Abstract
Heteroblastic and homoblastic woody plants from the New Zealand flora provide a rich playground for testing hypotheses relating to phase change and flowering.
Keywords: ABC model, Clianthus, Eucalyptus, flowering, gibberellin, growth habit, heteroblasty, homoblasty, Metrosideros, microRNA, Pachycladon, phase change, Phormium, photoperiod, reproduction, Sophora
A brief introduction to the New Zealand flora
Over a decade ago we were invited by Georges Bernier to provide a ‘minireview of research activity’, which we presented as ‘A woody perennial perspective of flowering’ (Clemens et al., 2002). That review focused on Metrosideros excelsa. Here, we cover some of the research carried out subsequently on this and other species, and look towards the future use of some of the peculiarities of the New Zealand flora in extending our knowledge of phase change.
The New Zealand flora, which has relatively low numbers of families and genera for the area of the country, has often been described as ‘depauperate’ and predominated by small white or yellow flowers together with a paucity of butterflies and long-tongued bees (Webb and Kelly, 1993; Lee et al., 2001; Craine et al., 2006). However, there are several tree genera with brightly coloured flowers that are bird pollinated (Kelly et al., 2010). These include Metrosideros (Myrtaceae), Sophora (Fabaceae), and Clianthus (Fabaceae).
The red-flowering M. excelsa (pōhutukawa) and Clianthus (two species of kakabeak), and the yellow-flowering Sophora (several species of kowhai) hold iconic status for both indigenous Māori of New Zealand and for the population as a whole. The pōhutukawa is popularly known as the New Zealand Christmas tree as it flowers about Christmas time in the Southern Hemisphere summer. A lone pōhutukawa holds particular significance to Māori (Simpson, 1994). The tree, reputed to be 800 years old grows at Cape Reinga, the northernmost tip of the North Island. Te Reinga translates as the ‘leaping place of spirits’. The legend is that, on death, the spirit of the person travels to this most northern point, descends by way of the roots of this tree to the underworld, and then travels to the homeland of Hawaiiki-a-nui.
The website maoriplantuse.landcareresearch.co.nz provides interesting details about these plants. All three had significant use in pre-European times with the red flowers of pōhutukawa and kakabeak used for ornamentation. Both pōhutukawa and kowhai were used medicinally and for wood, although the kowhai seeds and wood are toxic. The yellow flowers of the kowhai were used as a dye as well as to signal the time to plant the staple root crop kūmara (sweet potato, Ipomoea batatas). Kakabeak may have been cultivated for food and it was widely grown in both pre- and post-European settlements (Colenso, 1885). Several kakabeak cultivars are grown as garden plants throughout New Zealand, for example, the red-flowered ‘Kaka King’, ‘White Heron’, and the pink ‘Flamingo’.
Unfortunately, all three genera now hold species that are nationally threatened (de Lange et al., 2013). Only 153 plants of kakabeak were detected in the wild in 2005 (Song et al., 2008b) growing in relatively inaccessible regions of the East Coast of the North Island. Indeed, in 1885, Colenso wrote that he had never seen Clianthus growing ‘truly wild and common’. Kakabeak is classified as critically threatened nationally and its continued survival in the wild is described as conservation-dependent (de Lange et al., 2013).
Banks and Solander were the first to collect kakabeak in 1769, but Clianthus puniceus was not described until 1835. Colenso grew kakabeak from locally cultivated plants in Northland and then later further south in Hawkes Bay. He contrasted the more northern form with the more southern form and described the latter as C. maximus (Colenso, 1885). In 1899, Kirk reduced C. maximus to a variety of C. puniceus. Subsequently, Heenan (2000) re-instated the two species, using C. puniceus to describe the one specimen from Kaipara Harbour (Northland), and C. maximus to describe the East Coast, including Hawkes Bay, populations. However, our data, based on the presence/absence of a seven-base-pair deletion in intron 2 of LEAFY, indicate that the Kaipara Harbour Clianthus is not a species distinct from the extant populations found in the wild in New Zealand. We suggested that it is more likely to be a translocation, and that there is a morphological gradation from north to south of a single species of Clianthus (Song et al., 2008b).
The kowhai is often referred to as New Zealand’s unofficial national flower. Several species are regarded as naturally uncommon (de Lange et al., 2013) but, like kakabeak, it is widely grown as a garden plant. By contrast, the pōhutukawa, unlike some other species of Metrosideros, is not formally regarded as threatened (de Lange et al., 2013), although its range has been greatly reduced owing to clearance and damage from introduced herbivores (Bylsma et al., 2014). The pōhutukawa has been widely planted as a road and park tree outside its natural range.
We were interested in the potential of these plants as cultivated species for cut flower or flowering plant production, but little work had been carried out relating to phase change, floral induction or flower development. A comparative analysis is offered in this commentary to contrast the differences in the timing of and cues for floral induction, and in floral initiation and floral organ differentiation. However, we show that, despite these contrasting behaviours, gene expression during flower development in all three species adhered to predictions based on the ABC model of flower development. The data provided are for plants studied in Palmerston North, New Zealand (40° 23' 6'' S, 175° 36' 51'' E). The comparative analysis of flowering in the three woody genera was first drawn together for a talk entitled ‘The ABCs of flowering in three iconic New Zealand species’ and was presented by PEJ as the 35th John Smaillie Tennant Lecture on the occasion of the 90th Birthday of the Department of Botany, University of Otago, New Zealand, 11 September 2014. However, in this commentary, we also introduce research on a wider range of species, and particularly on phase change.
Morphological analysis
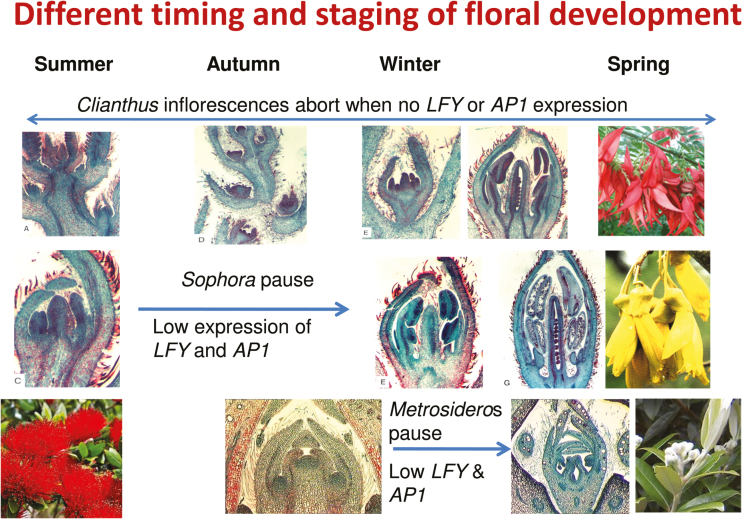
Over an annual cycle it was clear that floral initiation by the kowhai and kakabeak (referred to below as Sophora and Clianthus, respectively) occurred soon after the previous season’s flowering in spring, but that of pōhutukawa (referred henceforth as Metrosideros) was several months later in autumn (Fig. 1). In Sophora, floral primordia were evident in spring (October), and floral organ initiation occurred rapidly and continued through to mid-summer (end of January). Development then paused through the autumn and early winter, but in mid-winter (July) rapid organogenesis occurred although the petals remained small until a period of rapid elongation just prior to flowering in early spring (late August/early September) (Song et al., 2008a).
Fig. 1.
Temporal pattern of floral development of Clianthus, Sophora, and Metrosideros over an annual cycle. Floral initiation in Sophora and Clianthus occurred soon after the previous season’s flowering in spring whereas that in Metrosideros was in autumn. The relationship of the stage of development to reduced expression of LEAFY- and APETALA1-equivalents is shown.
While Clianthus initiated inflorescences continuously, and within these floral primordia were evident, organogenesis did not occur until late autumn/early winter. Organogenesis continued through to flowering in early spring (September) and, as with Sophora, petals were slow to elongate (Song et al., 2011). In contrast to both Sophora and Clianthus, cymule primordia were not detected in Metrosideros until the autumn, and then further organ initiation and differentiation was delayed until late spring (Sreekantan et al., 2001).
Environmental responsiveness
Experimentation showed that Metrosideros is a facultative short-day plant (Henriod et al., 2000), with flower numbers being influenced by cool (mean 15 °C), not cold (mean 10 °C) temperatures and irradiance, as well as by bud size (Henriod et al., 2003). While Clianthus inflorescences were continuously produced, most aborted and only those produced during a few weeks in autumn continued to elongate and commence organ initiation (Song et al., 2011). Floral initiation was, therefore, not a short-day response but it is likely that the subsequent organ initiation was a response to the cooler temperatures of late autumn. Similarly, floral initiation in Sophora was not a response to short-day signals, but flower development required cooler conditions: floral initiation and organ differentiation occurred in spring but organogenesis in Sophora was delayed until mid-winter (Song et al., 2008a).
Lee et al. (2001) suggest that there is a relative lack of cold tolerance in most of the modern New Zealand woody flora, which is of warm-temperate/subtropical affinity. They suggest that much of New Zealand’s floral richness was lost as the climate cooled during the Late Miocene-Pliocene (Lee et al., 2001), so it is interesting to identify a requirement for cooler temperatures for full floral development and the consequent deferment of flowering until early spring. However, this is not a vernalization requirement, as floral initiation started in all three species prior to winter chilling.
The ABC model
Within the three New Zealand species, the extended period of floral development, the different timing of organ initiation and development, and the ‘pause’ in development during the winter months provided an opportunity to assess the expression of LEAFY, and the relevant equivalents of the A (APETALA1), B (PISTILLATA), and C (AGAMOUS) genes associated with floral morphogenesis. We showed that the A-, B-, and C-equivalents in Sophora and Clianthus were expressed in sepals, petals, stamens, and carpels (Song et al., 2008a, 2011) as predicted by the floral organ identity model (Bowman et al., 1991; Coen and Meyerowitz, 1991).
In Arabidopsis all organs within a whorl develop simultaneously in the order of sepal, petal, stamen, and carpel. By contrast, in both Sophora and Clianthus this is not the case. Following sepal and petal initiation, only the outer stamen primordia initiate, followed then by the carpel, and then the inner stamens; in addition, the carpel primordium enlarges rapidly whereas petal enlargement is significantly delayed (Song et al., 2008a, 2011). One might then predict more ‘C’ gene expression before that of ‘B’. A close inspection of the expression profiles shows that the expression of StAG and CmAG increases more rapidly than that of StPI and CmPI, respectively, tempting the suggestion of a causative relationship between early carpel primordial enlargement and precocious expression of the ‘C’ gene (Song et al., 2008a, 2011).
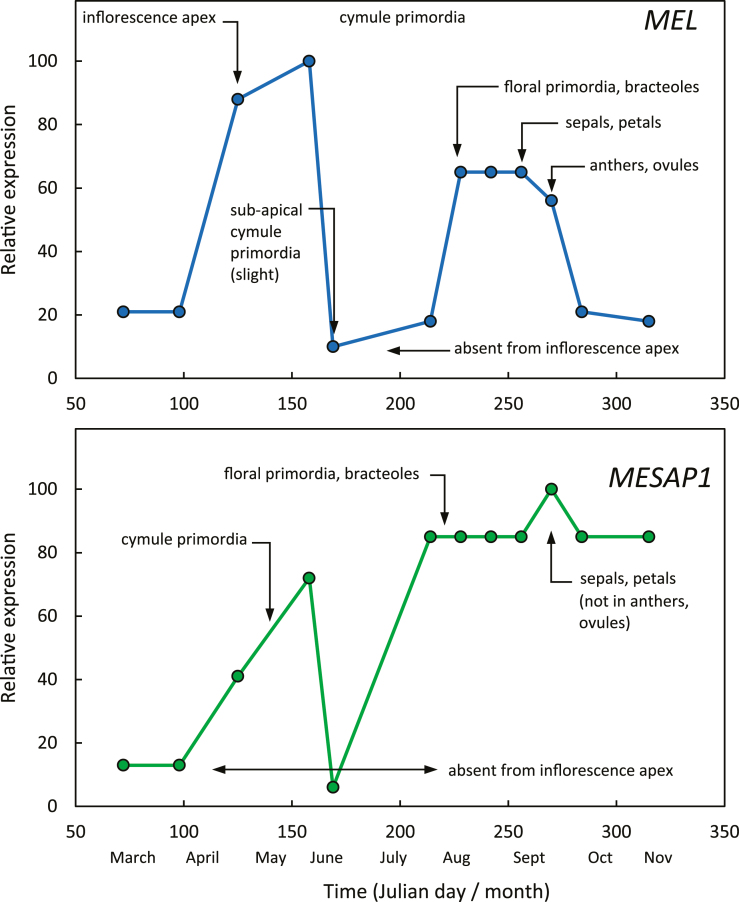
Combining the in situ hybridization, northern, RT-qPCR, and morphological data for Metrosideros (Sreekantan et al., 2004; Jaya et al., 2011), both MeLEAFY (MEL) and MeAP1 (MESAP1) were expressed as the cymule primordia developed during the autumn, were not expressed during much of the winter period, but increased in expression towards the end of winter. MESAP1 was expressed in sepals and petals but not anthers or ovules, as might be expected of an ‘A’ class gene (Fig. 2).
Fig. 2.
Expression of Metrosideros excelsa LEAFY (MEL) and APETALA1 (MESAP1) over time and space during floral development. Data compiled from Sreekantan (2002).
In Sophora, where floral organ initiation occurred throughout the summer, both StLFY and StAP1 were expressed, but expression had declined by the end of summer by which time floral development had synchronized but then paused. Floral organ differentiation coincided with the increase in expression of StLFY, StAP1, StAG, and StPI (Song et al., 2008a). In Clianthus, low level CmLFY expression occurred while inflorescences were proliferating, but floral development coincided with marked increases in CmLFY, CmAP1, CmAG, and CmPI (Song et al., 2011).
In all three species, floral organ initiation required the expression of both LFY and AP1 (Fig. 1). The ‘pause’ in flower development in Sophora and Metrosideros occurred at a different developmental stage (following floral organ initiation and cymule primordia initiation, respectively), and coincided with low expression of both LFY and AP1. Subsequent floral development in all three species required the expression of LFY and the ‘A’, ‘B’, and ‘C’ genes, respectively. This confirmed the dual role initially suggested by Bowman et al. (1993) for AP1 in floral meristem specification and floral organ development and expanded by Kaufmann et al. (2010) for AP1, suggesting that its actions are initially that of a repressor of floral repressors but then as a subsequent activator of regulatory genes involved in floral organ formation. While Mizzotti et al. (2014) question the universality of class A-function genes beyond Arabidopsis, AP1 in our three woody species conforms to such a gene, although functional analysis has not been carried out due to our respecting the three species as tāonga (‘treasures’) to Māori, and so not transforming the AP1 gene into Arabidopsis for functional analysis.
The gibberellin pathway
In Arabidopsis, gibberellin is required for flowering under non-inductive short days (Blázquez et al., 1998). We have shown that gibberellin has a promotive effect on flowering in two monocots: Chionochloa macra (Martin et al., 1993) and Phormium cookerianum (the endemic New Zealand mountain flax; Harris et al., 2009). In mountain flax, floral induction was unaffected by either temperature or daylength – size being the determinant of competence to flower. However, GA3 promoted flowering of small and medium-sized fans that would otherwise not have flowered. Expression of LFY correlated positively with size of fan and ability to flower and the application of GA3 accelerated the increase in LFY (Harris et al., 2009). However, the final level of LFY did not correlate with the proportion that flowered, a pattern similar to LtLFY in Lolium temulentum (Gocal et al., 2001) and RFL in rice (Kyozuka et al., 1998).
In contrast to the monocots, we have shown that the application of gibberellins caused floral buds of Metrosideros to abort (Clemens et al., 1995), a similar situation subsequently shown in other woody dicots, particularly fruit trees (Goldberg-Moeller et al., 2013, and references therein). Gibberellin caused an increase in LFY expression in non-flowering juveniles but not of AP1 (Sreekanatan et al., 2004). Our analysis of all three flowering woody species indicates that flowering will only progress if both LFY and AP1 are expressed. Interestingly, GA1 was not detectable in individual buds of reproductively competent plants in July, during the mid-winter ‘pause’ in floral development in Metrosideros, but was at its highest level during spring in actively growing vegetative shoots and in flower buds when LFY expression had again increased (Sreekantan et al., 2004). In their dual model for GA activity during Arabidopsis flowering, Yamaguchi et al. (2014) suggest that LFY is initially up-regulated by GA, but LFY subsequently directs the destruction of GA, thus allowing the accumulation of DELLA proteins required for the subsequent up-regulation of AP1 and for flowering to progress (see Fig. 4F in Yamaguchi et al., 2014). While the application of GA did not promote the juvenile to adult vegetative transition in Metrosideros (Clemens et al., 1995), it did inhibit the progression of floral buds to flowers, which would indicate that an over-supply of GA is inhibitory for the progression of floral development, as suggested by Yamaguchi et al. (2014). To determine where the GA1 that we detected is acting in individual flower buds harvested 2–3 weeks before anthesis, careful dissection and analysis of the floral buds will be required. However, at this stage of development, all floral organs had differentiated and were rapidly expanding, and the gibberellin may be involved in anther development (Sakata et al., 2014, and references therein).
Phase change
While the long juvenile phase exhibited in woody species is a serious constraint to plant breeding and to the production of floricultural crop plants raised from seed, it offers opportunities to characterize and elucidate the control of phenotypic transitions occurring in leaf and habit in the vegetative plant (vegetative phase change) alongside, and not necessarily coupled with, reproductive transition. As observed by Zotz et al. (2011), most plant species show a relatively subtle and gradual change in character between juvenile and adult vegetative states. However, there is a significant number of species in which there is a more-or-less abrupt demarcation between the two states (Zotz et al., 2011). Goebel (1900) described such species as ‘heteroblastic’, and those with an abrupt change in habit were described by Philipson (1964) as exhibiting ‘habit heteroblasty’. However, in the more recent literature the term ‘heteroblasty’ has come to be used for even the most gradual of morphological changes (e.g. Poethig, 2013) where the term ‘homoblastic’ (sensu strictoGoebel, 1900) should be used. Zotz et al. (2011) suggest the terminology used by Goebel (1900) should be retained and demonstrate schematically the abrupt or gradual changes taking place against a size/age profile (see Fig. 2 in Zotz et al., 2011). According to Cockayne (1911), ‘About two hundred species of New Zealand vascular plants, belonging to thirty-seven families, show a more or less well-marked distinction between the juvenile and adult stages of development, while in perhaps one hundred species the differences are very great indeed’. As New Zealand plant biologists, where the term heteroblasty describes a significant aspect of our flora, we fully support the use of Goebel’s original terminology (Horrell et al., 1990b; Darrow et al., 2002; Jaya et al., 2010b; Sooda et al., 2011; see also Appendix 1 in Zotz et al., 2011).
The confusion in the literature highlights the complexity of the situation, but now that a marker of the vegetative phase has been identified (Wu et al., 2009), some of these anomalies may be tested. In the annual model species (Arabidopsis thaliana and Zea mays), microRNA156 (miR156) is considered to be a master regulator of vegetative phase change (Poethig, 2013). MicroRNAs are short, single-stranded RNAs that regulate target gene expression at the post-transcriptional level. MicroRNA156 and miR172 have distinctive roles in maintaining the juvenile vegetative phase and initiating reproduction, respectively (Zhu and Helliwell, 2011; Spanudakis and Jackson, 2014). Transgenic experiments clearly show that high levels of miR156 maintain the juvenile vegetative state and delay flowering, whereas high levels of miR172 promote flowering and vice versa: plants with reduced miR156 flower earlier; those with reduced miR172 flower later (Yu et al., 2015, and references therein). While models show that a balance between miR156 and miR172 controls the timing of the juvenile to adult vegetative phase change (Zhu and Helliwell, 2011; Teotia and Tang, 2015), there appears to be little experimental work confirming this within the same plant (Wu et al., 2009) or in woody perennial species (Wang et al., 2011). As we have shown with the ABC genes, New Zealand native plants should also be ideal subjects to test the utility of microRNAs and their downstream regulated genes as markers of the different states, as well as contributing information relating to the juvenile to adult vegetative phase transition and the transition from non-reproductive to reproductive.
In addition to heteroblasty, the New Zealand flora has an unusually high proportion of species that exhibit a pronounced divaricating habit in the juvenile (Wardle, 1991; Goldberg et al., 2008). Divaricate plants typically have a wide branching angle, closely interlaced, springy, branches, and small leaves concentrated in the interior of the plant (Kelly, 1994). Tree species exhibiting the divaricating habit usually convert to an arborescent habit (tree) prior to flowering. However, some species appear to have ‘lost’ the ‘adult’ tree form (Cockayne, 1911) and yet are capable of flowering.
For example, different species of Sophora exhibit different life strategies: S. prostrata Buchan, S. microphylla Ait., and S. tetraptera J. Mill differ markedly from each other in their juvenile forms (Cockayne, 1911; Godley and Smith, 1978). Sophora prostrata grows as a prostrate, divaricating shrub throughout its ontogeny whereas S. tetraptera does not have a divaricating juvenile vegetative phase and forms a small tree with spreading branches about 12 m in height (Allan, 1961). Sophora microphylla on the other hand is variable in form depending on location, and may or may not have a juvenile divaricating form. However, the adult is a tree up to 10 m in height (Salmon, 1980; Carswell et al., 1996). Such inter- and intra-specific variation provides opportunities to test the miR156/miR172 model beyond the classical perennial phase change models (Wang et al., 2011). Moreover, and similar to a response in ivy (Rogler and Hackett, 1975; Horrell et al., 1990a), the application of gibberellin can induce a change from the adult form and foliage of several heteroblastic species to that more indicative of the divaricating juvenile (Horrell et al., 1990b), providing further material for microRNA analysis.
We also have the opportunity to use species in which experimental manipulations have been shown to affect both the vegetative to adult transition and also the transition to flowering. In contrast to heteroblastic species, Metrosideros excelsa exhibits homoblastic behaviour in the strict sense of Goebel (1900), since vegetative phase change occurs gradually between juvenile and adult states (Clemens et al., 1999). Experimentally, vegetative phase change can be hastened by growing plants with a single stem (Clemens et al., 1999; Kubien et al., 2007; Jaya et al., 2011), whereas flowering can be advanced by subsequently allowing such plants to branch (Jaya et al., 2011). In contrast to manipulating phase change by transgenic means (Lauter et al., 2005; Wu and Poethig, 2006; Wu et al., 2009; Wang et al., 2011), an ability experimentally to manipulate the transition of the juvenile to adult phase change, as well as the timing of reproduction, provides useful material to elaborate on the role of both miR156 in maintaining the juvenile phase and miR172 in the transition to the adult phase and to reproduction. Moreover, Metrosideros also exhibits ‘rejuvenation’ when elite selections are micropropagated from reproductive plants (Oliphant et al., 1991; Clemens et al., 1995). Such micropropagated plants are slow to return to flowering. In this case, the presence of miR156, or perhaps a change in the ratio between miR156 and miR172 might confirm whether this is a complete rejuvenation as both truly juvenile and truly adult and reproductive material are available for comparison.
In contrast to Metrosideros, experimentally single-stemmed plants of another member of the Myrtaceae, Eucalyptus occidentalis, do not undergo the transition from juvenile to adult vegetative foliage (Jaya et al., 2010a, b, 2011). Moreover, the plants bearing juvenile foliage flowered just as rapidly as branched plants with adult foliage. The floral transition in these plants is clearly independent of vegetative phase change. Eucalyptus occidentalis provides a unique opportunity to assess the independence of vegetative phase transitions and the transition from vegetative to reproductive, utilizing miR156 and miR172 as markers. The model proposed by Wang (2014) shows the potential for maintenance of juvenility within the leaf and transition to reproduction in the shoot apical meristem based on the different targets of downstream factors (see Fig. 2 in Wang, 2014).
Species that are distinctly heteroblastic (Goebel, 1900sensu stricto; Zotz et al., 2011) such as Elaeocarpus hookerianus Raoul (pokaka), but which exhibit a third ‘adolescent’ state (Day et al., 1997), may well be ideal plants to confirm the models that show both miR156 and miR172 expressing as plants transition from the juvenile vegetative to the adult vegetative states. Are both miR156/miR172 present in the non-reproductive adult vegetative state and miR172 expressing alone in the reproductive state (Teotia and Tang, 2015)?
Interestingly, analyses of cytokinins in leaves from juvenile and mature forms of E. hookerianus indicate that the divaricating juvenile form contained more active forms and fewer storage forms of cytokinin than did leaves from the transitional and adult arborescent forms (Day et al., 1995). Further, in Sophora, the divaricate form showed more active forms relative to storage forms of cytokinin (Carswell et al., 1996). We highlight this because, recently, Zhang et al. (2015) suggested that, as leaves age, the decline in shoot regenerative capacity in tissue culture is related to the decrease in miR156 and the up-regulation of a previously repressed gene that directly impacts on the cytokinin signalling pathway, thereby reducing plant responsiveness to cytokinin in culture in the older leaves (Zhang et al., 2015). It will be of interest to determine if similar correlations exist between cytokinin forms and/or sensitivity and miR156 in the New Zealand native species.
Another endangered species (Molloy et al., 1999; Luo et al., 2003), Pachycladon exile (Heenan) Heenan & A.D. Mitch. (Family Brassicaceae), provides a perennial herb related to Arabidopsis lyrata, which concurrently has primary and secondary axes that are floral, but tertiary axes that remain vegetative, green, and perennating (Sooda et al., 2011). Are such perennating structures vegetatively juvenile (high miR156?) and not competent to respond to flowering signals, or adult (high miR172) but somehow repressed, or carrying an intermediate level of both miR156 and miR172, i.e. vegetatively adult but not competent to respond reproductively?
Conclusions
The New Zealand flora provided flowers with quite different temporal patterns of development on to which could be overlaid the ABC model of floral development. Similarly, the flora offers species with which to assess the definitive nature of miR156 and miR172 and their downstream target genes in driving vegetative phase change in heteroblastic and homoblastic species, and their floral transition.
Work on these woody perennials emblematic of New Zealand was triggered by a call from industry for their greater global floricultural prominence. Work on woody perennials is famously dogged by slow maturation, long developmental cycles once a reproductive state is reached, and branch superstructures that might not lend themselves to commercial development. Paradoxically, these are the very phenomena that allowed us to examine and separate the morphological and genetic information we sought. Nonetheless, the combination of laboratory and horticultural skills, along with the intellectual endeavour necessary to obtain our results, are a testament to the dedication of students and co-workers who worked in a cultural and regulatory environment where genetic modification of these native tāonga could not be countenanced. We would like to dedicate this work to two colleagues who, in different ways, supported this work, Dr Garry Burge and Professor Michael McManus, both of whom have passed away.
Acknowledgements
Financial support was obtained principally from the New Zealand Public Good Science Fund Native Ornamental Plants Programme (Crop & Food Research Limited sub-contracts CO2626, CO2X0202, CO2X0015), the Agricultural and Marketing Research and Development Trust, the British Council Higher Education Links Scheme, and Massey University. This commentary was written while PEJ was on sabbatical leave from the University of Canterbury.
References
- Allan HH. 1961. Flora of New Zealand. I. Wellington:New Zealand Government Printer. [Google Scholar]
- Blázquez MA , Green R , Nilsson O , Sussman MR , Weigel D. 1998. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. The Plant Cell 10, 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL , Alvarez J , Weigel D , Meyerowitz EM , Smyth DR. 1993. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119, 721–743. [Google Scholar]
- Bowman JL , Smyth DR , Meyerowitz EM. 1991. Genetic interactions among floral homeotic genes of Arabidopsis. Development 112, 1–20. [DOI] [PubMed] [Google Scholar]
- Bylsma RJ , Clarkson BD , Efford JT. 2014. Biological flora of New Zealand 14: Metrosideros excelsa, pōhutukawa, New Zealand Christmas tree. New Zealand Journal of Botany 52, 365–385. [Google Scholar]
- Carswell FE , Day JS , Gould KS, Jameson PE. 1996. Cytokinins and the regulation of plant form in three species of Sophora. New Zealand Journal of Botany 34, 123–130. [Google Scholar]
- Clemens J , Henriod RE , Bailey DG , Jameson PE. 1999. Vegetative phase change in Metrosideros. Shoot and root restriction. Plant Growth Regulation 28, 207–214. [Google Scholar]
- Clemens J , Henriod R , Sismilich M , Sreekantan L , Jameson PE. 2002. A woody perennial perspective of flowering. In: Bernier G, ed. The Flowering Newsletter 33, Liège: University of Liège, 17–22. [Google Scholar]
- Clemens J , Jameson PE , Bannister P , Pharis RP. 1995. Gibberellins and bud break, vegetative shoot growth and flowering in Metrosideros collina cv. Tahiti. Plant Growth Regulation 16, 161–171. [Google Scholar]
- Cockayne L. 1911. Observations concerning evolution, derived from ecological studies in New Zealand. Transactions and Proceedings of the Royal Society of New Zealand 44, 1–50. [Google Scholar]
- Coen ES , Meyerowitz EM. 1991. The war of the whorls: genetic interactions controlling flower development. Nature 353, 31–37. [DOI] [PubMed] [Google Scholar]
- Colenso W. 1885. On Clianthus puniceus, Sol. Transactions and Proceedings of the New Zealand Institute 18, 291–295. [Google Scholar]
- Craine JM , Lee WG , Walker S. 2006. The context of plant invasions in New Zealand: evolutionary history and novel niches. In: Allen RB , Lee WG, eds. Biological invasions in New Zealand . Ecological Studies, Vol. 186. Berlin, Heidelberg: Springer-Verlag, 167–177. [Google Scholar]
- Darrow HE , Bannister P , Burritt DJ , Jameson PE. 2002. Are juvenile forms of New Zealand heteroblastic trees more resistant to water loss than their mature counterparts? New Zealand Journal of Botany 40, 313–325. [Google Scholar]
- Day J , Jameson PE , Gould KS. 1995. Cytokinins associated with metamorphic vegetative growth in Elaeocarpus hookerianus. Australian Journal of Plant Physiology 22, 67–73. [Google Scholar]
- Day J , Gould KS , Jameson PE. 1997. Vegetative architecture of Elaeocarpus hookerianus. Transition from juvenile to adult. Annals of Botany 79, 617–624. [Google Scholar]
- de Lange PJ , Rolfe JR , Champion PD , Courtney SP , Heenan PB , Barkla JW , Cameron EK , Norton DA , Hitchmough RA. 2013. Conservation status of New Zealand indigenous vascular plants, 2012. Wellington:New Zealand Department of Conservation. [Google Scholar]
- Gocal GFW , King RW , Blundell CA , Schwart OM , Andersen CH , Weigel D. 2001. Evolution of floral meristem identity genes. Analysis of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiology 125, 1788–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godley EJ , Smith DH. 1978. Kowhais and their flowering. Annual Journal of the Royal New Zealand Institute of Horticulture 5, 24–31. [Google Scholar]
- Goebel K. 1900. Organography in plants. I. General organography. English translation by Balfour IB. Oxford:Clarendon Press. [Google Scholar]
- Goldberg J , Trewick SA , Paterson AM. 2008. Evolution of New Zealand’s terrestrial fauna: a review of molecular evidence. Philosophical Transactions of the Royal Society B 363, 3319–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg-Moeller R , Shalom L , Shlizerman L , Samuels S , Zur N , Ophir R , Blumwald E , Sadka A. 2013. Effects of gibberellin treatment during flowering induction period on global gene expression and the transcription of flowering-control genes in Citrus buds. Plant Science 198, 46–57. [DOI] [PubMed] [Google Scholar]
- Harris JC , Song J , Jameson PE , Clemens J. 2009. Autonomous, environmental and exogenous gibberellin regulation of floral development and isolation of a putative partial FLORICAULA/LEAFY homologue in Phormium cookianum (Agavaceae). Plant Growth Regulation 58, 191–199. [Google Scholar]
- Heenan PB. 2000. Clianthus (Fabaceae) in New Zealand: a reappraisal of Colenso’s taxonomy. New Zealand Journal of Botany 38, 361–371. [Google Scholar]
- Henriod RE , Jameson PE , Clemens J. 2000. Effects of photoperiod, temperature and bud size on flowering in Metrosideros excelsa (Myrtaceae). Journal of Horticultural Science and Biotechnology 75, 55–61. [Google Scholar]
- Henriod RE , Jameson PE , Clemens J. 2003. Effect of irradiance during floral induction on floral initiation and subsequent development in buds of different size in Metrosideros excelsa (Myrtaceae). Journal of Horticultural Science and Biotechnology 78, 204–212. [Google Scholar]
- Horrell BA , Jameson PE , Bannister P. 1990. a Responses of ivy (Hedera helix L.) to combinations of gibberellic acid, paclobutrazol and abscisic acid. Plant Growth Regulation 9, 107–117. [Google Scholar]
- Horrell BA , Jameson PE , Bannister P. 1990. b Plant growth regulation and phase change in some New Zealand heteroblastic species. New Zealand Journal of Botany 28, 187–193. [Google Scholar]
- Jaya E , Kubien DS , Jameson PE , Clemens J. 2010. a Vegetative phase change and photosynthesis in Eucalyptus occidentalis: architectural simplification prolongs juvenile traits. Tree Physiology 30, 393–403. [DOI] [PubMed] [Google Scholar]
- Jaya ESKD , Clemens J , Song J , Zhang H , Jameson PE. 2010. b Quantitative expression analysis of meristem identity genes in Eucalyptus occidentalis: AP1 is an expression marker for flowering. Tree Physiology 30, 304–312. [DOI] [PubMed] [Google Scholar]
- Jaya E , Song J , Clemens J , Jameson PE. 2011. Effect of environment and shoot architecture on floral transition and gene expression in Eucalyptus occidentalis and Metrosideros excelsa. Plant Growth Regulation 64, 53–61. [Google Scholar]
- Kaufmann K , Wellmer F , Muino JM , et al. 2010. Orchestration of floral initiation by APETALA1. Science 328, 85–89. [DOI] [PubMed] [Google Scholar]
- Kelly D. 1994. Towards a numerical definition for divaricate (interlaced small-leaved) shrubs. New Zealand Journal of Botany 32, 509–518. [Google Scholar]
- Kelly D , Ladley JJ , Robertson AW , Anderson SH , Wotton DM , Wiser SK. 2010. Mutualisms with the wreckage of an avifauna: the status of bird pollination and fruit-dispersal in New Zealand bird pollinators. New Zealand Journal of Ecology 34, 66–85. [Google Scholar]
- Kubien DS , Jaya E , Clemens J. 2007. Differences in the structure and gas exchange physiology of juvenile and adult leaves in Metrosideros excelsa. International Journal of Plant Sciences 168, 563–570. [Google Scholar]
- Kyozuka J , Konishi S , Nemoto K , Izawa T , Shimamoto K. 1998. Down-regulation of RFL, the FLO/LFY homolog of rice, accompanied with panicle branch initiation. Proceedings of the National Academy of Sciences, USA 95, 1979–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauter N , Kampani A , Carlson S , Goebel M , Moose SP. 2005. MicroRNA172 down-regulates glossy15 to promote vegetative phase change in maize. Proceedings of the National Academy of Sciences, USA 102, 9412–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DE , Lee WG , Mortimer N. 2001. Where have all the flowers gone? Depletion and turnover in the New Zealand Cenozoic angiosperm flora in relation to palaeogeography and climate. Australian Journal of Botany 49, 341–356. [Google Scholar]
- Luo C , Bicknell RA , Heenan PB. 2003. Embryology of two threatened species of Pachycladon (Brassicaceae). New Zealand Journal of Botany 41, 171–178. [Google Scholar]
- Molloy BJP , Edgar E , Heenan PB , de Lange PJ. 1999. New species of Poa (Poaceae) and Pachycladon (Brassicaceae) from limestone, North Otago, South Island, New Zealand. New Zealand Journal of Botany 37, 41–50. [Google Scholar]
- Martin M , Jameson PE , Mark AF , Yeung EC , Pharis RP. 1993. Early panicle development in Chionochloa macra induced to flower by 2,2-dimethyl gibberellin A4 or long days. New Zealand Journal of Botany 31, 193–201. [Google Scholar]
- Mizzotti C , Galliani BM , Masiero S. 2014. The backstage of the ABC model: the Antirrhinum majus contribution. Plant Biosystems 148, 176–186. [Google Scholar]
- Oliphant JL , Clemens J , Baird HE. 1991. Accelerated maturation after micropropagation of Metrosideros cultivars by plant growth regulators and other cultural practices. Acta Horticulturae 314, 221–225. [Google Scholar]
- Philipson WR. 1964. Habit in relation to age in New Zealand trees. Journal of Indian Botanical Science 42, 167–179. [Google Scholar]
- Poethig RS. 2013. Vegetative phase change and shoot maturation in plants. Current Topics in Developmental Biology 105, 125–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler CE , Hackett WP. 1975. Phase change in Hedera helix – induction of mature to juvenile phase change by gibberellin A3. Physiologia Plantarum 34, 141–147. [Google Scholar]
- Sakata T , Oda S , Tsunaga Y , et al. 2014. Reduction of gibberellin by low temperature disrupts pollen development in rice. Plant Physiology 164, 2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon JT. 1980. The native trees of New Zealand. Auckland: Heinemann Reed. [Google Scholar]
- Simpson PG. 1994. Pōhutukawa and biodiversity. Conservation Advisory Science Notes No. 100. Wellington:Department of; Conservation [Google Scholar]
- Song J , Clemens J , Jameson PE. 2008. a Quantitative expression analysis of the ABC genes in Sophora tetraptera, a woody legume with an unusual sequence of floral organ development. Journal of Experimental Botany 59, 247–259. [DOI] [PubMed] [Google Scholar]
- Song J , Clemens J , Jameson PE. 2011. Expression of floral identity genes in Clianthus maximus during mass inflorescence abortion and floral development. Annals of Botany 107, 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J , Murdoch J , Gardiner SE , Young A , Jameson PE , Clemens J. 2008. b Molecular markers and a sequence deletion in intron 2 of the putative partial homologue of LEAFY reveal geographical structure to genetic diversity in the acutely threatened legume genus Clianthus. Biological Conservation 141, 2041–2053. [Google Scholar]
- Sooda A , Song J , Jameson PE , Clemens J. 2011. Phase change and flowering in Pachycladon exile and isolation of LEAFY and TERMINAL FLOWER1 homologues. New Zealand Journal of Botany 49, 281–293. [Google Scholar]
- Spanudakis E , Jackson S. 2014. The role of microRNAs in the control of flowering time. Journal of Experimental Botany 65, 365–380. [DOI] [PubMed] [Google Scholar]
- Sreekantan L. 2002. Molecular studies of flowering in Metrosideros excelsa (Myrtaceae). PhD thesis, Massey University. [Google Scholar]
- Sreekantan L , Clemens J , McKenzie MJ , Lenton JR , Croker SJ , Jameson PE. 2004. Flowering genes in Metrosideros fit a broad herbaceous model encompassing Arabidopsis and Antirrhinum. Physiologia Plantarum 121, 163–173. [DOI] [PubMed] [Google Scholar]
- Sreekantan L , McKenzie MJ , Jameson PE , Clemens J. 2001. Cycles of floral and vegetative development in Metrosideros excelsa (Myrtaceae). International Journal of Plant Sciences 162, 719–727. [Google Scholar]
- Teotia S , Tang G. 2015. To bloom or not to bloom: role of microRNAs in plant flowering. Molecular Plant 8, 359–377. [DOI] [PubMed] [Google Scholar]
- Wang J-W. 2014. Regulation of flowering time by the miR156-mediated age pathway. Journal of Experimental Botany 65, 4723–4730. [DOI] [PubMed] [Google Scholar]
- Wang J-W , Park MY , Wang L-J , Koo Y , Chen X-Y , Weigel D , Poethig RS. 2011. MiRNA control of vegetative phase change in trees. PLoS Genetics 7, e1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle P. 1991. Vegetation of New Zealand. Cambridge: Cambridge University Press. [Google Scholar]
- Webb CJ , Kelly D. 1993. The reproductive biology of the New Zealand flora. Trends in Ecology and Evolution 8, 442–447. [DOI] [PubMed] [Google Scholar]
- Wu G , Park MY , Conway SR , Wang JW , Weigel D , Poethig RS. 2009. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G , Poethig RS. 2006. Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133, 3539–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi N , Winter CM , Wu M-F , Kanno Y , Yamaguchi A , Seo M , Wagner D. 2014. Gibberellin acts positively then negatively to control onset of flower formation in Arabidopsis. Science 344, 638–641. [DOI] [PubMed] [Google Scholar]
- Yu S , Lian H , Wang J-W. 2015. Plant developmental transitions: the role of microRNAs and sugars. Current Opinion in Plant Biology 27, 1–7. [DOI] [PubMed] [Google Scholar]
- Zhang T-Q , Lian H , Tang H , et al. 2015. An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. The Plant Cell 27, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q-H , Helliwell CA. 2011. Regulation of flowering time and floral patterning by miR172. Journal of Experimental Botany 62, 487–495. [DOI] [PubMed] [Google Scholar]
- Zotz G , Wilhelm K , Becker A. 2011. Heteroblasty – a review. Botanical Review 77, 109–151. [Google Scholar]