Introduction
Generalized granuloma annulare (GGA) is a widespread inflammatory condition that is often resistant to current treatment modalities. Apremilast, a phosphodiesterase 4 (PDE-4) inhibitor, may represent a novel treatment option due to its ability to downregulate inflammatory pathways while maintaining a favorable safety profile. We report 2 cases of GGA treated with apremilast.
Granuloma annulare (GA) is a relatively common benign inflammatory skin condition. The generalized variant, defined as involving the trunk and extremities, makes up one-fourth of cases.1 Localized GA often spontaneously resolves; however, GGA is likely to persist despite therapy.2 Presently, there are no controlled clinical trials suggesting an optimal treatment, and current therapies are based on case reports and expert opinion.
Apremilast is an oral PDE-4 inhibitor approved for the treatment of moderate to severe plaque psoriasis and psoriatic arthritis, and recently approved for oral ulcers associated with Behçet disease. The exact pathogenesis of GA is unknown, but it is believed to be a type 1 T helper (Th1)-type delayed hypersensitivity reaction to a variety of antigens.3 Apremilast may be used as a treatment in GA due the ability to reduce Th1 cytokines such as tumor necrosis factor-α and interferon-γ.4,5 In the following cases, the use of apremilast as a potential treatment for GGA is assessed.
Case reports
Patient 1
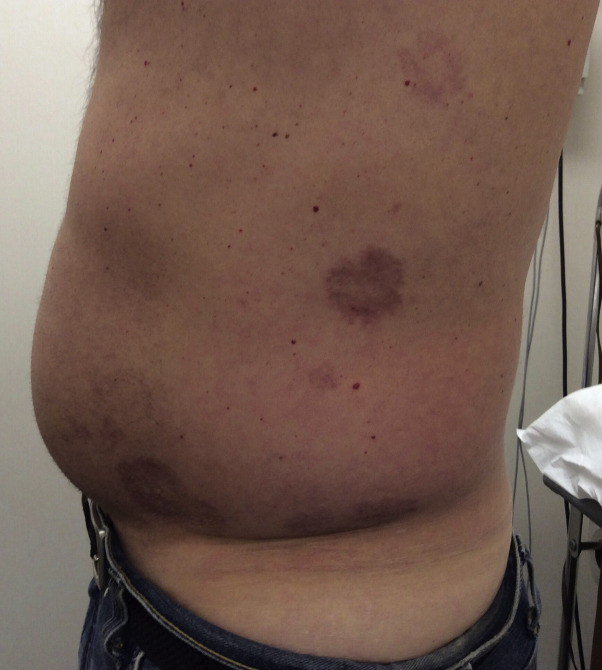
A 45-year-old man with a medical history of type 2 diabetes mellitus, dyslipidemia, and hypertension presented with a 1-year history of annular plaques on the abdomen and bilateral lower extremities. The specimen from a biopsy performed on the left abdomen was consistent with GA. Despite treatment with high-potency topical corticosteroids and intralesional corticosteroids, the lesions were persistent and progressive (Fig 1). After more than 1 year of unsuccessful corticosteroid treatment, the decision was made to start apremilast.
Fig 1.
Generalized granuloma annulare involving the abdomen. Pre-apremilast clinical presentation.
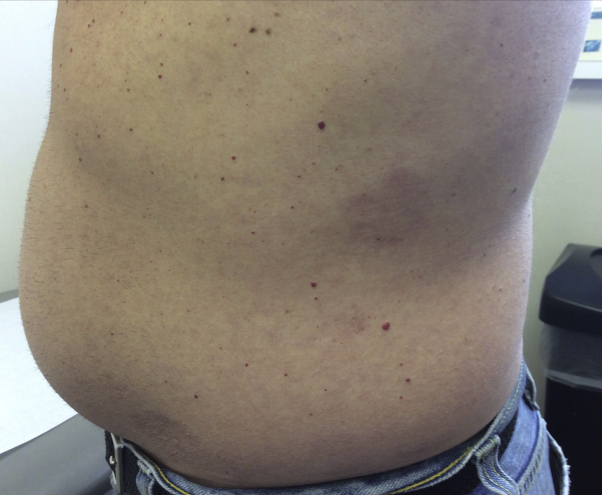
At this time, all topical and intralesional corticosteroid medications were stopped. He was started on an initial dose of 10 mg of apremilast, increasing by an increment of 10 mg daily until the maintenance dosage of 30 mg twice daily was achieved on day 6. At the 3-month follow-up, there was noticeable improvement in erythema and induration of lesions. Improvement was maintained at 1 year (Fig 2) and at the 18-month follow-up. No new lesions appeared during the course of treatment. The patient denied any adverse effects and is still tolerating apremilast therapy.
Fig 2.
Patient 1. Generalized granuloma annulare involving the abdomen. Improvement one year after apremilast initiation.
Patient 2
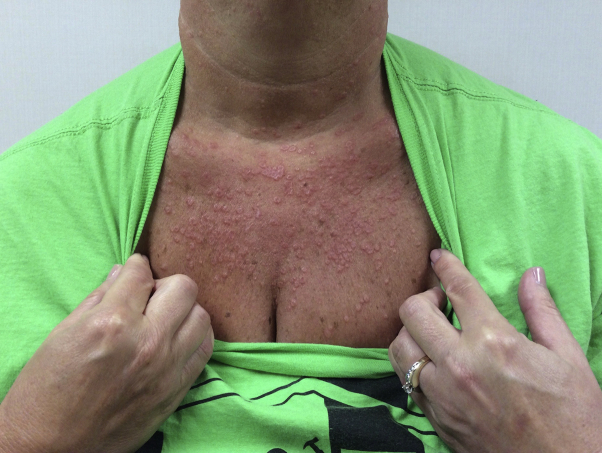
A 53-year-old woman with no medical history presented with a 2-month history of pink papules on the chest and upper extremities. The specimen from a shave biopsy performed on the chest was consistent with GA. The patient began treatment with clobetasol 0.025% cream twice daily to the affected areas. At the 2-month follow-up, significantly more pink annular papules and plaques had developed on the chest and extremities despite topical treatment (Fig 3).
Fig 3.
Patient 2. Generalized granuloma annulare involving the chest. Pre-apremilast clinical presentation.
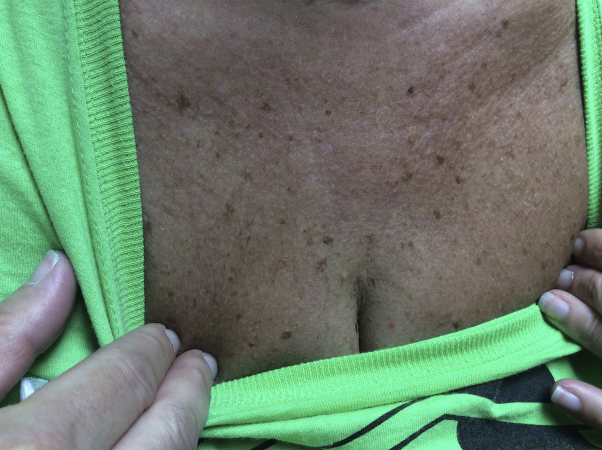
At this time, the clobetasol was discontinued and the patient was started on oral apremilast. The dosing of apremilast was 10 mg the first day, increasing by an increment of 10 mg daily until the maintenance dosage of 30 mg twice daily was achieved on day 6. After 3 months of apremilast therapy, the lesions of the chest had largely resolved, with only mild erythema being present. The lesions on the chest were clinically resolved 4 months after apremilast therapy was initiated (Fig 4). Lesions on the upper extremities still persisted; however, there was noticeable improvement. The patient denied any adverse effects while using apremilast and is still tolerating the medication.
Fig 4.
Patient 2. Generalized granuloma annulare involving the chest. Improvement four months after apremilast initiation.
Discussion
Case reports have described various treatments for GGA, including, but not limited to, topical and systemic corticosteroids, retinoids, phototherapy, calcineurin inhibitors, dapsone, hydroxychloroquine, tissue necrosis factor-α inhibitors, nicotinamide, allopurinol, hydroxyurea, rifampicin-ofloxacin-minocycline combination, tetracycline, photodynamic therapy, vitamin E, cyclosporine, pentoxifylline, zidovudine, interferon-α, antidiabetics, excimer laser, and pulsed dye laser.2,6 Because this is a benign condition, safety profile and cost need to be considered when choosing a treatment regimen.
The dosage chosen in these patients was the dosage approved by the United States Food and Drug Administration for psoriasis. Studies show serious adverse effects due to apremilast are low and are comparable to placebo; there is no increase in serious adverse effects with longer use of apremilast. Furthermore, apremilast has not been shown to cause reactivation tuberculosis. The most common adverse effects noted with apremilast are diarrhea, upper respiratory infection, nausea, nasopharyngitis, and headache.5
To our knowledge, the use of apremilast for GGA has not been reported. Use of apremilast to treat other inflammatory conditions, such as lichen planus, hidradenitis suppurativa, and Behçet disease, has been documented.7,8 PDE-4 is a dominant hydrolyzer for intracellular cyclic adenosine monophosphate.4 By inhibiting PDE-4, cyclic adenosine monophosphate levels increase, which causes a downregulation of Th1, Th17, and Th22 cytokines.4,5,7
GGA is known to be highly associated with dyslipidemia.3,9 Apremilast may have additional benefits in the treatment of GGA due to the ability to decrease triglyceride synthesis in the liver, increase lipolysis, and increase cholesterol efflux capacity, thus improving the lipid profile.10 Improvement of dyslipidemia was associated with concomitant improvement of GGA in 1 case study.3
This case report is limited by the lack of standardized assessment tools for GA. In addition, the patients' serum lipid composition was never tested. It is worth mentioning, although unlikely, the act of biopsying alone may be therapeutic.3 Both of the patients were biopsied several months before initiating apremilast, so improvement related to the biopsy is improbable.
This series describes the first documented use of apremilast for GGA. It appears apremilast may be an effective treatment. Further studies are necessary to better define the role of apremilast in the treatment of GGA.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Altman is a paid speaker for Celgene Corporation. Dr Blum has no conflicts of interest to disclose.
IRB approval status: Not applicable.
References
- 1.Piette E.W., Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75(3):457–465. doi: 10.1016/j.jaad.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Lukács J., Schliemann S., Elsner P. Treatment of generalized granuloma annulare – a systematic review. J Eur Acad Dermatol Venereol. 2015;29(8):1467–1480. doi: 10.1111/jdv.12976. [DOI] [PubMed] [Google Scholar]
- 3.Piette E.W., Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75(3):467–479. doi: 10.1016/j.jaad.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Schafer P.H., Parton A., Capone L., Cedzik D. Apremilast is a selective PDE4 inhibitor with regulatory effects on innate immunity. Cell Signal. 2014;26(9):2016–2029. doi: 10.1016/j.cellsig.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Papp K., Reich K., Leonardi C.L. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 6.Min M.S. Treatment of recalcitrant granuloma annulare (GA) with adalimumab: a single-center, observational study. J Am Acad Dermatol. 2015;74(1):127–133. doi: 10.1016/j.jaad.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Paul J., Foss C.E., Hirano S.A., Cunningham T.D., Pariser D.M. An open-label pilot study of apremilast for the treatment of moderate to severe lichen planus: a case series. J Am Acad Dermatol. 2013;68(2):255–261. doi: 10.1016/j.jaad.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Weber P., Jafari M., Yawalka N., Hunger R. Apremilast in the treatment of moderate to severe hidradenitis suppurativa: a case series of 9 patients. J Am Acad Dermatol. 2017;76(6):3. doi: 10.1016/j.jaad.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Wu W., Robinson-Bostom L., Kokkotou E., Jung H.-Y., Kroumpouzos G. Dyslipidemia in granuloma annulare: a case-control study. Arch Dermatol. 2012;148(10):1131–1136. doi: 10.1001/archdermatol.2012.1381. [DOI] [PubMed] [Google Scholar]
- 10.Gualtierotti R., De Lucia O. Efficacy and metabolic effect on serum lipids of apremilast in psoriatic arthritis: a case report. J Clin Med. 2019;8(3):398. doi: 10.3390/jcm8030398. [DOI] [PMC free article] [PubMed] [Google Scholar]