Abstract
Systemic sclerosis is an autoimmune disorder characterized by inflammation and a progressive fibrosis affecting the skin and visceral organs. Over the last two decades, it became clear that oxidative stress plays a key role in its pathogenesis. In this review, we highlighted the role of ROS in the various pathological components of systemic sclerosis, namely the inflammatory, the autoimmune and the fibrotic processes. We also discussed how these pathological processes can induce ROS overproduction, thus maintaining a vicious circle. Finally, we summarized the therapeutic approaches targeting oxidative stress tested in systemic sclerosis, in cells, animal models and patients.
1. Introduction
1.1. Systemic sclerosis
Systemic sclerosis (SSc, also called scleroderma) is a rare and heterogeneous connective tissue disease with a prevalence of 50–300 cases per million people, greater in population of European ancestry and lower in Asian groups. The disease affects mostly women (male: female ratio: 3–14:1) with an annual incidence ranging from 10.9 cases/million up to 43 cases/million) [1]. This chronic inflammatory disease is characterized by vascular abnormalities, autoimmunity and fibrosis of the skin and visceral organs. Microangiopathy occurs at an early step of the SSc as almost all patients develop Raynaud phenomenon and many show other manifestations of small vessel disease, including ischemic digital ulcers, pulmonary arterial hypertension and renal arterial involvement associated with malignant hypertension and renal failure. Autoimmunity is characterized by the presence in almost all patients of autoantibodies directed against nuclear antigens with some specificity being associated with extension of skin fibrosis and certain type of visceral involvement. Other autoantibodies directed against membrane antigens from fibroblasts and endothelial cells are also frequently observed. Scleroderma is characterized by an excessive production of extracellular matrix proteins (e. g. collagen) causing progressive interstitial and perivascular fibrosis of skin and visceral organs (mainly lung, kidney and digestive tract). The extension of skin fibrosis characterizes the form of the disease as diffuse or limited. The pathophysiology of SSc is very complex, with an interaction of genetic and environmental factors. The disease needs a trigger, such as an infection or exposure to toxic and occurs on a susceptible genetic background. Furthermore, several types of cells interact during SSc development such as innate and adaptive immune cells, fibroblasts, endothelial and smooth muscle cells and are dysregulated in scleroderma.
Despite an estimated heritability quite low (about 5%) in a study including 42 sets of twins [2], having an affected first-degree relative increased the risk of SSc 13 times compared to the general population [3], thus indicating that genetic factors play an important role in SSc. As in other complex diseases, numerous genetic susceptibility loci have been identified, and for each, the relative odd ratio was quite low. However, most identified associated genes are concentrated in a few specific pathways involved in immunity, such as the HLA system, T-cell and B-cell co-stimulatory molecules, the type I interferon, the Interleukin-12 and the TNF pathway and family as well as molecules involved in the debris clearance, autophagy and various detoxification mechanisms, [4].
Exposure to toxic or infectious agents remains the major environmental factors that trigger the disease. Their pathophysiological consequences seem to involve the induction of an oxidative burst that first impacts endothelial cells leading to vascular hyperreactivity, endothelial cells apoptosis and ischemia reperfusion events which may participate in a vicious circle of ROS overproduction causing autoimmunity by ROS-induced antigen post-translational modifications. Overproduction of ROS and activation of endothelial and immune cells lead to chronic inflammation and activate fibroblasts causing aberrant wound healing and fibrosis of the skin and visceral organs. Data from animal model support this sequence of events. Indeed, a chronic oxidative stress of the skin induced by direct exposure to pro-oxidative agents such as hydroxyl radicals, hypochlorous acid or bleomycin is sufficient to induce all the feature of the disease with fibrosis of the skin and visceral organs, vascular involvement and autoimmunity [5].
1.2. ROS in SSc
In patients with SSc, an oxidative stress, as defined by an imbalance between an oxidant and an anti-oxidant states, is classically observed. Indeed, according to a recent meta-analysis, several oxidative stress biomarkers, such as malondialdehyde (MDA- a marker of lipid peroxydation), nitric oxide and endogenous nitric oxide inhibitor asymmetric dimethylarginine (ADMA) are found higher in the blood of SSc patients than in controls [6]. By contrast, anti-oxidative biomarkers, such as superoxide dismutase and vitamin C, are lower in SSc patients’ blood than in controls [6]. Oxidative-induced post-translational protein modifications, such as advanced oxidation protein products (AOPP) are also increased in the plasma of SSc patients compared to non-SSc controls [7]. In SSc, the oxidative stress biomarkers were also found elevated in other biological samples apart from blood. SSc patients have higher urinary levels of 8-Oxo-2′-deoxyguanosine (8-oxodG) and isoprostanes that are produced in vivo by free radical-catalyzed peroxidation of arachidonic acid compared to controls [8]. In addition and relevant to the visceral involvement of the disease especially in the lung, patients with systemic sclerosis exhale more hydrogen peroxide (H2O2) [9] and nitric oxide [10] compared to healthy controls. Skin autofluorescence, a method for noninvasive assessment of advanced glycation endproducts as an indirect evidence of oxidative stress, was also measured and shown to be increased in patients with SSc compared to healthy controls [11].
In addition to these easily measurable biomarkers, the cell types involved in SSc pathophysiology also show evidence of an endogenous oxidative stress. Fibroblasts extracted from either fibrotic or non fibrotic skin of patients with SSc showed higher ROS levels as compared to skin fibroblasts from healthy control [12], [13], suggesting that oxidative stress may be an early event in the disease pathogenesis. In addition to fibroblasts, high levels of ROS have also been measured ex vivo in different cell types from SSc patients like monocytes [14], T lymphocytes [15] and erythrocytes [16] compared to healthy donor cells.
In addition, hydrogen peroxide production was shown to be higher in endothelial cells and fibroblasts incubated with serum samples from SSc patients compared to healthy controls. The levels of ROS induced by SSc sera on endothelial cells and fibroblasts were increased in patients with diffuse SSc compared to patients with a limited form of the disease and was shown to be mediated by the presence of AOPP whose levels are increased in diffuse SSc and independent to levels and types of SSc auto-antibodies present in patients sera [7]. These data are consistent with the role of ROS-induced oxidized proteins on the systemic spreading of the disease.
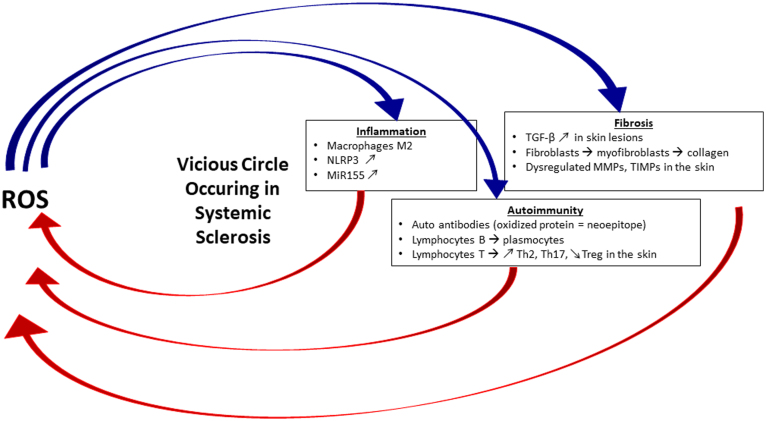
In this review, we will highlight the role of ROS in the various pathological components of SSc, namely the inflammatory, the autoimmune and the fibrotic processes, and in turn emphasize how these situations can influence the oxidative status of the cell involved in SSc (Fig. 1). We will then feature the therapeutic approaches targeting oxidative stress in SSc.
Fig. 1.
The vicious circle between ROS and the pathological components of Systemic Sclerosis.
The above figure summarizes the interplay of reactive oxygen species and the key players in the 3 different pathological components of SSc, namely inflammation, autoimmunity and fibrosis. For more details, see the corresponding subsections in the text.
2. ROS and inflammation in SSc
2.1. ROS and Macrophages in SSc
Macrophages are central in inflammation with the first responder being the pro-inflammatory M1 macrophages (classically activated) that are reacting to pathogen or sterile tissue injury through phagocytosis and by secreting pro-inflammatory cytokines and recruiting other immune cells, followed by the alternatively activated M2 macrophages that are responsible for the inflammation resolution with the pro-fibrotic wound-healing properties. As this dual model is oversimplified, and macrophages are able to display both M1 and M2 markers as well as numerous intermediate states, it is not surprising that signature of both M1 and M2 macrophages were described in SSc [17], [18]. However, several studies point toward the involvement of the “M2-type” monocyte/macrophages from the blood and affected tissues in the fibrotic tissue of SSc patients [19], [20]. Compelling evidence of macrophage involvement also comes from gene expression analysis in an hypotheses-free approach, of SSc tissues compared to one another or to healthy skin that revealed macrophage signatures [20], [21].
Oxidative stress is important for macrophage polarization, especially for M2 differentiation that is blocked by an inhibition of superoxide production [22]. Interestingly, STAT6 was shown to be involved in M2 macrophage polarization [23], and can be activated by ROS in T cells [24]. Thus it can be hypothesized that ROS may be responsible for the M2 polarization via STAT6 induction in SSc. Consistent with this hypothesis, leflunomide, a STAT6 inhibitor, prevented SSc symptoms in the HOCl and bleomycin induced mouse models of SSc, and restored polarization of macrophages from M2 into M1 [25].
2.2. ROS and inflammasome in SSc
In addition to macrophages, other cells are involved in inflammation (fibroblasts, endothelial cells) through their inflammasome, that was also described as involved in SSc [26]. In particular, it was shown that NRLP3 was overexpressed in SSc skin biopsies [27], and was involved in collagen synthesis by fibroblasts through the microRNA miR-155 induction [28]. NLRP3 absence (knockout model) prevents skin fibrosis development in the bleomycin-induced SSc in mice [29].
Oxidative stress could also modulate NLRP3 activation [30]. Indeed, the crystal structure of NLRP3 suggests that this molecule is highly sensitive to altered redox states [31]. Indeed, in podocytes, NLRP3 is activated through NADPH oxidase activation and superoxide production, whereas a SOD mimetic and the H2O2 scavenger catalase reduced NLRP3 activation [32]. In endothelial cells, a positive correlation was also observed between ROS production and NLRP3 activation [33]. Thus, if this hypothesis has not been explored so far, it is tempting to speculate that NLRP3 activation in SSc may be due to oxidative stress. Interestingly, silencing miR-155 in an endothelial cell line decreased ROS production [34]. Treatment of liver cells with catalase decreased miR-155 expression and substantially increased Nrf2 expression, whereas blocking Nrf2 increased miR-155 expression [35]. These data suggest that miR-155 could inhibit Nrf2 leading to ROS overproduction and NLRP3 activation, this way contributing to SSc pathogenesis.
3. ROS and autoimmunity in SSc
3.1. ROS and autoantibodies in SSc
It has been shown that 95% of patients with systemic sclerosis have circulating antinuclear autoantibodies (AAbs) in the blood. The most frequent are the anti-topoisomerase I (ATA) and the anti-centromere (CENP) AAbs. These AAbs are a serological hallmark of SSc and are used as biomarkers for establishing an early and accurate diagnosis [36]. However, most of the autoantibodies used for diagnosis don’t have proven pathogenic features but some are associated with specific clinical involvement. Of note, the ATA is associated with a diffuse form of SSc with increased mortality with pulmonary fibrosis, musculoskeletal and cardiac involvement [37]. Several other AAbs not specific for nuclear antigens have been described in SSc such as anti-fibroblasts and anti-endothelial cells, anti-fibrillin-1, anti-PDGF receptor, anti-endothelin type A receptor and anti-angiotensin II type I receptors, but their detection is highly variable among patients. Some of them are thought to be involved in the pathogenesis of SSc, but the specific involvement of each one is beyond the scope of this review (see [38] for more details about this aspect).
As already mentioned above, it has been suggested that oxidative stress plays an important role in the pathophysiology of scleroderma, and this role could include its impact on the autoimmune component of the disease. A study in mice showed that oxidation of the DNA topoisomerase I autoantigen is sufficient to break the tolerance towards this antigens leading to autoimmunity with the production of anti-topoisomerase I autoantibody and the accentuation of the disease by increasing fibroblast proliferation and type I collagen mRNA synthesis, as well as by increasing H2O2 production by endothelial cells [5]. This suggests that H2O2-induced protein oxidation can create neoepitopes that can lead to autoantibodies production. This was substantiated by a study from Casiola-Rosen L et al. showing that oxidation of DNA topoisomerase I by the Fenton reaction was able to modify this antigen and to increase its immunoreactivity towards anti-DNA topisomerase I Abs found in sera from SSc patients [39].
In addition, some autoantibodies in SSc recognize antioxidant enzymes. As an example, anti-peroxiredoxin and anti-methionine sulfoxide reductase A (MSRA) are detected at high level in 33% of SSc patients [40], [41]. The anti-peroxiredoxin I is associated with the severity of the disease. Peroxiredoxin have a peroxidase activity that can notably reduce hydrogen peroxide. The presence of antiperoxiredoxin I autoantibodies in the serum of SSc patient induce a 59% inhibition of peroxiredoxin I enzymatic activity. Anti-MSRA autoantibody is associated with pulmonary fibrosis and longer disease duration. MSRs have many functions, including the inactivation of oxidized proteins by methionine reduction. The anti-MSRA autoantibody is also able to inhibit the target enzyme activity by about 50%. Altogether, these autoantibodies inhibit the function of the recognized anti-oxydant enzymes and could then participate in the chronic oxidative stress observed in SSc.
Anti-PDGF receptors AAbs are also observed in a large proportion of patients with diffuse SSc. These AAbs are able to activate the PDGF receptors on fibroblasts and to activate the downstream MAP kinase pathways leading to overproduction of ROS that amplify ERK phosphorylation and participate in myofibroblasts conversion and collagen synthesis [42]. Furthermore, other AAbs found in SSc patients are also able to induce ROS production, maintaining this vicious circle in SSc. It is the case of an agonistic anti-ICAM-1 antibody, which binds to the adhesion molecule ICAM-1 on the surface of human endothelial cells inducing ROS production in vitro [43].
3.2. ROS, T and B cells in SSc
In addition to antigens, ROS can also have a direct impact on B and T cells differentiation and activation. Indeed, a study has showed that imbalance between ROS production and antioxidant systems activity have high impacts on the survival and the differentiation of B lymphocytes [44]. Particularly, normal plasmocytes differentiation is associated with a silencing of several anti-oxidant enzymes, such as Gpx1 and Catalase [45]. Therefore, the oxidative stress observed in SSc could promote the differentiation of B-cell into plasmocytes and lead to an increased antibody production, and amongst them, autoantibodies. Consistent with this idea, the level of immunoglobulin light chains in sera was showed to be increased by 1.3 fold in SSc patients compared to healthy controls in 2 independent studies [46], [47].
T-cells have also been described as involved in SSc [48]. A compelling evidence of their involvement is shown by an attenuation of the disease following depletion of CD3 + T cells in the bleomycin-induced SSc mouse model [49]. Data from human also strongly support a role for T cell in SSc, as exemplified by the clinical improvement induced by treatments targeting those cells like anti-CD52, anti-human thymocyte globulin and anti-CD25 Abs [50], [51], [52]. Th2-type cytokines such as IL-4 and IL-13 are increased in SSc [48]. These cytokines are first secreted by alternately activated macrophages, which induce lymphocytes differentiation into the Th2 subset responsible for activating macrophages inducing an activation loop between those two immune cells. Consistent with these data, an increase number of circulating Th2 lymphocytes subset is also observed in SSc, and correlates with the severity of lung fibrosis [53]. Surprisingly, ROS limits Th2 differentiation. Indeed a depletion of the Gpx1 anti-oxidant enzymes induces a decrease differentiation of T lymphocytes into the Th2 subset [54] but in the context of SSc, ROS seems to be unable to limit Th2 differentiation. An increase number of Th17 lymphoctes has also been shown in the blood, in the dermis and in the lung of patients with SSc. While variable data were found for circulating Treg cells [55] they were found decreased in the skin lesions of SSc patients [56]. As in SSc, a Th17/Treg imbalance has been observed in other inflammatory autoimmune skin diseases and restoring Th17 and Treg levels is a strategy used for therapeutic purpose in psoriasis [57]. Oxidative stress could favor Th17 differentiation while limiting Treg differentiation as suggested by the increase of Treg cells and the decrease of Th17 cells observed following a treatment with the potent antioxidant proanthocyanidins in mice [58], [57]. Therefore, targeting the oxidative stress in SSc may restore the Th17/Treg imbalance and subsequently decrease both inflammation and autoimmunity observed in SSc.
4. ROS and fibrosis in SSc
4.1. ROS, fibroblasts and TGF-β signaling in SSc
Fibrosis is an important clinical hallmark of systemic sclerosis and remains the major cause of organ failure and mortality of this disease [59]. This excessive deposition of collagen and extracellular matrix (ECM) components in tissues is the consequence of an overactivation of fibroblasts and their differentiation into myofibroblasts in response to chronic inflammation or cell injury [60]. In SSc, fibrotic process is thought to be initiated by vascular injury with ischemia reperfusion phenomena which is accompanied by an overactivation of NADPH oxidases (NOX) in endothelial cells and an important release of ROS [61]. Increased production of ROS is responsible for fibroblasts activation and triggers the production of pro-inflammatory cytokines from immune cells such as IL-1β that in turn modulate the activity of ROS [62], [63]. Also, fibroblasts from SSc have been shown to be a potent source of ROS through an up-regulation of the isoforms 2 and 4 of NOX protein suggesting the existence of an active loop that maintain an overproduction of ROS in SSc [64].
Emerging evidence indicates that ROS modulate the TGF-β pathway by MAPK activation and incidentally through ERK mediated SMAD phosphorylation [65]. TGF-β is a potent pro-fibrotic cytokine that is involved in most fibrotic processes. TGF-β drives the chronic activation of fibroblasts and their differentiation into ECM-producing myofibroblats in SSc [66] and increases the synthesis of IL-13 by T cells that in turns stimulate the collagen production by fibroblasts from SSc patients [67]. Elevated expression of the three isoforms of TGF-β has been shown in the skin of SSc patients and the blockade of the TGF-β receptor impairs the SMAD signaling pathway and attenuates bleomycin-induced pulmonary fibrosis in mice [68]. However, data on circulating level of the active isoform of TGF-β are controversial as it was showed to be decreased in SSc patients in one study [69] and increased in another study [70]. Accumulating data support the notion that TGF-β increases the generation of ROS by impairment of mitochondrial function with increase of O2 consumption [71], NADPH oxidases induction [72], and suppression of glutathione synthesis [73] thus leading to a subsequent oxidative stress imbalance. This interplay between TGF-β and ROS is a major cause of the vicious circle that maintains and enforces the fibrotic process in SSc.
4.2. ROS, MMPs and TIMPs in SSc
Altered degradation of ECM may also be involved in fibrotic disorders. ECM components remodeling is mediated by matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases, TIMPs). One study showed that serum levels of MMP-9 and TIMP-1 were 3- to 4-fold more elevated in SSc patients compared to controls and correlated with circulating TGF-β and disease severity [74]. Another study showed that skin lesions from patients with diffuse cutaneous SSc have decreased MMP-9 but elevated TIMP-1 expression compared to controls [75] suggesting that the data are dependent on the stage of the disease and the explored tissue. MMP10 was showed to be overexpressed in the serum and pulmonary arteries of patients with SSc-associated pulmonary hypertension [76]. MMP-12 levels were shown to be increased in patients with SSc and associated with severity of skin and pulmonary fibrosis as well as with peripheral vascular damage [77]. Serum MMP-13 levels is lower in SSc patients, compared to healthy controls [78]. MMPs and TIMPs involvement in SSc is further substantiated by several genetic associations of SNP in MMP and TIMP genes (for review, see [79]). The influence of ROS in the MMP and TIMP production was not investigated in the context of SSc. However, ROS seems to play a key role in modulating the activity of MMPs through stimulation of MAPK pathway [80], [81], [82]. Indeed, increased ROS levels in endothelial and keratinocyte cell lines were associated with the activation of the MAPKs (ERK and JNK), and the induction of MMP-9 at the mRNA and protein levels. Molecules targeting the ROS (ROS scavenger, SOD, NOX inhibitor) prevented the MAPK activation and thus the resulting MMP-9 induction. The stimuli used in these studies are different (heat shock, cadmium), but they demonstrate a general feature implicating ROS in the regulation of MMPs expression and activity. Furthermore, the study using Cadmium may be especially relevant for SSc as it was demonstrated that SSc patients exhibit higher levels of cadmium in their hair in a case control study [83]. ROS are also involved in the activation of latent MMPs pro-enzymes through the proteolysis of the cystein-zinc active pro-enzymatic site [84] and mediate the IL-1 β-dependent MMP-9 induction [85]. ROS may then be involved in the imbalance in MMPs and their inhibitors in SSc patients and participate in the observed ECM remodeling with a maintained collagen production in diseased fibroblasts. All these data support a role of oxidative stress at multiple levels in SSc fibrosis.
5. Targeting oxidative stress to treat SSc
As oxydative stress impacts all aspects of the pathophysiology of SSc, it constitutes an interesting therapeutic target (Table 1). Despite a reduced concentration of classical antioxidants, such as antioxidant vitamins (ascorbic acid, α-tocopherol, and β-carotene) and minerals (zinc, selenium) in SSc patients, there is little evidence that their supplementation have beneficial effect on SSc development [86]. A 6-month supplementation in α-tocopherol and ascorbic acid lead to a reduced skin thickening in a small group of patients with early diffuse SSc [87]. Other studies performed on shorter time period (2 h to 10 weeks) failed to demonstrate any beneficial effects of these antioxidants [88], [89], [90]. Amongst the possible explanations for these lacks of efficacy, the advanced stage of the disease, a malabsorption issues and an increased clearance in SSc patients were mentioned. Epigallocatechin-3-gallate (EGCG) is an antioxidant polyphenol present in green tea extracts (Camellia sinensis). It was shown to decrease oxidative stress in SSc fibroblasts [91], and to reduce bleomycin-induced pulmonary fibrosis in rats [92]. However, its clinical value remains to be explored. Pantethine, a derivative of vitamin B5, was shown to reduce oxidative stress in endothelial cells in vitro and in fibroblasts both in vitro and ex vivo from mice with HOCl-induced SSc [93]. In vivo, pantethine also reduced HOCl induced skin and lung fibrosis.
Table 1.
Summary of the therapies targeting the oxidative stress for systemic sclerosis.
| Molecules or strategy | Experimental conditions | Results | References |
|---|---|---|---|
| α-tocopherol and ascorbic acid | SSc patients (6-month treatment in early diffuse SSc patients) | Reduced skin thickening | Ostojic and Damjanov, 2011 |
| SSc patients (shorter treatment, more advanced disease) | No beneficial effects | Cracowski and al, 2005 / Herrick and al, 2000/ Mavrikakis and al, 2003 | |
| Epigallocatechin-3-gallate (EGCG) | SSc human fibroblasts | Decreased oxidative stress | Dooley and al, 2010 |
| Bleomycin treated rats | Decreased fibrosis | Sriram and al, 2009 | |
| Pantethine | Endothelial cells and fibroblasts from HOCl-induced SSc mice | Decreased oxidative stress | Kavian and al, 2015 |
| HOCl-induced SSc mice | Decreased fibrosis in skin and lung | ||
| Propylthiouracil (PTU) | HOCl-induced SSc mice | Decreased aortic thickening and reduced myofibroblast differentiation | Bagnato and al, 2015 |
| N-acetylcysteine (NAC) | SSc patients (intravenous treatment) | Reduced ischemic ulcers | Rosato et al., 2009 |
| Sambo and al, 2001a | |||
| Improved lung function | Rosato et al., 2011 | ||
| Inhibit fibroblast proliferation and collagen synthesis | Sambo and al, 2001b/ Servettaz and al, 2007 | ||
| Activated lung macrophages from SSc patients | Decreased peroxynitrite production | Failli and al, 2002 | |
| Bleomycin-induced SSc mice | Decrease oxidative stress and fibrosis | Zhou and al, 2013 | |
| NOX inhibition (NOX4 siRNA and pan-Nox inhibitor) | Fibroblasts from SSc patients | Decrease ROS and collagen production | Piera-Velazquez and al, 2015 |
| Bleomycin-induced SSc mice | Decrease skin fibrosis and myofibroblasts activation | Dosoki and al, 2017 | |
| Dimethyl fumarate (DMF) | Bleomycin-induced SSc mice | Reduced skin fibrosis | Toyama et al., 2018 |
| HOCl-induced SSc mice | Decreased fibrosis and immune activation | Kavian and al, 2018 | |
| Fibroblasts from the skin of HOCl-induced mice | Increased Glutathione, and reduced ROS production and cell proliferation | ||
| SSc patients | Not available yet | ClinicalTrials.gov NCT02981082 |
Propylthiouracil (PTU), an antithyroid drug and an inhibitor of lipid peroxidation, was showed to prevent aortic thickening and myofibroblast differentiation, thus reducing macrovascular alterations in the HOCl induced SSc mice model [94]. However, data on SSc patients are lacking.
N-acetylcysteine (NAC), a scavenger of free radicals and a precursor of the major antioxidant glutathione, was shown to be beneficial for SSc patients when administered intravenously in several studies [95], [96], [97], but given orally, it failed to have favourable effects [98]. NAC inhibits fibroblast proliferation and collagen synthesis [7], [99] and reduces peroxynitrite production by activated lung macrophages from SSc patients in vitro [100]. In the bleomycin-induced SSc mice model, NAC treatment diminished oxidative stress and attenuates skin fibrosis [101].
As NOX-4 expression and production were found increased in SSc skin and cultured SSc skin fibroblasts, the effect of NOX-4 inhibition were also investigated. A small-molecule NOX-4 inhibitor decreased collagen and fibronectin production by normal and SSc fibroblasts, and NOX-4 siRNA knockdown reduced ROS and collagen production by SSc fibroblasts [72]. In the bleomycin-induced SSc mouse model, pan-Nox pharmacological inhibition or genetic silencing of Nox-4 by siRNA attenuated skin fibrosis and myofibroblast activation [102]. Nox-4 knockdown also reduced skin collagen synthesis, α-SMA and fibronectin 1 expression in vivo. Following the demonstration of a positive effect of a NOX-1 and NOX-4 inhibitor (GKT831, Genkyotex) in several mouse models of fibrosis and encouraging data on its safety [103], its clinical investigation in the context of SSc are expected to begin in the upcoming years.
Dimethyl Fumarate (DMF), a FDA-approved anti-oxidative and anti-inflammatory agent, was shown to have beneficial effect on SSc in two independent studies in mice [104], [105]. It prevented bleomycin-induced skin fibrosis in mice, and blocked the pro-fibrotic effects of transforming growth factor-β (TGF-β) in SSc skin fibroblasts, through its action on the TGF-β/Akt1 pathway leading to degradation of TAZ and subsequent inhibition of the TAZ/YAP transcriptional targets [105]. The increased level of TAZ/YAP detected in SSc skin biopsies suggests that this treatment could also work in patients. In addition, DMF acts through its agonist effect on Nrf2, the master transcriptional regulator of anti-oxidant genes. In HOCl-induced SSc mice, DMF reduced fibrosis and immune activation [104]. The ex vivo treatment of skin fibroblasts from HOCl-induced SSc mice with DMF restores GSH intracellular content, decreases ROS production and cell proliferation. Nrf2 regulates GSH gene and Nrf2 knockout mice display an aggravated SSc phenotype upon HOCl treatment. DMF induced Nrf2 gene expression as well as its target genes in the HOCl-induced SSc mouse model, as well as in the SSc skin fibroblast derived from patients (but not from normal human skin fibroblasts). As a drop in NRF2 expression and target genes mRNA levels was observed in skin fibroblasts of SSc patients, it also suggests that DMF treatment could be beneficial in patients. A Phase 1 double-blinded, placebo-controlled clinical trial on Systemic Sclerosis patients with Pulmonary Hypertension is ongoing and should be completed in 2020 [106].
6. Conclusion
Systemic sclerosis is a connective tissue disease characterized by vascular abnormalities, fibrosis of the skin and visceral organs and autoimmunity mainly against nuclear antigens. ROS play a crucial role in the pathophysiology of scleroderma, as they remain one of the major environmental triggers of the disease through toxic exposure of infection. ROS acts on all cellular targets of SSc. They activate endothelial cells leading to vascular hyperreactivity, endothelial cells apoptosis and impaired angiogenesis. They act on differentiation and proliferation of fibroblasts and on their synthesis of ECM proteins leading to fibrosis. They favor autoimmunity and chronic inflammation through the genesis of neoepitopes and the activation of B and T lymphocytes and macrophages. ROS appears as the master regulator of this severe disease and because scleroderma is a prototypical inflammatory and fibrotic disease that includes for example psoriasis, rheumatoid arthritis, Crohn's disease, ulcerative colitis, myelofibrosis, interstitial lung disease or liver cirrhosis, the pathological features observed in scleroderma may be extended for many of those frequent disorders as well as therapeutic opportunities using ROS modulators.
References
- 1.Gabrielli A., Avvedimento E.V., Krieg T. Scleroderma. N. Engl. J. Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Feghali-Bostwick C., Medsger T.A., Wright T.M. Analysis of systemic sclerosis in twins reveals low concordance for disease and high concordance for the presence of antinuclear antibodies. Arthritis Rheum. 2003;48:1956–1963. doi: 10.1002/art.11173. [DOI] [PubMed] [Google Scholar]
- 3.Arnett F.C. Familial occurrence frequencies and relative risks for systemic sclerosis (scleroderma) in three United States cohorts. Arthritis Rheum. 2001;44:1359–1362. doi: 10.1002/1529-0131(200106)44:6<1359::AID-ART228>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Bossini-Castillo L., López-Isac E., Mayes M.D., Martín J. Genetics of systemic sclerosis. Semin. Immunopathol. 2015;37:443–451. doi: 10.1007/s00281-015-0499-z. [DOI] [PubMed] [Google Scholar]
- 5.Servettaz A. Selective oxidation of DNA topoisomerase 1 induces systemic sclerosis in the mouse. J. Immunol. Baltim. Md 1950. 2009;182:5855–5864. doi: 10.4049/jimmunol.0803705. [DOI] [PubMed] [Google Scholar]
- 6.Luo J.-Y., Liu X., Jiang M., Zhao H.-P., Zhao J.-J. Oxidative stress markers in blood in systemic sclerosis: a meta-analysis. Mod. Rheumatol. 2017;27:306–314. doi: 10.1080/14397595.2016.1206510. [DOI] [PubMed] [Google Scholar]
- 7.Servettaz A. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann. Rheum. Dis. 2007;66:1202–1209. doi: 10.1136/ard.2006.067504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avouac J., Borderie D., Ekindjian O.G., Kahan A., Allanore Y. High DNA oxidative damage in systemic sclerosis. J. Rheumatol. 2010;37:2540–2547. doi: 10.3899/jrheum.100398. [DOI] [PubMed] [Google Scholar]
- 9.Łuczyñska M. Elevated exhalation of hydrogen peroxide in patients with systemic sclerosis. Eur. J. Clin. Invest. 2003;33:274–279. doi: 10.1046/j.1365-2362.2003.01138.x. [DOI] [PubMed] [Google Scholar]
- 10.Tiev K.P. Exhaled nitric oxide, but not serum nitrite and nitrate, is a marker of interstitial lung disease in systemic sclerosis. Nitric Oxide Biol. Chem. 2009;20:200–206. doi: 10.1016/j.niox.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Murray A.K., Moore T.L., Manning J.B., Griffiths C.E.M., Herrick A.L. Noninvasive measurement of skin autofluorescence is increased in patients with systemic sclerosis: an indicator of increased advanced glycation endproducts? J. Rheumatol. 2012;39:1654–1658. doi: 10.3899/jrheum.111359. [DOI] [PubMed] [Google Scholar]
- 12.Tsou P.-S. Effect of oxidative stress on protein tyrosine phosphatase 1B in scleroderma dermal fibroblasts. Arthritis Rheum. 2012;64:1978–1989. doi: 10.1002/art.34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourji K. High reactive oxygen species in fibrotic and nonfibrotic skin of patients with diffuse cutaneous systemic sclerosis. Free Radic. Biol. Med. 2015;87:282–289. doi: 10.1016/j.freeradbiomed.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Sambo P. Monocytes of patients wiht systemic sclerosis (scleroderma spontaneously release in vitro increased amounts of superoxide anion. J. Invest. Dermatol. 1999;112:78–84. doi: 10.1046/j.1523-1747.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 15.Amico D. Intracellular free radical production by peripheral blood T lymphocytes from patients with systemic sclerosis: role of NADPH oxidase and ERK1/2. Arthritis Res. Ther. 2015;17:68. doi: 10.1186/s13075-015-0591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devrim E. Malondialdehyde and nitric oxide levels in erythrocytes from patients with systemic sclerosis. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2008;17:349–350. doi: 10.1159/000129620. [DOI] [PubMed] [Google Scholar]
- 17.Stifano G., Christmann R.B. Macrophage involvement in systemic sclerosis: do we need more evidence? Curr. Rheumatol. Rep. 2016;18:2. doi: 10.1007/s11926-015-0554-8. [DOI] [PubMed] [Google Scholar]
- 18.Trombetta A.C. A circulating cell population showing both M1 and M2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir. Res. 2018;19:186. doi: 10.1186/s12931-018-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frantz C., Pezet S., Avouac J., Allanore Y. Soluble CD163 as a potential biomarker in systemic sclerosis. Dis. Markers. 2018;2018:8509583. doi: 10.1155/2018/8509583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taroni J.N. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 2017;9:27. doi: 10.1186/s13073-017-0417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assassi S. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheumatol. Hoboken NJ. 2015;67:3016–3026. doi: 10.1002/art.39289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor N. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J. Immunol. Baltim. Md 1950. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H.J. Exogenous hydrogen peroxide induces lipid raft-mediated STAT-6 activation in T Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;42:2467–2480. doi: 10.1159/000480210. [DOI] [PubMed] [Google Scholar]
- 25.Morin F. Leflunomide prevents ROS-induced systemic fibrosis in mice. Free Radic. Biol. Med. 2017;108:192–203. doi: 10.1016/j.freeradbiomed.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Henderson J., O’Reilly S. Inflammasome lights up in systemic sclerosis. Arthritis Res. Ther. 2017;19:205. doi: 10.1186/s13075-017-1420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Godínez M.A. Expression of NLRP3 inflammasome, cytokines and vascular mediators in the skin of systemic sclerosis patients. Isr. Med. Assoc. J. IMAJ. 2015;17:5–10. [PubMed] [Google Scholar]
- 28.Artlett C.M., Sassi-Gaha S., Hope J.L., Feghali-Bostwick C.A., Katsikis P.D. Mir-155 is overexpressed in systemic sclerosis fibroblasts and is required for NLRP3 inflammasome-mediated collagen synthesis during fibrosis. Arthritis Res. Ther. 2017;19:144. doi: 10.1186/s13075-017-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Artlett C.M. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum. 2011;63:3563–3574. doi: 10.1002/art.30568. [DOI] [PubMed] [Google Scholar]
- 30.Abais J.M., Xia M., Zhang Y., Boini K.M., Li P.-L. Redox regulation of NLRP3 inflammasomes: ros as trigger or effector? Antioxid. Redox Signal. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae J.Y., Park H.H. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J. Biol. Chem. 2011;286:39528–39536. doi: 10.1074/jbc.M111.278812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abais J.M. Contribution of endogenously produced reactive oxygen species to the activation of podocyte NLRP3 inflammasomes in hyperhomocysteinemia. Free Radic. Biol. Med. 2014;67:211–220. doi: 10.1016/j.freeradbiomed.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y. Matrine suppresses AGE-induced HAEC injury by inhibiting ROS-mediated NRLP3 inflammasome activation. Eur. J. Pharmacol. 2018;822:207–211. doi: 10.1016/j.ejphar.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y. MicroRNA-155 Regulates ROS Production, NO Generation, apoptosis and multiple functions of human brain Microvessel endothelial cells under physiological and pathological conditions. J. Cell. Biochem. 2015;116:2870–2881. doi: 10.1002/jcb.25234. [DOI] [PubMed] [Google Scholar]
- 35.Wan C. Role of miR-155 in fluorooctane sulfonate-induced oxidative hepatic damage via the Nrf2-dependent pathway. Toxicol. Appl. Pharmacol. 2016;295:85–93. doi: 10.1016/j.taap.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Kayser C., Fritzler M.J. Autoantibodies in systemic sclerosis: unanswered questions. Front. Immunol. 2015;6:167. doi: 10.3389/fimmu.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehra S., Walker J., Patterson K., Fritzler M.J. Autoantibodies in systemic sclerosis. Autoimmun. Rev. 2013;12:340–354. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Choi M.Y., Fritzler M.J. Progress in understanding the diagnostic and pathogenic role of autoantibodies associated with systemic sclerosis. Curr. Opin. Rheumatol. 2016;28:586–594. doi: 10.1097/BOR.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Casciola-Rosen L., Wigley F., Rosen A. Scleroderma autoantigens are uniquely fragmented by metal-catalyzed oxidation reactions: implications for pathogenesis. J. Exp. Med. 1997;185:71–79. doi: 10.1084/jem.185.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iwata Y. Autoantibody against peroxiredoxin I, an antioxidant enzyme, in patients with systemic sclerosis: possible association with oxidative stress. Rheumatol. Oxf. Engl. 2007;46:790–795. doi: 10.1093/rheumatology/kem010. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa F. Autoantibody against one of the antioxidant repair enzymes, methionine sulfoxide reductase A, in systemic sclerosis: association with pulmonary fibrosis and vascular damage. Arch. Dermatol. Res. 2010;302:27–35. doi: 10.1007/s00403-009-0996-9. [DOI] [PubMed] [Google Scholar]
- 42.Baroni S.S. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N. Engl. J. Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
- 43.Wolf S.I., Howat S., Abraham D.J., Pearson J.D., Lawson C. Agonistic anti-ICAM-1 antibodies in scleroderma: activation of endothelial pro-inflammatory cascades. Vasc. Pharmacol. 2013;59:19–26. doi: 10.1016/j.vph.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bertolotti M., Sitia R., Rubartelli A. On the redox control of B lymphocyte differentiation and function. Antioxid. Redox Signal. 2012;16:1139–1149. doi: 10.1089/ars.2011.4252. [DOI] [PubMed] [Google Scholar]
- 45.Bertolotti M. B- to plasma-cell terminal differentiation entails oxidative stress and profound reshaping of the antioxidant responses. Antioxid. Redox Signal. 2010;13:1133–1144. doi: 10.1089/ars.2009.3079. [DOI] [PubMed] [Google Scholar]
- 46.Bosello S. Free light chains of immunoglobulins in patients with systemic sclerosis: correlations with lung involvement and inflammatory milieu. J. Clin. Pathol. 2018;71:620–625. doi: 10.1136/jclinpath-2017-204656. [DOI] [PubMed] [Google Scholar]
- 47.Lanteri A. Serum free light chains of immunoglobulins as biomarkers for systemic sclerosis characteristics, activity and severity. Autoimmun. Rev. 2014;13:974–980. doi: 10.1016/j.autrev.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 48.O’Reilly S., Hügle T., van Laar J.M. T cells in systemic sclerosis: a reappraisal. Rheumatol. Oxf. Engl. 2012;51:1540–1549. doi: 10.1093/rheumatology/kes090. [DOI] [PubMed] [Google Scholar]
- 49.Huaux F. Eosinophils and T lymphocytes possess distinct roles in bleomycin-induced lung injury and fibrosis. J. Immunol. Baltim. Md 1950. 2003;171:5470–5481. doi: 10.4049/jimmunol.171.10.5470. [DOI] [PubMed] [Google Scholar]
- 50.Becker M.O. The monoclonal anti-CD25 antibody basiliximab for the treatment of progressive systemic sclerosis: an open-label study. Ann. Rheum. Dis. 2011;70:1340–1341. doi: 10.1136/ard.2010.137935. [DOI] [PubMed] [Google Scholar]
- 51.Isaacs J.D. Monoclonal antibody therapy of diffuse cutaneous scleroderma with CAMPATH-1H. J. Rheumatol. 1996;23:1103–1106. [PubMed] [Google Scholar]
- 52.Stratton R.J., Wilson H., Black C.M. Pilot study of anti-thymocyte globulin plus mycophenolate mofetil in recent-onset diffuse scleroderma. Rheumatol. Oxf. Engl. 2001;40:84–88. doi: 10.1093/rheumatology/40.1.84. [DOI] [PubMed] [Google Scholar]
- 53.Tang J., Lei L., Pan J., Zhao C., Wen J. Higher levels of serum interleukin-35 are associated with the severity of pulmonary fibrosis and Th2 responses in patients with systemic sclerosis. Rheumatol. Int. 2018;38:1511–1519. doi: 10.1007/s00296-018-4071-8. [DOI] [PubMed] [Google Scholar]
- 54.Won H.Y. Glutathione peroxidase 1 deficiency attenuates allergen-induced airway inflammation by suppressing Th2 and Th17 cell development. Antioxid. Redox Signal. 2010;13:575–587. doi: 10.1089/ars.2009.2989. [DOI] [PubMed] [Google Scholar]
- 55.Mo C., Zeng Z., Deng Q., Ding Y., Xiao R. Imbalance between T helper 17 and regulatory T cell subsets plays a significant role in the pathogenesis of systemic sclerosis. Biomed. Pharmacother. Biomedecine Pharmacother. 2018;108:177–183. doi: 10.1016/j.biopha.2018.09.037. [DOI] [PubMed] [Google Scholar]
- 56.Klein S. Reduction of regulatory T cells in skin lesions but not in peripheral blood of patients with systemic scleroderma. Ann. Rheum. Dis. 2011;70:1475–1481. doi: 10.1136/ard.2009.116525. [DOI] [PubMed] [Google Scholar]
- 57.Lai R. Proanthocyanidins: novel treatment for psoriasis that reduces oxidative stress and modulates Th17 and Treg cells. Redox Rep. Commun. Free Radic. Res. 2018;23:130–135. doi: 10.1080/13510002.2018.1462027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park M.-K. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3(+) regulatory and IL-17(+) pathogenic T cell in autoimmune arthritis. Immunol. Lett. 2011;135:50–58. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 59.Herrick A.L. Systemic sclerosis: clinical features and management. Med. (Baltim.) 2018;46:131–139. [Google Scholar]
- 60.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pendyala S. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid. Redox Signal. 2009;11:747–764. doi: 10.1089/ars.2008.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lum H., Roebuck K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001;280:C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 63.Naik E., Dixit V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spadoni T. A reactive oxygen species-mediated loop maintains increased expression of NADPH oxidases 2 and 4 in skin fibroblasts from patients with systemic sclerosis. arthritis Rheumatol. Hoboken NJ. 2015;67:1611–1622. doi: 10.1002/art.39084. [DOI] [PubMed] [Google Scholar]
- 65.Rhyu D.Y. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J. Am. Soc. Nephrol. JASN. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 66.Midgley A.C. Transforming growth factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast differentiation is mediated by hyaluronan (HA)-facilitated epidermal growth factor receptor (EGFR) and CD44 co-localization in lipid rafts. J. Biol. Chem. 2013;288:14824–14838. doi: 10.1074/jbc.M113.451336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baraut J. Transforming growth factor-β increases interleukin-13 synthesis via GATA-3 transcription factor in T-lymphocytes from patients with systemic sclerosis. Arthritis Res. Ther. 2015;17:196. doi: 10.1186/s13075-015-0708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Higashiyama H. Inhibition of activin receptor-like kinase 5 attenuates bleomycin-induced pulmonary fibrosis. Exp. Mol. Pathol. 2007;83:39–46. doi: 10.1016/j.yexmp.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Dziadzio M., Smith R.E., Abraham D.J., Black C.M., Denton C.P. Circulating levels of active transforming growth factor beta1 are reduced in diffuse cutaneous systemic sclerosis and correlate inversely with the modified Rodnan skin score. Rheumatol. Oxf. Engl. 2005;44:1518–1524. doi: 10.1093/rheumatology/kei088. [DOI] [PubMed] [Google Scholar]
- 70.Dantas A.T. Reassessing the role of the active TGF-β1 as a biomarker in systemic sclerosis: association of serum levels with clinical manifestations. Dis. Markers. 2016;2016:6064830. doi: 10.1155/2016/6064830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abe Y., Sakairi T., Beeson C., Kopp J.B. TGF-β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway. Am. J. Physiol. Ren. Physiol. 2013;305:F1477–F1490. doi: 10.1152/ajprenal.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piera-Velazquez S., Makul A., Jiménez S.A. Increased expression of NAPDH oxidase 4 in systemic sclerosis dermal fibroblasts: regulation by transforming growth factor β. arthritis Rheumatol. Hoboken NJ. 2015;67:2749–2758. doi: 10.1002/art.39242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu R.-M. Transforming growth factor β suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic. Biol. Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu M.-H. Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis. Am. J. Pathol. 2005;167:1061–1069. doi: 10.1016/S0002-9440(10)61195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meng C., Chen X., Li J., Wu Y., Liu H. Expression of MMP-9 and TIMP-1 in lesions of systemic sclerosis and its implications. J. Huazhong Univ. Sci. Technol. Med. Sci. Hua Zhong Ke Ji Xue Xue Bao Yi Xue Ying Wen Ban. Huazhong Keji Daxue Xuebao Yixue Yingdewen Ban. 2008;28:480–482. doi: 10.1007/s11596-008-0424-y. [DOI] [PubMed] [Google Scholar]
- 76.Avouac J. Role of Stromelysin 2 (Matrix Metalloproteinase 10) as a Novel Mediator of Vascular Remodeling Underlying Pulmonary Hypertension Associated With Systemic Sclerosis. Arthritis Rheumatol. Hoboken NJ. 2017;69:2209–2221. doi: 10.1002/art.40229. [DOI] [PubMed] [Google Scholar]
- 77.Manetti M. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann. Rheum. Dis. 2012;71:1064–1072. doi: 10.1136/annrheumdis-2011-200837. [DOI] [PubMed] [Google Scholar]
- 78.Asano Y. Clinical significance of serum levels of matrix metalloproteinase-13 in patients with systemic sclerosis. Rheumatol. Oxf. Engl. 2006;45:303–307. doi: 10.1093/rheumatology/kei143. [DOI] [PubMed] [Google Scholar]
- 79.Peng W. Matrix metalloproteinases: a review of their structure and role in systemic sclerosis. J. Clin. Immunol. 2012;32:1409–1414. doi: 10.1007/s10875-012-9735-7. [DOI] [PubMed] [Google Scholar]
- 80.Shin M.H. Reactive oxygen species produced by NADPH oxidase, xanthine oxidase, and mitochondrial electron transport system mediate heat shock-induced MMP-1 and MMP-9 expression. Free Radic. Biol. Med. 2008;44:635–645. doi: 10.1016/j.freeradbiomed.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 81.Yoo H.G. IL-1beta induces MMP-9 via reactive oxygen species and NF-kappaB in murine macrophage RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2002;298:251–256. doi: 10.1016/s0006-291x(02)02431-2. [DOI] [PubMed] [Google Scholar]
- 82.Lian S. Cadmium induces matrix metalloproteinase-9 expression via ROS-dependent EGFR, NF-кB, and AP-1 pathways in human endothelial cells. Toxicology. 2015;338:104–116. doi: 10.1016/j.tox.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 83.Marie I. Systemic sclerosis and exposure to heavy metals: a case control study of 100 patients and 300 controls. Autoimmun. Rev. 2017;16:223–230. doi: 10.1016/j.autrev.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Nelson K.K., Melendez J.A. Mitochondrial redox control of matrix metalloproteinases. Free Radic. Biol. Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 85.Gurjar M.V., Deleon J., Sharma R.V., Bhalla R.C. Role of reactive oxygen species in IL-1 beta-stimulated sustained ERK activation and MMP-9 induction. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H2568–H2574. doi: 10.1152/ajpheart.2001.281.6.H2568. [DOI] [PubMed] [Google Scholar]
- 86.Grygiel-Górniak B., Puszczewicz M. Oxidative damage and antioxidative therapy in systemic sclerosis. Mediat. Inflamm. 2014;2014:389582. doi: 10.1155/2014/389582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ostojic P., Damjanov N. Effects of micronutrient antioxidants (alpha-tocopherol and ascorbic acid) on skin thickening and lung function in patients with early diffuse systemic sclerosis. Rheumatol. Int. 2011;31:1051–1054. doi: 10.1007/s00296-010-1398-1. [DOI] [PubMed] [Google Scholar]
- 88.Cracowski J.-L. Effects of short-term treatment with vitamin E in systemic sclerosis: a double blind, randomized, controlled clinical trial of efficacy based on urinary isoprostane measurement. Free Radic. Biol. Med. 2005;38:98–103. doi: 10.1016/j.freeradbiomed.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 89.Herrick A.L. A double-blind placebo-controlled trial of antioxidant therapy in limited cutaneous systemic sclerosis. Clin. Exp. Rheumatol. 2000;18:349–356. [PubMed] [Google Scholar]
- 90.Mavrikakis M.E. Ascorbic acid does not improve endothelium-dependent flow-mediated dilatation of the brachial artery in patients with Raynaud's phenomenon secondary to systemic sclerosis. Int. J. Vitam. Nutr. Res. Int. Z. Vitam. - Ernahr. J. Int. Vitaminol. Nutr. 2003;73:3–7. doi: 10.1024/0300-9831.73.1.3. [DOI] [PubMed] [Google Scholar]
- 91.Dooley A. Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatol. Oxf. Engl. 2010;49:2024–2036. doi: 10.1093/rheumatology/keq208. [DOI] [PubMed] [Google Scholar]
- 92.Sriram N., Kalayarasan S., Sudhandiran G. Epigallocatechin-3-gallate exhibits anti-fibrotic effect by attenuating bleomycin-induced glycoconjugates, lysosomal hydrolases and ultrastructural changes in rat model pulmonary fibrosis. Chem. Biol. Interact. 2009;180:271–280. doi: 10.1016/j.cbi.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 93.Kavian N. Pantethine prevents murine systemic sclerosis Through the Inhibition of microparticle Shedding. arthritis Rheumatol. Hoboken NJ. 2015;67:1881–1890. doi: 10.1002/art.39121. [DOI] [PubMed] [Google Scholar]
- 94.Bagnato G. Propylthiouracil modulates aortic vasculopathy in the oxidative stress model of systemic sclerosis. Vasc. Pharmacol. 2015;71:79–83. doi: 10.1016/j.vph.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 95.Rosato E. Long-term N-acetylcysteine therapy in systemic sclerosis interstitial lung disease: a retrospective study. Int. J. Immunopathol. Pharmacol. 2011;24:727–733. doi: 10.1177/039463201102400319. [DOI] [PubMed] [Google Scholar]
- 96.Rosato E., Borghese F., Pisarri S., Salsano F. The treatment with N-acetylcysteine of Raynaud's phenomenon and ischemic ulcers therapy in sclerodermic patients: a prospective observational study of 50 patients. Clin. Rheumatol. 2009;28:1379–1384. doi: 10.1007/s10067-009-1251-7. [DOI] [PubMed] [Google Scholar]
- 97.Sambo P. Intravenous N-acetylcysteine for treatment of Raynaud's phenomenon secondary to systemic sclerosis: a pilot study. J. Rheumatol. 2001;28:2257–2262. [PubMed] [Google Scholar]
- 98.Correa M.J.U., Mariz H.A., Andrade L.E.C., Kayser C. Oral N-acetylcysteine in the treatment of Raynaud's phenomenon secondary to systemic sclerosis: a randomized, double-blind, placebo-controlled clinical trial. Rev. Bras. Reumatol. 2014;54:452–458. doi: 10.1016/j.rbr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 99.Sambo P. Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway. Arthritis Rheum. 2001;44:2653–2664. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 100.Failli P. Effect of N-acetyl-L-cysteine on peroxynitrite and superoxide anion production of lung alveolar macrophages in systemic sclerosis. Nitric Oxide Biol. Chem. 2002;7:277–282. doi: 10.1016/s1089-8603(02)00120-9. [DOI] [PubMed] [Google Scholar]
- 101.Zhou C.-F. N-acetylcysteine attenuates subcutaneous administration of bleomycin-induced skin fibrosis and oxidative stress in a mouse model of scleroderma. Clin. Exp. Dermatol. 2013;38:403–409. doi: 10.1111/ced.12033. [DOI] [PubMed] [Google Scholar]
- 102.Dosoki H. Targeting of NADPH oxidase in vitro and in vivo suppresses fibroblast activation and experimental skin fibrosis. Exp. Dermatol. 2017;26:73–81. doi: 10.1111/exd.13180. [DOI] [PubMed] [Google Scholar]
- 103.Teixeira G. Therapeutic potential of NADPH oxidase 1/4 inhibitors. Br. J. Pharmacol. 2017;174:1647–1669. doi: 10.1111/bph.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kavian N. The Nrf2-antioxidant response element signaling pathway controls fibrosis and autoimmunity in scleroderma. Front. Immunol. 2018;9:1896. doi: 10.3389/fimmu.2018.01896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toyama T. Therapeutic targeting of TAZ and YAP by dimethyl fumarate in systemic sclerosis fibrosis. J. Invest. Dermatol. 2018;138:78–88. doi: 10.1016/j.jid.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DMF in Systemic Sclerosis - ClinicalTrials.gov. Available at: 〈https://clinicaltrials.gov/ct2/show/NCT02981082〉. (accessed 18 October 2018).