As microbiologists and public health officials scramble for weapons to combat antibiotic resistance, they may end up including an unlikely ally in their arsenal: other bacteria. The prime candidate right now is a predatory bacterium known as Bdellovibrio bacteriovorus.
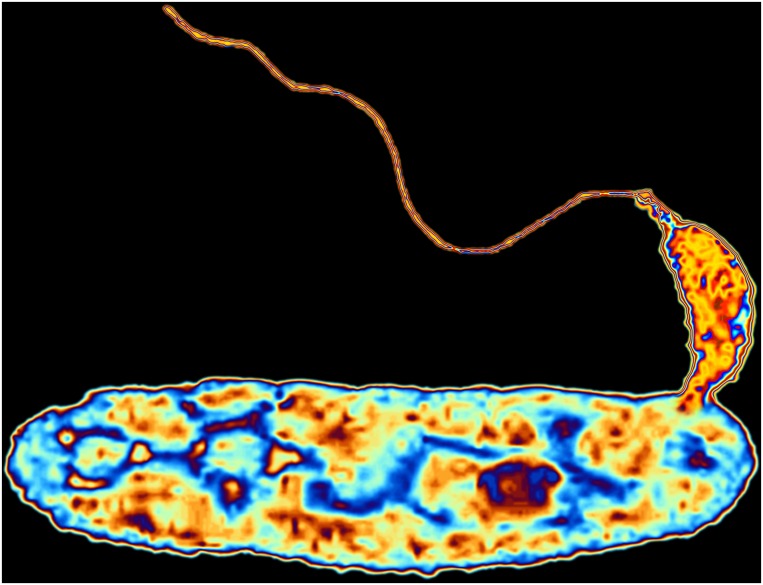
Researchers have started to evaluate predatory bacteria such as Bdellovibrio—shown here in a false-color transmission electron microscopy image at 50,000× magnification—as a means of treating intractable, antibiotic-resistant infections. Image credit: Science Source/ALFRED PASIEKA.
Found in soils and water globally, B. bacteriovorus are free-living, harmless bacteria—harmless to humans, at least. In the microbial world, they’re voracious predators of gram-negative bacteria, a group that includes Salmonella, Escherichia coli, and several other pathogens. Bdellovibrio punch a hole in the membranes of their prey, enter and consume their contents, then burst out again to find their next meal. “They’re a very efficient killing machine,” says Daniel Kadouri, an associate professor at the Rutgers School of Dental Medicine in Newark, NJ. “They act kind of like the creature in the movie Alien.”
Researchers are now beginning to evaluate Bdellovibrio and other similar predatory bacteria as a route to treating intractable, antibiotic-resistant infections. They’re starting to learn how these predators work in different organs and against different pathogens. As they examine various infection scenarios, they are also discovering how these microbes work in concert with the native microbiome and immune cells.
Pills full of predatory bacteria won’t replace antibiotics at pharmacies anytime soon. But studying these microbes could help prepare for a future when many antibiotics fail to treat multi-drug-resistant infections. “Right now, the idea that a single injection of live bacteria could be used as therapy seems quite extreme to people,” says microbiologist Elizabeth Sockett of the University of Nottingham in the United Kingdom. “But as we run out of other options, it might become a more testable—and more attractive—alternative.”
Predator Versus Pathogen
Bdellovibrio were accidentally discovered in the 1960s by scientists hunting in soil for bacteria-killing viruses known as bacteriophages (or, simply, phages). Viruses are nonmotile and grow quickly, forming clear patches on plates of bacteria. Bdellovibrio took longer to show up in cultures, but the patches they created expanded as the predators swarmed over their sessile prey (1).
“We got to this point because we looked at antibiotics as silver bullets, not recognizing that they produce huge amounts of selective pressure. Now, the community needs to start building an arsenal beyond just antibiotics.”
—Daniel Kadouri
Since their discovery, researchers have wondered about possible uses for these predators. Kadouri began studying Bdellovibrio in 2003 as a means to break up stubborn biofilms formed by Pseudomonas aeruginosa, a gram-negative microbe that causes infections in burns, wounds, the lung, eye, and other areas. At that time, antibiotic resistance was a concern mainly associated with gram-positive bacteria. “We had an abundance of antibiotics that were effective against gram-negative species,” Kadouri says. “Biofilms were the main problem.”
Although free-living Pseudomonas were susceptible to antibiotics, those sheathed in a biofilm’s protective layers proved hard to eliminate because drugs couldn’t reach them. In early experiments, Kadouri found that predatory bacteria could penetrate biofilms and break them up, making pathogens susceptible to antibiotics. Bdellovibrio species were effective against some biofilms whereas another group known as Micavibrio proved more potent against other bacterial prey. Unlike B. bacteriovorus, Micavibrio and some other Bdellovibrio species behave like leeches, sticking to the outside of host cells to suck their contents out. “Each one has a different host specificity, and some have different mechanisms of killing their prey as well,” Kadouri says.
B. bacteriovorus, for example, creates a porthole in a host’s cell membrane to enter its body. Once inside, it seals the hole up and then releases enzymes to digest the host’s contents. It replicates within its prey and then reemerges to invade new hosts. This process—wherein the host species’ contents never spill out—proves useful from a therapeutic standpoint, Sockett says, because it avoids spilling the pathogen’s innards, which could trigger a damaging inflammatory response if released.
In 2009, Sockett, Kadouri, and others were studying how predatory bacteria worked when another group of researchers unveiled a looming crisis: they found a severely ill patient’s infections were caused by New Delhi metallo-beta-lactamase 1 (NDM-1), a drug-resistant strain of the gram-negative pathogen Klebsiella pneumoniae. “The field changed when NDM-1 and other drug-resistant strains started to emerge,” Kadouri says. “The community started to understand that we were going to have a big problem with drug-resistant gram-negative bacteria.” Researchers began to revisit phage therapy and other alternatives to antibiotics that were once considered too unusual to be clinically useful.
Inside Animal Infections
In one preliminary study, Kadouri and his colleagues found that injecting Bdellovibrio into veins had no effect on an acute bloodstream Klebsiella infection in rats (2). The chances of predators surviving immune responses, making contact with prey, and consuming them in a systemic infection such as this one are slim, explains microbiologist Nancy Connell of the Johns Hopkins Center for Health Security, who coauthored the study. But the study helped clarify that predatory bacteria were nontoxic and would not incite a systemic immune response. “The value of these bacteria is more likely to be in localized infection sites in small regions or wounds,” she says. In a recent study of localized infection, the researchers found that B. bacteriovorus could reduce Yersinia pestis levels in rats’ lungs by 86% within a day of infection (3).
Microbiologist Robert Shanks, an associate professor at the University of Pittsburgh in Pennsylvania, first heard of predatory bacteria as a postdoctoral researcher working in the same lab as Kadouri. Now, Shanks studies Bdellovibrio’s potential to treat eye infections. These infections, caused by P. aeruginosa, Serratia marcescens, and other drug-resistant gram-negative pathogens, can occur on the eye’s surface in contact lens wearers or deep within the eye when microbes cross the blood-brain barrier.
Shanks and his team have found Bdellovibrio can consume several pathogens isolated from human infections and appear to be nontoxic—and even noninflammatory—in rabbit studies. Preliminary data from their team show that “the predatory bacteria [speed] up clearance of the pathogen from the ocular surface,” Shanks says. “They don’t work as well as an antibiotic, but they are much better than no antibiotic.” Although the predatory bacteria don’t completely eliminate pathogens in their studies, they may drive pathogen levels low enough to give the immune system a fighting chance, he says.
Sockett’s studies in zebrafish larvae show that Bdellovibrio work best in conjunction with an immune system response. When the researchers dosed larvae with a drug-resistant strain of the human pathogen Shigella flexneri and then treated them with predatory bacteria, they found that Bdellovibrio eradicated the pathogen in about half the animals treated. Larval survival increased from 25% to 67% as a result. The effect was a result of predatory bacteria and innate immune cells working together, the researchers found (4).
Because Bdellovibrio are not normally present in large numbers in animals, they don’t trigger an inflammatory response. As motile, gram-negative bacteria, Bdellovibrio bear flagella as well as large molecules known as lipopolysaccharides (LPS) on their surface. These chemicals usually signal “Invader!” and spur an inflammatory response from immune cells. But Bdellovibrio’s versions of these proteins don’t bind to immune cell receptors.
Nonetheless, the bacteria do interact with immune cells before eventually being consumed by them, Sockett’s team found. “The bacteria can live inside macrophages—they last for about 48 hours alive, but they’re not eating the contents of immune cells,” she explains.
This passive uptake suggests that macrophages might present predatory bacteria to antibody-forming cells—so repeated exposures could cause humans to develop immune reactions against Bdellovibrio. In one recent study, Sockett and her colleagues found more than 90% of human serum samples from a biobank carried low levels of antibodies to two Bdellovibrio species. Environmental exposure—to strains found in soil and water—may have caused such antibodies to form, Sockett says (5).
Whether these antibodies could change how predatory bacteria work in humans is still uncertain. But the antibodies do mean that such bacteria can likely only be used once. “We like to think of these as a fire extinguisher, a high-pressure environment where we’re short of other options,” Sockett says. “You use it once, but after that there’s immune recognition. Maybe because conventional medicine still works, we aren’t quite at the point of demand for that kind of single-use therapy.”
A Post-antibiotic Future
Even a single dose of Bdellovibrio directed toward a local infection is a long way off. Unlike the bacteriophages, which are also being studied as an alternative to conventional antibiotics, much remains unknown about predatory bacteria. Their broad-ranging, nonspecific activities seemingly have both pros and cons. Most gram-negative species are susceptible to predatory bacteria, and acquiring resistance, which can be a concern with phage therapy, is unlikely because predatory bacteria’s broad-ranging killing mechanisms don’t target specific prey proteins that can evolve resistance. But that also means Bdellovibrio could consume beneficial microflora. Preliminary studies by Connell, Kadouri (6), and Sockett (7) have found mixed effects. The predators increase numbers of gram-positive species in the gut microbiome of chickens and rats, but whether this imbalance has ill effects on animal health is uncertain. However, Connell points out that in terms of impact on the microbiome, “antibiotics are even worse.”
In many parts of Europe and Asia, bacteriophages have been used to treat patients’ infections for more than a century. Several patients from the United States and Canada have traveled to these regions to receive treatment for recalcitrant infections. And outside the clinic, phages are used in agricultural products within the United States. They’re now making their way into clinical trials in the United States and have been applied under the US Food and Drug Administration’s compassionate use guidelines. Phages are rarely affected by antibiotics and, as bacterial viruses (as opposed to viruses that attack human cells), are unlikely to provoke inflammation.
Predatory bacteria have no such track record yet, and researchers have more to learn about how they work. “It’s about understanding host responses and safety,” Sockett says. “That’s not to say that they are unsafe—it’s just that we don’t know enough about what might change when we apply these bacteria.”
Using live bacteria as antibacterial therapy also poses manufacturing and regulatory challenges. Because Bdellovibrio grows only on other bacteria and carries its own LPS—a molecule that’s currently used as a marker of contamination in drug manufacture—new standards will have to be created for predatory bacteria–based remedies. “Regulatory agencies are starting to look at new therapies in ways that they didn’t need to before,” Kadouri says. “Suddenly people are studying phage[s], predatory bacteria, etc. These are things that don’t fit the guidelines for chemical drugs.”
But the rampant rise of antibiotic resistance means unconventional therapies should be explored, Kadouri adds. Rather than using single compounds, it’s time to treat infectious disease the way we treat cancer, he asserts—with a combination of immunotherapy, radiation therapy, chemotherapy, and more. “We got to this point because we looked at antibiotics as silver bullets, not recognizing that they produce huge amounts of selective pressure,” Kadouri says. “Now, the community needs to start building an arsenal beyond just antibiotics.”
References
- 1.Stolp H., Starr M. P., Bdellovibrio bacteriovorus gen. et sp. N., a predatory, ectoparasitic and bacteriolytic microorganism. Antonie Van Leeuwenhoek 29, 217–248 (1963). [DOI] [PubMed] [Google Scholar]
- 2.Shatzkes K., et al. , Examining the efficacy of intravenous administration of predatory bacteria in rats. Sci. Rep. 7, 1864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo R., et al. , Susceptibility of virulent Yersinia pestis bacteria to predator bacteria in the lungs of mice. Microorganisms 7, 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willis A. R., et al. , Injections of predatory bacteria work alongside host immune cells to treat Shigella infection in zebrafish larvae. Curr. Biol. 26, 3343–3351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghunathan D., et al. , Engulfment, persistence and fate of Bdellovibrio bacteriovorus predators inside human phagocytic cells informs their future therapeutic potential. Sci. Rep. 9, 4293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shatzkes K., et al. , Effect of predatory bacteria on the gut bacterial microbiota in rats. Sci. Rep. 7, 43483 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atterbury R. J., et al. , Effects of orally administered Bdellovibrio bacteriovorus on the well-being and Salmonella colonization of young chicks. Appl. Environ. Microbiol. 77, 5794–5803 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]