Abstract
MYH9-related disease is a rare genetic disorder characterized by macrothrombocytopenia, with frequent proteinuric nephropathy, hearing loss, and cataract. Although proteinuric nephropathy usually progresses to renal failure, there is no established treatment for the nephropathy. We herein describe the case of a 19-year-old man carrying an E1841K MYH9 mutation, who developed persistent proteinuria. The patient was diagnosed with early-stage MYH9-related nephropathy based on the histological examination of a kidney biopsy specimen. The patient was treated with enalapril, which significantly reduced the proteinuria with no decline in his renal function. The early administration of renin-angiotensin system blockade therapy may have beneficial effects on MYH9-related nephropathy in patients with E1841K mutations. We also briefly summarize previously published cases of MYH9-related nephropathy treated with renin-angiotensin system (RAS) blockade therapy.
Keywords: MYH9-related disease, nephropathy, proteinuria, end-stage kidney disease, focal segmental glomerulosclerosis (FSGS), treatment
Introduction
MYH9-related disease is a rare autosomal dominant disorder caused by mutations of the MYH9 gene, which encodes non-muscle myosin heavy chain IIA (NMMHC-IIA) (1). MYH9-related disease includes 4 syndromes: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome. The prevalence of MYH9-related disease in Japan is estimated to be approximately 1 in 100,000 (2). At birth, patients with MYH9-related disease have only a variable degree of macrothrombocytopenia and neutrophilic inclusions; however, later in life, some patients may develop non-hematological manifestations, including proteinuric nephropathy, sensorineural hearing loss, and cataract.
Proteinuric nephropathy occurs in approximately 30% of patients with MYH9-related disease, with most developing progressive renal impairment and end-stage renal disease (ESRD) (3). The mechanism of renal disease progression after the onset of proteinuria remains unclear, and there is no established treatment for MYH9-related nephropathy. We herein describe the case of a patient carrying an E1841K MYH9 mutation, who was histologically diagnosed with early-stage MYH9-related nephropathy. In this patient, renin-angiotensin system (RAS) blockade therapy had the beneficial effect of reducing proteinuria. We also briefly summarize previously published cases of MYH9-related nephropathy treated with RAS blockade therapy.
Case Report
A 19-year-old Japanese man was referred to our hospital for the evaluation of persistent proteinuria. He was first noted to have transient proteinuria at 10 years of age and developed persistent proteinuria at 18 years of age. He was previously healthy with no significant medical history. His mother was diagnosed with Sjögren syndrome at 18 years of age and had mild renal insufficiency with normal urinalysis results (serum creatinine, 0.99 mg/dL). She was known to have thrombocytopenia (platelet count, 50,000 /μL) with large platelets and sensorineural deafness.
The patient was 161.5 cm tall, weighed 46 kg, and his blood pressure was 120/75 mmHg. A urinalysis revealed 3+ proteinuria with granular casts but no hematuria. His spot urine protein to creatinine ratio was 0.78 g/gCr, and 24-hour urine protein was 0.52 g/day. The patient's renal function was normal, with a serum creatinine level of 0.84 mg/dL (estimated glomerular filtration rate, 99 mL/min/1.73 m2). The results of liver function tests were within the normal ranges, and his serum albumin level was 4.5 g/dL. A complete blood count revealed mild thrombocytopenia (96,000 /μL) with normal hemoglobin and white blood cell counts. The platelet histogram showed a wide curve, and the mean platelet volume was increased to 13.8 fL (reference range, 7-11 fL), indicating the presence of giant platelets (Fig. 1a). On a peripheral smear, giant platelets and neutrophil cytoplasmic inclusions (Döhle-like bodies) were observed (Fig. 1b). The patient's coagulation test results were normal. Autoimmune disease tests were negative, and the patient's complement levels were normal. His hemoglobin A1c level was 5.1%. The patient's plasma renin activity and aldosterone concentration were not determined. Renal ultrasound revealed normal-sized kidneys with preserved cortical thickness; the renal length was 9.7 cm on the right side and 10.1 cm on the left side.
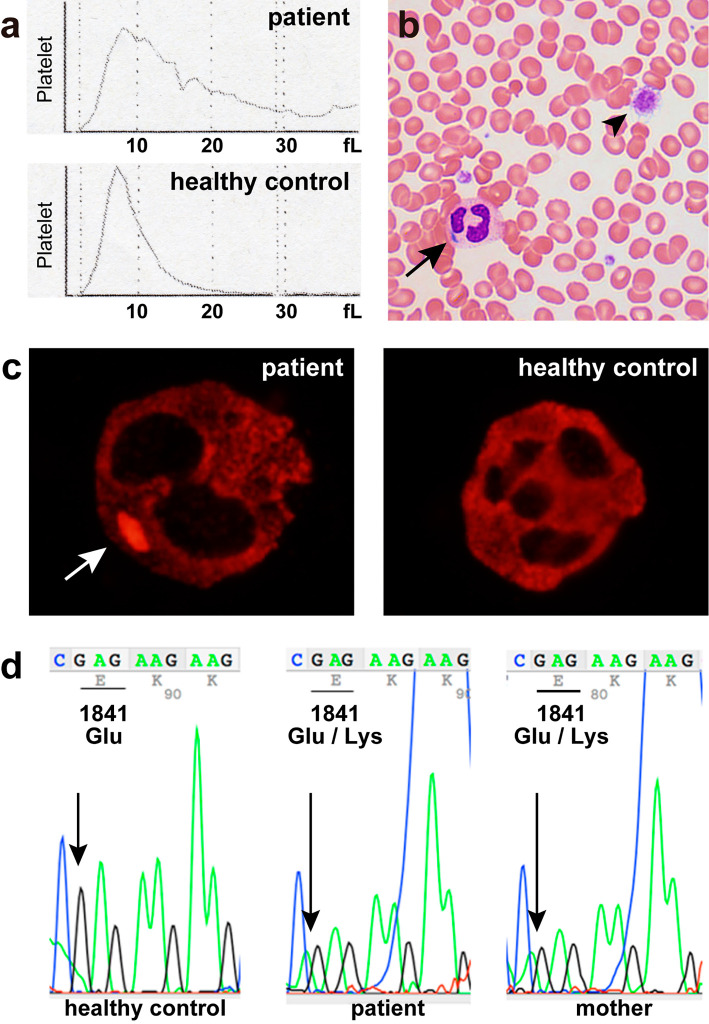
Figure 1.
(a) Platelet histograms from the patient and a healthy control. The patient’s histogram shows a broad distribution of platelets with no return to baseline, indicating the presence of abnormally sized platelets. The normal platelet histogram shows a narrow curve with a sharp peak that returns to baseline at 20 fL. (b) A peripheral blood smear shows a giant platelet (arrowhead) and Döhle-like body in the cytoplasm of a neutrophil (arrow) (Giemsa staining, original magnification ×600). (c) NMMHC-IIA localization in the neutrophils of the patient and a healthy control. NMMHC-IIA forms a cytoplasmic aggregate in a neutrophil of the patient (arrow). In the normal neutrophil, NMMHC-IIA is uniformly distributed in the cytoplasm (original magnification ×1,000). (d) A sequence analysis of the MYH9 gene reveals a heterozygous missense mutation of E1841K in the patient and his mother.
The presence of thrombocytopenia with giant platelets and Döhle-like bodies in the neutrophils strongly suggested MYH9-related disease. Immunostaining of a blood sample for NMMHC-IIA (the protein encoded by the MYH9 gene) revealed abnormal cytoplasmic aggregates of NMMHC-IIA in the neutrophils, corresponding to the Döhle-like bodies (Fig. 1c) (4). An audiological evaluation confirmed mild bilateral sensorineural deafness. Ophthalmological evaluation revealed no abnormalities. After obtaining written informed consent from the patient and his mother, a molecular genetic analysis was performed. Direct sequencing of the MYH9 gene revealed a heterozygous E1841K mutation in exon 38 in both the patient and his mother (Fig. 1d). Based on these findings, we diagnosed the patient with MYH9-related disease.
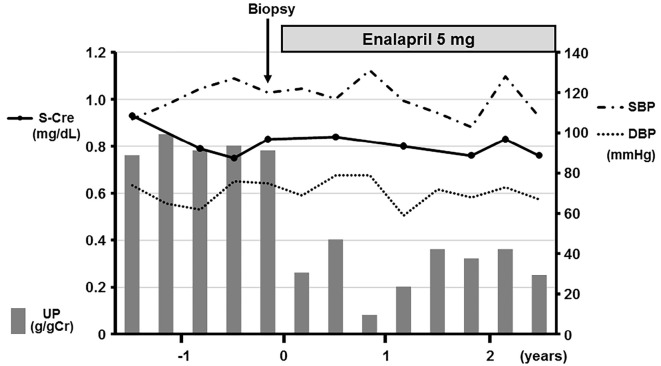
A kidney biopsy was performed to evaluate the underlying cause of proteinuria. The patient's platelet count before the biopsy was 102,000 /μL; thus, prophylactic platelet transfusion was not performed. Light microscopy revealed 15 glomeruli, none of which were globally or segmentally sclerosed. The glomeruli showed no significant abnormalities (Fig. 2a and b). No crescents were seen, and the blood vessels and interstitium were unremarkable (Fig. 2b). Immunofluorescence studies were all negative; however, electron microscopy revealed segmental thinning and thickening of the glomerular basement membrane (GBM) and moderate podocyte foot process effacement (Fig. 2c and d). Electron-dense deposits were not present in the glomeruli. These findings suggested a diagnosis of MYH9-related nephropathy. Enalapril (5 mg, per day) was administered. No adverse effects, including hypotension, were noted. At the 2.5-year follow-up, his proteinuria had decreased to 0.25 g/gCr and his serum creatinine level was stable at 0.76 mg/dL (Fig. 3).
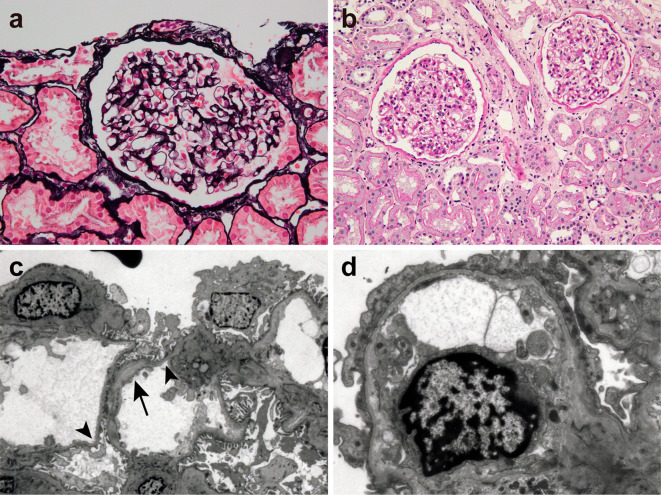
Figure 2.
Kidney biopsy findings consistent with MYH9-related nephropathy. (a) The glomerulus exhibits no apparent abnormalities (periodic acid-methenamine-silver staining, original magnification ×400). (b) A light micrograph shows normal glomeruli, renal tubules, and blood vessels (periodic acid-Schiff staining, original magnification ×200). (c) An electron micrograph shows thinning (arrowhead) and thickening (arrow) of the glomerular basement membrane (original magnification ×1,500). (d) A podocyte shows moderate foot process effacement. Electron-dense deposits are not present (original magnification ×5,000).
Figure 3.
The clinical course of the patient. UP: urinary protein, S-Cre: serum creatinine, SBP: systolic blood pressure, DBP: diastolic blood pressure
Discussion
Renal manifestations in MYH9-related disease typically begin with the early onset of proteinuria with or without microhematuria and most cases progress to ESRD (3). Kidney biopsy is contraindicated in patients with thrombocytopenia; thus, the pathogenesis of MYH9-related nephropathy is still not fully understood. NMMHC-IIA, the protein encoded by the MYH9 gene, is an actin-binding protein that also plays an important role in cell adhesion and the maintenance of tissue architecture (5). In the human kidney, NMMHC-IIA is mainly expressed in podocytes and also in renal tubular cells and endothelial cells of the interlobular arteries and arterioles (6). NMMHC-IIA is a key component of the podocyte foot process contractile apparatus, and its mutants are believed to impair the podocyte cytoskeleton, leading to foot process effacement and subsequent focal segmental glomerulosclerosis (FSGS) (6). In fact, mouse models of MYH9-related disease manifest with podocyte foot process effacement, proteinuria, FSGS, and glomerulosclerosis (7,8). In a few previous reports, the examination of kidney biopsy specimens from patients with MYH9-related nephropathy revealed similar glomerular lesions, which were often accompanied by GBM abnormalities including segmental thinning, splitting, and thickening (9). Although the exact mechanism underlying the development of GBM abnormalities in MYH9-related nephropathy remains unclear, mutated NMMHC-IIA is considered to modulate podocyte function, including GBM synthesis and organization (6,9). In the present case, the examination of the kidney biopsy specimen revealed moderate foot process effacement with segmental GBM thinning and thickening, which was consistent with previous histological findings in patients with MYH9-related nephropathy. There was no evidence of other glomerular disease, including immune complex-mediated glomerulonephritis. Based on these findings and the detection of an MYH9 mutation in the genetic test, the patient was diagnosed with MYH9-related nephropathy. We considered that our case represented an early stage of MYH9-related nephropathy due to the absence of FSGS lesions.
The genotype-phenotype correlations have been identified in MYH9-related disease (3). MYH9 is a large gene consisting of 41 exons, and more than 40 different mutations have been found to date (10). Among them, mutations affecting the following 6 residues are common and are responsible for 80% cases of MYH9-related disease: S96 and R702, located in the motor domain; R1165, D1424, and E1841, located in the coiled-coil domain; and R1933, located in the non-helical portion of the tail domain (10). Mutations in the motor domain are associated with severe nephropathy. In an event-free survival analysis, all patients with R702 mutations developed proteinuric nephropathy before 45 years of age and their renal function progressively declined, with a median time of 5 years from the onset of the proteinuria to ESRD (3). There is almost no variability in the clinical course of nephropathy in patients with R702 mutations; thus, this gene mutation is considered the only critical determinant of progression to ESRD in these patients.
In contrast, mutations in the coiled-coil domain, including E1841K mutations, are associated with a lower incidence of proteinuric nephropathy, with an incidence of 0.46 cases per 100 person-years in patients with an E1841K mutation (3). There is considerable clinical variability among individuals carrying the same mutation, even within the same family (3). In the present case, the patient's mother carried the same E1841K mutation, but she had no signs of proteinuric nephropathy. Moreover, in patients with a mutation in the coiled-coil domain of the MYH9 protein, the clinical course of proteinuric nephropathy also varies in severity; more than half of patients did not progress to ESRD after the onset of proteinuric nephropathy (3). In the analysis of a large family with 10 members carrying an E1841K mutation, 4 of 7 members with proteinuric nephropathy did not develop ESRD (11). These findings suggest that an additional factor, which remains unidentified, is required for the onset and progression of proteinuric nephropathy in patients with a mutation in the coiled-coil domain of the MYH9 protein.
Recently, interesting results regarding the progression of proteinuric nephropathy have been reported based on studies of mice with an E1841K mutation (7). At baseline, mice homozygous for an E1841K mutation (E1841K mutant mice) exhibited higher albuminuria with mild foot process effacement in comparison to wild-type mice but did not develop FSGS spontaneously during the follow-up period. Hypertension induced by angiotensin II resulted in severe foot process effacement and FSGS in E1841K mutant mice, whereas it had only a very mild effect on renal pathology in wild-type mice, despite similar blood pressure levels after angiotensin II infusion. Treatment with candesartan during angiotensin II infusion significantly prevented FSGS development in E1841K mutant mice. These results suggest that, in the setting of an E1841K mutation, the progression of nephropathy from podocyte injury to FSGS may require a second stimulus, and avoidance of this “second stimulus” in the early stages of nephropathy could prevent progression to ESRD (7). In the present case, the short-term renal effects of the early administration of RAS blockade therapy were favorable, with a significant reduction in proteinuria; however, longer follow-up would be needed to properly assess the effects of this therapy on the renal outcomes in our patient. Previously published cases of MYH9-related nephropathy treated with RAS blockade therapy are summarized in Table (6,12-17). Only 1 case carried the same E1841K mutation that was found in our patient. The plasma renin activity and aldosterone concentration were not measured before the initiation of therapy in any cases. Interestingly, RAS blockade therapy seemed effective for reducing the proteinuria and maintaining the renal function in all patients with a single missense mutation in the coiled-coil domain of the MYH9 protein. RAS blockade therapy may render podocytes less susceptible to a second stimulus; however, whether these agents act through their antiproteinuric effect, antihypertensive effect, or other unidentified mechanisms remains unclear. Further research is needed to confirm its efficacy and identify the underlying mechanism of its effect.
Table.
Case Reports of MYH9-related Nephropathy Treated with RAS Blockade Therapy.
| Case | Age*/Sex | Genotype | Domain | U-P* | U-B* | S-Cre* (mg/dL) | Treatment (mg/day) | Follow-up period | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Present case 2019 | 19/M | E1841K | coiled-coil | 0.78 g/gCr | (-) | 0.84 | enalapril 5 | 29 months | U-P: decreased to 0.25 g/gCr S-Cre: stable |
| [12], 2018 | 13/M | R702C | motor | <1 g/gCr | NA | normal | telmisartan 40 | ESRD at the age of 19 years | |
| 11/M | R702C | motor | 1-2 g/gCr | NA | normal | lisinopril 10 valsartan 160 | ESRD at the age of 15 years | ||
| 11/M | R702C | motor | 1 g/gCr | NA | normal | lisinopril 10 valsartan 160 | ESRD at the age of 17 years | ||
| [13], 2015 | 36/F | E1841K | coiled-coil | 0.926 g/day | NA | 0.7 | ARB | 1 year | U-P: decreased to 0.224 g/gCr S-Cre: stable |
| [14], 2013 | 22/M | Q1068_ L1074del | coiled-coil (deletion) | 2.18 g/day | (-) | 1.55 | candesartan 6 | ESRD at the age of 27 years | |
| [15], 2011 | 17/M | S96L | motor | (+) | (+) | 3.0 | ACEI | ESRD at the age of 23 years | |
| 12/F | S96L | motor | (+) | (+) | NA | ACEI | CKD4 at the age of 19 years | ||
| 1/M | R718W | motor | (+) | (-) | 0.3 | ACEI+ARB | ESRD at the age of 7 years | ||
| [6], 2010 | 3/F | R702C | motor | 0.5-0.7 g/gCr | (-) | CKD1 | valsartan 20 | 10 months | U-P: decreased to 0.1 g/gCr |
| NA/M | R702C | motor | NA | NA | NA | ARB and/or ACEI | The effect was transient. ESRD at the age of 20 years | ||
| NA/M | R702C | motor | NA | NA | NA | ARB and/or ACEI | The effect was not conclusive. | ||
| [16], 2008 | 39/M | D1424H | coiled-coil | 1.231 g/day | NA | 1.8 | ramipril 10 telmisartan 80 | 68 months | U-P: decreased to 0.09 g/gCr S-Cr: stable |
| 42/M | D1424H | coiled-coil | 1.570 g/day | NA | 1.2 | ramipril 10 telmisartan 80 | 16 months | U-P: decreased to 0.137 g/gCr S-Cr: stable | |
| 18/M | D1424H | coiled-coil | 0.768 g/day | NA | 0.9 | ramipril 10 losartan 50 | 11 months | U-P: decreased to 0.143 g/gCr S-Cr: stable | |
| 38/F | N93K | motor | 1.280 g/day | NA | 0.9 | ramipril 5 | 40 months | U-P: decreased to 0.39 g/gCr S-Cr: stable | |
| [17], 2004 | 27/F | K910Q + D1424H | coiled-coil (double mutations) | non-nephrotic | (+) | 3.3 | ACEI | ESRD |
RAS: renin-angiotensin system, U-P: urinary protein, U-B: urinary occult blood, S-Cre: serum creatinine, ESRD: end-stage renal disease, ARB: angiotensin II receptor blocker, ACEI: angiotensin-converting enzyme inhibitor, CKD: chronic kidney disease, NA: not available
* At the time of starting RAS blockade therapy
In summary, the early administration of RAS blockade therapy may have potential benefits in MYH9-related nephropathy in cases involving E1841K mutations. Early genotype identification would enable the better management of patients with MYH9-related nephropathy.
Written informed consent was obtained from the patient for the publication of his clinical data.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Toshiko Toyota for her excellent technical assistance.
References
- 1. Kunishima S, Kojima T, Matsushita T, et al. . Mutations in the NMMHC-A gene cause autosomal dominant macrothrombocytopenia with leukocyte inclusions (May-Hegglin anomaly/Sebastian syndrome). Blood 97: 1147-1149, 2001. [DOI] [PubMed] [Google Scholar]
- 2. Information Center for Specific Pediatric Chronic Diseases, Japan. May-Hegglin anomaly [Internet]. [cited 2019 Mar 26]. Available from: https://www.shouman.jp/disease/details/09_18_030/ (in Japanese).
- 3. Pecci A, Klersy C, Gresele P, et al. . MYH9-related disease: a novel prognostic model to predict the clinical evolution of the disease based on genotype-phenotype correlations. Hum Mutat 35: 236-247, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunishima S, Matsushita T, Kojima T, et al. . Immunofluorescence analysis of neutrophil nonmuscle myosin heavy chain-A in MYH9 disorders: association of subcellular localization with MYH9 mutations. Lab Invest 83: 115-122, 2003. [DOI] [PubMed] [Google Scholar]
- 5. Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778-790, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sekine T, Konno M, Sasaki S, et al. . Patients with Epstein-Fechtner syndromes owing to MYH9 R702 mutations develop progressive proteinuric renal disease. Kidney Int 78: 207-214, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Cechova S, Dong F, Chan F, Kelley MJ, Ruiz P, Le TH. MYH9 E1841K mutation augments proteinuria and podocyte injury and migration. J Am Soc Nephrol 29: 155-167, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Conti MA, Malide D, et al. . Mouse models of MYH9-related disease: mutations in nonmuscle myosin II-A. Blood 119: 238-250, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kopp JB. Glomerular pathology in autosomal dominant MYH9 spectrum disorders: what are the clues telling us about disease mechanism? Kidney Int 78: 130-133, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balduini CL, Pecci A, Savoia A. Recent advances in the understanding and management of MYH9-related inherited thrombocytopenias. Br J Haematol 154: 161-174, 2011. [DOI] [PubMed] [Google Scholar]
- 11. Hao J, Kunishima S, Guo X, Hu R, Gao W. A large family with MYH9 disorder caused by E1841K mutation suffering from serious kidney and hearing impairment and cataracts. Ann Hematol 91: 1147-1148, 2012. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto J, Hamasaki Y, Takahashi Y, et al. . Management of patients with severe Epstein syndrome: a review of four patients who received living-donor renal transplantation. Nephrology (Carlton) 24: 450-455, 2019. [DOI] [PubMed] [Google Scholar]
- 13. Oh T, Jung Seo H, Taek Lee K, et al. . MYH9 nephropathy. Kidney Res Clin Pract 34: 53-56, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishida M, Mori Y, Ota N, Inaba T, Kunishima S. Association of a novel in-frame deletion mutation of the MYH9 gene with end-stage renal failure: case report and review of the literature. Clin Nephrol 80: 218-222, 2013. [DOI] [PubMed] [Google Scholar]
- 15. Han KH, Lee H, Kang HG, et al. . Renal manifestations of patients with MYH9-related disorders. Pediatr Nephrol 26: 549-555, 2011. [DOI] [PubMed] [Google Scholar]
- 16. Pecci A, Granata A, Fiore CE, Balduini CL. Renin-angiotensin system blockade is effective in reducing proteinuria of patients with progressive nephropathy caused by MYH9 mutations (Fechtner-Epstein syndrome). Nephrol Dial Transplant 23: 2690-2692, 2008. [DOI] [PubMed] [Google Scholar]
- 17. Capria M, Andreucci M, Fuiano L, et al. . Double nucleotidic mutation of the MYH9 gene in a young patient with end-stage renal disease. Nephrol Dial Transplant 19: 249-251, 2004. [DOI] [PubMed] [Google Scholar]