Abstract
We applied the indirect cohort method to estimate effectiveness of 10-valent pneumococcal conjugate vaccine (PCV10) among young children in Brazil. Cases of invasive pneumococcal disease ([IPD] i.e. Streptococcus pneumoniae detected in normally sterile fluid) identified through laboratory-based surveillance and previously enrolled in a matched case-control effectiveness study were included. We estimated PCV10 effectiveness using multivariable logistic regression comparing PCV10 vaccination among children with vaccine-type or vaccine-related IPD versus children with non-vaccine-type disease. The adjusted effectiveness of ≥1 doses against vaccine-type (72.8%, 95% confidence interval [CI] [44.1, 86.7]) and vaccine-related (61.3%, 95%CI [14.5, 82.5]) IPD were similar to the effectiveness observed in the original case-control study (which required enrollment >1,200 controls). We also found significant protection of ≥1 doses against individual vaccine serotypes (14, 6B, 23F, 18C) and against vaccine-related serotype 19A. The indirect cohort methods leverages existing surveillance is a feasible approach for evaluating pneumococcal conjugate vaccines, particularly in resource-limited settings.
Keywords: pneumococcal conjugate vaccine, invasive pneumococcal disease, vaccine effectiveness, Brazil, case-control studies
Introduction
Streptococcus pneumoniae is a leading cause of pneumonia, sepsis and meningitis worldwide1. Pneumococcal conjugate vaccines (PCV) are important for reducing pneumococcal morbidity and mortality2. A 7-valent PCV (PCV7), available since 2000, was shown to be highly effective against invasive pneumococcal disease (IPD) caused by serotypes included in the vaccine as well as 6A, a vaccine-related serotype2. More recently, 10-valent (PCV10) and 13-valent PCVs with substantially better serotype coverage for IPD in the developing world have been increasingly introduced in low- and middle-income counties, where the burden of pneumococcal disease is greatest3.
In March 2010, Brazil became the first country to introduce the newly available PCV10 in a national immunization program. PCV10 was licensed based on immunogenicity data4, and at the time of introduction protection against clinical outcomes was unknown. A case-control study conducted in Brazil using age- and neighborhood-matched controls identified through a national birth registry demonstrated PCV10 effectiveness of an age-appropriate number of doses against vaccine-type IPD (83.8%, 95% confidence interval [CI] 65.9 to 92.3) and IPD caused by vaccine-related serotypes (77.9%, 95%CI 41.0 to 91.7)5; the study also reported significant protection against individual vaccine serotypes 14 (87.7%, 95%CI 60.8 to 96.1)and 6B (82.8%, 95%CI 23.8 to 96.1), and vaccine-related serotype 19A (82.2%, 95%CI 10.7 to 96.4). Those results were useful for the Brazilian Ministry of Health to justify the investment in PCV10 introduction. However case-control studies can be costly and complex to implement; such evaluations are not feasible for many resource-poor settings.
The Indirect cohort, or ‘Broome’ method, was developed to examine effectiveness of polysaccharide pneumococcal vaccine6. It is a case-only analysis in which the vaccination status of vaccine-type IPD case-patients is compared with that of non-vaccine-type. This method was used to evaluate PCV7 effectiveness in the United States7, England and Wales8, and Germany9. In the United States, the results of the indirect cohort were consistent with those of a case-control vaccine-effectiveness study that enrolled age- and geographically-matched controls identified through birth registries10. We conducted an indirect cohort analysis with data from Brazil to compare with results from the case-control study and to provide further insight into PCV10 protection against vaccine-type and vaccine-related IPD.
Methods
Methods for identifying and gathering data on cases have been described elsewhere5. Briefly, cases were identified through laboratory-based surveillance in 10 states in Brazil from March 2010 to December 2012. Cases were defined as S. pneumoniae detected from a normally sterile site (e.g. blood or cerebrospinal fluid) in a child age-eligible to receive ≥1 PCV10 dose. Initially cases were identified by culture only; however starting in December 2010, some study sites detected cases using polymerase chain reaction (PCR). Pneumococcal isolates submitted to Brazil’s national reference laboratory were serotyped using the Quellung reaction; cases detected by non-culture methods were serotyped by PCR5. After obtaining written informed consent from the parent or guardian of the child, epidemiologic data were gathered through in-person interviews conducted by study personnel using a standardized questionnaire. Vaccination histories were abstracted from case-patients’ immunization cards. The recommended PCV10 schedule included three primary doses (at 2, 4, and 6 months) and a booster dose (12 months). Catch-up schedules for children aged 3–11 months at the time of introduction included one to three primary doses (based on age) plus a booster dose; a single dose was recommended for children aged 12–23 months.
Cases were considered vaccine-type if due to serotypes included in PCV10 (1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F or 23F), and vaccine-related if in the same serogroup as a vaccine-type (i.e. 6A, 6C, 6D, 7C, 9N, 18A, 18B, 19A and 23A). All others were classified as non-vaccine-type. Vaccine doses received ≥14 days before the child sought medical care were included in the analysis. Children with the recommended number of PCV10 doses for their age were considered up-to-date. Those who had received a pneumococcal vaccine other than PCV10 were excluded. We used chi square to compare proportions and Wilcoxon-Mann-Whitney test to compare medians. We calculated odds of receipt of ≥1 PCV10 doses and up-to-date PCV10 vaccination (compared with 0 doses) among vaccine-type or vaccine-related cases versus non-vaccine-type cases and used logistic regression to estimate vaccine effectiveness as 1- odds ratio for PCV10 vaccination x100%. To adjust for confounders, we started with basic models that included vaccination status, date of medical attention, and age at illness as independent variables (latter two included as continuous variables). Additional covariates were included one by one in basic models for effectiveness against vaccine-type and vaccine-related disease; any that altered the odds ratio by 10% or more were included in multivariable analysis.
Results
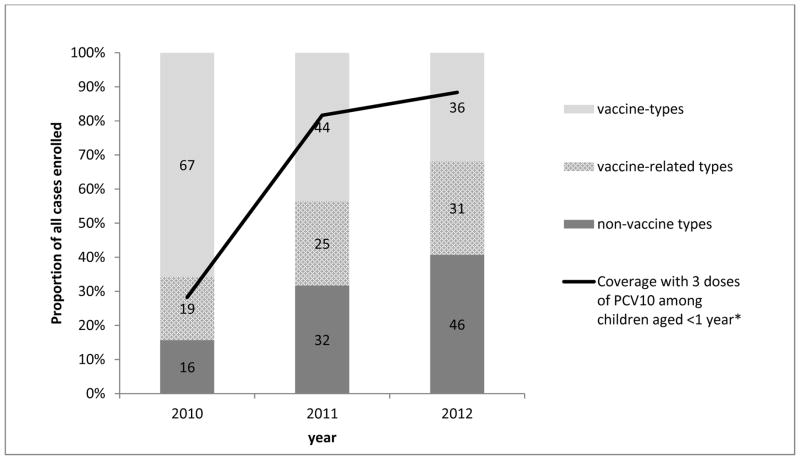
A total of 398 IPD cases were identified; 15 (3.7%) declined participation, 26 (6.5%) were not located, 32 (8.0%) had undetermined serotype, and 9 (2.3%) had received other pneumococcal vaccines. Among 316 cases included in analysis, 147 (46.5%) were vaccine-type, 75 (23.7%) were vaccine-related, and 94 (29.7%) were non-vaccine-type. The proportion of vaccine-type cases declined from 2010 to 2012, as vaccine coverage increased (Figure). Median ages of case-patients with vaccine-type, vaccine-related, and non-vaccine-type IPD were similar (Table 1). Case-patients with vaccine-type disease were less likely than those with non-vaccine-type to attend daycare, have received routine vaccination against diphtheria-tetanus-pertussis-Haemophilus influenzae type B (Hib) and have a mother with <12 years of education. Receipt of ≥1 PCV10 doses was significantly higher among non-vaccine-type cases (83.0%) compared with both vaccine-type cases (41.5%, p<0.0001) and vaccine-related cases (64.0%, p=0.005).
Figure.
Proportion of invasive pneumococcal disease cases due to vaccine serotypes, vaccine-related serotypes and non-vaccine serotypes enrolled in study by year and coverage with 3 doses of PCV10 among children aged <1 year. The numbers within each section of the bar represent the number of isolates.
*National coverage data for 3 doses of PCV10 among children aged <1 year obtained from http://pni.datasus.gov.br
Table 1.
Characteristics of cases of invasive pneumococcal disease cases due to vaccine serotypes, vaccine-related serotypes and non-vaccine serotypes
| Vaccine-type cases n=147 |
Vaccine-related cases n=75 |
Non-vaccine type cases n=94 |
|||
|---|---|---|---|---|---|
| n (%) | p value* | n (%) | p value* | n (%) | |
| Age in months, median; (interquartile range) | 13 (6 to 24) | 0.943 | 15 (8 to 22) | 0.489 | 12 (7, 24) |
| Pneumonia/bacteremia | 75 (51.0) | 0.995 | 38 (50.1) | 0.959 | 48 (51.1) |
| Meningitis | 72 (49.0) | 0.995 | 37 (49.3) | 0.959 | 46 (48.9) |
| Death | 36 (24.5) | 0.855 | 14 (18.7) | 0.288 | 24 (25.5) |
| Any chronic illness | 44 (29.9) | 0.459 | 17 (22.7) | 0.666 | 24 (25.5) |
| Asthma | 24 (16.3) | 0.449 | 9 (8.0) | 0.318 | 12 (12.8) |
| Premature birth (<37 weeks) | 20 (13.6) | 0.348 | 13 (17.3) | 0.136 | 9 (9.6) |
| Low birth weight | 19 (12.9) | 0.289 | 12 (16.0) | 0.134 | 8 (8.5) |
| Use of immunosuppressant drugs | 14 (9.5) | 0.829 | 3 (4.0) | 0.224 | 8 (8.7) |
| Exclusive breastfeeding until 3 months of age | 82 (55.8) | 0.677 | 40 (53.3) | 0.500 | 55 (58.1) |
| Date care (daily attendance) | 44 (29.9) | 0.005 | 38 (50.7) | 0.718 | 45 (47.9) |
| Maternal education <12 years | 20 (13.6) | 0.017 | 17 (22.7) | 0.638 | 24 (25.8) |
| Low household income | 58 (39.5) | 0.077 | 41 (54.7) | 0.641 | 48 (51.1) |
| Crowding | 85 (57.8) | 0.303 | 45 (60.0) | 0.246 | 48 (51.1) |
| Other children <5 years in the home | 76 (51.7) | 0.166 | 32 (42.7) | 0.988 | 40 (42.6) |
| Smoker in the home | 59 (40.1) | 0.537 | 20 (26.7) | 0.188 | 34 (36.2) |
| ≥1 dose diphtheria-pertussis-tetanus-Hib vaccine | 132 (89.8) | 0.017 | 71 (94.7) | 0.263 | 92 (97.9) |
| ≥1 dose PCV10 | 61 (41.5) | <.0001 | 48 (64.0) | 0.005 | 78 (83.0) |
| Up-to-date PCV10 status | 32 (21.8) | <.0001 | 22 (29.3) | 0.016 | 40 (42.6) |
Compared with non-vaccine type cases
The adjusted effectiveness of ≥1 doses against vaccine-type disease was 72.8% (95%CI 44.1 to 86.7), and against vaccine-related disease was 61.3% (95%CI 14.5 to 82.5) (Table 2). One or more doses were significantly protective against vaccine serotypes 14 (75.4%, 95%CI 14.5 to 82.5), 6B (69.7%, 95%CI 16.5 to 89.0), 23F (76.6%, 95%CI 14.6 to 93.6), and 18C (86.6%, 95%CI 30.6 to 97.4), as well as vaccine-related serotype 19A (71.3%, 95%CI 16.6 to 90.1). The effectiveness of an up-to-date schedule was generally similar to that of ≥1 doses, although confidence intervals for up-to-date schedule were wider and included zero for serotypes 6B, 18C, 19F and 19A. No significant protection of either schedule was shown for vaccine serotype 19F or vaccine-related serotype 6A.
Table 2.
Crude and adjusted estimates of PCV10 effectiveness against invasive pneumococcal disease for ≥1 doses and for up-to-date schedule for age†
| Serotype | Cases with ≥1 dose PCV10/total (%) | Effectiveness ≥1 doses | Cases UTD for PCV10/total (%) | Effectiveness up-to-date schedule‡ | ||
|---|---|---|---|---|---|---|
| Crude | Adjusted§ | Crude | Adjusted§ | |||
| Vaccine-types | 61/147 (41.5) | 85.4 (72.7, 92.3) | 72.8 (44.1, 86.7) | 32/147 (21.8) | 85.1 (69.8, 92.7) | 73.9 (41.9, 88.3) |
| Vaccine-related types | 48/75 (64.0) | 63.5 (25.4, 82.2) | 61.3 (14.5, 82.5) | 22/75 (29.3) | 67.4 (26.9, 85.5) | 64.8 (15.3, 85.4) |
| Individual vaccine serotypes | ||||||
| 14 | 28/72 (38.9) | 86.9 (73.3, 93.6) | 75.4 (43.2, 89.4) | 16/72 (22.2) | 85.5 (67.2, 93.6) | 75.8 (37.4, 90.7) |
| 6B | 16/32 (50.0) | 79.5 (50.7, 91.5) | 69.7 (16.5, 89.0) | 9/32 (28.1) | 77.5 (38.7, 91.7) | 65.0 (−8.5, 88.7) |
| 23F | 7/18 (38.9) | 86.9 (61.2, 95.6) | 76.6 (14.6, 93.6) | 2/18 (11.1) | 92.7 (63.5, 98.6) | 86.6 (22.9, 97.7) |
| 18C | 6/9 (33.3) | 89.7 (54.6, 97.7) | 86.6 (30.6, 97.4) | 3/9 (33.3) | 80.0 (10.2, 95.5) | 76.4 (−26.3, 95.6) |
| 19F | 4/8 (50.0) | 79.5 (9.3, 95.4) | 46.3 (−253.1, 91.8) | 1/8 (12.5) | 90.0 (3.5, 99.0) | 77.6 (−188.9, 98.3) |
| Individual vaccine-related serotypes | ||||||
| 19A | 15/26 (57.7) | 72.0 (28.0, 89.1) | 71.3 (16.6, 90.1) | 12/26 (38.5) | 63.6 (−2.3, 87.1) | 63.4 (−16.8, 88.6) |
| 6A | 16/24 (66.7) | 59.0 (−12.1, 85.0) | 51.0 (−52.2, 84.2) | 6/24 (25.0) | 70.0 (−0.3,91.0) | 62.2 (−42.2, 89.9) |
0 doses used as reference group for all analyses.
Partially vaccinated were excluded from the analysis of the effectiveness of an up-to-date schedule.
Adjusted for date of admission/medical attention, age at illness, daycare attendance and receipt of at least one diphtheria-tetanus-pertussis vaccine dose
Discussion
The results of this analysis were consistent with those of the case-control study in Brazil5, for which >1,200 community controls were enrolled. Recruitment of appropriate control subjects can be time- and resource-intensive, and may introduce bias11. Indirect cohort studies can be carried out entirely within a surveillance system for IPD, as long as serotyping is routinely performed and complete immunization histories are obtained for all cases. The ‘control’ group (i.e. non-vaccine-type disease) identified from IPD surveillance is likely to be relatively similar to cases in terms of access to care and IPD risk factors such as co-morbid conditions. Analogous ‘test-negative’ designs have been used to evaluate effectiveness of rotavirus and influenza vaccines12, and studies of Hib vaccine effectiveness against meningitis have similarly used children with pneumococcal meningitis as controls13. Our results support a growing body of evidence that the indirect cohort is a feasible, methodologically sound approach to estimating PCV effectiveness.
For relatively infrequent serotypes, the indirect cohort approach can provide better statistical power to estimate serotype-specific protection than studies using individually matched controls. This analysis yielded a more precise estimate of effectiveness against serotype 23F than previously reported5, and provided the first estimate of PCV10 protection against serotypes 18C and 19F. As with the case-control study in Brazil, this analysis provided evidence of PCV10 effectiveness against vaccine-related serotype 19A, with significant protection from ≥1 doses, and a suggestion of effectiveness (albeit with a confidence interval that includes zero) for an up-to-date schedule. We did not find evidence of PCV10 effectiveness against serotype 6A; however the analysis may have been underpowered to measure a moderate cross-protective effect for this vaccine-related serotype
The indirect cohort method is based on an assumption that vaccination does not impact the risk for non-vaccine-type disease among vaccinated individuals6. Following PCV7 introduction, an increase in non-vaccine-type disease, primarily serotype 19A, was observed in many settings2. The potential for bias due to increased risk for non-vaccine-type disease among vaccinated individuals was explored in indirect cohort analyses from England and Wales and the United States; both concluded that while effectiveness may be overestimated, the error in the estimate is likely to be <10%7,8. Based on studies of PCV impact on carriage with vaccine-type and non-vaccine-type serotypes14, an increase in carriage of non-PCV10 serotypes is likely among children receiving PCV10. However it is unknown whether the risk of non-PCV10-type disease will also increase among vaccinated individuals. Continued monitoring for emerging serotypes is needed in Brazil and other countries using PCV10.
Our findings are subject to additional limitations. As this study included cases from only 10 states, the findings may not be generalizable to other areas of Brazil or other countries. Some cases were not included due to inability to locate the child or a vaccination history, refusal to participate, or lack of serotype information; it is unknown how their exclusion may have impacted results. There may have been differences between vaccinated and unvaccinated children that we were not able to adjust for in the analysis.
Conclusion
Using the indirect cohort method we demonstrate high effectiveness of PCV10 against vaccine-type and vaccine-related IPD. Case-only analyses provided VE estimates similar to those from a matched case-control study that enrolled children without disease. These findings support the use of indirect cohort to measure PCV effectiveness against vaccine-type and vaccine-related IPD. This method is a feasible approach to evaluate PCV in resource-constrained settings, and may be useful for evaluating effectiveness against individual serotypes.
Acknowledgments
We would like to thank the children and their parents whose participation made this study possible. We would also like to the thank the surveillance units, hospital staff, meningitis and pneumonia surveillance personnel, and public health laboratory staff all the local, state and federal levels
Role of the funding source: Surveillance for invasive pneumococcal disease was funded by the Brazilian Ministry of Health, with support from the Pan American Health Organization and the U.S. Centers for Disease Control and Prevention. Health secretariats in participating states provided assistance with data collection. Participating hospitals were responsible for obtaining pneumococcal isolates. Support for the national reference laboratory for invasive pneumococcal diseases was provided by the Pan American Health Organization through the regional laboratory network project, SIREVA II. Authors who are employees of the funders and collaborating institutions took full responsibility for the design of the study, data collection, data analysis and the final decision to publish.
Footnotes
Conflict of interest statement: MC de Cunto Brandileone (member of the Brazilian Pneumococcal Conjugate Vaccine Effectiveness Study Group, not a named author) has received consulting fees from Pfizer, GlaxoSmithKline, Sanofi Pasteur and Novarti s and travel grants from Pfizer and GlaxoSmithKline. The other authors declared that they have no competing interests.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374(9693):893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Fitzwater SP, Chandran A, Santosham M, Johnson HL. The worldwide impact of the seven-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2012;31(5):501–8. doi: 10.1097/INF.0b013e31824de9f6. [DOI] [PubMed] [Google Scholar]
- 3.Progress in introduction of pneumococcal conjugate vaccine - worldwide, 2000–2012. MMWR Morb Mortal Wkly Rep. 2013;62(16):308–11. [PMC free article] [PubMed] [Google Scholar]
- 4.Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines. 2009;8(11):1479–500. doi: 10.1586/erv.09.113. [DOI] [PubMed] [Google Scholar]
- 5.Domingues CMAS, Verani J, Montenegro Renoiner E, de Cunto Brandileone MC, Flannery B, de Oliveira L, Santos J, de-Moraes J. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. The Lancet Respiratory Medicine. 2014;2(6):464–471. doi: 10.1016/S2213-2600(14)70060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. The New England journal of medicine. 1980;303(10):549–552. doi: 10.1056/NEJM198009043031003. [DOI] [PubMed] [Google Scholar]
- 7.De Serres G, Pilishvili T, Link Gelles R, Reingold A, Gershman K, Petit S, Farley M, Harrison L, Lynfield R, Bennett N, Baumbach J, Thomas A, Schaffner W, Beall B, Whitney C, Moore M. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine. 2012;30(27):4067–4072. doi: 10.1016/j.vaccine.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Andrews N, Waight P, Borrow R, Ladhani S, George R, Slack MPE, Miller E. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS ONE. 2011;6(12):e28435–e28435. doi: 10.1371/journal.pone.0028435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruckinger S, van der Linden M, Reinert R, von Kries R. Efficacy of 7-valent pneumococcal conjugate vaccination in Germany: An analysis using the indirect cohort method. Vaccine. 2010;28(31):5012–5016. doi: 10.1016/j.vaccine.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 10.Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, Reingold A, Thomas A, Glode MP, Zell ER, Jorgensen JH, Beall B, Schuchat A. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 11.Measuring impact of Streptococcus pneumoniae and Haemophilus influenzae type b conjugate vaccination. Department of Immunization, Vaccines and Biologicals, World Health Organization; 2012. [Google Scholar]
- 12.De Serres G, Skowronski DM, Wu XW, Ambrose CS. The test-negative design: validity, accuracy and precision of vaccine efficacy estimates compared to the gold standard of randomised placebo-controlled clinical trials. Euro surveillance. 2013;18(37) doi: 10.2807/1560-7917.es2013.18.37.20585. [DOI] [PubMed] [Google Scholar]
- 13.O’Loughlin RE, Edmond K, Mangtani P, Cohen AL, Shetty S, Hajjeh R, Mulholland K. Methodology and measurement of the effectiveness of Haemophilus influenzae type b vaccine: systematic review. Vaccine. 2010;28(38):6128–36. doi: 10.1016/j.vaccine.2010.06.107. [DOI] [PubMed] [Google Scholar]
- 14.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–55. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]