Summary
Several Phyllosticta species are known as pathogens of Citrus spp., and are responsible for various disease symptoms including leaf and fruit spots. One of the most important species is P. citricarpa, which causes a foliar and fruit disease called citrus black spot. The Phyllosticta species occurring on citrus can most effectively be distinguished from P. citricarpa by means of multilocus DNA sequence data. Recent studies also demonstrated P. citricarpa to be heterothallic, and reported successful mating in the laboratory. Since the domestication of citrus, different clones of P. citricarpa have escaped Asia to other continents via trade routes, with obvious disease management consequences. This pathogen profile represents a comprehensive literature review of this pathogen and allied taxa associated with citrus, focusing on identification, distribution, genomics, epidemiology and disease management. This review also considers the knowledge emerging from seven genomes of Phyllosticta spp., demonstrating unknown aspects of these species, including their mating behaviour.
Taxonomy
Phyllosticta citricarpa (McAlpine) Aa, 1973. Kingdom Fungi, Phylum Ascomycota, Class Dothideomycetes, Order Botryosphaeriales, Family Phyllostictaceae, Genus Phyllosticta, Species citricarpa.
Host range
Confirmed on more than 12 Citrus species, Phyllosticta citricarpa has only been found on plant species in the Rutaceae.
Disease symptoms
P. citricarpa causes diverse symptoms such as hard spot, virulent spot, false melanose and freckle spot on fruit, and necrotic lesions on leaves and twigs.
Useful websites
DOE Joint Genome Institute MycoCosm portals for the Phyllosticta capitalensis (https://genome.jgi.doe.gov/Phycap1), P. citriasiana (https://genome.jgi.doe.gov/Phycit1), P. citribraziliensis (https://genome.jgi.doe.gov/Phcit1), P. citrichinaensis (https://genome.jgi.doe.gov/Phcitr1), P. citricarpa (https://genome.jgi.doe.gov/Phycitr1, https://genome.jgi.doe.gov/Phycpc1), P. paracitricarpa (https://genome.jgi.doe.gov/Phy27169) genomes.
All available Phyllosticta genomes on MycoCosm can be viewed at https://genome.jgi.doe.gov/Phyllosticta.
Keywords: Citrus, Guignardia, mating type, systematics
Introduction
Phyllosticta species have been associated with Citrus spp. worldwide (Baayen et al., 2002; Baldassari et al., 2008; Brentu et al., 2012; Carstens et al., 2012; Er et al., 2014; Everett and Rees‐George 2006; Glienke et al., 2011; Glienke‐Blanco et al., 2002; Guarnaccia et al., 2017a; Wikee et al., 2013a; Wulandari et al., 2009). Citrus black spot (CBS) is an economically important foliar and fruit disease caused by P. citricarpa (Baldassari et al., 2008; Kotzé, 1981). The pathogen affects the rind of the fruit, causing different symptoms on almost all commercial citrus cultivars, with some lemons, limes, mandarins and late‐maturing sweet oranges being the most susceptible (Kiely, 1948a, 1948b, 1949; Kotzé, 1981, 2000; Snowdon, 1990). CBS disease was reported for the first time in Australia (Benson, 1895), and is currently present in warm, summer rainfall areas of Asia, Africa, South America and North America (Kotzé, 1981; Schubert et al., 2012; Yonow et al., 2013).
Phyllosticta citricarpa was previously not readily distinguishable from P. capitalensis, a morphologically similar but nonpathogenic species, considered as the asexual morph of Guignardia mangiferae (Baayen et al., 2002; Everett and Rees‐George, 2006; Glienke et al., 2011). After the advent of DNA‐based characterization, a robust multilocus analysis was developed to distinguish P. citricarpa from P. capitalensis and other Phyllosticta species, resolving the identification and detection of P. citricarpa (Glienke et al., 2011).
Phyllosticta capitalensis is a cosmopolitan fungus reported from a broad range of plant hosts (Baayen et al., 2002; Bezerra et al., 2012; Everett and Rees‐George, 2006; Glienke‐Blanco et al., 2002; Johnston, 1998; Meyer et al., 2006; Okane et al., 2001; Rakotoniriana et al., 2008; Rodrigues and Samuels, 1999; Rodrigues et al., 2004; Wikee et al., 2011; Yuan et al., 2009), and has been found on citrus associated with both CBS‐affected and asymptomatic plants (Baayen et al., 2002; Everett and Rees‐George, 2006; Glienke et al., 2011). Further Phyllosticta species have been reported as pathogens of citrus, namely P. citriasiana and P. citrimaxima, responsible for citrus tan spot disease on Citrus maxima in Asia (Wikee et al., 2013b; Wulandari et al., 2009), P. citrichinaensis on different citrus species in Asia and P. paracitricarpa on Citrus sinensis fruit in China (Wang et al., 2012). The last species was also detected from citrus leaf litter in Europe (Guarnaccia et al., 2017a). Nevertheless, P. citribraziliensis and P. paracapitalensis were reported as endophytes on different citrus hosts (Glienke et al., 2011; Guarnaccia et al., 2017a).
The occurrence of two opposite mating types genes (MAT1‐1‐1 and MAT1‐2‐1) has been reported in P. citricarpa populations, confirming the heterothallic behaviour of the fungus and the necessity of crossing between opposite mating types in order to produce viable progeny (Amorim et al., 2017; Wang et al., 2013, 2016). Carstens et al. (2017) conducted the first study on the global mating type distribution of P. citricarpa. Despite the importance of homo‐ or heterothallism and knowledge of mating type distribution, little is known about the sexual cycle of most Phyllosticta species associated with citrus.
This review focuses on the mating‐type behaviour of P. citricarpa and other related species associated with citrus, and improved methods for their detection. Currently available knowledge regarding the pathogen’s geographical distribution, disease epidemiology and management is summarized. Furthermore, key insights emerging from the genomes of six Phyllosticta spp. recently sequenced are highlighted. The purpose of this review is to provide a basis for future studies aimed at understanding the rapid detection of Phyllosticta species associated with citrus, their mating type distribution and global movement.
Taxonomy and Identification
The name previously applied to the sexual morph of Phyllosticta is Guignardia (van der Aa, 1973; Rossman et al., 2015). Guignardia was introduced by Viala and Ravaz (1892) as a replacement for Laestadia (Bissett, 1986). Guignardia bidwellii and related species were included in Botryosphaeria by Petrak (1957), and this proposal was supported by Barr (1970, 1972). Later, van der Aa (1973) monographed the genus Phyllosticta, and all species were re‐evaluated by van der Aa and Vanev (2002). Schoch et al. (2006) placed Phyllosticta in the order Botryosphaeriales. However, several authors showed that Botryosphaeriaceae contained both Botryosphaeria and Phyllosticta spp., although this relationship remained poorly resolved (Crous et al., 2006; Liu et al., 2012; Schoch et al., 2006).
The older name Phyllosticta was retained over that of Guignardia after the end of fungal dual nomenclature (Glienke et al., 2011; Hawksworth et al., 2011; Marin‐Felix et al., 2019; Rossman et al., 2015; Sultan et al., 2011; Wikee et al., 2011, 2013b; Wingfield et al., 2012; Wong et al., 2012; Wijayawardene et al., 2014). Wikee et al. (2013b) redefined Phyllosticta and resurrected the family name Phyllostictaceae, showing that it clusters separately from the Botryosphaeriaceae within the Botryosphaeriales.
The in vitro production of pycnidial conidiomata containing aseptate, hyaline conidia, encased in a mucoid layer and bearing a single apical appendage (van der Aa, 1973), is the most characteristic feature used to recognize species of Phyllosticta. However, the mucoid layer and appendage are not always present or visible. The sexual morph presents erumpent, globose to pyriform ascomata, often irregularly shaped, unilocular and with a central ostiole. Asci are eight‐spored, bitunicate, clavate to broadly ellipsoid, with a wide, obtusely rounded or slightly square apex. Ascospores are ellipsoid to limoniform, sometimes slightly elongated, aseptate, hyaline, showing a large central guttule and a mucoid cap at both ends. Spermatia produced in culture are hyaline, aseptate, cylindrical to dumbbell‐shaped with guttules at each end (van der Aa, 1973).
Studies incorporating DNA sequence data (Baayen et al., 2002; Glienke et al., 2011; Wikee et al., 2011; Wulandari et al., 2009) resolved different Phyllosticta species associated with citrus that were described based on phylogenetic data, morphology and culture characteristics (Crous et al., 2012; Su and Cai, 2012; Wang et al., 2012; Wong et al., 2012; Zhang et al., 2012).
Phyllosticta citricarpa and P. capitalensis have several morphological and physiological differences: (i) colonies of P. citricarpa produce a yellow halo when grown on oatmeal agar (OA), (ii) P. capitalensis generally grows faster, (iii) conidia of P. capitalensis are normally embedded within a thicker mucoid sheath than observed in P. citricarpa, and (iv) there is a higher level of hydrolytic enzyme production in P. citricarpa than in P. capitalensis (Baayen et al., 2002; Glienke et al., 2011; Romão et al., 2011). Apart from colony characteristics on OA, morphology is unreliable to distinguish these species, and the identification of P. citricarpa has been confused with P. capitalensis, which is easily isolated as an endophyte from a broad range of hosts, and commonly co‐occurs with P. citricarpa on citrus.
Phyllosticta capitalensis was originally described on Stanhopea (Orchidaceae) from Brazil by Hennings (1908). The name P. capitalensis was later applied to an endophytic species occurring in ericaceous plants in Japan by Okane et al. (2001), who also described its sexual morph as a new species, G. endophyllicola. The name to apply to the sexual morph has been much debated in the past. Baayen et al. (2002), based on DNA sequence data of the rDNA internal transcribed spacer (ITS) region, linked P. capitalensis to G. mangiferae, a species originally reported from Mangifera indica in India, separating P. capitalensis from G. endophyllicola. Later, Glienke et al. (2011), on the basis of a multilocus phylogenetic analysis, revealed that P. capitalensis sensu lato was genetically distinct from a reference isolate of G. mangiferae, and concluded that the correct name for the common endophyte is P. capitalensis, since G. mangiferae is a distinct taxon on mango. Phyllosticta citricarpa is clearly distinct from P. capitalensis based on sequences of the ITS region, with each species showing low levels of intraspecific variation (Hu et al., 2014; Okane et al., 2003; Rodrigues et al., 2004).
Significant progress in species differentiation was achieved with the introduction of multilocus DNA phylogenetic analyses. Several studies have been performed with multigene sequencing of a large number of Phyllosticta species (Glienke et al., 2011; Wang et al., 2012; Wulandari et al., 2009). Wulandari et al. (2009) revealed three Phyllosticta clades associated with citrus in Thailand using three partial DNA regions (ITS, tef1, actA), namely, P. capitalensis, P. citricarpa and P. citriasiana. Similarly, Wang et al. (2012) described P. citrichinaensis and distinguished two subclades within P. citricarpa, which were differentiated as two species in a later study (Guarnaccia et al., 2017a) and are described in greater detail below. In Brazil, Glienke et al. (2011) distinguished a new endophytic species associated with Citrus sp., P. citribraziliensis, combining four partial regions of DNA (ITS, tef1, actA, gapdh). The most recent study focusing on Phyllosticta taxonomy (Guarnaccia et al., 2017a) revealed two new species, namely P. paracapitalensis, from asymptomatic leaves of citrus species in Italy and Spain, and P. paracitricarpa, from leaf litter of C. limon in Greece. This study was based on the use of molecular data in which six gene regions (ITS, actA, tef1, gapdh, LSU, rpb2) were analysed.
The phylogeny of Phyllosticta based on the ITS and actA genomic loci has been reported as sufficient to differentiate most taxa, except those closely related to P. capitalensis (Wikee et al., 2013b), such as P. paracapitalensis, which can be distinguished by adding sequences of tef1 and rpb2 (Guarnaccia et al., 2017a). The use of a three‐gene analysis (ITS, actA, tef1) performed in a previous study by Wang et al. (2012) showed two poorly supported subclades within P. citricarpa. Highly supported independent lineages were obtained in the phylogenetic tree through the addition of a further three genomic loci (gapdh, LSU and rpb2) to confirm that the two subclades actually represent two distinct species, P. citricarpa and P. paracitricarpa (Guarnaccia et al., 2017a).
Distribution and Host Associations
Since the fifth century BC, the movement of citrus plants from Asia, where citrus and CBS disease are endemic, occurred to countries around the Mediterranean Sea (Mabberley, 2004; Nicolosi, 2007; Ramon‐Laca, 2003; Wu et al., 2018). Phyllosticta spp. may therefore have been introduced to and may be present in all citrus‐growing countries.
The first report of P. citricarpa causing CBS disease was in Australia in the late 19th century, specifically in coastal regions of New South Wales (Benson, 1895). Subsequently, P. citricarpa has been recorded in many citrus‐growing areas of Australia, Africa, Asia, Central, North and South America, and Europe (Supporting Information Table S1). Phyllosticta capitalensis has been reported worldwide as a common endophyte of citrus and other hosts (Baayen et al., 2002; Wikee et al., 2013a). Recent studies also revealed the existence of other Phyllosticta species on Citrus. In Brazil, Glienke et al. (2011) described P. citribraziliensis from Citrus sp. In Asia, P. citriasiana (China, Thailand, Vietnam) and P. citrimaxima (Thailand) were found on C. maxima (Wikee et al., 2013b; Wulandari et al., 2009) and P. citrichinaensis was described by Wang et al. (2012) as a pathogen of several Citrus spp. in China.
In 2016, knowledge about the diversity and distribution of fungi in association with citrus was enhanced when a survey performed in citrus‐growing areas of Europe revealed the presence of different pathogens, saprobes and endophytes, including four Phyllosticta spp. (Crous et al., 2016, 2017; Guarnaccia and Crous, 2017, 2018; Guarnaccia et al., 2017a, 2017b; Sandoval‐Denis et al., 2018). Two of these species, P. paracapitalensis and P. paracitricarpa, were described as new and were isolated from fresh asymptomatic leaves in Italy and Spain, respectively, and from leaf litter of lemon in Greece. Moreover, P. capitalensis and P. citricarpa were reported by molecular analysis for the first time associated with citrus in Europe from asymptomatic leaves in Greece, Italy, Malta, Portugal and Spain, respectively, and from leaf litter in Italy, Malta and Portugal. The six‐gene phylogenetic analysis performed by Guarnaccia et al. (2017a) revealed that a strain previously identified as P. capitalensis from Citrus aurantiifolia cultivated in New Zealand (Everett and Rees‐George, 2006) also clustered with P. paracapitalensis. Similarly, two Chinese strains appearing as P. citricarpa subclade‐II sensu Wang et al. (2012) grouped with P. paracitricarpa.
Eight Phyllosticta species are now associated with citrus: P. citricarpa and P. capitalensis are present on all continents where citrus species are cultivated. Phyllosticta paracapitalensis was reported from Europe and New Zealand, while P. paracitricarpa is present in Asia and Europe. Phyllosticta citrichinaensis, P. citriasiana and P. citrimaxima were found only in Asia, and the endophyte P. citribraziliensis has been reported only in South America (Glienke et al., 2011; Guarnaccia et al., 2017a; Wang et al., 2012; Wikee et al., 2013b; Wulandari et al., 2009). Current knowledge about the distribution of these Phyllosticta species reveals that most of them are present in Asia, where citrus originated (Wu et al., 2018). However, several incursions have occurred along with the global establishment of citrus cultivation and given the long‐range dispersal of these species via infected citrus plant propagation material, it would be expected that P. citricarpa and/or other Phyllosticta species may be present in many citrus‐producing countries.
Epidemiology and Symptoms
Citrus black spot disease has been reported in most of the major citrus‐producing countries, exclusively in areas with warm, summer rainfall climates (Carstens et al., 2012; Magarey et al., 2015; Martínez‐Minaya et al., 2018; Paul et al., 2005; Yonow et al., 2013). Phyllosticta citricarpa produces ascospores in pseudothecia and conidia in pycnidia as inoculum sources. In general, spore production and release occur during the rainy season (Dummel et al., 2015; Fourie et al., 2013; Kiely, 1948a; Kotzé, 1963, 1996, 2000; Reis et al., 2006).
Pseudothecia develop 40–180 days after leaf fall, and under favourable conditions such as alternate wetting and drying of leaves and mild to warm temperature fluctuations, inducing pseudothecium maturation and ascospore discharge (Dummel et al., 2015; Fourie et al., 2013; Hu et al., 2014; Huang and Chang, 1972; Kiely, 1948a; Kotzé, 1963; Lee and Huang, 1973; McOnie, 1964a; Reis et al., 2006; Truter, 2010).
Conidia are considered particularly important in the case of co‐existence of mature diseased fruit in a tree with young growing fruit, in areas with a large number of dead twigs and in citrus species in which hard spots with pycnidia are formed on leaves (Fig. 1) (Baldassari et al., 2006; Spósito et al., 2008, 2011). Ascospores are found only on fallen leaves (leaf litter) and not on fruit, green leaves and twigs attached to the canopy. Conidia can be found in dead twigs (not on green twigs), green leaves (mainly lemons) and diseased fruit as well as on fallen leaves (Baldassari et al., 2006; Kiely, 1948a; Kotzé, 1963).
Figure 1.
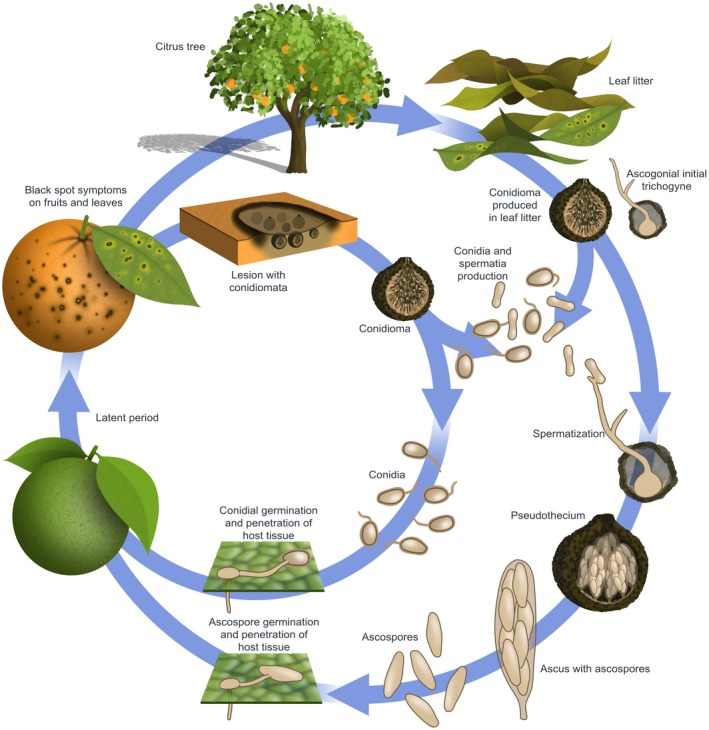
Cycle of citrus black spot disease, caused by Phyllosticta citricarpa.
An interesting case is the finding of only one mating type in the population from Florida. In this population, the primary inoculum is represented by conidia, which are related to the short‐distance dispersal of the disease (Hendricks et al., 2017; Wang et al., 2016). Thus, there is no production of pseudothecia and the asexual overwintering of the pathogen could occur endophytically as latent infections in leaves or twigs, in leaf or twig lesions, leaf litter, and on infected, out‐of‐season fruit on the tree (Kiely, 1948b; Spósito et al., 2007, 2011; Wager, 1952; Whiteside, 1967). Similarly, P. citricarpa strains were isolated from asymptomatic leaves and no CBS symptoms were ever found in Europe (Guarnaccia et al., 2017a).
Windborne ascospores are generally dispersed under field conditions over longer distances (c. 25 m) and are associated with the CBS spread between trees (Spósito et al., 2007), while conidia are dispersed over short distances (less than 80 cm) by washing‐down, being responsible for spread of the pathogen within a tree canopy (Kotzé, 1981; Spósito et al., 2011) or inter‐canopy (Hendricks et al., 2017).
Conditions required for infection by ascospores or conidia are at least 15 or 12 h leaf or fruit wetness at optimal temperatures (27 °C), respectively, while longer wetness periods are required at sub‐ or super‐optimal temperatures in the range of 15 to 35 °C (Kiely, 1948a, 1948b, 1949; Kotzé, 1963, 1981; McOnie, 1967; Noronha, 2002; Wang and Dewdney, 2014). The spores germinate and produce appressoria in the presence of moisture, and start infection by penetrating the cuticle and expanding into a small mass of mycelium between the cuticle and the epidermis wall, as a latent infection (McOnie, 1967).
Symptoms of CBS occur months later, in general after a latent period. In sweet orange fruit, false melanose lesions appear on green fruit from 40 to 110 days after inoculation, while hard spots are observed from 110 to 360 days. Some factors influence the length of the latent period, such as the inoculum concentration and fruit diameter (Frare et al., 2019). In addition, increasing temperatures from 20 to 27 °C when the fruit are mature can stimulate the appearance of CBS symptoms and lead to expression of a significant number of fruit lesions. High light intensity induces more fruit lesion development, thus the canopy side most exposed to light shows more symptoms. Under dry conditions CBS symptom expression may be increased. Age and stress are also linked to CBS development, since symptoms are more severe on older trees than on healthy, young trees (Kiely, 1948b; Kotze, 1981).
Phyllosticta citricarpa affects fruit, leaves and twigs of several citrus hosts causing diverse symptoms (Kiely, 1948a, 1949; Kotzé, 1981, 2000; Snowdon 1990). Hard spot, characterised by sunken, pale brown necrotic lesions with a dark reddish brown raised border, often containing pycnidia, is the most common symptom (Fig. 2). Further symptom types have been described: virulent spot, which are sunken necrotic lesions without defined borders mostly on mature fruit; false melanose, consisting of small black pustules usually in a tear stain pattern; freckle, cracked or speckled spot. Leaf and twigs symptoms rarely occur on orange, mandarin and other commercial citrus species, but they are frequently present on lemons. They appear as round, small, sunken necrotic lesions with a yellow halo (Kotzé, 1981).
Figure 2.
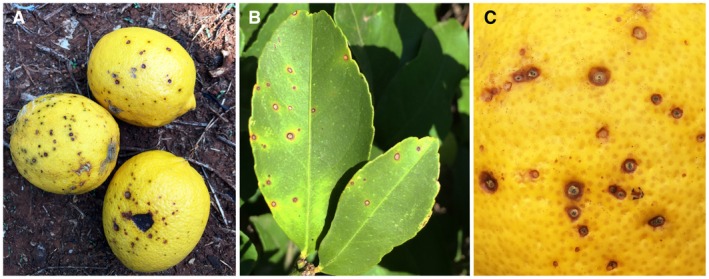
Citrus black spot symptoms caused by Phyllosticta citricarpa. (a) Typical hard and virulent spot with sunken necrotic lesions on Citrus limon fruit. (b) Hard spot lesions with a dark brown raised border on C. limon leaves. (c) Freckle spot containing pycnidia on C. sinensis rind.
In Asia, P. citriasiana and P. citrimaxima were reported to cause citrus tan spot on C. maxima fruit and leaves (Wikee et al., 2013b; Wulandari et al., 2009): shallow lesions with a small central depressed grey to tan area, with a dark brown margin. This symptom usually starts appearing with fruit ripening. Phyllosticta citrichinaensis causes a brown spot and red‐brown protuberant freckle on citrus leaves and fruit (Wang et al., 2012). Phyllosticta paracitricarpa was isolated from fruit spots on C. limon and C. sinensis in China, and its potential pathogenicity was demonstrated only through a preliminary pathogenicity test performed on detached mature orange fruit by Guarnaccia et al. (2017a).
The remaining Phyllosticta species recovered from citrus are considered as nonpathogenic. Phyllosticta capitalensis has been reported from 21 different plant families (Baayen et al., 2002; Bezerra et al., 2012; Everett and Rees‐George, 2006; Glienke‐Blanco et al., 2002; Johnston, 1998; Meyer et al., 2006; Okane et al., 2001; Rakotoniriana et al., 2008; Rodrigues and Samuels, 1999; Rodrigues et al., 2004; Yuan et al., 2009) and from symptomatic and asymptomatic citrus plants (Baayen et al., 2002; Everett and Rees‐George, 2006; Glienke et al., 2011). This species was not associated with lesion formation in a pathogenicity test on detached mature sweet orange fruit (Guarnaccia et al., 2017a). Phyllosticta citribraziliensis was described from healthy citrus leaves by Glienke et al. (2011). Phyllosticta paracapitalensis is known as an endophyte from C. limon, C. floridana and C. aurantiifolia (Guarnaccia et al., 2017a).
Sexual Reproduction and Genetics
The MAT1 mating type locus regulates sexual reproduction in ascomycetes by controlling mechanisms that lead to fertilization (Coppin et al., 1997; Debuchy et al., 2010). Two mating type idiomorphs are associated with MAT1, known as MAT1‐1, containing the MAT1‐1‐1 gene, and MAT1‐2, containing the MAT1‐2‐1 gene (Coppin et al., 1997; Debuchy and Turgeon, 2006; Debuchy et al., 2010; Pöggeler, 2001; Turgeon and Yoder, 2000). Numerous homothallic fungal species have been shown to possess both MAT genes, while heterothallic species have MAT1‐1 and MAT1‐2 idiomorphs in different individuals, and fertilization requires the fusion of a male (spermatium) and a female (ascogonium) elements (Alexopoulos et al., 1996; Coppin et al., 1997; Ni et al., 2011). Although it is not well known, the presence of spermatia and ascogonia is crucial in the P. citricarpa life cycle (Baayen et al., 2002; Kiely, 1948a), as well as for other members of Dothideomycetes. Kiely (1948a) was the first to reflect on the role of spermatia as male element due to the occurrence of spermatogonia prior to the formation of pseudothecial ascomata.
The heterothallic nature of P. citricarpa was recently confirmed through full sequence of target loci characterization, showing the presence of separated MAT1‐1 or MAT1‐2 idiomorphs (Amorim et al., 2017; Wang et al., 2016). Studies were performed during the last couple of decades with the aim of developing reliable methods to induce the sexual morph of P. citricarpa in vitro. The inoculation of a single mycelium plug or of a mycelial and spore suspension on a medium containing lemon leaf and malt extracts was reported as successful by Moran Lemir et al. (2000). However, this process was not reproducible during subsequent studies (Baldassari et al., 2008; Wang et al., 2016). Amorim et al. (2017) conducted in vitro crossings with isolates of different mating types on various media and incubated plates at different conditions, but did not observe any sexual structures. The first successful and reproducible mating of P. citricarpa in culture was reported by Tran et al. (2017), involving direct physical contact between two isolates of complementary mating types. The spermatia function as male gametes and their fertilization of receptive organs during sexual reproduction of P. citricarpa via spermatization was demonstrated. Spermatia were shown to be incapable of germinating and forming colonies on media, demonstrating their role in sexual reproduction rather than vegetative growth. This study confirmed that the mating type genes of P. citricarpa are functional and that sexual reproduction can occur under laboratory conditions. Furthermore, Tran et al. (2017) conducted multilocus genotyping using simple‐sequence repeat (SSR) markers to determine the genotypes and hybrid nature of the cultures derived from ascospores obtained in vitro. These results demonstrate that new recombinant genotypes were formed in the F1 progeny, confirming that genetic recombination occurs. Tran et al. (2018) also tested the pathogenicity of P. citricarpa ascospores, obtaining typical CBS symptoms on Troyer citrange leaves and Murcott tangor fruit with ascospores produced in vitro from characterized P. citricarpa isolates, confirmed with recovering and genotyping the inoculated recombinant ascospore isolates from obtained symptoms. These recent findings are crucial for new research projects aimed at understanding pathogenicity, as well as management of diseases caused by Phyllosticta species.
In Australia, Brazil, China and South Africa, MAT genotyping of populations revealed an almost equal mating type distribution, confirming the occurrence of sexual reproduction in these populations (Amorim et al., 2017; Carstens et al., 2017; Tran et al., 2017; Wang et al., 2016; Zhang et al., 2015). Interestingly, in Florida in the USA, only one mating type has been identified (Carstens et al., 2017; Wang et al., 2016). Both MAT1‐1 and MAT1‐2 containing isolates occur in Europe, but both mating types were not recovered together in the same geographic location, nor were pseudothecial ascomata ever observed (Guarnaccia et al., 2017a).
The first SSR markers for P. citricarpa were developed by Wang et al. (2016) from two published genome sequences of P. citricarpa and consist of seven polymorphic loci. One population from Australia and one from Florida were genotyped using these markers. Two to four alleles per locus were identified in the Australian population, and 11 multilocus genotypes (MLGs) among 24 Australian isolates. The populations from Florida consisted of a single MLG, confirming the results obtained in previous studies based on MAT genotyping (Wang et al., 2016; Zhang et al., 2015). Population genetic inferences were strengthened by the development of eight new polymorphic SSR markers by Carstens et al. (2017), which were added to the seven markers by Wang et al. (2016). Thus, a total of 15 SSR markers was used to genotype several populations from Australia, Brazil, China, Florida (USA) and South Africa (Carstens et al., 2017). The high level of gene and genotypic diversity within P. citricarpa populations of China and Australia, as well as the presence of several private alleles, demonstrated a longer evolutionary history with the citrus host in these countries, compared to South Africa, Brazil and the USA. The US population was confirmed to be clonal (Carstens et al., 2017). Populations from Australia, Brazil, South Africa and the USA shared an MLG, suggesting a long‐distance human‐mediated dispersal. Moreover, the clonal mating type (MAT1‐2‐1) present in the US population suggested a human introduction, with subsequent asexual reproduction of the pathogen in Florida (Carstens et al., 2017; Hendricks et al., 2017; Wang et al., 2016).
Another recent study based on the same 15 SSR markers used by Carstens et al. (2017) revealed that the P. citricarpa populations from Italy and Malta versus Portugal represented two separate clones, differing from each other in their MLGs and mating type (Guarnaccia et al., 2017a). The origin of P. citricarpa in Europe is still unclear because these populations also differed from one another in their connectivity and differentiation level from the other populations from Australia, Brazil, China, South Africa and the USA. The genotyping performed in that study showed that the populations from Portugal and Australia were more strongly connected to each other than to other populations, whilst very little connectivity was evident between the Portuguese population and those from the other continents, including the population from Italy and Malta. Similarly, the Italy/Malta population appeared to be distinct from the other known populations. These results suggest at least two separate introductions into Europe.
Genomics
Seven genomes and transcriptomes of Phyllosticta spp., including two strains of P. citricarpa, have been sequenced as part of the 1000 Fungal Genomes initiative of the United States Department of Energy Joint Genomics Institute (JGI; http://1000.fungalgenomes.org) (Table 1). The QIAGEN Genomic‐tip 100/G (QIAGEN Benelux B.V., Venlo, Netherlands) and the QIAGEN RNeasy Midi kit have been used, respectively, for DNA and RNA isolation. All of these genomes, except for P. citriasiana (strain CBS 120486) were sequenced using PacBio technology and assembled with Falcon v. 1.8.8 (https://github.com/PacificBiosciences/FALCON). The genome of P. citriasiana was sequenced using the Illumina platform and assembled with AllPathsLG release v. R49403 (Gnerre et al., 2011). All transcriptomes were sequenced using Illumina and assembled with Trinity v. 2.3.2 (Grabherr et al., 2011). All genomes were annotated using the JGI Annotation Pipeline (Grigoriev et al., 2014). The average Phyllosticta genome is about 30.9 Mbp with about 5.2% repeats and c.11 300 genes (Table 1, Fig. 3). Core Eukaryotic Genes Mapping Approach (CEGMA) (Parra et al., 2007) analysis showed that these assemblies capture more than 99.5% of conserved eukaryotic genes. In all these genomes, >70% of genes had more than one exon (median = 2), similar to other Dothideomycetes, with an average gene density of 365.33 Mbp. Of the 11 300 genes in a Phyllosticta genome, more than two‐thirds (67.6%) are shared with all other Phyllosticta strains in this study and an additional 20% are found in more than one Phyllosticta genome. The largest families include transporters, protein kinases and transcription factors. On average, 12% of these genes were unique to each species in our study (Fig. 3), with the largest fraction of species‐specific genes in P. capitalensis at the base of the Phyllosticta tree, and fewer differences within the group of two P. citricarpa isolates and P. paracitricarpa. However, further studies are needed to explore the variation within the P. citricarpa and P. paracitricarpa populations.
Table 1.
Assembly statistics, gene content and annotation characteristics of seven Phyllosticta spp. genomes sequenced for this study.
| P. capitalensis CBS 128856 | P. citriasiana CBS 120486 | P. citribraziliensis CBS 100098 | P. citrichinaensis CBS 130529 | P. paracitricarpa CBS 141357 | P. citricarpa CBS 127454 | P. citricarpa CBS 141350 | |
|---|---|---|---|---|---|---|---|
| Assembly length | 32 461 131 | 32 696 106 | 31 670 975 | 29 162 704 | 29 529 839 | 28 952 665 | 32 267 666 |
| # contigs (scaffolds) | 14 | 741 (133) | 32 | 25 | 134 | 152 | 82 |
| Contig (scaffold) N50 | 5 | 56 (14) | 7 | 4 | 23 | 23 | 13 |
| Contig (scaffold) L50, bp | 2 860 346 | 163 917 (807 147) | 1 720 616 | 2 710 567 | 510 184 | 440 231 | 900 945 |
| % GC, ignoring Ns | 54.58 | 53.05 | 54.17 | 55.07 | 54.35 | 54.6 | 52.56 |
| % repeats | 2.07 | 9.24 | 6.16 | 4.34 | 2.62 | 2.76 | 9.46 |
| Gene density (Mbp) | 371.61 | 347.69 | 350.51 | 380.35 | 375.25 | 382.97 | 348.93 |
| # genes | 12 063 | 11 368 | 11 101 | 11 092 | 11 081 | 11 088 | 11 259 |
| % spliced | 70.73 | 72.69 | 74.17 | 71.41 | 72.12 | 72.75 | 71.92 |
| % CEGMA coverage | 99.78 | 99.78 | 99.78 | 100 | 99.78 | 99.56 | 100 |
| # unique domains hit (HMMpfam) | 3764 | 3742 | 3741 | 3713 | 3692 | 3700 | 3733 |
Figure 3.
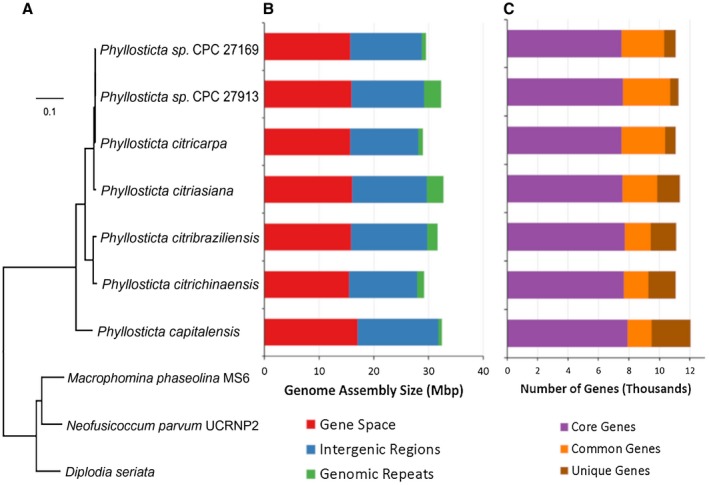
Phylogeny, assembly characteristics and gene composition of Phyllosticta genomes. (A) Genome based mid‐point rooted phylogenetic tree using 3455 single copy proteins identified using Markov Cluster Algorithm (mcl 10‐148, 1.008). Protein sequences were aligned with MAFFT (v. 7.123b) and the resulting alignment was cleaned using Gblocks 0.91b. The final alignment had 177824 distinct alignment patterns and was used for tree building using RAxML (v. 7.6.3) with 100 rapid bootstrap inferences, GAMMA model of rate heterogeneity and ML estimate of α‐parameter. All nodes showed 100% bootstrap support. (B) Genome size and repeat content. Predicted genes (CDS) covers about half of the assembled genome while genomic repeats cover between 2% and 10% of these assemblies. (C) Number of predicted genes. Core Genes are found in all Phyllosticta genomes, Common Genes are found in more than one Phyllosticta genome while Unique Genes are found only in one genome.
In order to provide a foundation for future studies, the mating type loci of the seven Phyllosticta genomes have been analysed with the aim of identifying which mating type(s) are present in Phyllosticta species and to characterize the mating type genes. Based on a comparison of the genes known to be in P. citricarpa (Amorim et al., 2017; Wang et al., 2016), the 40SS9, PH Domain, MAT1‐1‐1, MAT1‐2‐9, MAT1‐2‐1, OML1 and APN2 protein sequences (see Table S3 for accessions) have been selected. Then, based on translated nucleotide blast (Altschul et al., 1997), the scaffolds were identified in each assembly that contain fragments of the mating type locus genes. Both strains of P. citricarpa (European and Australian strains) and the P. paracitricarpa strain have heterothallic idiomorphs containing the MAT1‐2‐1 gene. In contrast, P. citriasiana and P. citribraziliensis strains have heterothallic idiomorphs containing the MAT1‐1‐1 gene. Phyllosticta citrichinaensis is homothallic, containing the full MAT1‐1 and MAT1‐2 loci on different scaffolds. Finally, P. capitalensis seems to contain a hybrid of both MAT1‐1 and MAT1‐2 loci, with the MAT1‐2‐1 and MAT1‐1‐1 co‐linearly present between the 40SS9 and OML1 genes. The scaffolds with the MAT locus fragments were visualized with SimpleSynteny (Veltri et al., 2016) (Fig. 4).
Figure 4.
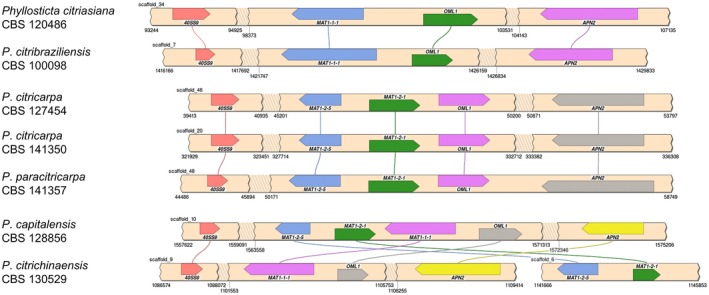
Syntenic relationships between mating type loci in different species of Phyllosticta. For each genome, the regions of the scaffolds containing mating type genes are illustrated as beige strips. Shaded breaks in the strips indicate larger genomic regions that do not contain our mating type genes, which are not visualized for clarity and brevity. The scaffold numbers and the relevant positions are annotated. Coloured boxes on the scaffolds indicate mating type genes, and the pointed end refers to the 3' end of the gene. Boxes with the same colour indicate the same mating type gene. Lines link the boxes to indicate their positions in the different genomes. Together, this figure visualizes the rearrangement of mating type loci across the Phyllosticta genomes.
In addition, new Phyllosticta‐specific mating type primers (Table 2) have been designed and a PCR method developed for rapid identification of the MAT1‐1‐1 and MAT1‐2‐1 genes in P. capitalensis, P. citriasiana, P. citribraziliensis, P. citricarpa, P. citrichinaensis and P. paracitricarpa strains (Supporting Information Fig. S1). The primer pair MAT111deg‐F2 and MAT111deg‐R3 amplified a fragment of approximately 1010 bp, whilst the primer pair MAT121deg‐F1 and MAT121deg‐R1 amplified a 300‐bp fragment of several tested isolates (Supporting Information Table S2).
Table 2.
Primers designed and used in this study.
| Primer | Sequence (5'–3') | Description |
|---|---|---|
| MAT111degF2 | GCTCTCAACTCTTTCATGGC | Phyllosticta spp. MAT1‐1‐1 specific |
| MAT111degR3 | TGGYKCGYYGCATCACGC | Phyllosticta spp. MAT1‐1‐1 specific |
| MAT121degF1 | AACAYRTCRARGCYCCGG | Phyllosticta spp. MAT1‐2‐1 specific |
| MAT121degR1 | YAABCCTGGRTTYTCCATCG | Phyllosticta spp. MAT1‐2‐1 specific |
Disease Management
Citrus tan spot caused by P. citriasiana and P. citrimaxima was only recently described and their economic importance is uncertain. Likewise, the economic importance of P. paracitricarpa is unknown. Control of these Phyllosticta species has not been studied, but it is likely to be effectively controlled by CBS management measures.
The management of CBS starts with planting disease‐free citrus trees, since infected propagation material is the most effective means of spreading P. citricarpa to new areas (Doidge, 1929; Kiely, 1948b; Kotze, 1981; Marchionatto, 1926; McOnie, 1964b; Silva‐Junior et al., 2016a; Wager, 1952). Regulatory measures are imposed by some countries to prevent the import and spread of P. citricarpa on infected propagation material into CBS‐free areas (EFSA, 2014; Kotzé, 1981). In addition, the movement of leaf litter from infected orchards through vehicle/machine movement is also important (Dewdney et al., 2018; Silva‐Junior et al., 2016a). Citrus fruit is not considered to be a realistic pathway for spread of P. citricarpa to new areas (USDA APHIS, 2010) for the following reasons: (i) the airborne ascospores cannot be produced on fruit, (ii) pycnidia are only produced in certain fruit lesion types (Brentu et al., 2012; FAO, 2014; Kotzé, 2000; Marques et al., 2012; OEPP/EPPO, 2009; Wager, 1952) and conidia are short‐lived with low germination ability (Kiely, 1948b), (iii) conidium dispersal from fruit lesions is by means of short‐distance (<1 m) wash‐down dispersal (Kiely, 1948b; McOnie, 1965; Spósito et al., 2008, 2011; Whiteside, 1967), (iv) standard packhouse treatments and cold storage effectively control P. citricarpa infections (Korf et al., 2001; Lucon et al., 2010; Rappussi et al., 2009, 2011; Schreuder et al., 2018; Seberry et al., 1967; Yan et al., 2016), and CBS lesions on fruit or discarded peel segments have a very low reproductive potential (Korf et al., 2001; Schreuder et al., 2018; Schutte et al., 2014), and (v) fallen leaves are not susceptible to infection (Truter et al., 2007). Inter‐state movement of commercial packhouse‐treated fruit from CBS present to CBS‐absent areas is therefore permitted in the USA, in line with their Pest Risk Analysis conclusion that fruit is not a realistic pathway (USDA APHIS, 2011). In European Union countries, where CBS disease has not been recorded, regulatory measures have been imposed on the import of citrus fruit from countries where CBS is present (EFSA, 2014). However, the technical justification for these measures has been contested (CBS Expert Panel, 2013, 2014, 2015).
Different chemical and cultural control measures are used for CBS management (Dewdney et al., 2018; Kotzé, 2000; Silva‐Junior et al., 2016a). The most effective strategy for control of CBS is the fungicide application during the period of fruit susceptibility (Lanza et al., 2018; Makowski et al., 2014; Schutte et al., 2003). The main fungicides used are strobilurins (quinone outside inhibitors, QoI), dithiocarbamates and fixed copper (multisite activity), and methyl benzimidazole carbamate (MBC), which may be applied singly or in mixtures with mineral oil (Dewdney et al., 2018; Kellerman and Kotze, 1977; Kotze, 1981, 2000; Miles et al., 2004; Schutte et al., 2003, 2012; Silva‐Junior et al., 2016a, 2016b). Sprays of QoI fungicides may reduce CBS symptoms by almost 100%, becoming one of the most valuable solutions for disease control (Dewdney et al., 2018; Fogliata et al., 2011; Miles et al., 2004; Schutte et al., 2003; Silva‐Junior et al., 2016a). The mode of action of QoI is highly specific; however, the presence of an intron in the cytochrome b gene reduces the risk of the G143A mutation, which causes QoI resistance (Hincapie et al., 2014; Stammler et al., 2013). Nonetheless, the number of QoI sprays per season should be restricted to prevent resistance development by another mechanism (Dewdney et al., 2018; Silva Junior et al., 2016a, 2016b).
In countries such as South Africa and Australia, the applications for control of CBS are restricted to the critical period of P. citricarpa infection, from petal fall stage, in October, to 120–150 days later, January–February (Kotzé 1981; Kiely, 1948b; Miles et al., 2004; Schutte et al., 1997, 2003). Typically, two to five spray rounds (dependent on citrus type and climate suitability of the production region for CBS) of mancozeb or copper‐based fungicides alone, or in mixture with MBC or QoI plus oil, are applied in South Africa, using high volumes from 6000 to 16 000 L/ha (Moyo et al., 2018; Schutte et al., 1997, 2003, 2012). In Brazil, where conditions are much more favourable for CBS (Magarey et al., 2015), a longer period of fruit protection is required (Lanza et al., 2018; Silva‐Junior et al., 2016a, 2016b). Two copper sprays are performed after petal fall, between September and November with a 21‐ to 28‐day interval, not only for CBS, but also to control other citrus diseases, followed by two to four QoI applications, performed with a 35‐ to 42‐day interval. This programme protects the fruit for 180–220 days until March or up to May. The spray volume is around 75 mL of spray mixture/m3 of the tree canopy, based on the tree‐row‐volume (TRV) concept (Lanza et al., 2018; Silva‐Junior et al., 2016a, 2016b). In the USA, the control programme has been based on fungicide efficacy results from other countries. Applications of copper and QoI fungicides are recommended from early May to mid‐September, but if favourable conditions occur in April, earlier sprays are recommended (Dewdney et al., 2018; Hincapie et al., 2014). Miles et al. (2004) demonstrated CBS control using the host defence promoter acibenzolar‐S methyl in the field, but it was not as effective as the registered fungicides.
In addition to chemical control, cultural control measures may be used to reduce the amount of P. citricarpa inoculum (Bellotte et al., 2009, 2013; Schutte and Kotzé, 1997), such as the removal of leaf litter with machines (Bellotte et al., 2009; Scaloppi et al., 2012; Spósito et al., 2011; Truter, 2010), the acceleration of leaf litter decomposition with urea, ammonium sulphate, sugarcane bagasse (Bellotte et al., 2009; Dewdney et al., 2018; Kotzé, 1981; van Bruggen et al., 2017), the mulching with plants that grow between rows of orchards to cover leaf litter (Bellotte et al., 2013; Schutte and Kotzé, 1997), the pruning of dead twigs (Silva et al., 2017; Silva‐Junior et al., 2016a), irrigation and balanced nutrition (Calavan, 1960; Dewdney et al., 2018; Kotzé, 1981), and the harvesting for optimal fruit quality and prevention of overlapping fruit sets (Kotzé, 1981; Spósito et al., 2008, 2011). Biological control of CBS with fungi, including P. capitalensis, and bacteria have shown inhibitory effect against P. citricarpa in vitro and in vivo (Almeida, 2009; Kupper et al., 2011; Pena et al., 2017; Santos et al., 2016; Tran et al., 2019); however, there is no biocontrol agent that controls CBS with fungicide‐like efficiency under field conditions. Genetic methods may improve the CBS resistance of citrus. Rodriguez et al. (2018) produced transgenic sweet orange trees with reduced production of d‐limonene in the fruit flavedo and enhanced resistance against P. citricarpa, but these materials need to be assessed under field conditions.
Postharvest treatments aimed at preventing decay and maintaining fruit quality have a strong effect on the CBS pathogen. Low temperatures reduce the postharvest development of symptoms on fruit (Agostini et al., 2006; Brodrick and Rabie, 1970) and standard packhouse treatments reduce the viability of the fungus in fruit lesions by three‐ to seven‐fold (Korf et al., 2001). Schreuder et al. (2018) demonstrated that the combination of standard packhouse fungicide treatments aimed at green mould or sour rot control (including pre‐packhouse drench, packhouse dip and brush application of a wax coating) followed by cold storage (common shipping protocol for exported fresh fruit) consistently showed moderate to high levels of control of CBS in lemons and oranges. Several other studies also reported control of latent P. citricarpa infections through application of various chemical or biological postharvest treatments (Lucon et al., 2010; Rappussi et al., 2009, 2011; Seberry et al., 1967; Yan et al., 2016). On the contrary, Agostini et al. (2006) reported no significant control with the postharvest fungicides evaluated in their study.
Conclusions and Future Research
Considerable taxonomic confusion has characterized studies about Phyllosticta spp. in the past, especially regarding the common mistake to distinguish P. capitalensis and P. citricarpa associated with symptoms on fruit, or the identification of P. citriasiana and P. citrichinaensis. After the advent of DNA‐based characterization, the taxonomy and classification of these species were clarified, offering new data and methods to improve rapid detection. Many genomes of several important plant pathogenic and endophytic fungi such as various Phyllosticta species have been sequenced. The genomes of P. citricarpa and P. capitalensis were already sequenced by Wang et al. (2016). The citrus pathogens P. citriasiana, P. citrichinaensis and P. paracitricarpa, and also the endophytic species P. citribraziliensis, are also now available for study.
Although the sexual morph of P. citricarpa was known (Wang et al., 2016), it was only recently successfully induced in vitro by Tran et al. (2017) through outcrossing of isolates carrying complementary MAT idiomorphs, thus demonstrating the heterothallic behaviour of P. citricarpa. In the same study, the sexual function of spermatia as male gametes in the life cycle of P. citricarpa, and the fertilization in this fungus occurring via spermatization, were also demonstrated (Tran et al., 2017). However, no research has to date focused on the sexual morph of the other Phyllosticta spp. associated with citrus, except for P. capitalensis that was demonstrated as being homothallic by Wang et al. (2016). Based on the whole genome analysis of several strains in this study, we identified P. citriasiana, P. citribraziliensis and P. paracitricarpa as heterothallic, and P. citrichinaensis as homothallic. We partially sequenced the MAT1‐1‐1 and MAT1‐2‐1 genes present in additional isolates of all the tested species by using new sets of specific primers designed in this study.
Future studies on the global distribution of mating types of P. citricarpa at the lesion, fruit and tree level, and movement of spermatia on the plant at the infection stage, are necessary to study their primary role in ascospore production, as well as to explain the occurrence of sexual reproduction of the fungus on leaf litter under orchard conditions. Such data would clarify the role of all the spore types in the disease cycle of CBS, aiding the development of a good and fast method for pathogenicity testing in order to screen various chemicals and/or biological agents. Larger populations of P. citriasiana, P. citrichinaensis and P. paracitricarpa are required to make it possible to obtain more details about the distribution of mating types for other pathogenic Phyllosticta spp.
The available genomic sequences for six species of the genus Phyllosticta provide an important resource to gain a better understanding regarding the distribution, sexual morph, rapid detection and marker development of these important species. However, more P. citricarpa genomes of both MAT idiomorphs and from diverse areas are essential. Also, the sequencing of P. paracapitalensis, which was unknown when the strains used in this study for the genome sequencing were selected, is still absent.
Further studies must be conducted on a wider global selection of P. citricarpa strains to improve our knowledge of its host association and distribution. A broader sampling is required to collect additional populations from Europe, Asia and Oceania for more detailed population studies. Population genetic inferences were strengthened by the development of several polymorphic SSR markers (Carstens et al., 2017; Wang et al., 2016), but a complete picture of the introduction and global movement pathways has not yet emerged. Therefore, more informative markers are needed to examine the population structure and migration pathways of P. citricarpa and other Phyllosticta spp. The development of new informative markers is crucial to accurately characterize clones and to track the global movement and introductions of P. citricarpa along with plant material from Asia, where Citrus is endemic, to other continents. These studies can also provide information on pathogenicity mechanisms, which remain very poorly understood.
Supporting information
Fig. S1 Different Phyllosticta isolates screened using the MAT111deg‐F2 and MAT111deg‐R3 primers (1010‐bp fragment; top part of both gel photos), and the same Phyllosticta isolates screened with the MAT121deg‐F1 and MAT121deg‐R1 primers (300‐bp‐fragment; lower part of both gel photos).
Table S1 Geographical distribution of Phyllosticta citricarpa.
Table S2 Collection and sequencing details of isolates included in this study.
Table S3 Accession numbers of mating type loci genes used in the synteny visualization.
Acknowledgements
We thank Chris Daum and his JGI team for sequencing the genomes and transcriptomes for this study, William Andreopoulos, Anna Lipzen, Kurt LaButti and Jasmyn Pangalinan for their contributions to assemblies, and Kerrie Barry for project management. The work conducted by the US Department of Energy Joint Genome Institute, a DOE Office of Science User Facility, is supported by the Office of Science of the US Department of Energy under contract no. DE‐AC02‐05CH11231.
References
- Agostini, J.P. , Peres, N.A. , Mackenzie, S.J. , Adaskaveg, J.E. and Timmer, L.W. (2006) Effect of fungicides and storage conditions on postharvest development of citrus black spot and survival of Guignardia citricarpa in fruit tissues. Plant Dis. 90, 1419–1424. [DOI] [PubMed] [Google Scholar]
- Alexopoulos, C. , Mims, C. and Blackwell, M. (1996) Introductory Mycology. 4th edn. New York: John Wiley and Sons Inc. [Google Scholar]
- Altschul, S.F. , Madden, T.L. , Schäffer, A.A. , Zhang, J. , Zhang, Z. , Miller, W. and Lipman, D.J. (1997) Gapped BLAST and PSI‐BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim, R. , Savi, D.C. , Ferreira‐Maba, L. , Aluizio, R. , Goulin, E.H. , Takita, M.A. , Machado, M.A. and Glienke, C. (2017) MAT gene idiomorphs suggest a heterothallic sexual cycle in the citrus pathogen Phyllosticta citricarpa . Eur. J. Plant Pathol. 147, 325–337. [Google Scholar]
- Baayen, R.P. , Bonants, P.J.M. , Verkley, G. , Carroll, G.C. , van der Aa, H.A. , De Weerdt, M. , Van Brouwershaven, I.R. , Schutte, G.C. , Maccheroni, W. , De Blanco, C.G. and Azevedo, J.L. (2002) Nonpathogenic isolates of the citrus black spot fungus, Guignardia citricarpa, identified as a cosmopolitan endophyte of woody plants, G. mangiferae (Phyllosticta capitalensis). Phytopathology, 92, 464–477. [DOI] [PubMed] [Google Scholar]
- Baldassari, R.B. , Reis, R.F. and De Goes, A. (2006) Susceptibility of fruits of the ‘Valencia’ and ‘Natal’ sweet orange varieties to Guignardia citricarpa and the influence of the coexistence of healthy and symptomatic fruits. Fitopatol. Bras. 31, 337–341. [Google Scholar]
- Baldassari, R.B. , Wickert, E. and De Goes, A. (2008) Pathogenicity, colony morphology and diversity of isolates of Guignardia citricarpa and G. mangiferae isolated from Citrus spp. Eur. J. Plant Pathol. 120, 103–110. [Google Scholar]
- Barr, M.E. (1970) Some amerosporous ascomycetes on Ericaceae and Empetraceae. Mycologia, 62, 377–394. [Google Scholar]
- Barr, M.E. (1972) Preliminary studies on the Dothideales in temperate North America. Contrib. Univ. Mich. Herb. 9, 523–638. [Google Scholar]
- Bassimba, D. , Nzambi, N. , Paixão, M.I.M.S. , Katula, I.D.G. and Vicent, A. (2018) First report of citrus black spot caused by Phyllosticta citricarpa in Angola. Plant Dis. 102, 683. [Google Scholar]
- Bellotte, J.A.M. , Kupper, K.C. , Rinaldo, D. , de Souza, A. and de Goes, A. (2013) The effects of inter-crop cultivation between rows of citrus crop on spreading of Guignardia citricarpa ascospores and in the citrus black spot occurrence. Rev Bras Frutic. 35, 102–111. [Google Scholar]
- Bellotte, J.A.M. , Kupper, K.C. , Rinaldo, D. , de Souza, A. , Pereira, F.D. and de Goes, A. (2009) Acceleration of the decomposition of Sicilian lemon leaves as an auxiliary measure in the control of citrus black spot. Trop. Plant Pathol. 34, 71–76. [Google Scholar]
- Benson, A.H. (1895) Black spot of the orange. Agric. Gaz. N.S.W. 4, 249–252. [Google Scholar]
- Bezerra, J.P.D. , Santos, M.G.S. , Svedese, V.M. , Lima, D.M.M. , Fernandes, M.J.S. , Paiva, L.M. and Souza‐Motta, C.M. (2012) Richness of endophytic fungi isolated from Opuntia ficus‐indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J. Microbiol. Biotechnol. 28, 1989–1995. [DOI] [PubMed] [Google Scholar]
- Bissett, J. (1986) Discochora yuccae sp. nov. with Phyllosticta and Leptodothiorella synanamorphs. Can. J. Bot. 64, 1720–1726. [Google Scholar]
- Brentu, F.C. , Oduro, K.A. , Offei, S.K. , Odamtten, G.T. , Vicent, A. , Peres, N.A. and Timmer, L.W. (2012) Crop loss, aetiology, and epidemiology of citrus black spot in Ghana. Eur. J. Plant Pathol. 133, 657–670. [Google Scholar]
- Brodrick, H.T. and Rabie, C.J. (1970) Light and temperature effects on symptom development and sporulation of Guignardia ciricarpa Kiely, on Citrus sinensis (Linn.) Osbeck. Phytophylactica, 2, 157–164. [Google Scholar]
- Calavan, E.C. (1960) Black spot of citrus. Citrus Grower, 323, 11–15. [Google Scholar]
- Carstens, E. , Le Roux, H.F. , Van Rooyen, L. , Coetzee, J. , Wentzel, R. , Laubscher, W. , Dawood, D. , Holtzhausen, M.A. , Schutte, G.C. , Fourie, P.H. and Hattingh, V. (2012) Citrus black spot does not occur in the Western Cape, Northern Cape and Free State provinces of South Africa. S. Afr. J. Sci. 108, 56–61. [Google Scholar]
- Carstens, E. , Linde, C. , Slabbert, R. , Miles, A. , Donovan, N. , Li, H.Y. , Zhang, K. , Dewdney, M.M. , Rollins, J.A. , Glienke, C. , Schutte, G.C. , Fourie, P. and McLeod, A. (2017) A global perspective on the population structure and reproductive system of Phyllosticta citricarpa . Phytopathology, 107, 758–768. [DOI] [PubMed] [Google Scholar]
- CBS Expert Panel (2013) Response to EFSA Panel on Plant Health, 2013 – Draft Scientific Opinion on the risk of Phyllosticta citricarpa (Guignardia citricarpa) for the EU territory with identification and evaluation of risk reduction options. http://www.citrusres.com/market-access.
- CBS Expert Panel (2014) Comments on the European Union Food Safety Authority’s Pest Risk Assessment for Phyllosticta citricarpa . http://www.citrusres.com/market-access.
- CBS Expert Panel (2015) Response to the EFSA 2015 statement on comments made by an international panel of scientists regarding EFSA’s 2014 risk assessment for Phyllosticta citricarpa . http://www.citrusres.com/market-access.
- Cobb, N.A. (1897) Letters on the diseases of plants: black‐spot of the orange. Agric. Gaz. N.S.W. 8, 229–231. [Google Scholar]
- Coppin, E. , Debuchy, R. , Arnaise, S. and Picard, M. (1997) Mating types and sexual development in filamentous ascomycetes. Microbiol. Mol. Biol. Rev. 61, 411–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Slippers, B. , Wingfield, M.J. , Rheeder, J. , Marasas, W.F.O. , Philips, A.J.L. , Alves, A. , Burgess, T. , Barber, P. and Groenewald, J.Z. (2006) Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 55, 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Summerell, B.A. , Shivas, R.G. , Burgess, T.I. , Decock, C.A. , Dreyer, L.L. , Granke, L.L. , Guest, D.I. , Hardy, G.E. , St, J. , Hausbeck, M.K. , Hüberli, D. , Jung, T. , Koukol, O. , Lennox, C.L. , Liew, E.C.Y. , Lombard, L. , McTaggart, A.R. , Pryke, J.S. , Roets, F. , Saude, C. , Shuttleworth, L.A. , Stukely, M.J.C. , Vánky, K. , Webster, B.J. , Windstam, S.T. and Groenewald, J.Z. (2012) Fungal planet description sheets: 107–127. Persoonia, 28, 138–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Wingfield, M.J. , Richardson, D.M. , Leroux, J.J. , Strasberg, D. , Edwards, J. , Roets, F. , Hubka, V. , Taylor, P.W.J. , Heykoop, M. , Martín, M.P. , Moreno, G. , Sutton, D.A. , Wiederhold, N.P. , Barnes, C.W. , Carlavilla, J.R. , Gené, J. , Giraldo, A. , Guarnaccia, V. , Guarro, J. , Hernández‐Restrepo, M. , Kolařík, M. , Manjón, J.L. , Pascoe, I.G. , Popov, E.S. , Sandoval‐Denis, M. , Woudenberg, J.H.C. , Acharya, K. , Alexandrova, A.V. , Alvarado, P. , Barbosa, R.N. , Baseia, I.G. , Blanchette, R.A. , Boekhout, T. , Burgess, T.I. , Cano‐Lira, J.F. , Čmoková, A. , Dimitrov, R.A. , My, D. , Dueñas, M. , Dutta, A.K. , Esteve‐Raventós, F. , Fedosova, A.G. , Fournier, J. , Gamboa, P. , Gouliamova, D.E. , Grebenc, T. , Groenewald, M. , Hanse, B. , Hardy, G.E.S.J. , Held, B.W. , Jurjević, Ž. , Kaewgrajang, T. , Latha, K.P.D. , Lombard, L. , Luangsa‐ard, J.J. , Lysková, P. , Mallátová, N. , Manimohan, P. , Miller, A.N. , Mirabolfathy, M. , Morozova, O.V. , Obodai, M. , Oliveira, N.T. , Ordóñez, M.E. , Otto, E.C. , Paloi, S. , Peterson, S.W. , Phosri, C. , Roux, J. , Salazar, W.A. , Sánchez, A. , Sarria, G.A. , Shin, H.‐D. , Silva, B.D.B. , Silva, G.A. , Smith, M.T. , Souza‐Motta, C.M. , Stchigel, A.M. , Stoilova‐Disheva, M.M. , Sulzbacher, M.A. , Telleria, M.T. , Toapanta, C. , Traba, J.M. , Valenzuela‐Lopez, N. , Watling, R. and Groenewald, J.Z. (2016) Fungal Planet description sheets: 400–468. Persoonia, 36, 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous, P.W. , Wingfield, M.J. , Burgess, T.I. , Carnegie, A.J. , St, J. , Hardy, G.E. , Smith, D. , Summerell, B.A. , Cano‐Lira, J.F. , Guarro, J. , Houbraken, J. , Lombard, L. , Martín, M.P. , Sandoval‐Denis, M. , Alexandrova, A.V. , Barnes, C.W. , Baseia, I.G. , Bezerra, J.D.P. , Guarnaccia, V. , May, T.W. , Hernández‐Restrepo, M. , Stchigel, A.M. , Miller, A.N. , Ordoñez, M.E. , Abreu, V.P. , Accioly, T. , Agnello, C. , Agustincolmán, A. , Albuquerque, C.C. , Alfredo, D.S. , Alvarado, P. , Araújo‐Magalhães, G.R. , Arauzo, S. , Atkinson, T. , Barili, A. , Barreto, R.W. , Bezerra, J.L. , Cabral, T.S. , Rodríguez, F.Camello , Cruz, R.H.S.F. , Daniëls, P.P. , da silva, B.D.B. , de Almeida, D.A.C. , de Carvalhojúnior, A.A. , Decock, C.A. , Delgat, L. , Denman, S. , Dimitrov, R.A. , Edwards, J. , Fedosova, A.G. , Ferreira, R.J. , Firmino, A.L. , Flores, J.A. , García, D. , Gené, J. , Giraldo, A. , Góis, J.S. , Gomes, A.A.M. , Gonçalves, C.M. , Gouliamova, D.E. , Groenewald, M. , Guéorguiev, B.V. , Guevara‐Suarez, M. , Gusmão, L.F.P. , Hosaka, K. , Hubka, V. , Huhndorf, S.M. , Jadan, M. , Jurjevi, Kraak, B. , Kuera, V. , Kumar, T.K.A. , Kušan, I. , Lacerda, S.R. , Lamlertthon, S. , Lisboa, W.S. , Loizides, M. , Luangsa‐Ard, J.J. , Lysková, P. , Maccormack, W.P. , Macedo, D.M. , Machado, A.R. , Malysheva, E.F. , Marinho, P. , Matoec, N. , Meijer, M. , Meši, A. , Mongkolsamrit, S. , Moreira, K.A. , Morozova, O.V. , Nair, K.U. , Nakamura, N. , Noisripoom, W. , Olariaga, I. , Oliveira, R.J.V. , Paiva, L.M. , Pawar, P. , Pereira, O.L. , Peterson, S.W. , Prieto, M. , Rodríguez‐Andrade, E. , Rojodeblas, C. , Roy, M. , Santos, E.S. , Sharma, R. , Silva, G.A. , Souza‐Motta, C.M. , Takeuchi‐Kaneko, Y. , Tanaka, C. , Thakur, A. , Smith, M.T.H. , Tkalec, Z. , Valenzuela‐Lopez, N. , Vanderkleij, P. , Verbeken, A. , Viana, M.G. , Wang, X.W. and Groenewald, J.Z. (2017) Fungal Planet description sheets: 625–715. Persoonia, 39, 625–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida, T.F. (2009) Mancha preta dos citros: expressao dos sintomas em frutos pela inoculacao com conidios e controle do agente causal (Guignardia citricarpa). PhD thesis, Universidade Estadual Paulista “Júslio De Mesquita Filho”. [Google Scholar]
- Debuchy, R. , Berteaux-Lecellier, V. , and Silar, P. (2010) Mating systems and sexual morphogenesis in ascomycetes In: Cellular and molecular biology of filamentous fungi (pp. 501–535). American Society of Microbiology. [Google Scholar]
- Debuchy, R. and Turgeon, B.G. (2006) Mating‐type structure, evolution, and function in euascomycetes In: The Mycota I: Growth, Differentiation and Sexuality (Kües U. and Fischer R., eds), pp. 293–323. Berlin, Heidelberg: Springer‐Verlag. [Google Scholar]
- Dewdney, M.M. , Schubert, T.S. , Estes, M.R. , Roberts, P.D. and Peres, N.A. (2018) Citrus black spot In: Florida Citrus Production Guide (Diepenbrock L.M., Dewdney M.M. and Vashisth T., eds), pp. 129–134. Gainesville: University of Florida, Institute of Food and Agricultural Services. [Google Scholar]
- Doidge, E.M. (1929) Some diseases of Citrus prevalent in South Africa. S. Afr. J. Sci. 26, 324. [Google Scholar]
- Dummel, D.M. , Agostini, J.P. and Moschini, R. (2015) Predictive model for ascospore release of Guignardia citricarpa using climatological data Proceedings of the XIIth International Citrus Congress, Valencia, Spain, B. Sabater‐Munoz et al. Acta Horticulturae, 1065, 953–963. [Google Scholar]
- EFSA (2014) Scientific Opinion on the risk of Phyllosticta citricarpa (Guignardia citricarpa) for the EU territory with identification and evaluation of risk. Eur. Food Saf. Auth. J. 12, 243. [Google Scholar]
- Er, H.L. , Hendricks, K. , Goss, E.M. , Smith, M. , Schubert, T.S. , Roberts, P.D. and van Bruggen, A.H.C. (2014) Isolation and biological characterization of Guignardia species from citrus in Florida. J. Plant Pathol. 96, 43–55. [Google Scholar]
- European Union (2000) Final report of a mission carried out in Brazil from 3 to 6 July 2000 in order to evaluate the pre‐export inspections on citrus fruit originating in Brazil and exported to the European Union. Available at http://ec.europa.eu/food/fs/inspections/pi/reports/brazil/pi_rep_braz_1180-2000_en.pdf. [Google Scholar]
- Everett, K.R. and Rees‐George, J. (2006) Reclassification of an isolate of Guignardia citricarpa from New Zealand as Guignardia mangiferae by sequence analysis. Plant Pathol. 55, 194–199. [Google Scholar]
- FAO (2014) ISPM No 27. 2006. Diagnostic protocols for regulated pests Intentional Standards for Phytosanitary Measures, Publication No 27: Annex 5. Phyllosticta citricarpa (McAlpine) Aa on fruit. Secretariat of the International Plant Protection, Food and Agriculture Organization of the United Nations, Rome. [Google Scholar]
- Fogliata, G.M. , Muñoz, M.L. , Rojas, A.A. and Ploper, D. (2011) Eficacia de tres estrobilurinas para el control de mancha rojiza (Guignardia mangiferae) y mancha negra (Guignardia citricarpa) en frutos de limón, en Tucumán. República Argentina. Rev. Ind. Agríc. Tucumán, 88, 37–45. [Google Scholar]
- Fourie, P.H. , Schutte, T. , Serfontein, S. and Swart, F. (2013) Modeling the effect of temperature and wetness on Guignardia pseudothecium maturation and ascospore release in citrus orchards. Phytopathology, 103, 281–292. [DOI] [PubMed] [Google Scholar]
- Frare, G.F. , Silva‐Junior, G.J. , Bassanezi, R.B. , Ramires, T.G. and Amorim, L. (2019) Sweet orange fruit age ad inoculum concentration affect expression of citrus black spot symptoms. Plant Dis. 10.1094/PDIS-03-18-0492-RE [DOI] [PubMed] [Google Scholar]
- Glienke, C. , Pereira, O. , Stringari, D. , Fabris, J. , Kava‐Cordeiro, V. , Galli‐Terasawa, L. , Cunnington, J. , Shivas, R. , Groenewald, J.Z. and Crous, P.W. (2011) Endophytic and pathogenic Phyllosticta species, with reference to those associated with citrus black spot. Persoonia, 26, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke‐Blanco, C. , Aguilar‐Vildoso, C.I. , Vieira, M.L.C. , Barroso, P.A.V. and Azevedo, J.L. (2002) Genetic variability in the endophytic fungus Guignardia citricarpa isolated from citrus plants. Genet. Mol. Biol. 25, 251–255. [Google Scholar]
- Gnerre, S. , MacCallum, I. , Przybylski, D. , Ribeiro, F.J. , Burton, J.N. , Walker, B.J. , Sharpe, T. , Hall, G. , Shea, T.P. , Sykes, S. , Berlin, A.M. , Aird, D. , Costello, M. , Daza, R. , Williams, L. , Nicol, R. , Gnirke, A. , Nusbaum, C. , Lander, E.S. and Jaffe, D.B. (2011) High‐quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc. Natl. Acad. Sci. U.S.A. 108, 1513–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr, M.G. , Haas, B.J. , Yassour, M. , Levin, J.Z. , Thompson, D.A. , Amit, I. , Adiconis, X. , Fan, L. , Raychowdhury, R. , Zeng, Q. , Chen, Z. , Mauceli, E. , Hacohen, N. , Gnirke, A. , Rhind, N. , di Palma, F. , Birren, B.W. , Nusbaum, C. , Lindblad‐Toh, K. , Friedman, N. and Regev, A. (2011) Full‐length transcriptome assembly from RNA‐seq data without a reference genome. Nat. Biotechnol. 29, 644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev, I.V. , Nikitin, R. , Haridas, S. , Kuo, A. , Ohm, R. , Otillar, R. , Riley, R. , Salamov, A. , Zhao, X. , Korzeniewski, F. , Smirnova, T. , Nordberg, H. , Dubchak, I. and Shabalov, I. (2014) MycoCosm portal: gearing up for 1000 fungal genomes. Nucleic Acids Res. 42, D699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia, V. and Crous, P.W. (2017) Emerging citrus diseases in Europe caused by Diaporthe spp. IMA Fungus. 8, 317–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia, V. and Crous, P.W. (2018) Species of Diaporthe on Camellia and Citrus in the Azores Islands. Phytopathol. Mediterr. 57, 307–319. [Google Scholar]
- Guarnaccia, V. , Groenewald, J.Z. , Li, H. , Glienke, C. , Carstens, E. , Hattingh, V. , Fourie, P.H. and Crous, P.W. (2017a) First report of Phyllosticta citricarpa and description of two new species, P. paracapitalensis and P. paracitricarpa, from citrus in Europe. Stud. Mycol. 87, 161–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia, V. , Groenewald, J.Z. , Polizzi, G. and Crous, P.W. (2017b) High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia, 39, 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth, D. , Crous, P.W. , Redhead, S.A. , Reynolds, D.R. , Samson, R.A. , Seifert, K.A. , Taylor, J.W. and Wingfield, M.J. (2011) The Amsterdam declaration on fungal nomenclature. IMA Fungus, 2, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks, K.E. , Christman, M. and Roberts, P.D. (2017) Spatial and temporal pattern of commercial citrus trees affected by Phyllosticta citricarpa in Florida. Sci. Rep. 7, 1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings, P. (1908) Fungi S. Paulenses IV a cl. Puttemans collecti. Hedwigia, 48, 1–20. [Google Scholar]
- Hincapie, M. , Wang, N.Y. , Peres, N.A. and Dewdney, M.M. (2014) Baseline sensitivity of Guignardia citricarpa isolates from Florida to azoxystrobin and pyraclostrobin. Plant Dis. 98, 780–789. [DOI] [PubMed] [Google Scholar]
- Hu, J. , Johnson, E.G. , Wang, N.Y. , Davoglio, T. and Dewdney, M.M. (2014) qPCR quantification of pathogenic Guignardia citricarpa and nonpathogenic G. mangiferae in Citrus. Plant Dis. 98, 112–120. [DOI] [PubMed] [Google Scholar]
- Huang, C.S. and Chang, S.L. (1972) Leaf infection with citrus black spot and perithecial development in relation to ascospore discharge of Guignardia citricarpa Kiely. J. Taiwan Agric. Res. 21, 256–263. [Google Scholar]
- Johnston, P.R. (1998) Leaf endophytes of manuka (Leptospermum scoparium). Mycol. Research. 102, 1009–1016. [Google Scholar]
- Kellerman, C.R. and Kotzé, J.M. (1977) The black spot disease of citrus and its control in South Africa. Proc. Int. Soc. Citricult. 3, 992–996. [Google Scholar]
- Kiely, T.B. (1948a) Preliminary studies on Guignardia citricarpa, N. SP.: The ascigerous stage of Phoma citricarpa McAlp, and its relation to black spot of citrus. Proc. Linn. Soc. NSW. 73, 249–292. [Google Scholar]
- Kiely, T.B. (1948b) Guignardia citricarpa (n.sp.) and its relationship to the black spot disease of Citrus in coastal orchards of New South Wales. J. Aust. I. Agric. Sci. 14, 81–83. [Google Scholar]
- Kiely, T.B. (1949) Black spot of citrus in New South Wales coastal orchards. Agric. Gaz. NSW. 60, 17–20. [Google Scholar]
- Korf, H.J.G. , Schutte, G.C. and Kotzé, J.M. (2001) Effect of packinghouse procedures of the viability of Phyllosticta citricarpa, anamorph of the black spot pathogen. Afr. Plant Prot. 7, 103–109. [Google Scholar]
- Kotzé, J.M. (1963) Studies on the black spot disease of citrus caused by Guignardia citricarpa Kiely, with particular reference to its epiphytology and control in Letaba. D.Sc. (Agric.) thesis. University of Pretoria, South Africa. [Google Scholar]
- Kotzé, J.M. (1981) Epidemiology and control of citrus black spot in South Africa. Plant Dis. 65, 945–950. [DOI] [PubMed] [Google Scholar]
- Kotzé, J.M. (1996) History and epidemiology of Citrus black spot in South Africa. Proc. Int. Soc. Citriculture. 1296–1299. [Google Scholar]
- Kotzé, J.M. (2000) Black spot In: Compendium of Citrus Diseases (Timmer L.W., Garnsey S.M. and Graham J.H., eds), pp 23–25. St. Paul, MN: American Phytopathological Society Press Inc. [Google Scholar]
- Kupper, K.C. , Correa, E.B. , Moretto, C. , Bettiol, W. and de Goes, A. (2011) Control of Guignardia citricarpa by Bacillus subtilis and Trichoderma spp. Rev. Bras. Frutic. 33, 1111–1118. [Google Scholar]
- Lanza, F.E. , Metzker, T.G. , Vinhas, T. , Behlau, F. and Silva‐Junior, G.J. (2018) Critical fungicide spray period for Citrus Black Spot control in São Paulo State. Brazil. Plant Dis. 102, 334–340. [DOI] [PubMed] [Google Scholar]
- Lee, Y.S. and Huang, C.S. (1973) Effect of climatic factors on the development and discharge of ascospores of the citrus black spot fungus. J. Taiwan Agric. Res. 22, 135–144. [Google Scholar]
- Liu, J.K. , Phookamsak, R. , Doilom, M. , Wikee, S. , Li, Y.M. , Ariyawansha, H. , Boonmee, S. , Chomnunti, P. , Dai, D.‐Q. , Bhat, J.D. , Romero, A.I. , Zhuang, W.‐Y. , Monkai, J. , Gareth Jones, E.B. , Chukeatirote, E. , Ko Ko, T.W. , Zhao, Y.‐C. , Wang, Y. and Hyde, K.D. (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers. 57, 149–210. [Google Scholar]
- Lucon, C.M.M. , Guzzo, S.D. , de Jesus, C.O. , Pascholati, S.F. and de Goes, A. (2010) Postharvest harpin or Bacillus thuringiensis treatments suppress citrus black spot in ‘Valencia’ oranges. Crop Protect. 29, 766–772. [Google Scholar]
- Mabberley, D.J. (2004) Citrus (Rutaceae): a review of recent advances in etymology, systematics and medical applications. Blumea, 49, 481–498. [Google Scholar]
- Magarey, R.D. , Hong, S.C. , Fourie, P.H. , Christie, D.N. , Miles, A.K. , Schutte, G.C. and Gottwald, T.R. (2015) Prediction of Phyllosticta citricarpa using an hourly infection model and validation with prevalence data from South Africa and Australia. Crop Prot. 75, 104–114. [Google Scholar]
- Makowski, D. , Vicent, A. , Pautasso, M. , Stancanelli, G. and Rafoss, T. (2014) Comparison of statistical models in a meta‐analysis of fungicide treatments for the control of citrus black spot caused by Phyllosticta citricarpa . Eur. J. Plant Pathol. 139, 79–94. [Google Scholar]
- Marchionatto, J.B. (1926) Fitoparasitos de la Argentina nuevos o poco conocidos in Physis. T. VIII, 367–372. [Google Scholar]
- Marin‐Felix, Y. , Hernández‐Restrepo, M. , Wingfield, M.J. , Akulov, A. , Carnegie, A.J. , Cheewangkoon, R. , Gramaje, D. , Groenewald, J.Z. , Guarnaccia, V. , Halleen, F. , Lombard, L. , Luangsa‐ard, J. , Marincowitz, S. , Moslemi, A. , Mostert, L. , Quaedvlieg, W. , Schumacher, R.K. , Spies, C.F.J. , Thangavel, R. , Taylor, P.W.J. , Wilson, A.M. , Wingfield, B.D. , Wood, A.R. and Crous, P.W. (2019) Genera of phytopathogenic fungi: GOPHY 2. Stud. Mycol. 92, 47–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques, J.P.R. , Spósito, M.B. , Mello, A.F.S. , Amorim, L. , Mondin, M. and Appezzato‐da‐Gloria, B. (2012) Histopathology of black spot symptoms in sweet oranges. Eur. J. Plant Pathol. 133, 439–448. [Google Scholar]
- Martínez‐Minaya, J. , Conesa, D. , López‐Quílez, A. and Vicent, A. (2018) Spatial and climatic factors associated with the geographical distribution of citrus black spot disease in South Africa. A Bayesian latent Gaussian model approach. Eur. J. Plant Pathol. 151, 991–1007. [Google Scholar]
- McOnie, K.C. (1964a) Source of inoculum of Guignardia citricarpa citrus black spot pathogen. Phytopathology, 54, 64–67. [Google Scholar]
- McOnie, K.C. (1964b) Apparent absence of Guignardia citricarpa Kiely from localities where citrus black spot is absent. S. Afr. J. Agric. Sci. 7, 347–354. [Google Scholar]
- McOnie, K.C. (1965) Source of infection for black spot of citrus. South Afr. Citrus J. 5, 5–9. [Google Scholar]
- McOnie, K.C. (1967) Germination and infection of citrus by ascospores of Guignardia citricarpa in relation to control of black spot. Phytopathology, 57, 743–746. [Google Scholar]
- Meyer, L. , Sanders, G.M. , Jacobs, R. and Korsten, L. (2006) A one‐day sensitive method to detect and distinguish between the citrus black spot pathogen Guignardia citricarpa and the endophyte Guignardia mangiferae . Plant Dis. 90, 97–101. [DOI] [PubMed] [Google Scholar]
- Miles, A.K. , Willingham, S.L. and Cooke, A.W. (2004) Field evaluation of strobilurins and a plant activator for the control of citrus black spot. Australas. Plant Pathol. 33, 371–378. [Google Scholar]
- Moran Lemir, A. , Stadnik, M. , Buchenauer, H. and Canton, N. (2000) In vitro production of ascospores and pathogenicity of Guignardia citricarpa, causal agent of citrus black spot. Summa Phytopathol. 26, 374–376. [Google Scholar]
- Moyo, P. , van Niekerk, J.M. and Fourie, P.H. (2018) Updated citrus black spot spray programmes 2018–2019. CRI Cutting Edge, 254, 1–21. [Google Scholar]
- Ni, M. , Feretzaki, M. , Sun, S. , Wang, X. and Heitman, J. (2011) Sex in fungi. Annu. Rev. Genet. 45, 405–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolosi, E. (2007) Origin and taxonomy In: Citrus Genetics, Breeding and Biotechnology. (Khan I.A., ed.), pp. 19–43. Wallingford, UK: CAB International. [Google Scholar]
- Noronha, M.D.A. (2002) Escala diagramatica para avaliacao da mancha preta em folhas de citros efeito da temperatura e da duracao do molhamento na prepenetracao de conidios de Guignardia citricarpa Kiely [Phyllosticta citricarpa (McAlp.) Van der Aa]. Agronomy: Concentration of Plant Pathology Masters Dissertation. Universidade de Sao Paulo. [Google Scholar]
- OEPP/EPPO . (2009) Data sheets on quarantine organisms PM 7/17, Guignardia citricarpa . EPPO Bull. 39, 318–327. [Google Scholar]
- Okane, I. , Nakagiri, A. and Ito, T. (2001) Identity of Guignardia sp. inhabiting ericaceous plants. Can. J. Bot. 79, 101–109. [Google Scholar]
- Okane, I. , Lumyong, S. , Nakagiri, A. and Ito, T. (2003) Extensive host range of an endophytic fungus, Guignardia endophyllicola (anamorph: Phyllosticta capitalensis). Mycoscience, 44, 353–363. [Google Scholar]
- Parra, G. , Bradnam, K. and Korf, I. (2007) CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics, 23, 1061–1067. [DOI] [PubMed] [Google Scholar]
- Paul, I. , Van Jaarsveld, A.S. , Korsten, L. and Hattingh, V. (2005) The potential global geographical distribution of citrus black spot caused by Guignardia citricarpa Kiely: Likelihood of disease establishment in the European Union. Crop Prot. 24, 297–308. [Google Scholar]
- Peña, L.C. , Jung, L.F. , Savi, D.C. , Servienski, A. , Aluizio, R. , Goulin, E.H. , Galli‐Terasawa, L.V. , de Noronha Sales Maia, B.H.L. , Annies, V. , Cavichiolo Franco, C.R. , Glienke, C. and Kava, V. (2017) A Muscodor strain isolated from Citrus sinensis and its production of volatile organic compounds inhibiting Phyllosticta citricarpa . J. Plant Dis. Protect. 124, 349–360. [Google Scholar]
- Petrak, F. (1957) Über die Gattungen Guignardia Viala and Ravaz und Discosphaerina v. Höhnel. Sydowia, 11, 435–445. [Google Scholar]
- Pöggeler, S. (2001) Mating‐type genes for classical strain improvements of ascomycetes. Appl. Microbiol. Biotechnol. 56, 589–601. [DOI] [PubMed] [Google Scholar]
- Rakotoniriana, E.F. , Munaut, F. , Decock, C. , Randriamampionona, D. , Andriambololoniaina, M. , Rakotomalala, T. , Rakotonirina, E.J. , Rabemanantsoa, C. , Cheuk, K. , Ratsimamanga, S.U. , Mahillon, J. , El‐Jaziri, M. , Quetin‐Leclercq, J. and Corbisier, A.M. (2008) Endophytic fungi from leaves of Centella asiatica: occurrence and potential interactions within leaves. Antonie Van Leeuwenhoek, 93, 27–36. [DOI] [PubMed] [Google Scholar]
- Ramon‐Laca, L. (2003) The introduction of cultivated citrus to Europe via northern Africa and the Iberian Peninsula. Econ. Bot. 57, 502–514. [Google Scholar]
- Rappussi, M.C.C. , Pascholati, S.F. , Benato, E.A. and Cia, P. (2009) Chitosan reduces infection by Guignardia citricarpa in postharvest ‘Valencia’ oranges. Braz. Arch. Biol. Technol. 52, 513–521. [Google Scholar]
- Rappussi, M.C.C. , Benato, E.A. , Cia, P. and Pascholati, S.F. (2011) Chitosan and fungicides on postharvest control of Guignardia citricarpa and on quality of ‘Pera Rio’ oranges. Summa Phytopathol. 37, 142–144. [Google Scholar]
- Reeder, R. , Kelly, P.L. and Harling, R. (2009) First confirmed report of citrus black spot caused by Guignardia citricarpa on sweet oranges (Citrus sinensis) in Uganda. Plant Pathol. 58, 399. [Google Scholar]
- Reis, R.F. , Timmer, L.W. and de Goes, A. (2006) Effect of temperature, leaf wetness and rainfall on the production of Guignardia citricarpa ascospores and on black spot severity on sweet orange. Fitopatol. Bras. 31, 29–34. [Google Scholar]
- Robbs, C.F. , Pimentel, J.P. and Ribeiro, R.L.D. (1980) A mancha preta dos frutos cítricos causada por Phoma citricarpa . Fitopatol. Bras. 5, 455. [Google Scholar]
- Rodrigues, K.F. and Samuels, G.J. (1999) Fungal endophytes of Spondias mombin leaves in Brazil. J. Basic Microbiol. 39, 131–135. [Google Scholar]
- Rodrigues, K.F. , Sieber, T.N. , Grünig, C.R. and Holdenrieder, O. (2004) Characterization of Guignardia mangiferae isolated from tropical plants based on morphology, ISSR‐PCR amplifications and ITS1‐5.8S‐ITS2 sequences. Mycol. Res. 108, 45–52. [DOI] [PubMed] [Google Scholar]
- Rodriguez, A. , Kava, V. , Latorre‐Garcia, L. , da Silva, G.J.J. , Pereira, R.G. , Glienke, C. , Ferreira‐Maba, L.S. , Vicent, A. , Shimada, T. and Pena, L. (2018) Engineering D‐limonene synthase down‐regulation in orange fruit induces resistance against the fungus Phyllosticta citricarpa through enhanced accumulation of monoterpene alcohols and activation of defence. Mol. Plant Pathol. 19, 2077–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romão, A.S. , Spósito, M.B. , Andreote, F.D. , Azevedo, J.L. and Araujo, W.L. (2011) Enzymatic differences between the endophyte Guignardia mangiferae (Botryosphaeriaceae) and the citrus pathogen G. citricarpa . Genet. Mol. Res. 10, 243–252. [DOI] [PubMed] [Google Scholar]
- Rossman, A.Y. , Adams, G.C. , Cannon, P.F. , Castlebury, L.A. , Crous, P.W. , Gryzenhout, M. , Jaklitsch, W.M. , Mejia, L.C. , Stoykov, D. , Udayanga, D. , Voglmayr, H. and Walker, D.M. (2015) Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus, 6, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval‐Denis, M. , Guarnaccia, V. , Polizzi, G. and Crous, P.W. (2018) Symptomatic citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia, 40, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos, P.J.C. , Savi, D.C. , Gomes, R.R. , Goulin, E.H. , da Senkiv, C. , Tanaka, F.A.O. , Almeida, A.M.R. , Galli‐Terasawa, L. , Kava, V. and Glienke, C. (2016) Diaporthe endophytica and D. terebinthifolii from medicinal plants for biological control of Phyllosticta citricarpa . Microb. Res. 186–187, 153–160. [DOI] [PubMed] [Google Scholar]
- Scaloppi, E.M.T. , Aguiar, R.L. , de Goes, A. and Sposito, M.B. (2012) Efeito do manejo cultural e químico na incidência e severidade da mancha‐preta dos citros. Rev. Bras. Frutic. 34, 102–108. [Google Scholar]
- Schoch, C.L. , Shoemaker, R.A. , Seifert, K.A. , Hambleton, S. , Spatafora, J.W. and Crous, P.W. (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia, 98, 1041–1052. [DOI] [PubMed] [Google Scholar]
- Schreuder, W. , du Plooy, W. , Erasmus, A. , Savage, C. , Basson, E. , Lennox, C. and Fourie, P.H. (2018) Postharvest fungicide treatments and cold storage control citrus black spot infections. Crop Prot. 112, 332–342. [Google Scholar]
- Schubert, T.S. , Dewdney, M.M. , Peres, N.A. , Palm, M.E. , Jeyaprakash, A. , Sutton, B. , Mondal, S.N. , Wang, N.Y. , Rascoe, J. and Picton, D.D. (2012) First report of Guignardia citricarpa associated with citrus black spot on sweet orange (Citrus sinensis) in North America. Plant Dis. 96, 1225. [DOI] [PubMed] [Google Scholar]
- Schutte, G.C. , Beeton, K.V. and Kotze, J.M. (1997) Rind stippling on Valencia oranges by copper fungicides used for control of citrus black spot in South Africa. Plant dis., 81, 851–854. [DOI] [PubMed] [Google Scholar]
- Schutte, G.C. and Kotzé, J.M. (1997) Grass mulching as part in integrated control programme for the control of citrus black spot. Citrus J. 7, 18–20. [Google Scholar]
- Schutte, G.C. , Mansfield, R.I. , Smith, H. and Beeton, K.V. (2003) Application of azoxystrobin for control of benomyl‐resistant Guignardia citricarpa on ‘Valencia’ oranges in South Africa. Plant Dis. 87, 784–788. [DOI] [PubMed] [Google Scholar]
- Schutte, G.C. , Kotze, C. , van Zyl, J. and Fourie, P. (2012) Assessment of retention and persistence of copper fungicides on orange fruit and leaves using fluorometry and copper residue analyses. Crop Prot. 42, 1–9. [Google Scholar]
- Schutte, G.C. , Kotze, C. and Korf, H.J.G. (2014) Influence of sunlight exposure on fertility of Phyllosticta citricarpa pycnidia in citrus black spot lesions on grapefruit and Valencia orange rinds. SA Fruit J. April: 54–57. [Google Scholar]
- Seberry, J.A. , Leggo, D. and Kiely, T.B. (1967) Effect of skin coatings on the development of black spot in stored Valencia oranges. Aust. J. Exp. Agric. Anim. Husb. 7, 593–600. [Google Scholar]
- Silva, A.O. , Savi, D.C. , Raiser, P.H.S. , Gonçalves, F.P. , Kava, V. , Galli‐Terasawa, L.V. and Glienke, C. (2017) Epidemiological aspects of Phyllosticta citricarpa colonization and viability in Citrus sinensis . J. Plant Dis. Prot. 124, 73–80. [Google Scholar]
- Silva‐Junior, G.J. , Feichtenberger, E. , Spósito, M.B. , Amorim, L. , Bassanezi, R.B. and Goes, A. (2016a) Pinta preta dos citros: a doença e seu manejo. v. 1. 208p. Araraquara, São Paulo, Brasil: Fundecitrus. [Google Scholar]
- Silva‐Junior, G.J. , da Scapin, M.S. , Silva, F.P. , Silva, A.R.P. , Behlau, F. and Ramos, H.H. (2016b) Spray volume and fungicide rates for citrus black spot control based on tree canopy volume. Crop Prot. 85, 38–45. [Google Scholar]
- Snowdon, A.L. (1990) A colour atlas of post‐harvest diseases and disorders of fruit and vegetables Vol. I. Barcelona, Spain: Wolfe Scientific Ltd. [Google Scholar]
- Spósito, M.B. , Amorim, L. , Ribeiro, P.J. Jr , Bassanezi, R.B. and Krainski, E.T. (2007) Spatial pattern of trees affected by black spot in citrus groves in Brazil. Plant Dis. 91, 36–40. [DOI] [PubMed] [Google Scholar]
- Spósito, M.B. , Amorim, L. , Bassanezi, R.B. , Bergamin Filho, A. and Hau, B. (2008) Spatial pattern of black spot incidence within citrus trees related to disease severity and pathogen dispersal. Plant Pathol. 57, 103–108. [Google Scholar]
- Spósito, M.B. , Amorim, L. , Bassanezi, R.B. , Yamamoto, P.T. , Felippe, M.R. and Czermainski, A.B.C. (2011) Relative importance of inoculum sources of Guignardia citricarpa on the citrus black spot epidemic in Brazil. Crop Prot. 30, 1546–1552. [Google Scholar]
- Stammler, G. , Schutte, G.C. , Speakman, J. , Miessner, S. and Crous, P.W. (2013) Phyllosticta species on citrus: risk estimation of resistance to QoI fungicides and identification of species with cytochrome b gene sequences. Crop Prot. 48, 6–12. [Google Scholar]
- Su, Y.Y. and Cai, L. (2012) Polyphasic characterisation of three new Phyllosticta spp. Persoonia, 28, 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan, A. , Johnston, P.R. , Park, D. and Robertson, A.W. (2011) Two new pathogenic ascomycetes in Guignardia and Rosenscheldiella on New Zealand’s pygmy mistletoes (Korthalsella: Viscaceae). Stud. Mycol. 68, 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, N.T. , Miles, A. , Dietzgen, R.G. , Dewdney, M.M. , Zhang, K. , Rollins, J.A. and Drenth, A. (2017) Sexual reproduction in the Citrus black spot pathogen, Phyllosticta citricarpa . Phytopathology, 107, 732–739. [DOI] [PubMed] [Google Scholar]
- Tran, N.T. , Miles, A.K. , Dietzgen, R.G. and Drenth, A. (2019) Phyllosticta capitalensis and P. paracapitalensis are endophytic fungi that show potential to inhibit pathogenic P. citricarpa on citrus. Australas Plant Path. 48, 281–296. [Google Scholar]
- Tran, N.T. , Miles, A. , Smith, M.W. , Dietzgen, R.G. and Drenth, A. (2018) Pathogenicity of Phyllosticta citricarpa ascospores on Citrus spp. Plant. Dis. 102, 1386–1393. [DOI] [PubMed] [Google Scholar]
- Truter, M. (2010) Epidemiology of Citrus Black Spot Disease in South Africa and Its Impact on Phytosanitary Trade Restrictions. PhD thesis. University of Pretoria, Pretoria, South Africa. [Google Scholar]
- Truter, M. , Labuschagne, P.M. , Kotzé, J.M. , Meyer, L. and Korsten, L. (2007) Failure of Phyllosticta citricarpa pycnidiospores to infect Eureka lemon leaf litter. Austral. Plant Pathol. 36, 87–93. [Google Scholar]
- Turgeon, B.G. and Yoder, O. (2000) Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genet. Biol. 31, 1–5. [DOI] [PubMed] [Google Scholar]
- USDA APHIS (United States Department of Agriculture Animal and Plant Health Inspection Service) (2010) Risk assessment of Citrus spp. fruit as a pathway for the introduction of Guignardia citricarpa Kiely, the organism that causes Citrus Black Spot disease. Center for Plant Health Science and Technology, Plant Epidemiology and Risk Analysis Laboratory, Raleigh, NC, USA. [Google Scholar]
- USDA APHIS (United States Department of Agriculture Animal and Plant Health Inspection Service) (2011) Federal domestic quarantine order Guignardia citricarpa Kiely Causal Agent of Citrus Black Spot (CBS) DA‐2011‐03.
- van Bruggen, A.H. , Sharma, K. and Shin, K. (2017) Sugar cane processing residue, bagasse, enhances decomposition of citrus leaves and could contribute to citrus black spot management. Crop Prot. 93, 89–97. [Google Scholar]
- van der Aa, H.A. (1973) Studies in Phyllosticta I. Stud. Mycol. 5, 1–110. [Google Scholar]
- van der Aa, H.A. and Vanev, S. (2002) A Revision of the Species Described in Phyllosticta. Utrecht, Netherlands: Centraalbureau voor Schimmelcultures. [Google Scholar]
- Veltri, D. , Wight, M.M. and Crouch, J.A. (2016) SimpleSynteny: a web‐based tool for visualization of microsynteny across multiple species. Nucleic Acids Res. 44(W1), W41–W45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viala, P. and Ravaz, L. (1892) Sur la dénomination botanique (Guignardia bidwellii) du black‐rot. Bull. Trimest. Soc. Mycol. Fr. 8, 63. [Google Scholar]
- Wager, V.A. (1952) The black spot disease of citrus in South Africa. Sci. B. Dep. Agric. Union S. Afr. 303, 1–52. [Google Scholar]
- Wang, N. and Dewdney, M.M. (2014) Nutritional and environmental effects on germination and appressorium formation of Guignardia citricarpa conidia . Phytopathology, 102(S4), 131. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Chen, G. , Huang, F. , Zhang, J. , Hyde, K.D. and Li, H. (2012) Phyllosticta species associated with citrus diseases in China. Fungal Divers. 52, 209–224. [Google Scholar]
- Wang, N.‐Y. , Rollins, J.A. and Dewdney, M.M. (2013) Characterization of the mating‐type locus (MAT) of Guignardia citricarpa, the fungal causal agent of citrus black spot. Phytopathology, 103, S2 156. [Google Scholar]
- Wang, N.‐Y. , Zhang, K. , Huguet‐Tapia, J.C. , Rollins, J.A. and Dewdney, M.M. (2016) Mating type and simple sequence repeat markers indicate a clonal population of Phyllosticta citricarpa in Florida. Phytopathology, 106, 1300–1310. [DOI] [PubMed] [Google Scholar]
- Whiteside, J.O. (1967) Sources of inoculum of the black spot fungus, Guignardia citricarpa, in infected Rhodesian orchards. Rhod. Zambia Malawi J. Agric. Res. 5, 171–177. [Google Scholar]
- Wijayawardene, N.N. , Crous, P.W. , Kirk, P.M. , Hawksworth, D.L. , Boonmee, S. , Braun, U. , Dai, D.Q. , D’souza, M.J. , Diederich, P. , Dissanayake, A. , Doilom, M. , Hongsanan, S. , Jones, E.B.G. , Groenewald, J.Z. , Jayawardena, R. , Lawrey, J.D. , Liu, J.‐K. , Lücking, R. , Madrid, H. , Manamgoda, D.S. , Muggia, L. , Nelsen, M.P. , Phookamsak, R. , Suetrong, S. , Tanaka, K. , Thambugala, K.M. , Wanasinghe, D.N. , Wikee, S. , Zhang, Y. , Aptroot, A. , Ariyawansa, H.A , Bahkali, A.H. , Bhat, D.J. , Gueidan, C. , Chomnunti, P. , De Hoog, G.S. , Knudsen, K. , Li, W.‐J. , McKenzie, E.H.C. , Miller, A.N. , Phillips, A.J.L. , Piątek, M. , Raja, H.A. , Shivas, R.S. , Slippers, B. , Taylor, J.E. , Tian, Q. , Wang, Y. , Woudenberg, J.H.C. , Cai, L. , Jaklitsch, W.M. and Hyde, K.D. (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers. 69, 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikee, S. , Udayanga, D. , Crous, P.W. , Chukeatirote, E. , McKenzie, E.H.C. , Bahkali, A.H. , Dai, D.Q. and Hyde, K.D. (2011) Phyllosticta – an overview of current status of species recognition. Fungal Divers. 51, 43–61. [Google Scholar]
- Wikee, S. , Lombard, L. , Crous, P.W. , Nakashima, C. , Motohashi, K. , Chukeatirote, E. , Alias, S.A. , McKenzie, E.H.C. and Hyde, K.D. (2013a) Phyllosticta capitalensis. A widespread endophyte of plants. Fungal Divers. 60, 91–105. [Google Scholar]
- Wikee, S. , Lombard, L. , Nakashima, C. , Motohashi, K. , Chukeatirote, E. , Cheewangkoon, R. , McKenzie, E.H.C. , Hyde, K.D. and Crous, P.W. (2013b) A phylogenetic re‐evaluation of Phyllosticta (Botryosphaeriales). Stud. Mycol. 76, 1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield, M.J. , De Beer, W.Z. , Slippers, B. , Wingfield, B.D. , Groenewald, J.Z. , Lombard, L. and Crous, P.W. (2012) One fungus, one name promotes progressive plant pathology. Mol. Plant Pathol. 13, 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, M.H. , Crous, P.W. , Henderson, J. , Groenewald, J.Z. and Drenth, A. (2012) Phyllosticta species associated with freckle disease of banana. Fungal Divers. 56, 173–187. [Google Scholar]
- Wu, G.A. , Terol, J. , Ibanez, V. , López‐García, A. , Pérez‐Román, E. , Borredá, C. , Domingo, C. , Tadeo, F.R. , Carbonell‐Caballero, J. , Alonso, R. , Curk, F. , Du, D. , Ollitrault, P. , Roose, M.L. , Dopazo, J. , Gmitter, F.G. Jr , Rokhsar, D.S. and Talon, M. (2018) Genomics of the origin and evolution of Citrus . Nature. 554, 311–316. [DOI] [PubMed] [Google Scholar]
- Wulandari, N.F. , To‐Anun, C. , Hyde, K.D. , Duong, L.M. , de Gruyter, J. , Meffert, J.P. , Groenewald, J.Z. and Crous, P.W. (2009) Phyllosticta citriasiana sp. nov., the cause of citrus tan spot of Citrus maxima in Asia. Fungal Divers. 34, 23–39. [Google Scholar]
- Yan, J. , Dewdney, M.M. , Roberts, P.D. and Ritenour, M.A. (2016) The effects of postharvest hot water and fungicide treatments on Guignardia citricarpa growth and the development of citrus black spot symptoms on ‘Valencia’ orange fruit. HortScience, 51, 1555–1560. [Google Scholar]
- Yonow, T. , Hattingh, V. and De Villiers, M. (2013) CLIMEX modelling of the potential global distribution of the citrus black spot disease caused by Guignardia citricarpa and the risk posed to Europe. Crop Prot. 44, 18–28. [Google Scholar]
- Yuan, Z. , Chen, Y. and Yang, Y. (2009) Diverse non‐mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): estimation and characterization. World J. Microbiol. Biotechnol. 25, 295–303. [Google Scholar]
- Zavala, M.G.M. , Er, H.L. , Goss, E.M. , Wang, N.Y. , Dewdney, M.M. and Van Bruggen, A.H.C. (2014) Genetic variation among Phyllosticta strains isolated from citrus in Florida that are pathogenic or nonpathogenic to citrus. Trop. Plant Pathol. 39, 119–128. [Google Scholar]
- Zhang, Y. , Crous, P.W. , Schoch, C.L. and Hyde, K.D. (2012) Pleosporales. Fungal Divers. 53, 1–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Wang, N.Y. , Dewdney, M.M. and Rollins, A. (2015) Lonely peninsula: The mating‐type and population of Phyllosticta citricarpa in Florida. Phytopathology, 105, S4, 157. [DOI] [PubMed] [Google Scholar]
- Zheng, X.M. (1983) Studies on the black spot of citrus (Guignardia citricarpa Kiely). J. S. Chin. Agric. Coll. 4, 53–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Different Phyllosticta isolates screened using the MAT111deg‐F2 and MAT111deg‐R3 primers (1010‐bp fragment; top part of both gel photos), and the same Phyllosticta isolates screened with the MAT121deg‐F1 and MAT121deg‐R1 primers (300‐bp‐fragment; lower part of both gel photos).
Table S1 Geographical distribution of Phyllosticta citricarpa.
Table S2 Collection and sequencing details of isolates included in this study.
Table S3 Accession numbers of mating type loci genes used in the synteny visualization.


