Abstract
Carotenoid-rich fractions (CRF) from pulp and peel of a new variety of purple tomato were investigated in comparison to a Red Cherry variety regarding carotenoids characterization, antioxidant capacity, and inhibition of proliferation of four tumor cell lines. CRF from peel of Purple tomato contains lutein, lycopene, and β-carotene up to 6, 1.5, and 2.5 times more than that of Red Cherry and it exhibited the highest antioxidant activity at 400 μg/mL, reaching 82% and 97% in DPPH and ABTS•+ assays, respectively. Besides that, the Purple peel showed the highest scavenging lipoperoxides capacity as well as displayed the highest ferric reducing antioxidant power compared to the other CRF. In turn, the Red Cherry pulp CRF showed the highest antiproliferative activity against four tumor cell lines (MCF-7, NCI-H460, HeLa, and HepG2) at non-toxic concentrations. High concentration of neurosporene, and lycopene in Red Cherry pulp CRF show to be related to the good antiproliferative activity found on it. Therefore, this new variety of nutrient-rich purple tomato could be explored as well as the commercial variety Red Cherry, since both are good sources of dietary carotenoids with health-promoting properties.
Keywords: Food science, Antioxidant, Phenolic compound, Food component analysis, Chemical composition of food, Chemical characterization of food, Food biochemistry, Lycopene, Carotene, Hepatotoxicity, Antiproliferative activity, Solanum lycopersicum L.
Food Science; Antioxidant; Phenolic Compound; Food Component Analysis; Chemical Composition of Food; Chemical Characterization of Food; Food Biochemistry; Lycopene; Carotene; Hepatotoxicity; Antiproliferative activity; Solanum lycopersicum L.
1. Introduction
The role of nutrition and diet in human health has attracted great interest in recent years. Several epidemiological studies have pointed that vegetable consumption contributes to reduce risks associated with various diseases such as atherosclerosis and cancer (Lenucci et al., 2006; Podsędek, 2007; Del Giudice et al., 2015). The beneficial effects attributed to vegetables and fruits are particularly related to the antioxidant activity of food constituents (Zanfini et al., 2010; Stačiċ et al., 2015; Soares et al., 2019). Antioxidants predominantly present in plants are vitamins C and E, phenolic compounds, and carotenoids (De Pascual-Teresa, Sanchez-Ballesta and García-Viguera, 2013). Regarding the last ones, their structure, such as size and presence of functional groups, is directly linked to their bioactive functions. A large range of fruits and vegetables is rich in bioactive compounds such as carotenoids including carrots, papaya, and tomatoes.
Tomatoes (Solanum lycopersicum L.) are among the most widely consumed crops. This fruit is consumed both in natura and processed in a wide range of products (Toor and Savage, 2005). Fresh fruits and tomato products contain 35%–96% total lycopene and 1%–22% cis-lycopene (Schierle et al., 1997; Holloway et al., 2000; Livny et al., 2002). Since tomato is a fruit rich in various carotenoid pigments, especially lycopene, it represents an important source of these molecules for human nutrition (Khachik et al., 2002).
Carotenoids play a major role in human nutrition and health, because they take part in pro-vitamin A and display anticancer properties (Fraser and Bramley, 2004). Moreover, carotenoids are excellent deactivators of reactive oxygen species (ROS) such as singlet oxygen (1O2) and peroxyl radical (ROO•) (Jomova and Valko, 2013; Burton and Ingold, 1984; Foote et al., 1970; Edge et al., 1997). Studies suggest that consumption of fresh market tomatoes and tomato products reduces the risk of chronic diseases such as cardiovascular diseases and cancer (Stačiċ et al., 2015; Palozza et al., 2011; Niranjana et al., 2015). In fact, a relation between the consumption of large amounts of tomato-based products and the reduction of prostate cancer cell proliferation has been associated to lycopene (Kim et al., 2004; Bommareddy et al., 2013).
Given that tomato is grown and consumed worldwide, it is a natural candidate for breeding for nutrient enrichment. Therefore, it has been genetically engineered to produce fruits with different physiological characteristics and improved nutritional properties. This study aimed to evaluate the composition of the carotenoid-rich fractions (CRF) of a new variety of nutrient-rich purple tomato, bred to display high concentrations of anthocyanin, ascorbic acid, and carotenoids. This non-transgenic variety was compared to a commercial Red Cherry variety regarding antioxidant activity of peel and pulp extracts as well as their antiproliferative activity against human tumor cell lines.
2. Material and methods
2.1. Plant material and processing
The nutrient-rich tomato near-isogenic lines (NILs) carrying the mutations anthocyanin fruit (Aft), atroviolacium (atv), aubergine (Abg), beta-carotene (B), old-gold crimsom (og), and high pigment 1 and 2 (hp1, hp2) from their original genetic backgrounds into Micro-Tom (MT) (Sestari et al., 2014), a new variety of nutrient-rich purple tomato (Solanum lycopersicum L. cv Micro-Tom), produced in a greenhouse at the Departamento de Ciências Biológicas, Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo, Piracicaba, SP, Brazil (22°42′30″S; 47°38′30″W), and the Red Cherry variety (Solanum lycopersicum cv cerasiforme), purchased in a local market, were employed in this study (Fig. 1). The purple tomato was considered ripe for harvest when all the fruits were black, approximately two weeks from the appearance of the fruit. Ripe tomatoes were manually separated into peel, pulp, and seeds, and only the peel and pulp were used. The amount of raw material used was 500 g of each variety. After lyophilization, the samples were packaged in plastic bags protected from light and air and kept at −80 °C until extraction. Just before extraction, the lyophilized tomatoes were milled to a powder in a stainless steel mill at 28,000 rpm (Basic IKA A11, IKA, Campinas, SP, Brazil).
Fig. 1.
Visual aspect of Purple (A) and Red Cherry (B) tomatoes.
2.2. Obtention of carotenoids-rich fractions (CRF)
Aliquots of 10 mL of acetone: hexane (4:6, v:v) were added to 100 mg of samples (taken from ultrafreezer and immediately milled) and stirred for 20 min in sealed vials, followed by filtration through a qualitative paper filter 80 g (J Prolab Ind. e Com. de Produtos para Laboratório Ltda, São José dos Pinhais, PR, Brazil). The process was performed two more times, and the CRF were pooled and dried under nitrogen flow. The masses of the peel and pulp CRF were determined, and they were stored at −80 °C until analysis (Nagata and Yamashita, 1992). For DPPH, ABTS•+, FRAP, TBARS, antiproliferative activity, and hepatotoxicity analyses, the CRF were firstly diluted with acetone: hexane (1:1; v:v) to a concentration of 1000 μg mL−1, and then diluted with ethanol p.a. to the final concentrations of 100, 200, and 400 μg mL−1. All extracts and analyses were performed in triplicate for each sample.
2.3. Characterization of carotenoids in CRF using HPLC
A Shimadzu liquid chromatograph Model LC20A (Shimadzu Co., Kyoto, Japan) equipped with a polymeric C30 column, 3 μm, 4.6 × 250 mm (YMC Co., Kyoto, Japan), and an ultraviolet-visible (UV-Vis) detector (SPD-20AV, Shimadzu) was used. The analyses were performed at a wavelength of 450 nm–470 nm, at 25 °C (CTO-20A, Shimadzu Co.) with the aid of an automatic injector (10 μL). The mobile phase used was composed of methanol (A) and tert-butyl methyl ether (B) at a flow rate of 0.8 ml min−1 as a linear gradient for 60 min, ranging from 90% to 40% of (A), followed by a rebalancing of the system to 90% of (A) for 15 min before another injection. The compounds were identified by their typical retention times in this method according to literature (Rodriguez-Amaya and Kimura, 2004). The quantification was performed using reference standards (β-carotene and lycopene, Sigma-Aldrich Co. LLC., St. Louis, MO, USA).
2.4. Evaluation of antioxidant activity
CRF were analyzed as antioxidant against the synthetic free radicals DPPH and ABTS. The DPPH method was performed as proposed by Brand-Willians et al. (1995) and the radical-scavenging activity was expressed as percentage of inhibition (%). ABTS•+ radical was produced according to Salvador et al. (2019). The analysis was carried out at 734 nm and the results were expressed as percentage of scavenging activity (%). Each CRF extract was analyzed in triplicate.
The assessment of endogenous lipid peroxidation was accomplished by detecting lipoperoxide derived from substances that react with thiobarbituric acid (Bird and Draper, 1984; Morais et al., 2006). Aliquots of 25 μL extracts or standards, 100 μL distilled water, and 125 μL 0.55% lyophilized egg yolk solution in sodium dodecyl sulfate as a source of lipids were added to falcon tubes. After that, 12.5 μL 0.07% 2,2-azo-bis-acid chloride (ABAP) aqueous solution, 375 μL 20% acetic acid solution (pH 3.5), and 375 μL thiobarbituric acid solution (TBA; 0.8% w:v) in sodium dodecyl sulfate solution (SDS; 0.55% w:v) were added and the resulting material was placed in a water bath (95 °C) for 1 h under stirring. After cooling, 1.2 mL n-butanol was added to each tube, centrifuged for 5 min at 8,000 rpm, and the supernatant was measured in a spectrophotometer at 532 nm. The same process was carried out using control tubes, containing all the reagents, except that the samples were not added, whereas in the white tube, ABAP was replaced with water. Analyses were carried out in quintuplicates. β-carotene and α-tocopherol were used as standards at same concentrations of samples (100, 200, and 400 μg mL−1). Inhibition of lipid peroxidation (%) was determined according to the equation 1 – (Abssample/Abscontrol) x 100.
CRF were analyzed by FRAP method according to Müller et al. (2010) with modifications. FRAP reagent was prepared as follows: 2.5 mL 10 mM 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ) solution in 40 mM HCl was added to 2.5 mL FeCl3.6H2O and 0.3 mL 25 mM acetate buffer (pH 3.6), the solution was incubated at 37 °C for 30 min. The analyses were performed in triplicate. The results are the mean of triplicates and expressed as μM Fe2+ per mg dry extract.
2.5. Evaluation of antiproliferative activity against human tumor cell lines
Human tumor cell lines MCF-7 (breast carcinoma), NCI-H460 (non-small cell lung cancer), HeLa (cervical carcinoma), and HepG2 (liver carcinoma cells) from the Leibniz-Institut Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ, Braunschweig, Germany) were used in this assay. Cells were maintained as adherent cell cultures in RPMI-1640 medium containing 10% inactivated fetal calf serum and 2 mM glutamine, at 37 °C in a humidified 5% CO2/95% air incubator. Cell lines were seeded at an appropriate density (1.0 × 104 cells/well) in 96-well plates. The sulforhodamine B test was conducted following the protocol described by Barros et al. (2013). Ellipticine and culture medium were used as positive and negative control, respectively, and the results are expressed in μgmL−1, growth inhibition of 50% (GI50, sample concentration capable of inhibiting the growth of cancer cells by 50%).
2.6. Hepatotoxicity (PLP2 cells)
A cell culture was prepared from fresh pig liver (Abreu et al., 2011). The cell culture was monitored every two or three days using a phase contrast microscope. Prior to cell confluence, cells were subcultured and inoculated into 96-well plates at a density of 1.0×104 cells/well in Dulbecco's Modified Eagle's medium (DMEM) with 10% inactivated fetal calf serum, 100 U.mL−1 penicillin, and 100 μg mL−1 streptomycin. Ellipticine and culture medium were used as positive and negative control, respectively, and the results are expressed in μg.mL−1, GI50.
2.7. Statistical analysis
Results are expressed as mean ± standard deviation. One-way analysis of variance (One-Way ANOVA) was performed for data analysis, and the Tukey's test was used for comparison of mean values. Pearson's correlation analysis was also performed to assess relationships between variables. Statistical analyses were carried out using Statistica 8.0 (StatSoft Inc., Tulsa, OK, USA). The results were considered statistically significant when p < 0.05.
3. Results and discussion
3.1. Yields of CRF and carotenoids characterization
Yields of CRF from Purple tomato were two-fold higher than Red Cherry (Table 1). Red Cherry variety did not have statistically significant difference in carotenoids yields between peel and pulp fractions.
Table 1.
Yield of Carotenoid-Rich Fractions (CRF) from the Purple and Red Cherry tomatoes.
| CRF | Yield (%) |
|
|---|---|---|
| Purple | Red Cherry | |
| Peel | 6.1 ± 0.7a | 3.1 ± 1.5a |
| Pulp | 7.1 ± 0.1b | 3.0 ± 0.8a |
Different letters between columns indicate significant difference (P < 0.05).
Lycopene (6), β-carotene (4), neurosporene (5), lutein (1), and isomers of β-carotene (3) were the major carotenoids present in CRF Purple peel and pulp. Lycopene was also the most abundant carotenoid in peel and pulp CRF of Red Cherry tomatoes, followed by neurosporene and β-carotene (Table 2). In Purple CRF, lutein, β-carotene, neurosporene, and lycopene were 1.7, 1.8, 1.8, and 2.1-fold higher in the peel than in the pulp, respectively. Instead, in CRF from Red Cherry, the concentrations of these compounds were higher in pulp. The qualitative chromatographic profile of Red Cherry was similar to that found for Purple tomatoes. Nonetheless, the former was lower in lutein and β-carotene.
Table 2.
Concentration of the carotenoids in Purple and Red Cherry tomatoes in mg 100g−1 in dry matter (DW).
| Variety | Lutein | 13 e 15-cis-β-carotene | Trans-β-carotene | Neurosporene | Lycopene | Total1 | |
|---|---|---|---|---|---|---|---|
| Purple | |||||||
| Peel | 7.24 ± 0.02a | 6.57 ± 0.10a | 10.14 ± 0.15a | 8.13 ± 0.20a | 12.95 ± 1.00a | 45.03 | |
| Pulp | 4.35 ± 0.08b | 3.65 ± 0.08b | 5.75 ± 0.03b | 4.52 ± 0.63b | 6.24 ± 0.19b | 24.51 | |
| Cherry | |||||||
| Peel | 1.25 ± 0.01d | 2.98 ± 0.31c | 3.90 ± 0.03c | 5.81 ± 0.04c | 8.47 ± 0.38c | 22.41 | |
| Pulp | 2.03 ± 0.02e | 2.86 ± 0.28c | 4.27 ± 0.06d | 7.30 ± 0.17d | 11.17 ± 0.55c | 27.63 | |
Different letters between columns indicate significant difference (P < 0.05).
Total is the sum of carotenoids quantified in the peel and pulp from Purple and Red Cherry tomatoes.
Carotenoids have been investigated in different varieties of tomatoes submitted to several agronomic traits, and a great diversity of concentration of these compounds has been reported, making the comparison of results difficult (Erba et al., 2013; D'Evoli et al., 2013; Acosta-Quezada et al., 2015). However, in a study of three varieties of tomatoes (Pera-Girona, Montserrat, and Caramba), the concentrations of lutein, β-carotene, and lycopene were inferior to those found in our study (Erba et al., 2013). In New Zealand, the assessment of three tomato cultivars led to the conclusion that the skin fraction of all cultivars had higher levels of lycopene compared to the pulp and seed fractions (Toor and Savage, 2005). This result may be associated with the fact that lycopene is mainly connected to the fiber fraction of the fruit (Sharma and Le Maguer, 1996). These findings support the results of CRF from Purple tomatoes in the present study, since the content of lycopene found in the peel fraction was higher than the pulp fraction (Table 2).
So far, few studies have been conducted with purple varieties of tomato aiming to combine genetic breeding with increased carotenoid concentration. Three mutations leading to uniformly purple fruits and enhanced nutrient contents from a Micro-Tom model cultivar were introduced into a commercial Cherry tomato cultivar. One of these mutations caused more chloroplast differentiation, which accounts for increased accumulation of carotenoids (Sestari et al., 2014). In fact, in the present study, CRF of peel from a new variety of Purple tomato was superior to CRF of peel from Red Cherry in the total sum of carotenoids content. The content of lutein in CRF of Purple tomato was two-fold higher in pulp and six-fold higher in peel compared to CRF of Red Cherry (Table 2). Since lutein is a xanthophyll present in green tissues, the high content found in CRF from Purple tomatoes is consistent with the presence of mutations enhancing chloroplast accumulation in the fruit (Sestari et al., 2014).
3.2. Antioxidant activity
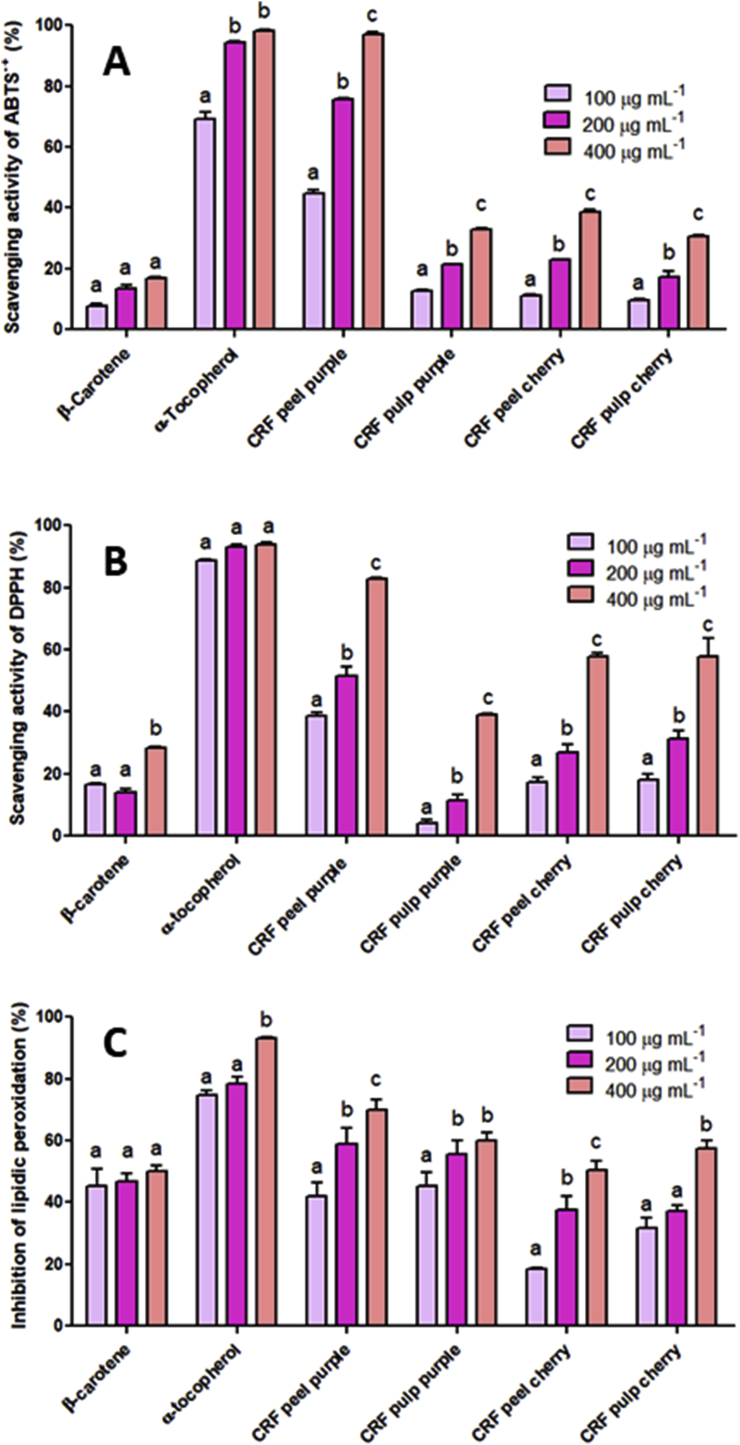
DPPH and ABTS•+ scavenging activities of CRF from Purple and Red Cherry tomatoes were compared to the standards β-carotene and α-tocopherol at the same concentrations (Fig. 2).
Fig. 2.
Free radical scavenging activity of DPPH (A), ABTS+•(B), and TBARS (C) of peel and pulp CRF from Purple and Red Cherry tomatoes. Different letters between columns in the groups indicate a significant difference (p < 0.05). CRF: carotenoid-rich fraction.
CRF of peel from Purple tomato displayed more efficient DPPH radical scavenging at all concentrations tested. DPPH scavenging activities were similar in Red Cherry peel and pulp fractions at each concentration, and 4.5-fold and 1.5-fold more efficient at 100 μg/mL and 400 μg/mL, respectively, than Purple pulp CRF. In a study using similar concentrations of extracts of five varieties of tomatoes, DPPH radical reduction capacity depended primarily on genetic characteristics (Stačiċ et al., 2015). In ABTS•+ assay, all fractions showed a concentration-dependent radical reduction capacity. Purple peel CRF at 400 μg/mL exhibited 97% inhibition, similar to that of α-tocopherol (98%) at the same concentration. Purple pulp CRF and Red Cherry pulp and peel CRF had similar behaviors compared at equal concentrations, and in none of them radical scavenging activity was above 40%. Given that hydrophobic carotenoids belong to a group of antioxidant compounds, their main mechanism of action is located in biological membranes (Young and Lowe, 2001).
In TBARS assay, both Purple CRF at 200 μg/mL had higher lipid oxidation inhibition (peel 60% and pulp 55.5%) than β-carotene standard (46.6%) (Fig. 2). At 400 μg/mL, Purple pulp and peel CRF and Red Cherry pulp CRF exhibited higher lipid oxidation inhibition than β-carotene, whereas Red Cherry peel CRF had similar inhibition (50%). Although in vitro tests indicate that the β-carotene incorporated into liposomes is an effective inhibitor of lipid peroxidation induced by oxidizing agents (Liebler et al., 1997), in humans this action is not yet clear (Young and Lowe, 2001). In experiments conducted in vivo, lycopene at 100, 200, 400, and 800 ppm was able to decrease lipid peroxidation as MDA as efficiently as BHT at 200 ppm (Basuny et al., 2009). The structural differences between carotenoids suggest different types of interaction with membrane lipids, which affect membrane fluidity and thus thermostability in different ways (Havaux et al., 1996).
The ability of Purple and Red Cherry CRF to reduce Fe3+-TPTZ to Fe2+-TPTZ, used as a criterion of antioxidant activity, is presented in Table 3. The standard β-carotene displayed the highest iron reducing capacity, following by Purple peel CRF, which is the fraction with the highest concentration of carotenoids (Table 2). Purple pulp and Red Cherry peel CRF showed no statistical differences regarding this parameter.
Table 3.
Antioxidant activity by FRAP method of Carotenoid-Rich Fractions (CRF) from the Purple and Red Cherry tomatoes.
| Samples | μM Fe2+.mg−1 dry extract |
|---|---|
| β-carotene | 12.92 ± 1.24a |
| α-tocopherol | 4.53 ± 0.22b |
| Purple peel | 1.25 ± 0.03c |
| Purple pulp | 0.50 ± 0.02d |
| Cherry peel | 0.41 ± 0.03d |
| Cherry pulp | 0.28 ± 0.01e |
Different letters between columns indicate significant difference (P < 0.05).
3.3. Antiproliferative and hepatotoxic activities
Carotenoids are one of several classes of biologically active compounds that display high antioxidant and anticancer activities (Niranjana et al., 2015). Epidemiological studies in humans suggest a protective effect of diets rich in carotenoids found in vegetables and fruits against cancer, but recent studies have demonstrated that isolatedly these phytonutrients are not able to exert beneficial health effects (Fiedor and Burda, 2014; Linnewiel-Hermoni et al., 2015). It has been proven that the combinations of carotenoids (lycopene, phytoene, and phytofluene) or carotenoids and polyphenols (carnosic acid and curcumin) act synergistically in cultures of prostate cancer cells by inhibiting the activity of the androgenic receptor and activating the electrophile/antioxidant response element (EpRE/ARE) transcription system (Linnewiel-Hermoni et al., 2015). In six tumor cell lines tested using lycopene for 48 h, reductions by 30% for T84 and HT-29 cells and 10% for MCF-7 cells were observed. After 96 h of treatment, only MCF-7 and HepG2 lines were responsive, with inhibition of 25% and 30% of cell viability, respectively, indicating that the effect of lycopene depends on time and cell type (Teodoro et al., 2012). In a study similar to the present one, with HeLa, MCF-7, and MRC-5 cell lines, a pronounced antiproliferative effect was observed in MRC-5 line and to a lesser extent in HeLa and MCF7 in concentrations ≥125 μg/mL hexane tomato waste extracts (Stačiċ et al., 2015). In the same study, antiproliferative activity against MCF-7 line was achieved by hexane tomato extracts at concentrations ranging from 600 to 1000 μg/mL. However, carotenoid-rich extracts obtained from Purple and commercial Red Cherry tomatoes at concentrations ranging from 51.22 to 182.31 μg/mL displayed better results (Table 4). This higher antiproliferative activity was attributed to the presence of a combination of lycopene and β-carotene (Stačiċ et al., 2015). Red Cherry pulp fraction achieved the highest antiproliferative activity against the four tumor cell lines tested in the present study, and the best result was against MCF-7 line. Purple peel and Red Cherry pulp fractions had similar good results against HepG2 line. Studies conducted with other breast (MCF-7, HBL-100, and MDA-MB-231) and prostate (LNCaP) cancer cell lines demonstrated inhibitory effects attributed to lycopene. In some cases, this carotenoid was a more effective anticancer agent than α- or β-carotene (Kim et al., 2004; Levy et al., 1995; Chalabi et al., 2004; Fornelli et al., 2007). The hepatotoxicity test demonstrated that Purple peel and pulp and Red Cherry peel fractions had toxic effects above 400 μg/mL and only Red Cherry pulp differed from this value, with a minimal toxic concentration of 293.93 μg/mL. These results show that the carotenoids present in the tomato varieties studied exhibited potential for the inhibition of tumor cell growth, with low toxicity to liver cells, indicating that they could be consumed at the concentrations studied.
Table 4.
Antiproliferative activity and hepatotoxicity of the Carotenoid-Rich Fractions (CRF) from the Purple and Red Cherry tomatoes.
| Samples/standard | Antiproliferative activity1 | Hepatotoxicity1 | |||
|---|---|---|---|---|---|
| MCF-7 | NCI-H460 | HeLa | HepG2 | PLP2 | |
| Purple Peel | 102.52 ± 5.64c | 156.82 ± 12.06b | 330.89 ± 11.52a | 87.20 ± 5.57b | >400 |
| Purple Pulp | 182.31 ± 17.90a | 203.05 ± 8.3a | >400 | 263.22 ± 11.75a | >400 |
| Cherry Peel | 139.44 ± 6.65b | 148.78 ± 13.92b | >400 | >400 | >400 |
| Cherry Pulp | 51.22 ± 1.16d | 94.80 ± 7.69c | 65.97 ± 3.24b | 81.69 ± 7.88b | 293.93 ± 12.08 |
| Ellipticine | 0.91 ± 0.04 | 1.03 ± 0.09 | 1.91 ± 0.06 | 1.14 ± 0.21 | 3.22 ± 0.67 |
Different letters between lines indicate significant difference (P < 0.05).
Antiproliferative activity and hepatotoxicity: values are expressed as GI50 (μg.mL−1).
3.4. Correlation between carotenoids and biological activities of purple and cherry tomatoes
Neurosporene and lycopene showed negative correlations with cell growth, indicating that these carotenoids had a high influence on antiproliferative activity of the tomato extracts (Table 5). Since the results of antiproliferative activity were achieved by GI50, a negative and strong correlation indicates that when these carotenoids are in high content, the GI50 for the cell evaluated tends to be low, evidencing the effect of these compounds. In fact, tomato powder containing phytofluene in addition to lycopene and other compounds was able to inhibit the prostate carcinogenesis in rats better than only lycopene (Meléndez-Martínez et al., 2015). Otherwise, a high positive correlation between lutein, β-carotenes, and the methods for evaluation of antioxidant activity was found (r > 0.7).
Table 5.
Correlations between carotenoids from CRF of Purple and Cherry tomatoes and their bioactivity activities.
| Correlation | Carotenoids |
||||||
|---|---|---|---|---|---|---|---|
| Lutein | 13 e 15-cis-β-carotene | Trans-β-carotene | Neurosporene | Lycopene | |||
| Bioactive activities | Anti-proliferative | MCF-7 | 0.10 | -0.03 | -0.02 | -0.83 | -0.81 |
| NCI-H460 | 0.43 | 0.27 | 0.30 | -0.64 | -0.61 | ||
| HeLa | 0.25 | 0.25 | 0.22 | -0.53 | -0.51 | ||
| HepG2 | -0.53 | -0.48 | -0.53 | -0.73 | -0.74 | ||
| Antioxidant | DPPH | 0.53 | 0.75 | 0.70 | 0.91* | 0.92* | |
| ABTS | 0.84 | 0.97** | 0.94* | 0.68 | 0.70 | ||
| FRAP | 0.92* | 1.00** | 0.98** | 0.54 | 0.57 | ||
| TBARS | 0.96** | 0.90* | 0.94* | 0.53 | 0.56 | ||
Values followed by (*) or (**) mean significative correlation at P < 0.1 or P < 0.05, respectively.
Anti-proliferative results used by correlation analysis were GI50.
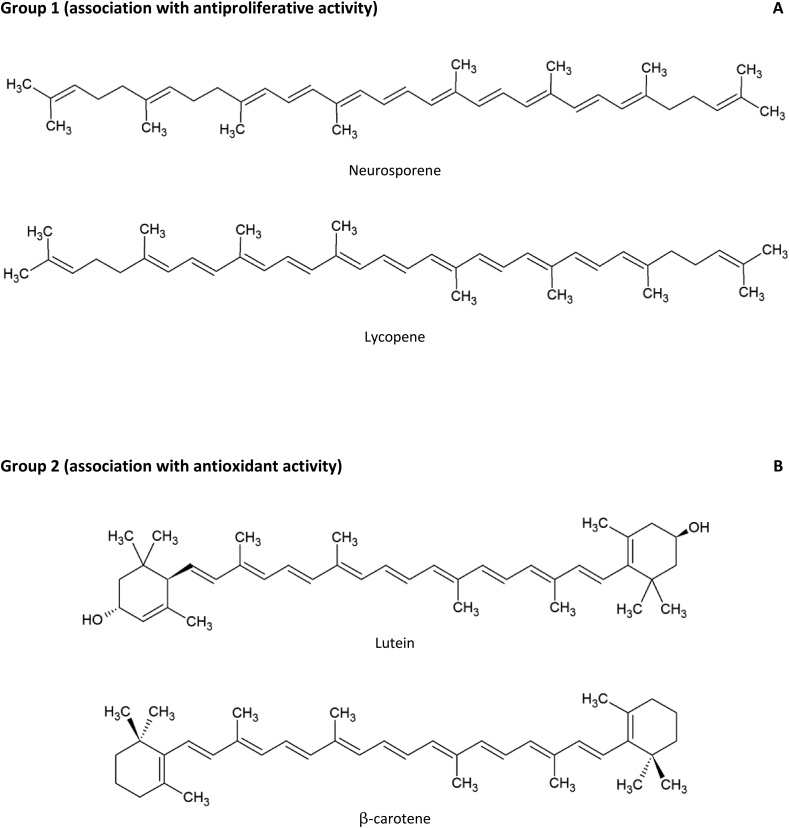
This correlation analysis allowed discriminating the carotenoids from Purple and Cherry tomatoes in two groups considering their bioactive activities and chemical structures. The first group, composed by neurosporene and lycopene, shares an influence on antiproliferative activity of the tomatoes and a linear polyunsaturated hydrocarbons structure. The second one includes lutein and β-carotenes, which impart more influence on antioxidant activity possibly by having β-rings at their two ends and hydroxyls (lutein) allowing donate protons or electrons (Fig. 3). Despite these features, both groups have a polyene chain which is characterized by conjugated double bonds and it is associated with the antioxidant activity of carotenoids (Jomova and Valko, 2013).
Fig. 3.
Structures representing the carotenoids identified in Purple and Red Cherry tomatoes. Group 1 showed association with antiproliferative activity (A) and group 2 imparted more influence on antioxidant activity of tomatoes (B).
4. Conclusion
In summary, Purple peel tomato displayed the best antioxidant activities which can be associated to its highest content of carotenoids, specially lutein and β-carotenes, compared to the other carotenoid-rich fractions. Additionally, the Purple peel CRF had antiproliferative activity against HepG2 line (liver hepatocelullar carcinoma) at non-toxic concentrations. Red Cherry tomato, in turn, stood out in antiproliferative activity against four tumor cell lines (MCF-7, NCI-H460, HeLa, and HepG2) with low hepatic toxicity. High concentrations of neurosporene and lycopene in Red Cherry pulp CRF show to be related to the good antiproliferative activity found on it. Therefore, the new variety of nutrient-rich Purple tomato and commercial variety Red Cherry are good sources of dietary carotenoids with diverse health-promoting properties, such as antioxidant and antiproliferative.
Declarations
Author contribution statement
Luciano H. Campestrini: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Priscilla S. Melo, Isabel C.F.R. Ferreira: Analyzed and interpreted the data; Wrote the paper.
Lazaro E.P. Peres: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Ricardo C. Calhelha: Performed the experiments.
Severino M. Alencar: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant number 2014/11150-6]; and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant numbers 306722/2012-7, 307040/2014-3, and 150015/2018-6) from Brazil; and Fundação para a Ciência e a Tecnologia (FCT) for CIMO [grant numbers Pest OE/AGR/UI0690/2014; SFRH/BPD/68344/2010] from Portugal.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abreu R.M.V., Ferreira I.C.F.R., Calhelha R.C., Lima R.T., Vasconcelos M.H., Adega F., Chaves R., Queiroz M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011;46:5800–5806. doi: 10.1016/j.ejmech.2011.09.029. [DOI] [PubMed] [Google Scholar]
- Acosta-Quezada P.G., Raigón M.D., Riofrio-Cuenca T., García-Martínez M.D., Plazas M., Burneo J.I., Figueroa J.G., Vilanova S., Prohens J. Diversity for chemical composition in a collection of different varietal types of tree tomato (Solanum betaceum Cav.), an Andean exotic fruit. Food Chem. 2015;169:327–335. doi: 10.1016/j.foodchem.2014.07.152. [DOI] [PubMed] [Google Scholar]
- Barros L., Pereira E., Calhelha R.C., Dueñas M., Carvalho A.M., Santos-Buelga C., Ferreira I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods. 2013;5:1732–1740. [Google Scholar]
- Basuny A.M., Gaafar A.M., Arafat S.M. Tomato lycopene is a natural antioxidant and can alleviate hypercholesterolemia. Afr. J. Biotechnol. 2009;8:6627–6633. [Google Scholar]
- Bird R.P., Draper H.H. Comparative studies on different methods of malondyhaldehyde determination. Methods Enzymol. 1984;90:299–305. doi: 10.1016/s0076-6879(84)05038-2. [DOI] [PubMed] [Google Scholar]
- Bommareddy A., Eggleston W., Prelewicz S., Antal A., Witczak Z., McCune D.F., Vanwert A.L. Chemoprevention of prostate cancer by major dietary phytochemicals. Anticancer Res. 2013;33:4163–4174. [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Techol. 1995 [Google Scholar]
- Burton G.W., Ingold K.U. β-carotene: an unusual type of lipid antioxidant. Science. 1984;224:569–573. doi: 10.1126/science.6710156. [DOI] [PubMed] [Google Scholar]
- Chalabi N., Le Corre L., Maurizis J.C., Bignon Y.J., Bernard-Gallon D.J. The effects of lycopene on the proliferation of human breast cells and BRCA1 and BRCA2 gene expression. Eur. J. Cancer. 2004;40:1768–1775. doi: 10.1016/j.ejca.2004.03.028. [DOI] [PubMed] [Google Scholar]
- De Pascual-Teresa S., Sanchez-Ballesta M.T., García-Viguera C. Anthocyanins. In: Ramawat K.G., Mérillon J.M., editors. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer; Heidelberg: 2013. pp. 1803–1819. [Google Scholar]
- Del Giudice R., Raiola A., Tenore G.C., Frusciante L., Barone A., Monti D.M., Rigano M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods. 2015;18:83–94. [Google Scholar]
- D’Evoli L., Lombardi-Boccia G., Lucarini M. Influence of heat treatments on carotenoid content of Cherry tomatoes. Foods. 2013;2:352–363. doi: 10.3390/foods2030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge R., McGarvey D.J., Truscott T.G. The carotenoids as anti-oxidants – a review. J. Photochem. Photobiol. B Biol. 1997;41:189–200. doi: 10.1016/s1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Erba D., Casiraghi M.C., Ribas-Agustí A., Cáceres R., Marfà O., Castellari M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013;31:245–251. [Google Scholar]
- Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote C.S., Chang Y.C., Denny R.W. Chemistry of singlet oxygen. X. Carotenoid quenching parallels biological protection. J. Am. Chem. Soc. 1970;92:5216–5218. doi: 10.1021/ja00720a036. [DOI] [PubMed] [Google Scholar]
- Fornelli F., Leone A., Verdesca I., Minervini F., Zacheo G. The influence of lycopene on the proliferation of breast cell line (MCF-7) Toxicol. In Vitro. 2007;21:217–223. doi: 10.1016/j.tiv.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Fraser P.D., Bramley P.M. The biosynthesis and nutritional uses of carotenoids. Progess Lipid Res. 2004;43:228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Havaux M., Tardy F., Ravenel J., Chanu D., Parot P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophyll content. Plant Cell Environ. 1996;19:1359–1368. [Google Scholar]
- Holloway D.E., Yang M., Paganga G., Rice-Evans C.A., Bramley P.M. Isomerization of dietary lycopene during assimilation and transport in plasma. Free Radic. Res. 2000;32:93–102. doi: 10.1080/10715760000300101. [DOI] [PubMed] [Google Scholar]
- Jomova K., Valko M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013;70:102–110. doi: 10.1016/j.ejmech.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Khachik F., Carvalho L., Bernstein P.S., Muir G.J., Zhao D.Y., Katz N.B. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp. Biol. Med. 2002;227:845–851. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- Kim L., Rao A.V., Rao L.G. Effect of lycopene on prostate LNCaP cancer cells in culture. J. Med. Food. 2004;5:181–187. doi: 10.1089/109662002763003320. [DOI] [PubMed] [Google Scholar]
- Lenucci M.S., Cadinu D., Taurino M., Piro G., Dalessandro G. Antioxidant composition in Cherry and high-pigment tomato cultivars. J. Agric. Food Chem. 2006;54:2606–2613. doi: 10.1021/jf052920c. [DOI] [PubMed] [Google Scholar]
- Levy J., Bosin E., Feldman B., Giat Y., Miinster A., Danilenko M., Sharoni Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either α-carotene or β-carotene. Nutr. Cancer. 1995;24:257–266. doi: 10.1080/01635589509514415. [DOI] [PubMed] [Google Scholar]
- Liebler D.C., Stratton S.P., Kaysen K.L. Antioxidant actions of β-carotene in liposomal and microsomal membranes: role of carotenoid-membrane incorporation and α-tocopherol. Arch. Biochem. Biophys. 1997;338:244–250. doi: 10.1006/abbi.1996.9822. [DOI] [PubMed] [Google Scholar]
- Linnewiel-Hermoni K., Khanin M., Danilenko M., Zango G., Amosi Y., Levy J., Sharoni Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015;572:28–35. doi: 10.1016/j.abb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Livny O., Kaplan I., Reifen R., Polak-Charcon S., Madar Z., Schwartz B. Lycopene inhibits proliferation and enhances gap-junction communication of KB-1 human oral tumor cells. J. Nutr. 2002;132:3754–3759. doi: 10.1093/jn/132.12.3754. [DOI] [PubMed] [Google Scholar]
- Meléndez-Martínez A.J., Mapelli-Brahm P., Benítez-González A., Stinco C.M. A comprehensive review on the colorless carotenoids phytoene and phytofluene. Arch. Biochem. Biophys. 2015;572:188–200. doi: 10.1016/j.abb.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Morais S.M., Catunda-Júnior F.E.A., Silva A.R.A., Martins-Neto J.S. Atividade antioxidante de óleos essenciais de espécies de Croton do nordeste do Brasil. Quím. Nova. 2006;29:907–910. [Google Scholar]
- Müller L., Gnoyke S., Popken A.M., Böhm V. Antioxidant capacity and related parameters of different fruit formulations. LWT Food Sci. Techol. 2010;43:992–999. [Google Scholar]
- Nagata M., Yamashita I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Jpn. Soc. Food Sci. Technol.Nippon Shokuhin Kagaku Kogaku Kaishi. 1992;39:925–928. [Google Scholar]
- Niranjana R., Gayathri R., Mol S.N., Sugawara T., Hirata T., Miyashita K., Ganesan P. Carotenoids modulate the hallmarks of cancer cells. J. Funct. Foods. 2015;18:968–985. [Google Scholar]
- Palozza P., Simone R.E., Catalano A., Mele M.C. Tomato lycopene and lung cancer prevention: from experimental to human studies. Cancers. 2011;3:2333–2357. doi: 10.3390/cancers3022333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podsędek A. Natural antioxidants and antioxidant capacity of Brassica vegetables: a review. LWT - Food Sci. Technol. 2007;40:1–11. [Google Scholar]
- Rodriguez-Amaya D.B., Kimura M. Vol. 2. International Food Policy Research Institute (IFPRI) and International Center for Tropical Agriculture (CIAT); Washington, DC and Cali: 2004. p. 57. (HarvestPlus Handbook for Carotenoid Analysis. HarvestPlus Technical Monograph). [Google Scholar]
- Salvador I., Massarioli A.P., Silva A.P.S., Malaguetta H., Melo P.S., Alencar S.M. Can we conserve trans-resveratrol content and antioxidant activity during industrial production of chocolate? J. Sci. Food Agric. 2019;99:83–89. doi: 10.1002/jsfa.9146. [DOI] [PubMed] [Google Scholar]
- Schierle J., Bretzel W., Bühler I., Faccin N., Hess D., Steiner K., Schüep W. Content and isomeric ratio of lycopene in food and human blood plasma. Food Chem. 1997;59:459–465. [Google Scholar]
- Sestari I., Zsögön A., Rehder G.G., Teixeira L.L., Hassimoto N.M.A., Purgatto E., Benedito V.A., Peres L.E.P. Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv Micro-Tom) as a tool to produce nutrient-rich fruits. Scientia Horticulture. 2014;175:111–120. [Google Scholar]
- Sharma S.K., Le Maguer M. Lycopene in tomatoes and tomato pulp fractions. Ital. J. Food Sci. 1996;8:107–113. [Google Scholar]
- Soares J.C., Rosalen P.L., Lazarini J.G., Massarioli A.P., Silva C.F., Nani B.D., Franchin M., Alencar S.M. Comprehensive characterization of bioactive phenols from new Brazilian superfruits by LC-ESI-QTOF-MS, and their ROS and RNS scavenging effects and anti-inflammatory activity. Food Chem. 2019;281:178–188. doi: 10.1016/j.foodchem.2018.12.106. [DOI] [PubMed] [Google Scholar]
- Stačiċ S., Ćetkoviċ G., Ćanadanoviċ-Brunet J., Djilas S., Mandić A., Četojević-Simin D. Tomato waste: carotenoids content, antioxidant and cell growth activities. Food Chem. 2015;172:225–232. doi: 10.1016/j.foodchem.2014.09.069. [DOI] [PubMed] [Google Scholar]
- Teodoro A.J., Oliveira F.L., Martins N.B., Maia G.A., Martucci R.B., Borojevic R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012;12 doi: 10.1186/1475-2867-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor R.K., Savage G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005;38:487–494. [Google Scholar]
- Young A.J., Lowe G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- Zanfini A., Corbini G., La Rosa C., Dreassi E. Antioxidant activity of tomato lipophilic extracts and interactions between carotenoids and α-tocopherol in synthetic mixtures. LWT - Food Sci. Technol. 2010;43:67–72. [Google Scholar]