In the United States, a rare disease is defined by a prevalence of < 200,000 patients. There are approximately 7,000 unique rare diseases affecting an estimated 25 to 30 million people in the United States, 5% to 10% of whom are diagnosed with a rare disease of the lung.1, 2 Despite recent scientific advances in understanding rare lung diseases pathogenesis and disease-modifying therapies,3 rare lung diseases remain an important public health concern because of high morbidity and mortality in the United States and worldwide.4 Outside of lung transplantation, there are no cures for rare lung diseases, and early disease recognition remains limited.
The Rare Lung Diseases Consortium (RLDC) held the RLDC 2018 conference on September 6 to 9, 2018, at the Northern Kentucky Convention Center.5 As was presented at that conference, this article summarizes the National Heart, Lung, and Blood Institute’s (NHLBI) Division of Lung Diseases’ (DLD) support of rare lung diseases research. The objective(s) of this article are to inform all stakeholders, including early career investigators, on the continued commitment of the NHLBI to fund rare lung diseases research; to recognize the NHLBI’s ability to support programs that use a unique structure or partnership to advance knowledge and potential treatments in rare lung diseases; and to highlight the potential impact of novel tools and technologies to advance our understanding of and treatments for rare lung diseases.
NHLBI Rare Lung Diseases Funding
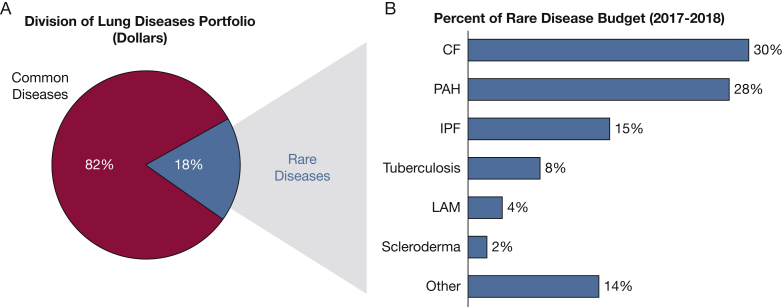
The lung is a complicated organ composed of several tissues and numerous cell types. This complexity manifests in many unique lung diseases represented in the NHLBI/DLD rare lung diseases portfolio. In fiscal years (FYs) 2017 and 2018, research projects focused on rare lung diseases received 18% of total DLD grant funding, with the largest percentage of rare lung diseases funding to research in cystic fibrosis (CF), pulmonary arterial hypertension, and idiopathic pulmonary fibrosis (IPF) (Fig 1). All data used for this study were obtained from the internal National Institutes of Health (NIH) administrative grants database known as Information for Management, Planning, Analysis, and Coordination II (IMPAC II), which stores all NIH application data across FYs. The NIH Query/View/Report System was used to search the IMPAC II database and for data extraction. The list of grants was identified through the Research, Condition, and Disease Categorization category of Rare Diseases. The list of diseases was manually curated using abstracts and award titles from all competing and noncompeting awards. In the United States, pulmonary TB is classified as a rare disease and therefore was included in the portfolio analysis. In contrast, although it has been categorized as a rare disease, ARDS likely has a prevalence in the United States of > 200,000, because of its incidence of approximately 200,0006 and long-term sequelae common in the surviving 60% to 70%.7 Therefore, research projects focused on acute lung injury and ARDS were excluded from this analysis.
Figure 1.
Division of Lung Diseases (DLD) portfolio for fiscal year (FY) 2017 and FY 2018. The pie chart (A) shows the percentage of grant funding allocated for common and rare diseases research. The bar chart (B) lists the rare lung diseases that make up ≥ 2% of the DLD rare disease portfolio (in dollars). CF = cystic fibrosis; IPF = idiopathic pulmonary fibrosis; LAM = lymphangioleiomyomatosis; Other = rare diseases or conditions that make up < 2% of the DLD rare lung disease portfolio and are listed here in alphabetical order: acute myeloid leukemia, alpha-1 antitrypsin deficiency, alveolar capillary dysplasia, amyotrophic lateral sclerosis, aspergillosis, bronchiolitis obliterans syndrome, congenital diaphragmatic hernia, chronic beryllium disease, chronic thromboembolic pulmonary hypertension, Duchenne muscular dystrophy, Hermansky-Pudlak syndrome, Hutchinson-Gilford progeria syndrome, Löfgren syndrome, lung transplantation, lysosomal storage disorder, non-TB mycobacterial lung disease, Pallister-Hall syndrome, pediatric ARDS, pleuropulmonary blastoma, pulmonary alveolar proteinosis, pulmonary ciliary dyskinesia, respiratory distress syndrome, Rett syndrome, rheumatoid arthritis-associated interstitial lung disease, sarcoidosis, sickle cell disease, sudden infant death syndrome, tetralogy of Fallot, thalassemia, tracheoesophageal fistula, X-linked myotubular myopathy; PAH = pulmonary arterial hypertension.
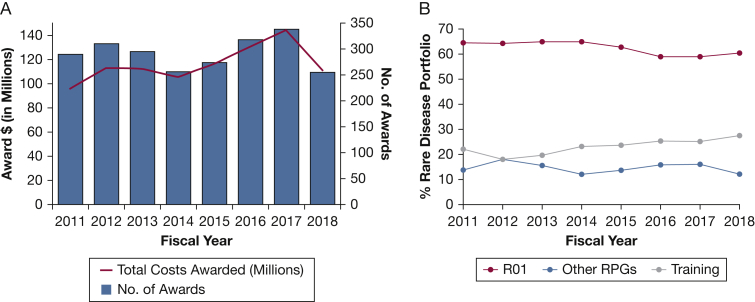
NHLBI funding for rare lung diseases research has ranged from $96 million in award dollars and 290 awards in FY 2011 to $145 million and 338 awards in FY 2017 (Fig 2A). From FY 2011 through FY 2018, 79% of rare diseases lung research grants have been awarded to established principal investigators and 21% to early stage investigators (Fig 2B). To help build the pipeline of early stage investigators and established principal investigators pursuing research in lung diseases, the DLD has been committed to supporting trainees through institutional programs and individual awards since its inception 50 years ago. Currently, through NIH- and NHLBI-supported grant mechanisms, several opportunities are available for MDs, PhDs, and MD-PhDs to pursue research training in rare lung diseases. The percentage of training awards in DLD’s rare lung diseases portfolio has ranged from 18% to 28% between FY 2011 and FY 2018 (Fig 2B). The number and mechanism of DLD-funded rare lung diseases training grants from FY 2011 through FY 2018 are shown in Table 1. Collectively, these data help demonstrate the NHLBI’s continued commitment to fund research project grants and training grants across a range of rare lung diseases.
Figure 2.
A-B, Division of Lung Diseases (DLD) rare disease portfolio fiscal years 2011 to 2018. A, Graph of the total costs awarded to DLD grants on rare diseases and the number of awards per year. B, Breakdown of the DLD rare disease portfolio by type of mechanism: R01, RPG excluding the R01, or Training Grants (including F, K, and T mechanisms). RPG activity codes are DP1, DP2, DP3, DP4, DP5, P01, P42, PN1, PM1, R00, R01, R03, R15, R22, R23, R29, R33, R34, R35, R36, R37, R50, R55, R56, R61, RC1, RC2, RC3, RC4, RF1, RL1, RL2, RM1, UA5, U01, U19, U34, UA5, UC1, UC2, UC3, UC4, UC7, UF1, UG3, UH2, UH3, UH5, UM1, and UM2. RPG = Research Project Grants.
Table 1.
Division of Lung Diseases’ Rare Lung Diseases Training Grant Mechanisms, Titles, and Cumulative Number of Awards by Mechanism (Fiscal Years 2011-2018)
| Grant Mechanism | Grant Mechanism Title | No. of Awards |
|---|---|---|
| F30 | Ruth L. Kirschstein National Research Service Award Individual Fellowship for Students at Institutions with NIH-Funded Institutional Predoctoral Dual-Degree Training Programs | 15 |
| F31 | Ruth L. Kirschstein National Research Service Award Individual Predoctoral Fellowship | 9 |
| F32 | Ruth L. Kirschstein National Research Service Award Individual Postdoctoral Fellowship | 20 |
| K01 | Mentored Career Development Award to Promote Faculty Diversity in Biomedical Research | 7 |
| K02 | Independent Scientist Award | 1 |
| K08 | Mentored Clinical Scientist Research Career Development Award | 43 |
| K12 | Mentored Clinical Scientist Development Program Awards | 1 |
| K23 | Mentored Patient-Oriented Research Career Development Award | 20 |
| K24 | Midcareer Investigator Award in Patient-Oriented Research | 9 |
| K25 | Mentored Quantitative Research Development Award | 4 |
| K99/R00 | NIH Pathway to Independence Award | 6 |
| T32 | Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant | 2 |
| R03 | Limited Competition: Small Grant Program for NHLBI K01/K08/K23 Recipients | 6 |
NHLBI = National Heart, Lung, and Blood Institute; NIH = National Institutes of Health.
NHLBI’s Rare Lung Diseases Programs and Clinical Trials
Although most of the NHLBI’s rare disease research grants are funded through applications to parent funding opportunity announcements, several institute-initiated rare diseases research programs are funded by the NHLBI and partnering institutes or centers. These include the Trans-Omics for Precision Medicine (TOPMed) program, the Pulmonary Vascular Disease Phenomics program, and the National Center for Advancing Translational Sciences Rare Diseases Clinical Research Network (RDCRN). Additionally, the NHLBI supports rare lung diseases clinical trials, including the Multicenter Interventional Lymphangioleiomyomatosis Early Disease (MILED) and Clinical Efficacy of Antimicrobial Therapy Strategy Using Pragmatic Design in Idiopathic Pulmonary Fibrosis (CLEAN-UP IPF) studies. These programs and trials, which are subsequently described, are select examples of the NHLBI’s ongoing commitment to supporting research to improve outcomes in patients with rare lung diseases.
The NHLBI-funded TOPMed program is generating a valuable resource to support rare and common heart, lung, blood, and sleep diseases. It is currently composed of a set of whole-genome sequence data from 70 well-phenotyped participant cohorts, along with a growing repository of proteomics, epigenomics, and metabolomics data on the same participants. At present, data from approximately 144,000 participants with heart, lung, blood, or sleep diseases, and healthy control subjects, have been obtained. Importantly, for rare lung diseases, the database includes 1,500 patients with IPF and 1,000 patients with pulmonary sarcoidosis. In addition, the TOPMed database is made up of a diverse population with 40% European, 32% African, 16% Hispanic, and 10% Asian ancestry, which makes it a suitable resource to select different racial and ethnic groups as control subjects in rare diseases studies. Some of the datasets are accessible through Database of Genotypes and Phenotypes, whereas the rest are in the process of being open to the public soon.
The DLD-funded Pulmonary Vascular Disease Phenomics program is a cooperative agreement among seven clinical centers seeking to understand the molecular underpinnings of pulmonary hypertension (PH) in all its forms. Systemic and site-specific multiomics data are being analyzed in context with deep clinical and imaging phenotype characteristics of well over 1,000 patients with PH across the five World Health Organization/World Symposium on Pulmonary Hypertension groups. The overall goal is to develop a more precision medicine-based classification schema that will allow for molecularly targeted use of current therapies and more rapid development of specific, hopefully disease-modifying new treatments.8 In the current World Health Organization/World Symposium on Pulmonary Hypertension classification, both group 1 pulmonary arterial hypertension and group 4 chronic thromboembolic pulmonary hypertension are considered rare lung diseases. In addition, rare lung diseases including IPF and sarcoidosis are significant risk factors for group 3 PH, and patients with PH complicating their rare lung disease often have a poorer prognosis.9
Through two consortia in the RDCRN, the NHLBI supports research activities for several rare lung diseases, including lymphangioleiomyomatosis (LAM), hereditary pulmonary alveolar proteinosis (PAP), Hermansky-Pudlak syndrome, primary ciliary dyskinesia (PCD), idiopathic bronchiectasis, and CF. As one example of a notable finding from the RDCRN’s Genetic Disorders of Mucociliary Clearance Consortium, researchers have discovered that a biallelic mutation in the RSPH1 gene is associated with a milder clinical PCD phenotype and higher nasal nitric oxide levels when compared with patients with PCD with typical ultrastructural defects or mutations in genes commonly associated with PCD.10 As a part of the RDCRN’s RLDC, the National Center for Advancing Translational Sciences and NHLBI cofund the Multi-Center International Durability and Safety of Sirolimus in Lymphangioleiomyomatosis (MIDAS) study, an observational study to determine the risks and benefits of long-term sirolimus therapy for patients with LAM.11 Multicenter Interventional Lymphangioleiomyomatosis Early Disease, a NHLBI-funded clinical trial currently enrolling patients, also uses the RCDRN infrastructure to determine if low-dose long-term sirolimus in patients with LAM with relatively preserved lung function will safely prevent progression of LAM disease.12
As another example of the NHLBI’s leadership in advancing clinical research in rare lung diseases, CLEAN-UP IPF is one of four clinical studies that exist in the Pulmonary Trials Cooperative, a nimble NHLBI-funded consortium design that facilitates real-world, pragmatic research in rare and common lung diseases by using one central Network Management Core to support clinical studies in more than one disease. CLEAN-UP IPF is a randomized phase III trial investigating whether adding antimicrobial therapy consisting of cotrimoxazole or doxycycline to standard IPF therapy can reduce the composite, clinically relevant, primary end point of time to first nonelective respiratory hospitalization or all-cause mortality.13 The premise for the CLEAN-UP IPF trial was derived from a prior NHLBI-funded study titled Correlating Outcomes with biochemical Markers to Estimate Time-progression (COMET), which suggested progression of IPF to be associated with the presence of specific members within the Staphylococcus and Streptococcus genera in the alveolar space.14
Novel Technologies to Study and Treat Rare Lung Diseases
As a part of the NIH’s Demystifying Medicine Series, Dr Francis Collins spoke of 10 dramatic advances he anticipated within the next 10 years (Demystifying Medicine), of which at least four can directly impact rare lung diseases research: single-cell analysis, gene therapy for rare diseases, harnessing CRISPR-Cas and other gene editing tools, and understanding and application of inducible pluripotent stem cells. In the following section, we elaborate on specific NIH/NHLBI programs in these areas that are advancing rare lung diseases research toward definitive therapies.
The NHLBI-funded Molecular Atlas Lung Development Program Consortium was established in 2014 with the overall goal of identifying lung cells with molecular profiles associated with morphology and spatial information during different stages of normal lung development. Numerous lung cell types have been characterized and mapped back to the three-dimensional structure of the lung at each stage of development by using state-of-the-art techniques, including single cell RNA sequencing, cell type-specific proteomics, and high-resolution imaging. Subsequent to its development as a part of Molecular Atlas Lung Development Program, the single cell RNA-sequencing platform was used on lung epithelial cells from healthy control subjects and patients with IPF, leading to identification of a previously not described, indeterminate (C2) cell type preferentially expressed in samples derived from patients with IPF.15 Another recent study reported the cellular heterogeneity at the perinatal stage and the adaptive response of the lung at birth.16 Single cell analysis is a powerful tool to begin to understand heterogeneity of individual molecular profiles, and may be vital to identify and characterize the putative cell(s) involved in the pathogenesis of a specific rare lung disease.
For patients with monogenic lung diseases, technologic advances in gene editing offer novel opportunities to replace missing or defective genes with normal genes. Among monogenic lung diseases, CF is a prime target for gene therapy with improved delivery system to the airway epithelium. The DLD supports a project that has demonstrated successful delivery of peptide nucleic acids, a common gene editing method, to target and correct the delta F508 gene mutation in the nasal epithelium of CF mice and improve nasal potential difference.17 Another DLD-supported project seeks to design mucous-penetrating nanoparticles that can more efficiently reach the airway epithelium, and therefore provide a potential platform for eventual delivery of corrective gene therapy to mitigate CF disease.18 The DLD also oversees a project seeking to identify lung-targeted delivery tools for CRISPR-mediated gene correction as a part of the NIH Common Fund Somatic Cell Genome Editing Program, designed to further develop gene editing tools and bridge knowledge gaps to safely and effectively target disease-relevant cells using organ-specific delivery systems.
Hereditary PAP is a rare lung disease caused by mutations in the CSF2RA or CSF2Rb genes that disrupt granulocyte-macrophage colony-stimulating factor receptor signaling in alveolar macrophages to impair macrophage-mediated surfactant clearance, leading to alveolar accumulation of surfactant and cellular debris and impaired gas exchange.19, 20 Current treatment consists of recurrent whole lung lavage, an invasive procedure to remove the excess surfactant and debris by repeatedly washing the lungs with saline and improve breathing and lung function.21, 22 In seeking to identify a better therapeutic option for hereditary PAP, a DLD-funded project has demonstrated durable efficacy after transplantation of gene-corrected macrophages with functional GM-CSF receptors in a validated PAP animal model.23 Subsequently, the same group transplanted macrophages derived from human inducible pluripotent stem cells into the lungs of humanized PAP mice, where they functioned as primary human alveolar macrophages, leading to reduced alveolar fluid turbidity and improved lung histopathology.24 Using a combination of strategies to deliver gene-corrected macrophages to the lungs may eventually lead to a cure for hereditary PAP.
Even with the promise of technologic advances that can contribute to development of novel therapies, many rare lung diseases are chronic and progressive with the only cure an orthotopic lung transplantation, a resource-limited therapy with an unacceptably high 45% to 50% 5-year mortality rate.25 Many new strategies in lung transplantation have been developed since the first human lung transplant in 196326; however, organ shortage continues to be a problem as the demand for lung transplant continues to exceed the supply of suitable donor lungs.27 The NHLBI is currently supporting clinical trials examining novel approaches to increase viable organ availability through interventions in donor ventilator management28 and ex vivo lung perfusion.29 In addition to funding to increase the availability of suitable donor lungs, a long-term goal is to develop a sustainable treatment that may be less resource constrained by using bioengineering technology to regenerate lung. As one step toward this goal, an NIH-funded study recently demonstrated that adding autologous lung and mesenchymal stromal cells to an acellular scaffold led to successful implantation, growth, structure, and function of a bioengineered lung into a pig.30
Summary
The NHLBI’s DLD oversees research programs encompassing numerous rare lung diseases and is firmly committed to supporting research across the spectrum of basic science to clinical observational studies and interventional trials conducted by investigators at all career stages. Although most rare lung diseases research funded by the DLD stems from investigator-initiated applications in response to parent funding opportunity announcements, several successful institute-initiated rare lung diseases research programs demonstrate the DLD’s sustained commitment toward improving outcomes in rare lung diseases. With the promise of exciting technologic advances, the existence of novel funding opportunities for a cadre of skilled investigators, and the collaborative efforts of relevant advocacy groups and consortia, the goal to accelerate scientific research and improve outcomes in rare lung diseases is being realized.
Acknowledgments
Author contributions: L. J. V. and N. R. A. confirm that the study objectives and procedures are honestly disclosed, and both L. J. V. and N. R. A. personally designed and drafted the manuscript. R. K. and K. B. personally reviewed the data; they are expert in the statistical methods applied for data analysis and confirm that the methods for data extraction and analysis are clearly described. R. K. performed analysis of data extracted from the NIH/ NHLBI database, and K. B. performed the detailed analysis. J. K. and L. A. R. personally contributed to scientific study design, review, and approval of the final manuscript. Both J. K. and L. A. R. confirm that the analysis, methods are well described, and that the results are clearly described.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. J. V., N. R. A., L. A. R., R. K., K. B., and J. K. make public statements related to the subject of the manuscript.
Additional information: Public NIH grant records and Research, Condition, and Disease Categorization data can be obtained from the NIH RePORTER website (https://projectreporter.nih.gov/) and via the NIH RePORT website (https://report.nih.gov/rcdc/), respectively. Under the Freedom of Information Act (FOIA), 5 U.S.C. 552, information on NIH-funded biomedical research grants not publicly available may be formerly requested through the FOIA Coordinator in the Office of Extramural Research at OERFOIA@mail.nih.gov.
Footnotes
Drs Vuga and Aggarwal contributed equally to this manuscript.
References
- 1.National Center for Advancing Translational Sciences. Rare diseases. https://rarediseases.info.nih.gov/diseases
- 2.Schraufnagel DE, ed. Breathing in America: Diseases, Progress, and Hope. https://www.thoracic.org/patients/patient-resources/breathing-in-america/resources/breathing-in-america.pdf. Accessed on June 3, 2019
- 3.Pfizer Pfizer's Rapamune® (SIROLIMUS) becomes first FDA-approved treatment for lymphangioleiomyomatosis (LAM), a rare progressive lung disease. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_s_rapamune_sirolimus_becomes_first_fda_approved_treatment_for_lymphangioleiomyomatosis_lam_a_rare_progressive_lung_disease
- 4.Kiley J.P. Advancing respiratory research. Chest. 2011;140(2):497–501. doi: 10.1378/chest.11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack F., Trapnell B., Sherman S. RLDC 2018 Cincinnati LAMposium. https://guidebook.com/guide/144460 Accessed on June 3, 2019.
- 6.Rubenfeld G.D., Caldwell E., Peabody E. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 7.Herridge M.S., Tansey C.M., Matte A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 8.Newman J.H., Rich S., Abman S.H. Enhancing insights into pulmonary vascular disease through a precision medicine approach. A Joint NHLBI-Cardiovascular Medical Research and Education Fund Workshop Report. Am J Respir Crit Care Med. 2017;195(12):1661–1670. doi: 10.1164/rccm.201701-0150WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose L., Prins K.W., Archer S.L. Survival in pulmonary hypertension due to chronic lung disease: influence of low diffusion capacity of the lungs for carbon monoxide. J Heart Lung Transplant. 2019;38(2):145–155. doi: 10.1016/j.healun.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles M.R., Ostrowski L.E., Leigh M.W. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014;189(6):707–717. doi: 10.1164/rccm.201311-2047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack F, University of Cincinnati . National Institutes of Health; Bethesda, MD: 2015. Safety and durability of sirolimus for treatment of LAM (MIDAS). NCT02432560. ClinicalTrials.gov.http://clinicaltrials.gov/ct2/show/NCT02432560 Updated April 11, 2018. [Google Scholar]
- 12.McCormack F. National Institutes of Health; Bethesda, MD: 2017. University of Cincinnati. Multicenter Interventional Lymphangioleiomyomatosis (LAM) Early Disease Trial (MILED). NCT03150914. ClinicalTrials.gov.http://clinicaltrials.gov/ct2/show/NCT03150914 Updated January 4, 2019. [Google Scholar]
- 13.Weill Medical College of Cornell University . National Institutes of Health; Bethesda, MD: 2016. CleanUP IPF for the Pulmonary Trials Cooperative (CleanUp-IPF). NCT02759120. ClinicalTrials.gov.http://clinicaltrials.gov/ct2/show/NCT02759120 Updated January 21, 2019. [Google Scholar]
- 14.Han M.K., Zhou Y., Murray S. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med. 2014;2(7):548–556. doi: 10.1016/S2213-2600(14)70069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y., Mizuno T., Sridharan A. Single-cell RNA sequencing identifies diverse roles of epithelial cells in idiopathic pulmonary fibrosis. JCI Insight. 2016;1(20):e90558. doi: 10.1172/jci.insight.90558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo M., Du Y., Gokey J.J. Single cell RNA analysis identifies cellular heterogeneity and adaptive responses of the lung at birth. Nat Commun. 2019;10(1):37. doi: 10.1038/s41467-018-07770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeer N.A., Anandalingam K., Fields R.J. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat Commun. 2015;6:6952. doi: 10.1038/ncomms7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastorakos P., da Silva A.L., Chisholm J. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A. 2015;112(28):8720–8725. doi: 10.1073/pnas.1502281112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T., Sakagami T., Rubin B.K. Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med. 2008;205(12):2703–2710. doi: 10.1084/jem.20080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki T., Maranda B., Sakagami T. Hereditary pulmonary alveolar proteinosis caused by recessive CSF2RB mutations. Eur Respir J. 2011;37(1):201–204. doi: 10.1183/09031936.00090610. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A., Abdelmalak B., Inoue Y., Culver D.A. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir Med. 2018;6(7):554–565. doi: 10.1016/S2213-2600(18)30043-2. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T., Sakagami T., Young L.R. Hereditary pulmonary alveolar proteinosis: pathogenesis, presentation, diagnosis, and therapy. Am J Respir Crit Care Med. 2010;182(10):1292–1304. doi: 10.1164/rccm.201002-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki T., Arumugam P., Sakagami T. Pulmonary macrophage transplantation therapy. Nature. 2014;514(7523):450–454. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Happle C., Lachmann N., Ackermann M. Pulmonary transplantation of human induced pluripotent stem cell-derived macrophages ameliorates pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2018;198(3):350–360. doi: 10.1164/rccm.201708-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young K.A., Dilling D.F. The future of lung transplantation. Chest. 2019;155(3):465–473. doi: 10.1016/j.chest.2018.08.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy J.D., Webb W.R., Dalton M.L., Jr., Walker G.R., Jr. Lung homotransplantation in man. JAMA. 1963;186:1065–1074. doi: 10.1001/jama.1963.63710120001010. [DOI] [PubMed] [Google Scholar]
- 27.Valapour M., Lehr C.J., Skeans M.A. OPTN/SRTR 2016 annual data report: lung. Am J Transplant. 2018;18(suppl 1):363–433. doi: 10.1111/ajt.14562. [DOI] [PubMed] [Google Scholar]
- 28.Ware L.B., Vanderbilt University Medical Center . National Institutes of Health; Bethesda, MD: 2018. Goal of Open Lung Ventilation in Donors (GOLD). NCT03439995. ClinicalTrials.gov.http://clinicaltrials.gov/ct2/show/NCT03439995 Updated August 16, 2018. [Google Scholar]
- 29.Lau C, University of Virginia . National Institutes of Health; Bethesda, MD: 2017. Study to evaluate adenosine 2A receptor agonist (Regadenoson) in patients undergoing lung transplantation. NCT03072589. ClinicalTrials.gov.http://clinicaltrials.gov/ct2/show/NCT03072589 Updated April 17, 2019. [Google Scholar]
- 30.Nichols J.E., La Francesca S., Niles J.A. Production and transplantation of bioengineered lung into a large-animal model. Sci Transl Med. 2018;10(452) doi: 10.1126/scitranslmed.aao3926. [DOI] [PubMed] [Google Scholar]