Highlights
-
•
Prescribing was as expected from clinical practice.
-
•
90% received ET prescriptions during the second year after diagnosis.
-
•
Prescribing dropped more than five years after diagnosis.
-
•
The majority of younger women (under 55) received tamoxifen.
-
•
The majority of older women (55+) received AIs.
Keywords: Breast neoplasm, Therapeutics, Tamoxifen, Aromatase inhibitors, Prescriptions
Abstract
Introduction
National cancer registration data were linked to the Primary Care Prescription Database (PCPD) in England. The level of endocrine therapy (ET) prescribed in women after a diagnosis of breast cancer was studied.
Materials and methods
Cancer registrations for women diagnosed with breast cancer during 1995–2015, who survived to 31st March 2015, were linked to ET prescriptions issued during April–July 2015.
Results
Among 369 277 survivors of breast cancer diagnosed during 1995–2015, 37% were prescribed ET during April–July 2015. Among women whose breast cancer diagnosis was after 31st July 2010, 81% of those recorded with oestrogen receptor positive (ER+ve) disease were prescribed ET compared with only 6% of those with ER-ve disease. Younger women usually received tamoxifen and older women usually received aromatase inhibitors.
Discussion
The pattern of ET use observed in these data corresponds to that expected. This provides confidence in the potential of the PCPD for epidemiological research.
1. Introduction
Most women diagnosed with oestrogen receptor positive (ER+ve) breast cancer in England are prescribed endocrine therapy (ET). ET decreases recurrence and increases survival [1] and is the current standard treatment for patients with ER+ve disease in both early and metastatic breast cancer [2]. Adherence to ET was reported to be between 40% and 70% over a five year treatment period [3,4].
In England ET is commonly prescribed for a duration of five years [5]. It is usually initiated in a hospital setting, but the majority of repeat prescriptions are issued in primary care. It has been possible to link cancer registrations in England with prescriptions issued in primary care using the Clinical Practice Research Datalink (CPRD)[6], but the CPRD covers only around 7% of the population. Furthermore, it includes only patients registered with participating general practices. If a patient moves, further prescriptions may not recorded. This has hampered studies of drugs that are used for long periods of time, such as ET in women with breast cancer [7].
The Primary Care Prescription Database (PCPD) is an administrative database used to calculate reimbursement for prescriptions dispensed by community pharmacies. It contains demographic and drug information categorised according to the British National Formulary (BNF) version 68 [8]. The NHS Business Services Authority (NHSBSA) has partnered with the National Cancer Registration and Analysis Service (NCRAS), allowing cancer registration data to be linked with the PCPD. To assess the epidemiological research potential of the PCPD we have evaluated the level of prescribing of ET and selected other drugs in women with breast cancer in England.
2. Materials and methods
Cancer registration is managed by NCRAS and data on all patients diagnosed with cancer in England are collected. Cancer registrations for women diagnosed with breast cancer (ICD-10 code C50) in England between 1st January 1995 and 31st July 2015 with no previous cancer were identified. Women who died before 1st April 2015, those living outside of England and those with a date of death before the prescription was processed were excluded. This is because, in the PCPD, the date of the prescription is the date it was processed by the NHSBSA and in very few cases (0.3% of prescription items in the entire dataset [8]), this is after the patient’s death. We collected information on dates of birth, stage, ER and progesterone receptor (PR) status.
Ages were calculated as of the 1st April 2015 and grouped. We calculated the difference in years between the date of breast cancer diagnosis and the end of the reference period (31st July 2015). The confidence intervals were determined using the Wilson score methodology [9].
The cancer registrations were linked to ET prescriptions in the PCPD during a reference period of April to July 2015 using pseudonymised identifiers derived from NHS number and date of birth. For women recorded with early stage ER+ve disease diagnosed during 2010–2015 the registrations were also linked to prescriptions of the following drugs if they were co-prescribed with ET during the reference period: analgesics, statins, oral hypoglycaemics, and anticoagulents. The same was done for oral bisphosphonates co-prescribed with aromatase inhibitors (AIs). To estimate prescribing in the general population, age-weighted averages of individuals prescribed these drugs were calculated. These averages were weighted by individuals in the PCPD by each year of age and were restricted to those having received any prescription during the reference period, excluding individuals prescribed AIs (for the oral bisphosphonates estimates) and ET (for all other estimates). Sex was not specified as it is not included in the PCPD.
3. Results
Among 369 277 survivors of breast cancer diagnosed during the years 1995–2015, 61% had ER unknown; 34% had ER+ve; 5% had ER-ve; and 0.02% had ER borderline disease. The average age of women with ER+ve disease was 64. Overall, 37% of the study population were prescribed ET during the reference period. The commonest drugs were tamoxifen (34% of prescriptions) and AIs (64% of prescriptions). For women recorded with ER+ve disease, 69% were prescribed ET compared with 42% of women with borderline ER, 5% of women with ER-ve disease, and 23% of women with unknown ER status.
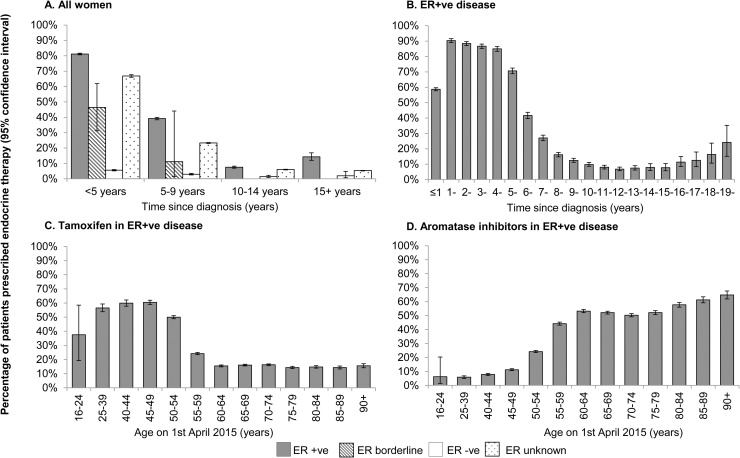
Among women recorded with ER+ve disease, 81% of those diagnosed after 31st July 2010 (i.e. within the previous five years at the time of the reference period) were prescribed ET compared with 46% of women with borderline ER status and only 6% of women with ER-ve disease (Fig. 1 panel A).
Fig. 1.
Prescriptions of endocrine therapy during April-July 2015 among women diagnosed with breast cancer in England during 1995–2015.
This figure presents the prescribing of endocrine therapy by ER status and time since diagnosis in Panel A. Panel B presents the prescribing of endocrine therapy by time since diagnosis in ER+ve women. Panels C and D present the prescribing of tamoxifen and aromatase inhibitors, respectively, by age. Confidence intervals were determined using the Wilson score methodology.
Among women recorded with ER+ve disease 59% of those diagnosed with breast cancer after 31st July 2014 (i.e. during the year prior to the reference period) were prescribed ET as were 90%, 88%, 87% and 85% respectively of women diagnosed during the 2nd, 3rd, 4th and 5th years previously (Fig. 1 panel B). For women diagnosed before this, the percentage of women still being prescribed ET during the reference period decreased progressively down to 7% during the 13th year before the reference period, and then increased slowly to 24% in the 20th year before the reference period.
For women recorded as ER+ve and aged less than 55, tamoxifen was the commonest drug and was prescribed to 55% of them (Fig. 1 panel C), while for ER+ve women aged 55 and older AIs were the commonest drug and were prescribed to 52% of them (Fig. 1 panel D).
Among women recorded with ER+ve disease, 22% of women who were prescribed AIs were co-prescribed oral bisphosphonates (3% in the general population). Other drugs commonly co-prescribed with ET to ER+ve women were analgesics (27% compared to 21% in the general population) and statins (24% compared to 24% in the general population). Much smaller percentages of women were co-prescribed oral hypoglycaemics (7% compared to 8% in the general population) or anticoagulents (4% compared to 4% in the general population).
4. Discussion
This proof of concept study has demonstrated the ability of the PCPD to identify prescriptions of ET issued in primary care to women in the years following a diagnosis of breast cancer. This is the first such data to become available for the entire population of England.
Most women with early breast cancer will not usually be prescribed ET until their other cancer treatments are completed, which usually takes a few months. Also, the initial ET prescription may be issued in hospital. Therefore, the best estimate of the overall proportion of ER+ve women who are prescribed ET is the percentage of women recorded in the PCPD who received it during the second year after their breast cancer diagnosis. During this period ET prescriptions were issued to 90% of women recorded as ER+ve.
Prescribing of ET decreased after around 5 years after breast cancer diagnosis which was expected as, during the time-period studied, guidelines for ET in early breast cancer recommended that it be prescribed for five years. ET is also recommended for metastatic breast cancer, so it was expected that ET prescriptions would be issued for a percentage of women in the second decade after breast cancer diagnosis, mostly for the treatment of metastatic disease.
A small proportion of ER-ve patients in the cohort were prescribed ET. A quarter of these women were recorded as PR positive, and the prescription of ET is in accord with evidence that tamoxifen increases survival in patients with ER-ve/PR+ve cancers [10].
The choice of ET type is related to menopausal status, with tamoxifen suitable at any age [5] but AIs suitable only for postmenopausal women or (much less commonly), younger women rendered postmenopausal. The patterns of ET prescriptions in the PCPD were in accordance with these scenarios.
The analysis of other drugs co-prescribed with endocrine therapy in ER+ve women with early breast cancer provides insight into the use of oral bisphosphonates in managing AI-associated bone loss [5] and of analgesics for the possible treatment of AI-induced arthralgia [11], while prescriptions of drugs associated with non-breast-cancer comorbidities such as statins and anticoagulents to treat cardiovascular disease and oral hypoglycaemics for diabetes were lower or similar to the general population.
ET has contributed substantially to patient survival [12,13] and prior to the linkage of PCPD to cancer registration data in England, ET prescribing in women with breast cancer could not be reliably captured for the entire population [14,7]. A key strength of the PCPD is its national and near complete coverage [8]. The coverage of the study population by the PCPD was high, with 86% of patients having received a prescription during the reference period. Some patients may have been missed due to not receiving a prescription during the short time frame covered by the reference period. An additional limitation of the PCPD is that age is missing for approximately 20% of prescriptions, potentially impacting the general population estimates for the co-prescribed drugs. Furthermore, as osteoporosis increases with age and women have higher rates of osteoporosis than men [15], our estimate of the prescribing rate of bisphosphonates is likely to underestimate the rate in older women and overestimate the rate in the entire population.
Prescribing was consistent with clinical practice and in accordance with ER status, patient age, and anticipated treatment duration. This study therefore provides confidence in the use of the PCPD for epidemiological purposes. A project is underway within PHE with the intention of making the prescriptions dataset available for request via the Office for Data Release [8] (ODR). This dataset has the potential for many applications, including providing a proxy for multimorbidity, as well as an insight into trends in prescribing both before and after cancer diagnosis and in end of life care. It could also be used to understand geographical differences in prescribing, adherence to guidance and to identify patterns in prescribing which could potentially highlight opportunities for earlier diagnosis. In the future, the PCPD should bridge a substantial gap in the knowledge of therapies that are not hospital based, especially those such as ET where primary care based prescription is the norm and continues over time.
Ethics approval and consent to participate
The authors declare that ethical approval and patient consent was not applicable.
Availability of data and material
The authors declare that there is no additional data available in relation to this study.
Conflict of interest
All authors declare that they have no conflicts of interest in relation to this work.
Funding
This work was supported by Cancer Research UK [grant number C8225/A21133].
There is no other funding to declare in relation to this work.
Authors’ contributions
DD, SD and KH designed the study and provided clinical support.
GE conducted the analysis with analytical support from KEH, JB and JC. All authors made substantial contributions to the interpretation of the findings.
All authors contributed to drafting the manuscript or revising it critically for important intellectual content and approved the final version submitted.
Acknowledgements
Data for this study is based on patient-level information collected by the NHS, as part of the care and support of cancer patients. The data is collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of Public Health England (PHE).
The authors acknowledge and thank the entire team and NHS Business Services Authority for supporting and facilitating this partnership.
Appendix.
Table A1, Table A2, Table A3, Table A4, Table A5, Table A6
Table A1.
Number of patients prescribed endocrine therapy by ER status during April–July 2015 among women diagnosed with breast cancer in England during 1995–2015.
| ER status | Patients prescribed ET | All patients |
|---|---|---|
| Positive | 85,692 | 124,240 |
| Negative | 976 | 20,258 |
| Borderline | 33 | 79 |
| Unknown | 51,089 | 224,700 |
| Total | 137,790 | 369,277 |
Table A2.
Number of ER+ve patients prescribed endocrine therapy by time since diagnosis during April–July 2015 among women recorded with ER+ve breast cancer in England during 1995–2015.
| Time since diagnosis | ER+ve patients prescribed ET | ER+ve patients |
|---|---|---|
| ≤5 years ago | 76,043 | 93,735 |
| 6 to 10 years ago | 9,025 | 23,014 |
| 11 to 15 years ago | 494 | 6,580 |
| >15 years ago | 130 | 911 |
Table A3.
Number of ER-ve patients prescribed endocrine therapy by time since diagnosis during April–July 2015 among women recorded with ER-ve breast cancer in England during 1995–2015.
| Time since diagnosis | ER-ve patients prescribed ET | ER-ve patients |
|---|---|---|
| ≤5 years ago | 842 | 15,065 |
| 6 to 10 years ago | 112 | 3,750 |
| ≥11 years ago | 22 | 1,443 |
Table A4.
Number of ER unknown patients prescribed endocrine therapy by time since diagnosis during April–July 2015 among women recorded with ER unknown breast cancer in England during 1995–2015.
| Time since diagnosis | ER unknown patients prescribed ET | ER unknown patients |
|---|---|---|
| ≤5 years ago | 24,095 | 36,051 |
| 6 to 10 years ago | 21,465 | 92,152 |
| 11 to 15 years ago | 3,506 | 58,307 |
| >15 years ago | 2,023 | 38,190 |
Table A5.
Number of ER+ve patients prescribed endocrine therapy by individual year since diagnosis during April–July 2015 among women recorded with ER+ve breast cancer in England during 1995–2015.
| Years since diagnosis | ER+ve patients prescribed ET | ER+ve patients |
|---|---|---|
| ≤1 | 12,844 | 21,870 |
| 1 | 19,432 | 21,512 |
| 2 | 17,361 | 19,633 |
| 3 | 14,967 | 17,257 |
| 4 | 11,439 | 13,463 |
| 5 | 5,473 | 7,746 |
| 6 | 1,599 | 3,838 |
| 7 | 881 | 3,257 |
| 8 | 507 | 3,136 |
| 9 | 341 | 2,743 |
| 10 | 224 | 2,294 |
| 11 | 167 | 2,086 |
| 12 | 131 | 1,922 |
| 13 | 105 | 1,404 |
| 14 | 50 | 638 |
| 15 | 41 | 530 |
| 16 | 48 | 421 |
| 17 | 27 | 216 |
| 18 | 24 | 147 |
| ≥19 | 31 | 127 |
Table A6.
Number of ER+ve patients prescribed tamoxifen and aromatase inhibitors by age during April–July 2015 among women recorded with ER+ve breast cancer in England during 1995–2015.
| Age as of April 2015 | ER+ve patients with a tamoxifen prescription | ER+ve patients with an aromatase inhibitor prescription | ER+ve patients |
|---|---|---|---|
| 16-39 | 1,622 | 169 | 2,881 |
| 40-44 | 2,836 | 370 | 4,734 |
| 45-49 | 6,113 | 1,133 | 10,107 |
| 50-54 | 8,139 | 3,944 | 16,290 |
| 55-59 | 3,495 | 6,392 | 14,467 |
| 60-64 | 2,381 | 8,197 | 15,403 |
| 65-69 | 3,152 | 10,233 | 19,648 |
| 70-74 | 2,381 | 7,346 | 14,623 |
| 75-79 | 1,466 | 5,340 | 10,242 |
| 80-84 | 1,157 | 4,533 | 7,857 |
| 85-89 | 713 | 3,055 | 4,989 |
| 90+ | 465 | 1,942 | 2,999 |
References
- 1.Moon Z., Moss-Morris R., Hunter M.S., Carlisle S., Hughes L.D. Barriers and facilitators of adjuvant hormone therapy adherence and persistence in women with breast cancer: a systematic review. Patient Prefer Adherence. 2017;11:305–322. doi: 10.2147/PPA.S126651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastiaannet E., Charman J., BørgeJohannesen T., Schrodi S., Siesling S., Eycken L. A European, Observational Study of Endocrine Therapy Administration in Patients With an Initial Diagnosis of Hormone Receptor-Positive Advanced Breast Cancer. Clinical Breast Cancer. 2017;(17):0–30728. doi: 10.1016/j.clbc.2017.11.019. pii: S1526-8209. [DOI] [PubMed] [Google Scholar]
- 3.Murphy C., Bartholomew L., Carpentier M., Bluethmann S., Vernon S. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast cancer research and treatment. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirgwin J., Giobbie-Hurder A., Coates A., Price K., Ejlertsen B., Debled M. Treatment adherence and its impact on disease-free survival in the Breast International Group 1-98 trial of tamoxifen and letrozole, alone and in sequence. Journal of clinical oncology. 2016;34(21):2452–2459. doi: 10.1200/JCO.2015.63.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early and locally advanced breast cancer: diagnosis and treatment. NICE Clinical Guideline. March 2017. NHS National Institute for Health Research.
- 6.Clinical Practice Research Datalink. www.cprd.com/home/ Accessed 2 May 2018.
- 7.Huiart L., Dell'Aniello S., Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. British journal of cancer. 2011;104(10):1558–1563. doi: 10.1038/bjc.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henson K., Brock R., Shand B., Coupland V., Elliss-Brookes L., Lyratzopoulos G. Cohort profile: prescriptions dispensed in the community linked to the national cancer registry in England. BMJ Open. 2018;8:e020980. doi: 10.1136/bmjopen-2017-020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Association of public health observatories. Technical Briefing – Commonly used public health statistics and their confidence intervals. https://fingertips.phe.org.uk/documents/APHO%20Tech%20Briefing%203%20Common%20PH%20Stats%20and%20CIs.pdf (Accessed 21 November 2018).
- 10.Yang L.-H., Tseng H.-S., Lin C., Chen L.-S., Chen S.-T., Kuo S.-J. Survival Benefit of Tamoxifen in Estrogen Receptor-Negative and Progesterone Receptor-Positive Low Grade Breast Cancer Patients. Journal of Breast Cancer. 2012;15(3):288–295. doi: 10.4048/jbc.2012.15.3.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne C. Management of arthralgias associated with aromatase inhibitor therapy. Current Oncology. 2007;14(Suppl 1):S11–S19. doi: 10.3747/co.2007.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H., Gray R., Braybrooke J., Davies C., Taylor C., McGale P. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. New England Journal of Medicine. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Breast Cancer Trialists’ Collaborative Group Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen patient-level meta-analysis of randomised trials. The Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barron T.I., Connolly R., Bennett K., Feely J., Kennedy M.J. Early discontinuation of tamoxifen. Cancer. 2007;109(5):832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 15.Alswat K.A. Gender Disparities in Osteoporosis. Journal of clinical medicine research. 2017;9(5):382–387. doi: 10.14740/jocmr2970w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that there is no additional data available in relation to this study.