Abstract
A 57-year-old man was diagnosed with IgA nephropathy. Hematuria and proteinuria were improved by tonsillectomy plus methylprednisolone pulse therapy. Lymphadenopathy, hypocomplementemia and pancytopenia were observed six years later, and urinalysis abnormalities recurred. A biopsy revealed mesangial proliferative glomerulonephritis with C3-dominant deposition. Human immunodeficiency virus (HIV) antibody demonstrated positive conversion. He was diagnosed with HIV-associated immune complex kidney disease (HIVICK). The hematuria, proteinuria and hypocomplementemia were improved by reducing the HIV viral load through antiretroviral therapy. When C3-dominant deposition is observed on a renal biopsy, HIVICK must be differentiated.
Keywords: human immunodeficiency virus, complement activation, renal biopsy
Introduction
The natural history of HIV infection has become a manageable chronic disease rather than an acute disease that rapidly progress toward acquired immunodeficiency syndrome (AIDS) thanks to advances in combination antiretroviral therapy (ART). However, with this improvement in the survival of HIV-infected patients, HIV complications other than infectious diseases, such as kidney, liver and cardiac disease, are now becoming clinically significant issues.
Patients with HIV are at risk for acute kidney injury and chronic kidney disease (CKD) (1). CKD associated with HIV infection includes collapsing the form of focal segmental glomerulosclerosis (FSGS), which is known as HIV-associated nephropathy (HIVAN), HIV-associated immune complex (IC) kidney disease (HIVICK), thrombotic microangiopathy (TMA) and nephrotoxicity associated with ART (2). HIVICK represents IC-mediated glomerulopathy or glomerulonephritis in patients infected with HIV. Mesangial proliferative glomerulonephritis (mesGN), lupus-like glomerulonephritis, post-infectious glomerulonephritis (PIGN) and membranoproliferative glomerulonephritis (MPGN) are described as major light microscopic patterns in the glomeruli (3).
We herein report a case of HIVICK with C3-dominant deposition induced by HIV infection after treatment of IgA nephropathy (IgAN).
Case Report
A 57-year-old man presented with proteinuria at approximately 3 g/day, hematuria of 20-29 red blood cells per high-power field and renal impairment (serum creatinine: 1.08 mg/dL). He underwent a percutaneous renal biopsy and was diagnosed with IgAN. After undergoing tonsillectomy with steroid pulse therapy following oral steroid therapy, his hematuria was negative, and his proteinuria decreased to 0.3 g/day. Six years later, he presented to the previous hospital complaining of systemic lymphadenopathy. The laboratory findings showed hypergammopathy of IgG and IgM. An axillary lymph node biopsy showed reactive lymphadenitis. After another year, he developed hematuria, proteinuria and hypocomplementemia and was then admitted to our hospital for a renal re-biopsy. He had suffered from herpes zoster twice. He had no family history of kidney disease.
On admission, his body temperature was 37.3°C, pulse rate was 76 beats per minute and blood pressure was 133/78 mmHg. He weighed 61.3 kg and reported a body weight loss of 17 kg within the preceding 9 months. Swollen cervical and axillar lymph nodes were palpable. No lung rale or heart murmurs were detected on chest auscultation. An examination of the abdomen was remarkable, showing pitting edema in both legs but no purpura.
A urinalysis revealed proteinuria (4.06 g/gCr), hematuria with 20-29 red blood cells per high-power field and 30-49 granular casts per whole field. The urinary N-acetyl-β-D-glucosaminidase (NAG) level was 30.6 U/gCr, and the urinary β2 microglobulin level was 20,212 μg/gCr. A blood examination showed pancytopenia, with a red cell count of 2.47 million/μL, a hemoglobin level of 8.0 g/dL, a white blood cell count of 1,390 /μL with 42% lymphocytes and a platelet count of 105,000 /μL. Laboratory findings showed renal dysfunction [serum creatinine of 1.41 mg/dL and an estimated glomerular filtration rate (eGFR) of 40.4 mL/min/1.73 m2] and hypoalbuminemia. Serum complement C3 and C4 levels and CH50 were decreased to 50.8 mg/dL, 9.1 mg/dL and 23 U/mL, respectively. The serum IgG and IgM levels increased to 4,093.6 mg/dL and 873.6 mg/dL, respectively. The serum IgA level was 211.9 mg/dL. Myeloreroxidase- or proteinase 3-antineutrophil cytoplasmic antibodies, antinuclear antibody, anti-streptolysis O antibody and cryoglobulin were all negative. The fractional excretion of sodium was 1.28%, with no findings of prerenal renal failure. He had a history of infection with hepatitis B virus, but the quantity of hepatitis B virus (HBV)-DNA was undetectable. Hepatitis C virus antibody was negative. Monoclonal proteins were not detected in the serum or urine by electrophoresis. Computed tomography (CT) of the body showed systemic lymphadenopathy and expansion of the inferior vena cava but no findings of hydronephrosis. The size and shape of the kidneys did not suggest atrophy.
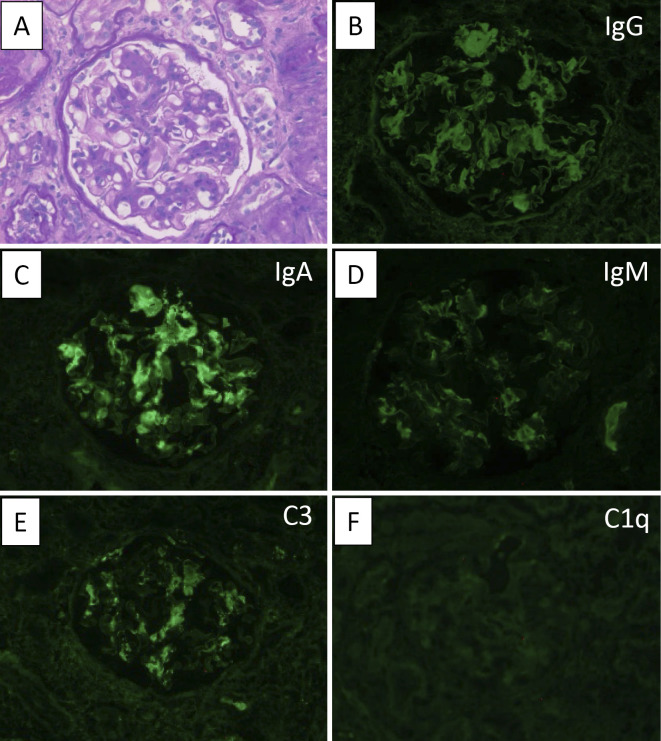
On the first biopsy, a total of 25 glomeruli were observed on light microscopy (LM), 13 of which exhibited global sclerosis. Mesangial cell proliferation and mesangial matrix expansion were observed (Fig. 1A), and 2 glomeruli exhibited fibrocellular crescent and adhesion. Interstitial fibrosis was mild. Immunofluorescence (IF) studies showed strong IgA (Fig. 1C) and moderate C3 (Fig. 1E) staining with granular mesangial deposits, whereas no deposition of C1q was noted (Fig. 1F). Based on these and other findings, the patient was diagnosed with IgAN. The histological findings in accordance with the Oxford-MEST score (4) and the histological grading criteria of the Japanese Society of Nephrology (5) were classified as M0, E1, S1, T1 and H3 (A/C), respectively.
Figure 1.
The findings of the first renal biopsy specimens. (A) Light microscopic findings by Periodic acid-Schiff (PAS) staining (original magnification ×400). (B-F) Immunofluorescence (IF) study of (B) IgG, (C) IgA, (D) IgM, (E) C3 and (F) C1q. IF studies showed strong staining of IgA, moderate staining of C3 and weak staining of IgG and IgM in the mesangial areas.
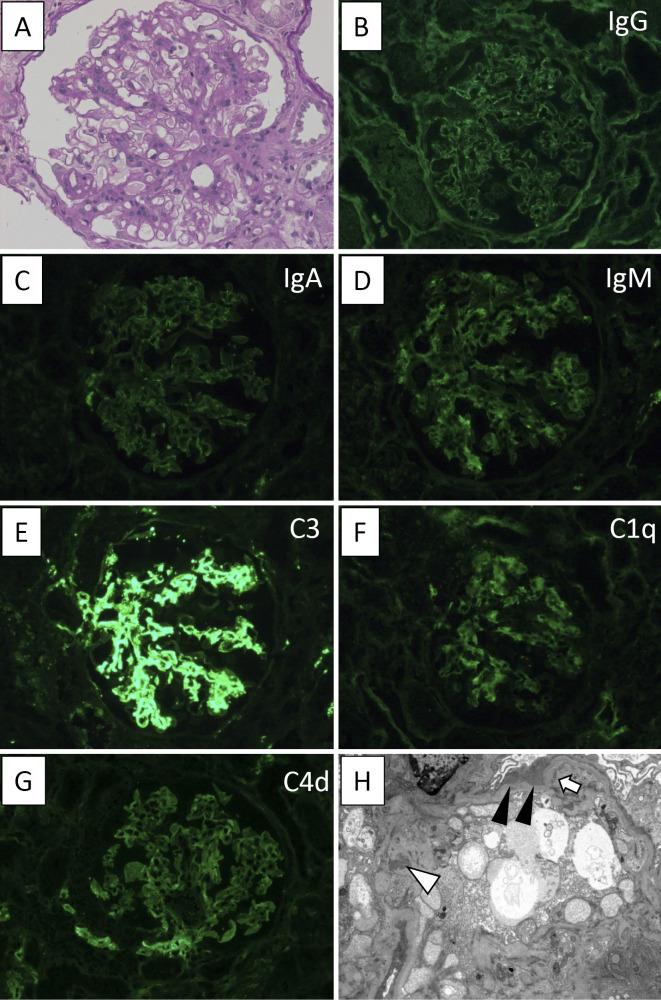
A second biopsy revealed a total of four glomeruli on LM, none of which exhibited global sclerosis. Mild interstitial fibrosis similar to that observed at the first biopsy and atherosclerosis were noted. Cell infiltration was focally detected in the renal interstitium, primarily composed of lymphocytes. Mesangial cell proliferation and mesangial matrix expansion were observed, but no findings of endocapillary proliferation, crescent formation or FSGS were shown (Fig. 2A). In contrast to the first biopsy, IF studies demonstrated weak granular deposition of IgM in the mesangial area and faint deposition of IgG (Fig. 2B) or IgA (Fig. 2C). Intense mesangial staining of C3 (Fig. 2E) and weak staining of C1q (Fig. 2F) and C4d (Fig. 2G) were noted, whereas no staining of C4 was seen. Kappa- and lambda-light chains were equally positive (Figure not shown). Electron microscopy (EM) showed diffuse foot process effacement and microvilli formation on podocytes. Furthermore, small electron-dense deposits were demonstrated mainly in the mesangial area and scantly in the subendothelial, subepithelial and intramembranous areas (Fig. 2H). Subepithelial hump-shaped deposits were not observed. Tubuloreticular inclusions (TRIs) were also not detected within the cytoplasm of glomerular endothelial cells. Based on these and other findings, he was diagnosed with mesGN with C3-dominant deposition.
Figure 2.
The findings of the second renal biopsy specimens. (A) Light microscopic findings by PAS staining (original magnification ×400). (B-G) IF study of (B) IgG, (C) IgA, (D) IgM, (E) C3, (F) C1q and (G) C4d. IF studies showed strong staining of C3; weak staining of IgM, C1q and C4d; and faint staining of IgG and IgA in the mesangial areas. (H) Electron microscopic findings. Small electron-dense deposits were noted mainly in the mesangial area (white arrowhead) and scantly in the subepithelial (black arrowhead) and subendothelial (arrow) areas (original magnification ×3,000).
A further examination revealed positive findings for HIV antibody despite negative findings having been noted at the time of tonsillectomy. He had no history of blood transfusion or drug abuse, but the patient reported having been engaged in a sexual relationship with a single male partner for the past four years at two years after the first biopsy. Laboratory test results were as follows: HIV-1 Western blotting (+), CD4 T lymphocyte counts 127 cells/μL, and HIV-1 RNA 1.1×106 copies/mL. Given these findings, the patient was diagnosed with HIV infection. An immunohistochemical study showed that HIV-1 p24 antigen (1:10 dilution, M0857; DAKO, Glostrup, Denmark) was negative on glomeruli and tubules (Figure not shown).
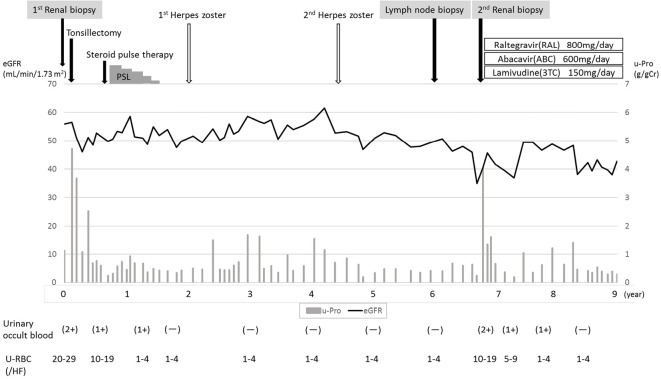
The patient was treated for HIV infection with raltegravir (800 mg), abacavir (600 mg) and lamivudine (150 mg) antiretroviral therapies (ART). After this treatment, the number of CD4+ cells increased while the HIV-RNA load decreased. Accordingly, his hematuria disappeared, and the urinary protein level markedly decreased; the renal function remained stable during treatment (Fig. 3). The serum levels of IgG and IgM decreased, and the serum complement C3 and C4 levels recovered to within normal limits while the serum circulating IC level decreased from 2.3 to <1.5 μg/mL. The serum of soluble C5b-9 (sC5b-9), which is generated by the assembly of C5b through C9 as a consequence of activation of the complement system, decreased from 6.94 to 0.48 μg/mL [healthy control: 0.52±0.20 μg/mL (mean±standard deviation)] (Table).
Figure 3.
The clinical course from the first biopsy to ART therapy. Following therapy for IgAN, the patient’s hematuria was negative, and his proteinuria had decreased. Roughly seven years later, he developed hematuria and proteinuria and was diagnosed with HIVICK. The urinary findings were improved by combination ART.
Table.
Laboratory Findings from the Second Biopsy to the HIV Therapy.
| Month | 0 (Admission) |
1 | 3 | 5 | 9 | 15 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG (mg/dL) | 4,093.6 | 3,786.2 | 3,192.1 | 2,694.1 | 2,144.4 | 2,050.7 | ||||||
| IgM (mg/dL) | 873.6 | 785.9 | 540.2 | 396.8 | 296.2 | 254.6 | ||||||
| C3 (mg/dL) | 57.4 | 109.4 | 135.4 | 117.2 | 127.3 | 177.5 | ||||||
| C4 (mg/dL) | 10.0 | 12.2 | 16.5 | 15.2 | 22.2 | 26.9 | ||||||
| CH50 (U/mL) | 26 | 38 | 50 | 47 | 46 | 74 | ||||||
| IC (µg/mL) | 2.3 | - | ≤1.5 | - | - | ≤1.5 | ||||||
| sC5b-9 (µg/mL) | 6.94 | - | - | - | - | 0.48 | ||||||
| HIV-RNA (copy/mL) | 1,100,000 | 100 | 59 | <20 | 170 | 86 |
Given the patient's HIV infection, mesGN with C3-dominant IC deposition, and clinical improvement with combination ART, his renal impairment was deemed to have been caused by HIVICK.
Discussion
We herein report a case of HIVICK with C3-dominant deposition induced by HIV infection after treatment of IgAN.
Proteinuria and hematuria were improved for a while after the combination of tonsillectomy and steroid pulse therapy (TSP) for IgAN. The pathological findings of the first biopsy exhibited not only active lesions but also chronic lesions, which might contribute to the sustained relatively high amount of proteinuria after TSP. There have been many reports of IgAN occurring in patients with HIV infection (2,3,6). TSP in the management of HIV-infected patients with IgAN has been reported to be effective in Japan (7,8). HIV antibodies were negative at the time of the first biopsy but turned positive by the second biopsy. The patient reported having been engaged in a sexual relationship with a single male partner for the past four years at two years after the first biopsy, suggesting that the patient had become infected with HIV at this time. Therefore, there is likely no relationship between HIV infection and the onset of IgAN in the present case.
CKD associated with HIV infection includes HIVAN, HIVICK, TMA and tubulointerstitial injury by nephrotoxicity of ART (2). HIVAN is pathologically characterized by the collapsing form of FSGS, podocyte proliferation and hypertrophy, endothelial cell TRI and microcystic tubular dilatation (9). No characteristic findings of HIVAN or TMA were observed on a renal biopsy. IgA immunostaining on the second renal biopsy was weak, suggesting that recurrence of IgAN was unlikely. HIVICK is characterized by the presence of glomerular IC deposition on a renal biopsy of HIV-positive patients (6). In contrast to the findings in the present case, patients with HIVICK have a lower viral load and higher CD4 and eGFR values than those with HIVAN (10). The mechanisms underlying the development of IC-mediated renal disease involves trapping circulating IC in glomerular tufts or the in situ formation of IC, causing complement activation and tissue injury (11). The presence of HIV antigens, including p24, was demonstrated in the circulating IC and IC eluted from renal biopsy tissue (12), but p24 antigen was not stained by immunohistochemistry in the present case. An immunohistochemical analysis using monoclonal anti-human parvovirus (HP) B19 antibody showed that one of the four patients with acute glomerulonephritis after human parvovirus B19 (HPB19) infection had positive staining in the glomeruli, although HPB19 DNA was identified on polymerase chain reaction in all kidney specimens (13). We were unable to analyze the HIV gene in the renal specimens of our patient.
The relationships between the pathological mechanisms and complement activation have been investigated for renal disease. Complement activation via the alternative pathway (AP) and lectin pathway is thought to contribute to the pathogenesis of IgAN (14). Because C3-dominant deposition was shown on IF of the renal biopsy in the present patient, we needed to exclude C3 glomerulopathy and PIGN (15). C3 glomerulopathy is a pathological entity characterized by the presence of glomerular deposits composed predominantly of C3 in the absence of significant amounts of immunoglobulin. The primary process of C3 glomerulopathy is complement activation via the AP; 59% of patients with C3 glomerulopathy had a low C3 value, and 23% also had a low C4 value. Immunohistology showed that staining for C3 and IgM was evident in 39% of patients (16). Our case was not diagnosed with C3 glomerulopathy due to the abnormal complement activation of AP for the following reasons: HIV infection was recognized and improved by ART with an impressive reduction in proteinuria and restoration of hypocomplementemia, mesGN was observed on LM (although MPGN is a typical finding of C3 glomerulopathy), and C4d was positive on IF despite being negative in almost all cases of C3 glomerulopathy (17). PIGN is characterized by endocapillary proliferative glomerulonephritis on LM and large subepithelial electron-dense deposits or “humps” on EM developing within a few weeks following recovery from infection. A total of 46% of HIV-infected patients with PIGN were confirmed to have a bacterial infection by positive blood culture (18). Complement activation by AP can be seen during infections that trigger the development of PIGN, and some cases of PIGN with persistent hematuria and proteinuria show an underlying abnormality in the regulatory factors of AP (19). The present case was not diagnosed with PIGN because no antecedent bacterial infection had been identified and the typical pathological findings of PIGN were not observed.
The complement system is directly or antibody-dependently activated by HIV infection. Not only is HIV resistant to lysis by activation of complement and C3 deposition on the viral surface, but the complement system contributes to HIV entry, infection and replication (20). The serum concentrations of C3 and C4 were low before HIV treatment in the present patient, and the IF study showed weak staining for IgM, C4 and C1q in the mesangial area, indicating that complement activation due to the classical pathway contributed to the pathogenesis of the renal lesions. In addition, staining for C3 was more intense than staining for other factors, suggesting that activation of the AP might also be involved in the renal pathogenesis. HIV-infected T and monocytic cell lines activate and fix C3 via the AP (21). Renal C3 deposition (12,22) and plasma C3 activation (23) were shown in patients with AIDS, but whether or not C3 was dominantly stained is unclear. Complement-regulating factors were not investigated in our case, and the mechanism underlying the activation of the AP by HIV is unclear.
Studies regarding the optimal treatment of patients with HIVICK are limited, and the clinical effects of ART in patients with HIVICK are unclear. Histological IC deposition reportedly improved in patients who commenced ART (24). The initiation of ART and subsequent suppression of HIV RNA resulted in improvements in the GFR and proteinuria (6). However, the use of ART is reportedly not associated with end-stage renal disease (ESRD) (10). In the present case, the serum complement levels improved while those of IC (C1q) and sC5b-9 decreased with ART, suggesting that IC formations contribute to the activity and consumption of complement. We were unable to perform a renal biopsy after ART due to the patient's refusal and therefore could not ascertain any histological improvement, but ART was clinically effective in improving the urinary disorder and renal function.
In conclusion, we encountered a case of HIVICK with C3-dominant deposition induced by HIV infection after treatment of IgAN. When C3-dominant deposition is demonstrated on a renal biopsy in patients with HIV infection, it is necessary to differentiate HIVICK.
Informed consent was obtained from the participants included in the article.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr. Michio Nagata (Department of Pathology, Institute of Basic Medical Sciences, University of Tsukuba) for providing critical advice for the assessment of the renal histological findings. We thank Dr. Tamaki Sasaki (Department of Nephrology and Hypertension, Kawasaki Medical School) for performing HIV-1 p24 antigen immunostaining.
References
- 1. Lucas GM, Ross MJ, Stock PG, et al. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 59: e96-e138, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiner NJ, Goodman JW, Kimmel PL. The HIV-associated renal diseases: current insight into pathogenesis and treatment. Kidney Int 63: 1618-1631, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Nobakht E, Cohen SD, Rosenberg AZ, Kimmel PL. HIV-associated immune complex kidney disease. Nat Rev Nephrol 12: 291-300, 2016. [DOI] [PubMed] [Google Scholar]
- 4. Cattran DC, Coppo R, Cook HT, et al. ; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society. . The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 76: 534-545, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Matsuo S, Kawamura T, Joh K, et al. Clinical guides for immunoglobulin A (IgA) nephropathy in Japan, third version. Nihon Jinzo Gakkai Shi 53: 123-135, 2011(in Japanese). [PubMed] [Google Scholar]
- 6. Booth JW, Hamzah L, Jose S, et al. Clinical characteristics and outcomes of HIV-associated immune complex kidney disease. Nephrol Dial Transplant 31: 2099-2107, 2016. [DOI] [PubMed] [Google Scholar]
- 7. Miyasato Y, Miyoshi T, Fujimoto D, Adachi M, Kitamura K, Mukoyama M. Successful treatment of rapidly progressive immunoglobulin A nephropathy with human immunodeficiency virus infection by steroid pulse therapy and tonsillectomy. Nephrology (Carlton) 21: 159-160, 2016. [DOI] [PubMed] [Google Scholar]
- 8. Tada M, Masumoto S, Hinoshita F. Clinical remission of IgA nephropathy in an HIV-positive patient after combined treatment with tonsillectomy and steroid pulse therapy. CEN Case Rep 4: 157-161, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol 11: 150-160, 2015. [DOI] [PubMed] [Google Scholar]
- 10. Foy MC, Estrella MM, Lucas GM, et al. Comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy . Clin J Am Soc Nephrol 8: 1524-1532, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Couser WG, Johnson RJ. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int 86: 905-914, 2014. [DOI] [PubMed] [Google Scholar]
- 12. Kimmel PL, Phillips TM, Ferreira-Centeno A, Farkas-Szallasi T, Abraham AA, Garrett CT. HIV-associated immune-mediated renal disease. Kidney Int 44: 1327-1340, 1993. [DOI] [PubMed] [Google Scholar]
- 13. Nakazawa T, Tomosugi N, Sakamoto K, et al. Acute glomerulonephritis after human parvovirus B19 infection. Am J Kidney Dis 35: E31, 2000. [DOI] [PubMed] [Google Scholar]
- 14. Mizuno M, Suzuki Y, Ito Y. Complement regulation and kidney diseases: recent knowledge of the double-edged roles of complement activation in nephrology. Clin Exp Nephrol 22: 3-14, 2018. [DOI] [PubMed] [Google Scholar]
- 15. Pickering MC, D'Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int 84: 1079-1089, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Medjeral-Thomas NR, O'Shaughnessy MM, O'Regan JA, et al. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol 9: 46-53, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sethi S, Nasr SH, De Vriese AS, Fervenza FC. C4d as a diagnostic tool in proliferative GN. J Am Soc Nephrol 26: 2852-2859, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murakami CA, Attia D, Carter-Monroe N, et al. The clinical characteristics and pathological patterns of postinfectious glomerulonephritis in HIV-infected patients. PLoS One 9: e108398, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sethi S, Fervenza FC, Zhang Y, et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int 83: 293-299, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Datta PK, Rappaport J. HIV and complement: hijacking an immune defense. Biomed Pharmacother 60: 561-568, 2006. [DOI] [PubMed] [Google Scholar]
- 21. Yefenof E, Asjo B, Klein E. Alternative complement pathway activation by HIV infected cells: C3 fixation does not lead to complement lysis but enhances NK sensitivity. Int Immunol 3: 395-401, 1991. [DOI] [PubMed] [Google Scholar]
- 22. Rao TK, Filippone EJ, Nicastri AD, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 310: 669-673, 1984. [DOI] [PubMed] [Google Scholar]
- 23. Tausk FA, McCutchan A, Spechko P, Schreiber RD, Gigli I. Altered erythrocyte C3b receptor expression, immune complexes, and complement activation in homosexual men in varying risk groups for acquired immune deficiency syndrome. J Clin Invest 78: 977-982, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabian J, Naicker S, Goetsch S, Venter WD. The clinical and histological response of HIV-associated kidney disease to antiretroviral therapy in South Africans. Nephrol Dial Transplant 28: 1543-1554, 2016. [DOI] [PubMed] [Google Scholar]