Key Points
A mucosally associated lymphoid tissue is identified in teleost pharyngeal cavity.
Trout pharyngeal bacteria are mainly coated with secretory IgT.
Teleost IgT plays a vital role in protecting the PC from invading pathogens.
Abstract
The pharyngeal organ is located at the crossroad of the respiratory and digestive tracts in vertebrate, and it is continuously challenged by varying Ags during breathing and feeding. In mammals, the pharyngeal mucosa (PM) is a critical first line of defense. However, the evolutionary origins and ancient roles of immune defense and microbiota homeostasis of PM are still unknown. In this study, to our knowledge, we are the first to find that diffuse MALT is present in PM of rainbow trout, an early vertebrate. Importantly, following parasitic infection, we detect that strong parasite-specific mucosal IgT and dominant proliferation of IgT+ B cell immune responses occurs in trout PM, providing, to our knowledge, the first demonstration of local mucosal Ig responses against pathogens in pharyngeal organ of a nonmammal species. Moreover, we show that the trout PM microbiota is prevalently coated with secretory IgT and, to a much lesser degree, by IgM and IgD, suggesting the key role of mucosal Igs in the immune exclusion of teleost pharyngeal bacteria. Overall, to our knowledge, our findings provide the first evidence that pharyngeal mucosal immunity appear earlier than tetrapods.
Introduction
The pharynx represents the intersection between the digestive and respiratory tracts in vertebrates (1), and the pharyngeal cavity (PC), for breathing and swallowing, is at risk for vast amounts of microbiota and foreign Ags from air, water, or food (2, 3). In terrestrial vertebrates, a unique choana connects the nasal cavity (NC) and PC (4, 5) and thus makes nasal and pharyngeal lymphoid tissue defined together as nasopharynx-associated lymphoid tissue (NALT), which acts as the first line of defense against external threats (6, 7). In mammals except rodents, NALT consists of organized lymphoid tissues containing adenoids, palatine tonsils, and lingual tonsils with highly organized germinal centers (GCs), known as Waldeyer’s ring in humans, as well as a diffuse network of immune cells surround the entrance to the pharynx (8–10). This offers mammals efficient innate and adaptive immunity to protect the upper respiratory tract (11, 12). So far, the pharyngeal tonsil has only been discovered in mammals and birds, whereas no evidence shows that the Waldeyer’s ring exists in the pharynx of birds (13, 14). In contrast, cold-blooded animals like reptiles, amphibians, and fish lack tonsils in their PC, and NALT has not been well investigated in these animals (10, 15). Although organized NALT structures in sarcopterygian fish like lungfish are found in the mucosa of the upper and lower jaw, they still lack B and T cell zones and GC formation (16). It is worth noting that teleost NALT has only been described as diffuse NALT in the NC, and it shares the main features of other teleost MALTs, including a dominant role of secreted IgT (sIgT) and IgT+ B cells in mucosal immunity (17, 18). However, because teleost fish lack the choana, and the PC is a separate compartment from the NC, whether MALT appears in the teleost PC remains an enigmatic question.
In aquatic vertebrates like teleost fish, the pharynx morphologically communicates with the gills behind the mouth and above the esophagus. Similar to mammals, teleost PC is also covered with the pharyngeal mucosa (PM), containing two main layers, the stratified squamous epithelium and the collagenous connective tissue (lamina propria [LP]) (19). Interestingly, mammalian PMs are known to contain mucus-producing cells in the epithelial layer as well as the mucus gland in the LP (20, 21), and secreted IgA (sIgA) found in pharyngeal mucus is produced by IgA-producing plasma cells in the PM generated from tonsil GCs (6, 22), which play a crucial role in humoral adaptive immunity in pharyngeal homeostasis (23–25). In contrast to mammals, teleost fish lack the mucus gland, and PM is only populated with abundant mucus secreting cells. However, so far, the pharyngeal molecular immunity mechanism within the early bony vertebrates has not been thoroughly investigated. Because teleost fish live in water medium, the PC may be subjected to more stimulation from waterborne Ags and evolutionary selective forces. Hence, we hypothesize that a mucosal immune system is indispensable in the pharynx for protecting the extensive and vulnerable mucosal surface.
Teleost fish represent the most ancient bony vertebrates, containing a specialized mucosal adaptive immune system and secretory Igs (26). So far, three teleost Ig classes (IgM, IgD, and IgT/IgZ) have been identified (27). IgM was thought to be the only functional Ig in teleosts in both the systemic and mucosal compartments. In fact, recent research suggests that IgM immune responses to infection in plasma are stronger than those in mucosal tissues (27, 28). Secreted IgD has been identified in catfish and trout (29, 30), but its function remains enigmatic. In contrast to IgM and IgD, teleost IgT (also called IgZ in some species) is the main mucosal Ig targeting mucosal pathogens and coating microbiota (28, 31–33), akin to IgA in mammals and IgX in amphibians (22, 28). We previously found that IgT is the main mucosal Ig protecting the teleost NC against pathogens (28) and that sIgA plays a major role in the PM of mammals. Thus, despite the independence of the PC from the NC in teleosts, we hypothesize that the role of teleost mucosal Ig in the PC involves a process of convergent evolution between tetrapods and nontetrapods.
To our knowledge, in this study, we are the first to show that the teleost PM is one of ancient MALTs, which can produce strong innate and adaptive immune responses to the infection with parasite Ichthyophthirius multifiliis. Moreover, we provide the first demonstration, to our knowledge, that sIgT is the main player involved in pathogen-specific immune responses. Critically, we show that IgT+ B cells are locally proliferated and generate parasite-specific IgT in the PM of rainbow trout, thus providing, to our knowledge, the first evidence of locally induced mucosal Igs responses in the PM of early vertebrates. Finally, we discover that teleost IgT plays a crucial role in immune exclusion by coating the symbiotic bacteria on the PM. Overall, our findings reveal that the presence of MALT in the PM of a nontetrapod species and sIgT plays a vital role in protecting the PC from invading pathogens.
Materials and Methods
Ethics statement
All animal procedures were performed in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of China and were approved by the Animal Experiment Committee of Huazhong Agricultural University (permit number HZAUFI-2016-007). All efforts were made to minimize the suffering of the animals.
Fish maintenance
Rainbow trout (mean weight, 200–300 g) used for pharyngeal bacteria isolation and rainbow trout (mean weight, 20–25 g) used in infection trials were obtained from a fish farm in Shiyan (Hubei, China) and maintained in aquarium tanks using a water recirculation system involving thermostatic temperature control and extensive biofiltration. Fish were acclimatized for 14 d at 15°C and fed daily with commercial trout pellets at a rate of 1% body weight per day, and feeding was terminated 48 h prior to sacrifice. Grass carp (Ctenopharyngodon idellus), southern catfish (Silurus meridionalis), mandarin fish (Siniperca chuatsi), and snakehead (Channa argus) were purchased from aquatic product market in Wuhan (Hubei, China).
I. multifiliis parasite isolation and infection
The method for I. multifiliis parasite isolation and infection were used as explained elsewhere (18). Briefly, heavily infected rainbow trout were anesthetized with overdose of MS-222 and transferred to a beaker with water to allow trophonts and tomonts exit the fish. After 4 h, the fish were removed, and the trophonts and tomonts were left in the water at 15°C for 24 h to let tomocyst formation and subsequent theront release. For parasite infection, two types of challenges with I. multifiliis parasite were performed. In the first one, fish were exposed by bath with a single dosage of ∼5000 theronts per fish for 3 h and then transferred into the aquarium containing new aquatic water. Tissue samples were taken at 0.5, 1, 4, 7, 14, 21, 28 and 75 d, and fluids (serum and pharyngeal mucus) were taken on day 28 (infected group) following I. multifiliis challenge. For the second type of challenge, fish were monthly exposed with ∼5000 theronts per fish (survivor group) for 3 h at 0, 30, and 60 d and then moved to tanks with clean water after each infection. Tissue samples and fluids (serum and pharyngeal mucus) were taken 15 d after the last challenge. Control fish (control group) were exposed to same tank water but without the parasites.
RNA isolation and quantitative real-time PCR analysis
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Tissue was homogenized by TissueLyser II (Jingxin Technology) with 5-mm stainless steel beads (QIAGEN) at 60 Hz for 1 min following the manufacturer’s instructions. The quantification and concentration of the extracted RNA was carried out by spectrophotometry (NanoPhotometer NP80 Touch), and the integrity of the RNA was determined by agarose gel electrophoresis. To normalize gene expression levels for each sample, equivalent amounts of total RNA (1000 ng) were used for cDNA synthesis as previously described (18). The synthesized cDNA was diluted four times and then used as a template for quantitative real-time PCR (qPCR) analysis. The qPCRs were performed on a 7500 Real-Time PCR System (Applied Biosystems) using the 2× SYBR Green qPCR Master Mix (YEASEN). All samples were performed in following conditions: 95°C for 5 min, followed by 40 cycles at 95°C for 10 s and at 58°C for 30 s. A sample from the serial dilution was run on a 1% agarose gel and stained with Red Gel Stain and viewed under UV light to confirm a band of the correct size was amplified. Trout elongation factor 1α (EF-1α) was used as control gene for normalization of expression. The relative expression level of the genes was determined using the Pfaffl method (34). The primers used for qPCR are listed in Supplemental Table I.
RNA sequencing library construction, sequencing, and bioinformatical analyses
The RNA sequencing (RNA-Seq) libraries from 12 samples (control fish of day 14; control fish of day 28; day 14 exposed to I. multifiliis; day 28 exposed to I. multifiliis) were generated similarly as in a previous study (35). Briefly, polyadenylated RNA fragments were purified by a Dynabeads mRNA Purification Kit, fragmented with RNA fragmentation buffer, and reverse transcribed into first-strand cDNA using random hexamer and SuperScript II Reverse Transcriptase, followed by second-strand cDNA synthesis using RNase H and DNA polymerase I. The resulting cDNA was end repaired, and a single “A” was added at the 3′ ends, and a unique identifier was labeled at the 5′ ends before ligating to Illumina paired-end sequencing adaptors. PCR was amplified using Phusion High-Fidelity Master Mix and Illumina primers with the condition of 98°C for 60 s, 15 cycles of 98°C for 10 s, and 65°C for 75 s and concluding with 65°C for 5 min. All RNA-Seq data were generated by Illumina paired-end sequencing with read length 150 bp. Reads were mapped to the Oncorhynchus mykiss genome using STAR (36) with default parameters. The mapped reads were analyzed by feature counts (37). Differential expression was estimated with edgeR package (38). We excluded the genes with low expression (count-per-million fewer than one in three or more samples) from downstream analysis. The resulting genes were considered as differentially expressed genes (DEGs) if false discovery rate ≤ 0.05 and log2 (fold-change) ≥ 1. To further understand the transcriptomic data, we carried out a gene ontology enrichment using KEGG Orthology-Based Annotation System (39) to determine the biological processes that were significantly upregulated following I. multifiliis infection. The National Center for Biotechnology Information Sequence Read Archive accession number for data reported in this manuscript is PRJNA560631 (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA560631/).
Collection of serum, pharyngeal mucus, and pharyngeal bacteria
For sampling, trout were sacrificed with an overdose of MS-222, and serum was collected and stored as described (31). To obtain pharyngeal mucus, we modified the method described previously (33). Briefly, trout pharyngeal tissue was excised rinsed with PBS three times to remove the remaining blood and then incubated for 12 h at 4°C, with slight shaking in protease inhibitor buffer (1×PBS, containing 1× protease inhibitor mixture [Roche], 1 mM PMSF [Sigma-Aldrich] [pH 7.2]) at a ratio of 1 g of pharyngeal tissue per milliliter of buffer. The suspension (including pharyngeal mucus and bacteria) was collected, vigorously vortexed, and then centrifuged at 400 × g for 10 min at 4°C to remove trout cells. To separate pharyngeal bacteria from mucus, the cell-free supernatant was thereafter centrifuged at 10,000 × g for 10 min. The resulting supernatant (containing pharyngeal mucus) was harvested, filtered with a 0.45-μm syringe filter (MilliporeSigma), and stored at 4°C prior to use the same day, whereas the pellet (containing pharyngeal bacteria) was washed three times with PBS (pH 7.2) and resuspended for further analysis.
Isolation of trout leukocytes from trout HK and pharyngeal tissue
The leukocytes from head kidney were obtained with 51/34% discontinuous Percoll density gradients as described previously (40). To obtain trout leukocytes from pharyngeal tissue, we modified the protocol from that used to isolate trout skin leukocyte as previously described (32). Briefly, rainbow trout were anesthetized with an overdose of MS-222, and blood was collected from the caudal vein. Pharyngeal tissue was obtained from each fish and washed carefully with cold PBS to avoid blood contamination. Thereafter, the pharyngeal tissue was cut into small pieces and then treated with modified DMEM (DMEM supplemented with 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin) containing 0.15 mg/ml collagenase IV (Invitrogen), for 30 min at 4°C with continuous shaking. The supernatant was collected, and the aforementioned procedure was repeated again. The pharyngeal tissue pieces were mechanically disaggregated on a 100-μm cell shredder, and the cell fraction was collected. Finally, all cell fractions obtained from the pharyngeal tissue after mechanical and enzymatic treatments were pooled and washed three times in fresh modified DMEM and layered over a 51/34% discontinuous Percoll gradient. After 30 min of centrifugation at 400 × g, cells lying at the interface of the gradient were collected and washed with modified DMEM medium.
SDS-PAGE and Western blot
Pharyngeal mucus and serum samples were collected as described above and boiled in SDS-PAGE Sample Loading Buffer (CWBiotech) at 95°C for 5 min and then resolved on 4–15% precast polyacrylamide gel (Bio-Rad Laboratories) under nonreducing or reducing conditions. The gels were transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories) with Trans-Blot SD (Bio-Rad Laboratories) by semidry method. For Western blot analysis, the membranes were blocked with 8% skim milk and incubated with anti-trout IgT (rabbit polyclonal Ab [pAb], 0.2 μg/ml), anti-trout IgM (mouse mAb, 0.2 μg/ml), or biotinylated anti-trout IgD (mouse mAb, 0.2 μg/ml) Abs or their respective isotypes control (IgG; BD Biosciences), followed by incubation with peroxidase-conjugated anti-rabbit, anti-mouse IgG (Invitrogen) or streptavidin (Invitrogen). For quantitative analyses of IgT, IgM, and IgD in pharyngeal mucus and serum, immunoreactive bands were first visualized with Clarity Western ECL Substrate (Bio-Rad Laboratories) and scanned by Amersham Imager 600 Imaging System (GE Healthcare), and then band densitometry was analyzed with ImageQuant TL software (GE Healthcare). Finally, the concentrations of IgT, IgM, and IgD were determined by plotting the obtained signal strength values on a standard curve generated for each blot using known amounts of purified trout IgT, IgM, or IgD.
The three Abs (anti-trout IgT [rabbit pAb], anti-trout IgM [mouse mAb], or biotinylated anti-trout IgD [mouse mAb]) we used in our experiments are gifted from our cooperative laboratory (Dr. O. Sunyer’s laboratory), and the specificities of these Abs have been confirmed in those papers (33).
Gel filtration
To analyze the monomeric or polymeric state of Igs in trout pharyngeal mucus, gel filtration was performed as described previously for gut and gill mucus (31, 33). Briefly, fractions containing the IgM, IgD or IgT were separated by gel filtration using a Superdex 200 FPLC column (GE Healthcare). The column was previously equilibrated with cold PBS (pH 7.2), and protein fractions were eluted at 0.5 ml/min with PBS using a fast 2protein liquid chromatography instrument with purifier systems (GE Healthcare). Identification of IgM, IgD, and IgT in the eluted fractions was performed by Western blot analysis using anti-IgM, anti-IgD, and anti-IgT Abs, respectively, as described above. A standard curve was generated by plotting the elution volume of the standard proteins in a Gel Filtration Standard (Bio-Rad Laboratories) against their known m.w., which was then used to determine the m.w. of the eluted IgT, IgM, and IgD by their elution volume.
Flow cytometry
For flow cytometry analysis, trout leukocytes suspensions were double stained with monoclonal mouse anti-trout IgT and anti-trout IgM (1 μg/ml each) at 4°C for 45 min. After washing three times, PE goat anti-mouse IgG1 and APC goat anti-mouse IgG2b (2 μg/ml each; BD Biosciences) were added and incubated at 4°C for 30 min to detect IgM+ and IgT+ B cells, respectively. Samples were washing three times prior to flow cytometric analysis. Pharyngeal bacteria were stained with anti-trout IgM or anti-trout IgT (1 μg/ml each), or their respective isotypes control (1 μg/ml each; BD Biosciences), at 4°C for 2 h with continuous shaking. After washing three times, secondary Abs Alexa Fluor 488 goat anti-mouse IgG1 or Alexa Fluor 488 goat anti-mouse IgG2b (1 μg/ml each; BD Biosciences) were added and incubated for 45 min at 4°C. To discriminate bacteria from debris, pharyngeal bacteria were labeled with BacLight Green Bacterial Stain (Invitrogen), following the manufacturer’s instructions. After three times washing, analysis of stained leukocytes and bacteria were analyzed with a CytoFLEX Flow Cytometer (Beckman Coulter) and FlowJo software (Tree Star).
Histology, light microscopy, and immunofluorescence microscopy studies
The pharyngeal tissues of grass carp, southern catfish, mandarin fish, snakehead, and rainbow trout were dissected and processed for routine histology as described previously (18). Briefly, all tissue samples were fixed in 4% neutral buffered formalin (1:10) overnight at 4°C and then transferred to 70% ethanol. Samples were embedded in paraffin and 5-μm sections were stained with H&E. Images were acquired in a microscope (Olympus) using the AxioVision software. For the detection of I. multifiliis parasite at the same time of IgT+ and IgM+ B cells, we used rabbit anti-trout IgT (0.5 μg/ml) polyclonal Ab and mouse anti-trout IgM (1 μg/ml) mAb to incubate sections overnight at 4°C. For the detection of I. multifiliis parasite at the same time of IgT+ and IgD+ B cells, we used rabbit anti-trout IgT (0.5 μg/ml) polyclonal Ab and mouse anti-trout IgD (2 μg/ml) mAb to incubated sections overnight at 4°C. After washing three times with PBS, secondary Abs Alexa Fluor 488-conjugated AffiniPure Goat Anti-Rabbit IgG or Cy3-conjugated AffiniPure Goat Anti-Mouse IgG (3 μg/ml each; Jackson ImmunoResearch Laboratories) were added and incubated at temperature for 40 min to detect IgT+ and IgM+ or IgT+ and IgD+ B cells, respectively. After washing three times with PBS, mouse anti–I. multifiliis polyclonal Ab (1 μg/ml) were added and incubated at 4°C for 6 h. After washing three times with PBS, secondary Ab Alexa Fluor 647 Goat anti-mouse (Jackson ImmunoResearch Laboratories) with 5 μg/ml were added and incubated at temperature for 40 min to detect I. multifiliis parasite. For the detection of pharyngeal trout polymeric IgR (tpIgR), we used the same methodology described to stain gill tpIgR using our rabbit anti-tpIgR (33). As controls, the rabbit IgG prebleed and the mouse-IgG1 isotype Abs were used at the same concentrations. All sections were stained with DAPI (1 μg/ml; Invitrogen) before mounting with fluorescent microscopy mounting solution. Pharyngeal bacteria were stained as previously described in the above section and stained bacteria were cytospinned on glass slides and mounted with fluorescent microscopy mounting solution. Images were acquired and analyzed using Olympus BX53 fluorescence microscope (Olympus) and the iVision-Mac scientific imaging processing software (Olympus).
Proliferation of B cells in the pharyngeal tissue of trout
For proliferation of B cells in trout, we modified the methodology as previously reported (31, 33). Briefly, survivor and control fish (∼30 g) were anesthetized with MS-222 and i.v. injected with 300 μg 5-ethynyl-2′-deoxyuridine (EdU) (Invitrogen) in 150 μl of PBS. After 24 h, leukocytes from pharyngeal tissue or head kidney were obtained as described above, and cells were incubated with mAb mouse anti-trout IgM or anti-trout IgT (1 μg/ml each) on ice for 1 h. After washing three times, Alexa Fluor 488 goat anti-mouse IgG1 or Alexa Fluor 488 goat anti-mouse IgG2b (3 μg/ml each; Invitrogen) were used as secondary Ab to detect IgM+ or IgT+ B cells, respectively. After 40 min incubation on ice, cells were washed three times with DMEM medium and fixed. EdU+ cell detection was performed according to the manufacturer’s instructions (Click-iT Plus EdU Alexa Fluor 647 Flow Cytometry Assay Kit; Invitrogen). Cells were then analyzed with a CytoFLEX Flow Cytometer (Beckman Coulter) and FlowJo software (Tree Star). For immunofluorescence analysis, we used rabbit anti-trout IgT (0.5 μg/ml) polyclonal Ab and mouse anti-trout IgM (1 μg/ml) mAb to incubate paraffin sections of pharyngeal tissues from control and survival fish previously injected with EdU overnight at 4°C. After washing with PBS, sections were incubated with Alexa Fluor 488-conjugated AffiniPure Goat anti-rabbit IgG or Cy3-conjugated AffiniPure Goat anti-mouse IgG (3 μg/ml each; Jackson ImmunoResearch Laboratories) for 2 h at room temperature. As primary control Abs, the rabbit prebleed, and the mouse-IgG1 isotype controls were used at the same concentrations. Stained cells were fixed and EdU+ cell detection was performed according to the manufacturer’s instructions (Click-iT EdU Alexa Fluor 488 Imaging Kit; Invitrogen). All sections were stained with DAPI (1 μg/ml; Invitrogen) before mounting with fluorescent microscopy mounting solution. Images were acquired and analyzed using Olympus BX53 fluorescence microscope (Olympus) and the iVision-Mac scientific imaging processing software (Olympus).
Tissue explants culture
To assess whether the parasite-specific IgT responses were locally generated in the pharyngeal tissue, we analyzed parasite-specific Ig titers from medium derived of cultured pharynx, head kidney, and spleen explants obtained from control and survivor fish, as described previously (31). Briefly, control and survivor fish were sacrificed with an overdose of MS-222, and blood was removed through the caudal vein to minimize the blood content in the collected organs. Thereafter, head kidney, spleen, and pharynx (∼30 mg each tissue) were collected and submerged in 70% ethanol for 1 min to eliminate possible bacteria on their surface. After washed twice with PBS, tissues were placed in a 24-well plate and cultured with 300 ml DMEM medium (Life Technologies), supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 200 μg/ml amphotericin B, and 250 μg/ml gentamicin sulfate, with 5% CO2 at 17°C. After 7 d culture, supernatants were harvested, centrifuged, and stored at 4°C prior to use the same day.
Binding of trout Igs to I. multifiliis
To assess whether infected and survivor fish had generated parasite-specific Igs, the capacity of IgT, IgM, and IgD from serum, pharyngeal mucus, or tissue (pharynx, spleen, and head kidney) explant supernatants to bind to I. multifiliis were measured using a pull-down assay as described previously (31, 32). Briefly, parasites (∼100 tomonts) were preincubated with a solution of 0.5% BSA in PBS (pH 7.2) at 4°C for 2 h. Subsequently, parasites were incubated with diluted pharyngeal mucus or serum or tissue (pharynx, head kidney, and spleen) explant supernatants from infected, survivor, or control fish overnight at 4°C with continuous shaking in a 300 μl volume. Dilutions were made with PBS containing 0.5% BSA (pH 7.2). After incubation, the tomonts were washed three times with PBS, and bound proteins were eluted with 2× Laemmli Sample Buffer (Bio-Rad Laboratories) and boiled for 5 min at 95°C. The eluted material was resolved on 4–15% SDS-PAGE Ready Gel (Bio-Rad Laboratories) under nonreducing conditions, and the presence of IgT, IgM, or IgD was detected by Western blotting using the anti-trout IgT, IgM, or IgD Abs, as described above.
Coimmunoprecipitate studies
To detect whether polymeric trout IgT present in the pharyngeal mucus was associated to a secretory component-like molecule derived from trout secretory component–like molecule (tSC), we used the same strategy previously described by us to detect the association of tpIgR to IgT in gut, gill, and nasal mucus (18, 31, 33). Briefly, 10 μg of anti-IgT were incubated with 400 μl of trout pharyngeal mucus overnight at 4°C. As negative control for anti-IgT, the rabbit IgG (purified from the prebleed serum of the rabbit) was used at the same concentrations. The 50 μl of Protein G Agarose (Invitrogen) was first washed five times with PBS and added into each reaction mixture and then incubated for 2 h at 4°C. Thereafter, the beads were washed five times with PBS, and subsequently bound proteins were eluted in 2× Laemmli Sample Buffer (Bio-Rad Laboratories). The eluted material was resolved by SDS-PAGE on 4–15% Tris-HCl Gradient Ready Gels (Bio-Rad Laboratories) under reducing (for tSC detection) or nonreducing (for IgT detection) conditions. Western blot was performed with anti-pIgR and anti-IgT Abs as described above.
Statistical analysis
Data were analyzed in Prism version 6.0 (GraphPad Software). An unpaired Student t test and one-way ANOVA with Bonferroni correction (Prism version 6.0; GraphPad Software) were used for analysis of differences between groups. Data are expressed as mean ± SEM. All p values <0.05 were considered statistically significant.
Results
Teleost PM shares universal features with teleost MALTs
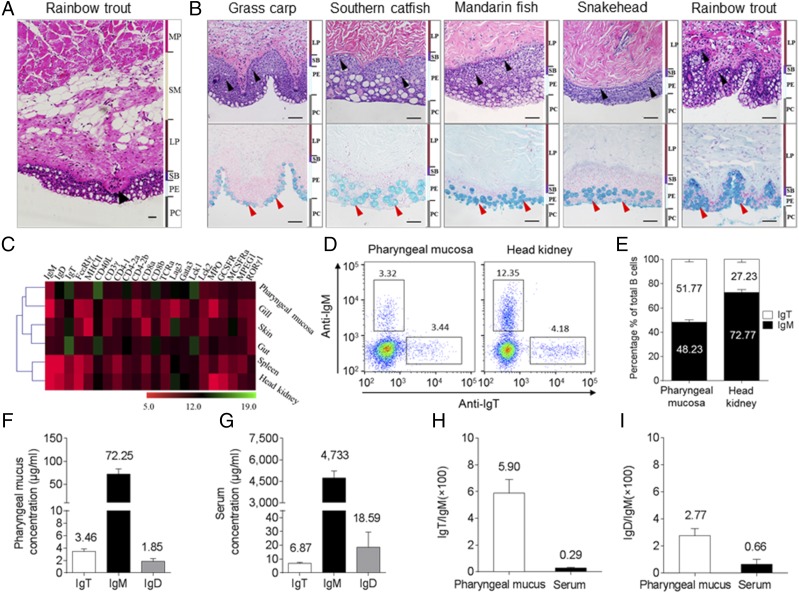
In this study, we examined the morphological structure of the PM in rainbow trout and found that, similar to the teleost gut, three typical layers are present in the pharynx: 1) mucosa (epithelium, stratum basale, and LP); 2) submucosa; and 3) muscularis propria (Fig. 1A). Interestingly, taste buds were found on top of the papillae of the submucosa (Fig. 1A), as discovered in mammals (41). To understand the histological organization of the teleost PM, we processed the paraffin sections of PM stained with H&E (Fig. 1B, upper) and Alcian blue (Fig. 1B, lower) obtained from five different teleost families: Cyprinidae, Siluridae, Percichthyidae, Channidae, and Salmonidae (Fig. 1B). We observed that the PMs of grass carp (C. idellus), southern catfish (S. meridionalis), mandarin fish (S. chuatsi), snakehead fish (Ophiocephalus argus Cantor), and rainbow trout (O. mykiss) all harbored abundant lymphoid cells scattered in the pharyngeal epithelium and LP (Fig. 1B, upper) and a large number of mucous cells in the pharyngeal epithelium (Fig. 1B, lower). These results were in agreement with the teleost MALTs (i.e., GALT, skin-associated lymphoid tissue, and NALT). Moreover, to understand the expression of immune-related genes in the PM from the control adult rainbow trout, we measured the levels of expression of 22 immune-related genes and cell markers in mucosal tissues (skin, gut, and gills) and systemic tissues (spleen and head kidney) as well as the PM by reverse transcription qPCR (Fig. 1C). By cluster analysis of the gene expression profiles of six candidate tissues, we found that PM is clustered with gills, skin, and gut (Fig. 1C), indicating an undiscovered mucosal immune function of the trout PM. Interestingly, although PM is not aggregated into one branch with the gut, the expression patterns of those genes in the PM particularly resembles the gut when compared with the other tissues. Subsequently, we analyzed the abundance of two main B cell subsets (IgM+ and IgT+ B cells) in the trout PM and head kidney by flow cytometry (Fig. 1D). Our results showed that the IgT+ B cells make up ∼51.77% of the total B cells in the PM of rainbow trout, whereas ∼48.23% of the total B cells are IgM+ B cells (Fig. 1E). Conversely, the percentage of IgT+ B cells (∼27.23%) in the head kidney of trout was ∼3-fold lower than that of IgM+ B cells (∼72.77%) (Fig. 1E). We next analyzed the concentrations of IgT, IgM, and IgD in the pharyngeal mucus and serum and found that the protein concentration of IgM was much higher than those of IgT and IgD in both the pharyngeal mucus and serum (Fig. 1F, 1G). However, the ratio of IgT/IgM and IgD/IgM in the pharyngeal mucus was ∼20-fold and ∼4-fold higher than those in the serum, respectively (Fig. 1H, 1I), which was similar to that we previously reported in trout nasal and gill mucus (11, 33). To understand the protein characterization of secretory Igs (sIgT, sIgM, and sIgD) in the pharyngeal mucus, we first collected the mucus and then loaded it into a gel filtration column. Similar to what we reported in trout nasal and gill mucus (11, 33), a great portion of IgT in the pharyngeal mucus was present in polymeric form, as it eluted at a fraction similar to that of trout IgM, a tetrameric Ig, but an extremely small portion of IgT was eluted in monomeric form (Supplemental Fig. 1A). Interestingly, immunoblot analysis showed that both the polymeric and monomeric IgT in the pharyngeal mucus migrated in the same position under nonreducing conditions (Supplemental Fig. 1B, right panel), suggesting that the subunits of polymeric IgT are associated by noncovalent interactions. In contrast, mucosal IgM and IgD migrated as a polymer and a monomer, respectively, under the same SDS-PAGE conditions (Supplemental Fig. 1B, left and middle panels).
FIGURE 1.
General organization of teleost PM lymphoid tissue. (A) H&E stain of the pharynx of a control adult rainbow trout (O. mykiss). Black arrowhead indicates taste bud. (B) H&E (upper) and Alcian blue (lower) stain of the pharynx from five different families of control adult teleost fish. From left to right: grass carp (C. idellus), southern catfish (S. meridionalis), mandarin fish (S. chuatsi), snakehead (O. argus Cantor), and rainbow trout (O. mykiss). Scale bar, 50 μm. Black and red arrowheads indicate lymphocytes and mucus cells, respectively. (C) Heat map illustrates results from qPCR of mRNAs for selected immune markers in trout skin, gut, gill, pharynx, spleen, and head kidney (n = 6). Data are expressed as mean cycle threshold (Ct) values ± SEM. (D) Flow cytometry analysis of pharynx (left) and head kidney (right) leukocytes stained with anti-IgM and anti-IgT Abs. Numbers in outlined boxes indicate the percentages of IgM+ (top left) and IgT+ (bottom right) B cells in the lymphocyte gate, respectively. (E) Frequency of IgM+ and IgT+ cells among total B cells present in trout pharynx and head kidney. (F and G) Immunoblot and densitometric analysis of the concentration of IgT, IgM, and IgD in pharyngeal mucus (F) and serum (G). (H and I) Ratio of IgT to IgM concentration (H) and IgD to IgM concentration (I) in pharyngeal mucus and serum, calculated from the values shown in (F) and (G), respectively. Results in (E)–(I) are expressed as mean ± SEM obtained from 12 individual fishes. MP, muscularis propria; PE, pharyngeal epithelium; SB, stratum basale; SM, submucosa.
Trout pharyngeal bacteria are mainly coated with IgT
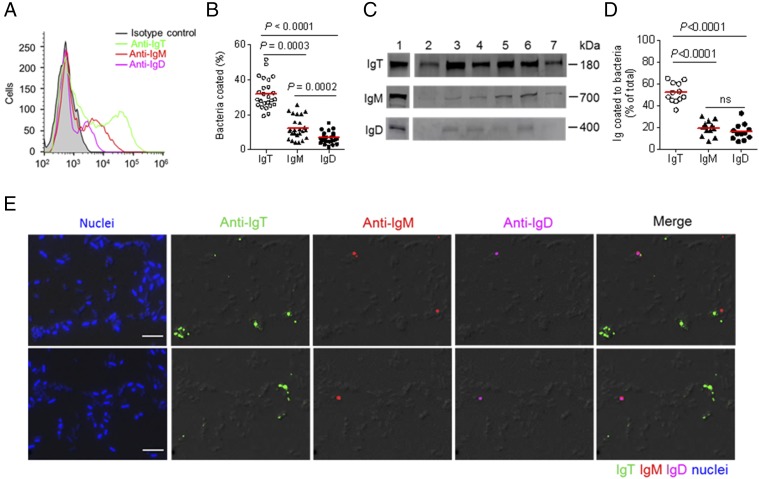
We previously reported that sIgT plays a dominant role in mucosal immune exclusion by coating a large fraction of microbiota on the mucosal surfaces of trout (31, 33). To test the participation of pharyngeal Igs in immune exclusion, using a previously reported method (32), we isolated the pharyngeal-associated bacteria and measured the levels of coating by trout Igs. Flow cytometry analysis showed that a high proportion of pharyngeal bacteria were stained for IgT (∼32%), whereas low proportions were coated with IgM (∼12%) and IgD (∼7%) (Fig. 2A, 2B). Interestingly, these results were similar to previous findings in trout skin (∼38% coated with IgT and ∼12% with IgM) (32). Moreover, immunoblot analysis showed that more than 50% of the IgT present in the pharyngeal mucus was used for bacteria coating, whereas only 17% of pharyngeal mucosal IgM, and only ∼14% of IgD were being used for that purpose (Fig. 2C, 2D). Importantly, using immunofluorescence microscopy, we further confirmed that a high percentage of trout pharyngeal bacteria were coated with mucosal IgT, and a much smaller percentage of bacteria were stained with IgM or IgD (Fig. 2E; isotype-matched control Abs, Supplemental Fig. 2A).
FIGURE 2.
A majority of trout pharyngeal bacteria are predominantly coated with IgT. (A) Representative flow cytometry histograms showing the staining of pharyngeal bacteria with IgT, IgM, and IgD. Bacteria were stained with isotype controls (shaded histograms), anti-trout IgT (green line), anti-trout IgM (red line), or anti-trout IgD (magenta line) mAbs, respectively. (B) Percentages of pharyngeal bacteria coated with IgT, IgM, or IgD (n = 25). The median percentage is showed by a red line. (C) Immunoblot analysis of IgT, IgM, or IgD coating on pharyngeal bacteria. Lane 1, 0.1 μg of purified IgT, IgM, or IgD; lanes 2–7, pharyngeal bacteria (n = 6). (D) Percentages of total pharyngeal mucus IgT, IgM, or IgD coating pharyngeal bacteria (n = 12). The median is shown by a red line. The statistical differences in (B) and (D) were evaluated by one-way ANOVA with Bonferroni correction. Data are representative of at least three independent experiments. (E) Differential interference contrast (DIC) images of pharyngeal bacteria stained with a DAPI/Hoechst solution (blue), anti-IgT (green), anti-IgM (red), or anti-IgD (magenta) and merging IgT, IgM, and IgD staining (Merge) (isotype-matched control Ab staining, Supplemental Fig. 2A). Scale bar, 5 μm.
Trout PM elicits strong immune responses to I. multifiliis parasite infection
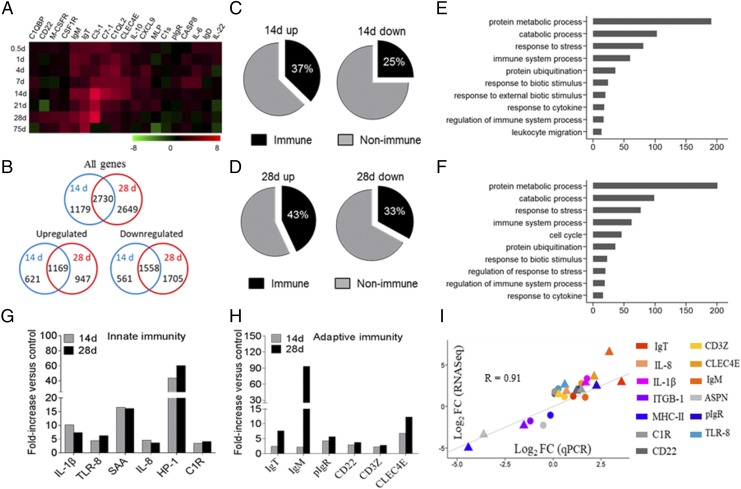
To evaluate the trout PM immune responses to pathogenic challenge, we selected the I. multifiliis parasite bath infection model, as it can elicit strong mucosal immune responses in teleosts (42, 43). At 14 d postinfection, the phenotype of small white dots appeared on the trout’s skin, gills, and pharyngeal surface (Supplemental Fig. 3B), and a pathological examination and immunofluorescence analysis showed that I. multifiliis had successfully invaded the trout PM (Supplemental Fig. 3C). Moreover, by qPCR, we detected the high expression of I. multifiliis/18S rRNA in the PM, gills, nose, and skin (Supplemental Fig. 3D). Interestingly, an increased expression trend (peaked on day 28) of I. multifiliis parasites in the trout PM was observed postinfection (Supplemental Fig. 3E). We next measured the expression of 19 immune-related genes and cell markers in the PM of trout by qPCR after I. multifiliis infection (primers used are shown in Supplemental Table I). Importantly, these results indicated that strong immune responses had occurred in the PM (Fig. 3A). Moreover, in agreement with the pathological changes in the PM (Supplemental Fig. 3A), days 14 and 28 were the most relevant in terms of the intensity of the immune response, and therefore, both were selected for subsequent RNA-Seq analysis. A total of 3909 (day 14) and 5379 (day 28) genes showed significantly differential expression, with 1790 and 2116 genes upregulated and 2119 and 3263 genes downregulated on days 14 and 28, respectively (Fig. 3B). Importantly, the resulting DEGs were subsequently filtered by the O. mykiss immune gene library, and more than 25% of DEGs were identified as immune-related genes, as shown in the pie charts (Fig. 3C, 3D). To further understand the transcriptomic data, we carried out gene ontology enrichment analysis to determine the biological processes that were significantly upregulated in the trout PM after I. multifiliis parasite infection. This overview indicated that biological processes representing genes were involved in protein metabolic process, immune system process, response to stress and cytokines, and protein ubiquitination (Fig. 3E, 3F). Importantly, we found on days 14 and 28 a significant modification in the expression of genes involved in 1) innate immunity (Fig. 3G; such as ILs, chemokines, complement factors, antimicrobial peptides, and TLR) and 2) adaptive immunity (Fig. 3H; including Ag-processing and presentation, BCR, and Igs). To validate the RNA-Seq results, 13 DEGs (10 that were upregulated and three that were downregulated) were detected by qPCR, and the results were significantly correlated with the RNA-Seq results at each time point (correlation coefficient R = 0.91, p < 0.001) (Fig. 3I).
FIGURE 3.
Kinetics of the immune response in trout pharynx following I. multifiliis parasites infection. (A) Heat map demonstrated results from qPCR of mRNAs for selected immune markers in I. multifiliis–infected fish versus control fish measured on days 0.5, 1, 4, 7, 14, 21, 28, and 75 postinfection in pharynx of rainbow trout (n = 9). Color value, log2 (fold change). (B) Venn diagrams of RNA-Seq experiment showed the overlap of genes upregulated or downregulated in the pharynx of rainbow trout at 14 or 28 d postinfection with I. multifiliis versus control fish (n = 9 per group). (C and D) Pie charts showed the percentages of immune and nonimmune genes upregulated or downregulated in the pharynx of rainbow trout at 14 (C) and 28 d (D) postinfection with I. multifiliis versus control fish by RNA-Seq studies. (E and F) Biological processes that were significantly altered of rainbow trout 14 (E) and 28 d (F) postinfection with I. multifiliis versus control fish revealed by RNA-Seq studies. Fold change differences between control and I. multifiliis–infected samples were calculated using cutoff of 2-fold. Significant differential expression at each time point (14 and 28 d) was established by unpaired t tests (p < 0.05). (G and H) Representative innate (G) and adaptive (H) immune genes modulated by I. multifiliis infection on day 14 or 28. (I) Confirmation of RNA-Seq studies by qPCR of 14 and 28 d infection with I. multifiliis. Circle, day 14 after infected with I. multifiliis; triangle, day 28 after infected with I. multifiliis; rectangle with colors, different colors represent different genes. The selected genes were the following: IgT, IgM, pIgR, Complement C1R (C1R), IL-8, IL-1β, integrin β-1 (ITGB1), MHC (MHC class II), CD22, CD3Z, C-type Lectin Domain Family 4 Member E (CLC4E), asporin (ASPN), and TLR-8.
Ig and B cell responses in PM to I. multifiliis parasite infection
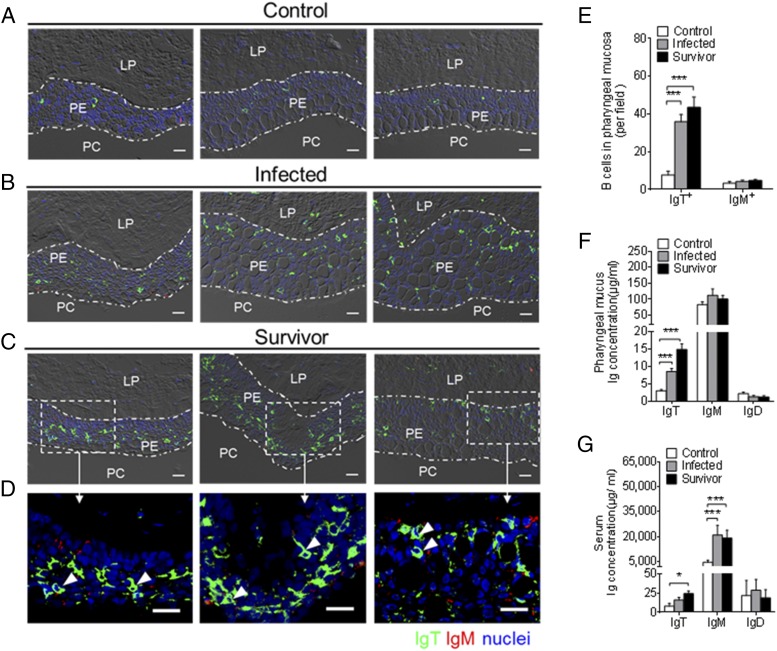
Using immunofluorescence microscopy, we detected that few IgT+ and IgM+ B cells permeated the PM of control fish (Fig. 4A; isotype-matched control Abs, Supplemental Fig. 2B). Interestingly, a moderate increase of IgT+ B cells was observed in the pharyngeal epithelium of 28-d-infected fish, which was ∼5-fold higher when compared with control fish (Fig. 4B, 4E). Notably, a large accumulation of IgT+ B cells was found in the pharyngeal epithelium of survivor fish (75 d postinfection), which was ∼6-fold higher when compared with those of control fish (Fig. 4C, 4E). It is worth mentioning that several of these IgT+ B cells were noticed to be secreting IgT (Fig. 4D, white arrows). In contrast, there was no significant difference in the number of IgM+ B cells in the infected and survivor fish when compared with that of control fish (Fig. 4A–C, 4E). In agreement with the increase of IgT+ B cells observed in the PM of infected and survivor fish, the IgT concentration in the pharyngeal mucus of the same fish groups was ∼3-fold and ∼5-fold higher, respectively, when compared with those of control fish (Fig. 4F). However, the protein concentration of IgM and IgD in the pharyngeal mucus remained unchanged after I. multifiliis parasite infection (Fig. 4F). Conversely, the IgM concentration was ∼4-fold higher in the serum of both infected and survivor fish when compared with control fish. The concentration of serum IgT was ∼2-fold and ∼3-fold higher in the infected and survivor groups, respectively (Fig. 4G). In contrast, the concentration of IgD remained unchanged in both the pharyngeal mucus and serum of the same fish (Fig. 4F, 4G).
FIGURE 4.
Accumulation of IgT+ B cells in the pharynx of trout infected with I. multifiliis. (A–C) Representative differential interference contrast (DIC) images of immunofluorescence staining on paraffinic sections of pharynx from uninfected control fish (A), 28 d–infected fish (B), and survivor fish (C). IgT+ and IgM+ B cells were stained with rabbit anti-trout IgT (green) and mouse anti-trout IgM (red), respectively; nuclei were stained with DAPI (blue) (isotype-matched control Ab staining, Supplemental Fig. 2B). (D) Enlarged images of the areas outlined in (C) are showing some IgT+ B cells possibly secreting IgT in pharyngeal epithelium (white arrowhead). Data are representative of at least three independent experiments (n = 9 per group). Scale bar, 20 μm. (E) Numbers of B cells in control, 28 d–infected, and survivor fish counted from (A)–(C). (F and G) Concentration of IgT, IgM, and IgD in pharyngeal mucus (F) and serum (G) from uninfected control fish, 28 d–infected fish, and survivor fish (n = 12). Data in (E)–(G) are representative of at least three independent experiments (mean ± SEM). *p < 0.05, ***p < 0.001 (one-way ANOVA with Bonferroni correction). PE, pharyngeal epithelium.
Pathogen-specific Ig responses in PM
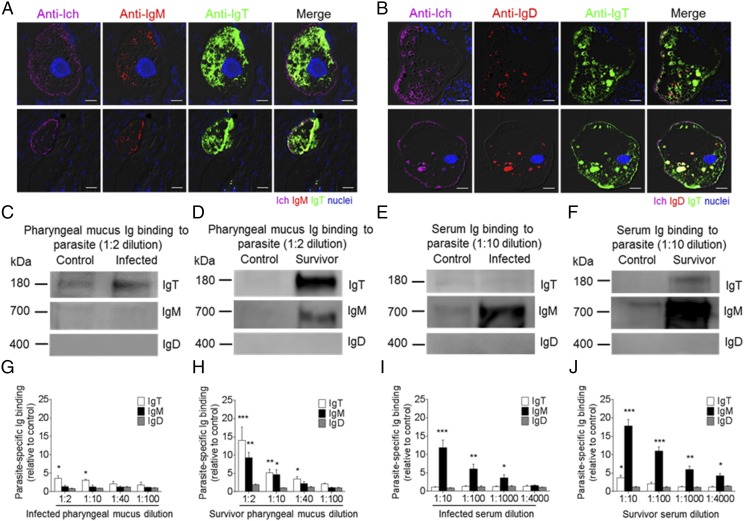
The accumulation of IgT+ B cells and the increase of IgT protein levels in the PM following I. multifiliis infection led us to hypothesize a key role of IgT in PM mucosal immunity. To verify this hypothesis, using immunofluorescence microscopy with anti–I. multifiliis Ab, I. multifiliis trophonts were easily observed in the trout PM 28 d postinfection (Fig. 5A, 5B; isotype-matched control Abs, Supplemental Fig. 2C). Strikingly, most pharyngeal parasites were predominantly coated with IgT, whereas only a few parasites were slightly coated with IgM, and almost no parasites were coated with IgD (Fig. 5A, 5B). Moreover, by a pull-down assay, we measured the capacity of pharyngeal Igs to bind the I. multifiliis parasite. Immunoblot analysis showed significant increases of parasite-specific IgT binding in up to 1:10 (∼3.1-fold) and 1:40 (∼3.5-fold) of the diluted pharyngeal mucus from infected (Fig. 5C, 5G) and survivor fish (Fig. 5D, 5H), respectively, when compared with that of control fish. However, we only found parasite-specific IgM binding in up to a 1:10 (∼5.2-fold) pharyngeal mucus dilution from survivor fish (Fig. 5D, 5H), whereas in serum, parasite-specific IgM binding in up to 1:1000 and 1:4000 serum dilutions from infected (Fig. 5E, 5I) and survivor fish (Fig. 5F, 5J) increased by ∼3.6-fold and ∼4.3-fold, respectively. In contrast, parasite-specific IgT binding was detected only in a 1:10 (∼3.8-fold) serum dilution of the survivor fish (Fig. 5E, 5F, 5I, 5J). Interestingly, no parasite-specific IgD binding was detected in either the pharyngeal mucus or serum from infected and survivor fish (Fig. 5C–J).
FIGURE 5.
Ig-specific responses in the pharynx from infected and survivor fish. (A and B) Four different microscope images of slides showing immunofluorescence staining of I. multifiliis parasites in pharyngeal paraffinic sections from trout infected with I. multifiliis after 28 d (n = 6). (A) From left to right: I. multifiliis (magenta), IgM (red), and IgT (green), with nuclei stained with DAPI (blue). (B) From left to right: I. multifiliis (magenta), IgD (red), and IgT (green), with nuclei stained with DAPI (blue); differential interference contrast (DIC) images showing merged staining (isotype-matched control Ab staining, Supplemental Fig. 2C). Scale bar, 20 μm. (C and D) Western blot analysis of IgT-, IgM-, and IgD-specific binding to I. multifiliis in pharyngeal mucus (dilution 1:2) from 28 d–infected (C) and survivor (D) fish. (E and F) Western blot analysis of IgT-, IgM-, and IgD-specific binding to I. multifiliis in serum (dilution 1:10) from 28 d–infected (E) and survivor (F) fish. (G and H) IgT-, IgM-, and IgD-specific binding to I. multifiliis in dilutions of pharyngeal mucus from infected (G) and survivor (H) fish, evaluated by densitometric analysis of immunoblots and presented as relative values to those of control uninfected fish (n = 12). (I and J) IgT-, IgM-, and IgD-specific binding to I. multifiliis in dilutions of serum from 28 d–infected (I) and survivor (J) fish, evaluated by densitometric analysis of immunoblots and presented as relative values to those of control uninfected fish (n = 12). Data are representative of at least three independent experiments (mean ± SEM). *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired Student t test).
Local proliferation of B cell and Ig responses in trout PM after I. multifiliis parasite infection
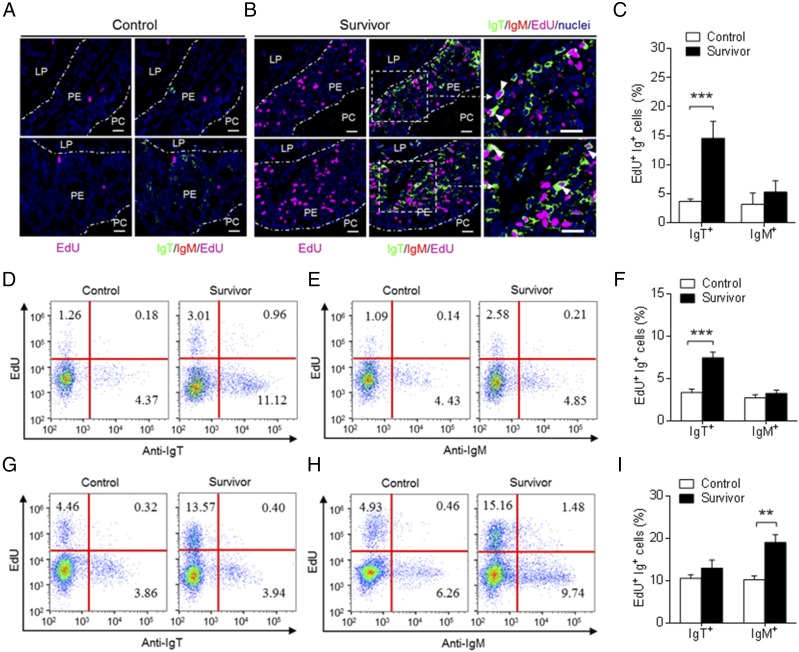
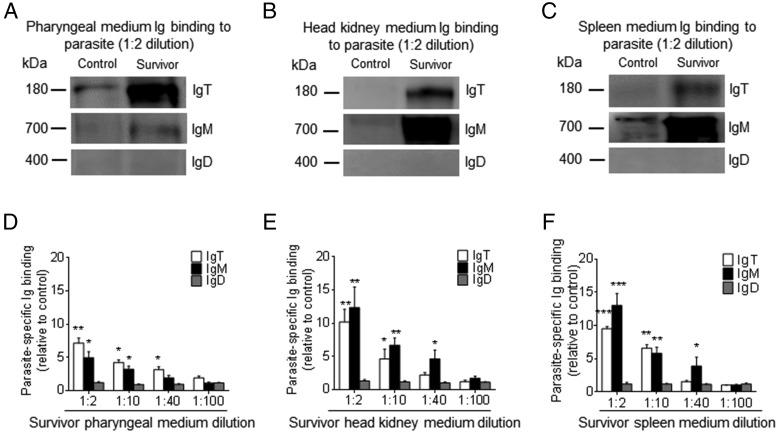
To determine whether the substantial increase of IgT+ B cells in the PM of survivor fish was derived from the process of local IgT+ B cell proliferation or the transportation of B cells from systemic lymphoid organs, we assessed the in vivo proliferative responses of IgT+ and IgM+ B cells from control and survivor fish. Using immunofluorescence microscopy, we detected a significantly higher percentage of EdU+ IgT+ B cells (∼14.56 ± 2.92% of all IgT+ B cells) of the PM in survivor fish when compared with that of control fish (∼3.69 ± 0.40% of all IgT+ B cells) (Fig. 6A–C), whereas there was no difference in the proliferation of IgM+ B cells between control and survivor fish (Fig. 6A–C). Importantly, similar results were obtained by flow cytometric analysis (Fig. 6D–F). The percentage of EdU+ IgT+ B cells was significantly higher in the PM of survivor fish (∼7.43 ± 0.68% of all IgT+ B cells) when compared with that of control fish (∼3.34 ± 0.39% of all IgT+ B cells) (Fig. 6D–F). However, we found no significant difference in the number of proliferated IgM+ B cells in the trout PM (Fig. 6D–F). In the head kidney, we found an ∼2-fold higher number of EdU+ IgM+ B cells in survivor fish (∼19.11 ± 1.84% of all IgM+ B cells) when compared with that of control fish (∼10.16 ± 0.92% of all IgT+ B cells), whereas no differences in proliferating IgT+ B cells were detected between control and survivor fish (Fig. 6G–I). Therefore, a certain percentage of IgT+ B cells are locally proliferated. To verify whether parasite-specific IgT in phalangeal mucus is locally synthesized in the PM or originated from systemic lymphoid organs, we further analyzed parasite-specific Ig titers from medium of cultured PM, head kidney, and spleen explants from control and survivor fish (Fig. 7). We detected parasite-specific IgT binding in up to 1:40 diluted medium (∼3.6-fold) of cultured PM explants of survivor fish, whereas low parasite-specific IgM titers were detected only at the 1:10 dilution in the same medium (Fig. 7A, 7D). On the contrary, the predominant parasite-specific IgM titers (up to 1:40) were found in the medium of survivor head kidney and spleen explants, and low parasite-specific IgT responses (up to 1:10) were detected in the same medium (Fig. 7B, 7C, 7E, 7F). Interestingly, we could not detect any parasite-specific IgD titers in the medium of cultured PM, head kidney, or spleen explants from control and survivor fish (Fig. 7A–F).
FIGURE 6.
Proliferative responses of IgT+ and IgM+ B cells in the pharynx of survived fish. (A and B) Immunofluorescence analysis of EdU incorporation by IgT+ or IgM+ B cells in the pharynx of control (A) and survivor fish (B). Pharynx paraffinic sections were stained for EdU (magenta), trout IgT (green), trout IgM (red), and nuclei (blue) detection (n = 9). White rectangle represents partial enlargement area. White arrowheads point to cells double stained for EdU and IgT. Data are representative of at least three independent experiments. Scale bar, 20 μm. (C) Percentage of EdU+ cells from the total pharynx IgT+ or IgM+ B cell populations in control or survivor fish counted from (A) and (B). (D and E) Representative flow cytometry dot plot showing proliferation of IgT+ (D) and IgM+ (E) B cells in pharynx leukocytes of control and survivor fish. The percentage of lymphocytes representing proliferative B cells (EdU+) is shown in each dot plot. (F) Percentage of EdU+ cells from the total pharynx IgT+ or IgM+ B cell populations in control and survivor fish (n = 9). (G and H) Representative flow cytometry dot plot showing proliferation of IgT+ (G) and IgM+ (H) B cells in head kidney leukocytes of control and survivor fish. The percentage of lymphocytes representing proliferative B cells (EdU+) is shown in each dot plot. (I) Percentages of EdU+ cells from the total head kidney IgT+ or IgM+ B cell populations in control and survivor fish (n = 9). Data in (C), (F), and (I) are representative of at least three independent experiments (mean ± SEM). **p < 0.01, ***p < 0.001 (unpaired Student t test). PE, pharyngeal epithelium.
FIGURE 7.
Local IgT-, IgM-, and IgD-specific responses to I. multifiliis parasite of survivor fish. Pharynx, head kidney, and spleen explants (∼30 mg each) from control and survivor fish were cultured in medium (400 μl) for 7 d. (A–C) Western blot analysis of IgT-, IgM-, and IgD-specific binding to I. multifiliis in the culture medium of pharynx (A), head kidney (B), and spleen (C) (dilution 1:2) from control and survivor fish. (D–F) IgT-, IgM-, and IgD-specific binding to I. multifiliis in dilutions of pharynx (D), head kidney (E), and spleen (F) culture medium from survivor fish, evaluated by densitometric analysis of immunoblots and presented as relative values to those of control fish (n = 12). Data are representative of at least three independent experiments (mean ± SEM). *p < 0.05, **p < 0.01, ***p < 0.001 (unpaired Student t test).
pIgR in PM of trout
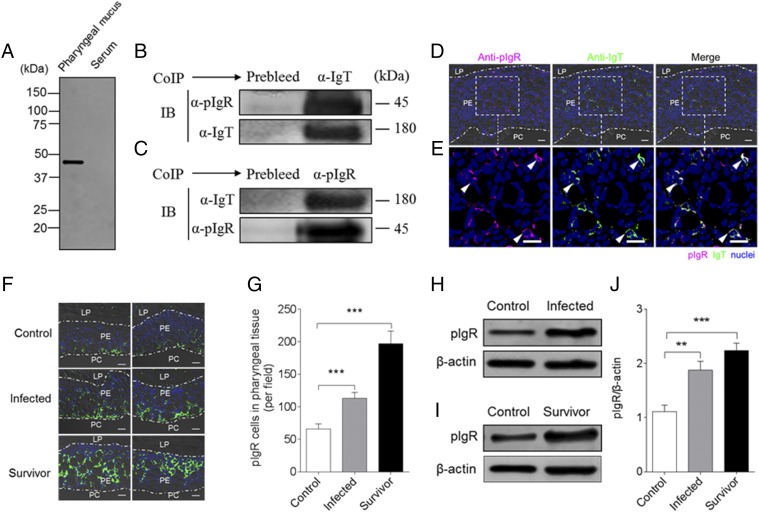
In mammals, sIgA is transported to PM surfaces mediated by the pIgR (8). Interestingly, previous studies showed that the secretory component of pIgR (tSC) is associated with IgT in the different mucosa of teleosts (18, 31–33). In the current study, using immunoblot analysis under reducing conditions, tSC was detected in the pharyngeal mucus, but not in the serum (Fig. 8A). To determine whether tSC (tpIgR) was associated with pharyngeal mucus IgT, we performed coimmunoprecipitation assays in pharyngeal mucus using Abs against tSC and trout IgT. We found that Abs against trout IgT were able to coimmunoprecipitate pIgR in the pharyngeal mucus (Fig. 8B) and that sIgT in the pharyngeal mucus could be immunoprecipitated by the anti-pIgR Ab (Fig. 8C). Moreover, immunofluorescence microscopy analysis showed that most of pIgR-containing cells were located in the pharyngeal epithelium of trout, and some of those pIgR-containing cells were stained with IgT (Fig. 8D, 8E; isotype-matched control Abs, Supplemental Fig. 2D). It is worth mentioning that a notable accumulation of pIgR-containing cells was observed in the PM of infected (∼2-fold) and survivor fish (∼4-fold) when compared with that of control fish (Fig. 8F, 8G). Critically, a significant increase of the pIgR protein levels was detected in the pharyngeal mucus in both infected (Fig. 8H, 7J) and survivor fish (Figs. 8I, 8J, 9).
FIGURE 8.
Trout pIgR associates with sIgT in pharyngeal mucus. (A) SDS-PAGE under reducing conditions of trout pharyngeal mucus and serum, followed by immunoblot analysis (IB) using anti-trout pIgR Ab. (B) Coimmunoprecipitation (CoIP) of pharyngeal mucus with rabbit anti-trout IgT Ab, followed by IB under reducing conditions (pIgR detection, upper panels) or nonreducing conditions (IgT detection, lower panels). (C) CoIP of pharyngeal mucus with rabbit anti-trout pIgR, followed by (IB) nonreducing conditions (IgT detection, upper panels) or under reducing conditions (pIgR detection, lower panels). IgG purified from rabbit’s serum before immunization (Prebleed) served as negative control for rabbit anti-trout IgT and rabbit anti-trout pIgR, respectively [left lane on each panel for (B) and (C)]. (D) Immunofluorescence staining for pIgR with IgT in pharynx paraffinic sections of rainbow trout. Differential interference contrast images of pharynx paraffinic sections were stained with anti-trout IgT (green), anti-trout pIgR (magenta), and DAPI for nuclei (blue) (n = 6) (isotype-matched control Abs for anti-pIgR in Supplemental Fig. 2D). (E) Enlarged sections of the areas outlined in (D) without differential interference contrast (DIC) showing some pIgR/IgT colocalization (white arrowhead). Data are representative of at least three independent experiments. Scale bar, 20 μm. (F) Two different DIC images of immunofluorescence staining for pIgR (green) on pharynx paraffinic sections from uninfected control fish (left), infected fish (middle), and survivor fish (right); nuclei are stained with DAPI (blue) (n = 9). Data are representative of at least three independent experiments. Scale bar, 20 μm. (G) Counts of pIgR cells per field (counted in 27 fields) in paraffinic sections of pharynx from uninfected control fish, infected fish, and survivor fish. (H) Representative IB of pIgR (upper panels) in pharyngeal mucus from uninfected control fish (left) and infected fish (right). (I) Representative IB of pIgR (upper panels) in pharyngeal mucus from uninfected control fish (left) and survivor fish (right). β-Actin was used as a loading control [lower lane on each panel for (H) and (I)]. (J) These data in (H) and (I) were quantified by densitometry, followed by normalization to the β-actin loading control (n = 9). Data are representative of at least three independent experiments (mean ± SEM). **p < 0.01, ***p < 0.001 (unpaired Student t test).
FIGURE 9.
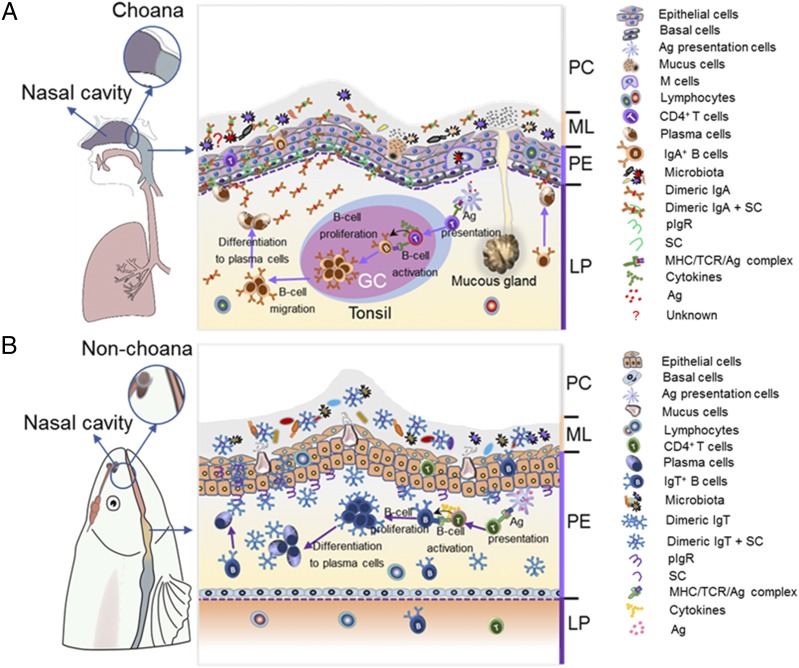
Schematic representation of mucosal homeostasis in PM of mammals (A) and fish (B). (A) Left, In mammals, they have evolved a unique choana that connects the NC and PC, thus forming a passageway to acquire oxygen from external air. (A) Right, All mucosal regions of mammalian PC are covered by PM, which contains two main layers, an outer layer of stratified squamous epithelium (pharyngeal epithelium) and an underlying layer of dense connective tissue (LP). It is populated with both mucus-secreting cells within pharyngeal epithelium (PE) and the mucus gland within LP, which secrete mucus together into mucous layer (ML). Moreover, mammalian PM has organized lymphoid structures (i.e., tonsils) with GC, and upon pathogenic infection, it can generate activated IgA+ B cells and then migrate into the LP, where they are further differentiated into plasmablasts or plasma cells. Thereafter, IgA containing the joining J chain plasma cells is secreted by plasma cells and transported via pIgR to the ML together with mucus in control of pathogens or the recognition of microbiota. (B) Left, In contrast, because teleost fish lacks the choana, the PC is a separate compartment from the NC. (B) Right, Fish PM has two similar layers, PE and LP. Interestingly, very abundant mucus-secreting cells are instead present, which produce pharyngeal mucus directly into the ML. Moreover, IgT+ B cells are found scattered mainly in the PE, where they increase in significant numbers upon pathogenic infection. Critically, sIgT is locally generated from these IgT+ B cells, and it is transported by pIgR produced by parenchymal cells within PE into pharyngeal secretions in control of pathogens or the recognition of microbiota. Overall, in pharyngeal mucosal site, teleost sIgT and mammal sIgA (similar, but slightly different, modes of production) play a conserved role in maintaining PM homeostasis.
Discussion
The mammalian pharynx has evolved tonsils strategically located to generate efficient mucosal immunity to protect the PC against airborne and alimentary Ags (6). However, in the ancient bony vertebrates like teleost fish, which lack tonsils in pharynx, whether they have pharyngeal immunity against waterborne Ags remains unknown. In this study, we showed the role of B cell and Ig responses in controlling pathogens in the PC of rainbow trout (O. mykiss), presenting molecular mechanisms for mucosal adaptive immunity similar to those within mammals. Thus, our findings revealed that the pharynx serves universally conserved immune defense functions in both primitive and modern bony vertebrates.
In this study, we showed for the first time, to our knowledge, that the trout PM contains diffuse MALT but lacks the organized lymphoid structures (i.e., tonsils), as well as a preponderance of IgT+ over IgM+ B cells. Interestingly, IgA+ B cells are fewer in number than IgG+ B cells in mammals (44), whereas IgA is the predominant Ig in the nasopharyngeal secretions of infants at 7 wk of age (45). In this study, the protein levels of pharyngeal mucus sIgT were found to be higher than those reported in the skin (∼11-fold) (32), gill (2-fold) (33), and nasal mucus (∼2-fold) (18) and comparable to those in the gut mucus (31). In line with the case of mammalian sIgA (46–48), IgT protein levels vary at individual mucosal surfaces in fish. Moreover, trout Ig protein characteristic analysis showed that most of the sIgT presents in the pharyngeal mucus as a polymer, similar to the characteristic of sIgA in the respiratory and gastrointestinal tract mucosal surfaces from humans (49). Unlike tetrameric IgM (31), the monomeric subunits of polymeric IgT are associated by noncovalent interactions, as previously found in other sources of mucus in teleosts (18, 31–33). Thus, the predominance of IgT+ B cells and the higher protein levels of IgT in the pharyngeal mucus suggest a possible role of IgT in pharynx mucosal immunity.
In mammals, sIgA acts as the first line of mucosal defense against microbiota by immune exclusion (50, 51). It has been estimated that up to 70% of bacteria in the gut lumen are coated with sIgA (less with IgG and IgM) (52), and IgA coating is thought to efficiently inhibit luminal bacteria colonization and invasion of the gut epithelium (53). Our previous findings in trout have shown that, similar to sIgA in mammals, sIgT is the main Ig isotype coating the bacteria in the mucosal surface of fish (18, 31–33). In this study, to our knowledge, we showed for the first time the presence of commensal bacteria in association with the teleost pharyngeal Igs. In the case of trout pharyngeal bacteria, a large population of bacteria was predominantly coated with sIgT and, to a lesser degree, with sIgM and sIgD. Although the PM was thought to be colonized by high densities of microbiota (54, 55), few studies have investigated the relationship between mucosal Igs and PM bacteria. Thus, our results, to our knowledge, provide the first demonstration of the dominant role of mucosal IgT in the control of pharyngeal commensal bacteria in vertebrates. Interestingly, a landmark study demonstrated that the members of the intestinal microbiota (Prevotellaceae, Helicobacter, and segmented filamentous bacteria), which are known to preferentially drive intestinal inflammation, are highly coated with IgA (56). In this study, we hypothesize that microbiota species in PM coated with sIgT may have a potential role in triggering inflammatory response. Therefore, future studies need to identify the type of PM microbiota species and address the potential role of PM microbiota species coated with sIgT.
In this study, we report for the first time, to our knowledge, that I. multifiliis can invade the trout PM and elicit strong local immune responses. By qPCR, we detected the I. multifiliis parasites loads in the trout pharynx began to increase significantly on day 7 postinfection, similar to what we have previously reported in the nose of trout (18). Notably, both innate and adaptive immune molecules are significantly upregulated in the trout PM postinfection. Critically, based on transcriptome sequencing analysis, the upregulated expression of immune-related molecules contains ILs, chemokines, complement factors, antimicrobial peptides, TLR, and Igs known to be crucial in the pharyngeal immune system of mammals (24, 57, 58). Our results concur with the previous studies in which a large number of immune-related genes were upregulated in nasal mucosa from rainbow trout postinfection with I. multifiliis parasites (18). Differently, we found that the greatest changes in expression of complement-related genes in nasal mucosa occurred on day 7 postinfection; however, it mainly occurred in PM on day 14 postinfection. In addition, we found that a large accumulation of IgT+, but not IgM+, B cells occurred in the pharyngeal epithelium of infected and survivor fish exposed to I. multifiliis, in accordance with the increased concentration of IgT, but not IgM or IgD, in the pharyngeal mucus of the same individuals. Similarly, numerous research studies on Ig and plasma cell responses in the mammalian PM have reported on pharyngeal secretions and various tonsils (11, 25, 59). Importantly, higher parasite-specific IgT titers than IgM were detected in the pharyngeal mucus as well as a much higher degree of IgT coating than IgM on the pharyngeal parasites, indicating an important role for sIgT in the control of I. multifiliis infection. As expected, we detected an overwhelming prevalence of IgM titers in the serum. Combined, our results support the idea that IgT and IgM responses are specialized in pharyngeal mucosal and systemic immunity, respectively. Interestingly, following intranasal immunization with lipoteichoic acid and cholera toxin B, Ag-specific IgA and IgG responses were evoked effectively in mammal PM and serum, respectively (12, 60). Thus, our results suggest a conserved role of fish sIgT and mammalian sIgA in the protection of the PM from pathogens through convergent evolution.
In mammals, most activated B cells in the PM migrate from tonsillar GCs (6, 8, 58), and the sIgA normally appearing in pharyngeal secretions is produced by local plasma cells that originated from various tonsils (6, 8). However, given the lack of tonsils in teleost fish, it is unclear whether the IgT+ B cell accumulations originated from local B cell proliferation in the PM or migration from other lymphoid organs. In this study, by in vivo proliferation assays, we showed for the first time, to our knowledge, dominant proliferative IgT+ B cell responses in the trout PM. In contrast, strong IgM+ B cell proliferative responses were detected in the head kidney of the same fish. Thus, this finding strongly suggests that the accumulation of IgT+ B cells in the pharyngeal epithelium is the result of local proliferation rather than migration from the head kidney. Interestingly, similar results were also discovered in the nose and gills (18, 33). In addition, we confirmed the local production of high pathogen-specific IgT titers in PM tissue explant cultures, suggesting the presence of parasite-specific plasma cells in the local PM. Similarly, in the PM from pharyngitis patients, a substantial sIgA seemed to be secreted locally by IgA-secreting plasma cells generated from lymphoid follicles and in the mucosa itself (23). Moreover, by in vitro culture of human tonsil lymphocytes following diphtheria toxoid stimulation, the specific IgA could be measured in the culture supernatant (61). Hence, our data indicate that the local mucosal B cells and secretion of mucosal Ig responses in the PM happen not only in tetrapod species, but also in nontetrapod species such as teleost fish.
To protect the mucosal surfaces from invading pathogens and maintain homeostasis, vertebrates have developed an exquisite mucosal immune system in which the secretory component or the pIgR produced by epithelial cells can transport sIgA to the mucosal surface (8, 62). However, little actual evidence exists in support of the direct interactions between sIgA and pIgR in the PM of vertebrates. Critically, we observed that pIgR-positive cells mainly existed in the epithelial layer of the trout PM, and trout secretory component was associated with sIgT in the pharyngeal mucus. Thus, our finding provides, to our knowledge, the first demonstration that sIgT was transported by pIgR to trout PM. More importantly, following I. multifiliis infection, we found a significant increase in the pIgR-positive cells and pIgR protein levels in the PM and pharyngeal mucus, respectively. These results are in agreement with previous findings on human intestinal epithelial cells treated with Saccharomyces boulardii and reovirus (63, 64). Moreover, prior studies have shown that pIgR-deficient mice cannot transport polymeric IgA into the intestinal lumen (65). Therefore, to gain insight into the role of pIgR in the homeostasis of PM, future studies are warranted to ascertain the specific contributions of pIgR to the pharyngeal immune responses of teleost fish against pathogens.
In conclusion, our findings indicate the presence of immune function in the PM of teleost fish. To maintain PC homeostasis, fish and mammals have evolved different structures and fascinating strategies in the PM. Mammals have a choana that connects the NC and PC, and their PM is known to contain both mucus glands and mucus-producing cells that produce the mucus that coats their pharyngeal epithelium (20, 21). Importantly, lymphoid structures (i.e., tonsils) are organized in the PM to defense against external pathogens. In that regard, activated IgA+ B cells generated from GCs migrate into the LP and pharyngeal epithelium, where they are further differentiated into plasmablasts or plasma cells to produce sIgA (6, 8, 58). Subsequently, the sIgA-containing J chain is transported via pIgR-expressing epithelial cells into secretions of PM in control of pathogens (62) (Fig. 9A). In contrast, teleost fish lack the choana and mucus glands, and the PM contains diffuse lymphoid cells without any organized lymphoid tissues. Critically, following pathogenic infection, IgT+ B cells in the PM epithelium can locally proliferate and differentiate into plasmablasts or plasma cells to produce sIgT and then transport via pIgR-expressing epithelial cells into the outer layer of the PM epithelium in control of pathogens and the recognition of microbiota (Fig. 9B). Thus, different types lymphoid structures (organized versus diffuse) and molecules (sIgA versus sIgT) evolved in PM of mammals and fish, but utilize functionally analogous strategies to maintain PC homeostasis. Overall, these data indicate that the mucosal adaptive immune response in the PM involves a process of convergent evolution between tetrapods and nontetrapods.
Supplementary Material
Acknowledgments
We thank Dr. J. Oriol Sunyer (University of Pennsylvania) for the generous gift of anti-trout IgM, anti-trout IgD, anti-trout IgT mAbs, anti-trout IgT, and anti-trout pIgR pAbs. We thank Dr. Fangkui Wang from the State Key Laboratory of Agricultural Microbiology (Huazhong Agricultural University) for technical help about flow cytometry analysis. We would also like to thank the staff of the Flow Cytometry and Cell Sorting Facility of Wuhan Institute of Biotechnology for immune cell analysis.
This work was supported by grants from the National Key Research and Development Program of China (2018YFD0900503 and 2018YFD0900400) (to Z.X.) and the National Natural Science Foundation of China (31873045) (to Z.X.).
The data presented in this article have been submitted to the National Center for Biotechnology Information Sequence Read Archive (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA560631/) under accession number PRJNA560631.
The online version of this article contains supplemental material.
- DEG
- differentially expressed gene
- EdU
- 5-ethynyl-2′-deoxyuridine
- GC
- germinal center
- LP
- lamina propria
- NALT
- nasopharynx-associated lymphoid tissue
- NC
- nasal cavity
- pAb
- polyclonal Ab
- PC
- pharyngeal cavity
- pIgR
- polymeric IgR
- PM
- pharyngeal mucosa
- qPCR
- quantitative real-time PCR
- RNA-Seq
- RNA sequencing
- sIgA
- secreted IgA
- sIgT
- secreted IgT
- tpIgR
- trout polymeric IgR
- tSC
- secretory component–like molecule.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Graham A., Richardson J. 2012. Developmental and evolutionary origins of the pharyngeal apparatus. Evodevo 3: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laitman J. T., Reidenberg J. S. 1993. Specializations of the human upper respiratory and upper digestive systems as seen through comparative and developmental anatomy. Dysphagia 8: 318–325. [DOI] [PubMed] [Google Scholar]
- 3.Casteleyn C., Breugelmans S., Simoens P., Van den Broeck W. 2011. The tonsils revisited: review of the anatomical localization and histological characteristics of the tonsils of domestic and laboratory animals. Clin. Dev. Immunol. 2011: 472460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janvier P. 2004. Wandering nostrils. Nature 432: 23–24. [DOI] [PubMed] [Google Scholar]
- 5.Zhu M., Ahlberg P. E. 2004. The origin of the internal nostril of tetrapods. Nature 432: 94–97. [DOI] [PubMed] [Google Scholar]
- 6.Brandtzaeg P. 2011. Immune functions of nasopharyngeal lymphoid tissue. Adv. Otorhinolaryngol. 72: 20–24. [DOI] [PubMed] [Google Scholar]
- 7.Debertin A. S., Tschernig T., Tönjes H., Kleemann W. J., Tröger H. D., Pabst R. 2003. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin. Exp. Immunol. 134: 503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry M., Whyte A. 1998. Immunology of the tonsils. Immunol. Today 19: 414–421. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti A., Chen K., Chorny A. 2011. Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 29: 273–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sepahi A., Salinas I. 2016. The evolution of nasal immune systems in vertebrates. Mol. Immunol. 69: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama Y., Harabuchi Y. 2002. Intranasal immunization with lipoteichoic acid and cholera toxin evokes specific pharyngeal IgA and systemic IgG responses and inhibits streptococcal adherence to pharyngeal epithelial cells in mice. Int. J. Pediatr. Otorhinolaryngol. 63: 235–241. [DOI] [PubMed] [Google Scholar]
- 12.Hotomi M., Yamanaka N., Shimada J., Suzumoto M., Ikeda Y., Sakai A., Arai J., Green B. 2002. Intranasal immunization with recombinant outer membrane protein P6 induces specific immune responses against nontypeable Haemophilus influenzae. Int. J. Pediatr. Otorhinolaryngol. 65: 109–116. [DOI] [PubMed] [Google Scholar]
- 13.Crole M. R., Soley J. T. 2012. Evidence of a true pharyngeal tonsil in birds: a novel lymphoid organ in Dromaius novaehollandiae and Struthio camelus (Palaeognathae). Front. Zool. 9: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickel R., Schummer A., Seiferle E. 1977. The Anatomy of the Domestic Birds. Parey, Berlin, Hamburg. [Google Scholar]
- 15.Shields J. W., Dickson D. R., Abbott W., Devlin J. 1979. Thymic, bursal and lymphoreticular evolution. Dev. Comp. Immunol. 3: 5–22. [DOI] [PubMed] [Google Scholar]
- 16.Tacchi L., Larragoite E. T., Muñoz P., Amemiya C. T., Salinas I. 2015. African lungfish reveal the evolutionary origins of organized mucosal lymphoid tissue in vertebrates. Curr. Biol. 25: 2417–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacchi L., Musharrafieh R., Larragoite E. T., Crossey K., Erhardt E. B., Martin S. A. M., LaPatra S. E., Salinas I. 2014. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 5: 5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Y. Y., Kong W., Yin Y. X., Dong F., Huang Z. Y., Yin G. M., Dong S., Salinas I., Zhang Y. A., Xu Z. 2018. Mucosal immunoglobulins protect the olfactory organ of teleost fish against parasitic infection. PLoS Pathog. 14: e1007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatchavalvanich K., Marcos R., Poonpirom J., Thongpan A., Rocha E. 2006. Histology of the digestive tract of the freshwater stingray Himantura signifer compagno and roberts, 1982 (Elasmobranchii, Dasyatidae). Anat. Embryol. (Berl.) 211: 507–518. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu T. 2013. Mucus, goblet cell, submucosal gland. In Nasal Physiology and Pathophysiology of Nasal Disorders. Springer, Heidelberg, Germany, p. 1–14. [Google Scholar]
- 21.Suzuki T., Sato T., Kano M., Ichikawa H. 2013. The distribution of galanin-immunoreactive nerve fibers in the rat pharynx. Neuropeptides 47: 231–236. [DOI] [PubMed] [Google Scholar]
- 22.van Kempen M. J., Rijkers G. T., Van Cauwenberge P. B. 2000. The immune response in adenoids and tonsils. Int. Arch. Allergy Immunol. 122: 8–19. [DOI] [PubMed] [Google Scholar]
- 23.Li Y. 1993. The relationship between SIgA and chronic granular pharyngitis. J. Laryngol. Otol. 107: 532–534. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka N., Fukuyama S., Ushikai M., Miyashita K., Kurono Y. 2003. Immune responses of palatine tonsil against bacterial antigens. Int. Congr. Ser. 1257: 141–144. [Google Scholar]
- 25.Brandtzaeg P. 2003. Immunology of tonsils and adenoids: everything the ENT surgeon needs to know. [Published erratum appears in 2004 Int. J. Pediatr. Otorhinolaryngol. 68: 387.] Int. J. Pediatr. Otorhinolaryngol. 67(Suppl. 1): S69–S76. [DOI] [PubMed] [Google Scholar]
- 26.Flajnik M. F., Kasahara M. 2010. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 11: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salinas I., Zhang Y. A., Sunyer J. O. 2011. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 35: 1346–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunyer J. O. 2013. Fishing for mammalian paradigms in the teleost immune system. Nat. Immunol. 14: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edholm E. S., Bengtén E., Stafford J. L., Sahoo M., Taylor E. B., Miller N. W., Wilson M. 2010. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J. Immunol. 185: 4082–4094. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez-Gomez F., Greene W., Rego K., Hansen J. D., Costa G., Kataria P., Bromage E. S. 2012. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. J. Immunol. 188: 1341–1349. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y. A., Salinas I., Li J., Parra D., Bjork S., Xu Z., LaPatra S. E., Bartholomew J., Sunyer J. O. 2010. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 11: 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z., Parra D., Gómez D., Salinas I., Zhang Y. A., von Gersdorff Jørgensen L., Heinecke R. D., Buchmann K., LaPatra S., Sunyer J. O. 2013. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 110: 13097–13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z., Takizawa F., Parra D., Gómez D., von Gersdorff Jørgensen L., LaPatra S. E., Sunyer J. O. 2016. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 7: 10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abyzov A., Mariani J., Palejev D., Zhang Y., Haney M. S., Tomasini L., Ferrandino A. F., Rosenberg Belmaker L. A., Szekely A., Wilson M., et al. 2012. Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492: 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R. 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao Y., Smyth G. K., Shi W. 2014. FeatureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. [DOI] [PubMed] [Google Scholar]
- 38.Robinson M. D., McCarthy D. J., Smyth G. K. 2010. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu J., Mao X., Cai T., Luo J., Wei L. 2006. KOBAS server: a web-based platform for automated annotation and pathway identification. Nucleic Acids Res. 34: W720–W724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J., Barreda D. R., Zhang Y. A., Boshra H., Gelman A. E., Lapatra S., Tort L., Sunyer J. O. 2006. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 7: 1116–1124. [DOI] [PubMed] [Google Scholar]
- 41.Travers S. P., Nicklas K. 1990. Taste bud distribution in the rat pharynx and larynx. Anat. Rec. 227: 373–379. [DOI] [PubMed] [Google Scholar]
- 42.Dickerson H., Clark T. 1998. Ichthyophthirius multifiliis: a model of cutaneous infection and immunity in fishes. Immunol. Rev. 166: 377–384. [DOI] [PubMed] [Google Scholar]
- 43.Dickerson H. W., Findly R. C. 2014. Immunity to Ichthyophthirius infections in fish: a synopsis. Dev. Comp. Immunol. 43: 290–299. [DOI] [PubMed] [Google Scholar]
- 44.Brandtzaeg P., Surjan L., Jr., Berdal P. 1978. Immunoglobulin systems of human tonsils. I. Control subjects of various ages: quantification of Ig-producing cells, tonsillar morphometry and serum Ig concentrations. Clin. Exp. Immunol. 31: 367–381. [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor C. E., Toms G. L. 1984. Immunoglobulin concentrations in nasopharyngeal secretions. Arch. Dis. Child. 59: 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell K. R., Shorr R., Cherry J. D., Hendley J. O. 1977. Improved method for collection of nasal mucus. J. Infect. Dis. 136: 109–111. [DOI] [PubMed] [Google Scholar]
- 47.Okada T., Konishi H., Ito M., Nagura H., Asai J. 1988. Identification of secretory immunoglobulin A in human sweat and sweat glands. J. Invest. Dermatol. 90: 648–651. [DOI] [PubMed] [Google Scholar]
- 48.Aufricht C., Tenner W., Salzer H. R., Khoss A. E., Wurst E., Herkner K. 1992. Salivary IgA concentration is influenced by the saliva collection method. Eur. J. Clin. Chem. Clin. Biochem. 30: 81–83. [PubMed] [Google Scholar]
- 49.Woof J. M., Russell M. W. 2011. Structure and function relationships in IgA. Mucosal Immunol. 4: 590–597. [DOI] [PubMed] [Google Scholar]
- 50.van der Waaij L. A., Limburg P. C., Mesander G., van der Waaij D. 1996. In vivo IgA coating of anaerobic bacteria in human faeces. Gut 38: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Egmond M., Damen C. A., van Spriel A. B., Vidarsson G., van Garderen E., van de Winkel J. G. 2001. IgA and the IgA Fc receptor. Trends Immunol. 22: 205–211. [DOI] [PubMed] [Google Scholar]
- 52.Phalipon A., Cardona A., Kraehenbuhl J. P., Edelman L., Sansonetti P. J., Corthésy B. 2002. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17: 107–115. [DOI] [PubMed] [Google Scholar]
- 53.Cerutti A., Rescigno M. 2008. The biology of intestinal immunoglobulin A responses. Immunity 28: 740–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baltimore R. S., Duncan R. L., Shapiro E. D., Edberg S. C. 1989. Epidemiology of pharyngeal colonization of infants with aerobic gram-negative rod bacteria. J. Clin. Microbiol. 27: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suárez-Arrabal M. C., Mella C., Lopez S. M., Brown N. V., Hall M. W., Hammond S., Shiels W., Groner J., Marcon M., Ramilo O., Mejias A. 2015. Nasopharyngeal bacterial burden and antibiotics: influence on inflammatory markers and disease severity in infants with respiratory syncytial virus bronchiolitis. J. Infect. 71: 458–469. [DOI] [PubMed] [Google Scholar]
- 56.Palm N. W., de Zoete M. R., Cullen T. W., Barry N. A., Stefanowski J., Hao L., Degnan P. H., Hu J., Peter I., Zhang W., et al. 2014. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158: 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perera R., Tsang P., Miller C., Li P., Lee M., Samaranayake L. 2011. Identification of differential pharyngeal cytokine profiles during HIV infection. BMC Proc. 5(Suppl. 1): P90. [Google Scholar]
- 58.Han Y., Bo Z. J., Xu M. Y., Sun N., Liu D. H. 2014. The protective role of TLR3 and TLR9 ligands in human pharyngeal epithelial cells infected with influenza a virus. Korean J. Physiol. Pharmacol. 18: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaur R., Kim T., Casey J. R., Pichichero M. E. 2012. Antibody in middle ear fluid of children originates predominantly from sera and nasopharyngeal secretions. Clin. Vaccine Immunol. 19: 1593–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell M. W., Moldoveanu Z., White P. L., Sibert G. J., Mestecky J., Michalek S M. 1996. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the Cholera toxin B subunit. Infect. Immun. 64: 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Platts-Mills T. A., Ishizaka K. 1975. IgA and IgA diphtheria antitoxin responses from human tonsil lymphocytes. J. Immunol. 114: 1058–1064. [PubMed] [Google Scholar]
- 62.Asano M., Komiyama K. 2011. Polymeric immunoglobulin receptor. J. Oral Sci. 53: 147–156. [DOI] [PubMed] [Google Scholar]
- 63.Buts J. P., Bernasconi P., Vaerman J. P., Dive C. 1990. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig. Dis. Sci. 35: 251–256. [DOI] [PubMed] [Google Scholar]
- 64.Kaetzel C. S. 2005. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 206: 83–99. [DOI] [PubMed] [Google Scholar]
- 65.Davids B. J., Palm J. E., Housley M. P., Smith J. R., Andersen Y. S., Martin M. G., Hendrickson B. A., Johansen F. E., Svärd S. G., Gillin F. D., Eckmann L. 2006. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J. Immunol. 177: 6281–6290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.