagnosis of vasculitis, presence of other autoantibodies, descriptions of lung pathology, and treatments.
ANCAs are not routinely measured at the UCSF ILD practice in the diagnostic evaluation of patients with suspected IPF; therefore, MPO and PR3 ANCAs were systematically measured from stored serum samples on all included UCSF patients using a clinical-grade enzyme-linked immunosorbent assay performed by a Clinical Laboratory Improvement Amendments-certified laboratory (Exsera BioLabs). At UC, ANCAs are included in the ILD autoimmunity serology panel; therefore, nearly all patients seen in the ILD clinic have ANCAs tested at the time of their initial evaluation. This is initially performed by immunofluorescence and, if positive (titer ≥ 20), serum is subsequently tested by enzyme-linked immunosorbent assay for MPO and PR3 antibodies. MPO and PR3 antibodies were considered positive at a titer of ≥ 20 International Units/μL in the discovery cohort and ≥ 1.0 antibody index in the replication cohort.
For the patients in the discovery cohort, chest CT scans obtained at the time closest to enrollment were scored by a chest radiologist (B. M. E.) using a standardized data collection form for specific features relevant to ILD and classified as definitive, possible, or inconsistent with usual interstitial pneumonia (UIP) based on 2011 IPF guidelines.4 Individual features (eg, reticulation) were scored for their extent/severity using a semi-quantitative scale as follows: none, mild (< 10% of lung involvement), moderate (10%-50% lung involvement), and severe (> 50% lung involvement). For patients in the discovery cohort who had surgical lung biopsies, a lung pathologist (K. D. J.) scored the specimens for specific features and provided histopathologic classification using a standardized data collection form. The radiologist and pathologist were blinded to ANCA status. Standardized CT scan and pathology scores were not available for the replication cohort.
Statistical Analysis
The proportion of patients with MPO, PR3, or either antibody was reported along with 95% binomial CIs. Given the low prevalence of either ANCA, all subsequent associations were analyzed by categorizing patients as positive or negative for either MPO or PR3 antibodies (ie, ANCA-positive vs ANCA-negative). Clinical and radiographic characteristics were compared between patients who were ANCA-positive and ANCA-negative using the Fisher exact test or t test as appropriate. Transplant-free survival between the two groups was visualized using Kaplan-Meier survival plots and compared using the log-rank test and Cox proportional hazards models (stratified by cohort), both unadjusted and adjusted for other baseline variables commonly associated with survival in IPF; these included age, sex, FVC % predicted, and diffusing capacity of the lung for carbon monoxide % predicted.
Results
Clinical Characteristics
Among 353 patients with IPF in the discovery cohort, 14 (4.0%, 95% CI, 2.2-6.5) were found to have ANCAs present at the time of study enrollment. Of the patients with ANCAs, eight of 14 (57%) had PR3 antibodies and six of 14 (43%) had MPO antibodies. The proportion of patients with positive ANCAs was similar in the replication cohort (20 of 392 [5.1%]; 95% CI, 3.1-7.8). Of these, two of 20 (10%) had PR3 antibodies, 12 of 20 (60%) had MPO antibodies, and six of 20 (30%) had nonspecific ANCA positivity (positive by immunofluorescence, but subsequent PR3 and MPO antibody testing negative). The comparison of clinical characteristics between patients with ANCA-positive and ANCA-negative IPF is summarized in Table 1. Compared with patients with ANCA-negative IPF, patients with ANCA-positive IPF were more likely to be women in both cohorts (discovery cohort: 47.1% vs 22.9%, P = .09; replication cohort: 50.0% vs 25.0%, P = .01). There was also a trend in the combined cohort toward there being more nonwhite patients in the ANCA-positive group than the ANCA-negative group. There were no differences in severity of lung disease at enrollment, as measured by baseline pulmonary function tests. Six of the 14 patients with ANCA-positive IPF in the discovery cohort, and all of the patients with ANCA-positive IPF in the replication cohort, had positive titers of other antibodies (Table 2).
Table 1.
Clinical Characteristics of Patients With Idiopathic Pulmonary Fibrosis Who Are Positive vs Negative for Antineutrophil Cytoplasmic Antibodies
| Characteristic | Combined |
Discovery |
Replication |
||||||
|---|---|---|---|---|---|---|---|---|---|
| ANCA-Negative (n = 711) | ANCA-Positive (n = 34) | P Value | ANCA-Negative (n = 339) | ANCA-Positive (n = 14) | P Value | ANCA-Negative (n = 372) | ANCA-Positive (n = 20) | P Value | |
| Age (y) | 70.3 ± 8.3 | 67.9 ± 8.9 | .09 | 70.6 ± 8.6 | 66.6 ± 7.8 | .09 | 70.1 ± 8.0 | 68.8 ± 9.6 | .48 |
| Female | 163 (22.9) | 16 (47.1) | .001 | 70 (20.6) | 6 (42.9) | .09 | 93 (25.0) | 10 (50.0) | .01 |
| Race/ethnicity | .06 | .21 | .05 | ||||||
| White | 590 (83.0) | 24 (70.6) | 272 (80.2) | 10 (71.4) | 318 (85.5) | 14 (70.0) | |||
| Asian or Pacific Islander | 36 (5.1) | 1 (2.9) | 27 (8.0) | 0 (0.0) | 9 (2.4) | 1 (5.0) | |||
| Black | 23 (3.2) | 4 (11.8) | 3 (0.9) | 0 (0.0) | 20 (5.4) | 4 (20.0) | |||
| Hispanic or Latino | 53 (7.5) | 4 (11.8) | 28 (8.3) | 3 (21.4) | 25 (6.7) | 1 (5.0) | |||
| Other or unknown | 9 (1.3) | 1 (2.9) | 9 (2.7) | 1 (7.1) | |||||
| FVC % predicted | 67.4 ± 18.1 | 66.3 ± 20.7 | .72 | 69.8 ± 18.1 | 70.5 ± 17.9 | .88 | 65.3 ± 17.9 | 63.3 ± 22.4 | .63 |
| Dlco % predicted | 48.4 ± 17.5 | 50.4 ± 22.2 | .54 | 47.4 ± 16.7 | 53.0 ± 26.2 | .25 | 49.4 ± 18.2 | 48.6 ± 19.3 | .86 |
| Ever smokera | 504 (70.9) | 26 (76.5) | .48 | 236 (69.6) | 12 (85.7) | .20 | 268 (72.0) | 14 (70.0) | .84 |
Data are presented as No. of patients (% total), mean ± SD, or as otherwise indicated. ANCA = antineutrophil cytoplasmic antibody; Dlco = diffusing capacity of lung for carbon monoxide.
Ever smoker (current or former) vs never smoker.
Table 2.
Clinical Characteristics, Treatment, and Outcomes of Patients With Antineutrophil Cytoplasmic Antibody-Positive Idiopathic Pulmonary Fibrosis
| Identification/ Cohort | Age (years)/Sex | ANCA Type | Vasculitis Diagnosis | Other Autoantibodies, Titers | Lung Biopsy Results | Outcome | Treatment | Months of Database Follow-Up | Months of Chart Follow-Up |
|---|---|---|---|---|---|---|---|---|---|
| 1/D | 72/F | PR3 | No | a | Emphysema, some areas of fibroblast foci, lung fibrosis with architectural distortion and subpleural accentuation. Nonclassifiable fibrosing interstitial pneumonitis. | Deceased | AZA | 25 | 0 |
| 2/D | 73/F | MPO | No | a | UIP | Deceased | AZA | 59 | 0 |
| 3/D | 54/F | MPO | MPA | ANA 1:160, RF 157 | a | Deceased | Steroids, AZA, CYC, MMF, RTX | 101 | 102 |
| 4/D | 65/M | PR3 | No | None | Moderate to severe fibrosis with subpleural and bronchiolocentric accentuation, fibroblast foci, and patchy airway-centered fibrosis. No well-developed microscopic honeycombing. | Transplant | Pirfenidone, nintedanib | 60 | 73 |
| 5/D | 63/F | MPO | MPA | ANA 1:160 | a | Alive | Steroids, RTX, NAC | 48 | 27 |
| 6/D | 67/M | MPO | No | ANA 1:40 | a | Alive | a | 42 | 0.0 |
| 7/D | 65/F | MPO | No | None | a | Alive | Nintedanib | 36 | 46 |
| 8/D | 67/M | MPO | No | ANA 1:640, RF 351 | a | Alive | Nintedanib | 25 | 34 |
| 9/D | 59/M | PR3 | No | ANA 1:160, anti-dsDNA, anti-CCP | UIP | Alive | a | 16 | 0 |
| 10/D | 55/F | PR3 | No | a | UIP | Alive | Pirfenidone | 12 | 18 |
| 11/D | 84/M | PR3 | No | ANA 1:40 | a | Alive | Nintedanib | 14 | 20 |
| 12/D | 65/M | PR3 | No | None | Emphysema, insufficient material | Alive | Pirfenidone, nintedanib | 11 | 18 |
| 13/D | 76/M | PR3 | No | None | UIP, scattered lymphoid aggregates with occasional germinal centers noted in the fibrotic regions. Pulmonary arteries show mild to moderate thickening. | Alive | Pirfenidone, nintedanib | 11 | 7 |
| 14/D | 67/M | PR3 | No | None | a | Alive | Nintedanib | 5 | 11 |
| 15/R | 84/M | NS | No | SSA | a | Alive | None | 4 | 7 |
| 16/R | 53/M | NS | No | ANA 1:160 | Extensive OP, superimposed on UIP | Alive | Steroids, AZA | 8 | 7 |
| 17/R | 65/M | PR3 | No | ANA 1:160 | UIP, emphysema, moderate to severe pulmonary hypertension | Alive | Nintedanib, pirfenidone | 26 | 32 |
| 18/R | 75/F | MPO | No | ANA 1:320, anti-CCP | a | Alive | Steroids, nintedanib | 14 | 16 |
| 19/R | 72/M | MPO | No | RF 166, Scl70 | UIP, centrilobular emphysema, scattered regions of dendriform calcifications | Alive | Steroids, MMF, pirfenidone | 0 | 15 |
| 20/R | 82/F | MPO | No | RF 37, SSA, SSB, anti-Ku | a | Alive | Steroids, MMF, pirfenidone | 3 | 2 |
| 21/R | 46/F | MPO | no | ANA 1:160, RF 62 | UIP with poorly formed granulomas consistent with chronic hypersensitivity pneumonitis, secondary pulmonary hypertension | Alive | Steroids, AZA, RTX, CYC | 26 | 22 |
| 22/R | 67/F | MPO | MPA | ANA 1:160 | UIP | Alive | Steroids, AZA, MMF, RTX | 0 | 4 |
| 23/R | 61/F | MPO | AAV | ANA 1:320 | UIP, NSIP in less involved areas, rare multinucleated giant cells | Alive | Steroids, AZA, MMF | 60 | 66 |
| 24/R | 71/M | NS | No | ANA 1:40 | a | Alive | Steroids | 2 | 12 |
| 25/R | 69/M | MPO | No | ANA 1:1,280, RF 78 | UIP, lymphoid follicles with germinal centers. Focal giant cells without granulomas | Transplant | None | 24 | 31 |
| 26/R | 70/M | NS | No | ANA 1:160 | a | Alive | Steroids, pirfenidone | 1 | 2 |
| 27/R | 74/M | NS | No | ANA 1:2,560 | UIP, few nonnecrotizing granulomas consistent with chronic hypersensitivity pneumonitis | Alive | Steroids, MMF | 11 | 15 |
| 28/R | 62/F | MPO | No | ANA 1:640 | UIP with extensive OP | Deceased | Steroids | 3 | 2 |
| 29/R | 76/F | MPO | No | ANA 1:80, RF, SSA | a | Alive | Steroids, AZA | 72 | 0 |
| 30/R | 55/F | NS | No | ANA 1:160 | a | Alive | Steroids, AZA | 29 | 48 |
| 31/R | 77/F | PR3 | No | ANA 1:320 | UIP | Alive | None | 80 | 85 |
| 32/R | 77/M | MPO | No | RF 23 | a | Deceased | None | 8 | 0 |
| 33/R | 71/M | MPO | No | ANA 1:160 | a | Deceased | Steroids | 5 | 5 |
| 34/R | 68/F | MPO | AAV | RF 20 | a | Deceased | Steroids, CYC | 45 | 9 |
AAV = ANCA-associated vasculitis; ANA = antinuclear antibody; Anti-CCP = anti-cyclic citrullinated peptide antibody; Anti-dsDNA = anti-double stranded DNA antibody; AZA, azathioprine; CYC = cyclophosphamide; D = discovery cohort; F = female; M = male; MMF = mycophenolate mofetil; Months of chart follow-up = time from study enrollment to most recent clinical documentation for review in the patient’s electronic medical record; Months of database follow-up = time since study enrollment to most recent vital status check; MPA = microscopic polyangiitis; MPO = myeloperoxidase; NAC = N-acetylcysteine; NS = nonspecific ANCA; NSIP = nonspecific interstitial pneumonia; OP = organizing pneumonia; PR3 = proteinase 3 antibody; R = replication cohort; RF = rheumatoid factor; RTX = rituximab; SSA = anti-Ro; SSB = anti-La; Treatment = medication recommended by consulting pulmonologist or medication that patient was recorded as taking; UIP = usual interstitial pneumonia. See Table 1 legend for expansion of other abbreviation.
No data available.
Radiologic Features
Radiographic UIP classification and specific chest CT scan findings are compared between patients with ANCA-positive and ANCA-negative IPF from the discovery cohort in Table 3. Compared with patients with ANCA-negative IPF, patients with ANCA-positive IPF were significantly more likely to have ground-glass opacities (33.3% vs 9.3%, P = .02) and moderate or severe (vs mild or absent) honeycombing (33.3% vs 10.5%, P = .04). There were no significant differences in 2011 UIP classification, distribution of fibrosis, or in the presence vs absence of honeycombing, traction bronchiectasis, consolidation, nodules, or small airways disease. CT scans were not available for scoring using the same methods in the replication cohort.
Table 3.
Chest CT Scan Findings of Patients With Antineutrophil Cytoplasmic Antibody-Positive vs Antineutrophil Cytoplasmic Antibody-Negative Idiopathic Pulmonary Fibrosis in the Discovery Cohort
| Feature | ANCA-Negative | ANCA-Positive | P Value |
|---|---|---|---|
| Total No. with CT scan scored | 313 | 12 | |
| UIP, definite or possiblea | 249 (79.6) | 9 (75.0) | .72 |
| Reticulation, moderate or severeb | 249 (79.6) | 8 (66.7) | .28 |
| Traction bronchiectasis present | 307 (98.1) | 12 (100.0) | > .99 |
| Moderate or severeb | 195 (62.3) | 4 (33.3) | .07 |
| Honeycombing present | 211 (67.4) | 9 (75.0) | .76 |
| Moderate or severeb | 33 (10.5) | 4 (33.3) | .04 |
| Fibrosis, cranial-caudal distribution | .64 | ||
| Diffuse | 14 (4.5) | 1 (8.3) | |
| Lower | 288 (92.0) | 11 (91.7) | |
| Middle or upper | 11 (3.5) | 0 (0.0) | |
| Fibrosis, axial distribution | > .99 | ||
| Central | 2 (0.6) | 0 (0.0) | |
| Diffuse | 23 (7.3) | 1 (8.3) | |
| Peripheral | 288 (92.0) | 11 (91.7) | |
| Ground-glass opacity present | 29 (9.3) | 4 (33.3) | .02 |
| Consolidation present | 11 (3.5) | 0 (0.0) | > .99 |
| Nodules present | 2 (0.6) | 0 (0.0) | > .99 |
| Small airways disease present | 68 (97) | 4/4 (100) | > .99 |
CT scans of the chest evaluated for UIP pattern and specific radiographic findings pertinent to interstitial lung disease. Values are No. (%) or as otherwise indicated. See Table 1 and 2 legends for expansion of abbreviations.
Definite or possible UIP pattern vs inconsistent with UIP pattern.
Moderate or severe vs mild or none.
Histopathologic Features
Eight patients with ANCA-positive IPF in the discovery cohort had lung biopsies, five of which were formally scored using a standardized data collection form. Ten patients with ANCA-positive IPF in the replication cohort had lung biopsies, and results were obtained from chart review and were not formally scored. Given the limited number of patients with lung biopsies, there were no statistical comparisons made between patients with ANCA-positive and ANCA-negative IPF. Summary of pathologic findings for patients with ANCA-positive IPF are included in Table 2. None of these patients had evidence of capillaritis or vasculitis on pathology.
Treatment and Outcomes
After a median follow-up time of 18.3 months by chart review, two of the six patients (33%) with MPO antibodies in the discovery cohort developed a clinical diagnosis of MPA, both at least 1 year after their diagnosis of IPF (Table 2). In the replication cohort, three of 12 patients (25%) with MPO antibodies subsequently developed clinical vasculitis (one developed MPA and two developed nonspecific ANCA-associated vasculitis) after a median follow-up of 10.5 months. Additionally, all patients who developed vasculitis in both the discovery and replication cohorts were women. The clinical manifestations of vasculitis seen in these patients were renal disease (three of five patients), mononeuritis multiplex (two of five patients), purpura (one of five patients), sinusitis (one of five patients), and inflammatory arthritis (one of five patients). None of the patients with PR3 or nonspecific ANCAs developed vasculitis during the follow-up period.
As outlined in Table 2, the choice of pharmacologic treatment in the discovery cohort appears to be based largely on time of study enrollment. The three patients with ANCA-positive IPF enrolled before 2012, when the results of the Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis trial had been published, were all recommended azathioprine, and most patients enrolled after this time were recommended antifibrotic agents alone.13 In the replication cohort, 15 of the 20 patients with ANCA-positive IPF received immunosuppressive medications, either with or without antifibrotic agents. Of the 15 patients who received immunosuppression, two were treated prior to the results of the Prednisone, Azathioprine, and N-Acetylcysteine for Pulmonary Fibrosis trial, eight were treated prior to multidisciplinary team diagnosis of IPF, two were treated because of concern for autoimmune features despite no CTD or vasculitis diagnosis, and three were treated after diagnosis of vasculitis.
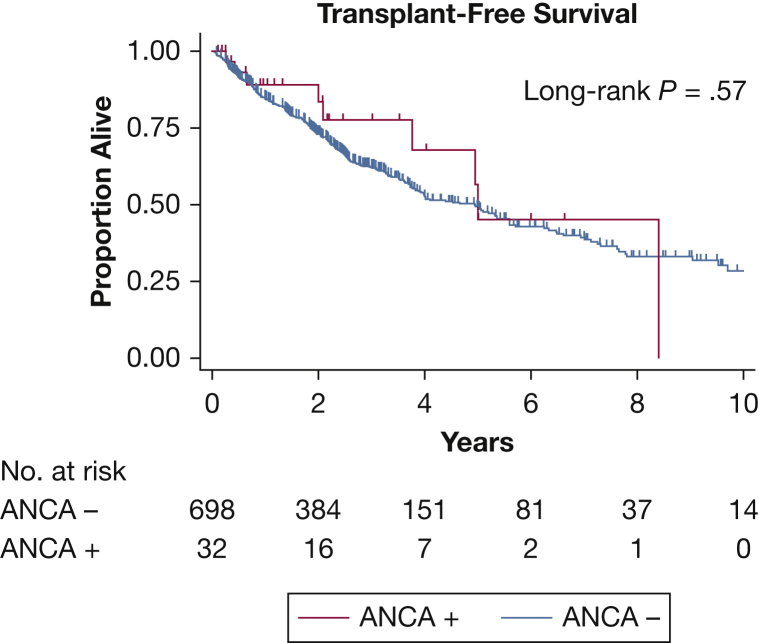
Including combined data from both cohorts, median transplant-free survival was 5.0 years (95% CI, 3.8-infinite) for patients with ANCA-positive IPF vs 4.9 years (95% CI, 3.8-5.6) for patients with ANCA-negative IPF (log-rank P = .57) (Fig 1). There was no significant difference in survival after controlling for age, sex, and baseline pulmonary function (hazard ratio, 0.84; 95% CI, 0.39-1.80; P = .65).
Figure 1.
Kaplan-Meier curve comparing transplant-free survival in patients with idiopathic pulmonary fibrosis that are positive vs negative for ANCA. ANCA = antineutrophil cytoplasmic antibody.
Discussion
This study demonstrates that a small percentage of North American patients with IPF are positive for ANCAs, but only a subset of patients with MPO antibodies appear to develop clinical vasculitis. It also provides further evidence that ANCA positivity is more common in patients with IPF compared with the general population, but suggests that it may be less common in North American populations than previously reported in Japanese IPF populations. This difference appears to be largely because of differences in prevalence of MPO antibody positivity. In this study, 1.7% to 3.1% of patients with IPF were MPO-positive, whereas prior studies performed in Japan found that MPO positivity was seen in about 4% to 10% of patients with IPF.7, 9, 12, 14 In contrast, the prevalence of PR3 positivity in the discovery cohort (2.3%) was similar to prior studies (2%-5%).8, 9
Clinically, patients with ANCA-positive IPF appear to be similar to patients with ANCA-negative IPF, except for a more even sex distribution in those who are ANCA-positive than those who are ANCA-negative. This mirrors the sex distribution in the ANCA-associated vasculidities where the percentage of men to women is also often equal or just slightly male predominant.15, 16 However, this finding contrasts prior studies of patients with ANCA-positive IPF, which found low proportions of women, similar to patients with ANCA-negative IPF.7, 8, 11, 12
To date, there have been no consistent chest CT scan findings that distinguish patients with ANCA-positive IPF from patients with ANCA-negative IPF. Nozu et al17 and Foulon et al11 found no significant difference in chest CT scan features between patients with ANCA-positive and ANCA-negative IPF. Ando et al7 found low attenuation areas (ie, mosaic perfusion) to be more common in patients with MPO-positive IPF; however, there were significantly more smokers in the MPO-positive IPF group. In this study, the most significant difference seen on chest CT scan of patients with ANCA-positive IPF was the increased prevalence of ground-glass opacities. This is consistent with the Hosoda et al findings12 of increased attenuation around honeycombing and cysts in patients who were MPO-positive with UIP patterns on surgical lung biopsy. The increased ground glass or areas of increased attenuation may reflect increased inflammation in the lungs of the patients with ANCA-positive IPF because Hosoda et al12 also found that patients who were MPO-positive had more prominent inflammatory cell infiltration and lymphoid follicles with germinal centers. We were unable to replicate these findings because of the low number of patients with ANCA-positive IPF with surgical lung biopsies.
Regarding outcomes of patients with ANCA-positive IPF, 25% to 33% of patients with MPO antibodies subsequently developed clinical manifestations of vasculitis. This is similar to prior studies of patients with MPO-positive IPF, which found that 16% to 26% of patients subsequently developed MPA; however, one study found a much higher percentage (83%).7, 9, 11, 12 The most common clinical manifestations of vasculitis seen in the two cohorts were renal disease (three of five patients) and mononeuritis multiplex (two of five patients). This is consistent with prior studies of patients with vasculitis and pulmonary fibrosis, which found the most common extrapulmonary manifestations of vasculitis to be renal involvement (57%-74%), peripheral nervous system involvement (32%-89%), and fever (52%-63%).9, 15, 18 Interestingly, all patients in this study who developed clinical vasculitis were MPO-positive and women, whereas none of the patients who were PR3-positive or men developed vasculitis during follow-up. The lack of development of vasculitis in patients with PR3-positive pulmonary fibrosis is consistent with prior studies.8, 9 None of the lung biopsies from the patients with ANCA-positive IPF showed any evidence of vasculitis at baseline. This finding is consistent with almost all prior studies; however, one study did report evidence of vasculitis in the lung biopsies of five of 15 patients with MPO-positive IPF.2, 7, 12, 14, 19
ILD is now recognized as a relatively common manifestation of MPA, seen in 2.7% to 45% of patients with MPA, with UIP pattern seen histologically in 45% to 100% of these patients.2, 18, 20, 21 Additionally, pulmonary fibrosis is a morbid manifestation of MPA. One study found fourfold higher mortality in patients with MPA with ILD compared with those without ILD.21 Another study of patients with MPA with and without ILD found that respiratory failure was the cause of death in all patients with MPA and ILD, whereas none of the patients with MPA without ILD died during follow-up.18 However, in the context of a diagnosis of IPF, this study did not find a statistically significant difference in survival between patients who were ANCA-positive and ANCA-negative, which may be because of the high mortality of IPF.7, 8, 11, 12
This study has several key strengths compared with prior studies of ANCA positivity in IPF. To our knowledge, it is the first study to systematically estimate the prevalence of ANCA positivity in two independent North American populations of patients with IPF. It is also the largest study to date examining the clinical associations of ANCA positivity in patients with IPF. It is also notable that both cohorts had remarkably similar findings despite differences in the methods and blinding to ANCA testing. There are, however, notable limitations to this study. First, the radiographic associations found in the discovery cohort could not be replicated; therefore, these results are provisional. Second, the limited duration of clinical follow-up for patients with ANCA-positive IPF may have resulted in an underestimation of the risk of developing vasculitis. Third, the incidence of ANCA seroconversion during follow-up could not be determined because only baseline serum samples were evaluated.
These findings have important clinical implications. Given the low prevalence of PR3 antibody positivity in North American patients with IPF and the lack of any associated impact on survival or development of vasculitis, these results do not support the routine measurement of PR3 antibodies in patients with suspected IPF. In contrast, MPO positivity, although uncommon in patients with IPF, when present, is associated with at least a 25% to 33% risk of developing clinical manifestations of vasculitis. Because the development of vasculitis has therapeutic implications and may impact morbidity, measurement of MPO antibodies may be indicated in patients with suspected IPF, especially women who have the highest risk of MPO positivity and vasculitis.
Acknowledgments
Author contributions: G. Y. L. is the guarantor of the content of the manuscript, including the data and analysis. G. Y. L., H. R. C., and B. L. contributed to conception and design. G. Y. L., I. B. V., N. A.-Z., B. M. E., K. D. J., P. J. W., A. A., M. E. S., and B. L. contributed to acquisition and/or interpretation of data. All authors contributed to drafting of the manuscript and/or critical revision of major intellectual content. All authors approved the final version of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This work was supported by the National Institutes of Health [Grant KL2TR001870] and the Nina Ireland Program for Lung Health.
References
- 1.Fischer A., Antoniou K., Brown K. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46(4):976–987. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 2.Katsumata Y., Kawaguchi Y., Yamanaka H. Interstitial lung disease with ANCA-associated vasculitis. Clin Med Insights Circ Respir Pulm Med. 2015;9(1):51–56. doi: 10.4137/CCRPM.S23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis W.D., Costabel U., Hansell D. An Official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G., Collard H., Egan J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vij R., Strek M.E. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143(3):814–824. doi: 10.1378/chest.12-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.S., Kim E.J., Lynch K.L. Prevalence and clinical significance of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir Med. 2013;107(2):249–255. doi: 10.1016/j.rmed.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ando M., Miyazaki E., Ishii T. Incidence of myeloperoxidase anti-neutrophil cytoplasmic antibody positivity and microscopic polyangitis in the course of idiopathic pulmonary fibrosis. Respir Med. 2013;107(4):608–615. doi: 10.1016/j.rmed.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Hozumi H., Enomoto N., Oyama Y. Clinical implication of proteinase-3-antineutrophil cytoplasmic antibody in patients with idiopathic interstitial pneumonias. Lung. 2016;194(2):235–242. doi: 10.1007/s00408-016-9851-x. [DOI] [PubMed] [Google Scholar]
- 9.Kagiyama N., Takayanagi N., Kanauchi T., Ishiguro T., Yanagisawa T., Sugita Y. Antineutrophil cytoplasmic antibody-positive conversion and microscopic polyangiitis development in patients with idiopathic pulmonary fibrosis. BMJ Open Respir Res. 2015;2(1) doi: 10.1136/bmjresp-2014-000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsiveriotis K., Tsirogianni A., Pipi E., Soufleros K., Papasteriades C. Antineutrophil cytoplasmic antibodies testing in a large cohort of unselected Greek patients. Autoimmune Diseases. 2011;2011:626495. doi: 10.4061/2011/626495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foulon G., Delaval P., Valeyre D. ANCA-associated lung fibrosis: analysis of 17 patients. Respir Med. 2008;102(10):1392–1398. doi: 10.1016/j.rmed.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Hosoda C., Baba T., Hagiwara E. Clinical features of usual interstitial pneumonia with anti-neutrophil cytoplasmic antibody in comparison with idiopathic pulmonary fibrosis. Respirology. 2016;21(5):920–926. doi: 10.1111/resp.12763. [DOI] [PubMed] [Google Scholar]
- 13.The Idiopathic Pulmonary Fibrosis Clinical Research Network Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T., Otani K., Egashira R. Interstitial pneumonia associated with MPO-ANCA: clinicopathological features of nine patients. Respir Med. 2012;106(12):1765–1770. doi: 10.1016/j.rmed.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 15.Comarmond C., Crestani C., Tazi A. Pulmonary fibrosis in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis. Medicine. 2014;93(24):340–349. doi: 10.1097/MD.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watts R.A., Mooney J., Skinner J., Scott D.G.I., MacGregor A.J. The contrasting epidemiology of granulomatosis with polyangiitis (Wegener’s) and microscopic polyangiitis. Rheumatology. 2012;51(5):926–931. doi: 10.1093/rheumatology/ker454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozu T., Kondo M., Suzuki K., Tamaoki J., Nagai A. A comparison of the clinical features of ANCA-positive and ANCA-negative idiopathic pulmonary fibrosis patients. Respiration. 2009;77(4):407–415. doi: 10.1159/000183754. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Casares M., Gonzalez A., Fielli M., Caputo F., Bottinelli Y., Zamboni M. Microscopic polyangiitis associated with pulmonary fibrosis. Clin Rheumatol. 2015;34(7):1273–1277. doi: 10.1007/s10067-014-2676-1. [DOI] [PubMed] [Google Scholar]
- 19.Homma S., Matsushita H., Nakata K. Pulmonary fibrosis in myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitides. Respirology. 2004;9(2):190–196. doi: 10.1111/j.1440-1843.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 20.Alba M.A., Flores-Suarez L.F., Henderson A.G. Interstital lung disease in ANCA vasculitis. Autoimmun Rev. 2017;16(7):722–729. doi: 10.1016/j.autrev.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schirmer J.H., Wright M.N., Vonthein R. Clinical presentation and long-term outcome of 144 patients with microscopic polyangiitis in a monocentric German cohort. Rheumatology. 2016;55(1):71–79. doi: 10.1093/rheumatology/kev286. [DOI] [PubMed] [Google Scholar]