Abstract
Gene transfer to and correction of hematopoietic stem cells (HSCs) are ideal strategies to cure a number of congenital and acquired disorders. However, transgene products may trigger immunological rejection of modified cells, limiting their therapeutic benefits. Preclinical and clinical data indicate that myeloablative total body irradiation (TBI) allows for efficient engraftment and tolerance to gene-modified HSCs. In contrast, myeloablative chemotherapy using busulfan or similar agents is only sufficient to induce tolerance to gene-modified HSCs producing no or non-immunogenic protein. If cells are modified to produce a protein that is xenogenic or congenitally absent in the patient, additional immunosuppression may be required to prevent an immunological reaction to the transduced cells. New gene editing and in vivo gene therapy techniques could pose additional immune concerns compared to ex vivo gene therapy methods. This review is intended to guide the design of conditioning and immunosuppression therapy in HSC-targeted gene therapy, as well as gene editing.
Keywords: conditioning, hematopoietic stem cells, gene therapy, immunoresponse
Introduction
Hematopoietic stem cell (HSC) transplantation is an established strategy to treat or cure a number of congenital and acquired disorders. However, allogeneic HSC transplantation is limited by a lack of suitable donors and significant risk of morbidity and mortality arising from opportunistic infection, graft failure, and graft-versus-host disease (GVHD). In recent years, autologous HSC-targeted gene therapy has emerged as an alternative to allogeneic HSC transplantation for primary immunodeficiencies, hemoglobinopathies, storage and metabolic disorders, congenital cytopenias, and stem cell deficiencies.1, 2, 3, 4, 5, 6, 7, 8 In HSC gene therapy, autologous bone marrow or mobilized peripheral blood cells are collected and purified to obtain a CD34+ fraction, and these cells are transduced with retrovirus-based vectors encoding the therapeutic gene of interest. Modified cells are then infused back to patients following preparative conditioning consisting of chemotherapy.9 Engrafted HSCs are capable of producing corrected blood cells of multiple lineages over the patient’s lifetime, thereby providing a one-time cure. By employing autologous cells, gene therapy also overcomes the need for a histocompatible donor, risk of GVHD, and requirement for long-term immunosuppression.
The first successful gene therapy trial was reported in 2000 for the treatment of X-linked severe combined immunodeficiency (X-SCID).6 The impressive results obtained in these early trials can be primarily attributed to the young age of the patient cohort and selective advantage of gene-modified cells in immunocompromised patients, allowing for phenotypic correction with even low levels of transduction. In contrast, gene therapy for other disorders must overcome additional barriers, such as the need for high-level gene marking and engraftment of gene-modified cells and the presence of a competent immune system. While the use of autologous cells in gene therapy avoids the more severe immune complications associated with allogeneic HSC transplantation, transgene-encoded proteins may be recognized as foreign by the patient’s immune system, potentially leading to the elimination of transduced cells. Indeed, immunoresponses to transgenic proteins and vector-derived elements have been reported in multiple preclinical and clinical settings.10, 11, 12, 13, 14 Therefore, predicting and ameliorating immunoresponses to gene-modified HSCs is necessary for successful gene therapy.
For the development of cellular immunity to genetically modified HSCs, peptides derived from intracellular transgene elements are processed and presented by major histocompatibility complex (MHC) class I molecules to T cells, thereby generating T cell immunoresponses to transgene-expressing cells. In addition, transgenic proteins can be processed by antigen-presenting cells and displayed by MHC class II, leading to activation of B-cell-mediated humoral responses with transgene-specific antibody production. When immunological rejection occurrs against transduced cells expressing intracellular GFP, both GFP-specific cytotoxic T lymphocyte (CTL) responses and antibody production are observed in a rhesus gene therapy model.15, 16, 17, 18 Both cellular and humoral immunoresponses are associated with HSC-targeted gene therapy; however, the type and strength of immunoresponses may depend on multiple factors, such as localization and immunogenicity of transgene products as well as tissue specificity and levels of transgene expression.
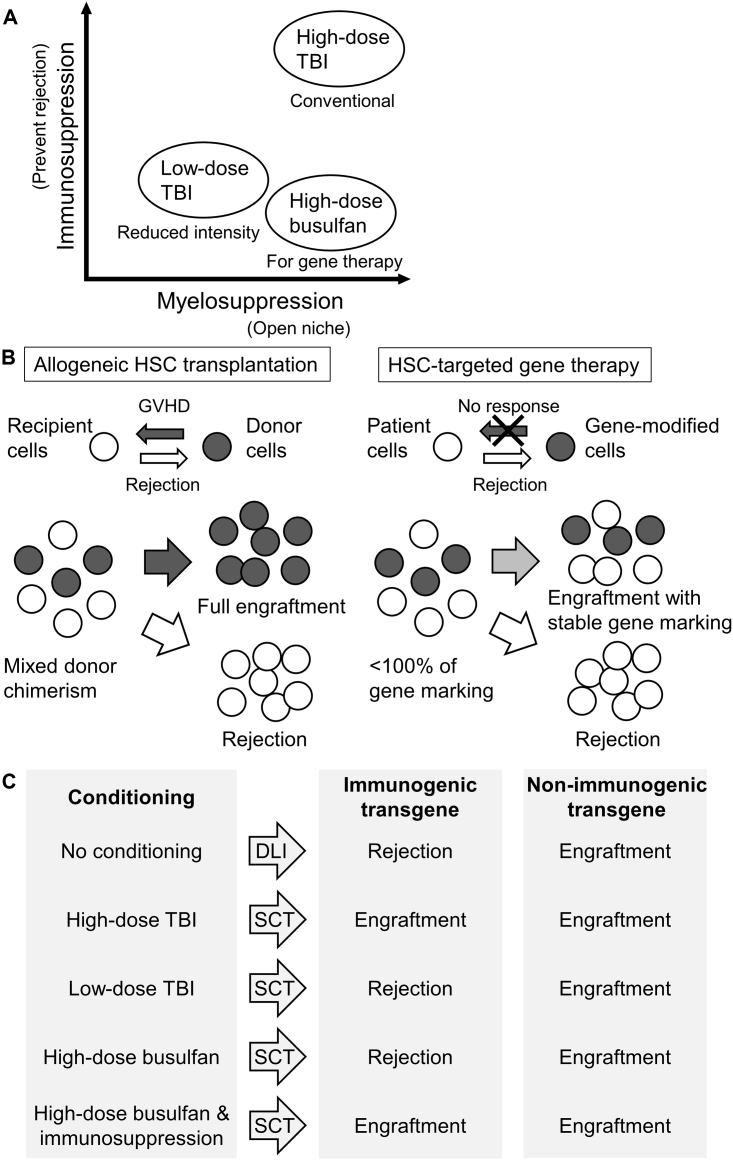
Specific tolerance to allografts or xenografts in recipients can be induced following transplantation of allogeneic or genetically modified HSCs.19, 20, 21, 22, 23, 24 The establishment of molecular chimerism in recipients leads to the development of tolerance through either central or peripheral mechanisms.19 However, reports of immunoresponses to genetically modified HSCs indicate that immunosuppression may be necessary to permit engraftment of HSCs before long-term tolerance to the transgene is established. High-dose total body irradiation (TBI), which is both myelosuppressive and immunosuppressive, has been shown to produce stable engraftment of gene-modified HSCs (Figure 1A).25, 26, 27 When employing busulfan conditioning, which is primarily myelosuppressive, the degree of dissimilarity between unmodified and modified cells appears to dictate the likelihood of a transgene-specific immunoresponse.17 Myeloablative busulfan conditioning alone permits engraftment of HSCs expressing minimally immunogenic proteins, but it does not necessarily prevent immunoresponses to xenogenic or congenitally absent proteins. In these situations, additional immunosuppression may be necessary.17
Figure 1.
Concept of Immunoresponse to Gene-Modified HSCs
(A) Intensities of myelosuppression and immunosuppression among conditioning regimens. (B) Immunoresponses between patient cells and gene-modified cells in HSC-targeted gene therapy compared to those between recipient and donor cells in allogeneic HSC transplantation. (C) Overall summary of donor lymphocyte injection (DLI) and HSC transplantation (SCT) with either immunologic or non-immunologic gene transduction following conditioning.
Conditioning for Robust Engraftment and Specific Tolerance to Transgenes
In allogeneic HSC transplantation, conditioning serves two purposes: myelosuppression, to open bone marrow niche space for transplanted cells to engraft, and immunosuppression, to prevent graft rejection (Figure 1A).28 Myelosuppression and immunosuppression are traditionally accomplished by TBI and chemotherapy drugs. Myeloablative doses of conditioning are used in allogeneic transplantation for the treatment of hematologic malignancies, in which it is necessary to deplete malignant cells as thoroughly as possible and to suppress the immune system for donor cell engraftment. Originally, conditioning was thought to be redundant in the context of gene therapy as this method employs autologous cells, which were thought to be incapable of triggering an immunoresponse. The first clinical trials did not use any myelosuppressive or immunosuppressive agents; however, these attempts uniformly failed to achieve any phenotypic correction due to no or extremely low engraftment of modified cells.29, 30, 31, 32
Engraftment of donor cells in allogeneic HSC transplantation can be enhanced by alloimmune reaction, whereas engraftment of donor cells in autologous gene therapy can only be facilitated by increasing the cell dose or removing resident HSCs (Figure 1B). Significant levels of gene marking have been obtained in mice transplanted without conditioning, but this approach required clinically prohibitive cell numbers.33, 34, 35 It is now accepted that some level of HSC depletion is needed for therapeutic levels of engraftment. The amount of myelosuppression and immunosuppression needed in gene therapy applications is dependent on the biology of the disease being targeted. For the treatment of immunodeficiencies, minimal myelosuppression can be used due to the survival advantage of corrected cells, but for hemoglobinopathies and lysosomal storage disorders, myeloablative doses of conditioning are needed.36 The necessity of immunosuppression in autologous gene therapy remains an open question. Autologous donor cells may be recognized as self by the patient’s immune system, or immunoresponses to the therapeutic transgene or other introduced elements may develop, leading to rejection of gene-modified cells.
Reports of anti-transgene immunity to lymphocytes transduced with herpesvirus thymidine kinase and hygromycin phosphotransferase fusion and neomycin phosphotransferase (neo) gene products in early clinical gene therapy trials prompted further investigations into the use of HSC gene therapy to induce specific tolerance to foreign transgenes.10,12 To examine the relationship between immunoresponses to transduced cells and their in vivo persistence, rhesus macaques were transplanted with autologous peripheral blood lymphocytes transduced with γ-retroviral vectors containing an expressed or non-expressed neo gene (Figure 1C). Consistent with earlier clinical observations, neo-expressing lymphocytes were eliminated from circulation, while lymphocytes containing the non-expressed version of the gene persisted up to 1 year. In animals that were transplanted with CD34+ cells transduced with a neo-expressing vector, infusion of neo-expressing lymphocytes did not lead to rejection of these cells.18 In another study, macaques demonstrating stable, multilineage engraftment of EGFP-expressing CD34+ cells following myeloablative conditioning were repeatedly immunized with soluble GFP protein. Anti-GFP humoral and cellular responses that increased with each immunization were observed in non-transplanted animals but not in transplanted animals with GFP marking.15 These findings support the notion that HSC transplantation can contribute to long-term tolerance to foreign transgenes.
In contrast, other groups have observed elimination of genetically modified HSCs due to anti-transgene immunity. A gene therapy study performed in a canine mucopolysaccharidosis type I (MPS I) model reported immunological elimination of transplanted bone marrow cells expressing a therapeutic α-l-iduronidase gene.13 The outcome in this study is likely explained by the lack of conditioning prior to transplantation. The rhesus macaques that developed tolerance to GFP following gene therapy received a myeloablative dose of TBI prior to transplantation, which may have permitted the initial engraftment of modified HSCs during the period when tolerance to the foreign protein product was being established. However, at least one study reported immunoresponses to GFP- and enhanced yellow fluorescent protein (YFP)-expressing HSCs in baboons that had undergone myeloablative TBI (10.2 Gy) prior to transplantation.37 The authors attributed this result to the use of a lentiviral vector for the transduction, but numerous other studies employing lentiviral vectors have achieved stable engraftment of GFP- and YFP-expressing cells in primate models following myeloablative TBI (Figure 1C).26,38 It is possible that sufficient immunosuppression was not achieved in these animals.
Reduced Intensity Conditioning (RIC) Does Not Prevent Immunoresponses to Foreign Transgenes in Large Animal Models
The toxicity associated with TBI prompted the development of RIC regimens in allogeneic transplantation and autologous gene therapy for inherited disorders, in which side effects are less tolerable than in the treatment of hematologic malignancies.36 Early experiments in mice showed that syngeneic Sca-1+ cells engraft efficiently using a RIC regimen.39,40 Another group demonstrated that in mice, radiation doses as low as 1 Gy permit engraftment and tolerance to neo- and GFP-expressing HSCs.41 In rhesus macaques, low-dose irradiation (5 Gy) followed by transplantation of CD34+ cells transduced with a neo-containing γ-retroviral vector resulted in up to 12% gene marking.42 Further experiments in nonhuman primates and clinical trials confirmed that when combined with high-efficiency gene-transfer methods, RIC could engraft HSCs at levels that would be therapeutically significant for some disorders.43 Despite these successes, other studies in large animals reported immunoresponses to transgenes when using lower doses of TBI.16 Rhesus macaques that underwent nonmyeloablative irradiation (2.4 Gy) prior to CD34+ cell transplantation with γ-retroviral GFP transduction developed strong GFP-specific CTL and antibody responses, leading to elimination of transduced cells.16 In contrast, the same group observed sustained circulation (4–6 months) of cells (5%–10%) expressing murine CD24 using a slightly higher nonmyeloablative regimen of 3.2 Gy.44 The different outcomes could be explained by the similarity between murine and rhesus CD24, compared to GFP, which is completely foreign.17
To determine the amount of radiation necessary for engraftment of and tolerance to gene-modified HSCs, TBI dose de-escalation was performed in a rhesus gene therapy model with lentiviral GFP transduction.45 Larger doses of TBI were associated with higher in vivo gene marking levels, evaluated by both GFP-positive percentages and vector copy numbers (VCNs). However, at levels utilized for reduced intensity conditioning (4 Gy), immunoresponses to GFP were observed (Figure 1C). GFP-positive percentages (%GFP) were transiently elevated to >90% in granulocytes at 1–3 months post-transplant and subsequently reduced to undetectable levels.45,46 When >90% GFP was detected in granulocytes, the GFP localization pattern (several punctate, intense GFP signals in granulocyte cytoplasm) differed from the even GFP signal observed in lentivirally transduced cells with stable GFP marking and could be due to internalization (phagocytosis) of GFP protein by granulocytes.17 A positive mixed lymphocyte reaction (MLR) assay to GFP-positive autologous cells and anti-GFP antibody production remained detectable after %GFP decreased to undetectable levels, indicating both cellular- and humoral-mediated immunity to GFP after 4 Gy TBI conditioning.45,46 Furthermore, lower doses of TBI were associated with increased anti-GFP antibody production.36 These data demonstrate that myeloablative doses of TBI are required for efficient engraftment of and tolerance to gene-modified HSCs, except in diseases in which gene-modified cells have a survival advantage. Despite the frequent observation of anti-transgene immunity when using low-dose TBI, the necessity of immunosuppression remained unclear in these studies because TBI is both potently myeloablative and immunosuppressive.
Busulfan Conditioning Allows for Engraftment of Gene-Modified Cells Expressing a Non-immunogenic Transgene
In allogeneic HSC transplantation for nonmalignant disorders and in current gene therapy trials, myeloablation is more commonly accomplished using chemotherapy drugs such as busulfan or melphalan rather than TBI due to their reduced toxicities. While TBI has both myeloablative and immunosuppressive effects, busulfan and its analogs are mainly myelosuppressive, with minimal effect on the lymphoid compartment. Therefore, these agents must be combined with additional immunosuppression in allogeneic transplantation settings.47,48 By the same rationale, busulfan conditioning alone should not abrogate immunoresponses to foreign transgenes in the context of autologous gene therapy. Studies performed using mouse and nonhuman primate models established that busulfan conditioning provides sufficient myelosuppression to allow engraftment of lentivirally transduced HSCs, with gene marking increasing in a dose-dependent manner.49, 50, 51, 52 None of these initial studies reported the development of an immunoresponse to modified cells.
Another study on rhesus macaques evaluated whether the combination of busulfan and fludarabine would permit engraftment of and tolerance to HSCs transduced with lentiviral vectors containing GFP, which is immunogenic in these animals without conditioning.16 Both humoral and cellular immunity were observed to GFP in busulfan-conditioned animals, but GFP-transduced CD34+ cells were not completely eliminated.51 Immunoresponses were not observed to control cells containing a non-expressed DNA sequence tag. Due to differences in rhesus and human pharmacokinetics, fludarabine was excreted rapidly, and therefore its effects on tolerance induction could not be assessed, emphasizing the importance of performing appropriate dosage studies to obtain clinically predictive results in animal studies.51 A more recent study in rhesus macaques determined that myeloablative busulfan conditioning alone was sufficient for engraftment of human γ-globin-transduced HSCs, but it did not prevent rejection of GFP-transduced cells (Figure 1C).17 Therefore, busulfan conditioning alone may be insufficient when transduced cells produce xenogenic or congenitally absent proteins.
Post-transplant Immunosuppression for Prevention of Transgene-Specific Immunity
Immunosuppressive agents are given in combination with busulfan conditioning in allogeneic transplantation to prevent GVHD. Commonly used drugs include cyclosporine, tacrolimus, sirolimus, mycophenolate mofetil, and abatacept, which all act by inhibiting T cell activation.53 A number of these agents have shown some efficacy in preventing and inhibiting immunoresponses to foreign transgenes in preclinical and clinical gene therapy.17,53, 54, 55, 56, 57 In dogs transplanted with GFP-transduced HSCs, post-transplant cyclosporine prevented the development of immunoreactions to transgene products, which led to the elimination of transduced cells in non-cyclosporine-treated animals.55 Similarly, addition of abatacept (CTL-associated antigen 4 immunoglobulin [CTLA4-Ig]) and sirolimus (mammalian target of rapamycin [mTOR]) to myeloablative busulfan conditioning permitted engraftment of GFP-transduced cells in rhesus macaques (Figure 1C).17 Whereas immunosuppressive drugs at least temporarily prevented the development of immunoresponses in unprimed hosts, these drugs did not prevent immunoresponses to transplanted HSCs in animals demonstrating preexisting anti-transgene immunity.13,54 These observations have implications for HSC-targeted gene therapy in disorders where patients may have preexisting immunity to the transgenic protein from enzyme-replacement therapy.58 Furthermore, in studies that have successfully inhibited immunoresponses to foreign transgenes using immunosuppressive drugs, responses have sometimes developed after drug delivery was terminated. Therefore, conventional immunosuppressive regimens appear insufficient for inducing long-term tolerance to certain transgenes, although modulations in drug dosage and duration could potentially achieve this goal.
Clinical Findings in Gene Therapy Trials
Following several early setbacks, HSC-targeted gene therapy has been used to treat β-thalassemia, sickle cell disease (SCD), X-SCID, adenosine deaminase (ADA) deficiency, Wiskott-Aldrich syndrome (WAS), X-linked chronic granulomatous disease (X-CGD), MPS I, leukocyte adhesion deficiencies I and II (LAD1/2), cerebral adrenoleukodystrophy (ALD), cystinosis, Fabry’s disease, and Fanconi anemia.1,30,59,60 The success observed in many of these trials has been contingent on continuous improvements over the years in HSC collection and transduction conditions,61 gene delivery vectors,45 and conditioning protocols.36 The first clinical trials used γ-retroviruses for gene transfer, but these vectors were found to carry a risk of insertional mutagenesis due to a preference for insertion near promoter regions, which can be activated by viral enhancers.62,63 Self-inactivating (SIN) lentiviral vectors derived from HIV-1 are now used almost exclusively in current clinical applications, owing to their reduced genotoxicity and ability to transduce nondividing cells.64 In addition, most clinical trials use myeloablative chemotherapy to condition patients prior to transplantation. A notable exception to both of these trends is in trials for immunodeficiencies, in which the efficacy and safety of improved γ-retroviral vectors and nonmyeloablative conditioning regimens have been established.3,4,65, 66, 67
While immunological rejection of transgenes has occurred in other gene therapy settings, this phenomenon has not been conclusively observed in HSC-targeted clinical gene therapy trials to date. That immunological rejection of transduced HSCs has been reported in preclinical but not clinical gene therapy may reflect differences between the inherent immunogenicity of the transgenes and conditioning regimens that tend to be used in these respective settings.17 Most preclinical gene therapy studies utilize GFP to measure gene marking, due to its increased ease and accuracy of interpretation compared to vector-encoded drug-resistance genes or PCR-based methods. However, GFP is known to be highly immunogenic in mice, dogs, and nonhuman primates.14,16,55,68 Therefore, many of these experiments rely on high-dose TBI to provide immunosuppression as well as myelosuppression, despite its lack of current use in human gene therapy. In clinical settings, the transgene is typically a human gene rather than a foreign gene such as GFP. A recent study in rhesus macaques showed that myeloablative busulfan was sufficient for long-term engraftment of human γ-globin-expressing lentivirally transduced CD34+ cells, but not GFP-expressing cells.17 Similarly, in gene therapy trials for SCD and β-thalassemia, rejection of therapeutic βT87Q-globin has not been observed, likely because it differs from intact β-globin by only one amino acid.7,8
With respect to the requirement for immunosuppression, it may be important to distinguish between disorders in which a protein is altered, as in SCD, and disorders in which a protein is congenitally absent, as in many metabolic disorders. In a recent gene therapy trial for ALD, a lysosomal storage disorder caused by mutations in the ABCD1 gene, myeloablative busulfan conditioning and immunosuppression with pre-transplant cyclophosphamide allowed for successful engraftment of CD34+ cells transduced with ABCD1 cDNA-containing lentiviral vectors.69 However, in a similar trial for the lysosomal storage disorder metachromatic leukodystrophy, patients were conditioned using myeloablative busulfan alone. All patients displayed reconstituted arylsulfatase A (ARSA) production following transplantation and even without additional immunosuppression tested negative for ARSA and HIV-1 p24-specific antibodies.60 Notably, a trial for X-CGD was amended to add sirolimus to a myeloablative busulfan regimen after one patient displayed rapid loss of transduced cells post-transplantation. The investigators found no direct evidence of immunity to the gp91phox transgene in this patient, although a patient subsequently transplanted on the amended protocol displayed higher levels of marked cells.70
Immunoresponses in Preclinical HSC-Targeted Gene Editing
Gene transfer using retrovirus-based vectors is effective and has been successfully used in multiple clinical settings.2,7,71, 72, 73, 74 Despite these benefits, viral vectors integrate at unpredictable locations in the genome, which can lead to insertional mutagenesis and altered transgene expression patterns.75 In contrast, gene editing technologies allow modifications to be performed at a predetermined site, potentially reducing the risk of mutagenesis and allowing for maintenance of endogenous gene expression patterns. Gene editing is accomplished using programmable endonucleases, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and the CRISPR/Cas9 system, which is the latest and most popular method. These tools work by inducing site-specific DNA cleavage, thereby activating double-stranded break repair via either the non-homologous end joining (NHEJ) or homology-directed repair (HDR) pathways. The use of nucleases greatly enhances rates of HDR with donor DNA, making correction of disease-causing mutations feasible.76 Strategies to disrupt genes using NHEJ and to correct or insert genes using HDR are both in preclinical development.77, 78, 79
In both HSC-targeted gene editing using programmable nucleases and in gene therapy using viral vectors, autologous CD34+ HSCs are genetically modified ex vivo prior to transplantation. Therefore, conditioning and immunosuppression regimens developed for gene therapy should theoretically permit engraftment of gene-edited CD34+ cells. Similar to virally transduced CD34+ cells, gene-edited CD34+ cells should be tolerated following myeloablative busulfan conditioning; however, if edited CD34+ cells produce a neo-antigen, additional immunosuppression may be needed to prevent rejection of edited cells. In addition to the nature of the genetic manipulation, the potential immunogenicity of gene editing tools themselves must also be considered. Nucleases including ZFNs, TALENs, and Cas9 are foreign proteins and therefore may stimulate immunoresponses. Because CD34+ cells are only temporarily exposed to these proteins in ex vivo gene editing strategies, the likelihood that enough protein will remain to stimulate an immunoresponse in vivo seems low. In gene therapy with ex vivo lentiviral transduction, viral proteins such as capsid, integrase, reverse transcriptase, and protease are also temporally exposed to CD34+ cells, and immunological reactions to these proteins do not form when myeloablative conditioning is used. Thus, additional immunosuppression may be unnecessary to prevent immunoresponses to the nucleases exposed in ex vivo gene-edited HSCs.
While gene editing tools are unlikely to stimulate immunoresponses when used for ex vivo gene modification, they are more likely to do so when used to directly edit cells in vivo, as observed during in vivo liver-directed gene therapy for protein deficiencies.80 At present, gene modification of HSCs using viral vectors or gene editing tools is only clinically approved for ex vivo gene transfer. However, in vivo genetic modification of HSCs is under active investigation.81 In gene editing with the CRISPR/Cas9 system, the Cas9 gene is typically delivered using a viral vector, which possibly results in continuous nuclease expression in immunocompetent hosts. Whether intracellularly expressed Cas9 would stimulate immunoresponses was previously unknown, but pre-existing anti-Cas9 immunity was recently discovered in healthy human blood samples.82 Direct lysing of Cas9-expressing cells was not shown in this study, but the presence of antibodies and cellular immunity to Cas9 implies that cells modified to express this protein could be destroyed.82,83 Furthermore, overcoming pre-existing immunity is more difficult than preventing it in naive individuals. Recognition by Cas9-specific antibodies as well as CTLs may potentially reduce efficiency of homing and engraftment of gene-edited HSCs. Experiments in immunocompetent large animal models are needed to assess these issues further, but any immunological challenges presented by gene editing should not be fundamentally different from those already observed in the gene therapy field. Therefore, similar strategies to mediate them can likely be employed, such as adjusting the conditioning and immunosuppression regimen or delivery route.
Conclusions
Immunological responses to foreign transgenes are well documented in preclinical and clinical gene therapy studies. At present, immunological rejection of genetically modified HSCs has not been conclusively reported in human patients, although there is evidence from large animals and suggestions in at least one clinical trial that this could occur in some settings. The infrequency with which this phenomenon is observed in HSC-targeted gene therapy can be explained by a number of factors, perhaps most importantly the ability of transduced HSCs to confer transgene-specific tolerance to successfully transplanted recipients. While the risk of rejection on the basis of transgene immunity appears to be lower in ex vivo HSC gene therapy than in other forms of gene therapy, the immunogenicity of the transgene in its disease-specific context must be carefully considered when designing conditioning and immunosuppressive regimens for clinical trials. Recent preclinical developments in HSC-targeted gene therapy, such as gene editing and in vivo gene therapy, are likely to pose additional immunological concerns than the ones directly assessed in the studies reviewed herein. Going forward, clinically predictive nonhuman primate experiments will continue to be critical for studying the potential immunogenicity of modified HSCs. The field of gene therapy has successfully overcome many barriers to clinical application, with patients beginning to see therapeutic benefits in recent years. There is every reason to believe that the potential immunogenicity of genetically modified HSCs can be countered with similar ingenuity.
Author Contributions
C.M.D., J.F.T., and N.U. wrote the paper.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK) at the NIH. We thank Tina Nassehi for editing our manuscript.
References
- 1.Morgan R.A., Gray D., Lomova A., Kohn D.B. Hematopoietic stem cell gene therapy: progress and lessons learned. Cell Stem Cell. 2017;21:574–590. doi: 10.1016/j.stem.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiuti A., Cattaneo F., Galimberti S., Benninghoff U., Cassani B., Callegaro L., Scaramuzza S., Andolfi G., Mirolo M., Brigida I. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N. Engl. J. Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 4.Aiuti A., Slavin S., Aker M., Ficara F., Deola S., Mortellaro A., Morecki S., Andolfi G., Tabucchi A., Carlucci F. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 5.Boztug K., Schmidt M., Schwarzer A., Banerjee P.P., Díez I.A., Dewey R.A., Böhm M., Nowrouzi A., Ball C.R., Glimm H. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo M., Hacein-Bey S., de Saint Basile G., Gross F., Yvon E., Nusbaum P., Selz F., Hue C., Certain S., Casanova J.L. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 7.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ribeil J.A., Hacein-Bey-Abina S., Payen E., Magnani A., Semeraro M., Magrin E., Caccavelli L., Neven B., Bourget P., El Nemer W. Gene therapy in a patient with sickle cell disease. N. Engl. J. Med. 2017;376:848–855. doi: 10.1056/NEJMoa1609677. [DOI] [PubMed] [Google Scholar]
- 9.Copelan E.A. Hematopoietic stem-cell transplantation. N. Engl. J. Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 10.Riddell S.R., Elliott M., Lewinsohn D.A., Gilbert M.J., Wilson L., Manley S.A., Lupton S.D., Overell R.W., Reynolds T.C., Corey L., Greenberg P.D. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat. Med. 1996;2:216–223. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 11.Bonini C., Ferrari G., Verzeletti S., Servida P., Zappone E., Ruggieri L., Ponzoni M., Rossini S., Mavilio F., Traversari C., Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 12.Liberatore C., Capanni M., Albi N., Volpi I., Urbani E., Ruggeri L., Mencarelli A., Grignani F., Velardi A. Natural killer cell-mediated lysis of autologous cells modified by gene therapy. J. Exp. Med. 1999;189:1855–1862. doi: 10.1084/jem.189.12.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutzko C., Kruth S., Abrams-Ogg A.C., Lau K., Li L., Clark B.R., Ruedy C., Nanji S., Foster R., Kohn D. Genetically corrected autologous stem cells engraft, but host immune responses limit their utility in canine alpha-l-iduronidase deficiency. Blood. 1999;93:1895–1905. [PubMed] [Google Scholar]
- 14.Stripecke R., Carmen Villacres M., Skelton D., Satake N., Halene S., Kohn D. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 15.Kung S.K., An D.S., Bonifacino A., Metzger M.E., Ringpis G.E., Mao S.H., Chen I.S., Donahue R. Induction of transgene-specific immunological tolerance in myeloablated nonhuman primates using lentivirally transduced CD34+ progenitor cells. Mol. Ther. 2003;8:981–991. doi: 10.1016/j.ymthe.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig M., Connole M., Glickman R., Yue S.P., Noren B., DeMaria M., Johnson R.P. Induction of cytotoxic T lymphocyte and antibody responses to enhanced green fluorescent protein following transplantation of transduced CD34+ hematopoietic cells. Blood. 2001;97:1951–1959. doi: 10.1182/blood.v97.7.1951. [DOI] [PubMed] [Google Scholar]
- 17.Uchida N., Nassehi T., Drysdale C.M., Gamer J., Yapundich M., Bonifacino A.C., Krouse A.E., Linde N., Hsieh M.M., Donahue R.E. Busulfan combined with immunosuppression allows efficient engraftment of gene-modified cells in a rhesus macaque model. Mol. Ther. 2019;27:1586–1596. doi: 10.1016/j.ymthe.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heim D.A., Hanazono Y., Giri N., Wu T., Childs R., Sellers S.E., Muul L., Agricola B.A., Metzger M.E., Donahue R.E. Introduction of a xenogeneic gene via hematopoietic stem cells leads to specific tolerance in a rhesus monkey model. Mol. Ther. 2000;1:533–544. doi: 10.1006/mthe.2000.0072. [DOI] [PubMed] [Google Scholar]
- 19.Nikolic B., Sykes M. Mixed hematopoietic chimerism and transplantation tolerance. Immunol. Res. 1997;16:217–228. doi: 10.1007/BF02786391. [DOI] [PubMed] [Google Scholar]
- 20.Evans G.L., Morgan R.A. Genetic induction of immune tolerance to human clotting factor VIII in a mouse model for hemophilia A. Proc. Natl. Acad. Sci. USA. 1998;95:5734–5739. doi: 10.1073/pnas.95.10.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schumacher I.K., Newberg M.H., Jackson J.D., Hammel J.M., Rubocki R.J., Engelhard V.H., Fox I.J. Use of gene therapy to suppress the antigen-specific immune responses in mice to an HLA antigen. Transplantation. 1996;62:831–836. doi: 10.1097/00007890-199609270-00022. [DOI] [PubMed] [Google Scholar]
- 22.Andersson G., Illigens B.M., Johnson K.W., Calderhead D., LeGuern C., Benichou G., White-Scharf M.E., Down J.D. Nonmyeloablative conditioning is sufficient to allow engraftment of EGFP-expressing bone marrow and subsequent acceptance of EGFP-transgenic skin grafts in mice. Blood. 2003;101:4305–4312. doi: 10.1182/blood-2002-06-1649. [DOI] [PubMed] [Google Scholar]
- 23.Scott D.W. Gene therapy for immunologic tolerance: using bone marrow-derived cells to treat autoimmunity and hemophilia. Curr. Stem Cell Res. Ther. 2011;6:38–43. doi: 10.2174/157488811794480753. [DOI] [PubMed] [Google Scholar]
- 24.Tian C., Bagley J., Kaye J., Iacomini J. Induction of T cell tolerance to a protein expressed in the cytoplasm through retroviral-mediated gene transfer. J. Gene Med. 2003;5:359–365. doi: 10.1002/jgm.363. [DOI] [PubMed] [Google Scholar]
- 25.Donahue R.E., Wersto R.P., Allay J.A., Agricola B.A., Metzger M.E., Nienhuis A.W., Persons D.A., Sorrentino B.P. High levels of lymphoid expression of enhanced green fluorescent protein in nonhuman primates transplanted with cytokine-mobilized peripheral blood CD34+ cells. Blood. 2000;95:445–452. [PubMed] [Google Scholar]
- 26.Uchida N., Weitzel R.P., Shvygin A., Skala L.P., Raines L., Bonifacino A.C., Krouse A.E., Metzger M.E., Donahue R.E., Tisdale J.F. Total body irradiation must be delivered at high dose for efficient engraftment and tolerance in a rhesus stem cell gene therapy model. Mol. Ther. Methods Clin. Dev. 2016;3:16059. doi: 10.1038/mtm.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bodine D.M., Moritz T., Donahue R.E., Luskey B.D., Kessler S.W., Martin D.I., Orkin S.H., Nienhuis A.W., Williams D.A. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993;82:1975–1980. [PubMed] [Google Scholar]
- 28.Tutschka P.J., Santon G.W. Bone marrow transplantation in the busulfin-treated rat. III. Relationship between myelosuppression and immunosuppression for conditioning bone marrow recipients. Transplantation. 1977;24:52–62. doi: 10.1097/00007890-197707000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Bordignon C., Notarangelo L.D., Nobili N., Ferrari G., Casorati G., Panina P., Mazzolari E., Maggioni D., Rossi C., Servida P. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA− immunodeficient patients. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 30.Kohn D.B., Weinberg K.I., Nolta J.A., Heiss L.N., Lenarsky C., Crooks G.M., Hanley M.E., Annett G., Brooks J.S., el-Khoureiy A. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat. Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunbar C.E., Kohn D.B., Schiffmann R., Barton N.W., Nolta J.A., Esplin J.A., Pensiero M., Long Z., Lockey C., Emmons R.V. Retroviral transfer of the glucocerebrosidase gene into CD34+ cells from patients with Gaucher disease: in vivo detection of transduced cells without myeloablation. Hum. Gene Ther. 1998;9:2629–2640. doi: 10.1089/hum.1998.9.17-2629. [DOI] [PubMed] [Google Scholar]
- 32.Malech H.L., Maples P.B., Whiting-Theobald N., Linton G.F., Sekhsaria S., Vowells S.J., Li F., Miller J.A., DeCarlo E., Holland S.M. Prolonged production of NADPH oxidase-corrected granulocytes after gene therapy of chronic granulomatous disease. Proc. Natl. Acad. Sci. USA. 1997;94:12133–12138. doi: 10.1073/pnas.94.22.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart F.M., Crittenden R.B., Lowry P.A., Pearson-White S., Quesenberry P.J. Long-term engraftment of normal and post-5-fluorouracil murine marrow into normal nonmyeloablated mice. Blood. 1993;81:2566–2571. [PubMed] [Google Scholar]
- 34.Wu D.D., Keating A. Hematopoietic stem cells engraft in untreated transplant recipients. Exp. Hematol. 1993;21:251–256. [PubMed] [Google Scholar]
- 35.Schiffmann R., Medin J.A., Ward J.M., Stahl S., Cottler-Fox M., Karlsson S. Transfer of the human glucocerebrosidase gene into hematopoietic stem cells of nonablated recipients: successful engraftment and long-term expression of the transgene. Blood. 1995;86:1218–1227. [PubMed] [Google Scholar]
- 36.Bernardo M.E., Aiuti A. The role of conditioning in hematopoietic stem-cell gene therapy. Hum. Gene Ther. 2016;27:741–748. doi: 10.1089/hum.2016.103. [DOI] [PubMed] [Google Scholar]
- 37.Morris J.C., Conerly M., Thomasson B., Storek J., Riddell S.R., Kiem H.P. Induction of cytotoxic T-lymphocyte responses to enhanced green and yellow fluorescent proteins after myeloablative conditioning. Blood. 2004;103:492–499. doi: 10.1182/blood-2003-07-2324. [DOI] [PubMed] [Google Scholar]
- 38.Uchida N., Bonifacino A., Krouse A.E., Metzger M.E., Csako G., Lee-Stroka A., Fasano R.M., Leitman S.F., Mattapallil J.J., Hsieh M.M. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34+ cells mobilized by G-CSF and plerixafor. Exp. Hematol. 2011;39:795–805. doi: 10.1016/j.exphem.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mardiney M., 3rd, Malech H.L. Enhanced engraftment of hematopoietic progenitor cells in mice treated with granulocyte colony-stimulating factor before low-dose irradiation: implications for gene therapy. Blood. 1996;87:4049–4056. [PubMed] [Google Scholar]
- 40.Mardiney M., 3rd, Jackson S.H., Spratt S.K., Li F., Holland S.M., Malech H.L. Enhanced host defense after gene transfer in the murine p47phox-deficient model of chronic granulomatous disease. Blood. 1997;89:2268–2275. [PubMed] [Google Scholar]
- 41.Kang E., Giri N., Wu T., Sellers S., Kirby M., Hanazono Y., Tisdale J., Dunbar C.E. In vivo persistence of retrovirally transduced murine long-term repopulating cells is not limited by expression of foreign gene products in the fully or minimally myeloablated setting. Hum. Gene Ther. 2001;12:1663–1672. doi: 10.1089/10430340152528156. [DOI] [PubMed] [Google Scholar]
- 42.Huhn R.D., Tisdale J.F., Agricola B., Metzger M.E., Donahue R.E., Dunbar C.E. Retroviral marking and transplantation of rhesus hematopoietic cells by nonmyeloablative conditioning. Hum. Gene Ther. 1999;10:1783–1790. doi: 10.1089/10430349950017464. [DOI] [PubMed] [Google Scholar]
- 43.Kang E.M., Hanazano Y., Frare P., Vanin E.F., De Witte M., Metzger M., Liu J.M., Tisdale J.F. Persistent low-level engraftment of rhesus peripheral blood progenitor cells transduced with the Fanconi anemia C gene after conditioning with low-dose irradiation. Mol. Ther. 2001;3:911–919. doi: 10.1006/mthe.2001.0337. [DOI] [PubMed] [Google Scholar]
- 44.Rosenzweig M., MacVittie T.J., Harper D., Hempel D., Glickman R.L., Johnson R.P., Farese A.M., Whiting-Theobald N., Linton G.F., Yamasaki G. Efficient and durable gene marking of hematopoietic progenitor cells in nonhuman primates after nonablative conditioning. Blood. 1999;94:2271–2286. [PubMed] [Google Scholar]
- 45.Uchida N., Hsieh M.M., Bonifacino A.C., Krouse A.E., Metzger M.E., Donahue R.E., Tisdale J.F. Development of a new generation, forward-oriented therapeutic vector for hemoglobin disorders. Blood. 2016;128:1172. doi: 10.1038/s41467-019-12456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchida N., Weitzel R.P., Evans M.E., Green R., Bonifacino A.C., Krouse A.E., Metzger M.E., Hsieh M.M., Donahue R.E., Tisdale J.F. Evaluation of engraftment and immunological tolerance after reduced intensity conditioning in a rhesus hematopoietic stem cell gene therapy model. Gene Ther. 2014;21:148–157. doi: 10.1038/gt.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slavin S., Nagler A., Naparstek E., Kapelushnik Y., Aker M., Cividalli G., Varadi G., Kirschbaum M., Ackerstein A., Samuel S. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 48.Bornhäuser M., Thiede C., Schuler U., Platzbecker U., Freiberg-Richter J., Helwig A., Plettig R., Röllig C., Naumann R., Kroschinsky F. Dose-reduced conditioning for allogeneic blood stem cell transplantation: durable engraftment without antithymocyte globulin. Bone Marrow Transplant. 2000;26:119–125. doi: 10.1038/sj.bmt.1702500. [DOI] [PubMed] [Google Scholar]
- 49.Kahl C.A., Tarantal A.F., Lee C.I., Jimenez D.F., Choi C., Pepper K., Petersen D., Fletcher M.D., Leapley A.C., Fisher J. Effects of busulfan dose escalation on engraftment of infant rhesus monkey hematopoietic stem cells after gene marking by a lentiviral vector. Exp. Hematol. 2006;34:369–381. doi: 10.1016/j.exphem.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Hayakawa J., Hsieh M.M., Uchida N., Phang O., Tisdale J.F. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells. 2009;27:175–182. doi: 10.1634/stemcells.2008-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tarantal A.F., Giannoni F., Lee C.C., Wherley J., Sumiyoshi T., Martinez M., Kahl C.A., Elashoff D., Louie S.G., Kohn D.B. Nonmyeloablative conditioning regimen to increase engraftment of gene-modified hematopoietic stem cells in young rhesus monkeys. Mol. Ther. 2012;20:1033–1045. doi: 10.1038/mt.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang E.M., Hsieh M.M., Metzger M., Krouse A., Donahue R.E., Sadelain M., Tisdale J.F. Busulfan pharmacokinetics, toxicity, and low-dose conditioning for autologous transplantation of genetically modified hematopoietic stem cells in the rhesus macaque model. Exp. Hematol. 2006;34:132–139. doi: 10.1016/j.exphem.2005.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos G.W., Tutschka P.J., Brookmeyer R., Saral R., Beschorner W.E., Bias W.B., Braine H.G., Burns W.H., Elfenbein G.J., Kaizer H. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N. Engl. J. Med. 1983;309:1347–1353. doi: 10.1056/NEJM198312013092202. [DOI] [PubMed] [Google Scholar]
- 54.Berger C., Huang M.L., Gough M., Greenberg P.D., Riddell S.R., Kiem H.P. Nonmyeloablative immunosuppressive regimen prolongs In vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J. Virol. 2001;75:799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beagles K.E., Peterson L., Zhang X., Morris J., Kiem H.P. Cyclosporine inhibits the development of green fluorescent protein (GFP)-specific immune responses after transplantation of GFP-expressing hematopoietic repopulating cells in dogs. Hum. Gene Ther. 2005;16:725–733. doi: 10.1089/hum.2005.16.725. [DOI] [PubMed] [Google Scholar]
- 56.Enssle J., Trobridge G.D., Keyser K.A., Ironside C., Beard B.C., Kiem H.P. Stable marking and transgene expression without progression to monoclonality in canine long-term hematopoietic repopulating cells transduced with lentiviral vectors. Hum. Gene Ther. 2010;21:397–403. doi: 10.1089/hum.2009.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horn P.A., Keyser K.A., Peterson L.J., Neff T., Thomasson B.M., Thompson J., Kiem H.P. Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood. 2004;103:3710–3716. doi: 10.1182/blood-2003-07-2414. [DOI] [PubMed] [Google Scholar]
- 58.Squeri G., Passerini L., Ferro F., Laudisa C., Tomasoni D., Deodato F., Donati M.A., Gasperini S., Aiuti A., Bernardo M.E. Targeting a pre-existing anti-transgene T cell response for effective gene therapy of MPS-I in the mouse model of the disease. Mol. Ther. 2019;27:1215–1227. doi: 10.1016/j.ymthe.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marktel S., Scaramuzza S., Cicalese M.P., Giglio F., Galimberti S., Lidonnici M.R., Calbi V., Assanelli A., Bernardo M.E., Rossi C. Intrabone hematopoietic stem cell gene therapy for adult and pediatric patients affected by transfusion-dependent ß-thalassemia. Nat. Med. 2019;25:234–241. doi: 10.1038/s41591-018-0301-6. [DOI] [PubMed] [Google Scholar]
- 60.Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. doi: 10.1016/S0140-6736(16)30374-9. [DOI] [PubMed] [Google Scholar]
- 61.Uchida N., Nassehi T., Drysdale C.M., Gamer J., Yapundich M., Demirci S., Haro-Mora J.J., Leonard A., Hsieh M.M., Tisdale J.F. High-efficiency lentiviral transduction of human CD34+ cells in high-density culture with poloxamer and prostaglandin E2. Mol. Ther. Methods Clin. Dev. 2019;13:187–196. doi: 10.1016/j.omtm.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hacein-Bey-Abina S., von Kalle C., Schmidt M., Le Deist F., Wulffraat N., McIntyre E., Radford I., Villeval J.L., Fraser C.C., Cavazzana-Calvo M., Fischer A. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- 63.Hacein-Bey-Abina S., Von Kalle C., Schmidt M., McCormack M.P., Wulffraat N., Leboulch P., Lim A., Osborne C.S., Pawliuk R., Morillon E. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 64.An D.S., Wersto R.P., Agricola B.A., Metzger M.E., Lu S., Amado R.G., Chen I.S., Donahue R.E. Marking and gene expression by a lentivirus vector in transplanted human and nonhuman primate CD34+ cells. J. Virol. 2000;74:1286–1295. doi: 10.1128/jvi.74.3.1286-1295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gaspar H.B., Cooray S., Gilmour K.C., Parsley K.L., Zhang F., Adams S., Bjorkegren E., Bayford J., Brown L., Davies E.G. Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction. Sci. Transl. Med. 2011;3:97ra80. doi: 10.1126/scitranslmed.3002716. [DOI] [PubMed] [Google Scholar]
- 66.Candotti F., Shaw K.L., Muul L., Carbonaro D., Sokolic R., Choi C., Schurman S.H., Garabedian E., Kesserwan C., Jagadeesh G.J. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120:3635–3646. doi: 10.1182/blood-2012-02-400937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fischer A., Hacein-Bey Abina S., Touzot F., Cavazzana M. Gene therapy for primary immunodeficiencies. Clin. Genet. 2015;88:507–515. doi: 10.1111/cge.12576. [DOI] [PubMed] [Google Scholar]
- 68.Skelton D., Satake N., Kohn D.B. The enhanced green fluorescent protein (eGFP) is minimally immunogenic in C57BL/6 mice. Gene Ther. 2001;8:1813–1814. doi: 10.1038/sj.gt.3301586. [DOI] [PubMed] [Google Scholar]
- 69.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., Armant M., Dansereau C., Lund T.C., Miller W.P. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N. Engl. J. Med. 2017;377:1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang E.M., Choi U., Theobald N., Linton G., Long Priel D.A., Kuhns D., Malech H.L. Retrovirus gene therapy for X-linked chronic granulomatous disease can achieve stable long-term correction of oxidase activity in peripheral blood neutrophils. Blood. 2010;115:783–791. doi: 10.1182/blood-2009-05-222760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., Veres G., Schmidt M., Kutschera I., Vidaud M., Abel U., Dal-Cortivo L., Caccavelli L. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 72.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 73.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw K.L., Garabedian E., Mishra S., Barman P., Davila A., Carbonaro D., Shupien S., Silvin C., Geiger S., Nowicki B. Clinical efficacy of gene-modified stem cells in adenosine deaminase-deficient immunodeficiency. J. Clin. Invest. 2017;127:1689–1699. doi: 10.1172/JCI90367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cesana D., Ranzani M., Volpin M., Bartholomae C., Duros C., Artus A., Merella S., Benedicenti F., Sergi Sergi L., Sanvito F. Uncovering and dissecting the genotoxicity of self-inactivating lentiviral vectors in vivo. Mol. Ther. 2014;22:774–785. doi: 10.1038/mt.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim H., Kim J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 77.Canver M.C., Smith E.C., Sher F., Pinello L., Sanjana N.E., Shalem O., Chen D.D., Schupp P.G., Vinjamur D.S., Garcia S.P. BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature. 2015;527:192–197. doi: 10.1038/nature15521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Traxler E.A., Yao Y., Wang Y.D., Woodard K.J., Kurita R., Nakamura Y., Hughes J.R., Hardison R.C., Blobel G.A., Li C., Weiss M.J. A genome-editing strategy to treat β-hemoglobinopathies that recapitulates a mutation associated with a benign genetic condition. Nat. Med. 2016;22:987–990. doi: 10.1038/nm.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pavel-Dinu M., Wiebking V., Dejene B.T., Srifa W., Mantri S., Nicolas C.E., Lee C., Bao G., Kildebeck E.J., Punjya N. Gene correction for SCID-X1 in long-term hematopoietic stem cells. Nat. Commun. 2019;10:1634. doi: 10.1038/s41467-019-09614-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mingozzi F., High K.A. Immune responses to AAV in clinical trials. Curr. Gene Ther. 2011;11:321–330. doi: 10.2174/156652311796150354. [DOI] [PubMed] [Google Scholar]
- 81.Richter M., Saydaminova K., Yumul R., Krishnan R., Liu J., Nagy E.E., Singh M., Izsvák Z., Cattaneo R., Uckert W. In vivo transduction of primitive mobilized hematopoietic stem cells after intravenous injection of integrating adenovirus vectors. Blood. 2016;128:2206–2217. doi: 10.1182/blood-2016-04-711580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Charlesworth C.T., Deshpande P.S., Dever D.P., Camarena J., Lemgart V.T., Cromer M.K., Vakulskas C.A., Collingwood M.A., Zhang L., Bode N.M. Identification of preexisting adaptive immunity to Cas9 proteins in humans. Nat. Med. 2019;25:249–254. doi: 10.1038/s41591-018-0326-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Crudele J.M., Chamberlain J.S. Cas9 immunity creates challenges for CRISPR gene editing therapies. Nat. Commun. 2018;9:3497. doi: 10.1038/s41467-018-05843-9. [DOI] [PMC free article] [PubMed] [Google Scholar]