Abstract
Background
The diagnosis of sarcoidosis is made by the combination of clinical features and biopsy results. The clinical features of sarcoidosis can be quite variable. We developed a Sarcoidosis Diagnostic Score (SDS) to summarize the clinical features of patients with possible sarcoidosis.
Methods
Biopsy-confirmed patients with sarcoidosis seen during a 7-month period at the University of Cincinnati sarcoidosis clinic were prospectively identified. Patients with nonsarcoidosis seen at the same clinic were used as control patients. Using a modified World Association of Sarcoidosis and Other Granulomatous Disorders organ assessment instrument, we scored all patients for presence of biopsy, ≥1 highly probable symptom, and ≥1 at least probable symptom for each area. Two sarcoidosis scores were generated: SDS biopsy (with biopsy) and SDS clinical (without biopsy).
Results
The 980 evaluable patients were divided into two cohorts: an initial 600 patients (450 with biopsy-confirmed sarcoidosis, 150 control patients) to establish cutoff values for SDS biopsy and an SDS clinical and a validation cohort of 380 patients (103 biopsy-confirmed patients with sarcoidosis and 277 control patients). The best cutoff value for SDS biopsy was ≥ 6 (sensitivity, 99.3%; specificity, 100%). For the total the 980 patients, an SDS clinical ≥ 3 had a sensitivity of 90.6%, specificity of 88.5%, and a likelihood ratio of 7.9. An SDS clinical score ≥ 4 had a lower sensitivity of (76.9%) but higher specificity (98.6%).
Conclusions
For sarcoidosis, the presence of specific clinical features, especially multiorgan involvement, can enhance the diagnostic certainty. The SDS scoring system quantitated the clinical features consistent with sarcoidosis.
Key Words: biopsy, diagnosis, epidemiology, sarcoidosis
Abbreviations: AUC, area under the curve; ILD, interstitial lung disease; NPV, negative predictive value; OLD, obstructive lung disease; PPV, positive predictive value; ROC, receiver operating characteristic; SDS, Sarcoidosis Diagnostic Score; WASOG, World Association of Sarcoidosis and Other Granulomatous Disorders
FOR EDITORIAL COMMENT, SEE PAGE 1006
The American Thoracic Society statement on sarcoidosis states: “The diagnosis is established when clinicoradiological findings are supported by histological evidence of noncaseating epithelioid cell granulomas. Granulomas of known causes and local sarcoid reactions must be excluded.”1 This definition points to the complementary information of clinical information and pathologic findings. Several authors have noted that the finding of a granuloma is insufficient to make the diagnosis of sarcoidosis.2, 3, 4 In some cases, the presence of some clinical features may be sufficient to make the diagnosis.1, 4 The clinical features of sarcoidosis are diverse, with multiple organ involvement commonly encountered,5, 6, 7 and with more organ involvement increasing the confidence in the diagnosis.3, 4
Criteria for individual organ involvement have been proposed.8, 9 The World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) recently developed criteria for sarcoidosis organ involvement as “highly probable,” “at least probable,” and “possible.”8 The WASOG instrument provides a structured system for identifying the clinicoradiologic findings in patients with sarcoidosis and may standardize organ involvement reporting in patients with sarcoidosis.10
We tested whether the WASOG organ involvement criteria could be used to develop a Sarcoidosis Diagnostic Score (SDS). The SDS could be calculated in two scenarios: a patient with positive biopsy results (SDS biopsy) or a patient with clinical criteria and no biopsy (SDS clinical). We developed the SDS scoring system using a cohort of patients seen prospectively at the University of Cincinnati sarcoidosis clinic and subsequently validated the SDS scores using a second cohort of patients.
Methods
This was a prospective study of patients seen at the University of Cincinnati Medical Center Sarcoidosis and Interstitial Lung Disease Clinic from January 4, 2017, to July 28, 2017. Patients were diagnosed with sarcoidosis on the basis of clinical features consistent with sarcoidosis and ≥1 biopsy demonstrating noncaseating granuloma with no alternative diagnosis identified.11 A control population consisted of patients with nonsarcoidosis who presented with signs or symptoms consistent with sarcoidosis, but in whom an alternative diagnosis was confirmed. For the control group, patients were classified as: interstitial lung disease (ILD), ocular inflammation (eye), obstructive lung disease (OLD), or other. Patients were referred for ILD, including 44 with an underlying autoimmune disease. Patients referred to the sarcoidosis clinic for ocular inflammation included those with uveitis (anterior, intermediate, and posterior) as well as episcleritis and optic neuritis. Three of the patients in the “other” group had granulomatous infections resulting from active histoplasmosis. None had active TB. Although these conditions can be due to sarcoidosis, these patients were found to have an alternative diagnosis. Information for each patient included age, self-declared race, sex, age at diagnosis, and diagnostic group. Data was stored on Research Electronic Data Capture).12 The protocol has been approved by the University of Cincinnati institutional review board (protocol number 2013-3320).
At each clinic visit, all patients underwent evaluation by either R. P. B. or E. E. L. for organ involvement or calcium metabolism abnormalities using the WASOG sarcoidosis organ instrument (e-Table 1).8 Organ involvement was classified as biopsy positive, highly probable, at least probable, or possible. We added two additional items, Lofgren syndrome and elevated alkaline phosphatase, to the WASOG instrument. Lofgren syndrome (hilar adenopathy with erythema nodosum or periarticular arthritis13) was considered the same as a positive biopsy. An alkaline phosphatase ≥ 3 times the upper limit of normal without other etiology was considered at least probable for liver involvement.9 No additional points were awarded for more than one manifestation in the individual category for each organ.
Patients were divided into two groups. An initial cohort was studied to establish the various weights for each category. A separate group of patients also seen in the clinic during this time served as the validation cohort.
For each patient, the totals from each category were summed and two different scores were created. A score that included the biopsy results was the SDS biopsy score; the score without biopsy was the SDS clinical. The SDS biopsy and SDS clinical scores were compared between the patients with sarcoidosis and the control patients and a cutoff value was determined to separate these two groups.
Statistics
Comparisons were made using nonparametric testing, including χ2, Kruskal-Wallis, and Mann-Whitney U test for unpaired samples. Receiver operator curves (ROC) were generated and the sensitivity and specificity for each were determined. The area under the curve (AUC), the SD along with the positive predictive value (PPV) and negative predictive value (NPV) were calculated. The best point system was determined on the basis of comparison of other point schemes. The likelihood ratios and OR were determined for the combined population. Statistical analysis was performed using MedCalc Statistical Software, version 16.2.1 (MedCalc Software bvba).
Results
The initial cohort consisting of 600 patients included 450 patients with biopsy-confirmed sarcoidosis and 150 patients with other diseases similar to sarcoidosis. Table 1 describes the demographics of each patient population. Patients with eye disease and those with sarcoidosis experienced an earlier onset of disease diagnosis (P < .00001) compared with those with ILD, OLD, and other groups. There was also a difference in race among the five categories (P < .00001), with a higher proportion of blacks in the eye group compared with the other four categories; however, there was no significant sex difference among the groups.
Table 1.
Demographic Features of Initial Cohort
| Demographics | Sarcoidosis | Control Patients |
Total | |||
|---|---|---|---|---|---|---|
| ILDa | Eye | OLD | Othera | |||
| No. | 450 (75.0) | 61 (10.2) | 16 (2.7) | 45 (7.5) | 28 (4.7) | 600 |
| Age at diagnosis, mean ± SD, yb | 45 ± 11.4 | 67.07 ± 10.77 | 46 ± 14.6 | 64 ± 16.6 | 67 ± 12.3 | 49 ± 14.9 |
| Blackb | 198 (44.0) | 5 (8.2) | 10 (62.5) | 8 (17.8) | 1 (3.6) | 222 (37.0) |
| White | 251 (55.8) | 50 (82.0) | 6 (37.5) | 35 (77.8) | 27 (96.4) | 369 (61.5) |
| Asian | 1 (0.2) | 4 (6.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (0.83) |
| Race not recorded | 0 (0.0) | 2 (3.3) | 0 (0.0) | 2 (4.4) | 0 (0.0) | 4 (0.67) |
| Women | 292 (64.9) | 31 (50.8) | 12 (75.0) | 31 (68.9) | 13 (46.4) | 379 (63.2) |
| Patients with ≥ 1 possible criterion | 450 (100.0) | 56 (91.8) | 16 (100.0) | 43 (95.6) | 20 (71.4) | 585 (97.5) |
Data are presented as No. (%) unless otherwise indicated. ILD = interstitial lung disease; OLD = obstructive lung disease.
Eye: ocular disease; other: bronchiectasis, pulmonary nodules, pneumonia, connective tissue disease, malignancy, pleural effusions, vasculitis, and unexplained dyspnea.
Difference between groups, P < .00001.
The control patients had been referred to the clinic for the possible diagnosis of sarcoidosis, most with signs included in the WASOG instrument. More than 95% of the control population (range, 71%-100%) experienced at least one possible feature consistent with sarcoidosis. This included highly probable symptoms, at least probable symptoms, and possible symptoms related to sarcoidosis.
Table 2 documents the prevalence of individual organ/manifestations of the 450 patients with sarcoidosis. For each manifestation, the total column indicates the percentage of patients with sarcoidosis with either biopsy confirmed, highly probable, or at least probable criteria. We did not include the possible category because we subsequently determined that the possible sarcoidosis criteria were not used in either the SDS biopsy or SDS clinical scores.
Table 2.
Organ Involvement/Manifestations for Patients With Sarcoidosis in Initial Cohorts
| Organ Involvement/Manifestation | Biopsy | Highly Probable | At Least Probable | Total Having ≥ 1 Feature |
|---|---|---|---|---|
| Lung | 296 (65.8) | 294 (65.3) | 148 (32.9) | 386 (85.8) |
| Skin | 80 (17.8) | 30 (6.7) | 112 (24.9) | 133 (29.6) |
| Eye | 11 (2.4) | 101 (22.4) | 22 (4.9) | 116 (25.8) |
| Liver | 33 (7.3) | NCE | 61 (13.6) | 61 (13.6) |
| Hypercalcemia/hypercalciuria/nephrolithiasis | NCE | 54 (12.0) | 3 (0.7) | 56 (12.4) |
| Extrathoracic lymph node | 45 (10.0) | NCE | 53 (11.8) | 53 (11.8) |
| Neurologic | 6 (1.3) | 31 (6.9) | 21 (4.7) | 51 (11.3) |
| Spleen | 3 (0.7%) | NCE | 37 (8.2) | 37 (8.2) |
| Cardiac | 0 (0.0) | NCE | 36 (8.0) | 36 (8.0) |
| Bones/joints | 9 (2.0) | 3 (0.7) | 18 (4.0) | 19 (4.2) |
| Ear/nose/throat | 13 (2.9) | NCE | 12 (2.7) | 12 (2.7) |
| Parotid/salivary | 2 (0.4) | 0 (0.0) | 7 (1.6) | 7 (1.6) |
| Bone marrow | 11 (2.4) | 6 (1.3) | NCE | 6 (1.3) |
| Renal | 7 (1.6) | NCE | 5 (1.1) | 5 (1.1) |
| Musclesa | 0 (0.0) | NCE | 0 (0.0) | 0 (0.0) |
| Lofgren syndrome | NCE | 15 (3.3) |
Data are presented as No. (%) unless otherwise indicated. NCE = no criterion established.
One patient with possible muscle disease.
The most common manifestation, lung involvement, was seen in 85% of patients with sarcoidosis, with the second most common phenotypes, cutaneous and ocular, each being seen in about one-quarter of patients. More than 10% of patients experienced involvement from either liver, extrathoracic lymph nodes, neurologic system, or hypercalcemia/hypercalciuria/nephrolithiasis.
Biopsy-confirmed sites for the initial 450 patients with cohort included lungs, skin, and extrathoracic lymph nodes. Highly probable and at least probable symptoms for the lung and skin were the most common criteria noted.
To develop the SDS clinical score, we assigned variable weights to each of the different criteria of highly probable, at least probable, and possible. Table 3 reveals the sensitivity, specificity, PPV, NPV, SE, and AUC for various values in each category. The combinations of 3, 2, and 0 and 4, 3, 0 were associated with both the highest and same values for PPV, NPV, and AUC. These scores had the same PPV, NPV, and AUC because there was no further reclassification of patients using either the two weighing factors.
Table 3.
Comparison of Weights for SDS Clinical
| Highly Probable |
At Least Probable |
Possible | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC | SE | Score ≥ |
|---|---|---|---|---|---|---|---|---|---|
| 4 | 3 | 0 | 89.80 | 88.00 | 95.74 | 74.20 | 0.954 | 0.00785 | 4 |
| 3 | 2 | 0 | 89.80 | 88.00 | 95.74 | 74.20 | 0.954 | 0.00785 | 3 |
| 4 | 2 | 0 | 89.80 | 88.00 | 95.74 | 74.20 | 0.951 | 0.00817 | 3 |
| 2 | 1 | 0 | 89.80 | 88.00 | 95.74 | 74.20 | 0.951 | 0.00817 | 2 |
| 5 | 3 | 1 | 89.80 | 87.30 | 95.50 | 74.05 | 0.953 | 0.00861 | 5 |
| 4 | 2 | 1 | 89.80 | 87.30 | 95.50 | 74.05 | 0.949 | 0.00905 | 4 |
| 5 | 2 | 1 | 89.80 | 87.30 | 95.50 | 74.05 | 0.947 | 0.00921 | 4 |
| 6 | 2 | 1 | 89.80 | 87.30 | 95.50 | 74.05 | 0.946 | 0.00936 | 4 |
| 4 | 3 | 2 | 82.40 | 93.30 | 97.36 | 63.86 | 0.940 | 0.0102 | 6 |
| 3 | 2 | 1 | 82.40 | 93.30 | 97.36 | 63.86 | 0.948 | 0.00917 | 4 |
Bold numbers indicate the proposed weights for further studies.
AUC = area under the curve; NPV = negative predictive value; PPV = positive predictive value.
Likewise, the SDS biopsy score was determined by varying the weights of biopsy, highly probable, at least probable, and possible. Table 4 shows the sensitivity, specificity, PPV, NPV, SE, and AUC for various values for each category. With SDS biopsy scores > 6, high sensitivity and specificity occurred in two combinations: 8, 3, 2, 0 and 7, 3, 2, 0. These two combinations relied exclusively on the biopsy result; therefore the sensitivity, specificity, PPV, and NPV were the same. The final score of 5, 3, 2, and 0 with a cutoff ≥ 6 required clinical as well as pathology findings to diagnose sarcoidosis. The score of 5, 3, 2, and 0 had a lower cutoff value than that for 5, 4, 3, and 0. We assigned for both the SDS clinical and SDS biopsy 3 points for highly probable, 2 points for at least probable, and 0 points for possible.
Table 4.
Comparison of Weights for SDS Biopsy
| Biopsy | Highly Probable | At Least Probable | Possible | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC | SE | Score ≥ |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 3 | 2 | 0 | 99.30 | 100.00 | 100.00 | 97.94 | 1.000 | 0.0000675 | 6 |
| 5 | 4 | 3 | 0 | 99.30 | 100.00 | 100.00 | 97.94 | 1.000 | 0.000161 | 8 |
| 6 | 3 | 2 | 0 | 100.00 | 100.00 | 100.00 | 100.00 | 1.000 | 0.000 | 6 |
| 6 | 4 | 3 | 0 | 99.30 | 100.00 | 100.00 | 97.94 | 1.000 | 0.000146 | 8 |
| 7 | 3 | 2 | 0 | 100.00 | 100.00 | 100.00 | 100.00 | 1.000 | 0.000 | 6 |
| 7 | 4 | 3 | 0 | 99.30 | 100.00 | 100.00 | 97.94 | 1.000 | 0.0000675 | 8 |
| 8 | 3 | 2 | 0 | 100.00 | 100.00 | 100.00 | 100.00 | 1.000 | 0.000 | 6 |
| 8 | 4 | 3 | 0 | 100.00 | 100.00 | 100.00 | 100.00 | 1.000 | 0.000 | 8 |
Bold numbers indicate the proposed weights for further studies.
See Table 3 legend for expansion of abbreviations.
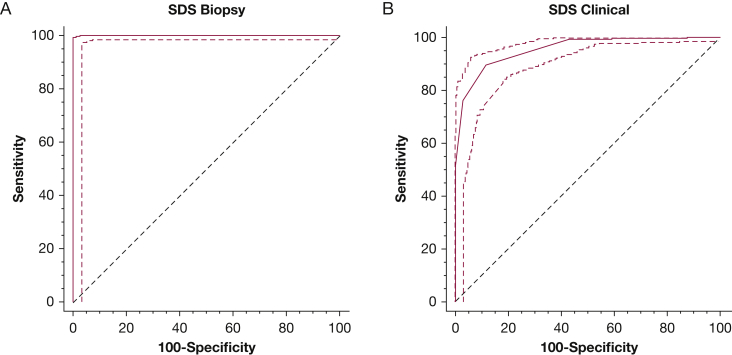
Figure 1 presents ROC used to determine the sarcoidosis category values for the initial population. For the SDS clinical score, patients with a score ≥ 3 experienced sarcoidosis with a sensitivity of 89.8%, specificity of 88.0%, and AUC of 0.954. For the SDS biopsy score, patients with a score ≥ 6 were diagnosed with sarcoidosis with a sensitivity of 99.3%, specificity of 100%, and AUC of 1.000.
Figure 1.
The receiver operator curve with 95% CI for (A) SDS biopsy and (B) SDS clinical. A, Using 6 points led to a sensitivity of 99.3%, specificity of 100%, and an AUC of 1.000. B, Using 3 points led to a sensitivity of 89.8%, specificity of 88.0%, and an AUC of 0.954. AUC = area under the curve; SDS = Sarcoidosis Diagnostic Score.
Next, a validation cohort of 380 patients, including 103 patients with sarcoidosis and 277 patients with nonsarcoidosis, was studied. Table 5 represents the demographics of these patients. The validation cohort was similar to the initial cohort except that only 83% of the control patients in the validation cohort had at least one possible symptom compared with 90% for the initial cohort. Using the assigned points of 5 for biopsy, 3 for highly probable, 2 for at least probable, and 0 for possible, we calculated the SDS clinical and SDS biopsy scores. The ROC calculations determined the same cutoff as the initial cohort. For SDS biopsy, the ROC identified 5 as the cutoff with both 100% sensitivity and specificity and the AUC was 1.000. For SDS clinical, the ROC again identified 3 as the cutoff, with a sensitivity of 94.2%, specificity of 88.8%, and AUC of 0.977.
Table 5.
Demographic Features of Validation Cohort
| Demographics | Sarcoidosis | Control Patients |
Total | |||
|---|---|---|---|---|---|---|
| ILD | Eye | OLD | Other | |||
| No., mean ± SD | 103 (27.1) | 78 (20.5) | 34 (8.9) | 141 (37.1) | 24 (6.3) | 380 |
| Age, mean ± SD | 45.51 ± 12.12 | 61.90 ± 15.63 | 44.21 ± 15.92 | 64.88 ± 13.78 | 59.17 ± 15.81 | NA |
| Black | 37 (35.9) | 12 (15.4) | 17 (50.0) | 15 (10.6) | 1 (4.2) | 82 (21.6) |
| White | 65 (63.1) | 63 (80.8) | 15 (44.1) | 118 (83.7) | 21 (87.5) | 282 (74.2) |
| Asian | 1 (1.0) | 0 (0.0) | 1 (2.9) | 1 (0.7) | 0 (0.0) | 3 (0.8) |
| Race not recorded | 0 (0.0) | 3 (3.8) | 1 (2.9) | 7 (5.0) | 2 (8.3) | 13 (3.4) |
| Women | 69 (67.0) | 52 (66.7) | 24 (70.6) | 89 (63.1) | 13 (54.2) | 247 (65.0) |
| Men | 34 (33.0) | 26 (33.3) | 10 (29.4) | 52 (36.9) | 11 (45.8) | 133 (35.0) |
| Patients with ≥ 1 possible criterion | 105 (100) | 70 (90.9) | 33 (100) | 116 (82.3) | 12 (50.0) | 336 (88.4) |
Data are presented as No. (%) unless otherwise indicated. NA = not available. See Table 3 legend for expansion of other abbreviations.
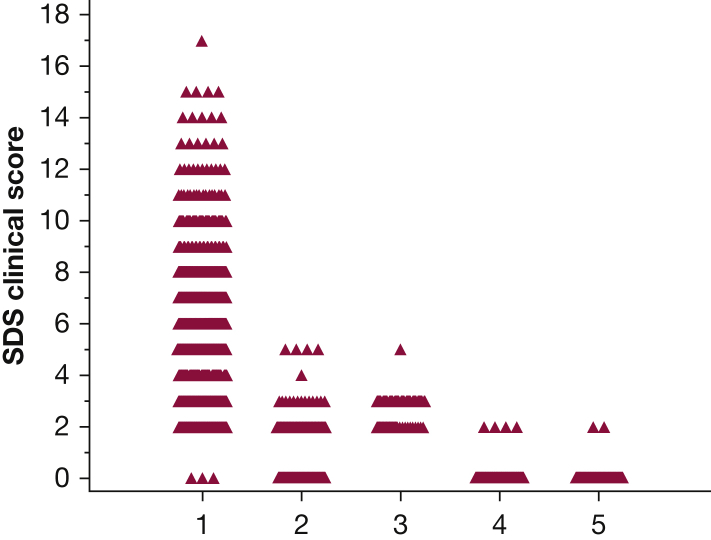
Combining the initial and validation cohort’s SDS clinical scores provided a total population of 553 patients with sarcoidosis and 427 control patients seen at least once during the 7 months of the study. Table 6 summarizes the mean and median scores for all five groups. Figure 2 demonstrates individual SDS clinical scores for all five groups. As can be seen, there were only five patients with nonsarcoidosis having an SDS clinical score of ≥ 4. An SDS clinical score of ≥ 3 had a sensitivity of 90.6% and specificity of 88.5%. An SDS clinical score ≥ 4 had a sensitivity of 76.9% and specificity of 98.6%. Table 7 summarizes the ORs and likelihood ratios for a patient having sarcoidosis using the calculated SDS clinical scores of 3, 4, 5, and 6. The higher the SDS clinical score, the higher the OR and likelihood ratio.
Table 6.
Summary of SDS Clinical and SDS Biopsy for the Whole Group
| Underlying Diagnosis | No. | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| SDS clinical | ||||||
| Sarcoidosis | 553 | 6.052 | 2.9246 | 5 | 0 | 17 |
| ILD | 139 | 1.604 | 1.1894 | 2 | 0 | 5 |
| Eye | 50 | 2.66 | 0.5928 | 3 | 2 | 5 |
| OLD | 186 | 0.043 | 0.2909 | 0 | 0 | 2 |
| Other | 52 | 0.0769 | 0.3884 | 0 | 0 | 2 |
| SDS biopsy | ||||||
| Sarcoidosis | 553 | 11.848 | 3.8106 | 11 | 5 | 29 |
| ILD | 139 | 1.604 | 1.1894 | 2 | 0 | 5 |
| Eye | 50 | 2.66 | 0.5928 | 3 | 2 | 5 |
| OLD | 186 | 0.043 | 0.2909 | 0 | 0 | 2 |
| Other | 52 | 0.0769 | 0.3884 | 0 | 0 | 2 |
See Table 3 legend for expansion of abbreviations.
Figure 2.
The individual SDS clinical scores for sarcoidosis, interstitial lung disease, eye, obstructive lung disease, or other groups. See Figure 2 legend for expansion of abbreviation.
Table 7.
OR and LR of Various SDS Clinical Scores
| SDS Clinical Score | OR | CI | LR |
|---|---|---|---|
| 3 | 74.3 | 49.2-112.3 | 7.9 |
| 4 | 233.0 | 101.6-534.1 | 54.7 |
| 5 | 203.7 | 82.8-501.3 | 60.4 |
| 6 | 839.7 | 52.2-13514.6 | 424.1 |
LR = likelihood ratio. See Table 3 legend for expansion of other abbreviation.
The control eye group of 50 patients referred to our clinic for possible sarcoidosis and were found to have alternative diagnosis including Vogt-Koyanagi-Harada, multiple sclerosis–associated uveitis, Bechet disease, Cogan syndrome, serpiginous choroiditis, HLA-B27 disease, Crohn disease, and idiopathic retinitis, optic neuritis, pars planitis, or pan uveitis. Forty-three of the patients in the ocular group were found to have an SDS clinical score of 3. In the WASOG instrument, uveitis is highly probable and awarded 3 points; however, only four eye patients experienced scores > 3: patient 817 had diffuse infiltrates (2 points) along with uveitis (3 points); patient 310 had uveitis and optic neuritis (3 points) along with scleritis (2 points); patient 28 had uveitis (3 points) and retinitis (2 points); and patient 36 had uveitis (3 points) and nodular tenosynovitis (2 points).
There were 139 total patients in the ILD group. None had any features consistent with highly probable lung involvement. Of the at least probable category, 89 had diffuse lung disease and 9 had upper lobe fibrosis. Subpleural honeycombing and/or traction bronchiectasis, which are not criteria in the WASOG instrument, was identified by radiology in 70 patients. For the ILD group, only two patients were awarded SDS clinical scores > 3: patient 49 had diffuse infiltrates (2 points) and spontaneous or inducible sustained venous thrombosis with no other risk factors (2 points) and patient 597 had diffuse infiltrates (2 points) along with optic neuritis (3 points).
Discussion
The diagnosis of sarcoidosis can be difficult for the clinician. Even in the setting of a biopsy revealing noncaseating granulomas, the clinical history and the exclusion of other granulomatous diseases are additional key ingredients. Teasing out a compatible clinical history of sarcoidosis can be challenging. The WASOG organ assessment tool was developed by consensus of an expert panel and provides a standardized formula that can categorize clinical findings into highly probable, at least probable, and possible groups. This investigation used these categories to generate an SDS for both a patient with biopsy consistent with sarcoidosis and a patient without a biopsy but compatible clinical findings. Sensitivity and specificity were enhanced with biopsy results. Although the presence of a granuloma is highly supportive of the diagnosis of sarcoidosis, other conditions such as TB and lymphoma can cause granulomas. The identification of a compatible clinical presentation increases the certainty of the diagnosis of sarcoidosis. Our cutoff of ≥ 6 meant that patients were not diagnosed on the basis of biopsy results alone. For the SDS clinical, a score ≥ 3 was only 88%. An SDS clinical score of ≥ 4 was associated with a higher specificity (98%), but lower sensitivity (77%).
This study evaluated a large number of patients seen at a tertiary referral sarcoidosis clinic in the United States. The age, sex, and reported race are similar to that reported in a prior study of 10 American centers.14 Approximately 45% of the patients were black, which is lower than that reported from another large American sarcoidosis clinic6; however, the percentage of black patients is compatible with a sarcoidosis clinic in the eastern United States.15 The proportion of organ involvement is also similar to previous large reports from US sarcoidosis clinics6; however, the results of this instrument may not be applicable to other countries, where the manifestations of sarcoidosis are different.16
The control group included patients referred to our center to be evaluated for possible sarcoidosis and in whom we were confident of a final diagnosis that was not sarcoidosis. As demonstrated in Table 1, the patients in the various control groups were similar to the patients with sarcoidosis. In addition, most had at least one feature suggesting sarcoidosis. In particular, a significant proportion of the patients with ILD had underlying autoimmune diseases, leading in some cases to extrapulmonary symptoms. Our clinic also evaluated a large number of patients who presented with ocular inflammation, in whom sarcoidosis is only one of the many possible causes. The SDS performed well for identifying patients with sarcoidosis vs other causes of ILD or ocular inflammation.
The ILD group included various other ILDs, such as idiopathic pulmonary fibrosis, connective tissue disease-associated pulmonary fibrosis, and nonspecific ILD. Many of the patients had features consistent with at least probable lung involvement, including diffuse infiltrates or upper lobe fibrosis. Subpleural honeycombing and/or traction bronchiectasis were identified in more than one-half of the ILD group. Subpleural honeycombing is seen in < 5% of patients with pulmonary sarcoidosis.17 Honeycombing, traction bronchiectasis, and basilar disease are not features included in the WASOG instrument. We also studied 186 patients with OLD, mostly asthma and chronic obstructive lung disease. OLD commonly occurs in sarcoidosis.18 In patients eventually diagnosed with sarcoidosis, delay in diagnosis has been attributed to signs and symptoms of OLD.19 Airway obstruction was rated as a possible diagnostic criterion for sarcoidosis in the WASOG organ assessment.20 We also evaluated a group of patients with chronic ocular inflammation who were referred for possible sarcoidosis; these patients were found to have an alternative diagnosis that has to be considered in patients with possible sarcoidosis.21
The American Thoracic Society statement is frequently cited to provide criteria for the diagnosis of sarcoidosis14, 22, 23; however, the statement is vague regarding clinical criteria for the diagnosis. To provide specific criteria for clinical features supportive of the diagnosis of sarcoidosis, we used the WASOG organ assessment instrument.8 Although others have provided specific examples of manifestations of sarcoidosis,3 these reports were mainly isolated examples without in-depth phenotypic evaluation. Specific diagnostic guidelines have been created for some organ systems, including eye,24 neurologic,25 and cardiac.26 Most of the criteria established in these documents are captured in the WASOG organ assessment instrument.
Our investigation incorporates two criteria not included in the WASOG instrument. The first was an elevated alkaline phosphatase ≥ 3 three times upper limit of normal with no other cause identified. This laboratory abnormality was included in the original A Case Control Etiologic Study of Sarcoidosis instrument.9 The other criterion was Lofgren syndrome consisting of bilateral hilar adenopathy and erythema nodosum and/or periarticular swelling.27 Lofgren syndrome was not included in the WASOG instrument because it was not specific for a single organ manifestation. Most experts consider Lofgren syndrome diagnostic for sarcoidosis.2, 14 In this study, we scored the presence of Lofgren syndrome the same as a positive biopsy. For patients with a positive biopsy or Lofgren syndrome, the presence of another clinical manifestation is highly supportive of the diagnosis.
For the initial cohort, there were several SDS scores with the same sensitivity, specificity, PPV, and NPV for both SDS clinical and SDS biopsy (Tables 3 and 4) that occurred because changes in the weighing resulted in reclassification of cases. For simplicity, we chose to use the same weight for highly probable (3) and at least probable (2) for both SDS clinical and SDS biopsy. When we analyzed the validation group, we found these weights confirmed what we found in the initial cohort.
The SDS clinical score may help the clinician in two ways. During initial evaluation, the presence of a SDS clinical score ≥ 3 will encourage the clinician to seek a biopsy to increase the confidence level regarding the diagnosis. For example, in a patient with ILD, the presence of extrapulmonary manifestation may lead to a bronchoscopy rather than a surgical lung biopsy. Likewise, clinicians may be more confident of the diagnosis of sarcoidosis in patients with a high SDS clinical score despite that a biopsy of an affected organ cannot be easily obtained. For example, patients referred for ocular inflammation or neurologic disease are often referred to our clinic to rule out sarcoidosis. Often, one has to rely on clinical presentation alone; however, one should be careful for those with SDS clinical scores of 3. For example, patients with uveitis may have score of 3 on the basis of presence of uveitis alone, but have another cause of their uveitis. The presence of extraocular disease (and a higher SDS clinical score) will enhance the certainty of the diagnosis. Further, multicenter studies should be performed to confirm that patients with an SDS clinical score of ≥ 4 have a high likelihood of sarcoidosis and may not need a biopsy.
The current study has several weaknesses. Patients were seen and organ involvement was scored by one of two experienced sarcoidologists (R. P. B. or E. E. L.) at one institution. It is unclear how well this instrument would perform when used at multiple centers and by less experienced health care providers. In addition, specific organ manifestations require specialized testing. Liver and spleen involvement may only be detected with abdominal imaging. Also, treatment may lessen the size or activity of lesion, making it undetectable. Although this study may help the clinician diagnose most cases of sarcoidosis, it is not sensitive enough to distinguish important sarcoidosis mimics such as berylliosis and immune deficiency syndrome.28, 29 We also did not evaluate any patients with active TB during our study. Because we limited the control groups to those in whom a specific diagnosis had been made, the proportion of patients with sarcoidosis studied is quite high (more than one-half of the cases in the study). This has a major effect on PPV and NPV. The SDS would be expected to be less specific if the proportion of patients with sarcoidosis studied was smaller; therefore, the value of the SDS clinical as a screening tool is most effective in those patients with an increased pretest probability for sarcoidosis. The likelihood ratio avoids some of the issues about underlying prevalence of disease; however, it has its limitations as well.30, 31
The SDS score may be improved with additional information. This includes the results of biomarkers such as an angiotensin-converting enzyme32 and chitotriosidase.33 Although no individual biomarker has been shown diagnostic for sarcoidosis, biomarkers may provide additional information using the SDS scoring system. Likewise, other features, such as duration of disease and response to antiinflammatory therapy, may also enhance the sensitivity and specificity of the SDS score. We were interested in developing a score that could be applied at time of diagnosis. The use of follow-up information may be useful to validate the SDS score. Other diagnostic tests such as BAL, endobronchial ultrasound, and PET scanning are already incorporated in the WASOG instrument.
In conclusion, we have developed the SDS, which standardizes the reporting of clinical disease manifestations. By calculating the score, one can determine the likelihood of the diagnosis of sarcoidosis. The SDS clinical provides a score made on the basis of the clinical features alone. The SDS biopsy adds the information from biopsy material and leads to a more specific diagnosis of sarcoidosis.
Acknowledgments
Author contributions: All authors were involved in designing the study. All have contributed to writing and reviewing manuscript and seen the final version. A. N. B. performed the data entry. R. P. B. and A. N. B. performed data analysis. R. P. B. was responsible for the overall study and maintaining the original data for this study.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Table can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Partial support was provided by National Institutes of Health [Grant 1UL1TR001425-01].
Supplementary Data
References
- 1.Statement on sarcoidosis. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Baughman R.P., Culver D.A., Judson M.A. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183(5):573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Judson M.A. The diagnosis of sarcoidosis. Clin Chest Med. 2008;29(3):415–427. doi: 10.1016/j.ccm.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Valeyre D., Bernaudin J.F., Uzunhan Y. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Respir Crit Care Med. 2014;35(3):336–351. doi: 10.1055/s-0034-1381229. [DOI] [PubMed] [Google Scholar]
- 5.Baughman R.P., Judson M.A., Teirstein A.S. Presenting characteristics as predictors of duration of treatment for sarcoidosis. QJM. 2006;99(5):307–315. doi: 10.1093/qjmed/hcl038. [DOI] [PubMed] [Google Scholar]
- 6.Judson M.A., Boan A.D., Lackland D.T. The clinical course of sarcoidosis: presentation, diagnosis, and treatment in a large white and black cohort in the United States. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29(2):119–127. [PubMed] [Google Scholar]
- 7.Pietinalho A., Ohmichi M., Hiraga Y. The mode of presentation of sarcoidosis in Finland and Hokkaido, Japan. A comparative analysis of 571 Finnish and 686 Japanese patients. Sarcoidosis. 1996;13(2):159–166. doi: 10.1007/BF00389839. [DOI] [PubMed] [Google Scholar]
- 8.Judson M.A., Costabel U., Drent M. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):19–27. [PubMed] [Google Scholar]
- 9.Judson M.A., Baughman R.P., Teirstein A.S. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86. [PubMed] [Google Scholar]
- 10.Zhou Y., Lower E.E., LI H.P. Cardiac sarcoidosis: the impact of age and implanted devices on survival. Chest. 2017;151(1):139–148. doi: 10.1016/j.chest.2016.08.1457. [DOI] [PubMed] [Google Scholar]
- 11.Hunninghake G.W., Costabel U., Ando M. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:149–173. [PubMed] [Google Scholar]
- 12.Obeid J.S., McGraw C.A., Minor B.L. Procurement of shared data instruments for Research Electronic Data Capture (REDCap) J Biomed Inform. 2013;46(2):259–265. doi: 10.1016/j.jbi.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grunewald J., Eklund A. Sex-specific manifestations of Lofgren's syndrome. Am J Respir Crit Care Med. 2007;175(1):40–44. doi: 10.1164/rccm.200608-1197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baughman R.P., Teirstein A.S., Judson M.A. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 15.Baughman R.P., Field S., Costabel U. Sarcoidosis in America. Analysis based on health care use. Ann Am Thorac Soc. 2016;13(8):1244–1252. doi: 10.1513/AnnalsATS.201511-760OC. [DOI] [PubMed] [Google Scholar]
- 16.Izumi T. Symposium: population differences in clinical features and prognosis of sarcoidosis throughout the world. Sarcoidosis. 1992;9:S105–S118. [Google Scholar]
- 17.van den Heuvel D.A., de Jong P.A., Zanen P. Chest computed tomography-based scoring of thoracic sarcoidosis: inter-rater reliability of CT abnormalities. Eur Radiol. 2015;25(9):2558–2566. doi: 10.1007/s00330-015-3685-4. [DOI] [PubMed] [Google Scholar]
- 18.Sharma O.P., Johnson R. Airway obstruction in sarcoidosis. A study of 123 nonsmoking black American patients with sarcoidosis. Chest. 1988;94(2):343–346. doi: 10.1378/chest.94.2.343. [DOI] [PubMed] [Google Scholar]
- 19.Judson M.A., Thompson B.W., Rabin D.L. The diagnostic pathway to sarcoidosis. Chest. 2003;123(2):406–412. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]
- 20.Judson M.A., Costabel U., Drent M. The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31(1):19–27. [PubMed] [Google Scholar]
- 21.Bradley D.A., Baughman R.P., Raymond L. Ocular manifestations of sarcoidosis. Semin Resp Crit Care Med. 2002;23:543–548. doi: 10.1055/s-2002-36518. [DOI] [PubMed] [Google Scholar]
- 22.Judson M.A., Baughman R.P., Costabel U. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–1307. doi: 10.1183/09031936.00000914. [DOI] [PubMed] [Google Scholar]
- 23.Baughman R.P., Drent M., Kavuru M. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 24.Herbort C.P., Rao N.A., Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS) Ocul Immunol Inflamm. 2009;17(3):160–169. doi: 10.1080/09273940902818861. [DOI] [PubMed] [Google Scholar]
- 25.Zajicek J.P., Scolding N.J., Foster O. Central nervous system sarcoidosis–diagnosis and management. QJM. 1999;92(2):103–117. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 26.Birnie D.H., Sauer W.H., Bogun F. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Grunewald J., Eklund A. Sex-specific manifestations of Lofgren's syndrome. Am J Respir Crit Care Med. 2007;175(1):40–44. doi: 10.1164/rccm.200608-1197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouvry D., Mouthon L., Brillet P.Y. Granulomatosis-associated common variable immunodeficiency disorder: a case-control study versus sarcoidosis. Eur Respir J. 2013;41(1):115–122. doi: 10.1183/09031936.00189011. [DOI] [PubMed] [Google Scholar]
- 29.Mayer A.S., Hamzeh N., Maier L.A. Sarcoidosis and chronic beryllium disease: similarities and differences. Semin Respir Crit Care Med. 2014;35(3):316–329. doi: 10.1055/s-0034-1377059. [DOI] [PubMed] [Google Scholar]
- 30.De Smet D., Martens G.A., Berghe B.V. Use of likelihood ratios improves interpretation of laboratory testing for pulmonary sarcoidosis. Am J Clin Pathol. 2010;134(6):939–947. doi: 10.1309/AJCPNC7STHG0FWMP. [DOI] [PubMed] [Google Scholar]
- 31.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17(8):646–649. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman J. The specificity and nature of serum-angiotensin-converting enzyme (serum ACE) elevations in sarcoidosis. Ann N Y Acad Sci. 1976;278:488–497. doi: 10.1111/j.1749-6632.1976.tb47061.x. [DOI] [PubMed] [Google Scholar]
- 33.Bargagli E., Bianchi N., Margollicci M. Chitotriosidase and soluble IL-2 receptor: comparison of two markers of sarcoidosis severity. Scand J Clin Lab Invest. 2008;68(6):479–483. doi: 10.1080/00365510701854975. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.