Significance
Mitochondrial damage and ensuring cell death underline many neurodegenerative diseases such as Parkinson’s disease and stroke. Using cellular assays that mimic the activation of mitochondrial apoptosis pathway, we screened out a small molecule that blocks apoptosis by protecting mitochondrial integrity and function. Specifically, Compound R6 prevents apoptosis by activating autophagy through mTOR inhibition; it also confers significant, dose-dependent neuroprotective effects in a rat cerebral ischemia/reperfusion injury model. Given increasing appreciation that mitochondrial damage affects the etiology of several common and devastating neurodegenerative diseases, Compound R6’s ability to pass the blood−brain barrier and confer strong antiapoptotic effects should encourage preclinical and medicinal chemistry research efforts, perhaps even extending—as with other known mTOR inhibitors—into evaluation of possible antiaging effects.
Keywords: mitochondria, mTOR, apoptosis, autophagy, stroke
Abstract
Apoptosis activation by cytochrome c release from mitochondria to cytosol is a normal cellular response to mitochondrial damage. Using cellular apoptosis assay, we have found small-molecule apoptosis inhibitors that protect cells from mitochondrial damage. Previously, we reported the discovery of a small molecule, Compound A, which blocks dopaminergic neuron death in a rat model of Parkinson’s disease through targeting succinate dehydrogenase subunit B (SDHB) of complex II to protect the integrity of the mitochondrial respiratory chain. Here, we report a small molecule, Compound R6, which saves cells from apoptosis via mammalian target of rapamycin (mTOR)-mediated induction of autophagy. Additionally, we show that Compound R6 protects mitochondrial integrity and respiration after induction of the intrinsic apoptosis pathway. Encouragingly, and supporting the potential further application of Compound R6 as a tool for basic and medicinal research, a pharmacokinetics (PK) profiling study showed that Compound R6 is metabolically stable and can pass the blood−brain barrier. Moreover, Compound R6 accumulates in the brain of test animals via intravenous and intraperitoneal administration. Finally, we found that Compound R6 confers significant neuroprotective effects on a rat cerebral ischemia/reperfusion model, demonstrating its potential as a promising drug candidate for neurodegenerative diseases.
Mitochondria in mammalian cells play many functional roles in maintaining the well-being of the organism, acting as the major bioenergy source, as well as a signaling compartment that can trigger apoptosis and inflammation (1, 2). Upon damage to mitochondria, intermembrane proteins are released into the cytosol. One such protein, cytochrome c, is able to initiate an apoptotic caspase-9/3 activation cascade, causing apoptotic cell death (3, 4). The release of cytochrome c during apoptosis is controlled by the Bcl-2 family of proteins that come in 3 known flavors: the mitochondrial outer membrane gatekeepers Bax and Bak; the antiapoptotic proteins like Bcl-2, Bcl-xL, and Mcl-1 that heterodimerize with Bax or Bak, and prevent them from forming oligomers on the mitochondria and altering mitochondrial outer membrane permeability; and the so-called BH-3-only proteins like Bim or Bid, which free Bax/Bak from Bcl-2/Bcl-xL/Mcl-1 by competitively binding to them (5, 6).
Our laboratory has been using the Bim protein driven by a doxycycline (Dox)-inducible promoter to specifically induce mitochondrial damage in U2OS cells (7). Upon addition of Dox to the culture medium, Bim is induced and the cells undergo apoptosis in a Bax/Bak-dependent manner. Using this system, we have identified the mitochondrial inner membrane protein OpaI, a dynamin-related GTPase for which loss of function mutations cause optical nerve atrophy, as well as the mitochondrial inner membrane protease OmaI, which cleaves OpaI upon Bax/Bak oligomerization, as important mediators of apoptosis that cause the mitochondrial cristae dilation to facilitate the release of cytochrome c from mitochondria.
In addition to OpaI and OmaI, we also identified a small-molecule compound, 1-(3,4-dimethoxybenzyl)-5-(2-(methylsulfonyl)-6-(trifluoromethyl)pyrimidin-4-yl)pyridin-2(1H)-one, named Compound A, that protects U2OS cells from Bim-induced apoptosis by covalently interacting with the a subunit of the mitochondrial electron transfer chain Complex II: component succinate dehydrogenase B (SDHB) (8). In the present study, we report the identification of a compound that specifically blocks Bim-induced apoptosis. Unlike Compound A, this compound is not a covalent modifier; it blocks apoptosis through mammalian target of rapamycin (mTOR)-mediated induction of autophagy. Moreover, this compound shows significant neuroprotective effects in a rat cerebral ischemia/reperfusion model, and, together with its ability to pass the blood−brain barrier and accumulate in the brain via both intravenous (IV) and intraperitoneal (IP) administration, it can be viewed as a promising drug candidate for treating neurodegenerative diseases.
Results
A Small Molecule Blocks Bim-Induced Intrinsic Apoptosis.
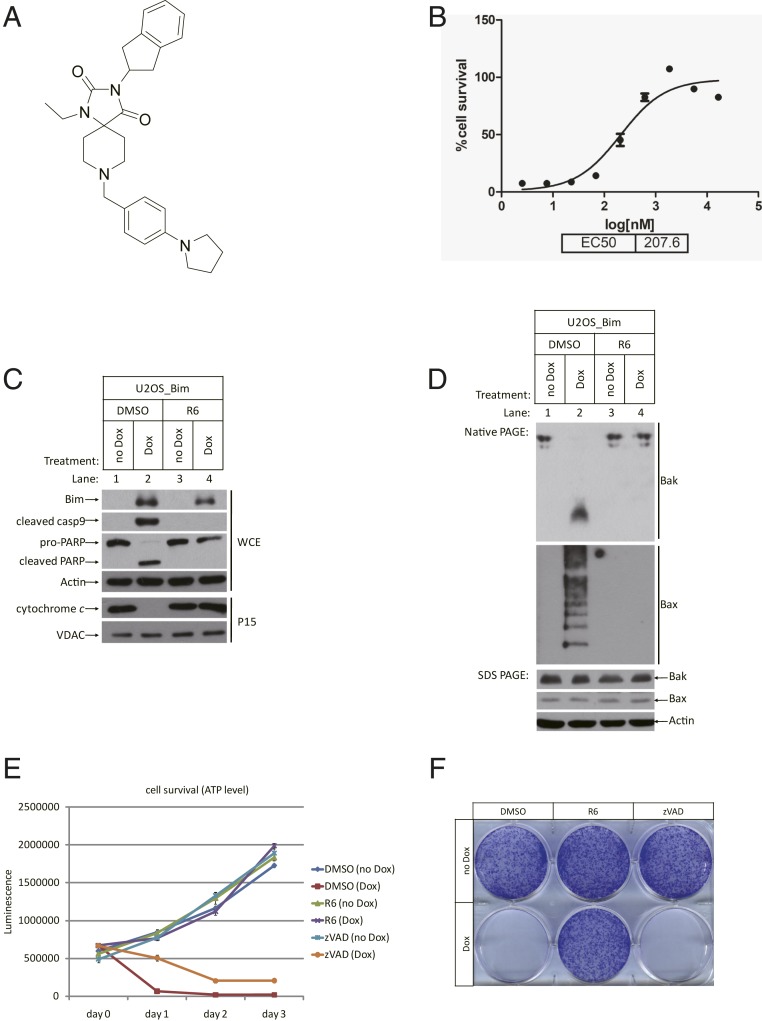
The U2OS_Bim cell line that we previously established responds to the addition of doxycycline (Dox) by undergoing apoptosis within a few hours (7). The addition of Dox induces the expression of Bim (henceforth Bim is referred to as BimEL, unless otherwise stated), which subsequently activates the intrinsic apoptosis pathway in this cell line. A chemical library of ∼50,000 compounds was screened for hits that could inhibit Dox-induced apoptosis in these U2OS_Bim cells. One of the hits, 3-(2,3-dihydro-1H-inden-2-yl)-1-ethyl-8-(4-(pyrrolidin-1-yl)benzyl)-1,3,8-triazaspiro[4.5]decane-2,4-dione, hereafter named Compound R6 (Fig. 1A), had a half-maximal inhibitory concentration around 207.6 nM (Fig. 1B). Compound R6, when present in the culture medium alongside Dox, blocked the release of cytochrome c from mitochondria and the subsequent activation of caspase-9, which is the well-characterized initiator caspase of the intrinsic apoptosis pathway (Fig. 1C and SI Appendix, Fig. S1A).
Fig. 1.
Compound R6 blocked Bim-induced intrinsic apoptosis. (A) Chemical structure of Compound R6. (B) U2OS_Bim cells were pretreated with Compound R6 starting from 50 μM, 3× dilute for 1 h, followed by treatment with or without Dox in each duplicate well for 24 h. Cell survival was determined by ATP level using CTG kit in all experiments. EC50 was then calculated by software GraphPad Prism. (C) U2OS_Bim cells were pretreated with DMSO or R6 (1 µM) for 1 h, followed by treatment with or without Dox for 6 h. Whole-cell extracts (WCE) or P15 fractions (mitochondria part) of the cells were analyzed by Western blotting using the indicated antibodies. Cleavage of caspase 9 and poly (ADP-ribose) polymerase (PARP) are biochemical markers for apoptosis. VDAC (voltage-dependent anion-selective channel) is a mitochondrial outer membrane protein used as control here. (D) Lysates of WCE were analyzed by Western blotting after blue native polyacrylamide gel electrophoresis (PAGE) or sodium dodecyl sulfate (SDS) PAGE using the indicated antibodies. Bax oligomerization always indicates the breakdown of mitochondrial outer membrane. R6 can protect the integrity of mitochondrial outer membrane after Bim induction. (E) U2OS_Bim cells were treated as indicated in 96-well plates. Cell survival was determined immediately after Dox treatment (day 0) or 1, 2, or 3 d later. Data are represented as the mean ± SD of duplicate wells. The same concentration of zVAD (20 µM) was used in all experiments. (F). U2OS_Bim cells were treated as indicated in a 6-well plate. After a week, colony formation was stained by crystal violet.
Unlike our previously reported apoptosis inhibitor Compound A (8), Compound R6 prevented the Bax protein from oligomerizing on the mitochondrial outer membrane, as analyzed by blue native gel electrophoresis (Fig. 1D). This result clearly indicates that Compound R6 functions upstream of any mitochondrial damage. Similar to Compound A, this compound protected the normal function of the mitochondria respiratory chain as measured by oxygen consumption (SI Appendix, Fig. S1B).
Another similarity with compound A is that the presence of Compound R6 also enabled Dox-treated cells to continue to proliferate (Fig. 1E). This capacity of Compound R6 to preserve cell proliferation contrasts with the well-known pan-caspase inhibitor z-VAD-FMK (zVAD), which acts downstream of mitochondrial damage and cannot prevent cells from eventual death even if the apoptotic morphological changes were abolished. Compound R6’s preservation of long-term cell viability was further confirmed in colony formation assays (Fig. 1F). U2OS_Bim cells readily formed cell colonies on the culture dish after 1 wk of culture with continuous cotreatment of Compound R6 and Dox, whereas hardly any colonies formed in cells with cotreatment of dimethyl sulfoxide (DMSO) or zVAD and Dox. All of these results support that Compound R6 functions upstream of mitochondrial damage after Bim induction.
Compound R6 Prevents Apoptosis by Inhibiting mTOR and Activating Autophagy.
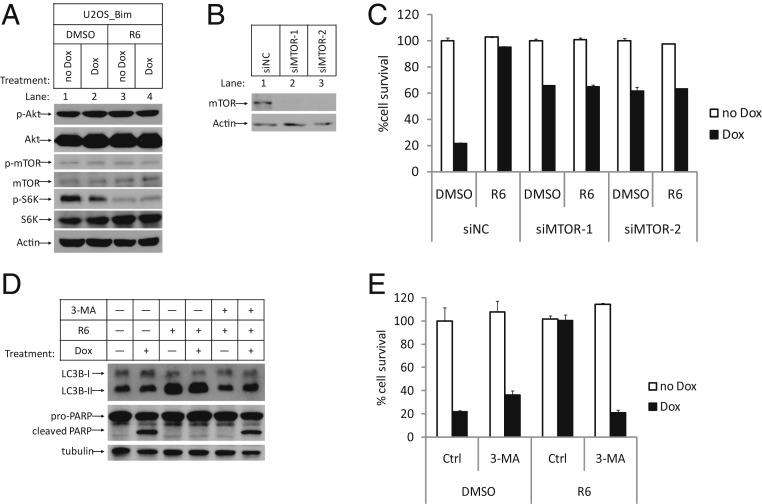
The unique ability of Compound R6 to prevent apoptosis upstream of Bax/Bak activation promoted us to investigate additional signaling pathways which may influence activation of the intrinsic apoptosis pathway. Interestingly, we noticed that Compound R6 was able to block phosphorylation of the threonine 389 residue of the ribosomal protein S6 kinase (S6K), a classical substrate of mTOR kinase (9–11). Moreover, this inhibition of mTOR does not seem to result from alteration of its upstream signaling events: We found no evidence for such inhibition of the activity of its upstream kinase Akt (Fig. 2A and SI Appendix, Fig. S2A). Nevertheless, a direct functional contribution of mTOR inhibition to block apoptosis was confirmed when we knocked down mTOR with 2 independent small interfering RNA (siRNA) oligos, which showed that restricting mTOR activity promoted cell viability upon Dox treatment. Importantly, these experiments also showed that treatment of mTOR knockdown cells with Compound R6 does not further increase these protective effects (Fig. 2 B and C). Moreover, the addition of the well-known mTOR inhibitor Rapamycin provided a similar level of protection as did mTOR knockdown and/or treatment with Compound R6 (12, 13) (SI Appendix, Fig. S2B).
Fig. 2.
Compound R6 worked through mTOR-mediated autophagy induction. (A) U2OS_Bim cells were treated in the same way as in Fig. 1C. WCE of the cells were analyzed by Western blotting using anti-p-Akt-S473 (p-Akt), anti-Akt (Akt), anti-p-mTOR-S2448 (p-mTOR), anti-mTOR (mTOR), anti-p-S6K-T389 (p-S6K), anti-S6K (S6K), and anti-β-Actin-HRP (Actin) antibodies. (B) U2OS_Bim cells transfected with indicated siRNAs were analyzed by Western blotting. (C) U2OS_Bim cells transfected with indicated siRNAs were treated with DMSO or R6 (1 µM) for 1 h, followed by treatment with or without Dox. Cell survival was determined after 24 h and represented as the mean ± SD of duplicate wells. (D and E) U2OS_Bim cells were pretreated with R6 and 3-MA (5 mM) together for 1 h, followed by treatment with or without Dox for 12 h. (D). WCE were analyzed by Western blotting using the indicated antibodies. (E). Cell survival was determined and represented as the mean ± SD of duplicate wells. Ctrl, control. siNC, negative control siRNA.
To expand the scope of our search for how mTOR inhibition of Compound R6 could lead to blocking of the intrinsic apoptosis pathway, we investigated whether Compound R6 affects the activity of autophagy, an activity known to be activated by mTOR inhibition (14). We monitored the cellular levels of the classic autophagy activity marker LC3-II in Compound R6-treated cells, and found that Compound R6 treatment induced accumulation of LC3-II. Also, the protective effects of Compound R6 were significantly diminished upon cotreatment with the autophagy inhibitor 3-MA or chloroquine (CQ) (15) (Fig. 2 D and E and SI Appendix, Fig. S2C), a finding that confirms compound R6 blocks apoptosis through induction of autophagy by inhibiting mTOR.
Compound R6 also Inhibits Intrinsic Apoptosis Specifically Induced by Serum Withdrawal plus a Bcl-2/Bcl-xL Dual Inhibitor.
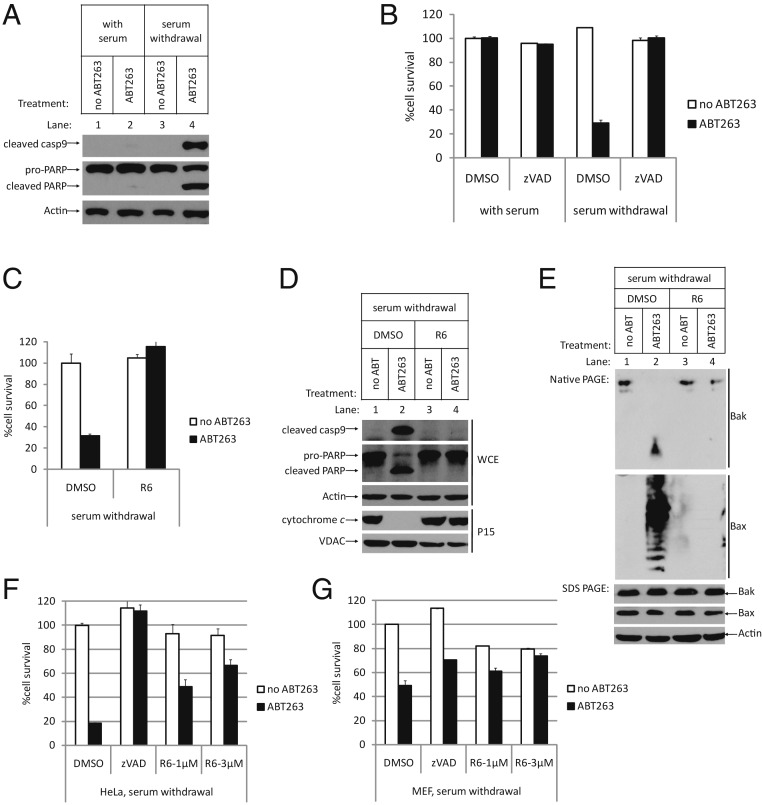
To explore whether autophagy induction by Compound R6 has general protection effects against intrinsic apoptosis, we used another intrinsic apoptosis induction system to test Compound R6. We initially confirmed that ABT263, a small-molecule Bcl-2/Bcl-xL dual inhibitor, can induce intrinsic apoptosis in serum withdrawal conditions in U2OS cells (Fig. 3 A and B). As expected, Compound R6 prevented apoptosis upstream of mitochondrial damage (16–18) (Fig. 3 C–E). Specifically, we observed that Compound R6 protected mitochondrial integrity upstream of Bax/Bak oligomerization, and it did not block cell proliferation—cells continued to grow when we added serum back to the medium after treatment (SI Appendix, Fig. S3). Moreover, when we applied similar treatments to other cell lines including HeLa cells and mouse embryonic fibroblasts (MEF), we again found that Compound R6 blocked apoptosis (Fig. 3 F and G), clearly indicating that the protective effect of Compound R6 is general in mammalian cells.
Fig. 3.
Compound R6 is a general inhibitor of the intrinsic apoptosis pathway. (A and B) In with-serum or serum withdrawal conditions, U2OS cells were pretreated with DMSO or zVAD for 1 h, followed by treatment with or without ABT263 (5 µM) for 8 h. (A) WCE were analyzed by Western blotting using the indicated antibodies. (B) Cell survival was determined and represented as the mean ± SD of duplicate wells. The same concentration of ABT263 was used in the following experiments. (C−E) In serum withdrawal condition, U2OS cells were treated as indicated. (C) Cell survival was determined after 8 h treatment and represented as the mean ± SD of duplicate wells. (D) WCE were analyzed by Western blotting after SDS PAGE using the indicated antibodies. (E) WCE were analyzed by Western blotting after blue native PAGE using the indicated antibodies. (F and G) HeLa cells or MEF cells were treated as indicated. Cell survival was determined and represented as the mean ± SD of duplicate wells. After apoptosis induction in MEF cells, cell survival rate in DMSO group is 49.16%, while that in zVAD group is 70.32%. In R6-treated group, cell survival rate in 1 µM group is 61.25%, and, in 3 µM group, it is 73.70%, more than that in the zVAD group.
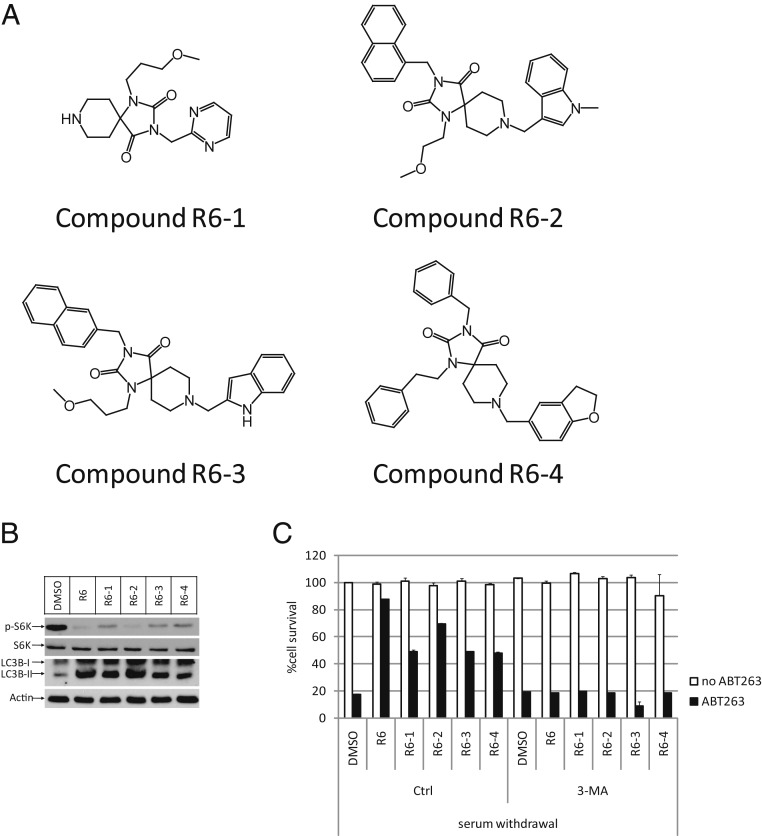
Next, we confirmed that Compound R6 also protected cells from apoptosis through autophagy induction by mTOR inhibition in this condition: Compound R6 could decrease the phosphorylation of S6K and increase the accumulation of LC3-II, and we observed that autophagy inhibitor 3-MA or CQ could diminish this protective effect from Compound R6. Moreover, there are 4 compounds which share structural similarity with Compound R6 that also showed apoptosis inhibition ability during the initial compound screen (Fig. 4A). We again tested these 4 analogs and found that they could also inhibit mTOR and up-regulate LC3-II accumulation, and cotreatment with 3-MA diminished the protective effect of each of them (Fig. 4 B and C). The consistency of these compounds’ behavior on autophagy induction and apoptosis inhibition reinforces our discovery of Compound R6’s capacity to block apoptosis through mTOR inhibition-mediated autophagy induction.
Fig. 4.
Four analogs of Compound R6 show consistency in autophagy induction and apoptosis inhibition. (A) Structures of Compound R6’s analogs. (B) In serum withdrawal condition, U2OS cells were pretreated with indicated compounds for 12 h and analyzed by Western blotting using anti-p-S6K-T389, anti-S6K, anti-LC3B, and anti-β-Actin-HRP antibodies. (C) In serum withdrawal condition, U2OS cells were treated as indicated. Cell survival was determined 8 h later and represented as the mean ± SD of duplicate wells.
Compound R6 Has Protective Effect against Neuronal Injury in a Rat Model of Transient Focal Cerebral Ischemia/Reperfusion Injury.
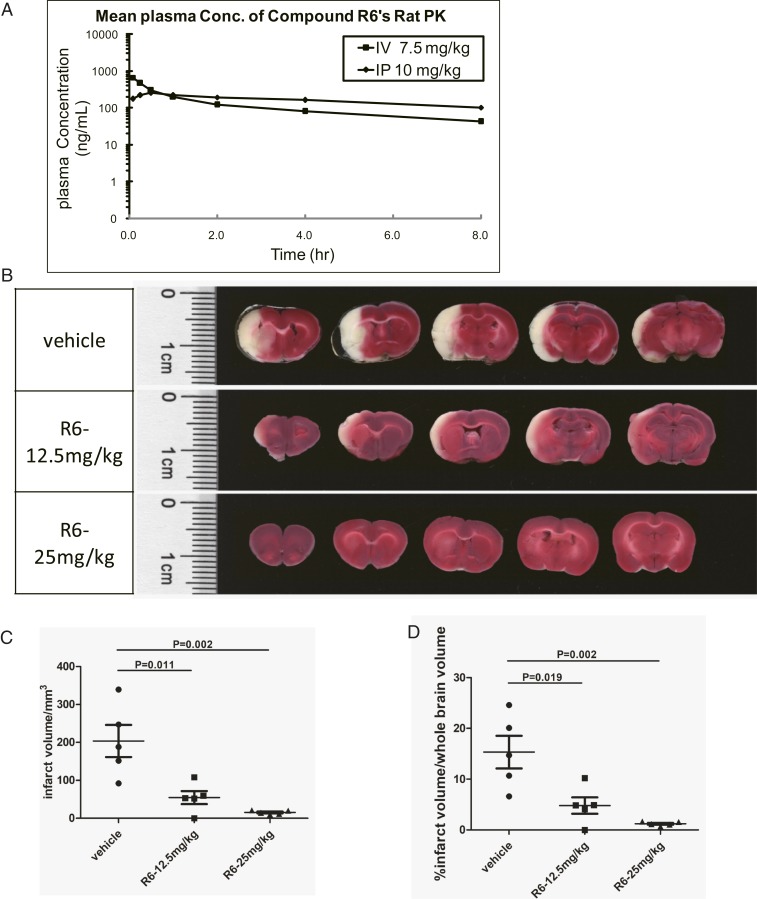
To explore the potential of Compound R6 as a drug lead, we profiled its IP/IV pharmacokinetics (PK) (Fig. 5A and SI Appendix, Table S1A), which revealed that its IP T1/2 was 6.36 h and its bioavailability (F%) was near 100%, results clearly underscoring Compound R6's apparent metabolic stability. Strikingly, these analyses also found that Compound R6 can pass the blood−brain barrier. Moreover, we observed that Compound R6 accumulated in the brain upon both IV and IP administration (SI Appendix, Table S1B), suggesting that Compound R6 should be viewed as a very promising drug lead for multiple brain diseases with known mitochondrial damage-related etiologies.
Fig. 5.
Compound R6 showed protective effects in a rat cerebral ischemia/reperfusion injury model. (A) PK profiling of Compound R6 by IV or IP injection. (B) R6 showed protective effects against ischemia/reperfusion injury in a dose-dependent manner. TTC was used to stain the sections from the MCA brains. The white color of the brain sections represents the infarct, and the red color represents the normal tissue. (C and D) Quantification of the infarct volume and the ratio of infarct volume to the whole brain volume; n = 5. P values were calculated by software GraphPad Prism.
One such disease is cerebral ischemia followed by reperfusion, which is caused by the loss of available oxygen resulting from temporary blockage of blood flow to certain parts of the brain. The exceptional pharmacological properties of Compound R6 allowed us to test its effect in a rat ischemic/reperfusion model wherein ischemia is caused by occlusion of the rat’s left-side middle cerebral artery (MCA) using a nylon suture; the subsequent reperfusion results from removal of the suture 1 h later (19–21). This ischemia/reperfusion injury caused severe damage to the left side of the rats’ brain, as shown by the infarct area in the control group (Fig. 5B). Treatment of Compound R6 by IP administration at 12.5 mg/kg or 25 mg/kg doses before the surgery significantly, and dose-dependently, decreased brain infarct volumes (Fig. 5 C and D). When the brain tissues were harvested from the vehicle or Compound R6-treated animals with ischemia/reperfusion injury and subjected to Western blotting analysis using anti-LC3 antibody, we noticed the increase of LC3-II levels in the Compound R6-treated animals, indicating that autophagy was indeed induced by the compound (SI Appendix, Fig. S4A).
Discussion
A Compound Blocks the Mitochondrial Pathway of Apoptosis by Inhibiting mTOR.
Our current study demonstrates that Compound R6 inhibits the mitochondrial pathway of apoptosis. In addition to our results for the U2O2_Bim cell line that we used for the initial screening, we also confirmed that Compound R6 can block apoptosis induced by serum withdrawal plus the addition of the Bcl-2/Bcl-xL inhibitor ABT263 in a variety of mammalian cell lines. We determined that Compound R6's protective effects are exerted upstream of mitochondria damage, since Bax oligomerization was prevented in the presence of Compound R6.
Although the direct target of Compound R6 is not yet known, it is plausible that its functional target is most likely mTOR, since the phosphorylation of S6K was shown to be dose-dependently inhibited by Compound R6, and cellular autophagy was activated upon Compound R6 treatment. Consistently, concurrent blockage of mTOR, either by siRNA knockdown or chemical inhibition using Rapamycin, offered similar protection against apoptosis.
So, how then does mTOR inhibition by Compound R6 block mitochondrial damage-induced apoptosis? We hypothesize that Compound R6—through its inhibition of mTOR—can prevent apoptosis through 2 independent mechanisms: 1) by slowing down protein synthesis so the continuous production of proapoptotic proteins such as Bim becomes attenuated, and 2) by inducing autophagy, which ultimately enables removal of mitochondria that show signs of damage like Bim accumulation or oligomerized Bax on outer membranes.
Compound R6 as a Drug Candidate for Neurodegenerative Diseases.
Compound R6 possesses unusual and potentially highly attractive pharmacological properties. In addition to its good pharmacodynamics properties, it passes the blood−brain barrier readily and accumulates in the brain tissue after both IV and IP administration. Compound R6 shows significant protective effects by decreasing the brain infarct volume in a rat cerebral ischemia/reperfusion injury model.
Mitochondrial damage is known to increase during the normal aging process and is also a common feature of many neurodegenerative diseases. Compound R6, and its potentially more potent derivatives, could confer therapeutic benefits for such diseases and may perhaps offer some aging-related effects.
Materials and Methods
All methods employed in this article are routinely used in our laboratories and are referenced (7, 8, 20), and a complete description of the methods is available in the SI Appendix, SI Materials and Methods.
Reagents.
Compound R6 was synthesized by Z.C. in Z.Z.’s laboratory at the National Institute of Biological Sciences, Beijing. The following small molecules were used: 3-MA (HY-19312; MCE), Rapamycin (HY-10219; MCE), and CQ (HY-17589; MCE). The following antibodies were used: anti-Bim (2819; CST), anti-cleaved caspase 9 (9501; CST), anti-PARP (9532; CST), anti-VADC (4866; CST), anti-Bax (5203; CST), anti-Bak (12105; CST), anti-phospho-S6K-T389 (9234; CST), anti-S6K (9202; CST), anti-LC3B (3868; CST), anti-phospho-mTOR (5536; CST), anti-mTOR (2972; CST), anti-cytochrome c (556433; BD), anti-β-Actin-HRP (D291-7; MBL), and anti-β-tubulin (BE3312-10; EASY BIO).
siRNA Oligos.
siMTOR-1: A double stranded RNA oligo with the sense strand (5′-3′): CCCGGATCATTCACCCTATTGdTdT and the antisense strand (5′-3′): CAATAGGGTGAATGATCCGGGdTdT was used to knockdown mTOC1 expression; siMTOR-2: An alternative double stranded RNA oligo with the sense (5′-3′): GCCTTGTTTGTGGCTCTGAATdTdT and antisense (5′-3′): ATTCAGAGCCACAAACAAGGCdTdT was also used to knockdown mTOC1 expression.
Transfection.
Transfection of siRNA into cells using Lipofectamine 3000 (Thermo) was performed according to the manufacturer’s instructions.
Cell Culture and Stable Cell Lines.
U2OS, HeLa, and MEF cells were cultured in DMEM containing 10% (V/V) fetal bovine serum. The U2OS_Bim cell line was generated as described in ref. 7.
Cell Survival Assay.
Cell survival was analyzed by measuring adenosine 5′-triphosphate (ATP) levels with a Cell Titer-Glo (CTG) kit from Promega, according to the manufacturer’s instructions.
Cellular Fractionation.
We modified the Cellular Fractionation method described in ref. 7. Briefly, harvested cell pellets were resuspended in cold buffer A (20 mM Hepes, 40 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1× mixture protease inhibitor [Roche], 1× PhosSTOP [Roche]) containing 250 mM sucrose, the volume of which was 5 times that of the cell pellet. After incubation on ice for 15 min, cells were broken by passage through a 22G needle 25 times. The resulting mixtures were centrifuged at 1,000 × g for 5 min at 4 °C. The S1 supernatants were then centrifuged at 15,000 × g for 30 min at 4 °C, and the pellet fraction (P15) was retained.
Blue Native PAGE.
Whole-cell extracts were prepared with a Native Sample Kit from Life Technologies (Thermo Fisher Scientific) according to the manufacturer’s instructions. Ten micrograms of total protein from each extract sample was loaded on 4 to 16% gradient native gels, and standard gel electrophoresis was performed.
Crystal Violet Staining.
We modified the Crystal Violet Staining described in ref. 8. Briefly, 3,000 cells per well were seeded in 6-well plates and treated on the following day, as indicated in the figure legends. Cells were washed twice with phosphate-buffered saline (PBS), followed by incubation with 0.05% crystal violet in H2O for 10 min at room temperature. Subsequently, the crystal violet solution was removed, and the samples were again washed twice with PBS, followed by removal of PBS and drying of the plate at room temperature.
Transient Focal Cerebral Ischemia/Reperfusion Injury.
Animal procedures were approved by the NIBS (National Institute of Biological Sciences, Beijing) local ethics committee. We modified the procedure described in ref. 20. Briefly, male Sprague–Dawley rats (weight 230 g to 250 g) were given Compound R6 by IP injection and then anesthetized with 2% isoflurane. The left common, external, and internal carotid arteries (CCA, ECA, and ICA) were exposed. A 4-0 monofilament nylon suture with a silicon-coated tip was introduced through an incision of the ECA into the ICA to occlude the origin of the MCA for 1 h. Animals were killed 24 h later. Brains were removed and sliced into sections of 2-mm thickness. Infarct size was examined via staining with 1.5% 2,3,5-triphenyltetrazolium chloride (TTC) at 37 °C for 10 min.
Supplementary Material
Acknowledgments
We thank Dr. John Hugh Snyder for critically reading the manuscript. We would also like to express our gratitude to the Imaging Center, Biological Resource Center, and Metabolomics Center at National Institute of Biological Sciences, Beijing, for technical support. This work was supported by an institutional grant from the Ministry of Science and Technology of China and Beijing Municipal Commission of Science and Technology.
Footnotes
The authors declare no competing interest.
Data deposition: All data discussed in the paper will be made available to readers.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1911246116/-/DCSupplemental.
References
- 1.Balaban R. S., Nemoto S., Finkel T., Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Wang X., The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001). [PubMed] [Google Scholar]
- 3.Li P., et al. , Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91, 479–489 (1997). [DOI] [PubMed] [Google Scholar]
- 4.Liu X., Kim C. N., Yang J., Jemmerson R., Wang X., Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell 86, 147–157 (1996). [DOI] [PubMed] [Google Scholar]
- 5.Czabotar P. E., Lessene G., Strasser A., Adams J. M., Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Annis M. G., et al. , Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 24, 2096–2103 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang X., Jiang H., Shen Z., Wang X., Activation of mitochondrial protease OMA1 by Bax and Bak promotes cytochrome c release during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 111, 14782–14787 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X., et al. , A small molecule that protects the integrity of the electron transfer chain blocks the mitochondrial apoptotic pathway. Mol. Cell 63, 229–239 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Pullen N., Thomas G., The modular phosphorylation and activation of p70s6k. FEBS Lett. 410, 78–82 (1997). [DOI] [PubMed] [Google Scholar]
- 10.Laplante M., Sabatini D. M., mTOR signaling in growth control and disease. Cell 149, 274–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanathan A., Schreiber S. L., Direct control of mitochondrial function by mTOR. Proc. Natl. Acad. Sci. U.S.A. 106, 22229–22232 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown E. J., et al. , A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature 369, 756–758 (1994). [DOI] [PubMed] [Google Scholar]
- 13.Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H., RAFT1: A mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78, 35–43 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Jung C. H., Ro S.-H., Cao J., Otto N. M., Kim D.-H., mTOR regulation of autophagy. FEBS Lett. 584, 1287–1295 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanida I., Ueno T., Kominami E., “LC3 and autophagy” in Autophagosome and Phagosome (Springer, 2008), pp. 77–88. [DOI] [PubMed] [Google Scholar]
- 16.Ley R., Balmanno K., Hadfield K., Weston C., Cook S. J., Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 278, 18811–18816 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Tse C., et al. , ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Vartak S. V., et al. , Novel BCL2 inhibitor, Disarib induces apoptosis by disruption of BCL2-BAK interaction. Biochem. Pharmacol. 131, 16–28 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Lin M. T., Beal M. F., Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Li L., et al. , Discovery of highly potent 2-sulfonyl-pyrimidinyl derivatives for apoptosis inhibition and ischemia treatment. ACS Med. Chem. Lett. 8, 407–412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Putcha G. V., et al. , Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29, 615–628 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.