Male hypogonadism (abnormally low levels of circulating serum testosterone resulting from a variety of medical and lifestyle issues) can affect males throughout their life span, often because of aging. The Leydig cells, located in the interstitial compartment of the testis and nestled between the seminiferous tubules, produce testosterone in response to luteinizing hormone (LH), which is produced and secreted by the pituitary gland (1). Testosterone, in turn, regulates the development and maintenance of the masculine phenotype and male reproductive function.
Although in recent years media and lay press reports on male hypogonadism usually focus on late-onset declines in circulating testosterone levels in older men, conditions impairing the hypothalamic–pituitary–gonadal axis in children or adolescents can result in delayed puberty for these congenital forms of male hypogonadism. Again, their treatment must continue throughout their life span. There is a significant need for new approaches to treat male hypogonadism. Current treatments (depending upon the etiology of the hypogonadism) are limited to injection of testosterone cyprionate, testosterone enanthate, or testosterone undeconoate, application of testosterone-containing gels or patches applied to the skin or substances applied to the gum and cheek, and a nasal spray or implantable pellet. Gonadotropins and/or testosterone may be used in boys to stimulate testicular and penile growth. The elegant studies of Li et al. (2) provide a bidirectional approach to specify different steroidogenic cell populations that share developmental origins, which in the future may provide a source of human Leydig-like cells to be used for transplantation for the clinical therapy of male hypogonadism. Transplantation of human autologous Leydig-like cells for androgen supplementation in males would revolutionize the treatment of male hypogonadism. (Fig. 1)
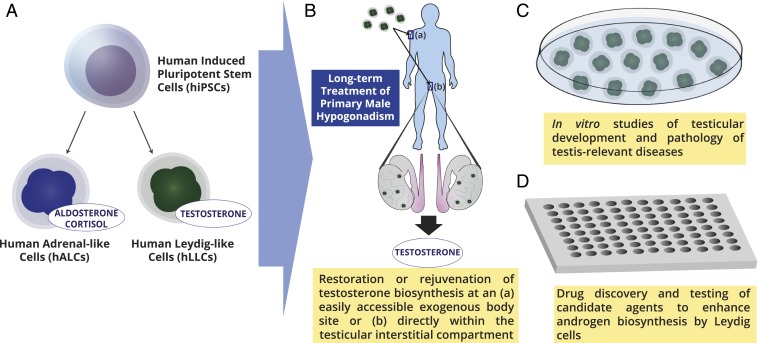
Fig. 1.
A look toward the future: Potential applications of human Leydig-like cells derived from human induced pluripotent stem cells. A shows the bidirectional differentiation of human adrenal-like cells that secreted aldosterone and cortisol and human Leydig-like cells secreting testosterone. B depicts the potential rejuvenation of testosterone biosynthesis in males with primary hypogonadism with transplantation of the human Leydig-like cells directly into the interstitial compartment of the testis or at a more readily accessible exogenous site. C shows the use of the Leydig-like cells for studies of testicular development and to help define the etiologies of specific testis-relevant diseases. D highlights the potential use of Leydig-like cells for drug discovery and testing of candidate drugs that enhance androgen biosynthesis. Image courtesy of Adlai Nelson (Weill Cornell Medicine, New York City, NY).
Decreased testosterone, which can result from defects in the hypothalamus or pituitary (neoplasms, trauma, genetic defects), results in hypogonadotropic hypogonadism (also referred to as central or secondary hypogonadism). When low circulating testosterone levels result from a testicular malfunction of steroidogenesis due to aging, genetic causes, injury, infection, or inflammation, it is referred to hypergonadotropic or primary hypogonadism (reviewed in ref. 3). With increasing male age (and in some infertile men), the Leydig cells become less responsive to LH, resulting in diminished androgen production (4). Treatment of the aging, hypogonadotropic male for symptoms of male age-related impaired hormone production by the Leydig cells remains controversial, and therapies are aimed at addressing relatively vague symptoms, predominantly sexual dysfunction (decreased bone mass, muscle strength, depression, lethargy, anemia, and libido are also problematic). The criteria used to determine the indications for replacement are controversial and the efficacy is uncertain. There are also potential risks, as well as known benefits, to treatment of late-onset male hypogonadism (3).
Adult-onset hypogonadism is a common condition in men. After the age of 40, there is a progressive gradual decline in serum total testosterone levels. Indeed, the Baltimore Longitudinal Study of Aging showed that the incidence of men with hypogonadism increases progressively with aging and by the time men are in their 80s, 50% of them are biochemically hypogonadal, although the exact incidence and steroid levels vary somewhat in different publications (reviewed in ref. 3). Importantly, because more than one-half of men maintain relatively normal levels of testosterone into old age, late-onset male hypogonadism is not a universal indication of aging. It is frequently associated with comorbidities (such as obesity, metabolic syndrome, type 2 diabetes mellitus) (3).
Treatment of human male hypogonadism is complicated. Because late-onset hypogonadism is associated with being overweight, being sedentary, and having chronic diseases, modifiable risk factors should first be minimized through exercise, weight loss, and treatment of comorbidities/impaired general health (3). Low testosterone is the result of lifestyle and poor health rather than the cause, but treatment remains controversial because there are also risks associated with exogenous testosterone administration and the optimal serum testosterone levels for men remains conflicted in the literature (3). Testosterone abuse among athletes and bodybuilders (anabolic steroid users) results from the use of steroids and other androgenic drugs to raise their circulating androgen levels far higher than normal. Risks of both therapeutic testosterone administration and abuse include infertility (impairment of spermatogenesis), acne, sleep apnea, gynecomastia, blood clots, stimulation of prostatic or of existing prostate cancer growth, and other issues.
Because available modalities of testosterone delivery to hypogonadal males are inconvenient, Leydig stem cell-based approaches were considered in the past for enhancement of testicular testosterone production for the treatment of male hypergonadotropic hypogonadism. In the early 2000s, Leydig cells that were microencapsulated in alginate-poly-l-lysine–encapsulated microspheres were administered to castrated rats to bring circulating testosterone levels to about 40% of normal levels for about 1.5 mo (5); however, if translated to clinical practice, this approach could provide only a short-term, temporary solution to a long-term problem for treatment of late-onset hypogonadism in men. Shortly thereafter, adult Leydig cell progenitors isolated from adult murine testes were enriched and transplanted into testes of mice with targeted deletion of the LH receptor that were hypogonadal and infertile. These cells partially restored circulating testosterone levels and restored spermatogenesis (6). Likewise, undifferentiated Leydig stem cells from 1-wk-old rat testes were isolated, characterized, expanded in vitro, and transplanted into rats with chemically induced Leydig cell depletion (7). In vitro and in vivo studies in animal models of stem Leydig and progenitor Leydig cells followed, but these studies did not lead down a path toward the development of a transplantable Leydig-like cell for eventual human application aimed at the long-term treatment of primary male hypogonadism.
The development of a well-defined approach to induce the differentiation of human induced pluripotent stem cells into human Leydig-like or adrenal-like cells that Li et al. (2) describe, offers the potential to develop methods to use these cells together with surgical delivery either to the normal location of Leydig cells within interstitial compartment of the testis or perhaps to a more easily accessible exogenous site in the body to achieve Leydig cell regeneration/rejuvenation. This application would provide a long-term method to treat late-onset hypogonadism, as well as some forms of central hypogonadism.
The authors define an approach that allowed the differentiation of human induced pluripotent stem cells into human Leydig-like cells and human adrenal-like cells and demonstrate their differential expression of the specific steroidogenic enzymes that are necessary for testosterone or aldosterone and corticosteroids biosynthesis, respectively (Fig. 1). Testosterone and aldosterone/cortisol were differentially present in the conditioned media from the Leydig-like and adrenal-like cells. The patterns of gene expression of the induced pluripotent stem cells markedly differed from the partially differentiated steroidogenic cells, which in turn shared a number of similarities. There were differences between the 2 steroidogenic cell types noted on a microarray analysis of the transcriptomes: adult Leydig cell marker proteins, such as the steroidogenic acute regulatory protein (STAR) and luteinizing hormone/choriogonadotropin receptor (LHCGR), were expressed by the human Leydig-like cells, whereas the melanocortin2 receptor (MC2R) was slightly up-regulated in the human adrenal-like cells, suggesting immaturity despite secreting aldosterone and cortisol (2). Protein expression studies showed expression of key proteins involved in steroidogenesis that are specific and/or differentially expressed in adrenal-like and Leydig-like cells. In-depth studies of Leydig cell ultrastructure, together with patterns of steroids produced, suggested that the human Leydig-like and adrenal-like cells require a few additional steps of differentiation to reach their full maturity.
Despite this revolutionary advance, more research is required to translate this work from the “bench to the bedside.” It remains to be demonstrated that these Leydig-like cells can ever fully function as normal human Leydig cells and maintain long-term steroidogenesis. Importantly, it would be expected that the transplanted cells would be part of a system under the control of the hypothalamic–pituitary–gonadal/“Leydig cell” axis resulting in normal, but not elevated levels of circulating testosterone, yet stimulation of these Leydig-like cells by LH remains to be shown. In all likelihood, these Leydig-like cells are not fully differentiated adult Leydig cells as the response to cAMP administration and their ultrastructural morphology suggest that further differentiation is required. Nevertheless, it appears that the quest to develop an autologous human Leydig cell for research, clinical, and drug discovery applications is close to completion (Fig. 1).
Acknowledgments
The author’s research is supported in part by the Frederick J. and Theresa Dow Wallace Fund of the New York Community Trust and National Institutes of Health Grants R01DK078121-10 (to D.J.L.) from the National Institute of Diabetes and Digestive and Kidney Diseases, 1P01HD087157 (to Dr. Martin Matzuk; D.J.L., Co-Project Leader), 1R01HD095341 (to Dr. Thomas Garcia; D.J.L., Co-Investigator), and 1U54HD100549-01 (to Drs. Lonny Levin and Jochen Buck; D.J.L., Core Leader) from the Eunice Kennedy Schriver National Institute of Child Health and Human Development.
Footnotes
Competing interest statement: 1) Celmatix: scientific advisory board: no financial compensation; 2) PuJiang Reproductive Medicine: forum lecturer at international conference; travel expenses; 3) Shanghai Andrology Society and Shanghai General Hospital: lecturer; travel and honorarium; 4) Suzhou University IVF and Fertility Center: lecturer: local travel expenses; 5) Hanzhou First Hospital of Zhejiang University: lecturer: local travel expenses; and 6) Fuji Film Irvine Scientific Chicago Seminar: lecturer, travel expenses, and $250 honorarium.
See companion article on page 23274.
References
- 1.Zirkin B. R., Papadopoulos V., Leydig cells: Formation, function, and regulation. Biol. Reprod. 99, 101–111 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L., Li Y., Sottas C., Culty M., Fan J., Hu Y., Cheung G., Chemes H. E., Papadopoulos V., Directing differentiation of human induced pluripotent stem cells toward androgen‐producing Leydig cells rather than adrenal cells. Proc. Natl. Acad. Sci. U.S.A. 116, 23274–23283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salonia A., et al. , Paediatric and adult-onset male hypogonadism. Nat. Rev. Dis. Primers 5, 38 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huhtaniemi I., Forti G., Male late-onset hypogonadism: Pathogenesis, diagnosis and treatment. Nat. Rev. Urol. 8, 335–344 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Machluf M., Orsola A., Boorjian S., Kershen R., Atala A., Microencapsulation of Leydig cells: A system for testosterone supplementation. Endocrinology 144, 4975–4979 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Lo K. C., Lei Z., Rao ChV., Beck J., Lamb D. J., De novo testosterone production in luteinizing hormone receptor knockout mice after transplantation of Leydig stem cells. Endocrinology 145, 4011–4015 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Ge R. S., et al. , In search of rat stem Leydig cells: Identification, isolation, and lineage-specific development. Proc. Natl. Acad. Sci. U.S.A. 103, 2719–2724 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]