Abstract
Background
Plasma brain natriuretic peptide (BNP) level is a prognostic biomarker in pulmonary arterial hypertension (PAH). Its impact on long-term overall survival (OS) was investigated in the Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management (REVEAL), a 5-year observational, multicenter, US registry of patients with PAH.
Methods
Patients were ≥ 18 years of age, met right heart catheterization criteria at rest, had World Health Organization group I PAH, and had BNP measurement at enrollment. Optimal BNP threshold was obtained via receiver operating characteristic curve analysis. OS was compared in patients with low (≤ 340 pg/mL) vs high (> 340 pg/mL) BNP at baseline; changes between baseline and last assessment were also examined. Patients were categorized based on baseline (low or high) and follow-up (low or high) BNP values; hazard ratios (HRs) for OS were estimated and compared using Cox regression.
Results
Overall, 1,426 patients were analyzed. Mortality risk was significantly higher in patients with baseline high vs low BNP (HR, 3.6; 95% CI, 3.0-4.2). BNP change analysis at ≤ 1 year postenrollment demonstrated that the low-low group had the lowest and the high-high group had the highest 5-year mortality risk (HR, 0.23; 95% CI, 0.19-0.27). Changes in BNP score also correlated with change of risk of death.
Conclusions
Baseline BNP threshold of 340 pg/mL strongly predicted survival up to 5 years in patients with PAH. A BNP reduction at 1 year since enrollment was associated with decreased mortality risk, whereas an increase in BNP at 1 year was associated with an increased mortality risk, supporting BNP as a surrogate marker of PAH survival.
Key Words: biomarkers, brain natriuretic peptide, mortality, pulmonary arterial hypertension, survival
Abbreviations: 6MWD, 6-min walk distance; BNP, brain natriuretic peptide; FC, functional class; HR, hazard ratio; NT-proBNP, N-terminal pro b-type natriuretic peptide; NYHA, New York Heart Association; OS, overall survival; PAH, pulmonary arterial hypertension; REVEAL, Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management; WHO, World Health Organization
Pulmonary arterial hypertension (PAH) is a rare, often fatal disease that is characterized hemodynamically by increased pulmonary artery pressure and pulmonary vascular resistance culminating in right ventricular failure and death.1, 2 Estimates of its prevalence range from 15 to 26 cases per 1 million people.1, 3
Much of what is known about this rare disease has been derived from patient registries.4, 5 The largest registry of patients with PAH established to date is the multicenter, observational US-based Registry to Evaluate Early and Long-term Pulmonary Arterial Hypertension Disease Management (REVEAL). Initiated in 2006, REVEAL has enrolled > 3,000 patients with World Health Organization (WHO) group I PAH.6 The data from REVEAL have allowed us to assess the relationship between patient and disease-specific parameters and clinical outcomes.
Previous analyses indicate that overall survival (OS) rates of patients with PAH remain poor.2, 7 The 5-year OS rate was 65.4% for previously diagnosed patients and 61.2% for newly diagnosed patients.7 Those with more advanced disease (New York Heart Association [NYHA] functional class [FC] III or IV) had even lower 5-year rates of OS (previously diagnosed patients: FC III 57.0% and FC IV 27.2%; newly diagnosed patients: FC III 60.0% and FC IV 43.8%).7
Plasma brain natriuretic peptide (BNP) is a hormone secreted mainly by the cardiac ventricles, with levels increasing in proportion to the degree of right ventricular dysfunction in patients with pulmonary hypertension.8 Plasma BNP level has been shown to be an independent predictor of mortality in patients with PAH.9, 10 A previous analysis from REVEAL indicated that a BNP level > 180 pg/mL was predictive of an increased risk of mortality at 1-year postenrollment. The 180 pg/mL BNP threshold was developed based on an earlier REVEAL data cut (n = 2,716) to predict 1-year survival and was used for calculation of a multivariable prognostic score—the REVEAL PAH risk score.10 Patients with a baseline plasma BNP > 180 pg/mL had a significantly lower survival rate than those with a baseline plasma BNP ≤ 180 pg/mL (hazard ratio [HR], 3.2; 95% CI, 2.7-3.8; P < .001).
Using the final REVEAL database of 3,515 patients followed over 5 years, the present analysis represents the largest and longest BNP cohort to date.
Methods
Study Design and Analytic Cohort
The study design, including inclusion and exclusion criteria and statistical methods, of the REVEAL Registry have been described previously.6 The study was conducted in accordance with the amended Declaration of Helsinki, and the protocol was reviewed and approved by the institutional review board at each participating center (e-Appendix 1), with written informed consent obtained from all patients.6
This analysis included patients who had a BNP measurement at enrollment, were ≥ 18 years of age, had WHO group 1 PAH (confirmed by right-sided heart catheterization), and had pulmonary arterial wedge pressure ≤ 15 mm Hg measured at rest at the time of diagnosis. There were no protocol-driven measurements of BNP. Where available, BNP level measured from clinical assessment was collected at enrollment. Results of any subsequent BNP measurements were collected on a quarterly basis thereafter; BNP assay methodologies from each institution were not collected.
Data included in this analysis are based on the final database as of February 4, 2013.
Statistical Methods
Data were summarized by descriptive statistics. Optimal BNP thresholds for predicting OS over 5 years were identified via receiver operating characteristics analysis (e-Fig 1). BNP values were categorized as high and low using these thresholds. Changes in BNP were also examined, with patients categorized as low-low (low BNP at baseline and last assessment), low-high (BNP increased from low at baseline to high at last assessment), high-low (BNP decreased from high at baseline to low at last assessment), and high-high (high BNP at baseline and last assessment). To minimize selection bias, patients who did not have a BNP value postenrollment were classified as no change and included in the low-low or high-high group, depending on their initial BNP value. HRs for OS and comparisons between groups based on the BNP threshold were calculated using Cox regression. Survival status was based on patient vital status up to the last follow-up visit; patients who received a transplant, either before or after study enrollment, were treated in the analysis the same as other patients. Correlation coefficients between plasma BNP level on a log transformed scale and other prognostic factors at baseline were calculated using Pearson correlation coefficient.
BNP Score
BNP scores were developed based on the change in HR from the Cox regression model. BNP values of < 50, 50 to < 80, 80 to < 200, 200 to < 500, 500 to < 1,100, and ≥ 1,100 pg/mL were assigned scores of 0, 1, 2, 3, 4, and 5, respectively. Absolute value change in BNP scores between baseline and postbaseline assessments at 1, 2, 3, 4, and/or 5 years (± 3 months) was 1 (1 score increase), 2 (2 score increase), or ≥ 3 (≥ 3 score increase), 0 (no change), –1 (1 score decrease), –2 (2 score decrease), or ≥ –3 (≥ 3 score decrease). BNP scores and change in BNP scores were used for correlation with OS.
Results
Disposition and Characteristics of Patients
A total of 1,426 patients were included in the analysis (e-Fig 2). Mean BNP level ± SD for all patients was 325.4 ± 614.0 pg/mL at baseline. A BNP threshold of 340 pg/mL was identified by receiver operating characteristics analysis as being predictive of OS up to 5 years postenrollment. In total, 1,051 patients had a low (≤ 340 pg/mL) BNP level (mean, 106.1 ± 88.6 pg/mL), and 375 patients had a high (> 340 pg/mL) BNP level (mean, 940 ± 949 pg/mL). Almost all baseline demographics and disease characteristics differed significantly between patients with a high vs low BNP as shown in Table 1. Patients with low BNP were younger, less likely to be newly diagnosed with PAH, and had better NYHA/WHO FC, longer 6-min walk distance (6MWD), and better hemodynamics by right heart catheterization. PAH etiology also differed significantly between patients with low BNP and patients with high BNP (P < .05) (Table 1). Compared with patients with high BNP, a greater proportion of patients with low BNP had idiopathic PAH (49.5% vs 42.9%), familial PAH (3.5% vs 2.1%), portopulmonary hypertension (5.7% vs 4.0%), and congenital heart disease (9.8% vs 5.6%), but a lower proportion had connective tissue disease (23.4% vs 36.8%) and PAH associated with drugs and toxins (5.1% vs 6.1%), respectively.
Table 1.
Characteristics of Patients With PAH and a BNP Assessment at Enrollment (N = 1,426)
| Characteristic | BNP at Enrollment |
P Value | |
|---|---|---|---|
| ≤ 340 pg/mLa (n = 1,051) | > 340 pg/mLa (n = 375) | ||
| Age, y | 50.9 ± 14.5 | 57.0 ± 14.3 | < .0001 |
| Sex | ≥ .05 | ||
| Male | 205 (19.5) | 86 (22.9) | |
| Female | 846 (80.5) | 289 (77.1) | |
| Race | ≥ .05 | ||
| White | 778 (74.0) | 276 (73.6) | |
| Black | 122 (11.6) | 59 (15.7) | |
| Hispanic | 97 (9.2) | 26 (6.9) | |
| Asian or Pacific Islander | 34 (3.2) | 7 (1.9) | |
| Native American or Native Alaskan | 7 (0.7) | 1 (0.3) | |
| Other | 5 (0.5) | 2 (0.5) | |
| Unknown | 8 (0.8) | 4 (1.1) | |
| BMI, kg/m2 | 28.7 ± 7.0 | 27.3 ± 6.9 | < .001 |
| Newly diagnosed | 211 (20.1) | 129 (34.4) | < .0001 |
| PAH etiology | < .05 | ||
| Idiopathic | 520 (49.5) | 161 (42.9) | |
| Familial | 37 (3.5) | 8 (2.1) | |
| Connective tissue disease | 246 (23.4) | 138 (36.8) | |
| Congenital heart disease | 103 (9.8) | 21 (5.6) | |
| Portopulmonary hypertension | 60 (5.7) | 15 (4.0) | |
| HIV | 18 (1.7) | 4 (1.1) | |
| Drugs and toxins | 54 (5.1) | 23 (6.1) | |
| Other | 13 (1.2) | 5 (1.3) | |
| NYHA/WHO functional class | < .0001 | ||
| I | 78 (8.3) | 5 (1.6) | |
| II | 375 (40.1) | 72 (22.6) | |
| III | 445 (47.6) | 193 (60.5) | |
| IV | 37 (4.0) | 49 (15.4) | |
| Disease characteristics | |||
| 6MWD, m | 382.6 ± 122.4 (n = 933) | 285.7 ± 122.1 (n = 305) | < .0001 |
| eGFR, mL/min/1.73 m2 | 78.5 ± 25.8 (n = 933) | 64.8 ± 24.4 (n = 347) | < .0001 |
| Resting mPAP, mm Hg | 48.9 ± 14.3 (n = 1,043) | 52.0 ± 12.6 (n = 373) | < .001 |
| mRAP, mm Hg | 8.3 ± 5.1 (n = 1,043) | 11.5 ± 5.7 (n = 346) | < .0001 |
| Cardiac index, L/min/m2 | 2.5 ± 0.9 (n = 881) | 2.1 ± 0.7 (n = 305) | < .0001 |
| PVRI, Wood units × m2 | 17.8 ± 11.5 (n = 870) | 22.1 ± 10.6 (n = 305) | < .0001 |
| Resting PAWP, mm Hg | 9.7 ± 4.0 (n = 1,032) | 9.8 ± 4.0 (n = 372) | ≥ .05 |
| Mixed venous oxygen saturation, % | 65.6 ± 8.6 (n = 730) | 58.5 ± 9.9 (n = 227) | < .0001 |
Data are presented as mean ± SD, No. (%), or as otherwise indicated. 6MWD = 6-min walk distance; BNP = brain natriuretic peptide; eGFR = estimated glomerular filtration rate; mPAP = mean pulmonary arterial pressure; mRAP = mean right atrial pressure; NYHA = New York Heart Association; PAH = pulmonary arterial hypertension; PAWP = pulmonary arterial wedge pressure; PVRI = pulmonary vascular resistance index; WHO = World Health Organization.
The BNP threshold of 340 pg/mL was determined by receiver operating characteristic curve analysis as being optimal for predicting 5-year overall survival.
Change in BNP Over Time
BNP assessment was performed annually on average for patients who had at least one postenrollment BNP assessment. The median time between enrollment and last BNP assessment was 3 years (interquartile range, 1-5 years). The change in BNP value over time for each of the 4 BNP shift groups (ie, low-low, low-high, high-low, high-high) is shown in e-Figure 3. For patients who had postbaseline values, (1) most had a low baseline BNP level (≤ 340 pg/mL) at enrollment and remained low at last assessment (n = 713, 62.9%), (2) BNP levels continued to increase over time (until approximately 3 years) in the group of patients who shifted from a low to high BNP (> 340 pg/mL) (n = 143, 12.6%), (3) BNP levels tended to decrease gradually over time (until approximately 3 years) in the subset of patients who shifted from a high to low BNP (n = 103, 9.1%), and (4) BNP levels remained high over time in the subset of patients who had high BNP levels (> 340 pg/mL) at baseline and at last assessment (n = 174, 15.4%).
When differences between BNP groups by baseline characteristics were examined (e-Table 1), compared with patients in the low-low BNP group, patients in the low-high group were significantly older (median, 55.6 vs 50.8 years; P = .0033), had lower median baseline BMI (26.3 vs 27.9 kg/m2; P = .0068), had lower median baseline glomerular filtration rate (68.9 vs 78.9 mL/min/1.73 m2; P = .0004), had lower median baseline mixed oxygen venous saturation (63.0% vs 67.0%; P = .0055), and had a difference in etiology, with a lower proportion diagnosed with idiopathic PAH (39.9% vs 51.0%) and a higher proportion diagnosed with PAH associated with connective tissue disease (35.7% vs 21.5%) (P = .0006), respectively. Compared with patients in the high-low group, patients in the high-high group were significantly older (median, 59.2 vs 54.7 years; P = .0017), had a shorter median baseline 6MWD (262.0 vs 327.0 m; P < .0001), had lower median baseline BMI (25.5 vs 27.4 kg/m2; P = .0111), had lower baseline median glomerular filtration rate (62.5 vs 67.4 mL/min/1.73 m2; P = .0449), and had a difference in etiology, with a higher proportion diagnosed with PAH associated with connective tissue disease (40.8% vs 26.2%) (P < .0001), respectively. A significantly higher proportion of patients in the high-low group were also using IV/subcutaneous prostacyclin compared with those in the high-high group (17.5% vs 7.7%, respectively; P = .0057).
Survival Analysis
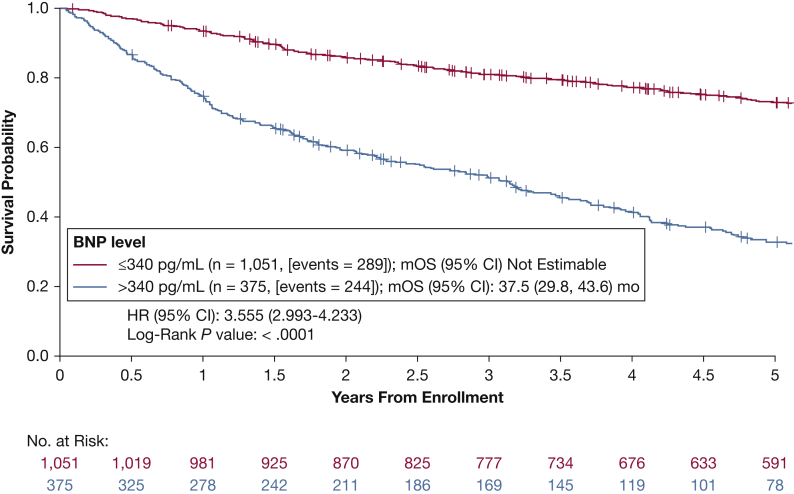
Five-year Kaplan-Meier survival curves for patients with measurements of BNP at enrollment are presented in Figure 1. Patients with a baseline BNP > 340 pg/mL had a significantly higher mortality risk than those with a baseline BNP ≤ 340 pg/mL (HR, 3.6; 95% CI, 3.0-4.2; P < .001). Kaplan-Meier 5-year survival estimates were 72.9% (95% CI, 70.0-75.6) for patients with a BNP ≤ 340 pg/mL and 32.5% (95% CI, 27.4-37.8) for patients with a BNP > 340 pg/mL. These findings were consistent regardless of underlying PAH etiology (e-Table 2). Overall, among those patients included in the survival analysis, 3.5% (n = 50) received a transplant during follow-up (median time to transplant from study enrollment, 2.43 years).
Figure 1.
Kaplan-Meier estimates of 5-y overall survival for patients with World Health Organization group 1 peripheral arterial hypertension stratified by baseline BNP value. BNP = brain natriuretic peptide; HR = hazard ratio; mOS = median survival time.
OS by Change in BNP
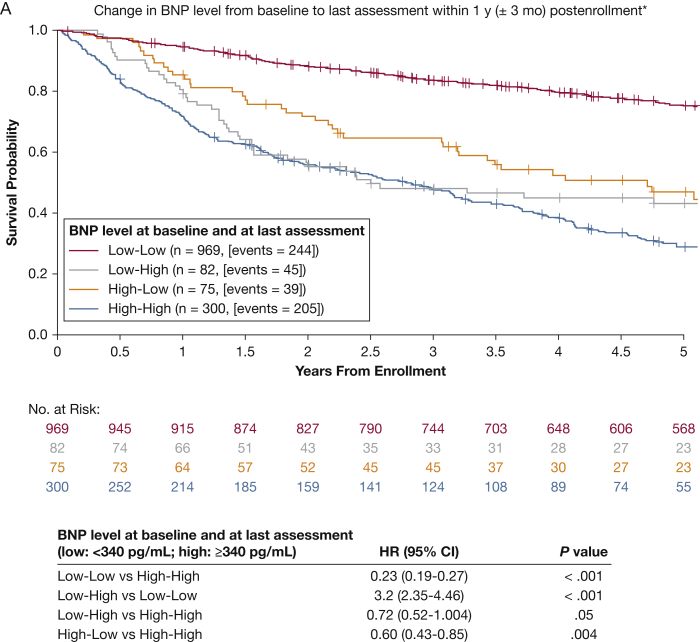
Analysis of the change in BNP from baseline to the last BNP assessment available within 1 year is presented in Figure 2A. Patients were categorized as low-low (BNP ≤ 340 pg/mL at baseline and 1 year), low-high (BNP increased from ≤ 340 pg/mL at baseline to > 340 pg/mL at 1 year), high-low (BNP decreased from > 340 pg/mL at baseline to ≤ 340 pg/mL at 1 year), and high-high (BNP > 340 pg/mL at baseline and 1 year). Of 1,426 patients, approximately 80% had at least one postenrollment BNP value. Patients without a BNP value postenrollment were included in the low-low or high-high group, depending on baseline BNP. For patients with a postenrollment BNP assessment within 1 year (including those patients without postenrollment values who were rolled into the low-low or high-high groups as previously specified), the low-low group (n = 969, 244 events) had the lowest mortality risk, and the high-high group (n = 300, 205 events) had the highest mortality risk. The HR for the comparison of the low-low group with the high-high group was 0.23 for patients with 1-year postenrollment BNP (95% CI, 0.19-0.27; P < .001). Changing from a high BNP at baseline to a low BNP within 1 year (n = 75, 39 events) decreased the overall risk of death by 40% (HR, 0.60; 95% CI, 0.43-0.85; P = .004). Compared with the low-low group, the low-high group (n = 82, 45 events) was associated with a significant increase in the risk of death for patients with 1-year postenrollment BNP (HR, 3.2; 95% CI, 2.35-4.46; P < .001). In contrast, patients in the low-high group had a decreased risk compared with the high-high group within 1-year postenrollment BNP (HR, 0.72; 95% CI, 0.52-1.004; P = .05).
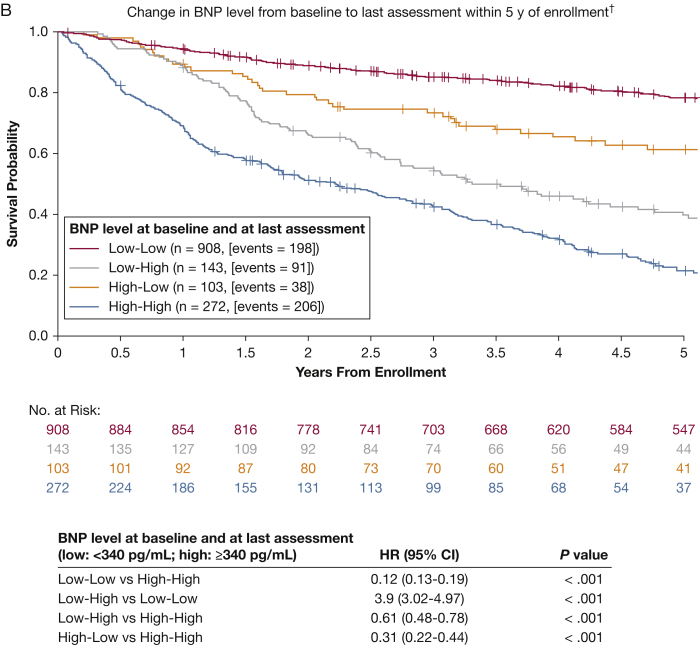
Figure 2.
A, B, Kaplan-Meier estimates of 5-y overall survival according to change in BNP value relative to baseline (N = 1,426). A, Change in BNP from baseline to last assessment within 1 y postenrollment. Patients who did not have any BNP assessment within 1 y postbaseline were classified as no change in BNP. B, Change in BNP from baseline to last assessment within 5 y of enrollment. Patients who did not have any BNP assessment postbaseline were classified as no change in BNP. See Figure 1 legend for expansion of abbreviations.
For patients with a BNP assessment within 5 years, the 5-year Kaplan-Meier estimates of OS between BNP groups were associated with similar, although more pronounced, trends (Fig 2B). Patients in the low-low group had a decreased risk of death compared with the high-high group (HR, 0.12; 95% CI, 0.13-0.19; P < .001). For patients in the high-low group, BNP values declined quickly and remained low throughout the 5-year period. Changing from high BNP to low BNP (n = 103, 38 events) within 5 years decreased the overall risk by 69% (HR, 0.31; 95% CI, 0.22-0.44; P < .001). Compared with the low-low group, the low-high group (n = 143, 91 events) was associated with a significant increase in mortality risk (HR, 3.9; 95% CI, 3.02-4.97; P < .001). Patients in the low-high group had a decreased risk compared with the high-high group (HR, 0.61; 95% CI, 0.48-0.78; P < .001).
A sensitivity analysis conducted in patients who had BNP levels available, at baseline and last assessment, yielded similar results (e-Appendix 2, e-Table 3).
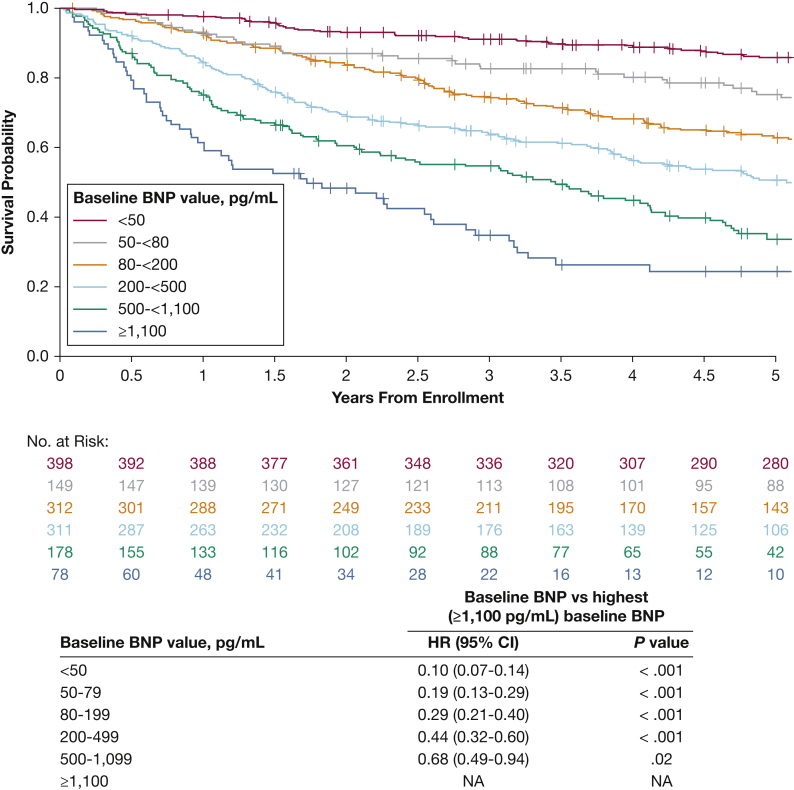
Overall, BNP scores alone were highly correlated with OS (e-Appendix 2, Fig 3), and risk of death correlated proportionally and significantly with the change in BNP score between baseline and postbaseline assessment within 5 years of enrollment (e-Appendix 2, e-Table 4).
Figure 3.
Kaplan-Meier estimates of 5-y overall survival, by BNP score at baseline. NA = not applicable. See Figure 1 legend for expansion of other abbreviations.
Other BNP Correlations at Baseline
A significant positive correlation was demonstrated between BNP and mean right atrial pressure (r = 0.3; P < .001) and NYHA/WHO FC (r = 0.3; P < .001) (Table 2). Glomerular filtration rate at enrollment (r = –0.3; P < .001), the most recent 6MWD test (r = –0.4; P < .001), and the most recent mixed venous oxygen saturation (r = –0.3; P < .001) were negatively correlated with BNP at baseline (Table 2).
Table 2.
Correlation Coefficients Between BNP Level and Other Parameters at Baseline
| Characteristic | No. | Coefficienta | P Valuea |
|---|---|---|---|
| BMI, kg/m2 | 1,399 | –0.11 | < .001 |
| Cardiac index, L/min/m2 | 1,077 | –0.14 | < .001 |
| GFR, mL/min/1.73 m2 | 1,279 | –0.32 | < .001 |
| mPAP at rest,b mm Hg | 1,416 | 0.13 | < .001 |
| mRAP, mm Hg | 1,337 | 0.30 | < .001 |
| NYHA/WHO FC | 1,252 | 0.30 | < .001 |
| PVR,c Wood units | 1,385 | 0.09 | < .001 |
| 6MWD,b m | 1,238 | –0.37 | < .001 |
| Mixed venous oxygen saturation,b % | 957 | –0.34 | < .001 |
| PAWPb at rest | 1,381 | 0.04 | .140 |
FC = functional class; GFR = glomerular filtration rate; PVR = pulmonary vascular resistance. See Table 1 legend for expansion of other abbreviations.
Pearson correlation coefficient and its P value of BNP on a log transformed scale and other parameters.
Most recent measurement.
Fick hierarchy: preferred analysis variable.
Discussion
This analysis is the largest and longest cohort to examine the role of BNP as a biomarker in PAH. Our findings suggest that BNP level strongly predicts 5-year OS in patients with PAH and that 340 pg/mL is a predictive BNP threshold.
Patients who remained in the low BNP group had the lowest mortality risk, and those remaining in the high BNP group had the highest mortality risk. BNP reduction within 1 year of enrollment was associated with a 40% decrease in the risk of mortality, whereas an increased BNP was associated with a 3.2-fold increase in risk of mortality. Use of two consecutive BNP assessments provided a more reliable prediction of OS, especially for improvement. This is supported by the observation that some patients could start and end with a BNP value ≤ 340 pg/mL, but have transiently high BNP values (e-Fig 4).
These data confirm and expand on previous REVEAL results, showing high levels of BNP at enrollment were a strong indicator of increased mortality.10, 11 Benza et al10 identified that a BNP threshold of 50 pg/mL predicted better survival, whereas a threshold of 180 pg/mL predicted poor survival at 1 year. The lower BNP threshold likely has a higher sensitivity but lower specificity for risk, whereas the BNP threshold in the current study likely has a higher specificity for risk. An updated analysis of the REVEAL risk score found that a threshold of 200 to 800 pg/mL imparts the same degree of risk.12 Overall, BNP levels above normal are associated with increased mortality risk. Additionally, these findings are consistent with those of a primary pulmonary hypertension cohort (N = 60) where both high baseline plasma BNP and increases in BNP over 3-month follow-up period were strong predictors of mortality.9 Further, a study of 55 patients with severe pulmonary hypertension (including 36 patients with idiopathic PAH) found that N-terminal pro b-type natriuretic peptide (NT-proBNP) was useful in long-term prognosis.13 A separate study of 109 patients with systemic sclerosis found that NT-proBNP levels > 395 pg/mL were associated with pulmonary hypertension, and baseline and serial changes in NT-proBNP over a 10-month follow-up period were highly predictive of 1-year survival.14 Results from the randomized clinical trial of Aspirin and Simvastatin for Pulmonary Arterial Hypertension (ASA-STAT) demonstrated that, among 65 patients with PAH, higher NT-proBNP levels at baseline were independently (irrespective of age, sex, PAH etiology, and 6MWD) associated with increased risk of death or lung transplantation.15 Importantly, whereas the follow-up period in these previous studies was within 1 year of enrollment, our current analysis demonstrates that BNP is useful in predicting PAH mortality risk for up to 5 years.
Limitations
Other parameters known to have an impact on BNP (ie, age, weight, renal function, NYHA/WHO FC, mean right atrial pressure, mean pulmonary arterial pressure, pulmonary vascular resistance, 6MWD, peak oxygen uptake, cardiac index)16, 17 were not accounted for in this analysis. Although, the BNP score may provide a simple tool to correlate a biomarker with clinical outcome, its prognostic strength is likely improved when combined with other known prognostic PAH factors.18 In the current study, 3.5% of patients received a transplant during follow-up, which may have affected our analysis; however, the trend between BNP change group and OS was consistent when these patients were excluded from analysis. Therefore, we do not think the predictive value of BNP on clinical outcome was affected by transplantation. In addition, this was an uncontrolled, observational registry analysis. As such, patient visits were not mandated, and study measurements were not mandated nor acquired at predefined intervals. Although statistically significant, correlations between BNP and hemodynamic parameters were low compared with other studies.8, 9, 19 These low correlations may be reflective of timing of assessments for hemodynamic variables which may not have been contemporaneous with corresponding BNP assessments; therefore, these data should be interpreted with caution. Because the REVEAL Registry is a US-based cohort, and reflected real-world clinical practice in the United States between 2006 and 2012, the generalizability of these observations to other treatment eras and countries is unknown. Survival analyses in PAH cohorts are prone to survivor bias when patients with different disease durations are pooled.20 Most patients included in the REVEAL Registry (86.5%) had established disease at the time of enrollment.10 However, time from diagnosis was not independently associated with mortality. Because treatment practice was evolving over the study period (between 2006 and 2013), and the data collection of PAH therapies was limited by patient start/stop date, this study was not able to clearly define differences between groups regarding monotherapy or combination therapy.
Conclusions
Plasma BNP provides a simple, noninvasive, and relatively inexpensive biomarker that can be monitored to help inform therapeutic decisions. A BNP threshold of 340 pg/mL strongly predicted 5-year OS. Importantly, a change in BNP (and its score) correlated with changes in mortality risk.
Acknowledgments
Author contributions: R. P. F. takes responsibility for the content of the manuscript, including the data and analysis. R. P. F., H. W. F., D. B. B., C. G. E., A. E. F., M. D. M., C. Z., D. R. M., M. S., and R. L. B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; contributed substantially to the study design (analysis plan), data analysis, reporting, interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. P. F. reports being on steering committee and/or advisory board for Abbott, Actelion, Arena Pharmaceuticals, Bayer, St. Jude Medical, and United Therapeutics; adjudication committee and data safety monitoring board with United Therapeutics; and consulting for Bayer. R. P. F. also holds a National Institutes of Health grant in pulmonary vascular disease (U01 HL125205), although not associated with this publication. H. W. F. reports being a steering committee and advisory board member for Actelion. H. W. F. also reports being a speaker for Gilead, Bayer, and Actelion; being a steering committee, board, or advisory committee member for Gilead, Bayer, Bellerophon, and United Therapeutics/Lung LLC; and research support from Gilead, Actelion, and United Therapeutics. D. B. B. reports grants for the conduct of clinical trials from Actelion, Gilead, United Therapeutics/Lung LLC, Bellerophon, Reatta, Arena, and Eiger; being a shareholder of Johnson & Johnson; being a consultant to Actelion, Gilead, United Therapeutics, Belleraphon, and Arena; and being a steering committee or advisory board member to Actelion, Gilead, United Therapeutics/Lung LLC, and Belleraphon. C. G. E. reports being a consultant to Actelion, Bellerophon, and Bayer. A. E. F. reports grants for the conduct of clinical studies from Bayer, Gilead, Actelion, Ikaria, United Therapeutics, and Intermune; and being a consultant for Bayer, Actelion, Gilead, Novartis, and Intermune. M. D. M. reports being on the steering committee for Lung Biotechnology and on the data monitoring committee for Pfizer. D. R. M. reports being an employee of ICON. C. Z. and M. S. report being salaried employees of Actelion Pharmaceuticals US and receive stocks or stock options in Actelion Pharmaceuticals US, Inc. R. L. B. reports being a member of the steering committee, board, or advisory committee for Bayer, Bellerophon, and United Therapeutics/Lung LLC; and receiving research support from Gilead, Actelion, United Therapeutics, and Bellerophon.
Role of sponsors: Actelion Pharmaceuticals US, Inc, is the sponsor of the REVEAL Registry and provided funding and support for the analysis presented.
Other contributions: Third-party medical editorial assistance was provided by BlueMomentum, an Ashfield Company, part of UDG Healthcare plc, and was funded by Actelion Pharmaceuticals US, Inc.
Additional information: The e-Appendixes, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Actelion Pharmaceuticals US, Inc, is the sponsor of the REVEAL Registry and provided funding and support for the analysis presented.
Supplementary Data
References
- 1.Simonneau G., Gatzoulis M.A., Adatia I. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25):D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N., Humbert M., Vachiery J.L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M., Sitbon O., Chaouat A. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 4.McGoon M.D., Benza R.L., Escribano-Subias P. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(25):D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Awdish R., Cajigas H. Definition, epidemiology and registries of pulmonary hypertension. Heart Fail Rev. 2016;21(3):223–228. doi: 10.1007/s10741-015-9510-y. [DOI] [PubMed] [Google Scholar]
- 6.McGoon M.D., Krichman A., Farber H.W. Design of the REVEAL registry for US patients with pulmonary arterial hypertension. Mayo Clin Proc. 2008;83(8):923–931. doi: 10.4065/83.8.923. [DOI] [PubMed] [Google Scholar]
- 7.Farber H.W., Miller D.P., Poms A.D. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148(4):1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N., Nishikimi T., Okano Y. Plasma brain natriuretic peptide levels increase in proportion to the extent of right ventricular dysfunction in pulmonary hypertension. J Am Coll Cardiol. 1998;31(1):202–208. doi: 10.1016/s0735-1097(97)00452-x. [DOI] [PubMed] [Google Scholar]
- 9.Nagaya N., Nishikimi T., Uematsu M. Plasma brain natriuretic peptide as a prognostic indicator in patients with primary pulmonary hypertension. Circulation. 2000;102(8):865–870. doi: 10.1161/01.cir.102.8.865. [DOI] [PubMed] [Google Scholar]
- 10.Benza R.L., Miller D.P., Gomberg-Maitland M. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL) Circulation. 2010;122(2):164–172. doi: 10.1161/CIRCULATIONAHA.109.898122. [DOI] [PubMed] [Google Scholar]
- 11.Benza R.L., Gomberg-Maitland M., Miller D.P. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest. 2012;141(2):354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 12.Benza R.L., Elliott C.G., Farber H.W. Updated risk score calculator for patients with pulmonary arterial hypertension (PAH) in the Registry To Evaluate Early And Long-Term PAH Disease Management (REVEAL) Am J Respir Crit Care Med. 2017;195:A6899. [Google Scholar]
- 13.Fijalkowska A., Kurzyna M., Torbicki A., Szewczyk G., Pruszczyk P., Szturmowicz M. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest. 2006;129(5):1313–1321. doi: 10.1378/chest.129.5.1313. [DOI] [PubMed] [Google Scholar]
- 14.Williams M.H., Handler C.E., Akram R. Role of N-terminal brain natriuretic peptide (N-TproBNP) in scleroderma-associated pulmonary arterial hypertension. Eur Heart J. 2006;27(12):1485–1494. doi: 10.1093/eurheartj/ehi891. [DOI] [PubMed] [Google Scholar]
- 15.Al-Naamani N., Palevsky H.I., Lederer D.J. Prognostic significance of biomarkers in pulmonary arterial hypertension. Ann Am Thorac Soc. 2016;13(1):25–30. doi: 10.1513/AnnalsATS.201508-543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuchte H.H., Holzapfel M., Baumgartner R.A. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43(5):764–770. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 17.Redfield M.M., Rodeheffer R.J., Jacobsen S.J., Mahoney D.W., Bailey K.R., Burnett J.C., Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5):976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 18.Benza R.L., Miller D.P., Barst R.J., Badesch D.B., Frost A.E., McGoon M.D. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest. 2012;142(2):448–456. doi: 10.1378/chest.11-1460. [DOI] [PubMed] [Google Scholar]
- 19.Nagaya N., Ando M., Oya H. Plasma brain natriuretic peptide as a noninvasive marker for efficacy of pulmonary thromboendarterectomy. Ann Thorac Surg. 2002;74(1):180–184. doi: 10.1016/s0003-4975(02)03654-8. [DOI] [PubMed] [Google Scholar]
- 20.Miller D.P., Gomberg-Maitland M., Humbert M. Survivor bias and risk assessment. Eur Respir J. 2012;40(3):530–532. doi: 10.1183/09031936.00094112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.