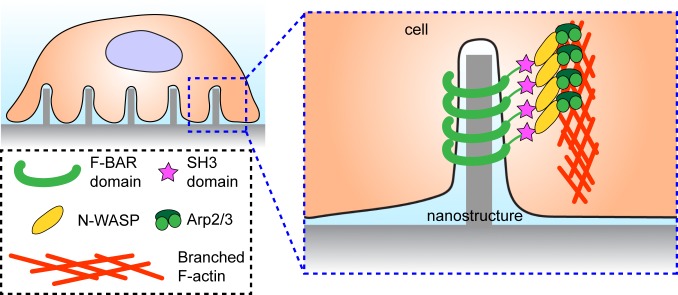
In recent years, it has become increasingly apparent that the material characteristics of a cell’s environment are an important aspect of cell functions, whether they are in the context of developmental biology (1, 2) or implantable devices (3, 4). While the importance of this is well known, the specific ways in which nanoscale topographical cues influence cell mechanical function have, until now, been mostly understood phenomenologically, or with respect to other processes such as endocytosis (5). To better understand these influences, Lou et al. (6) perform a careful investigation of cytoskeletal responses to nanoscale surface protrusions (Fig. 1). They accomplish this by designing slabs with surface decorations of nanopillars or nanobars (5). By systematically varying the radius of curvature in each case, they are able to elucidate a pathway that describes how the cytoskeletal protein actin accumulates and ultimately forms branching structures in response to the membrane curvature induced by the protrusions.
Fig. 1.
F-actin formation around high-curvature nanostructures.
Cells interact with their local mechanical environments in a number of ways, most of which are mediated by the actin cytoskeleton (7). The cytoskeleton governs cell shape and motility, as well as the generation, transmission, and transduction of mechanical forces. The essential components are filamentous actin (F-actin), which forms the structural basis for the cytoskeleton, and myosin, which acts as a motor along actin filaments. An immense variety of other proteins act in concert with actin and myosin to control and functionalize the cytoskeleton. For instance, different actin structures can emerge from different nucleating proteins—assembly mediated by formin proteins promotes elongated filaments, while the Arp2/3 complex induces branched actin structures (7), typically found within the lamellipodium of the leading edge in motile cells. Interactions with the environment require interfaces between the cytoskeleton and extracellular matrix that come in the form of focal adhesions. Finally, environmental sensing constitutes another set of functional molecules, including, among them, the BAR domain in relevant proteins, which is involved in sensing membrane curvature.
Environmental cues are responsible for many important biological phenomena in vitro and in vivo. Constraints on stem cell shape, enforced by controlling the adhesive area of cells, have been found to determine the fate of mesenchymal stem cells (8). Similarly, it has been observed that a crucial alignment of cytoskeleton and the underlying cell matrix defined the anterior−posterior axis of fruit fly embryos, which are a commonly used model system for developmental biology (1). A clinically important example is wound healing, in which cells must mechanically coordinate with each other and their local environments to close gaps (9). It is therefore apparent that material interfaces have an important impact on biological function. This impact is further underlined in the context of implantable devices, where the interfaces are between biological and nonbiological materials.
By their very nature, devices implanted in an organism have effects that come from the implantation itself, as well as the device. In one well-known example, the integration of titanium implants with bone tissue can be improved by modulating the surface roughness of the titanium (4). Likewise, on a smaller length scale, individual cells are responsive to the nanoscale surface characteristics of synthetic substrates (10). From this, we can gather that the importance of local structure to cell function is a consideration that spans several length scales. A natural question to arise, then, centers around how to study these phenomena. What is the specific way in which surface topography induces cell- and tissue-level physiology, and how can we leverage it to aid in designing therapeutic devices?
The work presented in PNAS by Lou et al. (6) presents a systematic study of the specific cellular response to structures along the surface. They began with nanopillars of varying radii grown on a SiO2 substrate, and observed that actin accumulates on the curved lateral surface of the pillars (Fig. 1), but not on the flat tops. Moreover, this accumulation was found to be more pronounced in the case of smaller pillars, suggesting that higher curvature has a stronger effect. To further separate out the role of curvature on this actin accumulation, they also created nanobars out of the same material with a stadium shape from the top-down perspective, that is, long, straight sides and curved ends, such that the bar width controlled the radius of curvature. Here, again, actin accumulated on the ends but not on the straight sides, providing evidence that it was the curvature induced by topographical features, rather than the topographic prominence, that induced cytoskeletal reorganization.
Further examination showed that this curvature-dependent accumulation was accompanied by Arp2/3 and related activator proteins (Fig. 1), rather than formins (6). This suggests that the cellular response to the nanostructures is defined by branched actin networks, rather than long filaments. This, in turn, draws an intriguing parallel with normal cell conditions, where protrusions along the front edge are typically curved and are also composed primarily of Arp2/3-mediated branched actin networks. In the case of the nanobars, however, the actin accumulation is also very dynamic; actin, Arp2/3, and related proteins all assemble and disassemble along the structures on the time scale of minutes.
The curvature dependence of the actin localization provides clues into the mechanism for its accumulation. To that end, the authors (6) consider proteins containing the curvature-sensing BAR domain (Fig. 1), and find that they too are present with the branched actin networks. They further emphasized this by fabricating another nanostructure, wedged nanobars, with a narrow, high-curvature end and a wide, low-curvature end. These wedged nanobars reaffirm the curvature dependence of the accumulation, as a BAR domain protein was found to localize primarily to the narrow end. Moreover, when expression of this protein was modified to remove the actin polymerization domain—while keeping the curvature sensing domain intact—the curvature-dependent actin accumulation was abolished. This suggests a mechanism for the accumulation, in which the BAR domain is involved in sensing the induced membrane curvature and activating Arp2/3 complex, which then nucleates the observed branched actin networks (Fig. 1).
The work presented in PNAS by Lou et al. presents a systematic study of the specific cellular response to structures along the surface.
The impacts of surface topography are not just limited to the immediate area around curved edges, as Lou et al. (6) also identified whole-cell effects. Namely, cell-scale actin bundles called stress fibers are found to cover much less cell area when they are grown on the nanopillars. Accompanying this effect is a change in the development cycle of focal adhesions, the cytoskeletal components that transmit forces from the cell to the substrate. It is tempting to conclude from this that the nanopillars somehow impair force transmission throughout the cell, but the authors find stronger evidence that it is rather a result of the broader reorganization of actin; with less available actin, the focal adhesions are unable to mature, but are likely to be able to transmit forces according to a previous study (11).
The arrays of nanopillars, nanobars, and wedged nanobars presented here (6) will provide a useful means for studying cell−surface topography interactions in a much more systematic and rigorous way than was previously possible. By controlling the radius of curvature for each of these structures, Lou et al. are able to identify a critical radius of ∼400 nm, below which the structures cause substantial changes in the actin cytoskeleton. An especially exciting possibility that emerges from the work of Lou et al. is that of using surface topography as a stimulus in its own right. Current fabrication techniques can yield surfaces with very precise topographical features, and, with the work presented here, we can now identify the requisite geometry of such features required to elicit a desired effect. Moreover, the curvature-sensing and actin accumulation pathway described here enables the rational design of surfaces with specific biochemical targets in mind. This will allow future work on implanted devices to either purposefully include a mechanical component to their purpose or else to understand and mitigate the topography-induced side effects that might accompany implantation.
Footnotes
The authors declare no competing interest.
See companion article on page 23143.
References
- 1.Cetera M., et al. , Epithelial rotation promotes the global alignment of contractile actin bundles during Drosophila egg chamber elongation. Nat. Commun. 5, 5511 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engler A. J., Sen S., Sweeney H. L., Discher D. E., Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Kotov N. A., et al. , Nanomaterials for neural interfaces. Adv. Mater. 21, 3970–4004 (2009). [Google Scholar]
- 4.Agarwal R., García A. J., Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 94, 53–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao W., et al. , Nanoscale manipulation of membrane curvature for probing endocytosis in live cells. Nat. Nanotechnol. 12, 750–756 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lou H.-Y., et al. , Membrane curvature underlies actin reorganization in response to nanoscale surface topography. Proc. Natl. Acad. Sci. U.S.A. 116, 23143–23151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fletcher D. A., Mullins R. D., Cell mechanics and the cytoskeleton. Nature 463, 485–492 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McBeath R., Pirone D. M., Nelson C. M., Bhadriraju K., Chen C. S., Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Brugués A., et al. , Forces driving epithelial wound healing. Nat. Phys. 10, 683–690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luu T. U., Gott S. C., Woo B. W. K., Rao M. P., Liu W. F., Micro- and nanopatterned topographical cues for regulating macrophage cell shape and phenotype. ACS Appl. Mater. Interfaces 7, 28665–28672 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oakes P. W., Beckham Y., Stricker J., Gardel M. L., Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J. Cell Biol. 196, 363–374 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]