Abstract
Transposable elements are one of the major contributors to genome-size differences in metazoans. Despite this, relatively little is known about the evolutionary patterns of element expansions and the element families involved. Here we report a broad genomic sampling within the genus Hydra, a freshwater cnidarian at the focal point of diverse research in regeneration, symbiosis, biogeography, and aging. We find that the genome of Hydra is the result of an expansion event involving long interspersed nuclear elements and in particular a single family of the chicken repeat 1 (CR1) class. This expansion is unique to a subgroup of the genus Hydra, the brown hydras, and is absent in the green hydra, which has a repeat landscape similar to that of other cnidarians. These features of the genome make Hydra attractive for studies of transposon-driven genome expansions and speciation.
Transposable elements (TEs) were originally discovered by Barbara McClintock in maize (1) and later found to comprise a significant fraction of plant and animal genomes (2). Well-known for their contribution to total genome size (most recently in refs. 3 and 4), transposons are also sources of regulatory element evolution, modulators of gene expression (5), and a potential basis of large-scale genomic rearrangements (6).
Hydra provides an intriguing system to study the evolutionary history of TEs. The genus is subdivided into 2 major groups: the brown hydras (comprised of the Vulgaris, Oligactis, and Braueri clades) and the algal symbiont-containing green hydra (comprised of the Viridissima clade) (7). Cnidarian genomes are typically smaller than 500 Mb in size (8), as in the Viridissima clade, which has a genome size of about 300 Mb. In contrast, the genomes of brown hydras are ∼1 Gb in size (9). High abundance of TEs in the Hydra vulgaris strain 105 genome (9) has led to the hypothesis that large genome size is due to their expansion in this taxon. However, genomic data from other Hydra lineages were required to determine the timing of TE expansion and to rule out other scenarios, such as genome duplication in brown hydras.
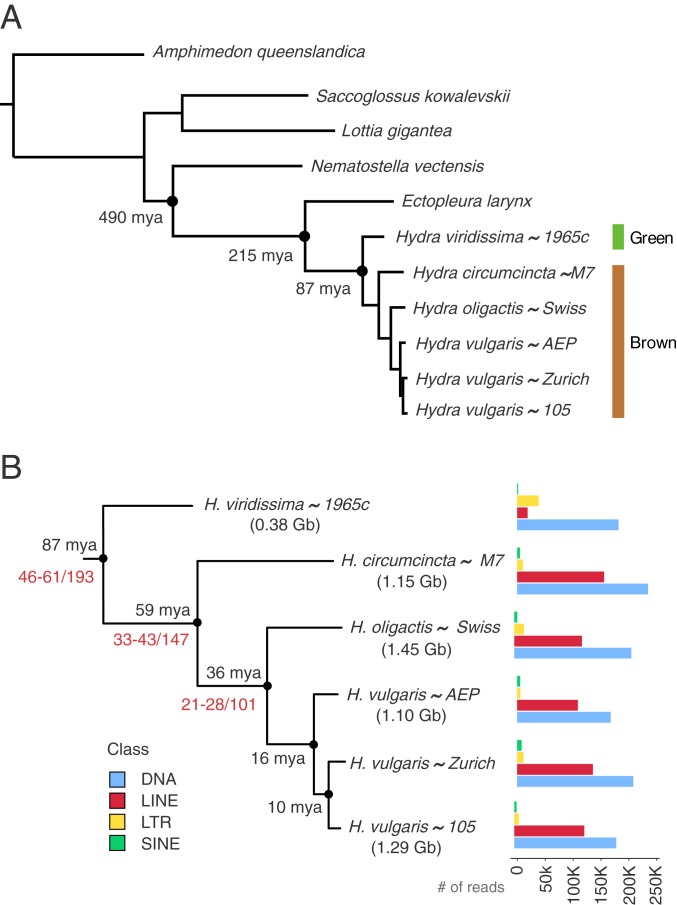
To address this question, we sequenced genomes and transcriptomes from 4 brown hydras and 1 Hydra viridissima strain (Materials and Methods). Using the H. vulgaris strain 105 gene set as a reference (10), we constructed single-ortholog gene families using mutual best BLAST (basic local alignment search tool) hits to a select set of species (Fig. 1A). We used RAxML (11) to construct a phylogeny (Fig. 1A). The branching order for the 4 Hydra clades was identical to that found previously (7). We used r8s (12) to estimate divergence times, setting the cnidarian–bilaterian divergence as a calibration point to 550 Mya (13). We obtained 87 Mya for the beginning of the Hydra radiation and 59 Mya for the timing of the brown/green hydra split. These estimates for Hydra radiation times are based on transcriptome data and fall between previously reported estimates (7, 14) (Fig. 1B).
Fig. 1.
Phylogeny of the genus Hydra. (A) RAxML phylogeny of Hydra and other metazoans. Dating estimates are provided for each node. (B) Branching pattern and divergence within the genus Hydra. Age estimates in black are from this study, those in blue are from ref. 7, and those in red are from refs. 14 and 24. Bar charts on the right indicate proportions of the major repeat element classes in each species. Genome sizes are from refs. 25 and 26.
Using transcriptome data, we searched for evidence of a genome duplication event in the brown hydras. We found that 75% (8,629 out of 11,543) of gene families had the same number of genes in both H. viridissima and H. vulgaris. Additionally, 84.7% and 81.1% of the gene families contained a single gene from H. vulgaris and H. viridissima, respectively. Thus, there was no evidence for genome duplication as the explanation for the large genome size in the brown hydras. However, a good reference assembly for the green hydra genome will be required to completely rule out a rediploidization scenario.
To test the contribution of TEs to the Hydra genome expansion, we used DNAPipeTE (15) to identify and assemble highly abundant DNA reads from a random sample of 1 million reads from each species. We found that all of the major TE classes are represented at similar levels in the Hydra genomes with the exception of long interspersed nuclear elements (LINEs). LINEs were strikingly enriched (>6-fold) in the brown hydra genomes (Fig. 1B). We found that 2 of the 3 major LINE classes are overrepresented, L2 and CR1 (16), comprising at least 8 to 12% of all sampled reads in brown hydras, compared to less than 0.5% in the green hydra.
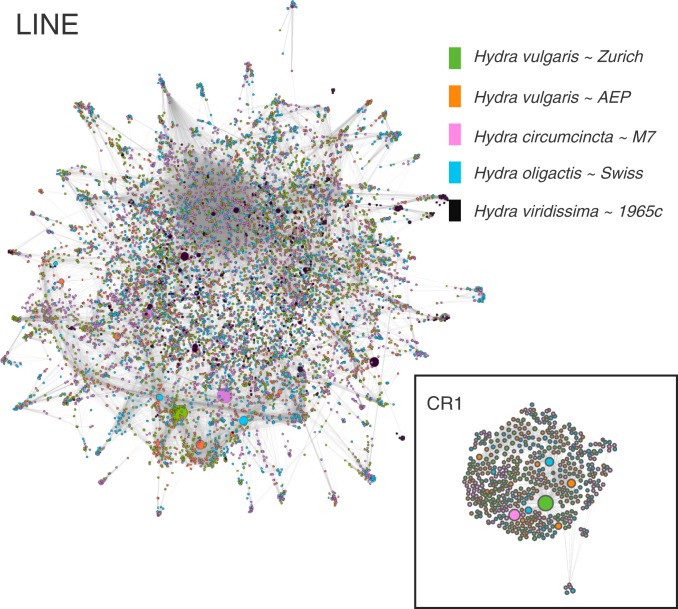
To determine the evolutionary history of LINEs in the brown hydras, we constructed a similarity graph based on BLASTN scores among all detected LINE consensus sequences. While we could identify contributions from all CR1/L2 families, we found that the expansion was largely limited to a specific region of the graph (Fig. 2). This indicates that the largest expansion happened in only one or a few highly related CR1 families (as defined by DNAPipeTE), together responsible for at least 28% of the expansion among the brown hydras.
Fig. 2.
Expansion of a specific CR1 retrotransposon family in the brown hydra lineage. Sequence similarity graph of LINE families for 5 Hydra species. The sizes of the circles correspond to the number of elements within each family, with species colored according to the key. Expansion is restricted to only one part of the graph (larger circles), stemming mostly from a single retrotransposon family (CR1, expanded panel).
We next investigated whether the CR1 expansion happened independently in each brown hydra lineage or at the base of the brown hydra clade. Based on similarity graphs of CR1 families (Fig. 2), we found that more than half of all DNAPipeTE CR1 families (e.g., 85 out of 116 CR1 families in Hydra circumcincta and 60 out of 105 CR1 families in Hydra oligactis) could be traced back to the last common brown hydra ancestor. Interestingly, the majority of CR1 sequences in the genomes apparently lack the ability to propagate autonomously, as they are relatively short (478 bp on average, estimated by RepeatCraft, ref. 17).
Taken together, our findings show that a single CR1 family dominated the CR1/L2 LINE expansion after the separation of the green and brown hydra lineages. Given the wide distribution of these elements across the genome, an alternative scenario of repetitive element excision events that happened only in the green hydra lineage seems unlikely. Moreover, the repeat content of the green hydra genome is similar to that of other cnidarians, for example Nematostella vectensis (18), suggesting that this was the ancestral state for Hydra and for the phylum Cnidaria in general.
The observed genome expansion pattern in the genus Hydra is strikingly different from the other reported recent expansions, such as in larvaceans (3) and rotifers (4), in which expansion is due to a combination of various repeat element classes. These observations highlight the diversity of genome expansion events and demonstrate the importance of carrying out genome comparisons across a taxonomic group. Our results also indicate that Hydra will be an attractive model system for a targeted study of repeat-driven genome expansion and the role of repeat expansions in speciation.
Materials and Methods
Hydra cultures were maintained using standard methods (19). DNA and RNA extractions were done using Qiagen kits. Library preparation and Illumina sequencing were done using standard methods. Sequences have been deposited in the NCBI Sequence Read Archive entry for the project (PRJNA114713). Transcriptomes were assembled with Trinity (20), filtered with CD-hit (21), and the peptides predicted with Transdecoder (22). Orthologous groups were constructed using OrthoFinder (23). The full analysis pipeline is available from https://github.com/niccw/hydracompgen.
Acknowledgments
This work was made possible, in part, through access to the Genomics High Throughput Facility Shared Resource of the Cancer Center Support Grant P30CA-062203 at the University of California, Irvine; and NIH shared instrumentation grants 1S10RR025496-01, 1S10OD010794-01, and 1S10OD021718-01. W.Y.W. and O.S. were supported by Austrian Science Fund Grant P30686-B29. D.E.M. and D.M.B. were supported by National Institute on Aging Grant 1R01AG037965-01 and a Templeton Foundation Immortality Project grant. D.E.M. was additionally supported by the Pomona College Sara and Egbert Schenck Memorial Fund. T.W.H. was supported by German Science Foundation Grants SFB-873-A1-3 and SFB-1324-A5-1. R.E.S. was supported by funds from the University of California, Irvine, School of Medicine. P.C. was supported by the National Science Foundation CAREER Award DEB 0953571. We thank Dr. A. Nawrocki for help in obtaining the Ectoplura larynx transcriptome.
Footnotes
The authors declare no competing interest.
Data deposition: Sequences have been deposited in the NCBI Sequence Read Archive entry for the project (PRJNA114713). The full analysis pipeline is available from https://github.com/niccw/hydracompgen.
References
- 1.McClintock B., The origin and behavior of mutable loci in maize. Proc. Natl. Acad. Sci. U.S.A. 36, 344–355 (1950). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canapa A., Barucca M., Biscotti M. A., Forconi M., Olmo E., Transposons, genome size, and evolutionary insights in animals. Cytogenet. Genome Res. 147, 217–239 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Naville M., et al. , Massive changes of genome size driven by expansions of non-autonomous transposable elements. Curr. Biol. 29, 1161–1168.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Blommaert J., Riss S., Hecox-Lea B., Mark Welch D. B., Stelzer C. P., Small, but surprisingly repetitive genomes: Transposon expansion and not polyploidy has driven a doubling in genome size in a metazoan species complex. BMC Genomics 20, 466 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feschotte C., Transposable elements and the evolution of regulatory networks. Nat. Rev. Genet. 9, 397–405 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim J. K., Simmons M. J., Gross chromosome rearrangements mediated by transposable elements in Drosophila melanogaster. BioEssays 16, 269–275 (1994). [DOI] [PubMed] [Google Scholar]
- 7.Martínez D. E., et al. , Phylogeny and biogeography of Hydra (Cnidaria: Hydridae) using mitochondrial and nuclear DNA sequences. Mol. Phylogenet. Evol. 57, 403–410 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Adachi K., Miyake H., Kuramochi T., Mizusawa K., Okumura S., Genome size distribution in phylum Cnidaria. Fish. Sci. 83, 107–112 (2017). [Google Scholar]
- 9.Chapman J. A., et al. , The dynamic genome of Hydra. Nature 464, 592–596 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Human Genome Research Institute , Hydra 2.0 genome Project Portal. https://research.nhgri.nih.gov/hydra/. Accessed 18 October 2019.
- 11.Stamatakis A., Ludwig T., Meier H., RAxML-III: A fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21, 456–463 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Sanderson M. J., r8s: Inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19, 301–302 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Parry L. A., et al. , Ichnological evidence for meiofaunal bilaterians from the terminal Ediacaran and earliest Cambrian of Brazil. Nat. Ecol. Evol. 1, 1455–1464 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Schwentner M., Bosch T. C. G., Revisiting the age, evolutionary history and species level diversity of the genus Hydra (Cnidaria: Hydrozoa). Mol. Phylogenet. Evol. 91, 41–55 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Goubert C., et al. , De novo assembly and annotation of the Asian tiger mosquito (Aedes albopictus) repeatome with dnaPipeTE from raw genomic reads and comparative analysis with the yellow fever mosquito (Aedes aegypti). Genome Biol. Evol. 7, 1192–1205 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metcalfe C. J., Casane D., Modular organization and reticulate evolution of the ORF1 of Jockey superfamily transposable elements. Mob. DNA 5, 19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong W. Y., Simakov O., RepeatCraft: A meta-pipeline for repetitive element de-fragmentation and annotation. Bioinformatics 35, 1051–1052 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putnam N. H., et al. , Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science 317, 86–94 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Lenhoff H. M., Brown R. D., Mass culture of hydra: An improved method and its application to other aquatic invertebrates. Lab. Anim. 4, 139–154 (1970). [DOI] [PubMed] [Google Scholar]
- 20.Grabherr M. G., et al. , Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 29, 644–652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L., Niu B., Zhu Z., Wu S., Li W., CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 28, 3150–3152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas B. J., et al. , De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 8, 1494–1512 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emms D. M., Kelly S., OrthoFinder: Solving fundamental biases in whole genome comparisons dramatically improves orthogroup inference accuracy. Genome Biol. 16, 157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nawrocki A. M., Collins A. G., Hirano Y. M., Schuchert P., Cartwright P., Phylogenetic placement of Hydra and relationships within Aplanulata (Cnidaria: Hydrozoa). Mol. Phylogenet. Evol. 67, 60–71 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Zacharias H., Anokhin B., Khalturin K., Bosch T. C. G., Genome sizes and chromosomes in the basal metazoan Hydra. Zoology (Jena) 107, 219–227 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Hemmrich G., Anokhin B., Zacharias H., Bosch T. C. G., Molecular phylogenetics in Hydra, a classical model in evolutionary developmental biology. Mol. Phylogenet. Evol. 44, 281–290 (2007). [DOI] [PubMed] [Google Scholar]