Significance
Ecological and evolutionary processes work together in the assembly of regional and local communities from available species pools based on species traits linked to ecological tolerances. Because phylogenies summarize the evolutionary history of the clades within them, phylogeny-based approaches are essential to understanding the origin of variation in species richness across environmental gradients. Here, we analyze, within a phylogenetic context, a comprehensive dataset on the distributions of seed plants in China. Specifically, we examined relationships between indices of phylogenetic structure and environment for individual clades, from taxonomic families to the root of the phylogeny of the Chinese seed plants. We found that phylogenetic niche conservatism plays an important role in the assembly of regional floras in China.
Keywords: environmental filtering, niche conservatism, phylogenetic diversity, phylogenetic relatedness, seed plants
Abstract
Species assemble into communities through ecological and evolutionary processes. Phylogenetic niche conservatism—the tendency of species to retain ancestral ecological distributions—is thought to influence which species from a regional species pool can persist in a particular environment. We analyzed data for seed plants in China to test hypotheses about the distribution of species within regional floras. Of 16 environmental variables, actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation most strongly influenced regional species richness, phylogenetic dispersion, and phylogenetic diversity for both gymnosperms (cone-bearing plants) and angiosperms (flowering plants). For most evolutionary clades at, and above, the family level, the relationships between metrics of phylogenetic dispersion (i.e., average phylogenetic distance among species), or phylogenetic diversity, and the 3 environmental variables were consistent with the tropical niche conservatism hypothesis, which predicts closer phylogenetic relatedness and reduced phylogenetic diversity with increasing environmental stress. The slopes of the relationships between phylogenetic relatedness and the 3 environmental drivers identified in this analysis were steeper for primarily tropical clades, implying greater niche conservatism, than for primarily temperate clades. These observations suggest that the distributions of seed plants across large-scale environmental gradients in China are constrained by conserved adaptations to the physical environment, i.e., phylogenetic niche conservatism.
The presence of a species in a local ecological assemblage, or community, reflects the interplay between ecological and evolutionary processes (1, 2). The species composition of a spatially defined assemblage depends on the regional species pool, whose composition reflects large-scale biogeographical processes that influence speciation, extinction, and dispersal. A local assemblage can include only species whose environmental tolerances allow them to maintain populations under the local abiotic and biotic conditions of the environment (3, 4). At a broad spatial scale, environmental filtering is thought to play a strong role in determining the species composition of an assemblage, whereby species that cannot survive and reproduce in the local physical environment are excluded (5). The ability of a species to tolerate a particular set of ecological conditions reflects its evolutionary history of adaptation. The phylogenetic niche conservatism (PNC) hypothesis states that species with shared evolutionary history (i.e., species in an evolutionary clade) tend to tolerate similar environmental conditions and thus exhibit similar geographic distributions (6, 7). Accordingly, PNC has important consequences for the assembly of both local communities and the regional species pools from which local communities are assembled (1). The PNC hypothesis [i.e., retention of ecological traits over evolutionary time among related clades or species (8, 9)] predicts that communities developing under more stressful (e.g., colder, drier, more seasonal) conditions should be more strongly structured by environmental filtering than communities in less stressful conditions. Thus, communities in more stressful environments should exhibit higher phylogenetic relatedness (clustering) and lower phylogenetic diversity.
Most of the major clades of present-day organisms first appeared when our planet was dominated by tropical environments (10, 11). For example, many taxonomic families of seed plants originated during the warm period between the beginning of the Cretaceous and the end of the Eocene (12), when global climate cooling (13) forced clades of warm-adapted plants at higher latitudes to retreat to lower latitudes, evolve tolerance of colder temperatures, or become extinct. Because ecological traits tend to be phylogenetically conserved (1, 6, 7), few members of clades cross major ecophysiological boundaries, particularly toward harsher (e.g., colder) environments (14). Thus, most tropical clades fail to disperse into extratropical regions because they lack ecological and physiological adaptations to survive winter temperatures below freezing (15, 16). Accordingly, species in regions with colder and drier (i.e., more stressful) climates should be more closely related phylogenetically (clustered) due to PNC. This expectation is often referred to as the “tropical niche conservatism” hypothesis because adaptations to tolerate freezing conditions rarely evolve in tropical clades (6, 7).
For seed plants (Spermatophyta), empirical studies have indeed shown that phylogenetic relatedness increases, and phylogenetic diversity decreases, with decreasing temperature and precipitation (e.g., refs. 17 and 18). However, most previous studies on phylogenetic structure of plant communities have focused exclusively on angiosperms (flowering plants). All of the families of gymnosperms—conifers and their relatives—originated long before global climate cooling began in the Eocene. In contrast to angiosperms, which diversified mainly during the late Cretaceous and Cenozoic, and whose patterns of phylogenetic structure along environmental gradients are generally consistent with the tropical niche conservatism hypothesis, gymnosperm diversity and dominance declined, and many gymnosperm clades went extinct, after the end of the Cretaceous (19). Several living clades of gymnosperms are confined to small refugia, suggesting that their current distributions might not be shaped primarily by climate. Thus, the relationship between phylogenetic structure and climate for gymnosperms might differ from that commonly observed for angiosperms.
Global climate cooling following the Eocene thermal maximum (∼50 million years ago; refs. 13 and 20) resulted in some lineages evolving tolerance of cold temperatures and extending their distributions into temperate climates. Nonetheless, many clades remain restricted to tropical climates. Because members of these latter clades are likely more sensitive to cold temperatures than are those that now inhabit temperate climates, one would expect the relationship between difference in temperature optima and phylogenetic relatedness to be steeper for clades that are primarily restricted to tropical climates, compared to those that are additionally distributed in temperate climates. We test this hypothesis here.
Previous studies have shown inconsistent patterns of phylogenetic relatedness along geographical and environmental gradients. For example, geographic patterns of phylogenetic relatedness differ strikingly between 2 reptile clades (Ctenophorus and Lerista) in Australia, where the net relatedness index (NRI) decreases to the north for Ctenophorus but increases for Lerista (figure 2 of ref. 21). Among angiosperm plants in North America, the relationship between phylogenetic relatedness and mean annual temperature is negative for some clades (e.g., lamiids and malvids) but positive for others (e.g., fabids) (18). At a lower taxonomic level, among plants in California, the relationship between phylogenetic relatedness and mean annual temperature is positive for the family Amaranthaceae, but negative for the Caryophyllaceae (22). If PNC were to influence the relationship between phylogenetic structure and environment, one would expect to find phylogenetic signal in this relationship (i.e., more closely related clades would exist under more similar ecological conditions).
In this study, we conduct analyses that address questions concerning geographical and ecological distributions associated with phylogenetic relationships of seed plants in China. We focus on 2 aspects of phylogenetic structure, namely, phylogenetic dispersion (relatedness) and phylogenetic diversity. Specifically, we address the following 4 questions:
-
1)
Are the relationships between phylogenetic metrics and environment consistent between gymnosperms and angiosperms? Are phylogenetic patterns in either of these 2 major clades, which diverged ∼352 million years ago (12), more consistent with the tropical niche conservatism hypothesis (e.g., decreasing phylogenetic relatedness with increasing temperature)? Given that no families of gymnosperms originated during the Cenozoic period of pronounced global climate cooling, whereas many families of angiosperms originated after establishment of the tropical−temperate climate gradient, we predict that patterns of phylogenetic−climatic relationship would be more consistent with the tropical niche conservatism hypothesis for angiosperms than for gymnosperms (hypothesis H1).
-
2)
Are the relationships between phylogenetic relatedness and climate variables stronger for families that originated in tropical climates than for those that originated in temperate climates? Considering that tropical plants are more sensitive to cold temperature than are temperate plants, we predict that the relationships between phylogenetic relatedness and climate variables are stronger for ancestrally tropical families (hypothesis H2).
-
3)
To what extent are the relationships between phylogenetic metrics and environmental variables for individual major clades (families, orders, and node-based clades above the ordinal level) consistent with the tropical niche conservatism hypothesis (i.e., phylogenetic relatedness increases, and phylogenetic diversity decreases, with decreasing temperature and water availability)? Given that predictions from the tropical niche conservatism hypothesis have been confirmed in previous studies on angiosperms as a whole (17, 18), these patterns should apply to most, if not all, major clades of seed plants (hypothesis H3).
-
4)
Do relationships between phylogenetic metrics and environmental variables exhibit phylogenetic signal? Under the tropical niche conservatism hypothesis, we would expect this to be the case (hypothesis H4).
Here, we extend a recent analysis of the phylogenetic structure of Chinese plants (17) by 1) including both gymnosperms and angiosperms; 2) examining the relationships between phylogenetic metrics and environmental variables separately for individual families, orders, and all node-based clades above the ordinal level; 3) examining the relationships separately for primarily tropical families and primarily temperate families; and 4) relating phylogenetic metrics to environmental variables that are thought to influence assembly of plant communities.
Results
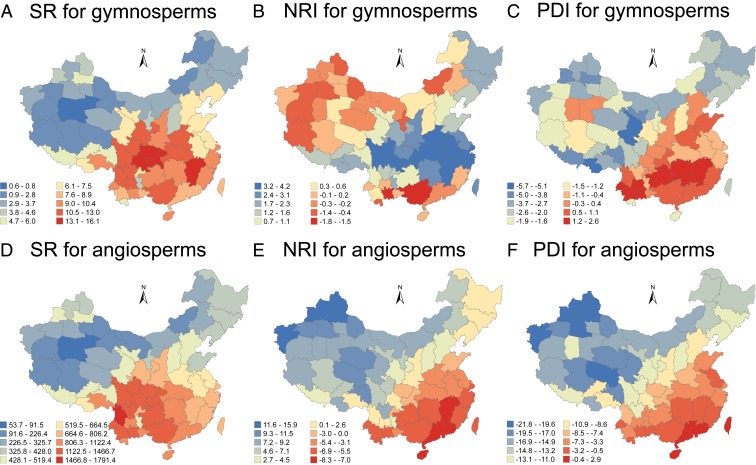
Geographic patterns of species richness and the phylogenetic diversity index (PDI) for gymnosperms and angiosperms are generally similar: Values decrease from southeastern China toward northeastern and northwestern China (Fig. 1). However, the geographic pattern of phylogenetic relatedness for gymnosperms was generally opposite to that for angiosperms. Specifically, the NRI calculated for gymnosperms was highest (i.e., exhibiting the most phylogenetic clustering) in central and eastern China and much lower (i.e., more phylogenetic overdispersion) in northwestern China; in contrast, for angiosperms, NRI was highest in northwestern China and lowest in southeastern China.
Fig. 1.
Geographic patterns of species richness (SR), net relatedness index (NRI), and phylogenetic diversity index (PDI) for Chinese gymnosperms (A–C) and angiosperms (D–F). Note that 1) SR and PDI increase, and NRI decreases, along the color gradient from blue to red, and 2) SR for each region was adjusted by the area size of the region (i.e., the number of species was divided by log10-transformed area in square kilometers).
When seed plants were analyzed as a whole, or when gymnosperms and angiosperms were analyzed separately, the 4 environmental variables representing climate change between glacial and interglacial periods were, in general, weakly correlated with species richness, NRI, and PDI (Table 1). The 3 environmental variables representing habitat heterogeneity were weakly or moderately correlated with species richness, NRI, and PDI (Table 1; see Methods for definitions of correlation strength). Of the 3 environmental variables measuring climate seasonality, precipitation seasonality was weakly to moderately correlated with species richness, NRI, and PDI, whereas the 2 variables representing temperature seasonality were, in general, moderately to strongly correlated with species richness, NRI, and PDI (Table 1). Of the 2 variables representing temperature seasonality, annual temperature range was more strongly correlated with species richness, NRI, and PDI than was bio4 (temperature SD) of the WorldClim variables. Of the 16 environmental variables examined in this study, actual evapotranspiration was most strongly correlated with species richness, NRI, and PDI, followed by annual precipitation and minimum temperature of the coldest month (Table 1 and SI Appendix, Table S1); the latter variable was strongly correlated with mean annual temperature and temperature annual range (r = 0.92 and −0.88, respectively). Accordingly, in most of the remaining analyses, we focus on 3 environmental variables: actual evapotranspiration, annual precipitation, and minimum temperature of the coldest month. These variables are directly relevant to the tropical niche conservatism hypothesis owing to the general increase in minimum temperature and annual precipitation toward tropical latitudes.
Table 1.
Pearson’s correlation coefficient between each of the 3 measures of assemblages (SR, NRI, and PDI) and each of the 16 measures of the environment
| Gymnosperm | Angiosperm | |||||
| Environmental variable | SR | NRI | PDI | SR | NRI | PDI |
| General environmental condition | ||||||
| Mean annual temperature | 0.56 | 0.16 | 0.67 | 0.56 | −0.80 | 0.83 |
| Min. temperature of coldest month | 0.69 | 0.23 | 0.65 | 0.73 | −0.84 | 0.89 |
| Annual precipitation | 0.69 | 0.39 | 0.52 | 0.74 | −0.86 | 0.88 |
| Precipitation of driest month | 0.52 | 0.40 | 0.49 | 0.51 | −0.75 | 0.75 |
| Actual evapotranspiration | 0.78 | 0.43 | 0.61 | 0.83 | −0.89 | 0.89 |
| Potential evapotranspiration | 0.41 | −0.03 | 0.62 | 0.44 | −0.67 | 0.72 |
| Climate seasonality | ||||||
| Temperature seasonality | −0.61 | −0.18 | −0.42 | −0.71 | 0.61 | −0.69 |
| Temperature annual range | −0.69 | −0.23 | −0.48 | −0.78 | 0.69 | −0.77 |
| Precipitation seasonality | −0.42 | −0.22 | −0.21 | −0.43 | 0.28 | −0.29 |
| Habitat heterogeneity | ||||||
| Topographic heterogeneity | −0.35 | −0.16 | −0.29 | −0.33 | 0.46 | −0.40 |
| Temperature heterogeneity | −0.40 | −0.21 | −0.39 | −0.37 | 0.55 | −0.49 |
| Precipitation heterogeneity | 0.29 | 0.30 | −0.08 | 0.29 | −0.30 | 0.32 |
| Quaternary climate change | ||||||
| Temperature anomaly | 0.01 | 0.43 | 0.07 | −0.09 | −0.14 | 0.04 |
| Temperature velocity | −0.13 | 0.25 | 0.06 | −0.26 | −0.11 | 0.03 |
| Precipitation anomaly | −0.27 | −0.08 | −0.46 | −0.35 | 0.16 | −0.21 |
| Precipitation velocity | 0.12 | 0.30 | 0.19 | 0.21 | −0.12 | 0.04 |
NRI, net relatedness index; PDI, phylogenetic diversity index; SR, species richness.
Overall, correlations between environmental variables and values of species richness, phylogenetic dispersion, and phylogenetic diversity were stronger for angiosperms than for gymnosperms, although the signs (directions) of the correlations for a given environmental variable were the same for gymnosperms and angiosperms with respect to species richness and PDI (Table 1). For NRI, however, the signs of the correlations with respect to a given environmental variable for gymnosperms were generally opposite to those for angiosperms, and the correlations for gymnosperms were weak in most cases (Table 1). For example, the correlation between NRI and actual evapotranspiration was negative for angiosperms (r = −0.89) but positive for gymnosperms (r = 0.43; Table 1). These results generally support hypothesis H1, that angiosperms more closely follow a pattern of tropical niche conservatism over the range of environments represented in eastern Asia.
When the relationships between phylogenetic relatedness and environment were examined for primarily tropical and primarily temperate families separately, the individual slopes of the relationships between phylogenetic relatedness and actual evapotranspiration, annual precipitation, and minimum temperature of the coldest month were steeper for primarily tropical families (Table 2). For example, the slope of the relationship between NRI and actual evapotranspiration for tropical families was −0.89, whereas for temperate families it was −0.67 (Table 2). This pattern is consistent with hypothesis H2, that the NRI decreases with increasing temperature and precipitation, and that these relationships are stronger for primarily tropical families.
Table 2.
Standardized coefficients of the regression of the NRI of angiosperm assemblages against each of the 3 most important measures of environment for tropical families and temperate families
| Variable | TROP | TEMP |
| Actual evapotranspiration | −0.89 | −0.67 |
| Minimum temperature of coldest month | −0.82 | −0.62 |
| Annual precipitation | −0.84 | −0.58 |
TEMP, temperate; TROP, tropical.
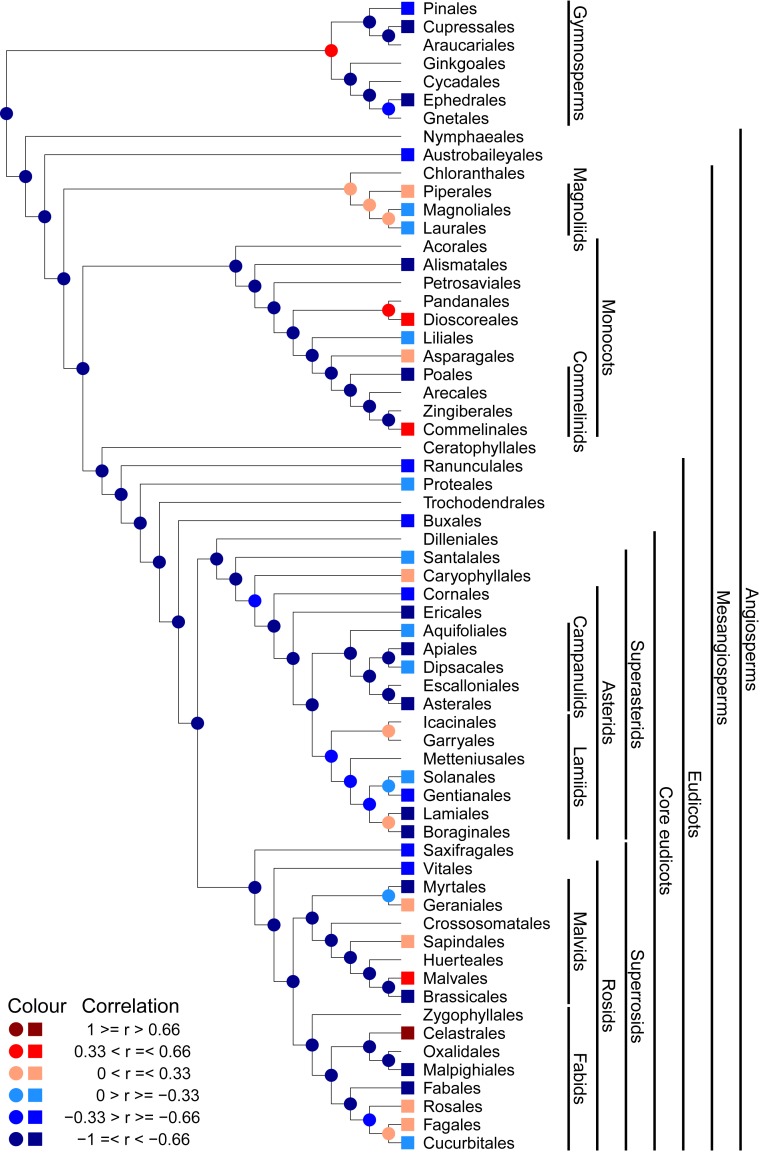
Of the 62 node-based clades above the ordinal level distributed across the phylogeny of the seed plants in China (Fig. 2), ∼84% had strongly or moderately negative relationships between NRI and actual evapotranspiration, minimum temperature of the coldest month, or annual precipitation; but fewer than 13% of the clades had strongly or moderately positive relationships between NRI and the 3 environmental variables (Fig. 2, Table 3, and SI Appendix, Figs. S2 and S3). For PDI, 84% of the nodes had strongly or moderately positive relationships, whereas only 7 to 10% of the nodes had strongly or moderately negative relationships (SI Appendix, Figs. S4–S6 and Table 3).
Fig. 2.
Correlation coefficient (indicated by color) between net relatedness index (NRI) and actual evapotranspiration for each node-based clade and tip in the order-level phylogeny for Chinese seed plants. For illustrative purposes, branch lengths are not shown in the phylogeny. Tips without symbols indicate that no correlation analysis was conducted because these clades had fewer than 15 species and/or occurred in fewer than 33 regions (Methods).
Table 3.
Percentage of nodes, or orders, in the order-level phylogeny of the seed plants in China for which NRI or PDI is strongly or moderately correlated with AET, Tmin, or AP (+ for positive correlation, r > 0.66; − for negative correlation, less than −0.66)
| Node | Order | |||||
| Phylogenetic metric/environmental variable | + | − | W | + | − | W |
| NRI | ||||||
| AET | 12.9 | 83.9 | 3.2 | 9.8 | 51.2 | 39.0 |
| Tmin | 1.6 | 83.9 | 14.5 | 17.1 | 53.7 | 29.3 |
| AP | 4.8 | 83.9 | 11.3 | 9.8 | 51.2 | 39.0 |
| PDI | ||||||
| AET | 83.9 | 9.7 | 6.5 | 46.3 | 22.0 | 31.7 |
| Tmin | 83.9 | 6.5 | 9.7 | 46.3 | 22.0 | 31.7 |
| AP | 83.9 | 8.1 | 8.1 | 43.9 | 22.0 | 34.1 |
AET, actual evapotranspiration; AP, annual precipitation; Tmin, minimum temperature of the coldest month. W indicates weak or no correlation, −0.33 ≤ r ≤ 0.33). Of the 63 orders of seed plants in China, only the 41 orders that each had ≥15 species and occurred in ≥50% of the 66 geographic regions were considered when calculating percentages for orders. Data presented in this table were obtained from Fig. 2 and SI Appendix, Figs. S2–S6.
Of the 63 order-level clades of seed plants in China, 41 were represented by more than 15 species and occurred in more than one-half of the 66 geographic regions. Of these 41 orders, the relationships between NRI and the 3 environmental variables (i.e., actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation) were strongly or moderately negative for 51 to 54% of the orders and were strongly or moderately positive for 10 to 17% of the orders (Fig. 2, Table 3, and SI Appendix, Figs. S2 and S3). The relationships between PDI and the 3 environmental variables were strongly or moderately positive for 44 to 46% of the orders and were strongly or moderately negative for 22% of the orders (SI Appendix, Figs. S4–S6 and Table 3).
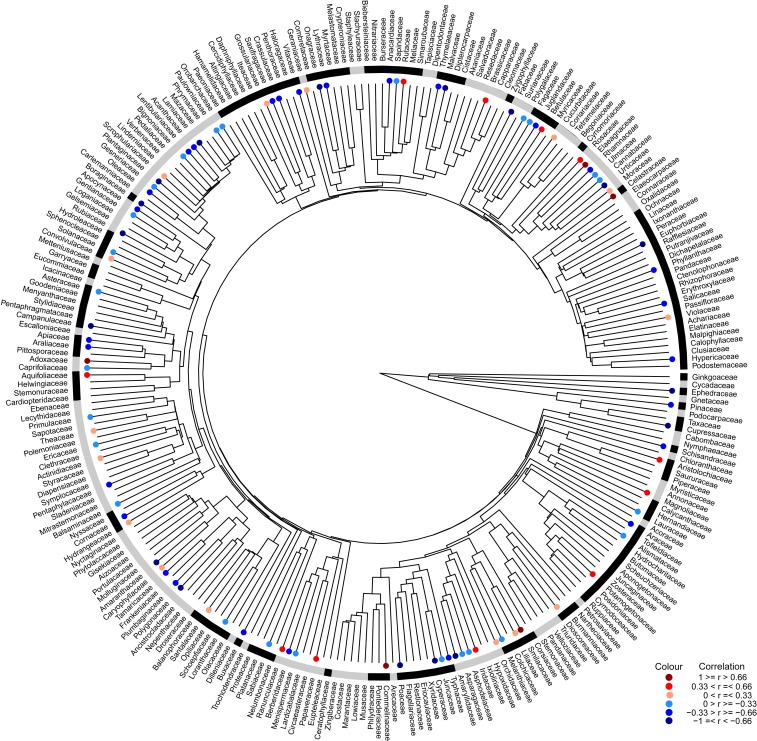
Of the 261 family-level clades of seed plants in China, 100 had ≥15 species and occurred in ≥50% of the 66 geographic regions. Of these 100 families, the relationships between NRI and the 3 environmental variables were strongly or moderately negative for 41 to 44% and were strongly or moderately positive for 13 to 17% (Fig. 3 and SI Appendix, Figs. S7 and S8 and Table S2). The relationships between PDI and the 3 environmental variables were strongly or moderately positive for 32 to 43%, and strongly or moderately negative for 18 to 21%, of the families (SI Appendix, Figs. S9–S11 and Table S2). Taken together, for most of the clades at and above the family level, phylogenetic relatedness increased and phylogenetic diversity decreased with decreasing actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation. This is consistent with hypothesis H3, the tropical niche conservatism hypothesis.
Fig. 3.
Correlation coefficient (represented by colored symbol) between the net relatedness index (NRI) and actual evapotranspiration for each of Chinese seed plant families. Families without a colored dot indicate that no correlation analysis was conducted because they each had fewer than 15 species and/or occurred in fewer than 33 regions (Methods). Families belonging to the same order were indicated with the same color (gray or black) in the outer ring.
When correlation coefficients for the relationships between the 2 phylogenetic metrics and the 3 environmental variables were related to ages of clades (families, orders, and node-based clades above the ordinal level), the correlations tended to be more negative at older ages for NRI and more positive at older ages for PDI (SI Appendix, Fig. S12). Thus, the relationship between each of the 2 phylogenetic metrics and each of the 3 environmental variables tended to be stronger at an older clade age. This result matches the relationships reported in Table 3.
Values of Blomberg’s K, a measure of phylogenetic signal, were typically near the midpoint of the measure’s range, from 0 to 1, for NRI, and they were higher for PDI than for NRI (Table 4). This provides support for hypothesis H4, that the relationship between a phylogenetic measure (NRI or PDI) and the environment itself exhibits phylogenetic signal, i.e., the relationship is determined, to some degree, by the evolutionary histories of different major clades.
Table 4.
Results of tests for phylogenetic signal in the relationship between each of 2 phylogenetic metrics (NRI and PDI) and each of 3 environmental variables (AET, Tmin, and AP)
| Environmental variable | |||
| Phylogenetic metric | AET | Tmin | AP |
| NRI | 0.539 | 0.492 | 0.546 |
| PDI | 0.630 | 0.558 | 0.630 |
AET, actual evapotranspiration; AP, annual precipitation; NRI, net relatedness index; PDI, phylogenetic diversity index; Tmin, mean temperature of the coldest month.
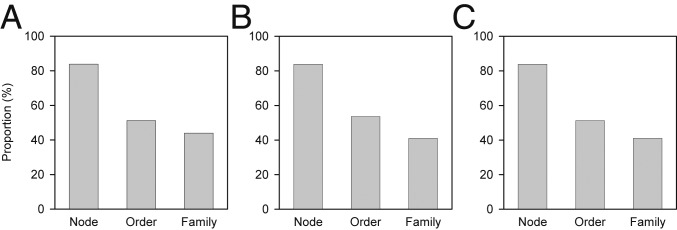
The proportion of strongly or moderately negative correlations between NRI and the 3 environmental variables was highest for node-based clades above the ordinal level and decreased through family-level clades (Fig. 4). For example, the proportion of significant negative correlations between NRI and actual evapotranspiration decreased from 86% for the node-based clades above the ordinal level to 46% for families (Fig. 4A).
Fig. 4.
Proportion of strongly or moderately negative correlations between net relatedness index (NRI) and actual evapotranspiration (A), mean temperature of the coldest month (B), or annual precipitation (C). Node refers to all node-based clades above the order level in the family-level phylogeny of the Chinese seed plants (Fig. 3). Individual correlations for node-based clades and orders are presented in Fig. 2 and SI Appendix, Figs. S2 and S3; individual correlations for families are presented in Fig. 3 and SI Appendix, Figs. S7 and S8.
Correlations between values of NRI or PDI calculated from the full assemblages of seed plant species in each of the 66 geographic regions of China and those derived from 1,000 assemblages each with 276 randomly selected species, which is the smallest number of species in any of the 66 geographic regions, were very strong (r = 0.95 for NRI and 0.89 for PDI; SI Appendix, Extended Text). Similarly, values of NRI or PDI of assemblages of the seed plant species derived from different-sized species pools were strongly correlated (r = 0.99–1.00; SI Appendix, Table S3). Thus, it is unlikely that variation in species richness among geographic regions and variation in species richness among clades would have affected the results of our analyses on the relationships between phylogenetic metrics (i.e., NRI and PDI) and the environmental variables examined.
Discussion
We analyzed a comprehensive dataset for seed plants in China to test 4 hypotheses set out in the Introduction. Our results were consistent with the predictions of these hypotheses. We discuss some of their implications below.
Contrasting Geographic Patterns in NRI between Gymnosperms and Angiosperms.
In general, the geographic pattern of NRI for gymnosperms contrasts with that for angiosperms. Specifically, NRI decreases from east to west in China for gymnosperms, except for the southernmost part of the region (Fig. 1), whereas NRI increases from the east to the west for angiosperms (Fig. 1). High NRI for gymnosperms in central and eastern China parallels the high species richness of gymnosperms in this region (Fig. 1A), where the climate is relatively warm and wet, but species are phylogenetically more clustered relative to the entire species pool of Chinese gymnosperms. This pattern is inconsistent with the tropical niche conservatism hypothesis, and likely reflects a complicated evolutionary history of gymnosperms in China.
The central and eastern part of China has been considered as a center of diversification and a refuge for gymnosperms. This area harbors all 6 Chinese endemic genera of gymnosperms (i.e., Ginkgo, Cathaya, Metasequoia, Nothotsuga, Pseudolarix, and Pseudotaxus), some of which are primarily or fully restricted to the area (23). For example, the well-known “living fossil” genus Metasequoia occurs naturally only in a small area of central China, although it has been planted widely outside the region. For angiosperms, the geographic patterns of species richness, phylogenetic dispersion, and phylogenetic diversity are generally consistent with the tropical niche conservatism hypothesis (Fig. 1). Our analyses suggest that simultaneously including both gymnosperms and angiosperms in a phylogenetically based analysis might obscure patterns evident in each group separately.
The Geographic Pattern of Species Richness Differs from Distributions of NRI and PDI for Angiosperms in Southern China.
In the broad area of southern China east of the Tibet-Qinghai Plateau, species richness is higher in the western part of the area than in its eastern part, and it is highest in the Hengduan Mountains of northwestern Yunnan Province (region YN6 in SI Appendix, Fig. S1 and Fig. 1D). In contrast, phylogenetic diversity is lower in the western part of the area than toward the east (Fig. 1F; compare Fig. 1 D and F for YN6), which is generally consistent with the pattern of phylogenetic dispersion across the area (Fig. 1E). This discrepancy between the geographic pattern of species richness and the patterns of phylogenetic diversity and dispersion suggests that some species-rich genera in the western part of the area (particularly in the Hengduan Mountains) diversified recently (e.g., Rhododendron, Pedicularis, Primula, and Gentiana) (23, 24) and thus have, on average, relatively short divergence times and correspondingly low phylogenetic diversity.
Determinants of Patterns of Species Richness and Phylogenetic Dispersion of Seed Plants across China.
We examined the relationships between each of 16 environmental variables and measures of species richness and of phylogenetic dispersion and diversity separately for gymnosperms and angiosperms (Table 1). In general, variation in habitat heterogeneity and Quaternary climate change among the 66 regions across China had little effect on variation in species richness, phylogenetic dispersion, and diversity. Among environmental variables, present-day actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation were most strongly correlated with species richness and with phylogenetic dispersion and diversity.
The NRI for gymnosperms was positively correlated with actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation. Thus, phylogenetic relatedness among gymnosperms in China cannot be explained by the tropical niche conservatism hypothesis, which predicts negative relationships between NRI and the 3 environmental variables. However, gymnosperms account for fewer than 1% of the species of seed plants in China. In contrast, NRI for angiosperms was strongly and negatively correlated with these 3 environmental variables, consistent with the tropical niche conservatism hypothesis.
The 3 environmental variables most strongly correlated with species richness and phylogenetic dispersion and diversity are also strongly correlated among each other (r = 0.88 to 0.96). Of these 3 variables, actual evapotranspiration is, on average, the strongest correlate for species richness and phylogenetic dispersion and diversity. Because actual evapotranspiration represents both temperature and water availability and measures energy–water balance, and because the minimum temperature of the coldest month is strongly correlated with mean annual temperature (r = 0.92), which is a measure of ambient energy, it makes sense that actual evapotranspiration is, at least statistically, the stronger determinant among the climate variables of species richness and phylogenetic dispersion for angiosperms.
Relationships of Both Phylogenetic Dispersion and Diversity to the Environment Tend to Be More Variable for Younger Clades.
Negative relationships between NRI and each of actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation were observed for nearly all node-based clades across the order-level phylogeny of the seed plants in China (Fig. 2). Similarly, relationships between PDI and the 3 environmental variables were positive for nearly all node-based clades across the phylogeny. This suggests that the mechanisms posited by the tropical niche conservatism hypothesis have consistently played a role in generating patterns of phylogenetic dispersion and diversity in both basal nodes and more derived nodes, i.e., over the long history of diversification of seed plants in China. These relationships tend to be more variable for clades having more recent origin (Figs. 2–4), suggesting that tropical niche conservatism has played a stronger and more consistent role for more basal clades. At the ordinal and family levels, the NRI decreases, and the PDI increases, toward more tropical environments (Figs. 2 and 3), consistent with tropical niche conservatism.
Evolutionary Conservatism in the Relationships between Phylogenetic Structure and Environment.
Relationships between phylogenetic structure and the environment exhibit evolutionary conservatism for the seed plants in China. Of the 6 analyses conducted to detect phylogenetic signal using Blomberg’s K, 5 produced values greater than 0.5 (Table 4). This suggests that the relationships between either NRI or PDI and environmental variables are, to some degree, phylogenetically conservative across the phylogeny of seed plants in China. That is, more closely related families tend to have more similar relationships between phylogenetic structure and environment. Of the 261 families of seed plants in China, only 100 met the sample criteria, i.e., ≥15 species occurring in ≥50% of the geographic regions in China, to be included in analyses to detect phylogenetic signal. Although Blomberg’s K should be independent of phylogeny size, power to detect significant structure might be reduced for small phylogenies (25), such as the phylogeny used in this analysis, having only 100 family-level tips.
Phylogenetic Dispersion of Typically Tropical Families Is More Sensitive to Stressful Environments than That of Typically Temperate Families.
China includes both tropical and temperate climates. The steeper slopes of the relationships between phylogenetic dispersion (NRI) and the 3 key environmental variables (actual evapotranspiration, minimum temperature of the coldest month, annual precipitation) for tropical families compared to temperate families (Table 2) are consistent with the species in tropical families being more sensitive to cold and arid environments than are those of temperate families. That more plant families are primarily confined to tropical climates than to temperate climates (26) suggests that few species in many of the plant families that originated under tropical climates have evolved tolerance of freezing temperatures. Steeper slopes of the relationships between NRI and climate variables (representing temperature and precipitation) observed for tropical families in China, compared with temperate families, suggest that families, genera, and/or species are more closely related (clustered) toward colder and drier climates for a given degree of climate shift.
Conclusion
Of the 16 environmental variables examined in this study, actual evapotranspiration, minimum temperature of the coldest month, and annual precipitation were most closely associated with patterns of species richness, phylogenetic dispersion, and phylogenetic diversity for both gymnosperms and angiosperms in China. However, patterns of phylogenetic dispersion differed between gymnosperms and angiosperms, in that NRI was positively correlated with actual evapotranspiration for gymnosperms but negatively correlated for angiosperms. For most clades at and above the family level, the relationships between metrics measuring phylogenetic dispersion and diversity and the 3 environmental variables were consistent with the tropical niche conservatism hypothesis (i.e., phylogenetic relatedness increases and phylogenetic diversity decreases with decreasing temperature and increasing aridity). The slopes of the relationships between phylogenetic relatedness and the environmental variables were steeper for tropical clades than for temperate clades, as would be expected from greater sensitivity of tropical species to stressful physical conditions. A small number of clades exhibited relationships between phylogenetic metrics and environments that were inconsistent with the tropical niche conservatism hypothesis. However, these inconsistencies occurred primarily in clades descended from shallow nodes (i.e., far from the root of the phylogeny) and their distribution across the phylogeny bears phylogenetic signal, suggesting that these clades might represent special cases. Overall, our results imply that patterns of phylogenetic structure of seed plants across geographic and environmental gradients in China primarily indicate PNC reflecting initial adaptation to warm, wet environments.
Methods
Regional Species Assemblage Data.
Geographical sample units of this study are provinces, or “subprovinces” of large provinces, in China. We combined Beijing and Tianjin Municipalities with Hebei Province, Shanghai Municipality with Zhejiang Province, and Hong Kong with Guangdong Province. We divided 7 large provinces (i.e., Gansu, Qinghai, Neimenggu, Sichuan, Yunnan, Xinjiang, and Xizang) into 44 subprovinces. A subprovince included one or more administrative prefectures within a province. In total, 66 provinces or subprovinces were used in this study, which we call “regions” for convenience (SI Appendix, Fig. S1). On average, each region occupied an area of ∼144,000 km2, equivalent to a square with 380 km on each side, or a circle with a diameter of 428 km.
Checklists of seed plants were compiled for each of the Chinese provinces based on published provincial and regional floras (27) and the Flora of China (23). Checklists of seed plants for each subprovince were compiled based on numerous distribution data, which included species checklists for administrative regions, distribution records for administrative regions and counties in provincial floras, species checklists of local floras (e.g., counties, nature reserves, forest parks), herbarium specimen records with the Global Biodiversity Information Facility (GBIF) (https://www.gbif.org/), and the National Specimen Information Infrastructure (NSII) (www.nsii.org.cn), and various distribution datasets (17). Our assessment of the completeness of the subprovincial species checklists, based on the relationship between species richness and the environment, showed that, on average, the completeness of the subprovincial species checklists did not differ from that of the provincial species checklists that were compiled with an aim of generating complete species checklists for each province (SI Appendix, S1). This suggests that the checklists of the subprovinces used in this study are sufficiently complete.
Botanical nomenclature of species was standardized according to The Plant List (version 1.1; www.theplantlist.org). Infraspecific taxa were combined with their respective species. Nonnative species were excluded. As a result, a total of 29,158 seed plant species in 2,919 genera were included in this study (192 species in 37 genera for gymnosperms; 28,966 species in 2,882 genera for angiosperms). We grouped the genera of the Chinese seed plants into families and orders based on the Angiosperm Phylogeny Website (http://www.mobot.org/MOBOT/research/APweb). Delineations of families and orders for angiosperms are consistent with those of the Angiosperm Phylogeny Group (ref. 28; i.e., APG IV). The Chinese seed plants belong to 261 families in 63 orders. Each family was associated with one of the 3 broad climate distributions: primarily tropical (n = 132; SI Appendix, S4), primarily temperate (=80; SI Appendix, S4), and broadly distributed in both tropical and temperate climates (=49), according to ref. 29 and the Angiosperm Phylogeny website (version 14; http://www.mobot.org/MOBOT/research/APweb/).
Phylogeny Reconstruction.
We used a recently published, dated megaphylogeny for seed plants, GBOTB (30), as a backbone to generate a phylogeny for the Chinese seed plant species. GBOTB was constructed using 79,881 taxa in GenBank and a backbone provided by Open Tree of Life, version 9.1 (30). This is the largest dated megaphylogeny for seed plants. Of the 261 families of seed plants in China, only 2 (i.e., Rafflesiaceae and Mitrastemonaceae) do not have representative species in GBOTB. We added them to GBOTB according to refs. 12 and 31. As a result, all families of Chinese seed plants were resolved in the expanded megaphylogeny. Of the 2,919 genera of the Chinese seed plants, 2,531 were included in GBOTB. Of those genera that occurred in China but were not included in GBOTB, 219 have closely related (often sister) genera in GBOTB based on the megaphylogenies of refs. 12 and 17. We treated them as sisters to their respective closely related genera in GBOTB. As a result, relationships of 2,750 (94.2%) of the Chinese seed plant genera were resolved. At the species level, 11,463 species of the seed plants in China were included in GBOTB. For the genera and species of the Chinese seed plants that are absent from GBOTB, we added them to their respective genera (in the case of species) and families (in the case of genera) using the Phylomatic and BLADJ approaches (32) implemented in the V.PhyloMaker software (33). Specifically, V.PhyloMaker sets branch lengths of added taxa of a family by placing the nodes evenly between dated nodes and terminals within the family, and adds a missing genus at the basal node within the family and a missing species at the basal node within its genus. We used build.nodes.1 and scenario 3 in V.PhyloMaker. Finally, we pruned the megaphylogeny to retain only the 29,158 Chinese seed plant species.
Phylogenetic Structure and Diversity Metrics.
The NRI is the most commonly used measure of phylogenetic relatedness (32); accordingly, we used NRI to quantify phylogenetic relatedness of species assemblages in this study. NRI measures the standardized effect size of mean phylogenetic distance (MPD), which estimates the average phylogenetic relatedness between all possible pairs of species in an assemblage. NRI is defined as follows (34):
where MPDobserved is the observed MPD, MPDrandomized is the expected (i.e., average) MPD of the randomized assemblages, and sdMPDrandomized is the SD of the MPD for the randomized assemblages. NRI is a richness-standardized index. A positive NRI value indicates that MPD is less than expected by chance (i.e., species are more closely related than expected by chance) and that species are phylogenetically clustered. Conversely, a negative NRI value results when the observed MPD is greater than expected by chance (i.e., species are more distantly related than expected by chance) and thus indicates phylogenetic overdispersion.
Faith’s phylogenetic diversity (PD) (35) is a commonly used measure for the phylogenetic diversity of species assemblages. PD measures the sum of all phylogenetic branch lengths that connect species in a species assemblage. In general, PD is strongly and positively correlated with species richness. To account for the effect of species richness, we used the standardized effect size of PD to quantify phylogenetic diversity in each species assemblage. This PDI is defined as follows (36):
where PDobserved is the observed PD, PDrandomized is the expected PD of the randomized assemblages, and sdPDrandomized is the SD of the PD for the randomized assemblages. Thus, PDI is also a richness-standardized index. Large PDI values indicate high phylogenetic diversity. PDI is strongly and negatively correlated with the commonly used nearest taxon index (NTI) (37). NRI captures structure deeper in the tree, whereas PDI captures more tip-level structure. We used the software PhyloMeasures (38) to calculate NRI and PDI. With PhyloMeasures, both indices were obtained using computationally efficient algorithms (39, 40). Specifically, PhyloMeasures calculates NRI and PDI based on exact solutions given a particular tree and species richness, rather than based on a resampling approximation of the mean and variance. Defining these statistical moments requires a null model and a defined species pool. Our null model considered all possible combinations of S species from the species pool (where S is the richness of a sample to be standardized) to be equally likely (38). We calculated NRI and PDI for each family and for each node-based clade from the family tips to the root of the phylogeny of the Chinese seed plants. In each calculation, the species pool included only the species of the concerned clade.
Environmental Data.
Current species distributions reflect contemporary climate conditions, long-term climate change (particularly since the Last Glacial Maximum), and topographic heterogeneity (41–43). We examined a variety of environmental variables (n = 16) to determine which are most closely associated with geographical patterns of species richness, NRI, and PDI across China. The 16 variables represented 4 major categories (i.e., “general” environmental condition, climate seasonality, habitat heterogeneity, and glacial–interglacial climate change).
The category of general environmental condition included 6 environmental variables: mean annual temperature, minimum temperature of the coldest month, annual precipitation, precipitation in the driest month, actual evapotranspiration, and potential evapotranspiration. Mean annual temperature and annual precipitation are among the most important climate variables governing species distributions at broad spatial scales (41) and are directly relevant to the tropical niche conservatism hypothesis (44). Minimum temperature of the coldest month and precipitation of the driest month measure stressful temperatures and water availability; these 2 climate variables are directly relevant to the stress-dominance hypothesis (5, 45) and mechanisms related to the tropical niche conservatism hypothesis. Actual evapotranspiration combines temperature and water availability into a single variable, and is a measure of energy–water balance (46). Actual evapotranspiration is considered as a productive energy metric (47). Potential evapotranspiration is a measure of ambient energy (48). Data for the 4 climate variables were obtained from the WorldClim website (http://worldclim.org/version2; corresponding to bio1, bio6, bio12, and bio14, respectively), using the data at a resolution of 30 arc-seconds. Data for the 2 evapotranspiration variables were obtained from the CGIAR website (http://www.cgiar-csi.org/data). The mean value of each of the 6 climate variables was calculated for each of the 66 geographic regions.
The category “climate seasonality” includes 2 variables measuring temperature seasonality (temperature variation over a year based on the SD of monthly temperature averages, and annual temperature range) and one variable for precipitation seasonality, which is the ratio of the SD of the monthly total precipitation to the mean monthly total precipitation. Data for these climate seasonality variables were obtained from the WorldClim website (http://worldclim.org/version2; corresponding to bio4, bio7, and bio14, respectively), using the data at a resolution of 30 arc-seconds. The mean value of each of the 3 climate variables was calculated for each of the 66 geographic regions.
The category of habitat heterogeneity includes variation (SD) in topography (elevation), mean annual temperature, and annual precipitation among grid cells of ∼1 km2 within each of the 66 geographic regions. These variables were obtained from the WorldClim website (http://worldclim.org/version2), using data at a resolution of 30 arc-seconds.
The category of Quaternary climate change includes 4 variables (i.e., temperature anomaly, precipitation anomaly, temperature velocity, precipitation velocity), which measure glacial–interglacial oscillations of temperature and precipitation since the Quaternary. Specifically, we used the differences in mean annual temperature and annual precipitation between the Last Glacial Maximum and the present as a temperature anomaly and a precipitation anomaly, respectively; we calculated temperature velocity and precipitation velocity as the ratio of the rate of climate change through time to the rate of climate change across space (49). The mean annual temperature and annual precipitation at the Last Glacial Maximum were computed from the average of the data simulated from CCSM3 (Community Climate System Model, version 3) (50, 51) and MIROC3.2 [Model for Interdisciplinary Research on Climate, version 3.2 (52)]. The mean value of each of the 4 climate variables was calculated for each of the 66 geographic regions.
Data Analysis.
All 16 environmental variables xi were standardized to vary from 0 to 1, using the formula (xi – minimum)/(maximum – minimum). We conducted correlation analyses to assess the relationship between each of the 2 phylogenetic metrics (i.e., NRI and PDI) and each of the 16 environmental variables, for seed plants as a whole, and for gymnosperms and angiosperms separately. We considered a correlation to be strong for |r| > 0.66, moderate for 0.66 ≥ |r| > 0.33, and weak for |r| ≤ 0.33. We conducted ordinary least-squares regression analysis to estimate the slope of the relationship between phylogenetic relatedness and each of the environmental variables for tropical families and temperate families separately. To test whether patterns of phylogenetic structure and diversity for each of the individual clades, from the root to each family in the phylogeny of the Chinese seed plants, are consistent with the tropical niche conservatism hypothesis, we calculated correlations between phylogenetic metrics and major temperature-related and precipitation-related climate variables for each clade having more than 15 species and occurring in more than 50% of the 66 geographic regions in China (SI Appendix, Fig. S1).
Blomberg’s K (25) has been widely used to assess phylogenetic signal (e.g., refs. 53–55). Accordingly, we used Blomberg’s K to evaluate phylogenetic signal in the correlation (strength and direction) between each of the 2 phylogenetic metrics (NRI and PDI) and environment for the families for which we conducted a correlation analysis (i.e., families with ≥15 species occurring in ≥50% of geographic regions). Blomberg’s K commonly varies from ∼0 (low phylogenetic signal) to ≥1.0 [high phylogenetic signal, marginally above the Brownian motion null (25)]. Thus, a larger value of Blomberg’s K indicates a stronger phylogenetic signal.
Species richness varies among the 66 geographic regions. Although the 2 phylogenetic metrics used in this study are richness-standardized indices, we conducted an analysis that more strictly assesses whether the variation in species richness among the regions might have affected our analyses of the relationships between phylogenetic metrics and environmental variables. Specifically, we used a randomization approach to generate assemblages with equal numbers of species in each of the 66 geographic regions. Given that the minimum number of seed plant species in a region was 279, we randomly selected 279 seed plant species from each geographic region and calculated NRI and PDI; we repeated this 1,000 times and averaged the 1,000 values for each of the 2 phylogenetic metrics. We then examined whether values of NRI and PDI are strongly correlated with their counterparts based on the full assemblage of each geographic region. A strong positive correlation between the 2 would indicate that variation in species richness among the regions would have no, or little, effect on our analysis.
Species richness also varies among clades across the phylogeny of the Chinese seed plants, causing variation in species pool size among clades when calculating NRI and PDI for each clade. To assess whether this could have affected our analyses of the relationships between phylogenetic metrics and environmental variables, we used a randomization approach to generate subsets of data with fixed numbers of species in each data subset. Specifically, we first set the number of species to 2,000, randomly selected 2,000 species from the full species pool of the Chinese seed plants, and calculated NRI and PDI for each geographic region based on the 2,000 selected species; we repeated this 1,000 times and averaged the values of the 1,000 randomizations for each of NRI and PDI. We then repeated this randomization approach for 4,000, 6,000, 8,000, and 10,000 species, and examined whether values of NRI and PDI are strongly correlated among the 5 sizes of species pools. A strong positive correlation in resultant values of NRI and PDI among different sizes of species pools would suggest no, or little, effect of differences in species pool size among different clades on our analysis.
Data Availability.
All data used in this study have been published and are accessible to readers from the cited sources.
Supplementary Material
Acknowledgments
We thank several reviewers for their constructive comments. This study was supported by the Key Projects of the Joint Fund of the National Natural Science Foundation of China (U1802232), the Major Program of the National Natural Science Foundation of China (31590823), and the Strategic Priority Research Program of Chinese Academy of Sciences (XDA20050203) (to Hang Sun); National Natural Science Foundation of China (31700165), Youth Innovation Promotion Association Chinese Academy of Sciences (2019382), Young Academic and Technical Leader Raising Foundation of Yunnan Province (2019HB039) (to T.D.); National Natural Science Foundation of China (31600340) (to Y.J.); and National Natural Science Foundation of China (31870506) and Natural Science Foundation of Jiangsu Province (BK20181398) (to L.M.).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1822153116/-/DCSupplemental.
References
- 1.Donoghue M. J., Colloquium paper: A phylogenetic perspective on the distribution of plant diversity. Proc. Natl. Acad. Sci. U.S.A. 105 (suppl. 1), 11549–11555 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricklefs R. E., A comprehensive framework for global patterns in biodiversity. Ecol. Lett. 7, 1–15 (2004). [Google Scholar]
- 3.Cardillo M., Phylogenetic structure of mammal assemblages at large geographical scales: Linking phylogenetic community ecology with macroecology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366, 2545–2553 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraterrigo J. M., Wagner S., Warren R. J., Local-scale biotic interactions embedded in macroscale climate drivers suggest Eltonian noise hypothesis distribution patterns for an invasive grass. Ecol. Lett. 17, 1447–1454 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Coyle J. R., et al. , Using trait and phylogenetic diversity to evaluate the generality of the stress-dominance hypothesis in eastern North American tree communities. Ecography 37, 814–826 (2014). [Google Scholar]
- 6.Wiens J. J., Donoghue M. J., Historical biogeography, ecology and species richness. Trends Ecol. Evol. (Amst.) 19, 639–644 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Latham R. E., Ricklefs R. E., Global patterns of tree species richness in moist forests: Energy-diversity theory does not account for variation in species richness. Oikos 67, 325–333 (1993). [Google Scholar]
- 8.Peterson A. T., Soberón J., Sanchez-Cordero V., Conservatism of ecological niches in evolutionary time. Science 285, 1265–1267 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Wiens J. J., et al. , Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310–1324 (2010). [DOI] [PubMed] [Google Scholar]
- 10.Behrensmeyer A. K., et al., Eds., Terrestrial Ecosystems Through Time. Evolutionary Paleoecology of Terrestrial Plants and Animals (University of Chicago Press, Chicago, 1992). [Google Scholar]
- 11.Graham A., Late Cretaceous and Cenozoic History of North American Vegetation (Oxford University Press, New York, 1999). [Google Scholar]
- 12.Zanne A. E., et al. , Three keys to the radiation of angiosperms into freezing environments. Nature 506, 89–92 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Condamine F. L., Sperling F. A. H., Wahlberg N., Rasplus J.-Y., Kergoat G. J., What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 15, 267–277 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Ricklefs R. E., Evolutionary diversification and the origin of the diversity-environment relationship. Ecology 87(suppl. 7), S3–S13 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Futuyma D. J., Evolutionary Biology (Sinauer Associates, Sunderland, MA, ed. 3, 1998). [Google Scholar]
- 16.Ricklefs R. E., Schluter D., “Species diversity: Regional and historical influences” in Species Diversity in Ecological Communities. Historical and Geographical Perspectives, Ricklefs R. E., Schluter D., Eds. (University of Chicago Press, Chicago, 1993), pp. 350–363. [Google Scholar]
- 17.Lu L.-M., et al. , Evolutionary history of the angiosperm flora of China. Nature 554, 234–238 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Qian H., Sandel B., Phylogenetic structure of regional angiosperm assemblages across latitudinal and climatic gradients in North America. Glob. Ecol. Biogeogr. 26, 1258–1269 (2017). [Google Scholar]
- 19.Davis C. C., Schaefer H., Plant evolution: Pulses of extinction and speciation in gymnosperm diversity. Curr. Biol. 21, R995–R998 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Graham A., The age and diversification of terrestrial New World ecosystems through Cretaceous and Cenozoic time. Am. J. Bot. 98, 336–351 (2011). [DOI] [PubMed] [Google Scholar]
- 21.Lanier H. C., Edwards D. L., Knowles L. L., Phylogenetic structure of vertebrate communities across the Australian arid zone. J. Biogeogr. 40, 1059–1070 (2013). [Google Scholar]
- 22.Qian H., Sandel B., Phylogenetic relatedness of native and exotic plants along climate gradients in California, USA. Divers. Distrib. 23, 1323–1333 (2017). [Google Scholar]
- 23.Wu C.-Y., Raven P. H., Hong D.-Y., Eds., Flora of China (Science Press and Missouri Botanical Garden Press, Beijing, St. Louis, 1994–2013), vol. 2-25. [Google Scholar]
- 24.Xing Y., Ree R. H., Uplift-driven diversification in the Hengduan Mountains, a temperate biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A. 114, E3444–E3451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blomberg S. P., Garland T. Jr, Ives A. R., Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 57, 717–745 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Heywood V. H., Brummitt R. K., Culham A., Seberg O., Flowering Plant Families of the World (Royal Botanic Gardens, Kew, England, 2007). [Google Scholar]
- 27.Liu Q.-R., Yu M., Ma J.-S., Review on the Chinese local floras. Guihaia 27, 844–849 (2007). [Google Scholar]
- 28.Angiosperm Phylogeny Group , An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 181, 1–20 (2016). [Google Scholar]
- 29.Wu Z., Zhou Z., Sun H., Li D., Peng H., The Areal-Types of Seed Plants and Their Origin and Differentiation (Yunnan Science and Technology Press, Kunming, China, 2006). [Google Scholar]
- 30.Smith S. A., Brown J. W., Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Gastauer M., Meira-Neto J. A. A., Avoiding inaccuracies in tree calibration and phylogenetic community analysis using Phylocom 4.2. Ecol. Inform. 15, 85–90 (2013). [Google Scholar]
- 32.Webb C., Ackerly D., Kembel S., Phylocom: Software for the Analysis of Phylogenetic Community Structure and Character Evolution, with Phylomatic, Version 4.2 (2011) https://phylodiversity.net/phylocom/. Accessed 20 October 2018.
- 33.Jin Y., Qian H., V.PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 42, 1353–1359 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webb C. O., Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 156, 145–155 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Faith D. P., Conservation evaluation and phylogenetic diversity. Biol. Conserv. 61, 1–10 (1992). [Google Scholar]
- 36.Kembel S. W., et al. , Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Mazel F., et al. , Influence of tree shape and evolutionary time-scale on phylogenetic diversity metrics. Ecography 39, 913–920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsirogiannis C., Sandel B., PhyloMeasures: A package for computing phylogenetic biodiversity measures and their statistical moments. Ecography 39, 709–714 (2016). [Google Scholar]
- 39.Tsirogiannis C., Sandel B., Cheliotis D., Efficient computation of popular phylogenetic tree measures. Lect. Notes Comput. Sci. 7534, 30–43 (2012). [Google Scholar]
- 40.Tsirogiannis C., Sandel B., Kalvisa A., New algorithms for computing phylogenetic biodiversity. Lect. Notes Comput. Sci. 8701, 187–203 (2014). [Google Scholar]
- 41.Rahbek C., Graves G. R., Multiscale assessment of patterns of avian species richness. Proc. Natl. Acad. Sci. U.S.A. 98, 4534–4539 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandel B., et al. , The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Qian H., Fridley J. D., Palmer M. W., The latitudinal gradient of species-area relationships for vascular plants of North America. Am. Nat. 170, 690–701 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Hawkins B. A., et al. , Community phylogenetics at the biogeographical scale: Cold tolerance, niche conservatism and the structure of North American forests. J. Biogeogr. 41, 23–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Normand S., et al. , Importance of abiotic stress as a range-limit determinant for European plants: Insights from species responses to climatic gradients. Glob. Ecol. Biogeogr. 18, 437–449 (2009). [Google Scholar]
- 46.Bini L. M., Diniz J. A. F., Hawkins B. A., Macroecological explanations for differences in species richness gradients: A canonical analysis of South American birds. J. Biogeogr. 31, 1819–1827 (2004). [Google Scholar]
- 47.Evans K. L., Greenwood J. J. D., Gaston K. J., Dissecting the species-energy relationship. Proc. Biol. Sci. 272, 2155–2163 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawkins B. A., Porter E. E., Water-energy balance and the geographic pattern of species richness of western Palearctic butterflies. Ecol. Entomol. 28, 678–686 (2003). [Google Scholar]
- 49.Loarie S. R., et al. , The velocity of climate change. Nature 462, 1052–1055 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 51.Otto-Bliesner B. L., et al. , Last Glacial Maximum and Holocene climate in CCSM3. J. Clim. 19, 2526–2544 (2006). [Google Scholar]
- 52.Hasumi H., Emori S., K-1 Coupled Model (MIROC) Description (The University of Tokyo, Tokyo, 2004). [Google Scholar]
- 53.Buckley L. B., et al. , Phylogeny, niche conservatism, and the latitudinal diversity gradient in mammals. Proc. Biol Sci. 277, 2131–2138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qian H., Zhang J., Using an updated time-calibrated family-level phylogeny of seed plants to test for non-random patterns of life forms across the phylogeny. J. Syst. Evol. 52, 423–430 (2014). [Google Scholar]
- 55.Li Y., et al. , Leaf margin analysis of Chinese woody plants and the constraints on its application to palaeoclimatic reconstruction. Glob. Ecol. Biogeogr. 25, 1401–1415 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study have been published and are accessible to readers from the cited sources.