Abstract
Serum perfluoroalkyl acids (PFAAs) have been linked to disruption of maternal thyroid hormone homeostasis, but results have varied between studies which we hypothesized was due to timing of the thyroid hormone measurements, variability in PFAA isomer patterns, or presence of other stressors.
In a longitudinal study design, we investigated the time-dependency of associations between PFAA isomers and thyroid hormones during pregnancy and post-partum while considering thyroid peroxidase antibody (TPOAb) status and mercury (Hg) co-exposure. In participants of a prospective Canadian birth cohort (n=494), free thyroxine (FT4), free triiodothyronine (FT3), thyroid stimulating hormone (TSH) and TPOAb were quantified in maternal plasma collected in each trimester and 3-months postpartum, and 25 PFAAs (15 linear and 10 branched) and Hg were quantified in samples collected during the second trimester.
Perfluorohexane sulfonate (PFHxS) and total branched isomers of perfluorooctane sulfonate (PFOS) were positively associated with TSH in mixed-effect models, with strongest associations early in gestation. Throughout pregnancy and post-partum, PFHxS was inversely associated with FT4, consistent with elevated TSH, while Hg was inversely associated with FT3. In TPOAb-positive women, negative associations were found between PFUnA and FT4, and 1m-PFOS and TSH, supporting previous studies that thyroid disorder increases susceptibility to PFAA-mediated hormone dysregulation. Hg did not confound associations but was a significant interaction term, revealing further positive associations between PFOS isomers (∑3m+4m-PFOS) and TSH.
Higher perfluoroalkyl sulfonate exposures were associated with higher TSH and/or lower FT4, strongly suggestive that PFHxS and branched PFOS isomers are risk factors for subclinical maternal hypothyroidism. Isomer-specific analysis is important in future studies, as crude measures of ‘total-PFOS’ masked the associations of branched isomers. A concerning result was for PFHxS which had consistent negative associations with FT4 at all time points and a positive association with TSH in early pregnancy when fetal development is most sensitive to disruption.
Keywords: Perfluoroalkyl acids, Perfluoroalkyl sulfonates, Perfluoroalkyl carboxylates, Thyroid hormones, Pregnancy, Longitudinal study design
1. Introduction
Thyroid hormones are important in critical periods of neurodevelopment, including neurogenesis, neuronal migration, proliferation, and myelination (Howdeshell, 2002; Préau et al., 2015). Maintenance of an adequate maternal concentration of thyroid hormones is, therefore, essential for healthy fetal and postnatal neurodevelopment (Williams, 2008). In humans, fetal production of thyroxine (T4) and triiodothyronine (T3) is not established until late in the first trimester (Burrow et al., 1994), and until this point the fetus relies on the maternal supply (Fitzpatrick and Russell, 2010). Thus, early stages of pregnancy exert stress on the hypothalamic–pituitary–thyroid axis (Glinoer, 1999). Alterations of maternal thyroid hormone status are linked to adverse birth outcomes and child development (Negro and Stagnaro-Green, 2014). Maternal hypothyroidism, defined by elevated thyroid stimulating hormone (TSH) with free T4 (FT4) in the reference range, has been associated with spontaneous abortion, preterm birth, placental abruption, low birth weight (Abalovich et al., 2002; Casey et al., 2005; Leung et al., 1993), and lower scores on neuropsychological tests in children (Haddow et al., 1999). Maternal hypothyroxinemia, defined as FT4 in the lowest 10th percentile without a compensatory increase in TSH, has been associated with lower psychomotor development and delayed mental and motor function in infants (Pop et al., 2003, 1999; Smit et al., 2000).
Perfluoroalkyl acids (PFAAs) are among the most prominent organic contaminants in human blood, with perfluorohexane sulfonate (PFHxS), perflurooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) present at highest concentrations in Canadian (Haines et al., 2017) and American (Olsen et al., 2017) populations. Historic and ongoing production of these compounds, combined with their environmental persistence and bioaccumulation potential has led to their global distribution and accumulation in people and wildlife (Reiner and Place, 2015). Dietary intake is a major pathway of PFAA exposure (Fromme et al., 2007; Haug et al., 2011; Rylander et al., 2010), particularly where fish and seafood are major dietary items (Berger et al., 2009; Haug et al., 2010), but PFAAs or related precursors may also be present in carpeting, textiles, indoor air and household dust (De Silva et al., 2012; Kato et al., 2009; Kubwabo et al., 2005; Makey et al., 2017; Shoeib et al., 2011).
In rodent developmental toxicology studies, PFOS generally elicits effects on thyroid hormone metabolism that are consistent with hypothyroxinemia (Lau et al., 2003; Luebker et al., 2005; Thibodeaux et al., 2003; Yu and Liu, 2009). In monkeys exposed to PFOS (Seacat et al., 2002) or PFOA (i.e., ammonium perfluorooctanoate) (Butenhoff et al., 2002), subtle alterations in T3 and T4 homeostasis were induced. There have been fewer studies of PFHxS, but one early rat study reported no developmental, nor reproductive toxicity, even at high doses (10 mg PFHxS/kg-day) (Butenhoff et al., 2009). More recent studies have reported that PFHxS-exposed pregnant rats and their offspring had reduced serum T4 measured post-pregnancy (Ramhøj et al., 2018), while in mice, dams had increased liver weight and slight reductions in mean live litter sizes, but no development effects were noted in pups (Chang et al., 2018).
Epidemiological studies of maternal thyroid disruption by PFAAs have been conducted in Asia, Europe and North America, but with differences in experimental design, including differences in measurement timing for thyroid hormones or PFAAs (Berg et al., 2015; Chan et al., 2011; de Cock et al., 2014; Kim et al., 2011; Lopez-Espinosa et al., 2012b; Wang et al., 2014, 2013; Webster et al., 2014). Positive associations are often reported between certain PFAAs and TSH (Berg et al., 2015; Wang et al., 2014, 2013; Webster et al., 2014), suggesting that PFAA exposure may be a risk factor for maternal hypothyroidism. However, as described in a recent review, associations of PFAAs with TSH were not always significant (p < 0.05); moreover, previously reported associations of PFAAs with T3 and T4 between studies have been inconsistent, and future well-designed investigations are suggested to confirm the nature of these relationships (Ballesteros et al., 2017). Due to the dynamic nature of thyroid hormone concentrations throughout normal pregnancy (Glinoer, 1997, 1999), we propose a longitudinal design with repeated measurements of thyroid hormones across the gestational period. A previous study made repeated measurement of thyroid hormones to assess relationship with PFAAs, but this included only one timepoint during pregnancy, with all other measurements after birth (Berg et al., 2015).
Additional stressors to the thyroid system may also contribute to variability between studies. In the US general population, PFAAs were associated with changes in thyroid hormone regulation, but only in a subset of participants considered both iodine deficient and had tested positive for thyroid peroxidase antibody (TPOAb) (Webster et al., 2016); a marker of autoimmune hypothyroidism (i.e., Hashimoto’s disease). Similarly, in a Canadian birth cohort, PFAAs (PFNA, PFOA and PFOS) were positively associated with TSH, but only in women with high TPOAb (Webster et al., 2014).
TPOAb has since been incorporated as a covariate into models of PFAAs and other contaminants to evaluate their effects on maternal thyroid hormone status (Berg et al., 2014; Preston et al., 2018), considered important since a high proportion of pregnant women with hypothyroidism (e.g., 31–77 %) have been identified to have elevated TPOAb (Abbassi-Ghanavati et al., 2010; Haddow et al., 1999). In the current study we tested a new hypothesis that co-exposure to mercury (Hg) may confound the effects of PFAAs. Maternal exposure to Hg has deleterious effects on cognition and motor development of offspring, and has been associated with changes in maternal thyroid hormone status, particularly T3 (Chen et al., 2013; Ursinyova et al., 2012). Moreover, like PFAAs, dietary intake of fish is a major source of Hg exposure (i.e., methylmercury). Previous epidemiological studies have not considered co-exposure of PFAAs with Hg, but for pregnant rats the combined exposure of PFOA and Hg caused non-additive changes in gene expression in brain regions of the offspring (Cheng et al., 2013).
The aim of the current investigation was to examine the longitudinal association between maternal PFAA exposure and thyroid hormone status in the prospective Canadian pregnancy cohort study known as APrON (Alberta Pregnancy Outcomes and Nutrition). With measurements of thyroid hormone status at three timepoints during pregnancy, and once in the post-partum period, while also considering Hg co-exposure and TPOAb status; this is a large (n=494) and highly detailed investigation of risk factors for maternal thyroid disruption. This is also the first such study to use an isomer-specific analysis for the PFAAs, which can have different pharmacokinetics and placental transfer (Beesoon et al., 2011; Benskin et al., 2009; De Silva et al., 2009), and isomer-specific associations have already been noted for birth weight and gestational age (Li et al., 2017).
2. Materials and Methods
2.1. Study Participants and Blood Sample Collection
Recruitment to the APrON longitudinal Canadian pregnancy cohort was between March 2009 and July 2012, including 2140 women from the Edmonton and Calgary metropolitan regions in Alberta, Canada. The study protocols were approved by the University of Calgary Health Research Ethics Board, and the University of Alberta’s Human Ethics Research Board. Participants provided written informed consent prior to sample or data collection. A full description of recruitment methods, rationale for APrON and a detailed description of the cohort is published elsewhere (Kaplan et al., 2014; Leung et al., 2016).
For the current study, women were included if residing in Calgary, recruited prior to 18 weeks of gestation, providing a blood sample during second trimester for PFAA analysis, and conceived naturally without the use of fertility hormones or assisted reproductive technique) were considered. Inclusion was further limited to non-smokers, women quitting smoking during pregnancy or quit when finding out they were pregnant (i.e., have a history of smoking), resulting in 494 eligible participants. Data was collected through in-person interviews, administration of a first-visit questionnaire, and follow-ups at each timepoint. Details of the assessments have been published (Kaplan et al., 2014), but included data collection on diet, physical activity, mental health, medical history, and demographics. For this investigation, potential covariates and confounders included maternal age, education, household income, ethnicity, parity, medical conditions, as well as a history of smoking, alcohol, and recreational drug use. A subset of participants (n=25) self-reported to be taking medication (e.g., Levothyroxine) for hypothyroidism were included, and adjusted for in final statistical models. Participants missing demographic data (n=16) were not included in final statistical models, and a flowchart and detailed description of the sample population and selected covariates is in the supplementary information (Fig. A1 and Table A1).
Except for the first trimester, plasma or sera were available for most women in each trimester of pregnancy: Timepoint 1 (< 13 weeks gestation, n=167), Timepoint 2 (14 to 27 weeks gestation, n=487), and Timepoint 3 (27 to 40 weeks gestation, n=465). Additional samples were available from 3 months post-partum, Timepoint 4 (n=479). Although the majority of PFAA analyses were measured in plasma, serum was utilized when plasma was not available (14 % of all samples), as the use of either plasma or sera as comparable matrices representing human exposure to PFAAs has been previously validated (Manzano-salgado et al., 2015). All collection and storage materials were tested for background contamination using HPLC-grade water as a surrogate matrix, and no contamination was detected in any materials. A complete description of blood collection, QA/QC protocols, and analyte recoveries are in the supplementary information (Appendix A).
2.2. Thyroid Hormone Analysis
Free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), and thyroid peroxidase antibodies (TPOAb) were measured at all time points (Timepoints 1 to 4) in plasma of participants. Chemiluminescent microparticle immunoassay kits were used on the Architect System (Abbott Diagnostics, Santa Clara, CA). Commercially available controls (Abbott Diagnostics) were included with each run (every 20 samples) to verify uniform precision between runs. In addition to commercial kits, an in-house reference sample (fasting plasma) was also included with each new lot number to ensure instrumental accuracy and consistency of calibration curves with a coefficient of variation < 1 % for thyroid hormones. Six sets of duplicates within each run were included as a QC check with an acceptable coefficient of variation of < 10 %.
2.3. Isomer-Specific PFAA Analysis.
Extraction of PFAAs was from maternal plasma collected at Timepoint 2 using a modified method from Glynn et al. (2012). Due to their long half-lives (Olsen et al., 2007), PFAA exposure is highly correlated across trimesters (Fisher et al., 2016), making single observations a robust measurement of PFAA exposure. An aliquot of 0.5 mL of plasma was placed in a 15 mL conical polypropylene centrifuge tube containing 1 ng of 8 isotopically labeled internal standards in methanol (MPFAC-MXA, Wellington Laboratories, Guelph, ON) (listed, Table. A2). Plasma extraction was by protein precipitation with 4 mL of acetonitrile (ACN) and sonication at room temperature for 10 min. The plasma/ACN mixture was centrifuged at 2000 rpm for 5 min in an Eppendorf Sorvall ST-40R tabletop centrifuge (Thermo-Fisher Scientific), the supernatant was transferred to a new 15 mL tube, and the pellet was discarded. The supernatant was then evaporated with nitrogen gas at 40 °C to a volume of 0.3 mL and reconstituted in 50:50 methanol:water to a final volume of 1 mL. This extract underwent dispersive cleanup (Powley et al., 2005) by transferring to a 1.7 mL Eppendorf tube containing 0.025 g of bulk graphitized carbon (Supelclean ENVI-Carb, Sigma Aldrich), that had been acidified with 50 µL of glacial acetic acid and vortexed for 10 s. The sample was centrifuged for 10 min at 10,000 rpm (Sorvall Legend Micro 21R, Thermo Scientific) and the top 0.5 mL was transferred to a glass auto-sampler vial.
A total of 25 PFAA analytes, including 16 perfluoroalkyl carboxylates (11 linear and 5 branched isomers) and 9 perfluoroalkyl sulfonates (4 linear and 5 branched isomers) were monitored in all maternal plasma samples at Timepoint 2. Analysis was by HPLC-MS/MS with a UFLC-XR Shimadzu HPLC coupled to an API 5000 triple quadrupole mass spectrometer (Applied Biosystems Sciex, Concord, ON) operating in negative ion mode with multiple reaction monitoring. Instrument parameters were modified from a previous method (Benskin et al., 2012). A detailed description of instrument parameters, median concentration, and detection limits for all PFAAs are provided in the supplementary material (Tables A2 and A3). An external solvent-based calibration curve was utilized and each linear and branched PFAA was quantified using the appropriate mass labeled internal standard. A 5 µL sample was injected onto an Ascentis Express F5 PFP analytical column (2.7 µm, 90 Å, 10 cm × 2.1 mm, Sigma-Aldrich) equipped with an Ascentis Express F5 PFP guard column (2.7 µm, 5.0 mm x 2.1 mm) at 40 °C. Upstream of the injector, two XTerra C18 columns (5 µm, 30 mm x 2.6 mm each, Waters) were in place to separate instrumental background PFAAs from PFAAs in the sample injected to the analytical column. A binary gradient elution was used, including (A) 5 mM aqueous formic acid and 5 mM ammonium formate, and (B) 100 % methanol at 0.2 mL/min. The elution gradient was initially 10 % B, 60 % B by 3 min, 88 % B by 14 min, and 100 % B by 14.5 min, held until 15 min and returned to initial conditions by 16 min with a further 5 min equilibration.
2.4. Total Hg Analysis
A 100 µL aliquot of maternal blood cell fraction from Timepoint 2 was diluted with 100 µL of deionized water and diluted with a basic solution containing 25 µg/L of iridium as internal standard, 10 µg/L gold, 0.5 g of EDTA in 1% v/v ammonia hydroxide, 2.5% butanol, and 0.05% v/v Triton X100. The resultant 50-fold dilution was then analyzed for total Hg using inductively coupled plasma mass-spectrometry (Agilent 8800 ICP-MS/MS). Helium was used as collision gas to remove interferences. Two sources of external quality controls were used (Seronorm™ and Clinchek™) at three levels and injected after every 10 samples. The analytical acceptability range was defined as within 20 % of the reference value according to manufacturer guidelines. Duplicates of each sample were run, and for every 10 samples a sample was randomly selected and spiked with the analytes as an additional QC check. Complete details of quality assurance and quality controls, including instrumental limits of detection and quantitation (LOD, LOQ), and Hg recovery are described in the supplementary material (Tables A6, A7, and A8).
2.5. Statistical Analysis
Thyroid hormones were measured over time for the same subject. These observations were not independent, violating the assumption of ordinal regression modeling, thus mixed effect modelling was employed to accommodate the correlation structure within observations of the same subjects. All analyses were performed using R.3.3.2. Log-transformation of both PFAAs and maternal thyroid hormones were considered, but the model fit was best when only the outcomes (thyroid hormones) were log-transformed. Plots of the residuals supported the normality of the residuals and confirmed the goodness of fit (data not shown). The effect of each potential covariate was evaluated in the separated mixed effect model in the presence of the main predictor, either PFAAs or Hg. Covariates with p < 0.2 were nominated to enroll in a multiple regression model. The fixed parameters of the multiple mixed models included the main predictor, the covariates from the first step (p < 0.2) and a time variable indicating Timepoints. We also estimated subject-specific trajectories by considering a time variable in the random part of the model. For all mixed effect modeling, three correlation structures (unstructured, autoregressive and compound symmetry) were attempted and the structure with the lowest Akaike information criterion – a method of assessing the quality of a model – was used for final model selection. Multicollinearity between covariates was tested and the goodness of fit for all the models was evaluated exploring the models’ residuals. Evaluation in statistical models was restricted to PFAAs that were detected in > 80 % of samples in the overall population. Values below detection limits were replaced with an imputed value of LOD/√2 (Hornung and Reed, 1990).
For each model of maternal PFAA or Hg exposure with thyroid hormone, in addition to the main effect, the outcome at each timepoint considered the significance of a time interaction (p < 0.05), accounting for the effect over each sequential Timepoint (e.g., the change in the effect of each PFAA on modeled thyroid hormone from Timepoint 1 to Timepoint 2, Timepoint 2 to Timepoint 3, etc.). At Timepoint 4, the significance of an additional interaction (p < 0.05) was considered to account for the change in effect during pregnancy to post-birth. The main effect, and the outcome at each timepoint were adjusted for all significant covariates for each thyroid hormone. Common significant covariates, including maternal age, ethnicity and history of smoking were identified for all measured thyroid hormones (FT3, FT4, and TSH). In addition to these, a diagnosed thyroid condition was included for FT4, and a history of drug and alcohol use was included for TSH.
3.0. Results
3.1. Population Description
Participants had a mean age of 32, the majority were Caucasian (88 %), had completed post-secondary education (99 %), and most (82 %) had annual household incomes above $77,000 CDN (Table A1). Most participants were either nulliparous or primiparous (91 %). A proportion (23 %) were self-reported to have a history of smoking, defined as having consumed >100 cigarettes over their lifetime. However, the self-reported proportion of current smokers was 2 % among pregnant participants. Overall, the described population had similar characteristics to a Canadian birth cohort study (n= 152) in the metropolitan area of Vancouver, Canada, that previously reported on PFAA-thyroid associations.
3.2. PFAA Concentrations in Maternal Plasma
Certain perfluoroalkyl carboxylates (linear-PFOA, perfluorononanoate (PFNA), and perfluorodecanoate (PFDA)) and perfluoroalkyl sulfonates (PFHxS, linear-PFOS, and most branched PFOS isomers (iso, 5m, 3m, and 4m)) were detected in > 99 % of plasma samples. Perfluoroheptanoate (PFHpA), perfluoroundecanoate (PFUnA), and perfluorododecanoate (PFDoA) were detected less frequently, in 66, 89, and 55 % of samples, respectively. Total PFOS had the highest median concentration at 4.77 ng/mL, followed in descending order by PFOA (2.11 ng/mL), PFHxS (1.03 ng/mL), PFNA (0.69 ng/mL), PFDA (0.25 ng/mL), PFHpA (0.08 ng/mL), PFUnA (0.06 ng/mL), and PFDoA (0.06 ng/mL) (Table 1). Among the major PFAAs, total PFOS, total PFOA, and PFHxS concentrations were comparable to recent measurements in Canadians (Fisher et al., 2016; Health Canada, 2013).
Table 1.
Concentrations, and detection frequency of PFAAs (ng/mL), Hg (µg/L), and isomers of PFOS in maternal plasma (n=494), including isomer proportions of total PFOS (%).
| AM (SD) | GM | Min | Median | Max | % > DL | Total PFOS (%)b | |
|---|---|---|---|---|---|---|---|
| PFHpA | 0.14 (0.33) | 0.04 | < LOD a | 0.08 | 3.87 | 66.6 | |
| PFOA | 2.88 (3.38) | 2.12 | 0.265 | 2.11 | 43.3 | 100 | |
| PFNA | 1.05 (1.27) | 0.76 | < LOD a | 0.69 | 15.8 | 99.0 | |
| PFDA | 0.38 (0.51) | 0.26 | < LOD a | 0.25 | 7.01 | 99.8 | |
| PFUnA | 0.21 (0.15) | 0.16 | < LOD a | 0.06 | 1.26 | 88.5 | |
| PFDoA | 0.05 (0.050) | 0.03 | < LOD a | 0.06 | 0.68 | 55.5 | |
| PFHxS | 1.52 (1.76) | 1.01 | 0.03 | 1.03 | 15.9 | 100 | |
| PFOS | 5.18 (2.69) | 4.54 | 0.66 | 4.77 | 17.9 | 100 | |
| Hg | 1.47 (1.32) | 0.98 | 0.03 | 1.06 | 8.68 | 97.6 | |
| Isomers of PFOS | |||||||
| Linear-PFOS | 2.72 (1.49) | 2.34 | 0.14 | 2.49 | 9.15 | 100 | 69.0 |
| ∑Br-PFOS | 1.22 (0.66) | 1.08 | 0.25 | 1.08 | 4.77 | 100 | 31.0 |
| 1m-PFOS | 0.08 (0.05) | 0.06 | < LOD a | 0.06 | 0.43 | 96.8 | 2.1 |
| ∑3m+4m-PFOS | 0.26 (0.14) | 0.23 | 0.05 | 0.23 | 1.19 | 100 | 6.1 |
| 5m-PFOS | 0.38 (0.23) | 0.32 | 0.06 | 0.33 | 1.63 | 100 | 9.8 |
| Iso-PFOS | 0.50 (0.28) | 0.43 | 0.11 | 0.42 | 1.92 | 99.6 | 12.7 |
Note: AM, arithmetic mean; GM, geometric mean; SD, standard deviation; DL, detection limit. Total populations samples were collected at Timepoints 1 (0.6 %), 2 (96 %), 3 (3 %), and 4 (0.4 %)
Value below methods quantitation limits (MQL; ng/mL), detailed in supplementary material
Total PFOS (%) applies to isomers of PFOS only
For isomers of PFOS, the median concentration of linear PFOS was 2.49 ng/mL (69 % of total PFOS) and total branched PFOS was 1.08 ng/mL (31 % of total PFOS) in maternal plasma (Table 1). Among PFOS branched isomers, the highest median concentration was for iso-PFOS (0.42 ng/mL, 12.7 % of total PFOS), followed by 5m-PFOS (0.33 ng/mL, 9.8 %), ∑3m-+4m-PFOS (0.23 ng/mL, 6.1 %), and 1m-PFOS (0.07 ng/mL, 2.1 %) (Table 1). Although a broad range of PFOA isomers were also monitored in maternal plasma, only linear-PFOA (2.11 ng/mL, 96.8 % of total PFOA) and iso-PFOA (0.07 ng/mL, 3.2 %) were detectable. However, due to the low frequency of detection of iso-PFOA, it was excluded from models of associations with thyroid hormones and further mention of PFOA refers only to linear PFOA.
Spearman correlations (listed in Table A9 of the supplementary information), indicated moderate correlations between numerous carboxylate PFAAs and sulfonate PFAAs within each family, but weak correlations between carboxylate and sulfonate groups; isomers of PFOS were moderately to strongly correlated (Table A9).
3.3. Total Hg Concentrations in Maternal Red Blood Cells
Total Hg was frequently detectable (98 %) in maternal blood cell fraction (Table 1). After adjusting for relative volumes of plasma and cell fraction (i.e., Hg concentration in RBC fraction were divided by 2), concentrations of total Hg in the current study (median of 0.490 µg/L) were comparable to whole blood concentrations in pregnant women from another recent Canadian cohort (0.491 µg/L, MIREC) (Arbuckle et al., 2016). Canadian cohort values were lower than recent cohort studies conducted in Norway (1.21 µg/L, MISA) (Veyhe et al., 2015) and Korea (3.19 µg/L, MOECH) (Jeong et al., 2017). In the current study, Hg was not strongly correlated with any PFAA analytes or their isomers, but significant low to moderate correlations were observed between Hg and PFDA, and Hg and PFUnA (Table A9).
3.4. Thyroid Hormone Concentrations in Maternal Plasma
Concentrations of thyroid hormones (TSH, FT4, and FT3) at all Timepoints are summarized in Table 2. TSH increased slightly mid-gestation (Timepoint 2), whereas decreasing trends for FT4 and FT3 were observed throughout the pregnancy (Timepoint 1 to 3). FT4 subsequently increased at 3-months postpartum (Timepoint 4). An increase in TSH between the first and second trimester, and parallel patterns of decreasing FT4 and FT3 have been noted previously, but levels generally remain in the reference range (Glinoer, 1997).
Table 2.
Thyroid hormone concentrations in maternal plasma over the 3 trimesters of pregnancy (Timepoints 1 to 3) and three months postpartum (Timepoint 4).
| Timepoint 1 (n=167) | Timepoint 2 (n=487) | Timepoint 3 (n=465) | Timepoint 4 (n=479) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| GM (GSD) | Median (range) | GM (GSD) | Median (range) | GM (GSD) | Median (range) | GM (GSD) | Median (range) | ||
| TSH | All | 1.04 (2.65) | 1.18 (0.15, 3.21) | 1.36 (1.96) | 1.38 (0.57, 3.15) | 1.20 (1.76) | 1.28 (0.52, 2.45) | 0.90 (3.20) | 1.14 (0.07, 2.28) |
| (mIU/L) | High TPOAb | *2.03 (3.48) | *2.07 (0.48, 8.01) | *1.60 (2.92) | *2.01 (0.59, 4.58) | 1.13 (2.53) | 1.42 (0.20, 2.57) | 0.36 (8.42) | 0.53 (0.07, 2.28) |
| Normal TPOAb | 0.94 (2.41) | 1.10 (0.15, 2.52) | 1.32 (1.79) | 1.33 (0.57, 2.76) | 1.22 (1.63) | 1.27 (0.54, 2.38) | 1.11 (1.74) | 1.17 (0.50, 2.28) | |
| FT4 | All | 15.1 (1.18) | 14.9 (11.8, 19.6) | 14.1 (1.16) | 14.0 (11.6. 17.5) | 12.9 (1.24) | 12.6 (10.0, 16.6) | 14.3 (1.18) | 14.03 (11.6, 19.4) |
| (pmol/L) | High TPOAb | 14.6 (1.24) | 14.2 (8.9, 19.6) | 14.8 (1.18) | 14.4 (11.3, 19.6) | 13.7 (1.30) | 13.4 (10.6, 18.2) | *16.5 (1.51) | *15.8 (11.6, 28.2) |
| Normal TPOAb | 15.2 (1.17) | 15.1 (12.1, 18.7) | 14.0 (1.15) | 14.0 (11.6, 17.0) | 12.7 (1.23) | 12.5 (10.0, 15.9) | 14.0 (1.18) | 13.9 (11.7, 17.0) | |
| FT3 | All | 4.64 (1.16) | 4.62 (3.72, 5.58) | 4.54 (1.14) | 4.53 (3.69, 5.51) | 4.43 (1.23) | 4.36 (3.62, 5.41) | 4.16 (1.18) | 4.13 (3.30, 5.30) |
| (pmol/L) | High TPOAb | 4.38 (1.13) | 4.38 (3.46, 4.99) | 4.53 (1.18) | 4.55 (3.61, 5.47) | 4.41 (1.13) | 4.47 (3.79, 5.11) | 4.55 (3.26) | 4.49 (3.16, 7.77) |
| Normal TPOAb | 4.68 (1.16) | 4.69 (3.81, 5.58) | 4.54 (1.14) | 4.53 (3.70, 5.51) | 4.43 (1.25) | 4.35 (3.61, 5.42) | 4.10 (1.14) | 4.10 (3.30, 5.05) | |
| TPOAb a | n (%) | 8 (5 %) | 73 (15 %) | 70 (15 %) | 67 (14 %) | ||||
Note: Sample collection: Timepoint 1 (< 13 weeks), Timepoint 2 (14 – 26 weeks), Timepoint 3 (27 – 40 weeks), Timepoint 4 (3 months postpartum) GM, geometric mean; GSD, geometric standard deviation
Range defined as the 5th percentile (lower) and 95th percentile (upper) of the sample population.
p < 0.05, level of significance (high vs. normal TPOAb)
Threshold indicating participants with high TPOAb, n (%) > 9 IU/mL
TPOAb data have been used as a binary variable (i.e., high/normal) in previous investigations of PFAAs and thyroid hormones. Cutoff values used have ranged from low (i.e., 9 mIU/mL) (Chen et al., 2013; Webster et al., 2016, 2014), to moderate (34 to 50 mIU/mL) (Abbassi-Ghanavati et al., 2010; Berg et al., 2015) to high (90 mIU/mL) (Pop et al., 1995). Here, we used 9 mIU/mL, to be consistent with previous PFAA studies (Chen et al., 2013; Webster et al., 2016, 2014), and because this value has been considered a threshold for eliciting disturbances in immunological function of thyroid tissue (National Center for Health Statistics, 2009). The range of women with elevated TPOAb in the current study was 5 to 15 % (Table 2), which is comparable but slightly wider than the reference range for euthyroid women (i.e., 6 – 12 %) based on previous studies of thyroid hormone status and TPOAb (Abbassi-Ghanavati et al., 2010; Loh et al., 2016; Meena et al., 2016; Springer et al., 2017). Including, and adjusting for hypothyroidism in models of the current population may have attributed to increased proportion of women with elevated TPOAb. In women categorized as high TPOAb, TSH was significantly higher early in pregnancy (Timepoint 1 and Timepoint 2), and FT4 was significantly higher postpartum (Timepoint 4) compared to normal TPOAb women (p < 0.05 using student t-test).
3.5. Associations between PFAA or Hg Exposure and Maternal Thyroid Hormones
Significant associations (p < 0.05) between PFAAs and thyroid hormones (TSH, FT4, FT3) are summarized in Table 3 (see Table A5 for all results). TSH was positively associated with several perfluoroalkyl sulfonates (PFHxS, ∑Br-PFOS, and 5m-PFOS) during pregnancy, but not with any perfluoroalkyl carboxylates, nor Hg (Table 3). The strength of the significant associations was dependent on time, with all three associations significant in early pregnancy (Timepoint 1), only PFHxS was significant in the second trimester (Timepoint 2), and none were significant late in pregnancy (Timepoint 3). The effect size of PFAAs with TSH were larger in comparison to PFHxS with FT4, suggesting that PFAAs may elicit a stronger influence on specific maternal thyroid hormones. At 3-months postpartum (Timepoint 4), a significant association with linear-PFOS was revealed, and association with 5m-PFOS was significant again, but with a decreased coefficient compared to earlier in gestation (Timepoint 1). For PFHxS, ∑Br-PFOS, and 5m-PFOS, the relative trends are similar over time (Fig. 1A–C), with strong dose-dependent positive associations at Timepoint 1 (Fig. 1A–C) which weaken by Timepoint 2, disappear by Timepoint 3, and reappear post-partum. It is concerning that the most significant and strongest associations occurred in the earliest stage of pregnancy when the fetus may be most susceptible to subclinical maternal hypothyroidism.
Table 3.
Mixed effects model of overall associations between exposure to PFAAs, Hg or PFAA and Hg co-exposure with maternal thyroid hormones.
| PFAA | Main Effect | Timepoint 1 | Timepoint 2 | Timepoint 3 | Timepoint 4 | |
|---|---|---|---|---|---|---|
| (n=478) | (n=151) | (n=471) | (n=449) | (n=463) | ||
| β (95 % CI) | β (95 % CI) | β (95 % CI) | β (95 % CI) | β (95 % CI) | ||
| TSH | PFHxS a,b,c | 0.144 (0.039, 0.249)** | 0.093 (0.025, 0.160)** | 0.042 (0.007, 0.078)* | −0.009 (−0.045, 0.028) | 0.049 (−0.001, 0.098) |
| ∑ Br-PFOS a,b,c | 0.286 (0.017, 0.555)* | 0.180 (0.007, 0.352)* | 0.073 (−0.021, 0.167) | −0.034 (−0.132, 0.065) | 0.122 (−0.011, 0.255) | |
| L-PFOS a,c | 0.005 (−0.031, 0.041) | 0.005 (−0.031, 0.041) | 0.005 (−0.031, 0.041) | 0.005 (−0.031, 0.041) | 0.062 (0.007, 0.118)* | |
| 5m-PFOS a,b,c | 0.851 (0.094, 1.608)* | 0.541 (0.055, 1.03)* | 0.230 (−0.035, 0.496) | 0.080 (−0.357, 0.196)s | 0.411 (0.034, 0.787)* | |
| ∑3m+4m PFOS(*Hg) a,d,e | 1.357 (0.060, 2.65)* | 0.998 (0.106, 1,89)* | 0.639 (0.036, 1.24)* | 0.281 (−0.343,0.905) | 0.920 (0.173, 1.67)* | |
| FT4 | PFHxS a | −0.006 (−0.012, −0.001)* | −0.006 (−0.012, −0.001)* | −0.006 (−0.012, −0.001)* | −0.006 (−0.012, −0.001)* | −0.006 (−0.012, −0.001)* |
| FT3 | 1m-PFOS a,c | −0.122 (−0.302, 0.059) | −0.122 (−0.302, 0.059) | −0.122 (−0.302, 0.059) | −0.122 (−0.302, 0.059) | 0.333 (0.082, 0.583)* |
| Hg | −0.016 (−0.023, −0.008)* | −0.016 (−0.023, −0.008)* | −0.016 (−0.023, −0.008)* | −0.016 (−0.023, −0.008)* | −0.016 (−0.023, −0.008)* | |
Significance level
p < 0.05
p < 0.01
model estimates were calculated using log-values of thyroid hormone concentrations where β = change in log(hormone) per unit (1ng/mL) increase in PFAA.
Effect of PFAAs on thyroid hormones after adjusted for moderately significant covariates (p < 0.2) in univariate models
Association includes time as a significant interaction (all Timepoints)
Association includes birth as a significant interaction (Timepoint 4 only)
Association includes Hg as a significant interaction
Interaction of Hg, effect sizes within the table were calculated when Hg set to a reference value
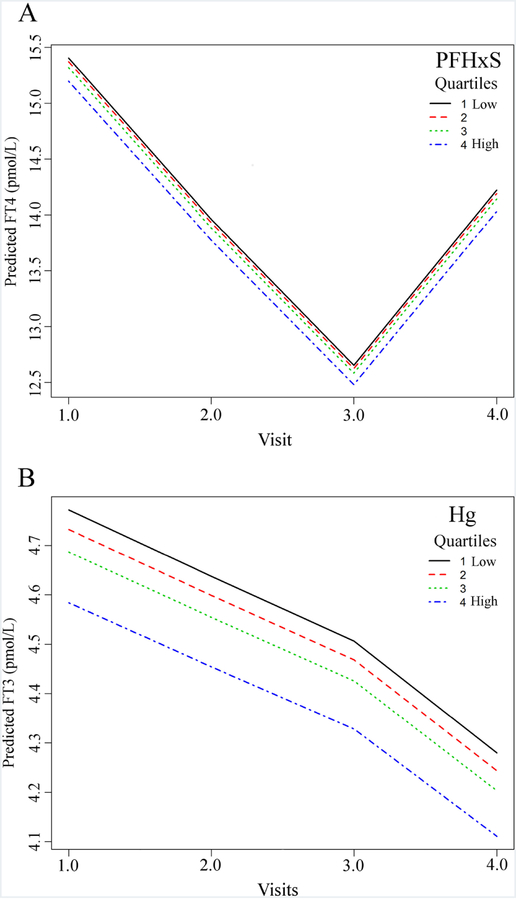
Figure 1.
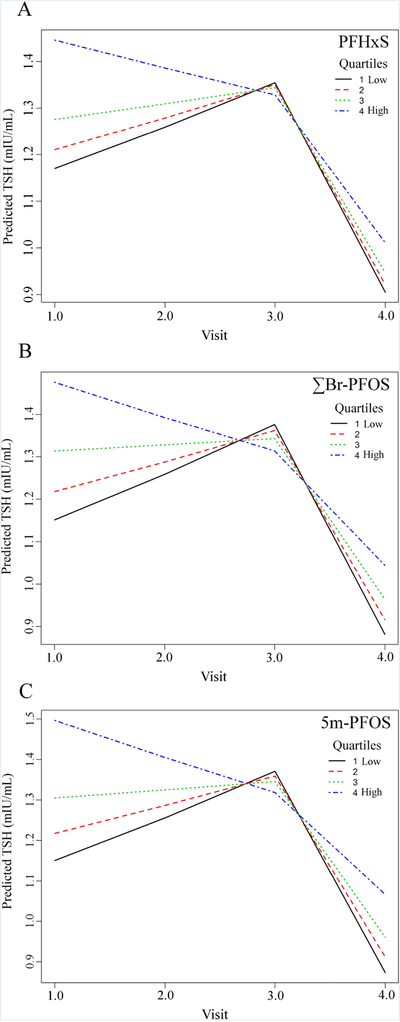
Change in predicted TSH (mIU/L) with each median quartile concentration (ng/mL) of PFHxS (A), ∑Br-PFOS (B), and 5m-PFOS (C), with binary predictors at a fixed reference level and mean maternal age. Includes an interaction term which best fits the directional change from increasing in TSH levels (mIU/mL) during pregnancy (Timepoints 1 – 3) to decreasing TSH postpartum (Timepoint 4).
For FT4, the only significant association was a negative association with PFHxS at all Timepoints (Table 3). This can be visualized over time for increasing inter-quartile concentrations of PFHxS (Fig. 2A), demonstrating the consistent dose-dependent association despite the expected decrease in FT4 during the first and second trimester, as well as the subsequent postpartum rise (Table 2). These significant associations for PFHxS and FT4 are consistent with the positive associations between PFHxS and TSH at Timepoints 1 and 2, because the function of elevated TSH is to stimulate the thyroid gland to produce more T4 in response to low circulating T4 and T3.
Figure 2.
Change in predicted FT4 (pmol/L) with each median quartile concentration (ng/mL) of PFHxS (A), and the change in predicted FT3 (pmol/L) with each median quartile concentration (µg/L) of Hg (B), with binary predictors at a fixed reference level and mean maternal age. Includes an interaction term which best fits the directional change of concentration of FT4 (A), and Hg (B) during pregnancy (Timepoints 1 to 3) to post pregnancy (Timepoint 4).
For FT3, the only significantly associations were for Hg and 1m-PFOS (Table 3). In the case of Hg, a significant negative association with FT3 was observed consistently across all Timepoints. This can be observed visually as a dose-response decline in FT3 with increasing median quartile concentration of Hg (Fig. 2B). Increasing 1m-PFOS concentrations were positively associated with FT3 at 3-months postpartum (Timepoint 4), but not at other Timepoints.
The significant associations between TSH and the branched isomers (∑Br-PFOS, 5m-PFOS), or between FT3 and 1m-PFOS, would likely have been missed in this study without the isomer-specific analysis. This is because the branched isomers are relatively minor, and the major isomer (Linear-PFOS, 69% of total PFOS) was not associated with TSH during pregnancy (Table 3), nor with FT4 or FT3 at any Timepoint. In fact, by summing all linear and branched isomers we confirmed that total PFOS was not associated with TSH, FT4, or FT3 (Table A5). A recent review pointed out that previous studies of PFOS and thyroid disruption have been inconsistent (Ballesteros et al., 2017), and we propose that ‘total-PFOS’ analytical methods have contributed to this.
Including an interaction term for Hg co-exposure did not influence any of the above noted associations for PFAAs. However, one significant new association was revealed, a positive association between ∑3m+4m-PFOS and TSH (p < 0.05) (Table 3) when including the interaction of Hg co-exposure. This indicates that increasing concentrations of ∑3m+4m-PFOS are associated with TSH, but that increasing concentrations of Hg interact to weaken the association (β = −0.30, p = 0.047). This new relationship agrees with the general association for ∑Br-PFOS and 5m-PFOS which were significantly positively associated with TSH in early pregnancy (Timepoint 1) and at 3-months postpartum (Timepoint 4) (Table 3).
3.6. Inclusion of TPOAb status
Including a TPOAb interaction term into the model (Table A5), whereby participants were classified as either normal (< 9 IU/mL) or high (> 9 IU/mL) at each time point, did not significantly alter any of the established main effect associations (Table 3). Nevertheless, new significant associations were revealed after inclusion of TPOAb status (Table 4). PFUnA was positively associated with FT4 in the high TPOAb group only. The association was consistent at all measured timepoints; thus, the result is unlikely spurious because it was consistent throughout pregnancy and at 3-months postpartum. The branched PFOS isomer,1m-PFOS, was also associated with TSH when including TPOAb status, but the result was time-dependent (Table 4). Specifically, in the normal TPOAb group, there was a significant positive association between 1m-PFOS and TSH at Timepoints 1 and 2, but not at Timepoints 3 and 4. The positive association of 1m-PFOS with TSH is not surprising, as the normal TPOAb group represents 85% of the population, and similar longitudinal trends were observed for the sum of all PFOS branched isomers (∑Br-PFOS), as well as 5m-PFOS. More unexpected was the opposite association in the high TPOAb group, whereby 1m-PFOS was inversely associated with TSH (Table 4). This association was not significant early in gestation (Timepoints 1 and 2) but became stronger over subsequent timepoints, with significant negative associations in late pregnancy and at 3-months postpartum (Timepoints 3 and 4). This vulnerable TPOAb subgroup may be more susceptible to the effects of environmental contaminants such as PFAAs, and these results suggest a unique response compared to the larger normal TPOAb population.
Table 4.
Mixed effects model coefficients for associations between PFAAs and thyroid hormones in adjusted models after including interaction term for TPOAb status
| Time | Normal TPOAb | High TPOAb | TPOAb Interaction c | ||
|---|---|---|---|---|---|
| β (95 % CI) | β (95 % CI) | (p-value) | |||
| FT4 | PFUnA a | all | −0.011 (−0.087, 0.066) | −0.240 (−0.456, −0.025)* | 0.049 |
| TSH | 1m-PFOS a,b | 1 | 1.97 (0.198, 3.74)* | −0.819 (−3.13, 1.49) | 0.008 |
| 2 | 1.36 (0.218, 2.51)* | −1.427 (−3.32, 0.468) | |||
| 3 | 0.754 (−0.273, 1.78) | −2.03 (−3.88, −0.190)* | |||
| 4 | 0.146 (−1.40, 1.69) | −2.64 (−4.83, −0.453)* | |||
Effect of PFAAs on thyroid hormones after adjusting for significant covariates in univariate models
Significant interaction with time, coefficient calculated for main effect (all) and for each Timepoint (#)
Level of significance (p-value) of TPOAb interaction term of normal vs. high TPOAb of the main effect (all Timepoints) using a threshold of 9 IU/mL
p < 0.05
p < 0.01 level of significance
4. Discussion
During pregnancy, specific thyroid hormones were influenced by background exposure to PFAA sulfonates (Table 3), as PFHxS, and branched-PFOS isomers were positively associated with TSH (p < 0.05) in a dose-dependent manner that were strongest in early pregnancy (1st trimester) and weakened over subsequent trimesters. In addition to associations of TSH, PFHxS was also negatively associated with FT4, and Hg was negatively associated with FT3. Including additional interactions did not further affect the established associations of PFAAs and thyroid hormones but newly significant associations were revealed when considering effect modification by Hg (e.g., ∑3m+4m PFOS with TSH, Table 3), and TPOAb (e.g., PFUnA with FT4 and 1m-PFOS with TSH, Table 4).
Maternal thyroid hormone homeostasis is under inherent stress during pregnancy. Increasing estrogen in early gestation coincides with increased T4-binding globulin, resulting in increased total T3 and T4 (Glinoer, 1999) which continues throughout pregnancy to meet maternal and fetal demand, even after onset of fetal thyroid function at 16–20 weeks (Moog et al., 2017). Elevated TPOAb, which occurs in 6 – 12 % of pregnant women, is considered a clinical marker of autoimmune thyroiditis (Hashimoto’s disease) and indicates additional stress on the thyroid that increases the risk of adverse birth outcomes (Abbassi-Ghanavati et al., 2010; Li et al., 2010). Thus, pregnant women may be vulnerable to thyroid disruption from the additional stress of environmental contaminants that act as endocrine disruptors, such as PFAAs and Hg. Epidemiological studies of pregnant women are complicated by gradual changes in maternal physiology, and by changing concentration of thyroid hormones throughout the pregnancy (Harada et al., 1979; Mandel et al., 2005; Tulchinsky and Little, 1994; Yazbeck and Sullivan, 2012), also observed here (Table 2). Given the susceptibility of pregnant women to thyroid disruption, while also considering the biological importance of understanding the timing of any disruption, longitudinal studies are particularly well suited to studies of maternal thyroid disruption.
In prospective cohorts, longitudinal approaches have several advantages over other study designs, including identification of the timing, trends, or recurrence of adverse outcomes (Caruana et al., 2015). This study on PFAAs is the first to measure thyroid outcomes at multiple time points during the gestational period, and important time-dependent associations were revealed for the first time (Table 3). Our statistical models using repeat measures accounted for changes in thyroid hormone levels over time, revealing significant relationships between PFHxS and TSH, or PFOS isomers and TSH that were strongest early in pregnancy, gradually disappeared by the third trimester, and in some cases, reappeared post-partum. These trends suggest a window of vulnerability in early pregnancy at a time when the fetus is almost entirely dependent on the maternal supply of thyroid hormones. In other cases, the longitudinal design revealed no trend over time but demonstrated consistent significant associations at each time point. For example, the negative associations between PFHxS and FT4, and between Hg and FT3 (Table 3). Such consistent results provide great internal validity that the associations are not spurious.
In the current study, overall results for PFHxS were particularly strong and compelling. Higher maternal PFHxS exposure was positively associated with TSH (time-dependent) and negatively associated with maternal FT4 (not time-dependent). This is consistent with previous findings of a positive association of PFHxS with TSH (Wang et al., 2014, 2013; Webster et al., 2014) and negative association with FT4I; an index of free T4 in circulation (Preston et al., 2018). Taking this into consideration with the current dataset showing a consistent dose-response between increasing PFHxS concentrations and declining FT4 at all Timepoints, evidence is building for a causal relationship (Bradford-Hill, 1965). Several mechanisms have been proposed to explain the thyroid disrupting effects of PFAAs, including, increased hepatic clearance and excretion of T4 through glucuronidation (Yu et al., 2009), deiodinase enzyme mediated conversion of T4 to T3 (Yu et al., 2009), and the competitive displacement of T4 from thyroid hormone binding proteins in serum (Jones et al., 2003; Weiss et al., 2009). In fact, compared to other PFAAs, PFHxS has the highest in vitro competitive binding potency to human transthyretin, an important human T4 serum transport protein (Weiss et al., 2009). Our findings are in support of this latter mechanism, as it has been proposed that competitive displacement of T4 by PFAAs leads to a transient increase in FT4, but that the resulting clearance from induction of T4 metabolism ultimately reduces FT4 at steady-state (Kim et al., 2011). Moreover, dose-dependent reductions in plasma FT4 have also been observed in chicken embryos exposed to PFHxS (Cassone et al., 2012). Notwithstanding such in-vitro results, this mechanism required relatively high concentrations of PFAAs, and the validity in-vivo at lower concentrations is questionable (Ren et al., 2016). Nevertheless, observed associations in this study and previous studies are consistent with this mechanism.
According to NHANES data between 1999–2000 (Calafat et al., 2007) and 2007–2008 (Webster et al., 2016), human exposure to PFOS drastically declined (geometric mean declined from 30.4 to 13.5 ng/mL) whereas PFHxS exposure was effectively unchanged (2.1 and 1.9 ng/mL, respectively) over the same time period. More recent monitoring of PFAAs in plasma samples collected from American Red Cross donors in 2015 found further decreased PFOS (4.3 ng/mL), and to a lesser extent, PFHxS (0.9 ng/mL) (Olsen et al., 2017), which was at comparable concentrations to the current study (Table 1). Human PFOS exposure has declined much faster than PFHxS, and PFHxS may soon be the most prominent PFAA in humans if it is not targeted for mitigation.
To our knowledge, this is the first epidemiological study to include an isomer-specific analysis of PFAAs for investigation of maternal thyroid hormone disruption. This is a limitation of previous work because PFOS and PFOA are present in human blood as various linear or branched isomers that are known to have different pharmacokinetics (Benskin et al., 2009; De Silva et al., 2009). Isomer-specific analysis is particularly important for PFOS which was historically manufactured as a mixture of linear (70%) and branched (total 30%) isomers. Populations around the world have wide-ranging proportions of branched PFOS isomers, for example the branched PFOS content in samples from China (52 %) (Zhang et al., 2013), is higher than in Norway (30–47 %) (Haug et al., 2009; Rylander et al., 2010) or Vietnam (17 %) (Rylander et al., 2009). Higher proportions of branched PFOS isomers have been detected in fetal cord blood (24 %, and 36–54 %) compared to paired maternal samples (17 %, and 27–44 %) (Beesoon et al., 2011; Chen et al., 2017) and branched PFOS isomers have higher transplacental transfer efficiencies than linear PFOS (Beesoon and Martin, 2015).Thus, the associations between branched PFOS isomers and maternal thyroid disruption (Table 3) could also be relevant to thyroid disruption in the developing fetus.
Interestingly, the strongest associations with TSH were for PFHxS and branched PFOS isomers, with no corresponding association for linear PFOS (Table 3). We postulate that PFHxS and branched PFOS isomers share optimal physical properties or molecular sizes which allows them to interact most strongly with receptor biomolecules that control thyroid hormone homeostasis. Compared to linear PFOS, PFHxS and branched PFOS molecules are shorter in length and less hydrophobic, for example eluting earlier than linear PFOS in reversed phase chromatography. PFHxS has already been demonstrated to be more optimal than PFOS for displacing T4 from transthyretin (Weiss et al., 2009), but the same studies have yet to be done for branched PFOS isomers. For all these reasons, future epidemiological and toxicological studies of PFOS should consider isomer-specific analysis and isomer-specific toxicity.
During pregnancy, thyroid hormone concentrations are dynamic but tightly controlled. PFAAs were significantly associated with altered maternal thyroid hormone concentrations (FT4 and TSH), but it is important to note that thyroid hormone levels remained in the reference range. Although reference ranges exist for the general population (e.g., 0.45 to 4.5 mU/L for TSH and 4.5 to 12.5 mg/dL FT4I ), there is uncertainty in the value of universal reference ranges of these hormones during pregnancy as concentrations vary by gestational age, number of fetuses, and between populations (Fitzpatrick and Russell, 2010). Furthermore, even subtle alterations in maternal thyroid hormone status may affect fetal development, as children of women with subclinical maternal hypothyroidism (Haddow et al., 1999) or maternal hypothyroxinemia (Pop et al., 2003) had decreased neurodevelopmental indices. Although only subclinical associations in maternal thyroid hormone status during pregnancy were detected here, the public health significance for fetal neurodevelopment can be important due to widespread exposure of the population to PFAAs and presence of cumulative risk factors (e.g., TPOAb).
The “multiple-hit hypothesis” proposed by Webster et al. (2014), states that populations with pre-existing thyroid related conditions may be more susceptible to the effects of thyroid disrupting environmental contaminants, supported by evidence of PFAA-induced thyroid dysregulation in a subset of people with high TPOAb and an iodine deficiency (Webster et al., 2016). Within the small subgroup of women with elevated TPOAb in the current study, PFUnA was negatively associated with FT4, and 1m-PFOS was negatively associated with TSH (Table 4). PFOS has previously been reported to have both positive (Webster et al., 2014) and negative (Preston et al., 2018) associations with TSH in high TPOAb status pregnant women. Although the mechanism of interaction between PFAAs, thyroid hormones, and TPOAb is not well understood, these results further support the multiple-hit hypothesis (Webster et al., 2014).
Total Hg analysis was included in the current study to control for a hypothesized confounding variable, but also to consider any relevant mixture effects on thyroid disruption. In the current Canadian cohort, blood Hg was not strongly correlated with serum PFAAs and was not considered a confounder, but Hg should be included in future studies where fish intake is higher. Nevertheless, Hg was negatively associated with free T3, similar to previous associations between Hg and total T3 (FT3 was not analyzed) in pregnant women from Quebec, Canada, (Takser et al., 2005) and Spain (Llop et al., 2015). In Slovakia, Hg in cord blood was inversely associated with total T3 and FT3 in 6-month old infants (Ursinyova et al., 2012). These findings are consistent with the proposed mechanism of action for Hg through enzymatic inhibition of deiodinase activity (enzyme D3, in particular), resulting in reduced T3 production (Mori et al., 2006; Soldin et al., 2008). A possible mixture effect between Hg and ∑3m-+4m-PFOS was observed in models of TSH, whereby the main effect of ∑3m-+4m-PFOS was positively associated with TSH when including Hg co-exposure, however, the ∑3m-+4m-PFOS-Hg interaction term was negative, indicating that at higher Hg concentrations association of these PFOS isomers with Hg became weaker. Thus, it is possible that Hg increases sensitivity to thyroid disruption by PFOS branched isomers but is dependent on the concentration of Hg.
Although this was among the largest and most detailed studies of PFAAs and thyroid disruption, we acknowledge some study limitations. Due to the logistical challenges of recruiting pregnant women early in pregnancy, first trimester (Timepoint 1) samples for thyroid hormone analysis were not always available, resulting in a smaller sample size when compared to the other timepoints. Participants all resided in the metropolitan area of Calgary, Canada, and were from rather narrow demographic (e.g., high socioeconomic status) that may not be representative of the overall population. This may limit the external validity of our findings when considering other global populations, but it does not decrease the internal validity of our findings. Nevertheless, with respect to PFAA exposure, the concentrations of PFAAs in maternal plasma in this study were almost identical to another Canadian birth cohort (Webster et al., 2014), and similar to other cohorts in Korea (Kim et al., 2011), China (Lin et al., 2016), Taiwan (Wang et al., 2014), and Norway (Berg et al., 2014). With respect to participant iodine sufficiency, this was determined using questionnaires, assuming sufficient if taking prenatal supplements that included iodine. In the general US population, thyroid hormone status was particularly susceptible to PFAAs for a subgroup with combined high TPOAb status and an iodine deficiency, (Webster et al., 2016), thus future studies might consider inclusion of urinary iodine. Finally, thyroid hormones were measured by immunoassay. Although this is one of the most commonly employed methods of quantifying thyroid hormones in human samples, radioimmunoassays, such as the one used in this study, are sensitive to serum binding protein concentration, not designed to be used under conditions when protein levels are subject to change (e.g., during pregnancy) (Soldin and Soldin, 2011). Similar problems resulted in reporting bias and underreporting of FT4 values in animal models (Chang et al., 2007), but have been deemed less problematic in human samples (Lopez-Espinosa et al., 2012a). Nonetheless, pregnancy cohort studies might benefit from using LC-MS/MS for its enhanced specificity and precision over immunoassays.
5. Conclusions
The prospective APrON birth cohort study allowed one of the largest and most detailed studies of maternal thyroid hormone disruption by PFAAs. With three sampling times throughout pregnancy and another sampling time after birth, the longitudinal analysis revealed new trends that are of biological significance, and which could explain variability among previous studies. By considering additional thyroid stressors, Hg and TPOAb status, the study also revealed interactions of relevance to the multiple hit hypothesis, and to the toxicology of environmental chemical mixtures in human blood, respectively. By using an isomer-specific method for PFAA analysis, the distinct toxicological behavior and relevance of branched PFOS isomers was revealed, which highlights the importance of isomer-specific PFAA methodologies in future epidemiology and toxicology studies. Building on previous literature, there is now very strong evidence in support of causal relationships between PFHxS and PFOS exposure and disruption of maternal thyroid homeostasis in early pregnancy. This information should be considered in future international decisions under the Stockholm Convention, where PFHxS has been nominated for inclusion as a Persistent Organic Pollutant (Stockholm Convention, 2018), and PFOS is already included but with many current exemptions under its listing in Annex B.
Supplementary Material
Highlights.
Perfluoroalkyl acids were associated with thyroid hormones during and after pregnancy
Associations were strongest in early pregnancy, a sensitive stage of neurodevelopment
Evidence mounting for cause-effect relationship for perfluorohexane sulfonate
Isomer-specific associations for branched perfluorooctane sulfonate, not linear
Associations influenced by mercury co-exposure and thyroid peroxidase antibodies
Acknowledgements
Funding: This work was supported by the U.S. National Institute of Environmental Health Sciences [National Institutes of Health, Development Grant, PI JW Martin, 1R21ES021295-01]; and the Canadian Institute of Health Research [Open Operating Grant, PI JW Martin, MOP 123535]. A.J.F.R was also supported by funding from the Stollery Children’s Hospital Foundation and supporters of the Lois Hole Hospital for Women through the Women and Children’s Health Research Institute. We thank all members and participants of the Alberta Pregnancy Outcomes and Nutrition (APrON) Cohort for their contributions to this project, and Alberta Innovates Health Solutions for initial funding of the APrON cohort.
Abbreviations
- ACN
acetonitrile
- APrON
Alberta pregnancy outcomes and nutrition
- FT3
free triiodothyronine
- FT4
free thyroxine
- Hg
mercury
- HPLC-MS/MS
high performance liquid chromatography mass spectrometry
- ICP-MS/MS
inductively coupled plasma mass-spectrometry
- LOD
limit of detection
- LOQ
limit of quantitation
- PFAAs
perfluoroalkyl acids
- PFDA
perfluorodecanoate
- PFDoA
perfluorododecanoate
- PFHpA
perfluoroheptanoate
- PFHxS
perlfuorohexane sulfonate
- PFNA
perfluorononanoate
- PFOA
perfluorooctanoate
- PFOS
perlfuorooctane sulfonate
- PFUnA
perfluoroundecanoate
- TPOAb
thyroid peroxidase antibody
- TSH
thyroid stimulating hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O, 2002. Overt and Subclinical Hypothyroidism Complicating Pregnancy. Thyroid 12, 63–68. 10.1089/105072502753451986 [DOI] [PubMed] [Google Scholar]
- Abbassi-Ghanavati M, Casey B, Spong C, McIntire DD, Halvorson LM, Cunningham FG, 2010. Pregnancy outcomes in women with thyroid peroxidase antibodies. Obstet. Gynecol 116, 381–386. 10.1097/AOG.0b013e3182040b53 [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Liang CL, Morisset AS, Fisher M, Weiler H, Cirtiu CM, Legrand M, Davis K, Ettinger AS, Fraser WD, 2016. Maternal and fetal exposure to cadmium, lead, manganese and mercury: The MIREC study. Chemosphere 163, 270–282. 10.1016/j.chemosphere.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa M-J, 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ. Int 99, 15–28. 10.1016/j.envint.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Beesoon S, Martin JW, 2015. Isomer-specific binding affinity of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) to serum proteins. Environ. Sci. Technol 49, 5722–5731. 10.1021/es505399w [DOI] [PubMed] [Google Scholar]
- Beesoon S, Webster GM, Shoeib M, Harner T, Benskin JP, Martin JW, 2011. Isomer Profiles of Perfluorochemicals in Matched Maternal, Cord, and House Dust Samples : Manufacturing Sources and Transplacental Transfer. Environ. Health Perspect 119, 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskin JP, De Silva AO, Martin LJ, Arsenault G, Mccrindle R, Riddell N, Mabury SA, Martin JW, Leah Martin, J., 2009. Disposition of perfluorinated acid isomers in sprague-dawley rats; part 1: Single dose. Environ. Toxicol. Chem 28, 542–554. [DOI] [PubMed] [Google Scholar]
- Benskin JP, Ikonomou MG, Woudneh MB, Cosgrove JR, 2012. Rapid characterization of perfluoralkyl carboxylate, sulfonate, and sulfonamide isomers by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1247, 165–70. 10.1016/j.chroma.2012.05.077 [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Hansen S, Elverland A, Veyhe A-S, Jorde R, Odland JØ, Sandanger TM, 2015. Assessing the relationship between perfluoroalkyl substances, thyroid hormones and binding proteins in pregnant women; a longitudinal mixed effects approach. Environ. Int 77, 63–9. 10.1016/j.envint.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Berg V, Nøst TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevåg OM, Odland JØ, Sandanger TM, 2014. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ. Int 69, 58–66. 10.1016/j.envint.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Berger U, Glynn A, Holmström KE, Berglund M, Ankarberg EH, Törnkvist A, 2009. Fish consumption as a source of human exposure to perfluorinated alkyl substances in Sweden - Analysis of edible fish from Lake Vättern and the Baltic Sea. Chemosphere 76, 799–804. 10.1016/j.chemosphere.2009.04.044 [DOI] [PubMed] [Google Scholar]
- Bradford-Hill A, 1965. The Enviroment and Disease: Association or Causation? Proc. R. Soc. Med 58, 295–300. https://doi.org/ DOI: 10.1016/j.tourman.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrow GN, Fisher DA, Larsen RP, 1994. Mechanisms of Disease - Maternal and Fetal Thyroid Function. N. Engl. J. Med 331, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, Jung R, Kennedy G, Lieder P, Olsen G, Thomford P, 2002. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol. Sci 69, 244–257. 10.1093/toxsci/69.1.244 [DOI] [PubMed] [Google Scholar]
- Butenhoff JL, Chang S-C, Ehresman DJ, York RG, 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod. Toxicol 27, 331–41. 10.1016/j.reprotox.2009.01.004 [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong L, Kuklenyik Z, Reidy JA, Needham LL, 2007. Polyfluoroalkyl Chemicals in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and Comparisons with NHANES 1999–2000 115, 1596–1602. 10.1289/ehp.l0598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruana EJ, Roman M, Hernández-Sánchez J, Solli P, 2015. Longitudinal studies. J. Thorac. Dis 7, E537–E540. 10.3978/j.issn.2072-1439.2015.10.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG, 2005. Subclinical Hypothyroidism and Pregnancy Outcomes. Obstet. Gynecol 105, 239–245. 10.1097/01.AOG.0000152345.99421.22 [DOI] [PubMed] [Google Scholar]
- Cassone CG, Vongphachan V, Chiu S, Williams KL, Letcher RJ, Pelletier E, Crump D, Kennedy SW, 2012. In ovo effects of perfluorohexane sulfonate and perfluorohexanoate on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol. Sci 127, 216–24. 10.1093/toxsci/kfs072 [DOI] [PubMed] [Google Scholar]
- Chan E, Burstyn I, Cherry N, Bamforth F, Martin JW, 2011. Perfluorinated acids and hypothyroxinemia in pregnant women. Environ. Res 111, 559–64. 10.1016/j.envres.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Chang S-C, Thibodeaux JR, Eastvold ML, Ehresman DJ, Bjork J. a, Froehlich JW, Lau CS, Singh RJ, Wallace KB, Butenhoff JL, 2007. Negative bias from analog methods used in the analysis of free thyroxine in rat serum containing perfluorooctanesulfonate (PFOS). Toxicology 234, 21–33. 10.1016/j.tox.2007.01.020 [DOI] [PubMed] [Google Scholar]
- Chang S, Butenhoff JL, Parker GA, Coder PS, Zitzow JD, Krisko RM, Bjork JA, Wallace KB, Seed JG, 2018. Reproductive and developmental toxicity of potassium perfluorohexanesulfonate in CD-1 mice. Reprod. Toxicol 78, 150–168. 10.1016/j.reprotox.2018.04.007 [DOI] [PubMed] [Google Scholar]
- Chen A, Kim SS, Chung E, Dietrich KN, 2013. Thyroid Hormones in Relation to Lead, Mercury, and Cadmium Exposure in the National Health and Nutrition Examination Survey, 2007 – 2008. Environ. Health Perspect 121, 2007–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Yin S, Kelly BC, Liu W, 2017. Isomer-Specific Transplacental Transfer of Perfluoroalkyl Acids: Results from a Survey of Paired Maternal, Cord Sera, and Placentas. Environ. Sci. Technol 51, 5756–5763. 10.1021/acs.est.7b00268 [DOI] [PubMed] [Google Scholar]
- Cheng J, Fujimura M, Zhao W, Wang W, 2013. Neurobehavioral effects, c-Fos/Jun expression and tissue distribution in rat offspring prenatally co-exposed to MeHg and PFOA: PFOA impairs Hg retention. Chemosphere 91, 758–64. 10.1016/j.chemosphere.2013.02.016 [DOI] [PubMed] [Google Scholar]
- de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M, 2014. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants – a Dutch prospective cohort study. Environ. Heal 13, 1–10. 10.1186/1476-069X-13-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva AO, Allard CN, Spencer C, Webster GM, Shoeib M, 2012. Phosphorus-containing fluorinated organics: Polyfluoroalkyl phosphoric acid diesters (diPAPs), perfluorophosphonates (PFPAs), and perfluorophosphinates (PFPIAs) in residential indoor dust. Environ. Sci. Technol 46, 12575–12582. 10.1021/es303172p [DOI] [PubMed] [Google Scholar]
- De Silva AO, Benskin JP, Martin LJ, Arsenault G, McCrindle R, Riddell N, Martin JW, Mabury S. a, 2009. Disposition of perfluorinated acid isomers in Sprague-Dawley rats; part 2: subchronic dose. Environ. Toxicol. Chem 28, 555–67. 10.1897/08-254.1 [DOI] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, Haines D, Davis K, Fraser WD, 2016. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ. Heal 15, 1–14. 10.1186/s12940-016-0143-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DL, Russell MA, 2010. Diagnosis and management of thyroid disease in pregnancy. Obstet. Gynecol. Clin. North Am 37, 173–193. 10.1016/j.ogc.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Fromme H, Schlummer M, Möller A, Gruber L, Wolz G, Ungewiss J, Böhmer S, Dekant W, Mayer R, Liebl B, Twardella D, 2007. Exposure of an adult population to perfluorinated substances using duplicate diet portions and biomonitoring data. Environ. Sci. Technol 41, 7928–33. [DOI] [PubMed] [Google Scholar]
- Glinoer D, 1999. What happens to the normal thyroid during pregnancy? Thyroid 9, 631–635. [DOI] [PubMed] [Google Scholar]
- Glinoer D, 1997. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocr. Rev 18, 404–33. [DOI] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, Darnerud PO, 2012. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ. Sci. Technol 46, 9071–9. 10.1021/es301168c [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allen WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ, 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med 341, 549–555. [DOI] [PubMed] [Google Scholar]
- Haines DA, Khoury C, Saravanabhavan G, Werry K, Walker M, Malowany M, 2017. Human biomonitoring reference values derived for persistent organic pollutants in blood plasma from the Canadian Health Measures Survey 2007–2011. Int. J. Hyg. Environ. Health 220, 744–756. 10.1016/j.ijheh.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Harada A, Hershman JM, Reed AW, Glenn D, Christine JD, Stanley D, Jewelewicz R, Pekary AE, 1979. Comparison of thyroid stimulators and thyroid hormone concentrations in the sera of pregnant women. J. Clin. Endocrinol. Metab 48, 793–797. [DOI] [PubMed] [Google Scholar]
- Haug LS, Huber S, Becher G, Thomsen C, 2011. Characterisation of human exposure pathways to perfluorinated compounds - comparing exposure estimates with biomarkers of exposure. Environ. Int 37, 687–93. 10.1016/j.envint.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Becher G, 2009. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ. Sci. Technol 43, 2131–2136. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsæter AL, Kvalem HE, Haugen M, Becher G, Alexander J, Meltzer HM, Knutsen HK, 2010. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int 36, 772–778. 10.1016/j.envint.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Canada Health, 2013. Second Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 2
- Hornung R, Reed L, 1990. Estimation of average concentration in presence of nondetectable values [Google Scholar]
- Howdeshell KL, 2002. A model of the development of the brain as a construct of the thyroid system. Environ. Heal 110, 337–348. 10.1289/ehp.02110s3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KS, Ha E, Shin JY, Park H, Hong YC, Ha M, Kim S, Lee SJ, Lee KY, Kim JH, Kim Y, 2017. Blood heavy metal concentrations in pregnant Korean women and their children up to age 5 years: Mothers’ and Children’s Environmental Health (MOCEH) birth cohort study. Sci. Total Environ 605–606, 784–791. 10.1016/j.scitotenv.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Jones PD, Hu W, De Coen W, Newsted JL, Giesy JP, 2003. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem 22, 2639–49. [DOI] [PubMed] [Google Scholar]
- Kaplan BJ, Giesbrecht GF, Leung BMY, Field CJ, Dewey D, Bell RC, Manca DP, O’Beirne M, Johnston DW, Pop VJ, Singhal N, Gagnon L, Bernier FP, Eliasziw M, Mccargar LJ, Kooistra L, Farmer A, Cantell M, Goonewardene L, Casey LM, Letourneau N, Martin JW, 2014. The Alberta Pregnancy Outcomes and Nutrition (APrON) cohort study: rationale and methods. Matern. Child Nutr 10, 44–60. 10.1111/j.1740-8709.2012.00433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Calafat AM, Needham LL, 2009. Polyfluoroalkyl chemicals in house dust. Environ. Res 109, 518–23. 10.1016/j.envres.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H, Giesy JP, 2011. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ. Sci. Technol 45, 7465–72. 10.1021/es202408a [DOI] [PubMed] [Google Scholar]
- Kubwabo C, Stewart B, Zhu J, Marro L, 2005. Occurrence of perfluorosulfonates and other perfluorochemicals in dust from selected homes in the city of Ottawa, Canada. J. Environ. Monit 7, 1074–8. 10.1039/b507731c [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson L. a, 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. II: postnatal evaluation. Toxicol. Sci 74, 382–92. 10.1093/toxsci/kfg122 [DOI] [PubMed] [Google Scholar]
- Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH, 1993. Perinatal outcome in hypothyroid pregnancies. Obstet. Gynecol 81, 349–353. [PubMed] [Google Scholar]
- Leung BMY, Giesbrecht GF, Letourneau N, Field CJ, Bell RC, Dewey D, Manca D, O’Beirne M, Pop VJ, Singhal N, Gagnon L, Bernier F, Eliasziw M, McCargar L, Kooistra L, Farmer A, Cantell M, Martin J, 2016. Perinatal nutrition in maternal mental health and child development: birth of a pregnancy cohort. Early Hum. Dev 93, 1–7. 10.1016/j.earlhumdev.2015.11.007 [DOI] [PubMed] [Google Scholar]
- Li M, Zeng XW, Qian Z. (Min), Vaughn MG, Sauvé S, Paul G, Lin S, Lu L, Hu LW, Yang BY, Zhou Y, Qin X. Di, Xu SL, Bao WW, Zhang YZ, Yuan P, Wang J, Zhang C, Tian YP, Nian M, Xiao X, Fu C, Dong GH, 2017. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ. Int 102, 1–8. 10.1016/j.envint.2017.03.006 [DOI] [PubMed] [Google Scholar]
- Li Y, Shan Z, Teng W, Yu X, Li Y, Fan C, Teng X, Guo R, Wang H, Li J, Chen Y, Wang W, Chawinga M, Zhang L, Yang L, Zhao Y, Hua T, 2010. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25–30 months. Clin. Endocrinol. (Oxf) 72, 825–829. 10.1111/j.1365-2265.2009.03743.x [DOI] [PubMed] [Google Scholar]
- Lin Y, Li J, Lai J, Luan H, Cai Z, Wang Y, Zhao Y, Wu Y, 2016. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing Prenatal Exposure Study. Sci. Rep 6, 1–9. 10.1038/srep21699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop S, Lopez-espinosa M, Murcia M, Alvarez-pedrerol M, 2015. Synergism between exposure to mercury and use of iodine supplements on thyroid hormones in pregnant women. Environ. Res 138, 298–305. 10.1016/j.envres.2015.02.026 [DOI] [PubMed] [Google Scholar]
- Loh TP, Tee JCS, Tee NWS, Cheng WL, Thevarajah M, Sabir N, Chew YY, Sethi SK, Khoo CM, 2016. Association between thyroid function tests and anti-thyroid peroxidase (TPO) antibodies in pregnancy. Endocrine 53, 865–867. 10.1007/s12020-015-0844-y [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Fitz-Simon N, Bloom MS, Calafat AM, Fletcher T, 2012a. Comparison between free serum thyroxine levels, measured by analog and dialysis methods, in the presence of perfluorooctane sulfonate and perfluorooctanoate. Reprod. Toxicol 33, 552–5. 10.1016/j.reprotox.2011.04.002 [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa M-J, Mondal D, Armstrong B, Bloom MS, Fletcher T, 2012b. Thyroid function and perfluoroalkyl acids in children living near a chemical plant. Environ. Health Perspect 120, 1036–1041. 10.1289/ehp.1104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebker DJ, Case MT, York RG, Moore JA, Hansen KJ, Butenhoff JL, 2005. Two-generation reproduction and cross-foster studies of perfluorooctanesulfonate (PFOS) in rats. Toxicology 215, 126–148. 10.1016/j.tox.2005.07.018 [DOI] [PubMed] [Google Scholar]
- Makey CM, Webster TF, Martin JW, Shoeib M, Harner T, Dix-Cooper L, Webster GM, 2017. Airborne precursors predict maternal serum perfluoroalkyl acid concentrations. Environ. Sci. Technol 51, 7667–7675. 10.1021/acs.est.7b00615 [DOI] [PubMed] [Google Scholar]
- Mandel SJ, Spencer CA, Hollowell JG, 2005. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid 15, 44–53. 10.1089/thy.2005.15.44 [DOI] [PubMed] [Google Scholar]
- Manzano-salgado CB, Casas M, Lopez-espinosa M, Kraus T, Schettgen T, Sunyer J, Vrijheid M, 2015. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ. Res 142, 471–478. 10.1016/j.envres.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Meena M, Chopra S, Jain V, Aggarwal N, 2016. The effect of anti-thyroid peroxidase antibodies on pregnancy outcomes in euthyroid women. J. Clin. Diagnostic Res 10, QC04–QC07. 10.7860/JCDR/2016/19009.8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moog NK, Entringer S, Heim C, Wadhwa PD, Kathmann N, Buss C, 2017. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100. 10.1016/j.neuroscience.2015.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Yoshida K, Hoshikawa S, Ito S, Yoshida M, Satoh M, Watanabe C, 2006. Effects of perinatal exposure to low doses of cadmium or methylmercury on thyroid hormone metabolism in metallothionein-deficient mouse neonates. Toxicology 228, 77–84. 10.1016/j.tox.2006.08.017 [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics, 2009. Laboratory Procedure Manual - Thyroid Peroxidase Antibodies [WWW Document]. Lab. Ref. Man URL https://wwwn.cdc.gov/nchs/data/nhanes/2007-2008/labmethods/thyrod_e_met_thyroid_peroxidase_antibodies.pdf (accessed 3.19.18).
- Negro R, Stagnaro-Green A, 2014. Diagnosis and management of subclinical hypothyroidism in pregnancy. BMJ 349, 1–10. 10.1136/bmj.g4929 [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froelich JW, Seacat AM, Butenhoff JL, Zobel LR, 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, Herron RM, Hanna H, Nobiletti JB, Rios JA, Reagen WK, Ley CA, 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environ. Res 157, 87–95. 10.1016/j.envres.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ, 2003. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin. Endocrinol. (Oxf) 59, 282–8. [DOI] [PubMed] [Google Scholar]
- Pop VJ, de Vries E, van Baar AL, Waelkens JJ, de Rooy HA, Horstein M, Donkers MM, Komproe IH, van Son MM, Vader HL, 1995. Maternal thyroid peroxidase antibodies during pregnancy: a marker for impaired child development? J. Clin. Endocrinol. Metab 80, 3561–3566. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Kuijpens JL, Van Baar AL, Verkerk G, Van Son MM, De Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL, 1999. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin. Endocrinol. (Oxf) 50, 147–155. [DOI] [PubMed] [Google Scholar]
- Powley CR, George SW, Ryan TW, Buck RC, 2005. Matrix effect-free amalytical methods for determination of perfluorinated carboxylic acids in environmental matrixes. Anal. Chem 77, 6353–6358. [DOI] [PubMed] [Google Scholar]
- Préau L, Fini JB, Morvan-Dubois G, Demeneix B, 2015. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim. Biophys. Acta - Gene Regul. Mech 1849, 112–121. 10.1016/j.bbagrm.2014.06.015 [DOI] [PubMed] [Google Scholar]
- Preston EV, Webster TF, Oken E, Henn BC, McClean MD, Rifas-Shiman SL, Pearce EN, Braverman LE, Calafat AM, Ye X, Sagiv SK, 2018. Maternal Plasma per- and Polyfluoroalkyl Substance Concentrations in Early Pregnancy and Maternal and Neonatal Thyroid Function in a Prospective Birth Cohort: Project Viva (USA). Environ. Health Perspect 126, 1–11. 10.1289/EHP2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramhøj L, Hass U, Boberg J, Scholze M, Christiansen S, Nielsen F, Axelstad M, 2018. Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause antiandrogenic effects in rats. Toxicol. Sci 163, 579–591. 10.1093/toxsci/kfy055 [DOI] [PubMed] [Google Scholar]
- Reiner JL, Place BJ, 2015. Perfluorinated Alkyl Acids in Wildlife, in: DeWitt JC (Ed.), Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances Springer International Publishing, pp. 127–150. 10.1007/978-3-319-15518-0_5 [DOI] [Google Scholar]
- Ren XM, Qin WP, Cao LY, Zhang J, Yang Y, Wan B, Guo LH, 2016. Binding interactions of perfluoroalkyl substances with thyroid hormone transport proteins and potential toxicological implications. Toxicology 366–367, 32–42. 10.1016/j.tox.2016.08.011 [DOI] [PubMed] [Google Scholar]
- Rylander C, Phi DT, Odland JO, Sandanger TM, 2009. Perfluorinated compounds in delivering women from south central Vietnam. J. Environ. Monit 11, 2002–2008. 10.1039/b908551c [DOI] [PubMed] [Google Scholar]
- Rylander C, Sandanger TM, Frøyland L, Lund E, 2010. Dietary patterns and plasma concentrations of perfluorinated compounds in 315 norwegian women: The NOWAC postgenome study. Environ. Sci. Technol 44, 5225–5232. 10.1021/es100224q [DOI] [PubMed] [Google Scholar]
- Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butenhoff JL, 2002. Subchronic toxicity studies on perfluorooctanesulfonate potassium salt in cynomolgus monkeys. Toxicol. Sci 68, 249–64. [DOI] [PubMed] [Google Scholar]
- Shoeib M, Harner T, Webster M, G., Lee SC, 2011. Indoor sources of poly- and perfluorinated compounds (PFCs) in Vancouver, Canada: implications for human exposure. Environ. Sci. Technol 45, 7999–8005. 10.1021/es103562v [DOI] [PubMed] [Google Scholar]
- Smit BJ, Kok JH, Vulsma T, Briet JM, Boer K, Wiersinga WM, 2000. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr 89, 291–295. 10.1111/j.1651-2227.2000.tb18424.x [DOI] [PubMed] [Google Scholar]
- Soldin OP, O’Mara DM, Aschner M, 2008. Thyroid hormones and methylmercury toxicity. Biol. Trace Elem. Res 126, 1–12. 10.1007/s12011-008-8199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldin OP, Soldin SJ, 2011. Thyroid hormone testing by tandem mass spectrometry. Clin. Biochem 44, 89–94. 10.1016/j.clinbiochem.2010.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer D, Jiskra J, Limanova Z, Zima T, Potlukova E, 2017. Thyroid in pregnancy: From physiology to screening. Crit. Rev. Clin. Lab. Sci 54, 102–116. 10.1080/10408363.2016.1269309 [DOI] [PubMed] [Google Scholar]
- Stockholm Convention, 2018. Candidate POPs. Perfluorohexane sulfonic acid (PFHxS) and its salts and PFHxS-related compounds [WWW Document]. United Nations Environ. Program URL http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-PUB-factsheet-PFHxS-201803.English.pdf (accessed 11.21.18).
- Takser L, Mergler D, Baldwin M, Grosbois S. De, Smargiassi A, Lafond J, 2005. Thyroid Hormones in Pregnancy in Relation to Environmental Exposure to Organochlorine Compounds and Mercury. Environ. Health Perspect 113, 1039–1045. 10.1289/ehp.7685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson L. a, Lau C, 2003. Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse. I: maternal and prenatal evaluations. Toxicol. Sci 74, 369–81. 10.1093/toxsci/kfg121 [DOI] [PubMed] [Google Scholar]
- Tulchinsky D, Little B, 1994. Maternal-Fetal Endocrinology, 2nd ed. W.B. Saunders Company, Philidelphia. [Google Scholar]
- Ursinyova M, Uhnakova I, Serbin R, 2012. The relation between human exposure to mercury and thyroid hormone status. Biol. Trace Elem. Res 148, 281–291. 10.1007/s12011-012-9382-0 [DOI] [PubMed] [Google Scholar]
- Veyhe AS, Hofoss D, Hansen S, Thomassen Y, Sandanger TM, Odland JØ, Nieboer E, 2015. The Northern Norway Mother-and-Child Contaminant Cohort (MISA) Study: PCA analyses of environmental contaminants in maternal sera and dietary intake in early pregnancy. Int. J. Hyg. Environ. Health 218, 254–264. 10.1016/j.ijheh.2014.12.001 [DOI] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen P, Lien G, Chen H, Tseng Y, 2014. Association between Maternal Serum Perfluoroalkyl Substances during Pregnancy and Maternal and Cord Thyroid Hormones : Taiwan Maternal and Infant Cohort Study. Environ. Health Perspect 122, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Starling AP, Haug LS, Eggesbo M, Becher G, Thomsen C, Travlos G, King D, Hoppin JA, Rogan WJ, Longnecker MP, 2013. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environ. Heal 12, 76 10.1186/1476-069X-12-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Rauch SA, Marie NS, Mattman A, Lanphear BP, Venners SA, 2016. Cross-Sectional Associations of Serum Perfluoroalkyl Acids and Thyroid Hormones in U.S. Adults: Variation According to TPOAb and Iodine Status (NHANES 2007–2008). Environ. Health Perspect 124, 935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW, 2014. Associations between Perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: A population-based cohort study. Environ. Res 133C, 338–347. 10.1016/j.envres.2014.06.012 [DOI] [PubMed] [Google Scholar]
- Weiss JM, Andersson PL, Lamoree MH, Leonards PEG, van Leeuwen SPJ, Hamers T, 2009. Competitive binding of poly- and perfluorinated compounds to the thyroid hormone transport protein transthyretin. Toxicol. Sci 109, 206–16. 10.1093/toxsci/kfp055 [DOI] [PubMed] [Google Scholar]
- Williams GR, 2008. Neurodevelopmental and neurophysiological actions of thyroid hormone. J. Neuroendocrinol 20, 784–794. 10.1111/j.1365-2826.2008.01733.x [DOI] [PubMed] [Google Scholar]
- Yazbeck CF, Sullivan SD, 2012. Thyroid disorders during pregnancy. Med. Clin. North Am 96, 235–56. 10.1016/j.mcna.2012.01.004 [DOI] [PubMed] [Google Scholar]
- Yu W, Liu W, 2009. Prenatal and postnatal impact of perfluorooctane sulfonate (PFOS) on rat development: a cross-foster study on chemical burden and thyroid hormone system. Environ. Sci. Technol 43, 8416–8422. [DOI] [PubMed] [Google Scholar]
- Yu W, Liu W, Jin Y-H, 2009. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ. Toxicol. Chem 28, 990–996. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Beesoon S, Zhu L, Martin JW, 2013. Isomers of perfluorooctanesulfonate and perfluorooctanoate and total perfluoroalkyl acids in human serum from two cities in North China. Environ. Int 53, 9–17. 10.1016/j.envint.2012.12.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.