Significance
Adaptive radiations, comprising many species derived from one or a small number of ancestral species in a geologically short time, are prominent components of the world’s biodiversity. Introgressive hybridization of divergent species has been important in increasing variation, leading to new morphologies and even new species, but how that happens throughout evolutionary history is not known. A long-term field study of Darwin’s finches on Daphne Major island, Galápagos, shows that introgression enhances variation and increases the potential for future evolution. We use a dated phylogeny to infer that populations became more variable in morphological traits through time, consistent with this enhancement effect, and then declined in variation after reaching a maximum. Introgression may be especially important with future climate change.
Keywords: speciation, hybridization, introgression, beaks, Darwin’s finches
Abstract
Adaptive radiations are prominent components of the world’s biodiversity. They comprise many species derived from one or a small number of ancestral species in a geologically short time that have diversified into a variety of ecological niches. Several authors have proposed that introgressive hybridization has been important in the generation of new morphologies and even new species, but how that happens throughout evolutionary history is not known. Interspecific gene exchange is expected to have greatest impact on variation if it occurs after species have diverged genetically and phenotypically but before genetic incompatibilities arise. We use a dated phylogeny to infer that populations of Darwin’s finches in the Galápagos became more variable in morphological traits through time, consistent with the hybridization hypothesis, and then declined in variation after reaching a peak. Some species vary substantially more than others. Phylogenetic inferences of hybridization are supported by field observations of contemporary hybridization. Morphological effects of hybridization have been investigated on the small island of Daphne Major by documenting changes in hybridizing populations of Geospiza fortis and Geospiza scandens over a 30-y period. G. scandens showed more evidence of admixture than G. fortis. Beaks of G. scandens became progressively blunter, and while variation in length increased, variation in depth decreased. These changes imply independent effects of introgression on 2, genetically correlated, beak dimensions. Our study shows how introgressive hybridization can alter ecologically important traits, increase morphological variation as a radiation proceeds, and enhance the potential for future evolution in changing environments.
By hybridizing 2 species of Australian fruit flies in the laboratory and following them for several generations, Lewontin and Birch (1) showed that introgression could account for an observed geographical expansion of a fruit fly, Dacus tryoni, into a new, hotter, and physiologically more stressful environment. In a parallel study of coregonid fish in Swedish lakes, Svärdson (2) found that introgressive hybridization of species with different numbers of gill rakers led to the production of new forms with the potential of evolving into new species. These 2 studies laid the foundation for modern investigations into the role of introgressive hybridization in the evolution of animal species (3, 4), and specifically into the possibility that interspecific gene exchange is important in initiating new evolutionary trajectories (5–8), and even new animal taxa (9–13), especially in adaptive radiations (7, 14–16). Together with studies of ongoing hybridization in a variety of animals (3, 17–19), they complement a large body of research into the better-known and widespread hybridization of plants (20–24). However, the link between hybridization in the present and the past has not been well established for adaptive radiations. Here we show, with an example of Darwin’s finches on the Galápagos Islands, how introgression affects morphological variances of hybridizing contemporary populations, and how variances change as a radiation proceeds.
Several field studies have documented hybridization of Darwin’s finches (25–27) or inferred it from phenotypic (28) and genetic data (29–32). On the small island of Daphne Major (0.34 km2) Geospiza fortis (medium ground finch, ∼17 g) interbreeds rarely with Geospiza scandens (cactus finch, ∼21 g), a common resident, and Geospiza fuliginosa (small ground finch, ∼12 g), an occasional immigrant; G. scandens and G. fuliginosa do not interbreed on this island. Significantly, none of them hybridize with the much larger Geospiza magnirostris (large ground finch, ∼30 g). We measured large samples of hybrid and nonhybrid finches annually from 1973 until 2012 (33). Within this period breeding was documented in detail from 1976 to 1998 (34). Hybridization was first observed in 1976 (35, 36), but it only led to breeding of the hybrids and backcrossing in 1983 after a climatically induced change in the vegetation that was favorable to hybrid survival. Observations on the dynamics of hybridization were supported by parentage determined by microsatellite DNA analysis (26). Fitness of hybrids was as high as, or possibly higher than, the fitness of parental species (33, 34). Species received new alleles more frequently by hybridizing than by breeding with conspecific immigrants (26). After this period (>1998) hybridization was inferred from microsatellite-determined admixtures (Methods) supplemented by observations of mixed pairs.
The principal finding from the first half of the study was an increase in phenotypic and quantitative genetic variation as a result of hybridization (5, 34, 37). This led to the following reasoning concerning hybridization in the past (38). Total effects of gene exchange are a function of both the frequency of interbreeding and the morphological effects at each interbreeding episode. When populations begin to diverge, an exchange of genes may be frequent but will have little effect on the variation of each. As morphological divergence proceeds further to a point at which the populations become biological species—they seldom interbreed but suffer little or no loss of fitness when they do—phenotypic and genetic effects of gene mixing are expected to be greater, and at some point reach a maximum. Thereafter, population variation declines, caused by strengthening of premating isolating mechanisms and hence increased rarity of interbreeding, and/or by the accumulation of incompatible alleles through mutations that reduce or prevent exchange.
Darwin’s finch populations possess several features that make them suitable for testing this hypothesis. These include a large number of phylogenetically young species for quantitative analysis, ages of the species as estimated by the dates of nodes in a phylogenetic tree, and measurements of population variation in ecologically significant (functional) traits. Eighteen species radiated rapidly from a common ancestor in little more than a million years (39, 40), and they comprise several populations of the same species on different islands. Dates of the last common ancestor of each species and a sister lineage have been estimated from whole-genome studies (40). Interspecific gene exchange has been a feature of the group’s history, beginning at some time after the first split, as inferred from the results of ABBA-BABA tests (40). Means and SDs of beak length and beak depth measurements of 99 populations have been published (ref. 41; see also ref. 42). Beaks are tools for dealing with food and are therefore ecologically significant traits (41, 43). They are also cues to species identity (44, 45) that, together with song (25, 46–49), are used in the choice of mates (49, 50) (SI Appendix, section 1). They are known to be subject to natural selection in this climatically varying environment (10, 34, 35, 51, 52). Selection episodically causes a large effect on means and small effects on variances (33, 35).
We first present a morphological analysis of contemporary hybridizing species on Daphne Major island, thereby providing a foundation for making inferences of hybridization in the past, and then test predictions of the hypothesis of past hybridization. We conclude by integrating morphological effects of past and present hybridization in the context of adaptive radiations.
Hybridization on Daphne Major Island
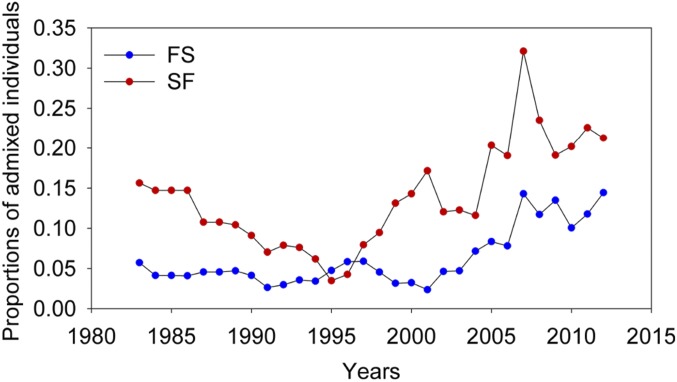
Backcrossing was determined by direct observation of breeding birds and microsatellite analysis of parentage from 1983 to 1998. Backcrossing occurred first in 1983 from G. scandens to G. fortis and then, beginning in 1987 and more extensively, from G. fortis to G. scandens (33). The incidence of hybridization can be inferred for the whole study period from the proportions of admixed individuals in populations of G. scandens and G. fortis (Fig. 1). The figure has 2 main features. First, proportions of hybrids (admixed individuals) were higher in the G. scandens samples than in the G. fortis samples throughout the 30-y period, except for 2 y. The contrast between species was evaluated statistically with newly produced admixed individuals (F1 and backcrosses) in 11 y of extensive breeding (Methods) because these are independent data. Frequencies of newly produced scandens × fortis hybrids (SF, i.e., higher assignment to G. scandens than to G. fortis) in the G. scandens samples significantly exceeded the frequencies of fortis × scandens hybrids (FS) in the G. fortis samples (paired t = 3.86, degrees of freedom = 10, P = 0.0032). Second, the proportion of admixed individuals increased across years, as shown by linear regression: For FS hybrids F = 18.42, P = 0.0020, and adjusted R2 = 0.64, and for SF hybrids F = 7.25, P = 0.0247, and adjusted R2 = 0.38. Increases reflect greater gains (births) of hybrids than losses (deaths and emigration). In contrast to these increases, the proportions of fortis × fuliginosa (Ff) hybrids in the G. fortis samples remained unchanged (F = 1.17, P = 0.3074) (SI Appendix, section 2) at an average frequency (0.08 ± 0.014 SE) no different from FS (0.06 ± 0.008 SE) and lower than SF (0.12 ± 0.020 SE) frequencies. These linear trends, and others below, are subject to the caveat that data for successive years are not independent samples, and the statistics are presented for their heuristic value.
Fig. 1.
Proportions of admixed individuals in samples of G. fortis (FS) and G. scandens (SF).
Morphological Change.
Morphological effects of introgression are manifested as changes in mean values of beak length and beak depth, and in variation of both (34). Expected changes are based on the standard quantitative genetics model of additive genetic variation and covariation underlying continuously varying traits (5, 53). The changes are expected to be linear through time in view of high hybrid fitness (Introduction), except when affected by natural selection on the means (33–35). In some years there is no breeding (33–35, 54) and hence no gains (recruitment) of hybrids to the population. This results in small perturbations to linearity driven solely by losses (mortality and emigration).
Means.
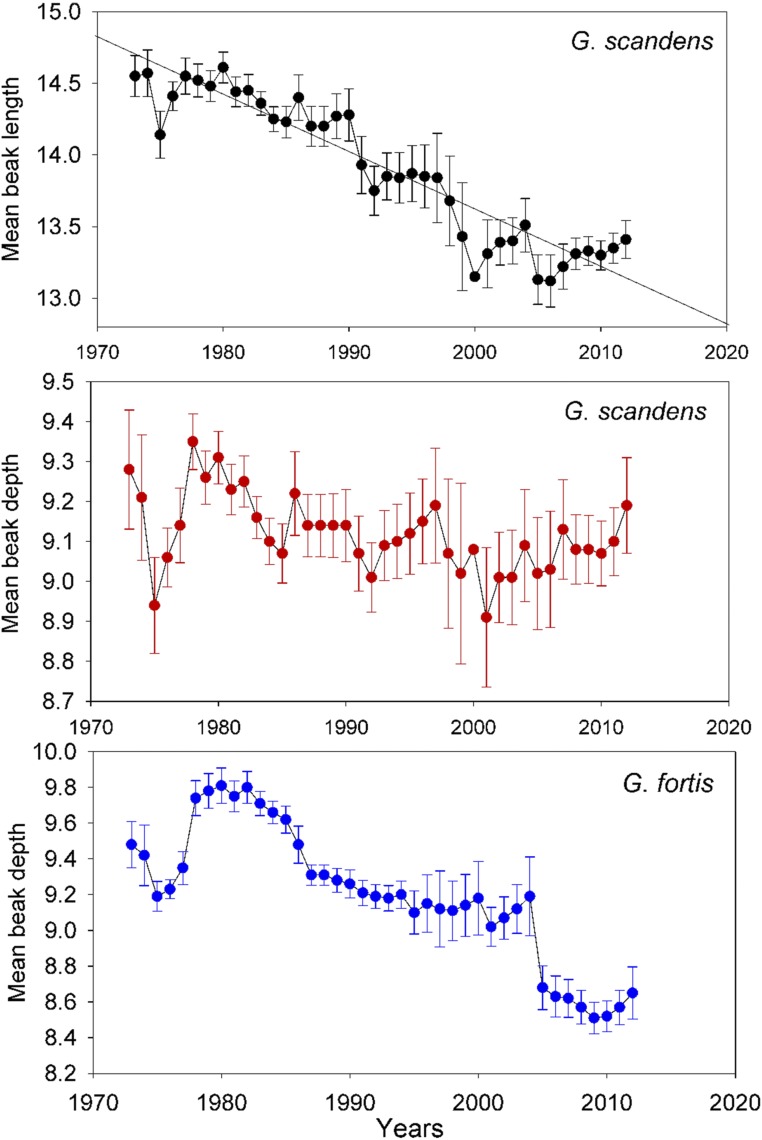
The average beak length of G. scandens, the larger species, should become smaller if introgressive hybridization of the 2 species is the sole cause of year-to-year changes. The predicted decline in average G. scandens beak length from 1987 onward is observed (Fig. 2). It is illustrated by a trend line fitted to the data by least squares regression (adjusted R2 = 0.78). In contrast to beak length, mean beak depth of G. scandens is not expected to decline regularly because it is much more similar to G. fortis than is beak length (Fig. 2). For example, overall averages with 95% confidence intervals for the 35 y are 9.18 ± 0.28 mm for G. fortis and 9.11 ± 0.07 mm for G. scandens. As expected, average beak depth of G. scandens fluctuates through random sampling without a net change (adjusted R2 = 0.02). The result of a systematic change in length but not in depth is a change in shape, in the direction of increasing bluntness.
Fig. 2.
Annual mean beak dimensions with 95% confidence limits. Mean beak length of G. fortis is almost identical to mean beak depth as a consequence of a strong phenotypic correlation (5). G. scandens samples vary from 15 (1974) to 364 (1983) with a mean of 135, and G. fortis samples vary from 31 (1974) to 933 (1976) with a mean of 311.
For G. fortis, the all-else-equal proviso is not applicable as they, unlike G. scandens, hybridize with rare immigrants of the small ground finch G. fuliginosa. Mean beak dimensions of G. fortis gradually declined until 2004, possibly more influenced by breeding with G. fuliginosa than with G. scandens (SI Appendix, Fig. S1). In 2004 the means decreased further and sharply (Fig. 2) when large birds (including FS hybrids) were at a selective disadvantage during a prolonged drought (54). After 2004 there was no further change. Stability could reflect the absence of hybridization, but the high proportion of admixed samples at this time (Fig. 1) when breeding was not studied in detail suggests instead that morphological effects of new hybrids (births) approximately balanced the effects of those that died or emigrated. Morphological effects of adding the relatively large FS hybrids may have been counterbalanced to some extent by addition of the relatively small Ff individuals (SI Appendix, Fig. S1).
Variation.
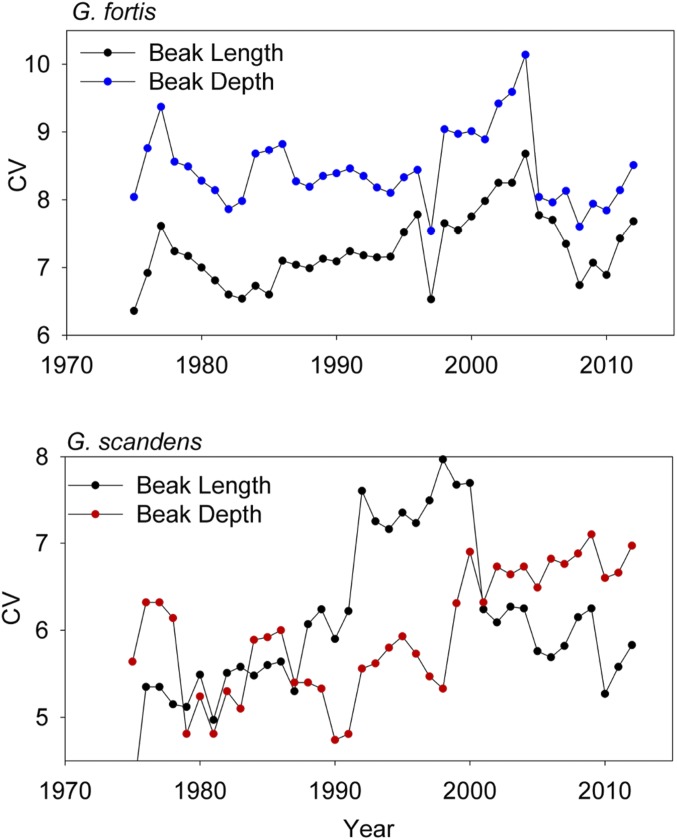
Variances are predicted to increase as a result of introgression, more in beak length than in beak depth because the species differ more in beak length (34). Since means decreased in both species, with few exceptions, coefficients of variation (CVs) should increase. The expected increases are observed (Fig. 3) and are closely matched by increases in variances (SI Appendix, section 3). Variation in G. fortis beak dimensions increased from 1983 up to 2004, as predicted, but then strong directional selection against large beak size (52) caused an associated and precipitous drop in the variance and CV of both dimensions. During the period before selection, CVs increased linearly with time both in beak length (F1,20 = 52.96, P < 0.0001, adjusted R2 = 0.712) and beak depth (F1,20 = 10.58, P = 0.004, adjusted R2 = 0.313). CVs of the 2 traits are strongly correlated across years (r = 0.74, P < 0.0001, n = 35 y).
Fig. 3.
Annual fluctuations in CVs.
CVs of the 2 traits in G. scandens are not correlated (r = 0.02, P < 0.90), and they fluctuated independently through time (Fig. 3). Beak-depth CVs increased linearly from the time of backcrossing (1987) onward (F1,24 = 91.79, P < 0.0001, adjusted R2 = 0.78), as predicted by the hypothesis of introgressive hybridization. In striking contrast, coefficients for beak length increased abruptly from 1990 to 1991 at a time of extensive breeding associated with El Niño conditions, remained elevated until 1999, which was a year post-El Niño without breeding, and then fell equally, and finally declined. Thus, variation in the 2 traits was positively associated from 1990 to 2000 and then negatively associated afterward. From 2000 onward, the CV of beak length regularly decreased (r = −0.66, P = 0.018) at the same time as the CV of beak depth increased (r = 0.67, P = 0.013).
The contrast in both means (Fig. 2) and variation (Fig. 3) implies that effects of introgression on beak length and beak depth were uncoupled. Uncoupling suggests a way in which predictions and discrepant observations are reconciled. In each species length and depth are genetically correlated, more strongly in G. fortis than in G. scandens (5). Phenotypic, and possibly genetic, correlations are weakened in F1 hybrids of species differing in beak proportions such as G. fortis and G. scandens (5). We interpret the contrast between strong trait covariation in G. fortis and weaker covariation in G. scandens as a reflection of greater introgression into G. scandens than into G. fortis (Fig. 1).
Two other factors might have contributed to increases in variation: conspecific gene flow and diversifying selection. In the nonbreeding season Daphne Major island receives immigrants, mainly G. fortis and G. fuliginosa, from Santa Cruz and Santiago, but they rarely stay to breed (26, 35). The frequency of G. fortis immigrants breeding with residents during the period of intense study of breeding was extremely low, and much lower than the frequency of hybridization (26). Immigrant G. fortis individuals were larger than average but not extreme and consequently had little effect upon the CVs. However, immigration might have been higher in the last 14 y when breeding was not studied in detail. CVs of G. scandens beak length declined at this time, even though admixed individuals were present (Fig. 1), which is contrary to expectation from the hybridization hypothesis (next section). There is no evidence of diversifying selection on this ecologically simple island (33).
Ad Hoc Explanations of Beak Length Variation in G. scandens
Differential gains of morphologically unusual hybrids could explain the unexpected increase in CV from 1990 to 1991, and differential losses could explain the decrease in CV from 2000 to 2001. The first explanation is tested by a comparison of beak lengths of hybrids present in 1990 (n = 7) with those newly recruited in 1991 (n = 5). In agreement with expectation, the mean beak length (±SE) of the 1991 recruits (11.79 ± 0.33 mm) was substantially smaller (t11 = 2.43, P = 0.0381) than the mean of the 1990 group (13.46 ± 0.60 mm).
The year 1999 was dry with little breeding and high mortality (33). The change in CV from the following year to the next was due to losses, 2 only, and not to gains. Further, the mean declined to a small extent (Fig. 3), and therefore the change in CV is not explained by the loss of (relatively small) hybrids. A change of variance without a change in mean is consistent with stabilizing selection. Hybridization appears not to have been relevant.
Hybridization as a Cause of Increases in Variance and Skewness.
Variation of a sample of measurements increases when hybrids are combined with nonhybrids. When a sample is split into hybrid and nonhybrids, these 2 components are predicted to differ in mean beak dimensions, more in length than in depth, and in a direction determined by the difference in means between species. We tested the prediction with samples of individuals (Table 1) produced in the 5 y of extensive breeding and largest numbers of admixed hybrids identified by microsatellites (2002, 2005, 2008, 2009, and 2010). Ff hybrids were excluded. Results agree with expectations (Table 1). For beak length, in all 5 y FS admixed individuals were larger on average than G. fortis, and SF admixed individuals were smaller on average than G. scandens (data combined, one-tailed binomial test, P = 0.001). Despite small samples of admixed individuals, 5 of the 10 individual comparisons were significantly different. For beak depth, in all 5 y FS admixed individuals were larger on average than G. fortis (P = 0.031), but SF admixed individuals were larger on average than G. scandens in only 3 comparisons (P = 0.812). The same procedure resulted in predictable changes in skewness. Frequency distributions are predicted by the hybridization hypothesis to be right-skewed, positively, in G. fortis toward the larger species, and left-skewed, negatively, in G. scandens (SI Appendix, section 4). Removal of hybrids should reduce, if not eliminate, skewness in frequency distributions of beak measurements (34). Reduction of skewness was observed for G. scandens beak length in all 5 y (P = 0.031) and in 4 y for G. fortis beak length. With data from the 2 species combined the one-tailed binomial probability is 0.011. Neither expectation was realized for beak depth (P = 0.188).
Table 1.
Beak dimensions of species compared with admixed individuals (FS or SF)
| Length | Depth | |||||||
| Year | Group | n | Mean | SE | P | Mean | SE | P |
| 2002 | G. fortis | 69 | 10.85 | 0.094 | 0.0078 | 9.10 | 0.099 | 0.2505* |
| FS | 7 | 11.75 | 0.371 | 9.70 | 0.469 | |||
| G. scandens | 67 | 13.48 | 0.099 | 0.0995* | 9.06 | 0.075 | 0.6363 | |
| SF | 7 | 12.31 | 0.597 | 8.94 | 0.196 | |||
| 2005 | G. fortis | 93 | 10.31 | 0.080 | <0.0001 | 8.62 | 0.062 | 0.1379* |
| FS | 10 | 12.11 | 0.257 | 9.88 | 0.343 | |||
| G. scandens | 29 | 13.17 | 0.124 | 0.1181* | 9.05 | 0.124 | 0.5354 | |
| SF | 10 | 12.65 | 0.286 | 9.20 | 0.182 | |||
| 2008 | G. fortis | 79 | 10.23 | 0.082 | 0.1186* | 8.47 | 0.074 | 0.1514* |
| FS | 5 | 11.34 | 0.563 | 9.22 | 0.422 | |||
| G. scandens | 97 | 13.41 | 0.077 | 0.0110 | 8.99 | 0.058 | 0.2540 | |
| SF | 19 | 12.65 | 0.261 | 9.16 | 0.172 | |||
| 2009 | G. fortis | 51 | 10.28 | 0.109 | 0.1406* | 8.37 | 0.106 | 0.3342 |
| FS | 5 | 11.50 | 0.666 | 8.72 | 0.320 | |||
| G. scandens | 40 | 13.46 | 0.142 | 0.2250* | 9.10 | 0.103 | 0.4676 | |
| SF | 5 | 12.42 | 0.726 | 9.32 | 0.213 | |||
| 2010 | G. fortis | 74 | 10.40 | 0.086 | 0.0262* | 8.46 | 0.072 | 0.2171 |
| FS | 10 | 11.36 | 0.359 | 8.73 | 0.268 | |||
| G. scandens | 59 | 13.28 | 0.125 | <0.0001 | 8.92 | 0.085 | 0.3132 | |
| SF | 15 | 11.91 | 0.317 | 8.73 | 0.177 | |||
P values are associated with one-tailed t tests that assume unequal variances or, when variances were equal, by F tests as indicated by an asterisk (*). Significance is indicated by boldface for emphasis. Original measurements were in millimeters.
Hybridization on Other Islands
Patterns of Variation in Time.
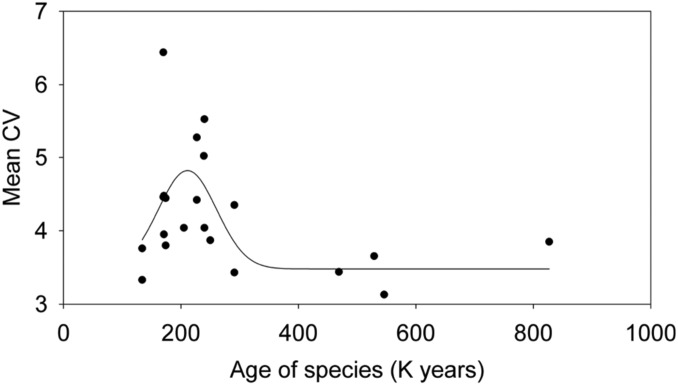
Variation is expected to increase and then to decrease in phylogenetic time according to the argument in the Introduction. Differences in average population variation of the 18 species (SI Appendix, section 5) fit the expected temporal pattern (Fig. 4). A maximum in average beak length and depth variation is reached after about 200,000 y. Roughly 100,000 y after that, variability has decreased to an apparent long-term average. Thus, there is a window of unknown width during which gene exchange has the greatest effect on phenotypic variation. This interpretation assumes that the pattern reflects a process: that each species has the potential to increase and then decrease in variation through time. Enhanced phenotypic variation probably reflects enhanced genetic variation because beak traits are highly heritable in the 3 species that have been studied in detail (25, 55, 56).
Fig. 4.
Average CVs of beak length for male specimens of each species of Darwin’s finches in relation to their ages. Points fitted by nonlinear, polynomial regression: peak, Gaussian, and 4 parameters. The adjusted R2 value is 0.23. Included are 2 pairs of populations, Certhidea fusca from Española and San Cristóbal and Geospiza septentrionalis from Wolf and Darwin. These are the only pairs of conspecific populations with estimated ages of independence (40). Morphological data are from ref. 41. The curve for beak depth variation is almost identical.
The low values of average coefficients of variation for species older than 400,000 y does not mean those species do not hybridize; it means only that introgression is not on a sufficient scale to affect morphological variation. Introgressive hybridization may persist for much longer. Price and Bouvier (57), surveying data from a large number of passerine bird species, estimated that infertility of hybrids arises on average 7 My after separation of the parental species from a common ancestor.
Unusually Variable Populations.
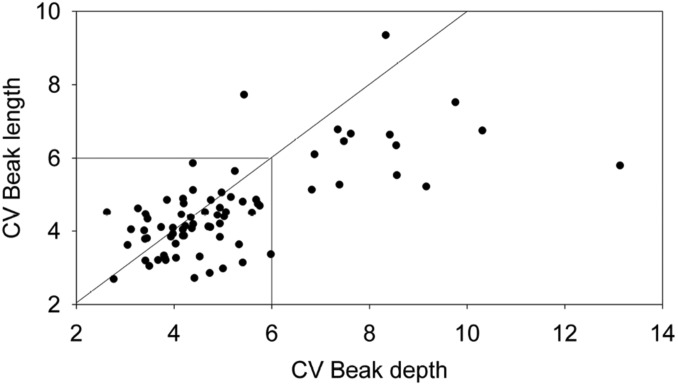
Coefficients of variation of individual populations are informative about hybridization even in the absence of age estimates (Fig. 5). A previous study of North American and South American emberizids, and Hawaiian cardueline finches, found that coefficients greater than 6.0 are rare and present in only 3 of 46 samples of beak length variation in 19 species and 2 of 44 samples of beak depth variation in 17 species (5, 25, 42). Coefficients exceeding 6.0 are much more common among Darwin’s finch populations. In the total dataset (SI Appendix, Table S1), 27 of 186 coefficients (14.5%) are >6.0. They are more frequent in beak depth than in beak length (Table 2) and are heterogeneous in 3 respects. First, all but 2 of them are in the ground finch genus Geospiza (Table 2). Second, most of them are from populations of one species, G. fortis. Third, populations of the same species differ strikingly in levels of variation. G. fortis is the best example. Most (8) of the 13 populations of this species are unusually variable in one or both beak dimensions. Coefficients of beak depth variation range from 3.4 (±0.08 SD) on Marchena to 10.3 (±1.43) on Santa Cruz. Thus, variation itself varies among species and islands.
Fig. 5.
CVs for beak dimensions of adult males of 75 populations of Darwin’s finches (41).
Table 2.
Populations with unusually variable beak length or depth by the criterion of CV >6.0 are shown in red
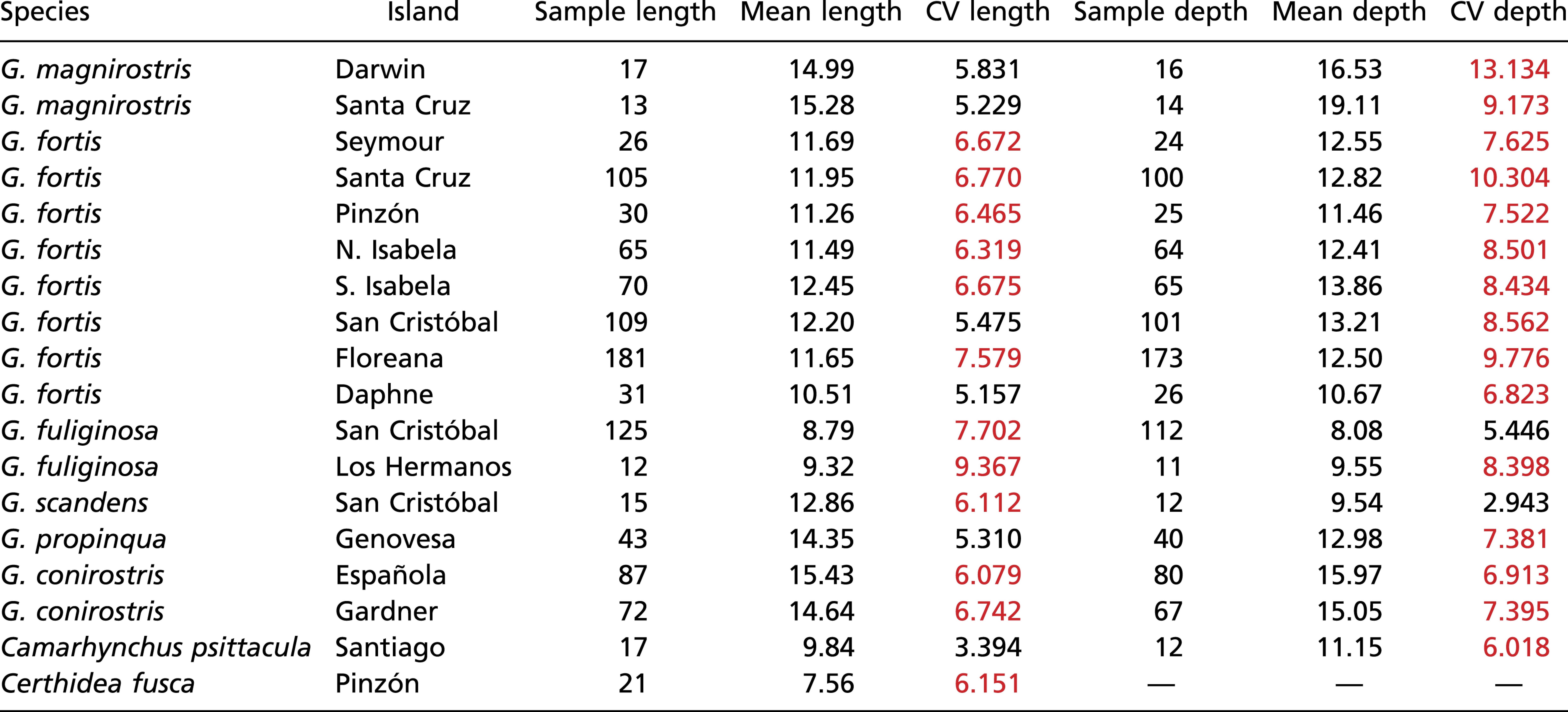 |
CV = 100 × SD/mean. Original measurements were in millimeters.
Comparative Tests.
When 2 hybridizing species differ more in one beak dimension than in the other, the hybridization hypothesis predicts the dimension that differs most will be the more variable. Field observations allow 2 tests.
G. fuliginosa and G. scandens on San Cristóbal.
Interbreeding was observed in the Bosque de Cactus in April 1997 and February 2018 (personal observation). The 2 species differ more in mean beak length (51.3%) than in mean beak depth (20.7%) and therefore are predicted to be unusually variable in beak length. This is observed in both species (Table 2).
Geospiza propinqua and G. magnirostris on Genovesa.
Hybridization was studied in the years 1978 to 1988 (25). The species differ more in average mean depth (63.4%) than in mean beak length (15.1%). They are predicted to be unusually variable in beak depth but not in length. This is observed in G. propinqua but not in G. magnirostris. A possible reason for the difference is unidirectional gene flow through differential backcrossing into G. propinqua. The direction of backcrossing would be influenced not only by paternal song but also by the size difference between the sexes. Males are generally larger than females (25, 42) and G. magnirostris are larger than G. propinqua, so G. propinqua males × G. magnirostris females are more size-compatible than G. magnirostris males × G. propinqua females. G. propinqua males × G. magnirostris pairs produce offspring that imprint on paternal song, and these backcross to G. propinqua (25, 49).
Indirect Evidence of Hybridization.
Populations of Geospiza conirostris and G. fuliginosa coexist on Española and Gardner with no other congeneric species. If they hybridize, they are expected to be unusually variable in both beak dimensions because on Española they differ substantially in both beak length (76.9%) and beak depth (92.9%): data for G. fuliginosa on Gardner are lacking. The expectation is realized in G. conirostris in both beak dimensions and on both islands, but not in G. fuliginosa. (SI Appendix, Table S1). The difference between the species can be rationalized as the result of unidirectional gene exchange. This hypothesis should be tested. Breeding patterns on these islands are almost unknown (58).
G. fortis has several unusually variable populations.
The species is morphologically intermediate between a smaller (G. fuliginosa) and a larger (G. magnirostris) species with similar but not identical beak proportions. If G. fortis receives genes from one or both of the others it should be unusually variable in beak depth, because this dimension is more strongly correlated with size (5) than is beak length, or in both beak dimensions, but not in beak length alone. Expected differences are observed. Six populations are unusually variable in both beak length and beak depth, and 2 populations are unusually variable in only beak depth. Five populations are not unusually variable in either beak dimension (SI Appendix, Table S1). There are no modern studies of them that could serve as a basis for an explanation. Sample sizes are small (n = 10 to 15) for 2 of them (Rábida and Fernandina). There are also no studies of the exceptionally variable population of G. magnirostris on Darwin whose variation is difficult to interpret (SI Appendix, section 6).
Solitary Finches.
Pinaroloxias inornata occurs on the strongly isolated Cocos Island (43). In the absence of other finch species, it is neither expected nor observed to be unusually variable (SI Appendix, Table S1). In the Galápagos, G. fuliginosa is the only species present on Los Hermanos, a cluster of 3 islets off the coast of Isabela. It is unusually variable in both beak depth and length. The variation could be the product of diversifying selection, but the islets are small and with little heterogeneity in vegetation, so the unusual variation is more likely to be the result of breeding with occasional G. fortis immigrants from nearby Isabela (59).
Conclusions and Discussion
Adaptive radiations provide rich material for understanding how and why natural and sexual selection cause populations to diverge, how barriers to interbreeding arise, why some taxa radiate and others do not (60), and how species accumulation through speciation is reduced by extinction (61–63). Introgressive hybridization in the early stages of a radiation may play a central role in both speciation (3, 4, 15, 64, 65) or its opposite, the collapse of the speciation process (38, 66–70). Those roles can be inferred as historical processes using genomic data (15, 16, 71) but can only be studied directly with currently hybridizing species, which is why there is a need to integrate the findings of hybridization in the past and the present in those radiating taxa where this is possible, such as cichlid fish (15, 16, 71, 72), estrildid finches (73), heliconiine butterflies (9, 74) and the Hawaiian silversword alliance (75–77). It is not possible in older radiations, such as Anolis lizards in the Caribbean that experience little or no contemporary hybridization (78), and Hawaiian honeycreeper finches that have lost many species through human-caused extinctions (79, 80).
Darwin’s finches are a model group for the study of adaptive radiation, and the finches on Daphne Major island are a model subgroup for studying evolution in contemporary time. Here we have combined the study of evolution in the past with evolution in the present by 1) quantifying contemporary hybridization and 2) examining the implication of increased variation in phylogenetically young species. Exchange of genes through introgressive hybridization provides a working hypothesis and an organizing framework for understanding levels of continuous morphological variation that are elevated above a background determined by mutation, drift, and intraspecific gene flow (6).
We found that population variation in Darwin’s finch beak traits increased with time since sharing a common ancestor with a sister species, reached a peak, and then fell. If the pattern of rise and fall of variation is due to a changing gene exchange, as hypothesized, there is a period of maximum genetic admixture and potential for genetic reorganization of important phenotypic traits. Consistent with this reasoning, hybridization of markedly different species elsewhere is known to result in the evolution of novel metabolic traits (8), reduced covariance of functionally related traits (81, 82), and enhanced evolvability (64). The period of maximum potential is likely to vary among taxa and environments and be dependent upon 3 rates: gene exchange, divergence, and evolution of genetic incompatibilities. This period will be reached quickly in explosive radiations like ant-nest beetles (83), cichlid fish (15, 16, 60, 71, 72), and lupins (84) and may last for longer in taxa that acquire genetic incompatibilities slowly, as in reptiles relative to other vertebrates such as mammals (85) and birds (57). At the time of maximum potential or soon after, a mosaic pattern of gene exchange arises, with some genes passing freely from one species to another and others being apparently filtered out for diverse reasons that are not well understood (12, 71, 86, 87). This may be a time of greatest ecological opportunity that helps to maintain the large variation, followed subsequently by a stronger regime of stabilizing selection, lower morphological variation, and a narrower ecological niche.
The long-term study of contemporary hybridization on Daphne provides a foundation for making inferences of hybridization in the past. The medium ground finch (G. fortis) hybridizes with a larger, nonsister species, the cactus finch (G. scandens). They shared a common ancestor 200,000 to 300,000 y ago (40), which places them close to the peak of population variation in relation to time that we attribute to introgressive hybridization (Fig. 4). The species on Daphne have unusually high coefficients of beak variation, ≥6.0 (Fig. 3), when compared with samples of other populations that have been studied from museum specimens only (Table 2 and Fig. 5).
The study of finches on Daphne revealed changing effects of hybridization on morphology. The strongest support for the introgressive hybridization hypothesis is the increased admixture in the G. scandens population, a decrease in average beak length toward the average G. fortis, and an increase in variation until the year 2000. The direction of backcrossing varied in time, with variances increasing and frequency distributions becoming skewed in a temporarily varying pattern. In a previous report we showed that hybridization of G. fortis and G. scandens resulted in convergence of beak shape in parallel to a convergence of the same magnitude in microsatellite DNA (33, 38). Here we have partitioned shape into the 2 most important components, length and depth. This has revealed unequal contributions to a change in shape in G. scandens but not in G. fortis. Beak length and depth covary strongly in G. fortis owing to a strong genetic correlation, whereas the genetic correlation is weaker in G. scandens (5). Trait independence in G. scandens following extensive hybridization is manifested as a change from a positive association of their coefficients of variation from 1990 to 2000 to a negative association afterward. Similarly, trait means varied concordantly only part of the time. Independent length and depth evolution may be caused by differential gene exchange, as well as by natural selection through subtle advantages that accrue from different functional associations with food (e.g., depth associated with efficiency of cracking seeds and length associated with efficiency of opening Opuntia echios fruits) (33). Hybridization may have facilitated the independence by weakening constraints from phenotypic and genetic correlations between the 2 traits (5). This implies an important role for introgression in the radiation, in combination with natural selection, because a major feature of the radiation is the large diversity of beak shapes (41, 43). The suggestion of selective gene transfer is supported by demonstrations of adaptive gene exchange in other systems (6, 72, 88–91).
The first genomics problem of hybridizing species is to identify and understand which genes are transferred and which are not. The second problem is to determine the functioning of transferred genes in a novel background. Bidirectional introgression in hybridizing bird species creates genomic novelty through rearrangements of ancestral variation (73), inversions (92), differential allelic exchange, and a mosaic of parental inheritance (12, 86, 93–96). These mechanisms are currently being assessed in Darwin’s finches, together with 2 questions that need to be answered in order to improve understanding of evolution by introgression. First, how does introgression affect expression of transcription factors at loci (HMGA2 and ALX1) that are known to be associated with beak size and shape variation (13, 97, 98), and second, how does introgression affect expression of signaling molecules, CaM (99) and BMP4 (100), that independently regulate the development of beak length and beak depth respectively during craniofascial development (101)?
The situation on Daphne is unusual for Darwin’s finches but not unique. Throughout the archipelago ground finch species interbreed and so do tree finch species, albeit rarely (27–29, 31). Nine populations of G. fortis and G. propinqua with unusually variable beaks (Table 2) are particularly interesting for what they imply about the ecology of variation. Each of them is intermediate in size between a coexisting larger and a smaller species. The middle species of a triplet has 2 size neighbors as potential breeding partners, whereas each of the outer, extreme species has only 1. Of the extreme species, only one small (G. fuliginosa) and one large (G. magnirostris) member of a triplet have unusually variable beaks. When the 3 species occupy dispersed positions on a single resource axis and are in potential competition the intermediate species is expected to be a generalist and the 2 outer species are expected to be specialists. The feeding ecology of the ground finches is consistent with these expectations (25, 33, 43, 102). Coexistence is dependent on the spacing of resource optima and their stability through time (59, 103). Given wide spacing, the intermediate species may be subject to diversifying selection, and even disruptive selection, for which there is evidence from studies of a population of G. fortis on Santa Cruz with a uniquely bimodal distribution of beak sizes (104, 105).
Hybridization is more than a factor of interest in past and present evolution; it is relevant to the future. The potential for evolutionary change is a function of quantitative genetic variation and covariation on the one hand and environmental variation on the other. The threat of environmental degradation caused by habitat destruction, alien introductions, and globally increasing temperatures and extreme events throws a spotlight on the sufficiency of genetic variation to allow evolutionary responses to environmental change (106–108). Hybridization, by elevating levels of quantitative genetic variation and reducing the strength of genetic correlations between traits, will increase the ability of some species to respond to new challenges (109). Hybrids are more live paths to the future than dead ends.
Methods
Sampling Design.
Field methods on Daphne Major island have been described extensively in previous publications (33, 35). Adults were captured in mist nets, 6 measurements were taken of body size and beak dimensions, and 1 numbered metal band and 3 color bands coded to correspond to the number were applied to the leg. Starting in 1988 a small drop of blood was taken by brachial wing puncture and stored in Drierite for later DNA extraction and microsatellite analysis (33). The percent of breeding birds that were banded was 90 to 100 in 1980 to 1994 and gradually declined thereafter. The Princeton University Animal Care Committee approved the research procedures.
Statistical Analysis.
As described before (33), the program STRUCTURE was used to assign individuals probabilistically to 3 groups, G. fortis (F), G. scandens (S), and G. fuliginosa (f) on the basis of allelic variation at 14 autosomal microsatellite loci. A probability criterion of ≥0.9 was used to classify an individual to 1 of the 3 species. In the few cases when the assignment probability fell below the criterion the individual was judged to be admixed and classified according to the magnitude of the probabilities of assignment to the 3 species. It was classified as a member of the G. fortis population if the assignment probability was highest to G. fortis (i.e., FS or Ff) and as a member of the G. scandens population if the assignment probability was highest to G. scandens (i.e., SF). Pedigrees, corrected for extrapair paternity, confirmed the reliability of the assignments. Ninety-seven percent of 167 G. fortis families produced only G. fortis offspring (n = 590), and 99% of 47 G. scandens families produced only G. scandens offspring (n = 175). Statistical analyses were performed in JMP (SAS Institute). All tests were 2-tailed, unless indicated otherwise.
Data Availability.
Data are available from the authors upon request.
Supplementary Material
Acknowledgments
We thank Eric Enbody for comments on the manuscript and the Charles Darwin Research Station for logistical support and the many assistants who helped in fieldwork. The research was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the National Science Foundation and was carried out with permission of the Galápagos National Parks Directorate, all of which we gratefully acknowledge.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1913534116/-/DCSupplemental.
References
- 1.Lewontin R. C., Birch L. C., Hybridization as a source of variation for adaptation to new environments. Evolution 20, 315–336 (1966). [DOI] [PubMed] [Google Scholar]
- 2.Svärdson G., “Significance of introgression in coregonid evolution” in Biology of Coregonid Fishes, Lindsey C. C., Woods C. S., Eds. (University of Manitoba Press, Winnipeg, 1970), pp. 39–59. [Google Scholar]
- 3.Abbott R., et al. , Hybridization and speciation. J. Evol. Biol. 26, 229–246 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Taylor S. A., Larson E. L., Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nat. Ecol. Evol. 3, 170–177 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Grant P. R., Grant B. R., Phenotypic and genetic effects of hybridization in Darwin’s finches. Evolution 48, 297–316 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Hedrick P. W., Adaptive introgression in animals: Examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 22, 4606–4618 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Seehausen O., Conditions when hybridization might predispose populations for adaptive radiation. J. Evol. Biol. 26, 279–281 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Pereira R. J., Barreto F. S., Burton R. S., Ecological novelty by hybridization: Experimental evidence for increased thermal tolerance by transgressive segregation in Tigriopus californicus. Evolution 68, 204–215 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Mavárez J., et al. , Speciation by hybridization in Heliconius butterflies. Nature 441, 868–871 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Grant P. R., Grant B. R., Synergism of natural selection and introgression in the origin of a new species. Am. Nat. 183, 671–681 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Lavretsky P., Engilis A. Jr, Eadie J. M., Peters J. L., Genetic admixture supports an ancient hybrid origin of the endangered Hawaiian duck. J. Evol. Biol. 28, 1005–1015 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Elgvin T. O., et al. , The genomic mosaicism of hybrid speciation. Sci. Adv. 3, e1602996 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamichhaney S., et al. , Rapid hybrid speciation in Darwin’s finches. Science 359, 224–228 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Seehausen O., Hybridization and adaptive radiation. Trends Ecol. Evol. 19, 198–207 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Malinsky M., et al. , Whole-genome sequences of Malawi cichlids reveal multiple radiations interconnected by gene flow. Nat. Ecol. Evol. 2, 1940–1955 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irisarri I., et al. , Phylogenomics uncovers early hybridization and adaptive loci shaping the radiation of Lake Tanganyika cichlid fishes. Nat. Comm. 9, 3159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kulmuni J., Pamilo P., Introgression in hybrid ants is favored in females but selected against in males. Proc. Natl. Acad. Sci. U.S.A. 111, 12805–12810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duckworth R. A., Semenov G. A., Hybridization associated with cycles of ecological succession in a passerine bird. Am. Nat. 190, E94–E105 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Wheatcroft D., Qvarnström A., Reproductive character displacement of female, but not male song discrimination in an avian hybrid zone. Evolution 71, 1776–1786 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Arnold M. L., Natural Hybridization and Evolution (Oxford University Press, Oxford, 1997). [Google Scholar]
- 21.Rieseberg L. H., et al. , Major ecological transitions in wild sunflowers facilitated by hybridization. Science 301, 1211–1216 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Stankowski S., Streisfeld M. A., Introgressive hybridization facilitates adaptive divergence in a recent radiation of monkeyflowers. Proc. Biol. Sci. 282, 20151666 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goulet B. E., Roda F., Hopkins R., Hybridization in plants: Old ideas and new techniques. Plant Physiol. 173, 65–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez-Gonzalez A., Lexer C., Cronk Q. C. B., Adaptive introgression: A plant perspective. Biol. Lett. 14, 20170688 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grant B. R., Grant P. R., Evolutionary Dynamics of a Natural Population (University of Chicago Press, Chicago, 1989). [Google Scholar]
- 26.Grant P. R., Grant B. R., Conspecific versus heterospecific gene exchange between populations of Darwin’s finches. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 1065–1076 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peters K. J., Myers S. A., Dudaniec R. Y., O’Connor J. A., Kleindorfer S., Females drive asymmetrical introgression from rare to common species in Darwin’s tree finches. J. Evol. Biol. 30, 1940–1952 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Bowman R. I., “The evolution of song in Darwin’s Finches” in Patterns of Evolution in Galapagos Organisms, Bowman R. I., Berson M., Leviton A. E., Eds. (American Association for the Advancement of Science, Pacific Division, San Francisco, CA, 1983), pp. 237–537. [Google Scholar]
- 29.Grant P. R., Grant B. R., Petren K., Hybridization in the recent past. Am. Nat. 166, 56–67 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Farrington H. L., Lawson L. P., Clark C. M., Petren K., The evolutionary history of Darwin’s finches: Speciation, gene flow, and introgression in a fragmented landscape. Evolution 68, 2932–2944 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Kleindorfer S., et al. , Species collapse via hybridization in Darwin’s tree finches. Am. Nat. 183, 325–341 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Lawson L. P., et al. , Slow motion extinction: Inbreeding, introgression, and loss in the critically endangered mangrove finch (Camarhynchus heliobates). Conserv. Genet. 18, 159–170 (2017). [Google Scholar]
- 33.Grant P. R., Grant B. R., 40 Years of Evolution. Darwin’s Finches on Daphne Major Island (Princeton University Press, Princeton, 2014). [Google Scholar]
- 34.Grant P. R., Grant B. R., Unpredictable evolution in a 30-year study of Darwin’s finches. Science 296, 707–711 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Boag P. T., Grant P. R., Darwin’s finches (Geospiza) on isla Daphne major, Galápagos: Breeding and feeding ecology in a climatically variable environment. Ecol. Monogr. 54, 463–489 (1984). [Google Scholar]
- 36.Grant P. R., Price T. D., Population variation in continuously varying traits as an ecological genetics problem. Am. Zool. 21, 795–811 (1981). [Google Scholar]
- 37.Grant P. R., Grant B. R., Hybridization of bird species. Science 256, 193–197 (1992). [DOI] [PubMed] [Google Scholar]
- 38.Grant P. R., Grant B. R., Markert J. A., Keller L. F., Petren K., Convergent evolution of Darwin’s finches caused by introgressive hybridization and selection. Evolution 58, 1588–1599 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Burns K. J., et al. , Phylogenetics and diversification of tanagers (Passeriformes: Thraupidae), the largest radiation of Neotropical songbirds. Mol. Phylogenet. Evol. 75, 41–77 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Lamichhaney S., et al. , Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 518, 371–375 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Lack D., The galapagos finches (geospizinae): A study in variation. Occas. Pap. Calif. Acad. Sci. 21, 1–159 (1945). [Google Scholar]
- 42.Grant P. R., Abbott I., Schluter D., Curry R. L., Abbott L. K., Variation in the size and shape of Darwin’s finches. Biol. J. Linn. Soc. Lond. 25, 1–39 (1985). [Google Scholar]
- 43.Grant P. R., Ecology and Evolution of Darwin’s Finches (Princeton University Press, Princeton, 1986). [Google Scholar]
- 44.Ratcliffe L. M., Grant P. R., Species recognition in Darwin’s finches (Geospiza, gould). I. Discrimination by morphological cues. Anim. Behav. 31, 1139–1153 (1983). [Google Scholar]
- 45.Ratcliffe L. M., Grant P. R., Species recognition in Darwin’s Finches (Geospiza, Gould). II. Geographic variation in mate preference. Anim. Behav. 31, 1154–1165 (1983). [Google Scholar]
- 46.Ratcliffe L. M., Grant P. R., Species recognition in Darwin’s Finches (Geospiza, Gould). III. Male responses to playback of different song types, dialects and heterospecific songs. Anim. Behav. 33, 290–307 (1985). [Google Scholar]
- 47.Grant P. R., Grant B. R., Mating patterns of finch hybrids determined by song and morphology. Biol. J. Linn. Soc. Lond. 60, 317–343 (1997). [Google Scholar]
- 48.Grant B. R., Grant P. R., Simulating secondary contact in allopatric speciation: An empirical test of premating isolation. Biol. J. Linn. Soc. Lond. 60, 317–343 (2002). [Google Scholar]
- 49.Grant B. R., Grant P. R., Cultural inheritance of song and its role in the evolution of Darwin’s finches. Evolution 50, 2471–2487 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Grant P. R., Grant B. R., Role of sexual imprinting in assortative mating and premating isolation in Darwin’s finches. Proc. Natl. Acad. Sci. U.S.A. 115, E10879–E10887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boag P. T., Grant P. R., Intense natural selection in a population of Darwin’s finches (geospizinae) in the Galápagos. Science 214, 82–85 (1981). [DOI] [PubMed] [Google Scholar]
- 52.Gibbs H. L., Grant P. R., Oscillating selection on Darwin’s finches. Nature 327, 511–513 (1987). [Google Scholar]
- 53.Falconer D. G., Mackay T. F. C., Introduction to Quantitative Genetics (Longman, Harlow, UK, ed. 4, 1996). [Google Scholar]
- 54.Grant P. R., Grant B. R., Evolution of character displacement in Darwin’s finches. Science 313, 224–226 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Grant P. R., Grant B. R., “Quantitative genetic variation in populations of Darwin’s Finches” in Adaptive Variation in the Wild, Mousseau T. A., Sinervo B., Endler J., Eds. (Oxford University Press, New York, 1999), pp. 3–41. [Google Scholar]
- 56.Keller L. F., Grant P. R., Grant B. R., Petren K., Heritability of morphological traits in Darwin’s finches: Misidentified paternity and maternal effects. Heredity 87, 325–336 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Price T. D., Bouvier M. M., The evolution of F1 postzygotic incompatibilities in birds. Evolution 56, 2083–2089 (2002). [PubMed] [Google Scholar]
- 58.Downhower J. F., Observations on the nesting of the small ground finch Geospiza fuliginosa and the large cactus finch Geospiza conirostris on Española, Galápagos. Ibis 120, 340–346 (1978). [Google Scholar]
- 59.Schluter D., Price T. D., Grant P. R., Ecological character displacement in Darwin’s finches. Science 227, 1056–1059 (1985). [DOI] [PubMed] [Google Scholar]
- 60.Seehausen O., African cichlid fish: A model system in adaptive radiation research. Proc. Biol. Sci. 273, 1987–1998 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rabosky D. L., Lovette I. J., Explosive evolutionary radiations: Decreasing speciation or increasing extinction through time? Evolution 62, 1866–1875 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Rabosky D. L., Glor R. E., Equilibrium speciation dynamics in a model adaptive radiation of island lizards. Proc. Natl. Acad. Sci. U.S.A. 107, 22178–22183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henao Diaz L. F., Harmon L. J., Sugawara M. T. C., Miller E. T., Pennell M. W., Macroevolutionary diversification rates show time dependency. Proc. Natl. Acad. Sci. U.S.A. 116, 7403–7408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parsons K. J., Son Y. H., Albertson R. C., Hybridization promotes evolvability in african cichlids: Connections between transgressive segregation and phenotypic integration. Evol. Biol. 38, 306–315 (2011). [Google Scholar]
- 65.Mallet J., Besansky N., Hahn M. W., How reticulated are species? Bioessays 38, 140–149 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dobzhansky T., Genetics and the Origin of Species (Columbia University Press, New York, ed. 2, 1941). [Google Scholar]
- 67.Rhymer J. M., Simberloff D., Extinction by hybridization and introgression. Annu. Rev. Ecol. Syst. 27, 83–109 (1996). [Google Scholar]
- 68.Taylor E. B., et al. , Speciation in reverse: Morphological and genetic evidence of the collapse of a three-spined stickleback (Gasterosteus aculeatus) species pair. Mol. Ecol. 15, 343–355 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Webb W. C., Marzluff J. M., Omland K. E., Random interbreeding between cryptic lineages of the common raven: Evidence for speciation in reverse. Mol. Ecol. 20, 2390–2402 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Vonlanthen P., et al. , Eutrophication causes speciation reversal in whitefish adaptive radiations. Nature 482, 357–362 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Salzburger W., Understanding explosive diversification through cichlid fish genomics. Nat. Rev. Genet. 19, 705–717 (2018). [DOI] [PubMed] [Google Scholar]
- 72.Meier J. I., et al. , Ancient hybridization fuels rapid cichlid adaptive radiations. Nat. Comm. 8, 14363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stryjewski K. F., Sorenson M. D., Mosaic genome evolution in a recent and rapid avian radiation. Nat. Ecol. Evol. 1, 1912–1922 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Enciso-Romero J., et al. , Evolution of novel mimicry rings facilitated by adaptive introgression in tropical butterflies. Mol. Ecol. 26, 5160–5172 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Barrier M., Robichaux R. H., Purugganan M. D., Accelerated regulatory gene evolution in an adaptive radiation. Proc. Natl. Acad. Sci. U.S.A. 98, 10208–10213 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Purugganen M. D., Robichaux R. H., Adaptive radiation and regulatory gene evolution in the Hawaiian silversword alliance (Asteraceae). Ann. Mo. Bot. Gard. 92, 28–35 (2005). [Google Scholar]
- 77.Lawton-Rauh A., Robichaux R. H., Purugganan M. D., Diversity and divergence patterns in regulatory genes suggest differential gene flow in recently derived species of the Hawaiian silversword alliance adaptive radiation (Asteraceae). Mol. Ecol. 16, 3995–4013 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Losos J. B., Lizards in an Evolutionary Tree: Ecology and Adaptive Radiation of Anoles (University of California Press, Berkeley, 2009). [Google Scholar]
- 79.Grant P. R., Population variation and hybridization: Comparison of finches from two archipelagos. Evol. Ecol. 8, 598–617 (1994). [Google Scholar]
- 80.Lerner H. R., Meyer M., James H. F., Hofreiter M., Fleischer R. C., Multilocus resolution of phylogeny and timescale in the extant adaptive radiation of Hawaiian honeycreepers. Curr. Biol. 21, 1838–1844 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Selz O. M., Lucek K., Young K. A., Seehausen O., Relaxed trait covariance in interspecific cichlid hybrids predicts morphological diversity in adaptive radiations. J. Evol. Biol. 27, 11–24 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Stelkens R., Seehausen O., Genetic distance between species predicts novel trait expression in their hybrids. Evolution 63, 884–897 (2009). [DOI] [PubMed] [Google Scholar]
- 83.Moore W., Robertson J. A., Explosive adaptive radiation and extreme phenotypic diversity within ant-nest beetles. Curr. Biol. 24, 2435–2439 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Hughes C., Eastwood R., Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the andes. Proc. Natl. Acad. Sci. U.S.A. 103, 10334–10339 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prager E. M., Wilson A. C., “Phylogenetic relationships and rates of evolution of birds” in Proceedings of the 16th International Ornithological Congress (Verlag der Deutsche Ornithologon-Gesellschaft, Berlin, 1995), pp. 1209–1214. [Google Scholar]
- 86.Parchman T. L., et al. , The genomic consequences of adaptive divergence and reproductive isolation between species of manakins. Mol. Ecol. 22, 3304–3317 (2013). [DOI] [PubMed] [Google Scholar]
- 87.Rifkin J. L., Castillo A. S., Liao I. T., Rausher M. D., Gene flow, divergent selection and resistance to introgression in two species of morning glories (Ipomoea). Mol. Ecol. 28, 1709–1729 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Song Y., et al. , Adaptive introgression of anticoagulant rodent poison resistance by hybridization between old world mice. Curr. Biol. 21, 1296–1301 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Norris L. C., et al. , Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc. Natl. Acad. Sci. U.S.A. 112, 815–820 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen D., Yu Y., Hahn M. W., Nakhleh L., Reticulate evolutionary history and extensive introgression in mosquito species revealed by phylogenetic network analysis. Mol. Ecol. 25, 2361–2372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bechsgaard J., Jorgensen T. H., Schierup M. H., Evidence for adaptive introgression of disease resistance genes among closely related Arabidopsis species. G3 (Bethesda) 7, 2677–2683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hooper D. M., Griffith S. C., Price T. D., Sex chromosome inversions enforce reproductive isolation across an avian hybrid zone. Mol. Ecol. 28, 1246–1262 (2019). [DOI] [PubMed] [Google Scholar]
- 93.Sardell J. M., Uy J. A. C., Hybridization following recent secondary contact results in asymmetric genotypic and phenotypic introgression between island species of Myzomela honeyeaters. Evolution 70, 257–269 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Kingston S. E., Parchman T. L., Gompert Z., Buerkle C. A., Braun M. J., Heterogeneity and concordance in locus-specific differentiation and introgression between species of towhees. J. Evol. Biol. 30, 474–485 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Runemark A., et al. , Variation and constraints in hybrid genome formation. Nat. Ecol. Evol. 2, 549–556 (2018). [DOI] [PubMed] [Google Scholar]
- 96.Marques D. A., Meier J. I., Seehausen O., A combinatorial view on speciation and adaptation. Trends Ecol. Evol. 34, 531–544 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Lamichhaney S., et al. , A beak size locus in Darwin’s finches facilitated character displacement during a drought. Science 352, 470–474 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Chaves J. A., et al. , Genomic variation at the tips of the adaptive radiation of Darwin’s finches. Mol. Ecol. 25, 5282–5295 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Abzhanov A., et al. , The calmodulin pathway and evolution of elongated beak morphology in Darwin’s finches. Nature 442, 563–567 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Abzhanov A., Protas M., Grant B. R., Grant P. R., Tabin C. J., Bmp4 and morphological variation of beaks in Darwin’s finches. Science 305, 1462–1465 (2004). [DOI] [PubMed] [Google Scholar]
- 101.Brugmann S. A., et al. , Comparative gene expression analysis of avian embryonic facial structures reveals new candidates for human craniofacial disorders. Hum. Mol. Genet. 19, 920–930 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grant P. R., Grant B. R., How and Why Species Multiply (Princeton University Press, Princeton, 2008). [Google Scholar]
- 103.Schluter D., Grant P. R., Determinants of morphological patterns in communities of Darwin’s finches. Am. Nat. 123, 175–196 (1984). [Google Scholar]
- 104.Hendry A. P., et al. , Possible human impacts on adaptive radiation: Beak size bimodality in Darwin’s finches. Proc. Biol. Sci. 273, 1887–1894 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huber S. K., De León L. F., Hendry A. P., Bermingham E., Podos J., Reproductive isolation of sympatric morphs in a population of Darwin’s finches. Proc. Biol. Sci. 274, 1709–1714 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bell G., Evolutionary rescue and the limits of adaptation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368, 20120080 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Razgour O., et al. , Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. U.S.A. 116, 10418–10423 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Radchuk V., et al. , Adaptive responses of animals to climate change are most likely insufficient. Nat. Commun. 10, 3109 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grant P. R., et al. , Evolution caused by extreme events. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372, 20160146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the authors upon request.