Significance
Certain adapted insect herbivores utilize plant toxins for self-defense against their own enemies. These adaptations structure ecosystems and limit our capacity to use biological control agents to manage specialized agricultural pests. We show that entomopathogenic nematodes that are exposed to the western corn rootworm, an important agricultural pest that sequesters defense metabolites from maize, can evolve resistance to these defenses. Resisting the plant defense metabolites likely allows the nematodes to infect and kill the western corn rootworm more efficiently. These findings illustrate how predators can counter the plant-based resistance strategies of specialized insect herbivores. Breeding or engineering biological control agents that resist plant defense metabolites may improve their capacity to kill important agricultural pests such as the western corn rootworm.
Keywords: tritrophic interactions, plant secondary metabolism, biological control, plant–herbivore interactions, coevolutionary arms race
Abstract
Plants defend themselves against herbivores through the production of toxic and deterrent metabolites. Adapted herbivores can tolerate and sometimes sequester these metabolites, allowing them to feed on defended plants and become toxic to their own enemies. Can herbivore natural enemies overcome sequestered plant defense metabolites to prey on adapted herbivores? To address this question, we studied how entomopathogenic nematodes cope with benzoxazinoid defense metabolites that are produced by grasses and sequestered by a specialist maize herbivore, the western corn rootworm. We find that nematodes from US maize fields in regions in which the western corn rootworm was present over the last 50 y are behaviorally and metabolically resistant to sequestered benzoxazinoids and more infective toward the western corn rootworm than nematodes from other parts of the world. Exposure of a benzoxazinoid-susceptible nematode strain to the western corn rootworm for 5 generations results in higher behavioral and metabolic resistance and benzoxazinoid-dependent infectivity toward the western corn rootworm. Thus, herbivores that are exposed to a plant defense sequestering herbivore can evolve both behavioral and metabolic resistance to plant defense metabolites, and these traits are associated with higher infectivity toward a defense sequestering herbivore. We conclude that plant defense metabolites that are transferred through adapted herbivores may result in the evolution of resistance in herbivore natural enemies. Our study also identifies plant defense resistance as a potential target for the improvement of biological control agents.
Despite the high abundance and diversity of arthropod herbivores, plants dominate terrestrial (agro)ecosystems (1). Predation by herbivore natural enemies and plant defenses are thought to contribute to this phenomenon (2, 3). Plants defend themselves against herbivores using a variety of strategies, including the production of specialized defense metabolites that are toxic and/or reduce their attractivity and digestibility (4–7). However, many herbivores evolved mechanisms to overcome the negative effects of plant defense metabolites, including behavioral avoidance, excretion, target site insensitivity, and detoxification through conjugation and breakdown (6, 8, 9). As a result, herbivores are often able to feed on defended plants and to ingest plant toxins without suffering major fitness consequences.
The ability to tolerate plant toxins has also enabled some specialized herbivores to coopt plant defense metabolites for self-defense against their own natural enemies (10, 11). Sequestration of plant toxins as a form of adaptation is relatively widespread in specialized insect herbivores (12, 13). Plant toxins may also accumulate in nonadapted insect herbivores, which are often inefficient at metabolizing and/or detoxifying plant defense compounds (14–16). Consequently, predators, parasites, and parasitoids are often exposed to plant toxins as they feed on herbivores. Despite the fact that plant toxin exposure of the third trophic level is common in nature, herbivore natural enemies succeed at controlling herbivores and reduce their negative impact on plant fitness and yield (3). How top-down control of herbivores is maintained in the face of the abundance, diversity, and ubiquity of plant defense metabolites is a potentially important open question in multitrophic interaction research and chemical ecology.
One possible explanation for the success of herbivore natural enemies attacking sequestering hosts is that similar to herbivores, they may have evolved the capacity to resist or tolerate plant defense metabolites (17). Different degrees of resistance to plant toxins have been observed in predators and parasitoids (18–21). However, whether plant defense metabolites can drive the evolution of resistance of members of the third trophic level, and to what extent resistance to plant defense metabolites improves the capacity of herbivore natural enemies to prey on adapted herbivores, is not well understood.
To address the questions above, we studied the impact of plant-derived benzoxazinoids on entomopathogenic nematodes. Benzoxazinoids are multifunctional defense metabolites that are produced by grasses such as wheat and maize (22) and protect them against generalist herbivores (23–25). The western corn rootworm (WCR; Diabrotica virgifera virgifera), a specialized maize herbivore and important agricultural pest, is fully resistant to benzoxazinoids (26). The rootworm larvae are attracted to benzoxazinoids (27) and accumulate them in their bodies (28). Entomopathogenic nematodes such as Heterorhabditis bacteriophora are common in natural and agricultural ecosystems across the globe and cooccur with the WCR in some areas. They are used as biological control agents against many different root pests, including the WCR (29, 30). Benzoxazinoid sequestration by the WCR reduces the capacity of a commercial H. bacteriophora strain to infect and kill the herbivore, suggesting that the corn rootworm coopts these plant defense metabolites for self-protection against entomopathogenic nematodes (28). Using natural variation and forward evolution, we investigated whether adapted H. bacteriophora nematodes are able to overcome this defense strategy of the WCR and whether their capacity to resist benzoxazinoids is associated with increased infectivity toward the WCR.
Results
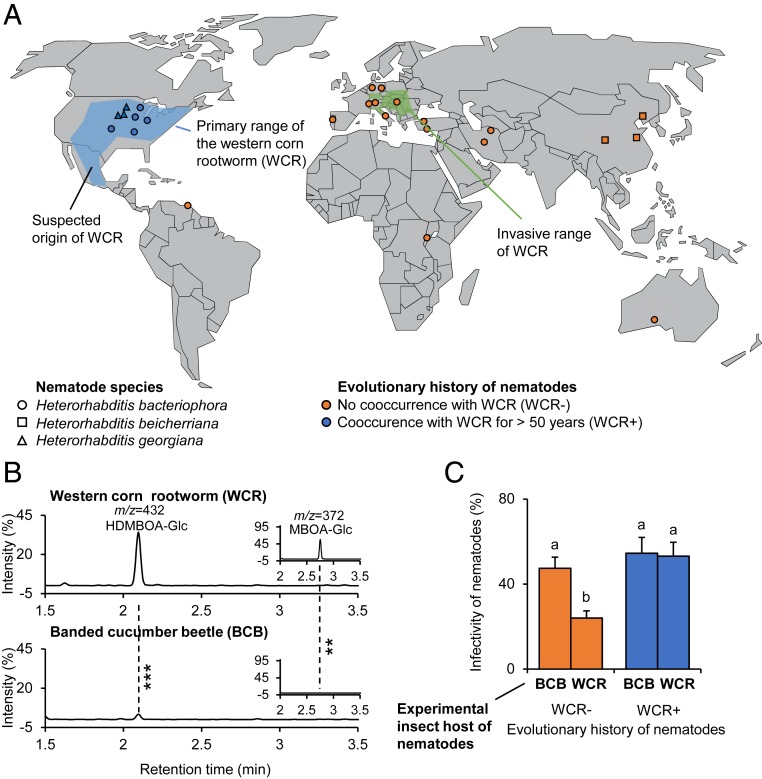
To test whether entomopathogenic nematodes that share an evolutionary history with the WCR may be able to resist benzoxazinoids, we established a global collection of 25 Heterorhabditis spp. strains, including strains collected from regions in which the WCR has been present for more than 50 y (henceforth called the primary range) and strains collected from other regions of the world in which the WCR is not present or has not been present until recently (Fig. 1A and SI Appendix, Table S1). Nematodes from the primary range were isolated from maize fields in which the WCR is present. They are thus likely to have encountered this herbivore in the past. Heterorhabditis bacteriophora has a broad host range, and the strains may also have infected other root herbivores occurring in maize fields, including wireworms and other rootworm species. Benzoxazinoids and their breakdown products can be found in the midgut of a wide range of insect herbivores (31, 32), but the WCR is the only herbivore known to selectively accumulate benzoxazinoids in its hemolymph (28). Nematode strains from other parts of the world never encountered the WCR, as they came from regions where the rootworm is not present, or they were isolated before the WCR invaded these regions (SI Appendix, Table S1).
Fig. 1.
Entomopathogenic nematodes from the primary range of the benzoxazinoid-sequestering WCR are more infective toward the WCR than the nonsequestering BCB. (A) World map showing the origin of the collected entomopathogenic nematode strains together with the primary and invasive ranges of the WCR, a specialized maize herbivore (WCR; data from 2012). Note that nematode strains from the invasive range do not share any evolutionary history with WCR, as they were collected before invasion. For detailed information about the different strains, refer to SI Appendix, Table S1. (B) Chromatograms of plant-derived benzoxazinoids in the body of WCR (Top) and the BCB (Bottom), a generalist root herbivore which does not sequester benzoxazinoids and is mainly present in Central America, Mexico, and the Southern United States, outside of the nematode sampling range. Asterisks indicate significant differences between herbivore species (**P < 0.01, ***P < 0.001). For quantitative comparisons, refer to SI Appendix, Fig. S1. (C) Infectivity of nematodes toward WCR and BCB. Infectivity is shown for nematodes with an evolutionary history with WCR of more than 50 y (blue) and nematodes without evolutionary history with WCR (orange). EMMeans and SEs derived from statistical models (Dataset S1) are shown. Different letters indicate significant differences between treatments (false discovery rate corrected P < 0.05).
Using internal transcribed spacer rRNA gene sequencing, 19 nematode strains within our collection were confirmed to be H. bacteriophora. Three strains from China were reclassified as Heterorhabditis beicheriana, and 3 strains from the United States were identified as Heterorhabditis georgiana, both of which are closely related to H. bacteriophora. In a first experiment, we compared the infectiveness of the different nematode strains toward larvae of the WCR and larvae of the banded cucumber beetle (BCB; Diabrotica balteata). In contrast to the WCR, the BCB feeds on many different plant species apart from maize and does not sequester benzoxazinoids (Fig. 1B and SI Appendix, Fig. S1) (28). The BCB occurs mainly in Central America, Mexico, and the Southern United States, outside of our collection range. Thus, none of the tested nematode strains are likely to share an evolutionary history with this herbivore.
Infectivity tests revealed a significant interaction between the host herbivore species and the evolutionary history of the nematodes (Fig. 1C and Dataset S1). Nematode strains from the primary range of the WCR were able to infect and kill the WCR and the BCB equally well. By contrast, the infectivity of nematodes from outside the primary range was significantly lower for the WCR than the BCB (Fig. 1C). Thus, nematodes that evolved in the presence of the WCR have an increased capacity to infect and kill this specific herbivore. This pattern was similar when only strains belonging to H. bacteriophora were considered (SI Appendix, Fig. S2). The infectivity of H. georgiana strains, all of which come from the primary range of the WCR, was higher toward the WCR than the infectivity of H. beicheriana strains, which come from Asia where the WCR is not present (SI Appendix, Fig. S2). Individual nematode strains varied in their capacity to infect the WCR and the BCB, suggesting that factors other than the potential evolutionary history with the WCR may also influence the evolution of infectivity.
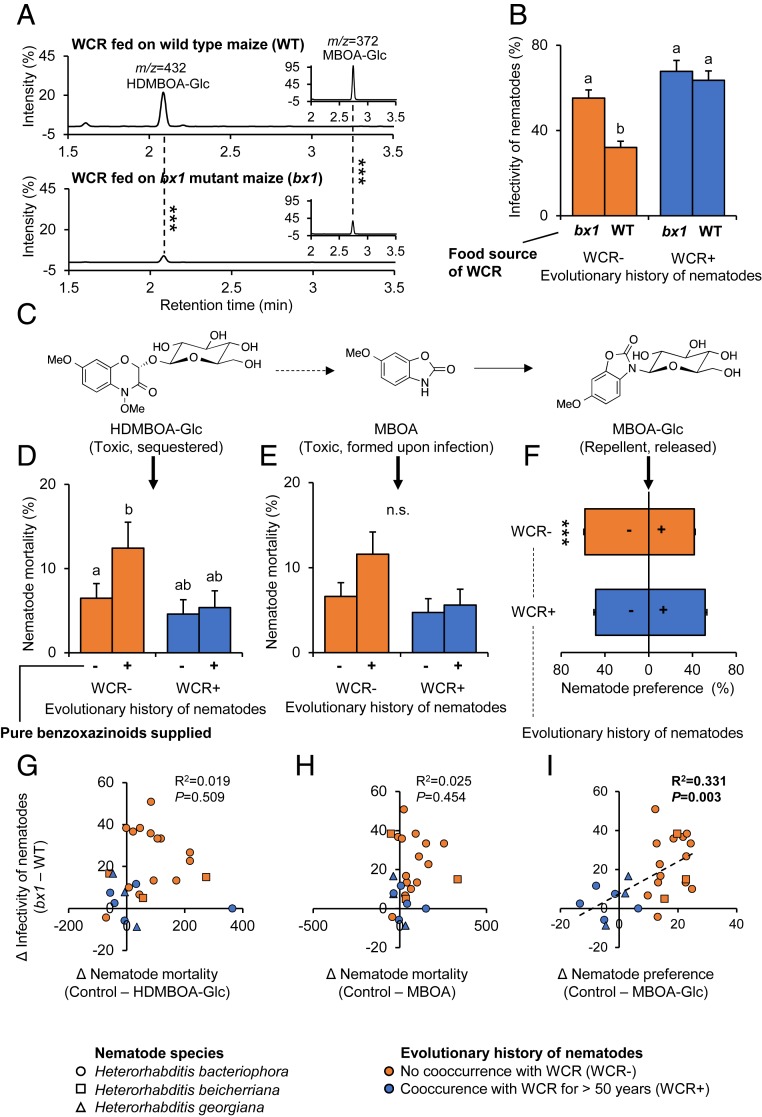
The BCB and WCR differ in many traits apart from benzoxazinoids that may explain the pattern observed in Fig. 1. To specifically test for the role of benzoxazinoids in determining the higher infectivity of nematode strains from the primary range of the WCR, we exposed the different nematode strains to WCR larvae that previously fed on wild-type (WT) or benzoxazinoid-deficient bx1 maize mutant plants. Bx1-mutant fed WCR larvae accumulate only low amounts of benzoxazinoids (Fig. 2A and SI Appendix, Fig. S3) (28). Nematodes from the primary range were able to infect WCR larvae equally well, independently of whether the larvae fed on benzoxazinoid-containing WT or benzoxazinoid-deficient bx1 mutant maize roots (Fig. 2B). By contrast, nematodes from other parts of the world suffered from a suppression of infectivity when exposed to larvae fed on WT plants compared to bx1 mutant fed larvae (Fig. 2B). This pattern was largely consistent across strains (SI Appendix, Fig. S4). The benzoxazinoid susceptibility of the different nematode strains (i.e., the difference in infectivity toward WT and bx1-fed WCR larvae) was negatively correlated to their infectivity toward the WCR (as measured in the previous experiment; Fig. 2) but not correlated to their infectivity toward the BCB (SI Appendix, Fig. S5). Thus, nematodes from the primary range are less susceptible to the benzoxazinoid-dependent defenses of the WCR than nematodes from other parts of the world, and this trait is associated with higher infectivity toward the WCR.
Fig. 2.
Nematodes from the primary range of the WCR are more resistant to sequestered benzoxazinoids. (A) Chromatograms of plant-derived benzoxazinoids in the body of WCR larvae fed on WT (Top) and benzoxazinoid-deficient bx1 mutant maize plants (Bottom). Asterisks indicate significant differences (***P < 0.001). For quantitative comparisons, refer to SI Appendix, Fig. S3. (B) Infectivity of nematodes that share an evolutionary history with the WCR (WCR+; blue) or not (WCR−; orange) toward WCR larvae fed on WT or bx1 mutant plants. Different letters indicate significant differences between treatments (false discovery rate corrected P < 0.05). (C) Benzoxazinoids found in WCR larvae. Two-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one O-glucoside (HDMBOA-Glc) accumulates in the larval body and is toxic for nematodes. Six-methoxy-2-benzoxazolinone (MBOA) is formed upon tissue disruption and nematode attack and is also toxic. Six-methoxy-2-benzoxazolinone N-glucoside (MBOA-Glc) is released by the larvae. It is not directly toxic but repels the nematodes. (D and E) Impact of physiologically relevant doses of HDMBOA-Glc (150 µg/mL) and MBOA (25 µg/mL) on nematode mortality. Different letters indicate significant differences between treatments (false discovery rate corrected P < 0.05). (F) Impact of physiological doses of MBOA-Glc (3 µg/mL) on nematode attraction. Asterisks indicate a significant effect of MBOA-Glc (***P < 0.001). EMMeans and SEs derived from statistical models (Dataset S1) are shown. (G–I) Linear correlations between benzoxazinoid dependent infectivity (data from B) and in vitro benzoxazinoid resistance (data from D–F). R2 and P values of linear regressions are shown. Dashed regression lines are shown for significant linear correlations. n.s., not significant.
Benzoxazinoids can protect the WCR from nematodes through a series of different, mutually nonexclusive mechanisms (Fig. 2C) (28). Six-methoxy-2-benzoxazolinone N-glucoside (MBOA-Glc) is an insect-specific conjugate formed from benzoxazinoid breakdown products that is released by the larvae and accumulates on their cuticule, thus making them less attractive to entomopathogenic nematodes. Two-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one O-glucoside (HDMBOA-Glc) is contained within the larval body and is directly toxic to the nematodes (28). Upon nematode infection, HDMBOA-Glc is broken down to 6-methoxy-2-benzoxazolinone (MBOA), which also reduces nematode survival (28). In choice experiments, MBOA-Glc reduced the attraction of nematodes that share no evolutionary history with the WCR, while nematodes from the primary range did not show a negative response toward MBOA-Glc (Fig. 2F and SI Appendix, Fig. S6). Physiological doses of HDMBOA-Glc induced mortality in strains that shared no evolutionary history with the WCR but not in strains from the primary range (Fig. 2D). No clear effects were found for MBOA-induced mortality (Fig. 2E). Simple linear regression analysis revealed a positive correlation between the avoidance of MBOA-Glc and the benzoxazinoid-specific suppression of nematode infectivity (Fig. 2I). Model selection applied on a multiple linear regression including all explanatory factors and their interactions resulted in a model with the behavioral response toward MBOA-Glc alone explaining 61% of benzoxazinoid-dependent nematode infectivity across the different strains (Dataset S1). Taken together, these results show that nematodes from the primary range of the WCR do not avoid an insect-specific benzoxazinoid metabolite and are more resistant to sequestered benzoxazinoids. Furthermore, the loss of behavioral avoidance of MBOA-Glc is associated with an increased capacity to infect benzoxazinoid-sequestering WCR larvae.
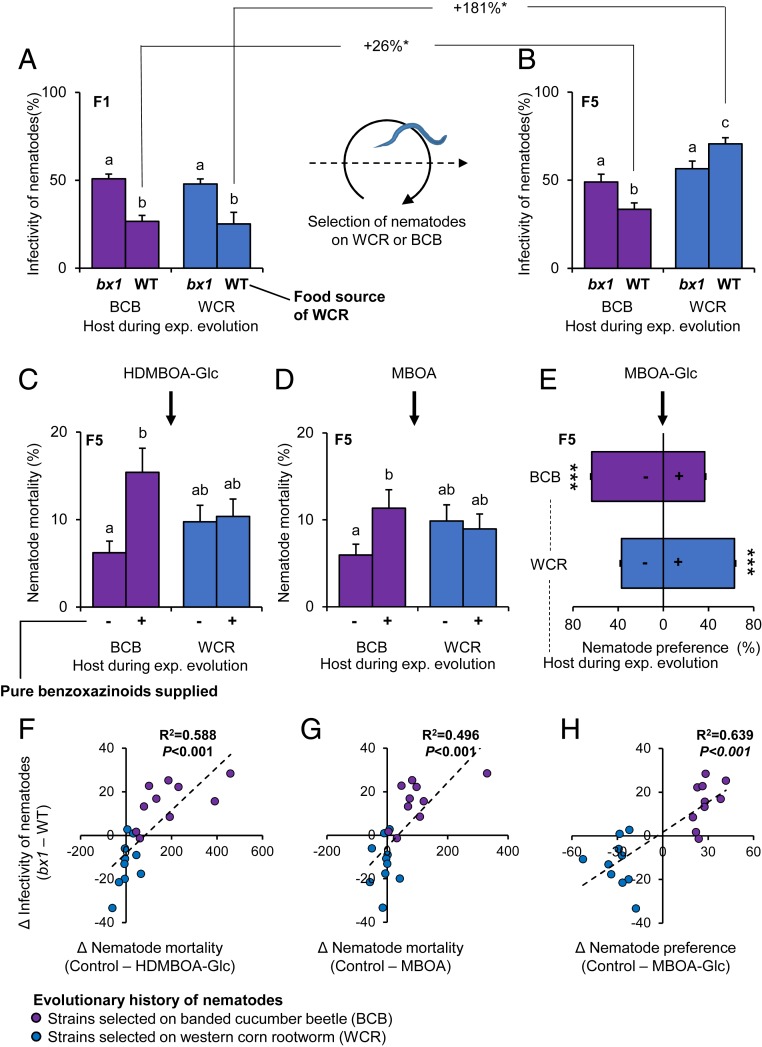
Benzoxazinoid tolerance in the nematode strains from maize fields of the primary range of the WCR may be confounded by population structure. Furthermore, their benzoxazinoid resistance may stem from exposure to benzoxazinoids exuded by maize roots rather than benzoxazinoids that are sequestered by the WCR. To test whether exposure to a benzoxazinoid-sequestering host can directly lead to the evolution of benzoxazinoid resistance, we designed a real-time evolution experiment. The benzoxazinoid-susceptible H. bacteriophora strain RW14 was multiplied and divided into 20 experimental (sub)populations. Ten populations were then reared on BCB larvae, and 10 populations were reared on WCR larvae, both of which were fed on WT maize roots. Using these 2 herbivore species allowed us to assess evolution on 2 natural hosts with different abilities to sequester benzoxazinoids. Subsequent infectivity tests were performed with WT and bx1 fed WCR larvae to specifically assess the evolution of benzoxazinoid resistance following exposure to the different herbivores. In the F1 generation, all experimental nematode populations showed reduced infectiveness toward WT-fed WCR larvae compared to bx1-fed larvae (Fig. 3A and SI Appendix, Fig. S7). After 5 generations of selection, nematodes reared on BCB larvae still showed reduced infectivity toward WT-fed WCR larvae, even though the effect was less strong than in the F1 generation (Fig. 3B and SI Appendix, Fig. S8). By contrast, nematodes reared on the WCR were overall better able to infect the WCR and no longer show a reduction in infectivity on WT-fed larvae compared to bx1-fed larvae (Fig. 3B and SI Appendix, Fig. S8). The nematodes were even more successful on WT-fed WCR larvae than on bx1 mutant-fed larvae (Fig. 3B). Thus, selection on a benzoxazinoid-sequestering host over 5 generations is sufficient for susceptible nematodes to evolve complete resistance to benzoxazinoid-dependent defenses.
Fig. 3.
Rapid evolution of benzoxazinoid resistance and infectivity in nematodes exposed to the WCR. (A) Infectivity of F1 nematodes reared on the WCR (WCR+; blue) or the BCB (purple) for 1 generation, exposed to WCR larvae fed on WT or bx1 mutant plants. Different letters indicate significant differences between treatments (false discovery rate corrected least square means, P < 0.05). (B) Infectivity of F5 nematodes reared on the WCR (WCR+; blue) or the BCB (purple) for 5 generations and exposed to WCR larvae fed on WT or bx1 mutant plants. Different letters indicate significant differences between treatments (false discovery rate corrected P < 0.05). Significant changes (*P < 0.05) in infectivity between F1 and F5 nematodes are indicated by asterisks. (C and D) Impact of physiologically relevant doses of HDMBOA-Glc (150 µg/mL) and MBOA (25 µg/mL) on nematode mortality. Different letters indicate significant differences between treatments (false discovery rate corrected P < 0.05). (E) Impact of physiological doses of MBOA-Glc (3 µg/mL) on nematode attraction. Asterisks indicate a significant effect of MBOA-Glc (P < 0.001). EMMeans and SEs derived from statistical models (Dataset S1) are shown. (F–H) Linear correlations between benzoxazinoid dependent infectivity (data from B) and in vitro benzoxazinoid resistance (data from C–E). R2 and P values of linear regressions are shown. Dashed regression lines are drawn for significant correlations.
To understand how the WCR-selected nematode populations may achieve higher infectiveness toward benzoxazinoid-containing WCR larvae, we subjected them to the same series of bioassays as the natural strain collection before (Fig. 3 C–E). Nematodes selected on BCB larvae avoided MBOA-Glc, while nematodes selected on WCR larvae were attracted by this compound (Fig. 3E and SI Appendix, Fig. S9). Furthermore, HDMBOA-Glc and MBOA reduced the survival of nematodes selected on the BCB but did not reduce survival of nematodes selected on the WCR (Fig. 3 C and D and SI Appendix, Fig. S9). Simple linear regression analysis revealed a correlation between the behavioral response to MBOA-Glc and benzoxazinoid-specific nematode infectivity as well as HDMBOA-Glc and MBOA toxicity and benzoxazinoid-specific nematode infectivity (Fig. 3 F–H). Multiple linear regression including all explanatory factors and their interactions, followed by model selection and averaging, resulted in a model with MBOA-Glc repellence and HDMBOA-Glc toxicity explaining 82% of the benzoxazinoid-dependent nematode infectivity across strains (Dataset S1). Thus, similar to the pattern observed for the natural strains, nematodes exposed to the WCR for 5 generations do no longer avoid an insect-specific benzoxazinoid breakdown product and become resistant to a sequestered benzoxazinoid and its breakdown product. Furthermore, their increased capacity to infect benzoxazinoid-sequestering WCR larvae is associated with both a loss of behavioral aversion and reduced benzoxazinoid toxicity.
Discussion
Herbivore natural enemies are often exposed to plant defense metabolites, either by coming into contact with plants directly or by preying on herbivores that contain plant defenses (33–36). Many herbivore natural enemies have been found to avoid plant defenses by rejecting herbivores that accumulate or sequester toxins (37–39). However, avoidance comes with significant costs, especially when mobility and host availability are limited. Soil-borne herbivore natural enemies such as entomopathogenic nematodes can typically only cover short distances (40) and may have a limited choice of hosts in agricultural environments. Thus, they are likely to be under considerable pressure to overcome, rather than to avoid, plant toxins, which may explain why nematodes that share an evolutionary history with the WCR show benzoxazinoid resistance in the form of a loss of behavioral aversion. Together with their increased capacity to withstand the toxic effects of benzoxazinoids, this behavioral shift likely allows the nematodes to prey successfully on benzoxazinoid-sequestering hosts such as the WCR. Correlation analysis suggests that behavioral responses to benzoxazinoids may be more important for benzoxazinoid-dependent nematode infectivity than metabolic resistance. Further experiments will be required to test this hypothesis. The exact contribution of the WCR to nematode evolution in the field relative to other soil-borne organisms that are commonly present in US maize fields and may modulate benzoxazinoid exposure also requires further study. Given the dominance of the WCR in maize fields in the areas from which the benzoxazinoid-resistant nematodes were sampled, however, we consider it likely that their resistance traits originate from the selection pressure exerted by the WCR.
The real-time evolution experiment further demonstrates that benzoxazinoid resistance in nematodes can evolve rapidly following exposure to the WCR. Entomopathogenic nematodes can also evolve higher abiotic stress tolerance and attraction to plant volatiles within a few generations when exposed to appropriate selection regimes (41, 42), suggesting that they possess mechanisms that allow them to adapt rapidly to their environment. To what extent this rapid evolution is driven by standing genetic variation, epigenetic effects, or changes in microbial communities, including changes in the bacterial symbionts of the nematodes (43, 44), remains to be investigated. The finding that nematodes selected on the BCB also become slightly less susceptible to benzoxazinoid-dependent defenses is noteworthy in the context of the current experiments as it may indicate that plant defense resistance in herbivore natural enemies can also evolve in the absence of a defense-sequestering herbivore, for instance, through exposure to residual plant defense levels in the soil as well as the gut and the frass of the herbivore. Surprisingly, nematodes that evolved with the WCR became even more infective on WT- than bx1 mutant-fed WCR larvae. This phenomenon is associated with a behavioral shift, resulting in nematodes being attracted to MBOA-Glc that is released by the WCR. It is possible that this change in behavior may be responsible for the observed increase in infectivity.
Whether evolved resistance to plant toxins is common in herbivore natural enemies remains to be determined. Given that exposure of natural enemies to plant toxins is frequent in nature (33–36) and that different natural enemies have been reported to resist and accumulate plant toxins (18–21), we expect evolved behavioral avoidance, metabolic resistance, and tolerance strategies to be widespread among members of the third trophic level. Host diversity and diet breadth will likely determine the prevalence and biochemical architecture of these traits in herbivore natural enemies (20).
The diversity of plant defense metabolites and arthropod herbivores in nature is thought to be the result of an ongoing coevolutionary arms race (45–49). Herbivore natural enemies have been shown to be negatively affected by plant defense metabolites in this context (50–52). The possibility that herbivore natural enemies may evolve resistance to increasingly toxic herbivores been considered (17, 18) but, to the best of our knowledge, not been addressed through manipulative experiments. Instead, adaptations of herbivore natural enemies to plant chemicals have been investigated in detail in the context of plant volatiles that serve as foraging cues for herbivore natural enemies (52–54), including volatiles that attracted entomopathogenic nematodes (55), and in the context of extrafloral nectar as food source (54, 56). Although conducted in an agricultural system, the present study does support an evolutionary link between the coevolutionary arms race of plants and herbivores and the third trophic level by showing that plant defense metabolites may influence the evolution of herbivore natural enemies as they are transferred through adapted herbivores. Trophic transfer of defense metabolites may promote the specialization and diversification of herbivore natural enemies (50). In wild systems, resistance to plant defenses by herbivore natural enemies may reduce the penalty for plants facing adapted herbivores (17), which again may reduce the negative selection pressure on basal plant defense metabolites and thereby contribute to within-plant chemical diversity. Detailed mechanistic and evolutionary studies, including broad phylogenetic analyses of multitrophic interaction networks in natural systems, could help to shed further light on these hypotheses.
Reducing the use of synthetic pesticides is an important aim in sustainable agriculture. The use of herbivore natural enemies as biological control agents is a promising strategy in this context (57). However, the efficacy of biocontrol agents is often limited, and a better understanding of the factors that determine their success is thus important to improve their use (58). Our study confirms that plant toxins can limit the capacity of natural enemies to control agricultural pests (17, 28) but also shows that plant toxin susceptibility shows pronounced heritable variation and can be offset rapidly through artificial selection. Plant toxin resistance thus represents a promising potential breeding target for the improvement of biological control agents.
Materials and Methods
Insects.
WCR (D. virgifera virgifera LeConte) eggs were supplied by the North Central Agricultural Research Laboratory, US Department of Agriculture, Agriculture Research Service, Brookings, SD. BCB (D. balteata LeConte) eggs were obtained from Syngenta Crop Protection AG. After hatching, WCR and BCB neonates were reared on freshly germinated WT (B73) maize seeds or on the benzoxazinoid mutant line bx1 (59). Third instar WCR and BCB larvae were used for all experiments. Galleria mellonella larvae were bought from Fischereibedarf Wenger and maintained at 8 °C until use for nematode rearing.
Nematodes.
Nematode collection, identification, and rearing.
We established a collection of 25 different Heterorhabditis spp. Detailed information on the different strains can be found in SI Appendix, Table S1. Strains were either obtained from collaborators or collected from the field. Field collections were realized by collecting 45 soil cores (10 cm depth, 2 cm diameter) for each individual location. Soil cores were pooled for each location, homogenized, and separated into 20 plastic containers (250 mL; Semadeni AG). Five G. mellonella larvae were then placed on the soil surface of each container. The containers were closed with a plastic lid and incubated in darkness under 24 ± 2 °C. Five to 10 d later, all nematode-infected G. mellonella larvae were individually transferred to white traps (60), and emerging nematodes were used to infect another set of G. mellonella larvae (61). Irrespective of whether they were isolated from the field or obtained from collaborators, all nematodes were identified by internal transcribed spacer rRNA gene sequencing as described previously (44, 62, 63). The collected nematode progeny was maintained in 250 mL flasks (Greiner Bio-One GmbH) at a density of 1 EPN per microliter tap water at 10 °C. Nematode strains were refreshed by multiplying them on G. mellonella larvae every 2 to 3 mo. Nematodes that were less than 1 mo old were used for all experiments.
Benzoxazinoid Analyses.
Benzoxazinoids from BCB larvae and WCR larvae fed on B73 or bx1 maize plants were extracted in 50% MeOH + 50% H2O+ 0.5% formic acid. Five flash frozen larvae (∼40 mg) were pooled and ground in 400 µL extraction buffer, and 5 replicates (each consisting of a pool of 5 larvae) were analyzed for each species and food source. The extracts were vortexed for 1 min and centrifuged twice at 17,000 g, at 4 °C. The supernatants were then analyzed on an Acquity UHPLC-MS system equipped with an electrospray source (Waters i-Class UHPLC-QDA). The method was modified from the one described previously (10). Briefly, the elution profile was 0 to 3.5 min, 99 to 72.5% A in B; 3.5 to 5.5 min, 100% B; 5.5 to 7.5 min, 99% A in B. The injection volume was 1 µL. DIMBOA, MBOA, DIMBOA-Glc, HDMBOA-Glc, and MBOA-Glc were all quantified in electrospray ionization in negative mode (ESI-) using selective ion recording and external standard curves.
Real-Time Evolution.
An experimental selection experiment was carried out using the RW14 nematode strain. A batch of newly hatched RW14 nematodes was aliquoted into 20 subpopulations, each consisting of 20,000 nematodes. Half the subpopulations were then reared on WCR larvae, and the other half were reared on BCB larvae. Infection was performed in Solo cups as described below in Nematode Infectivity. For each population, 30 Solo cups containing 5 larvae each were infected with 500 nematodes. Five days later, all of the infected larvae of the same subpopulation were collected together and transferred to white traps for collecting nematode progenies. In between each generation of selection, the populations were amplified in G. mellonella larvae by infecting 15 larvae per population with 200 nematodes each. The different populations were selected on WCR and BCB larvae for a total of 10 generations (5 generations within the selection host and 5 amplification steps in between), and F1 and F5 nematodes (referring to the number of generations on the selection host) of 10 independently selected subpopulations were phenotyped.
Nematode Infectivity.
To quantify the infectivity of the nematodes, 3 to 5 WCR or BCB larvae were placed into individual Solo cups (30 mL; Frontier Scientific Services, Inc.) containing a 5 mm layer of moist, autoclaved sand (Selmaterra, Bigler Samen AG). Five hundred nematodes in 500 µL tap water were applied into each Solo cup. After incubating the cups at 28 ± 0.5 °C for 5 d, the percentage of nematode-infected larvae in each Solo cup was determined. The infection status of the larvae was assessed visually. Nematode-infected larvae show a characteristic red/orange color, which was used as a marker of infection. Larvae were reared on either WT (B73) or bx1 mutant plants. Each Solo cup was treated as an independent replicate. The exact numbers of independent biological replicates for the individual experiments and treatments are provided in SI Appendix, Table S2.
Nematode Behavior.
To test the effect of MBOA-Glc on nematode behavior, we used the approach developed earlier (28). Briefly, a 5-mm agarose (5 g/L; Sigma Aldrich Chemie, Switzerland) layer was poured into a Petri dish (9 cm diameter; Greiner Bio-One GmbH). Three 5-mm-diameter wells were created equidistantly in the agar layer: 1 in the center of the plate, 1 on the right, and 1 on the left side of the plate. One hundred nematodes in 100 µL of water were dispensed the central well. The remaining 2 wells were filled either with 50 µL BCB exudates and 50 µL tap water or with 50 µL BCB exudates + 50 µL MBOA-Glc (3 ng/µL in tap water). BCB exudates were used to elicit nematode search behavior. Exudates were obtained by rinsing third instar BCB larva with 50 µL tap water. Nematode preference was recorded 24 h after the start of the experiment by counting the number of nematodes within different sectors of the Petri dishes. The dishes were divided into 4 sectors of equal size, with 2 opposite sectors containing the treatment wells. Nematodes within the treatment sectors were counted. Each Petri dish was treated as independent biological replicates, and 18 to 20 replicates were carried out for the different experiments and treatments (SI Appendix, Table S2).
Nematode Performance.
The effects of benzoxazinoids on nematode survival were tested as described (28). Briefly, 4,000 nematodes were incubated in 4 mL tap water containing either MBOA (25 µg/mL) or HDMBOA-Glc (150 µg/mL). These concentrations represent physiologically relevant doses (28). Nematodes were kept in 50 mL flasks (Greiner Bio-One GmbH) and incubated at 28 °C. Nematodes incubated in tap water were used as controls. The number of dead and living nematodes was recorded 7 d after incubation. Flasks were treated as independent biological replicates, and 8 to 10 replicates were carried out for the different experiments and treatments (SI Appendix, Table S2).
Statistical Analyses.
Generalized linear mixed models (distribution, binomial; link function, logit) were used to analyze mortality, infectivity, and preference bioassays. Effects of nematode origin/experimental evolution host, different treatments, and their interactions were used as main factors, and nematode strains/populations were used as a random factor. Wald tests were performed to assess significance of treatment effects. Pairwise comparisons of estimated marginal means (EMMeans) corrected by the false discovery rate method were used as post hoc tests (64). To determine correlations between benzoxazinoid resistance traits and benzoxazinoid dependent infectivity of the different nematode strains and populations the delta between BX+ (WT-fed WCR larvae) and BX- (bx1 mutant-fed larvae) infectivity (i.e., benzoxazinoid-dependent infectivity) was calculated based on EMMeans. Then, different types of benzoxazinoid resistance were determined for each strain by calculating the respective deltas as well. Linear models were then employed using benzoxazinoid-dependent infectivity as response variable and the different resistance deltas as explanatory variables. A complementary approach to test for multiple explanatory variables and their interactions was executed using model selection on a multiple linear regression including all resistance deltas and their interactions as explanatory variables. The Aikake information criterion corrected for small sample sizes (AICc) was used to rank submodels. Models showing a delta-AICc ≤ 2 were combined using a model averaging procedure, which allows computing the relative importance of each resistance delta in the averaged model. The packages used for the different analyses were RVAideMemoire, car, emmeans, MASS, lme4, lmerTest and MuMIn (65). All of the analyses were conducted in R 3.5.1.
Data and Materials Availability.
Some of the nematode strains were obtained under a materials transfer agreement. The data generated for this manuscript are available on Dryad, https://doi.org/10.5061/dryad.nk98sf7pf. R codes and results of statistical analyses are available in the form of an R markdown file (Dataset S1).
Supplementary Material
Acknowledgments
We thank the following scientists and institutions for sharing nematode strains: Ralf-Udo Ehlers (e-nema GmbH, Germany), Raquel Campos-Herrera (Instituto de Ciencias de la Vid y del Vino, Universidad de La Rioja, La Rioja, Spain), Stefan Toepfer (Plant Protection and Soil Conservation Directorate, CABI International, Hódmezővásárhely, Hungary), Alper Susurluk (Department of Plant Protection, Uludag University, Bursa, Turkey), Selcuk Hazir (Department of Biology, Adnan Menderes University, Aydin, Turkey), Javad Karimi (Biocontrol and Insect Pathology Laboratory, Department of Plant Protection, School of Agriculture, Ferdowsi University of Mashhad, Mashhad, Iran), David Clarke (School of Microbiology, University College Cork, Cork, Ireland), and Juan Ma (Institute of Plant Protection, Hebei Academy of Agricultural and Forestry Science/Integrated Pest Management Centre of Hebei Province, Baoding, PR China). We thank Fausto Prada, Lisa Thönen, Virginia Hill, Anja Boss, and Wei Huang for their assistance with laboratory experiments; David Ermacora for nematode rearing; and Anouk Guyer, Zixiao Zhao, and various interns from the B.E.H. laboratory for field assistance. We also thank Lance Meinke (University of Nebraska), Joe Spencer (Illinois Natural History Survey), Sarah Zukoff (Kansas State University), Ken Ostlie (University of Minnesota), and Billy Fuller and Brad McMannus (South Dakota State University) for identifying fields for nematode collection. This project was supported by the Swiss National Science Foundation (Grants 155781, 160786, and 157884) and by the University of Bern through the Interfaculty Research Cooperation One Health.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1912599116/-/DCSupplemental.
References
- 1.Bar-On Y. M., Phillips R., Milo R., The biomass distribution on Earth. Proc. Natl. Acad. Sci. U.S.A. 115, 6506–6511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson G. H., Frederick E. S., Lawrence B. S., Community structure, population control, and competition. Am. Nat. 94, 421–425 (1960). [Google Scholar]
- 3.Vidal M. C., Murphy S. M., Bottom-up vs. top-down effects on terrestrial insect herbivores: A meta-analysis. Ecol. Lett. 21, 138–150 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Farmer E. E., Ed., English, Leaf Defence (Oxford University Press, Oxford, 2016). [Google Scholar]
- 5.Wittstock U., Gershenzon J., Constitutive plant toxins and their role in defense against herbivores and pathogens. Curr. Opin. Plant Biol. 5, 300–307 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Stahl E., Hilfiker O., Reymond P., Plant-arthropod interactions: Who is the winner? Plant J. 93, 703–728 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Schuman M. C., Baldwin I. T., The layers of plant responses to insect herbivores. Annu. Rev. Entomol. 61, 373–394 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Karban R., Agrawal A. A., Herbivore offense. Annu. Rev. Ecol. Syst. 33, 641–664 (2002). [Google Scholar]
- 9.Heckel D. G., “Insect detoxification and sequestration strategies” in Annual Plant Reviews Volume 47: Insect-Plant Interactions, Voelckel C., Jander G., Eds. (Wiley Blackwell, Chichester, UK, 2014), pp. 77–114. [Google Scholar]
- 10.Petschenka G., Agrawal A. A., How herbivores coopt plant defenses: Natural selection, specialization, and sequestration. Curr. Opin. Insect Sci. 14, 17–24 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Erb M., Robert C. A. M., Sequestration of plant secondary metabolites by insect herbivores: Molecular mechanisms and ecological consequences. Curr. Opin. Insect Sci. 14, 8–11 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Nishida R., Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 47, 57–92 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Opitz S. E. W., Müller C., Plant chemistry and insect sequestration. Chemoecology 19, 117–154 (2009). [Google Scholar]
- 14.Johnson K. S., Comparative detoxification of plant (Magnolia virginiana) allelochemicals by generalist and specialist Saturniid silkmoths. J. Chem. Ecol. 25, 253–269 (1999). [Google Scholar]
- 15.Kumar P., Rathi P., Schöttner M., Baldwin I. T., Pandit S., Differences in nicotine metabolism of two Nicotiana attenuata herbivores render them differentially susceptible to a common native predator. PLoS One 9, e95982 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ode P. J., Plant toxins and parasitoid trophic ecology. Curr. Opin. Insect Sci. 32, 118–123 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Ode P. J., Plant chemistry and natural enemy fitness: Effects on herbivore and natural enemy interactions. Annu. Rev. Entomol. 51, 163–185 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Fink L. S., Brower L. P., Birds can overcome the cardenolide defence of monarch butterflies in Mexico. Nature 291, 67–70 (1981). [Google Scholar]
- 19.Barbosa P., et al. , Plant allelochemicals and insect parasitoids effects of nicotine on Cotesia congregata (say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae). J. Chem. Ecol. 12, 1319–1328 (1986). [DOI] [PubMed] [Google Scholar]
- 20.Reudler J. H., Biere A., Harvey J. A., van Nouhuys S., Differential performance of a specialist and two generalist herbivores and their parasitoids on Plantago lanceolata. J. Chem. Ecol. 37, 765–778 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasteels J. M., Chemical defence, offence and alliance in ants–aphids–ladybirds relationships. Popul. Ecol. 49, 5–14 (2007). [Google Scholar]
- 22.Frey M., Schullehner K., Dick R., Fiesselmann A., Gierl A., Benzoxazinoid biosynthesis, a model for evolution of secondary metabolic pathways in plants. Phytochemistry 70, 1645–1651 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Wouters F. C., Blanchette B., Gershenzon J., Vassão D. G., Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 15, 1127–1151 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B., et al. , Convergent evolution of a metabolic switch between aphid and caterpillar resistance in cereals. Sci. Adv. 4, eaat6797 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu L., et al. , Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9, 2738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robert C. A. M., et al. , A specialist root herbivore exploits defensive metabolites to locate nutritious tissues. Ecol. Lett. 15, 55–64 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Hu L., et al. , Plant iron acquisition strategy exploited by an insect herbivore. Science 361, 694–697 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Robert C. A. M., et al. , Sequestration and activation of plant toxins protect the western corn rootworm from enemies at multiple trophic levels. eLife 6, e29307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurtz B., Toepfer S., Ehlers R. U., Kuhlmann U., Assessment of establishment and persistence of entomopathogenic nematodes for biological control of western corn rootworm. J. Appl. Entomol. 131, 420–425 (2007). [Google Scholar]
- 30.Gray M. E., Sappington T. W., Miller N. J., Moeser J., Bohn M. O., Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annu. Rev. Entomol. 54, 303–321 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Glauser G., et al. , Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J. 68, 901–911 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Maag D., et al. , 3-β-D-Glucopyranosyl-6-methoxy-2-benzoxazolinone (MBOA-N-Glc) is an insect detoxification product of maize 1,4-benzoxazin-3-ones. Phytochemistry 102, 97–105 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Ugine T. A., Krasnoff S. B., Grebenok R. J., Behmer S. T., Losey J. E., Prey nutrient content creates omnivores out of predators. Ecol. Lett. 22, 275–283 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Coll M., Guershon M., Omnivory in terrestrial arthropods: Mixing plant and prey diets. Annu. Rev. Entomol. 47, 267–297 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Zemenick A. T., Kula R. R., Russo L., Tooker J. A., A network approach reveals parasitoid wasps to be generalized nectar foragers. Arthropod-Plant Interact. 13, 239–251 (2019). [Google Scholar]
- 36.Calvert W. H., Hedrick L. E., Brower L. P., Mortality of the monarch butterfly (Danaus plexippus L.): Avian predation at five overwintering sites in Mexico. Science 204, 847–851 (1979). [DOI] [PubMed] [Google Scholar]
- 37.Skelhorn J., Rowe C., Avian predators taste-reject aposematic prey on the basis of their chemical defence. Biol. Lett. 2, 348–350 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar P., Pandit S. S., Steppuhn A., Baldwin I. T., Natural history-driven, plant-mediated RNAi-based study reveals CYP6B46’s role in a nicotine-mediated antipredator herbivore defense. Proc. Natl. Acad. Sci. U.S.A. 111, 1245–1252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye M., et al. , An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci. Adv. 4, eaar4767 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaya H. K., Gaugler R., Entomopathogenic nematodes. Annu. Rev. Entomol. 38, 181–206 (1993). [Google Scholar]
- 41.Hiltpold I., Baroni M., Toepfer S., Kuhlmann U., Turlings T. C. J., Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J. Exp. Biol. 213, 2417–2423 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Mukuka J., Strauch O., Hoppe C., Ehlers R.-U., Improvement of heat and desiccation tolerance in Heterorhabditis bacteriophora through cross-breeding of tolerant strains and successive genetic selection. BioControl 55, 511–521 (2010). [Google Scholar]
- 43.Bilgrami A. L., Gaugler R., Shapiro-Ilan D. I., Adams B. J., Source of trait deterioration in entomopathogenic nematodes Heterorhabditis bacteriophora and Steinernema carpocapsae during in vivo culture. Nematology 8, 397–409 (2006). [Google Scholar]
- 44.Machado R. A. R., et al. , Whole-genome-based revisit of Photorhabdus phylogeny: Proposal for the elevation of most Photorhabdus subspecies to the species level and description of one novel species Photorhabdus bodei sp. nov., and one novel subspecies Photorhabdus laumondii subsp. clarkei subsp. nov. Int. J. Syst. Evol. Microbiol. 68, 2664–2681 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Züst T., et al. , Natural enemies drive geographic variation in plant defenses. Science 338, 116–119 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Agrawal A. A., Hastings A. P., Johnson M. T. J., Maron J. L., Salminen J. P., Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 338, 113–116 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Futuyma D. J., Agrawal A. A., Macroevolution and the biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. U.S.A. 106, 18054–18061 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wheat C. W., et al. , The genetic basis of a plant-insect coevolutionary key innovation. Proc. Natl. Acad. Sci. U.S.A. 104, 20427–20431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehrlich P. R., Raven P. H., Butterflies and plants: A study in coevolution. Evolution 18, 586–608 (1964). [Google Scholar]
- 50.Singer M. S., Stireman J. O., The tri-trophic niche concept and adaptive radiation of phytophagous insects. Ecol. Lett. 8, 1247–1255 (2005). [Google Scholar]
- 51.Kaplan I., Thaler J. S., Plant resistance attenuates the consumptive and non-consumptive impacts of predators on prey. Oikos 119, 1105–1113 (2010). [Google Scholar]
- 52.Hopkins R. J., van Dam N. M., van Loon J. J. A., Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 54, 57–83 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Turlings T. C. J., Erb M., Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 63, 433–452 (2018). [DOI] [PubMed] [Google Scholar]
- 54.Heil M., Rattke J., Boland W., Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science 308, 560–563 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Rasmann S., Turlings T. C. J., Root signals that mediate mutualistic interactions in the rhizosphere. Curr. Opin. Plant Biol. 32, 62–68 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Heil M., Extrafloral nectar at the plant-insect interface: A spotlight on chemical ecology, phenotypic plasticity, and food webs. Annu. Rev. Entomol. 60, 213–232 (2015). [DOI] [PubMed] [Google Scholar]
- 57.van Lenteren J. C., The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 57, 1–20 (2012). [Google Scholar]
- 58.Lommen S. T. E., de Jong P. W., Pannebakker B. A., It is time to bridge the gap between exploring and exploiting: Prospects for utilizing intraspecific genetic variation to optimize arthropods for augmentative pest control—A review. Entomol. Exp. Appl. 162, 108–123 (2017). [Google Scholar]
- 59.Maag D., et al. , Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize. Plant J. 88, 976–991 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Heungens K., Cowles C. E., Goodrich-Blair H., Identification of Xenorhabdus nematophila genes required for mutualistic colonization of Steinernema carpocapsae nematodes. Mol. Microbiol. 45, 1337–1353 (2002). [DOI] [PubMed] [Google Scholar]
- 61.McMullen J. G., 2nd, Stock S. P., In vivo and in vitro rearing of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae). J. Vis. Exp. 91, 52096 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campos-Herrera R., Johnson E. G., EL-Borai F. E., Stuart R. J., Graham J. H., Duncan L. W., Long-term stability of entomopathogenic nematode spatial patterns in soil as measured by sentinel insects and real-time PCR assays. Ann. Appl. Biol. 158, 55–68 (2011). [Google Scholar]
- 63.Vrain T. C., Wakarchuk D. A., Levesque A. C., Hamilton R. I., Intraspecific rDNA restriction fragment length polymorphism in the Xiphinema americanum group. Fundam. Appl. Nematol. 15, 563–573 (1992). [Google Scholar]
- 64.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300 (1995). [Google Scholar]
- 65.Herve M. R., Package ‘RVAideMemoire’, diverse basic statistical and graphical functions (Version 0.9-52, The Comprehensive R Archive Network, Vienna, Austria, 2015). https://CRAN.R-project.org/web/packages/RVAideMemoire. Accessed 27 March 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.