Abstract
Lipoxidation is a well-known reaction between electrophilic carbonyl species, formed during oxidation of lipids, and specific proteins that, in most cases, causes an alteration in proteins function. This can occur under physiological conditions but, in many cases, it has been associated to pathological process, including cancer. Lipoxidation may have an effect in cancer development through their effects in tumour cells, as well as through the alteration of immune components and the consequent modulation of the immune response. The formation of protein adducts affects different proteins in cancer, triggering different mechanism, such as proliferation, cell differentiation and apoptosis, among others, altering cancer progression. The divergent results obtained documented that the formation of lipoxidation adducts can have either anti-carcinogenic or pro-carcinogenic effects, depending on the cell type affected and the specific adduct formed. Moreover, lipoxidation adducts may alter the immune response, consequently causing either positive or negative alterations in cancer progression. Therefore, in this review, we summarize the effects of lipoxidation adducts in cancer cells and immune components and their consequences in the evolution of different types of cancer.
Abbreviations: ACR, acrolein; ADCC, antibody-dependent cellular cytotoxicity; AKR, aldo-keto reductases; AP-1, activator protein-1; ARE, antioxidant response element; ASK1, apoptosis signal regulating kinase; COX-2, cyclooxygenase-2; CTLs, cytotoxic T lymphocytes; cyPG, cyclopentenone prostaglandins; 15d-PGJ2, 15-deoxyΔ12–14 PGJ2; EGFR, epidermal growth factor receptor; ERK, extracellular signal-regulated kinase; PDGFR, platelet-derived growth factor receptor; GCL, glutamate cysteine ligase; GSH, glutathione; GST, glutathione S-transferasaes; 4-HHE, 4-hydroxy-hexenal; HNE, 4-hydroxy-2-nonenal; hPGD2s, hematopoietic prostaglandin D2 synthase; IκB, inhibitor of kappaB; IKK, IĸB kinase; iNOS, inducible nitric oxide synthase; JNKs, c-Jun N-terminal kinase; Keap1, Kelch-like ECH associating protein 1; LKB1, liver kinase B1; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MSA, mouse serum albumin; NFκB, nuclear factor-κB; NK, natural killer; PGA1, prostaglandin A1; PGA2, prostaglandin A2; PGD2, prostaglandin D2; Pin1, peptidylprolyl cis/trans-isomerase A1; PKB, protein kinase B; PPARs, peroxisome proliferator activated receptors; PUFAs, polyunsaturated fatty acids; RAGE, receptor for advanced glycation end products; RNS, reactive nitrogen species; ROS, reactive oxygen species; Th, T helper; TKRs, tyrosine kinase receptors; Tregs, Foxp3+ regulatory T cells; XIAP, X-linked inhibitor of apoptosis protein
Keywords: Lipoxidation, Protein adducts, Cancer, Immune system
Graphical abstract
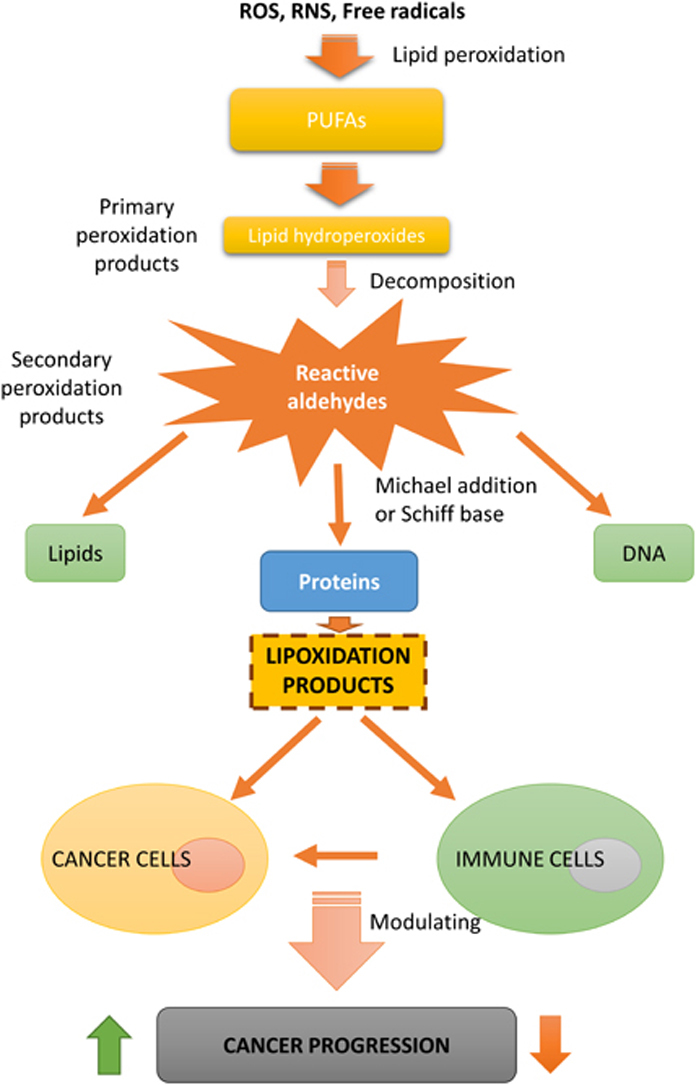
Diagram explaining the formation of lipoxidation adducts and their possible effects on the progression of cancer. Lipoxidation adducts influence tumour progression by acting directly on cancer cells functions and/or indirectly through the modulation of the immune response.
Highlights
-
•
Lipoxidation in tumour cells may lead to mechanism that interfere with cancer.
-
•
Lipoxidation adducts can have either anti-carcinogenic or pro-carcinogenic effects.
-
•
The triggered effects depend on the affected cell and the specific adduct formed.
-
•
Lipoxidation affecting immune components may influence cancer progression.
-
•
Lipoxidation may inhibit tumour progression through the inhibition of NFκB pathway.
1. Introduction
Oxidative stress is usually associated with an increase of reactive oxygen species (ROS), or a decrease on the antioxidant defences which, in turn, can favour the peroxidation of the polyunsaturated fatty acids (PUFAs) in membrane lipid bilayers, leading eventually to the formation of highly reactive aldehydes [1]. These electrophilic reactive aldehydes can spread from the site of origin and react with major biomolecules, like proteins, even at distant sites [2], causing a lipoxidation process. Lipoxidation is a well-known reaction between electrophilic carbonyl lipids species formed during oxidation of lipids and specific proteins [3].
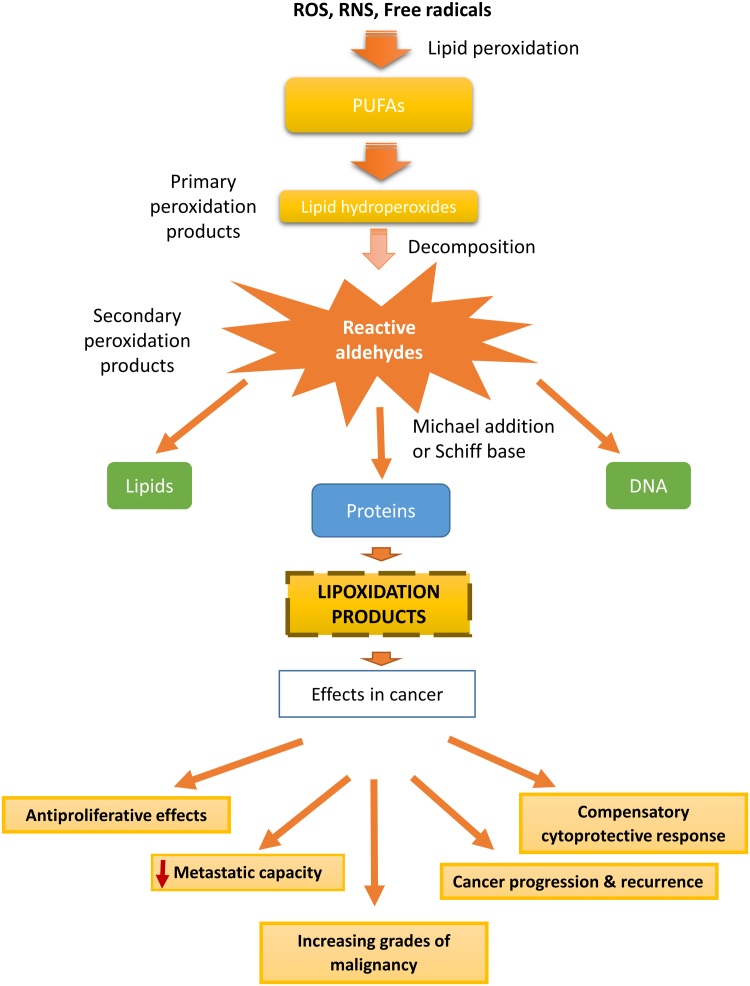
Lipid oxidation products may accumulate and covalently modify proteins, driving not only to physiological but also to pathological process through altering protein structure and function or changing signalling pathways. This has an effect in different pathologies such as cancer, in which lipid oxidation products may influence cancer progression either directly, through the modulation of cancer cells behaviour, or through the modulation of the immune response (Fig. 1) [4].
Fig. 1.
Diagram illustrating the formation of lipoxidation adducts and their possible effects on the progression of cancer.
The biological effects of reactive lipid carbonyl species generated by lipid peroxidation process are modulated by their local concentration and availability, which depends on the initial lipid targeted by peroxidation, as well as on the presence of cellular detoxifying and conjugating systems, and the cell ability to degrade modified proteins [5]. Also, quite important as well, depending on the type of protein modified, different effects can occur in the physiologic or the pathophysiologic signalling [6].
Oxidative modified molecules, including lipoxidation adducts, are also reported to have a significant role in the modulation of inflammation and immune response. They can induce adaptive immunity and have been implicated in the pathogenesis of various diseases [7]. In fact, it has been reported that the covalent reaction of electrophilic aldehydic products with proteins might lead to the formation of immunogenic biomolecules [8], and these lipoxidation products may alter the cellular signalling in the immune response in several pathologies, including cancer [9]. Moreover, it is well established that the immune system plays a very important role in cancer progression. In this regard, several studies in the past few years have demonstrated a dual role of leukocytes themselves contributing to either “pro-tumour” microenvironment or to “anti-tumour” microenvironment [10].
In this review, we will discuss and summarize the most recent advances in lipoxidation formation and its influence on the pathophysiology of cancer. We will also highlight the effect of lipoxidation in tumour and immune cells during cancer progression.
2. Chemistry of lipoxidation adducts and its relevance in disease pathophysiology
The unsaturated fatty acid are main targets of oxygen radicals leading to the formation of primary peroxidation products. These oxidized lipids can be decomposed to form secondary peroxidation products (carbonyl-based derivatives), and can react by addition reactions of the carbonyl groups (electrophiles) with amino and thiol groups (nucleophiles), leading to the formation of lipid-protein adducts or lipoxidation products [11] (Fig. 1). The aldehydes and other electrophilic carbonyl species generated will depend on the initial PUFA targeted by peroxidation. In this sense, the peroxidation of n-3 PUFAs (α-linolenic acid and docosahexaenoic acid) generates mainly 4-hydroxy-hexenal (4-HHE), while the peroxidation of n-6 PUFAs, such as linoleic acid and arachidonic acid, generates mostly 4-hydroxy-2-nonenal (HNE), which is the most intensively studied electrophilic reactive aldehyde [12], [13], [14]. The type of adducts that can be generated depends on the reactivity of the oxidized lipid species. In addition, the reaction of these compounds with a protein can take place by two principal reactions: (i) the addition of the aldehydic group to an amino group of the protein (e.g. lysine) forming a Schiff´s base adduct by loss of water and (ii) by a Michael addition to a nucleophile by the active C˭C double bond [3], [9]. While Schiff base formation is reversible, Michael adducts are quite stable, thus the formation of the latter seems to be preferred in vivo. It is also important to consider that lipoxidation depends on the balance of the rate of formation of the lipid oxidation product, its reactivity, and the rate of detoxification by enzymes such as glutathione peroxidases [15], glutathione S-transferasaes (GST) [16], or aldo-keto reductases (AKR) [17].
Lipoxidation can occur in healthy individuals [18], [19], since protein modification by reactive electrophilic species not only may inhibit protein function, but also, in a small number of cases, may cause a gain of function, even leading to beneficial effects [20], [21], [22].
Nevertheless, the importance of lipoxidation and its pathophysiological relevance have been broadly discussed in several works [14], [23], [24], [25], [26]. In fact, the measurement of global protein adducts, such as HNE-protein adducts, is commonly used as a biomarker of inflammation/oxidative stress/lipid peroxidation under various pathological conditions [27]. The accumulation of lipid peroxidation products, and therefore of lipoxidation adducts, has been involved in ageing and in well-defined diseases of liver, kidney, neurological and cardiovascular systems, endocrine and metabolic disorders, diabetes and its complications, and other oxidative stress related pathologies [28].
Furthermore, lipoxidation is highly associated with chronic degenerative diseases such as cancer. These topics will be discussed in the following section.
3. Lipoxidation in cancer: Effect on tumour and immune cells
Carcinogenesis and cancer therapies are strongly influenced by oxidative stress and by lipid peroxidation [28] and, consequently, by lipoxidation adducts. The most reported reactive carbonyl products formed during lipid peroxidation are malondialdehyde (MDA), acrolein (ACR), 4-hydroxy-hexenal (4-HHE) and 4-hydroxy-2-nonenal (HNE) [29], and several studies reported the formation of protein adducts with several proteins in different types of cancer [30], [31], [32], [33]. In fact, the greater reactivity of HNE, one of the major products of lipid peroxidation, with proteins, gave rise to the assumption that HNE has a cytotoxic and carcinogenic effect through the modulation of proteins involved in DNA repair [34]. Moreover, other works demonstrated that oxidative stress and electrophilic lipid peroxidation products, such as HNE, also play important roles in the induction of cell cycle arrest, differentiation process, and apoptosis in cancer cells [35]. However, some studies show controversial results regarding the influence of HNE, or HNE-adducts in different types of human cancers [36], [37], [38], [39], and the pattern of HNE histological appearance has been shown to be dependent on the histological origin of cancer [40].
Likewise, cancer cells are sensitive to lipid oxidation products since these products act as second toxic messengers of free radicals, as well as signalling molecules and growth regulating factors that influence important processes for cancer progression such as proliferation, differentiation and apoptosis [28]. But there are discrepancies in the appearance of lipoxidation adducts in distinct cancer types. For example, in hepatoma cells, it was shown that the majority of HNE was converted to the HNE-GSH conjugate, which was rapidly and efficiently exported from the cell [41]. However, in astrocytic and ependymal glial tumours, HNE-protein adducts were detected in mitotic, necrotic and apoptotic cells, and were associated with increasing grades of malignancy [42].
The disparity observed in the formation of lipoxidation adducts in various tumours may be explained by: a) the different membrane composition of lipids in different cancer cell types, as well as different cholesterol/PUFAs ratios, which determine different tendencies to form lipid peroxidation products and, therefore, different electrophilic lipids and, thus, different lipoxidation adducts [43]; b) the higher expression of detoxification enzymes and antioxidant proteins observed in some tumour cells, what results in a more efficient and rapid metabolism of lipid peroxidation products [44]; c) the different effects, either physiological or pathological, triggered by some lipid peroxidation products, that act through the antioxidant response element (ARE) to induce the expression of key metabolizing enzymes, such as GST [45], influencing on Keap1–Nrf2–ARE pathway [46], [47]; d) the local of formation and e) the targeted protein or enzyme that are adducted to the electrophilic lipid.
3.1. Effect of lipoxidation in tumour cells
As it was mentioned above, the level of oxidative stress and, consequently, the level of lipoxidation products vary among cancer types in relation with cell type. In liver cancer, it was found lower levels of lipid peroxidation products in hepatoma cells when compared to normal liver cells [48], [49], probably leading to lower levels of lipoxidation products, what can be explained, in part, by the observed increase in the activity of enzymes metabolizing toxic aldehydes during rat liver carcinogenesis [50], thus rendering the cancer cells more protected against the cytotoxic effect of lipoxidation products.
Several enzymes involved in tumour resistance due to their ability to metabolize electrophilic lipids are, at the same time, targets for lipoxidation themselves. This is the case of AKR that catalyse the reduction of ketones and aldehydes [51] or GST enzymes that are involved in drug detoxification [3]. AKR1B10, a member of AKR family, is overexpressed in several types of tumours, and it may contribute to tumorigenesis through various mechanisms, in addition to be involved in chemoresistance [52], [53]. This protein is a selective target for lipoxidation and inhibition by A-class cyclopentenone prostaglandins (cyPG) and it has been demonstrated that low concentrations of prostaglandin A1 (PGA1) potentiate the intracellular accumulation and G2/M cell cycle arresting effect of the topoisomerase inhibitor doxorubicin in A549 lung cancer cells [54], [55]. Due to their electrophilic nature, cyPG may form Michael adducts with GSH both enzymatically, through the action of GSTs, and non-enzymatically [56], [57]. Likewise, it has been found HNE adducts with GST detected by immunoprecipitation of GST followed by Western blot analysis using anti-HNE antibody [58]. On the top of that, GSTP1-1, a very important enzyme in tumour chemoresistance, can be covalently bound by various electrophilic lipids, including PGA1 and PGA2, causing its inactivation [22], [59], [60]. Hence, lipoxidation of GSTP1-1 could help to overcome the resistance of certain tumour cells to chemotherapy or radiation [55], [61].
On the other hand, lipoxidation adducts were found in renal [62], and colon cancer cells [63], as well as in astrocytic and ependymal glial tumours, in which the incidence of HNE-positive tumour cells increased with increasing grades of malignancy [42]. Although the amount of lipoxidation products in cancer cells, like HNE-protein adducts, has been often assayed as a means of assessing the level of oxidative stress, only in some cases the identification and the consequences of HNE-protein adduct formation on cancer cell growth or behaviour have been reported [14].
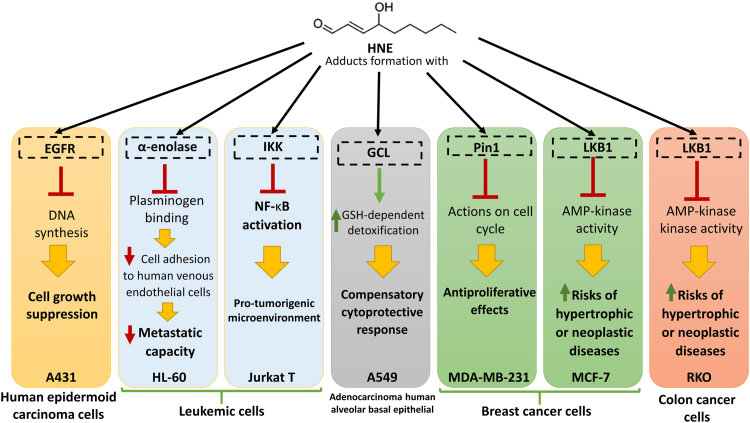
We have summarized the effect of HNE-protein adducts in distinct cancer cell lines, such as human epidermoid carcinoma, leukemic cells, adenocarcinoma human alveolar basal epithelial, breast cancer cells, or colon cancer cells, reported by different studies [64], [65], [66], [67], [68], [69], [70], [71], in Fig. 2. Both endogenous and exogenous HNE lead to lipoxidation adducts with many diverse proteins such as epidermal growth factor receptor (EGFR), α-enolase, peptidylprolyl cis/trans-isomerase A1 (Pin1), liver kinase B1 (LKB1), IĸB kinase (IKK), or glutamate cysteine ligase (GCL), triggering different effects very important in avoiding cancer progression, such as suppression of cell growth, reduction of metastatic capacity or anti-proliferative effects, but also in other cases triggering effects that favour cancer progression, as the modulation of tumour microenvironment to become more pro-tumorigenic or the cytoprotective response in cancer cells (Fig. 2).
Fig. 2.
Summary of the possible effects of HNE-protein adducts on different proteins and different cancer cell lines.
Moreover, other studies have shown that the formation of HNE protein adducts in renal and colon cancer tissues has been related to the growth and progression of kidney and colon cancer [30], although the progression of colon cancer results in loss of lipoxidation adducts in the malignant tissue and increase of reactive aldehydes in the surrounding area [31]. In accordance with these results, a different study in prostate cancer showed that ACR protein adducts could be associated with tumour progression and recurrence [32]. Moreover, tumour tissues in lung cancer showed lower antioxidant capacity than healthy tissues, which was accompanied by lower levels of fatty acids and higher levels of reactive aldehydes detected in the necrotic and stromal cells in these tumours, thus favouring the formation of lipoxidation products like the HNE-His protein adducts observed in necrotic lung cancer tissues [33].
Protein adducts are also involved in the inactivation of the proteasome [72], which is responsible for the intracellular degradation of proteins, whether they are damaged or no longer required for cellular processes [73]. Proteasome is then essential for many cellular pathways, including cell cycle, regulation of gene expression and resistance to oxidative stress. Therefore, protein lipoxidation adducts could alter carcinogenesis through their effect in the inactivation of the proteasome since cross-linked proteins are able to inhibit the proteasome, and further impair cellular protein turnover [74]. In fact, there are some studies showing that proteasome inhibitors induce apoptosis in leukemic cell lines, turning the proteasome into one of the possible targets with potential for therapeutic agents against cancer [75], [76], [77].
It is important to remark that, in several cases, the progression of malignancy is accompanied by reductions of oxidative stress, due to the upregulation of antioxidant capacity [78], and the induction of the Nfr2/Keap1 pathway, which negatively regulates the HNE intracellular concentration [79]. This also matches with the results showing that the adaptation to intrinsic oxidative stress in cancer cells can confer drug resistance. Thus, anticancer drugs and radiotherapy can induce oxidative stress and trigger cancer cells to undergo apoptosis, however some cancer cells escape from this process through the adaptation to intrinsic oxidative stress [34]. On the other hand, despite the reduction of intrinsic oxidative stress, the level of lipoxidation products in cancer cells may increase, due to the inflammatory response present in the tissues surrounding cancer lesions [14].
Transcription factors of the peroxisome proliferator activated receptors (PPARs) family play a key role both in tumour biology and in immune function [80]. The mechanisms reported so far suggest that each PPAR isotype is associated with pathways that relate to carcinogenesis due to direct effect in the cancer cells themselves, since they are involved in the control of cell proliferation, cell differentiation and apoptosis [81], [82]. But in addition to these functions, PPARs may act on the tumour environment by regulating inflammatory processes [83], [84], [85]. This family of nuclear receptors is also a target of lipoxidation processes. It has been demonstrated that 15-deoxyΔ12–14 PGJ2 (15d-PGJ2) binds covalently to a cysteine residue located in the PPARγ ligand binding pocket [86], [87], [88]. Further on, it was shown that 15d-PGJ2 activates PPARδ’s transcriptional activity through formation of a covalent adduct between its endocyclic enone at C9 and Cys249 in the receptor's ligand-binding domain [89]. In addition, HNE has been reported as an endogenous ligand for PPARβ/δ that causes its activation [90].
The divergent results obtained documented that the formation of lipoxidation adducts can have either anti-carcinogenic or pro-carcinogenic effects, depending on the cell type affected and the specific adduct formed [14]. The abundance of a protein, as well as the high reactivity and accessibility of some nucleophilic sites, may determine if a protein becomes, or not, a lipoxidation target [91], [92]. Moreover, depending on the nature/structure of the lipid oxidation product, which could have different structural features and, as well, different reactivity, it may lead to the formation of different types of lipoxidation adducts and thus to different functional consequences in the targeted protein [22], [93], [94]. In fact, it has been shown that biotinylated cyPG mimic many of the effects of cyPG in cellular models, including inhibition of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and induction of HO-1 and Hsp70 expression, but they are unable to elicit PPAR activation in vitro or in intact cells [95], [96]. Hence, by adding a bulky moiety to the carboxyl group of cyPG, it may be possible to dissociate some biological actions [97]. More studies are needed to disclose these effects depending on the type of cancer, their stage, the implicated targeted protein, or the reactive species involved.
3.2. Effect of lipoxidation on immune cells and their correlation with cancer
Chronic inflammatory processes induce oxidative/nitrosative stress and, as consequence, lipid peroxidation products and lipoxidation processes. In addition, it has been described that different chronic inflammatory conditions pre-dispose susceptible cells to malignant transformation and cancer progression [28], so that it has been estimated that chronic infection and associated inflammation contribute to about one in four of all cancer cases worldwide [98].
ROS, reactive nitrogen species (RNS) and lipid peroxidation products can modulate signalling molecules [99] and alter functions of proteins involved in inflammation and carcinogenesis [100], such as the nuclear transcription factor NFκB or stress response enzymes, namely iNOS and COX-2 [101], [102]. Furthermore, it has been reported that non-enzymatic oxidative modification of proteins, including lipoxidation, renders proteins immunogenic and leads to the generation of antibodies against oxidatively modified proteins [8], [103].
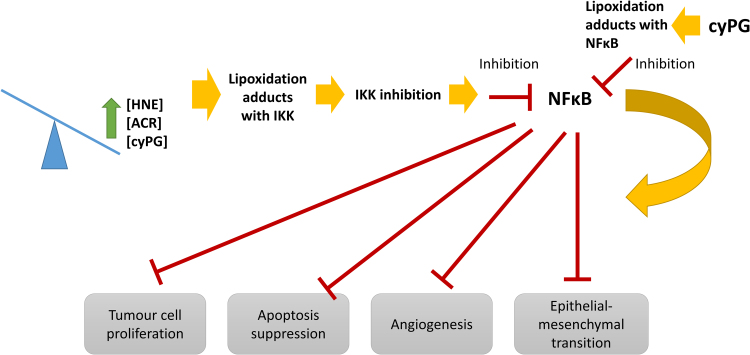
In fact, aldehydes exert a dual effect on inflammatory signalling, mainly depending on the concentration levels. On the one hand, at low concentrations, HNE activates PKCβ-signalling, inducing the production and secretion of CCL2 (MCP-1) by macrophages [104]. On the other hand, high concentrations of reactive aldehydes, such as HNE or ACR, inhibit the activation of NFκB, either via a direct inhibitory effect on proteasome, or via inhibition of the phosphorylation of inhibitor of kappaB (IκB) and its subsequent proteolysis [105], or a modification of IκB kinase (IKK) β -sub-unit by aldehydes [106] that has also been found to be a target of cyPG (Fig. 3) [107]. Moreover, 4-HHE activates the IKK, via the IKK/NFκB inducing kinase (NIK) pathway, through the increase in the activity of p38 MAPK and ERK1/2 kinase, resulting in NFκB activation [108]. In contrast, it has been described that cyPG can directly modify NFκB subunits p65 and p50, leading to NFκB inhibition by blocking its ability to bind DNA, studied by immunohistochemistry and Western blot analysis (Fig. 3) [109], [110]. Moreover, it has been proposed a role for 15d-PGJ2 in the control of lymphocyte proliferation and activation through mechanisms relying on NFκB inhibition, studied in knockout mice for hematopoietic prostaglandin D2 synthase (hPGD2s), which metabolizes cyclooxygenase (COX)-derived PGH2 to PGD2 and 15d-PGJ2 [111]. Furthermore, it was shown that 15d-PGJ2 controlled the balance of pro- vs. anti-inflammatory cytokines regulating leukocyte influx and macrophage efflux through draining lymphatics [112]. This is very important for cancer progression since NF-κB activation promotes the accumulation of pro-inflammatory cytokines at the tumour site, contributing to the pro-tumorigenic microenvironment. The activation of this transcription factor has been associated with tumour cells proliferation, suppression of apoptosis, angiogenesis and epithelial-mesenchymal transition, which facilitates distant metastasis [113].
Fig. 3.
Effects of NFκB inhibition mediated by lipoxidation adducts. High concentration of aldehydes, such as HNE or acrolein, or high concentration of cyclopentenone prostaglandins (cyPG) inhibits IKK activity through the formation of lipoxidation products. IKK inhibition results in the suppression of NFκB activity, hindering the effects triggered by NFkB, such as tumour cells proliferation, suppression of apoptosis, angiogenesis and epithelial-mesenchymal transition, which facilitates distant metastasis. Moreover, cyPG can directly modify NFκB subunits leading to NFκB inhibition, and therefore, the suppression of NFkB effects.
Additionally, it has been demonstrated that PPAR-α ligands and PPAR-γ ligands (15d-PGJ2) inhibit cell growth and induce monocytic differentiation in human promyelocytic leukemia cells (HL-60 cells), and HNE, which alone induces granulocytic-like differentiation of HL-60 cells, potentiates the monocytic differentiation induced by 15d-PGJ2. Moreover, HNE treatment significantly inhibits U937 (human histiocytic lymphoma) cell growth and potentiates the inhibition of cell growth in PPAR-γ ligand-treated cells [68]. And, in addition, it has been reported that HNE can form adducts with cysteine residues in the extracellular domain of TLR4 peptides, demonstrated by LC–MS/MS analysis, inhibiting its activation [114]. Hence, the formation of lipoxidation adducts with HNE can differentially regulate the activation of TLR4 and subsequently provoking an effect in the immune response.
It has been shown that both MDA-adducted mouse serum albumin (MSA) and HNE–MSA were able to significantly promote CD4+ T cell proliferation, leading to the hypothesis that lipoxidation adducts, could serve as an immunological trigger in the activation of CD4+ T cells. Moreover, it has been suggested that lipid peroxidation derived aldehydes preferentially promote Th1 differentiation, analysed by flow cytometry and ELISA in splenic lymphocytes from trichloroethene-treated mice [115]. In that sense, we could consider lipoxidation adducts a positive factor since Th1 cells have been associated with the promotion of anti-tumour responses: Th1 cells enhance the cytotoxic functions of NK and CD8+ cells, upregulate MHC Class I expression in tumour cells, and support CD8+ cell proliferation through the secretion of IL-2 [116].
Regarding monocytes function, it has been suggested that synthetic MDA-Lys, used as a prototype of advanced lipoxidation end products, can promote monocyte activation and vascular complications via the induction of inflammatory pathways and networks. In a candidate gene profiling approach, MDA-Lys increased the expression of key NFκB-dependent genes, such as MCP-1, iNOS, RAGE, IP-10, CCR-2, IL-6, IL-8, and COX-2 that are associated with monocyte activation. Antibody array profiling revealed that MDA-Lys can upregulate the chemokines CCL11 (eotaxin), TNFSF14, and CCL18. In addition, key factors that were noted to be induced by MDA-Lys, such as MCP-1, eotaxin, IL-6, IL-8, β1- and β2-integrins, and COX-2, are associated with monocyte activation, adhesion, and migration [117].
Neutrophils mediate key components of the cellular immune response which involves cellular adhesion, migration, phagocytosis and degradation and turnover of phagocytic metabolites [118]. It has been demonstrated, by mass spectrometry analyses, the existence of lipoxidation adducts of HNE with proteins involved in key pathways of neutrophil oxidative burst, phagocytosis, redox homeostasis and glucose metabolism. The same study also confirmed the formation of neutrophil protein-HNE adducts using candidate proteins found to be modified, by mass spectrometry. Taken together, these data suggest that HNE induces a pleiotropic mechanism to inhibit neutrophil function [119].
In addition, it has been reported that HNE seems to be an important cell growth regulating factor, acting as a signalling molecule interacting with the growth regulating effects of various cytokines [120], [121], [122], [123]. HNE, as a second messenger of ROS, activates activator protein 1 (AP-1) that promotes TGFβ synthesis and fibrogenesis. Hence, HNE could, at the same time, support fibrogenesis and inhibit the cancer growth.
The regulation of the immune system is very important in determining cancer progression [10]. Therefore, lipoxidation products may have an effect in cancer development by affecting immune components and modulating the immune response.
3.3. Overview of tumour immunology at tumour microenvironment and its relation with reactive aldehydes and lipoxidation
There are few studies on the role of lipoxidation adducts with respect to tumour immunology, but considering what is known about lipid peroxidation products, their influence in immunology, as described above, and the influence of immune microenvironment in tumour progression [10], [124], [125], [126], altogether it suggests that lipoxidation is a very important process in this field. Moreover, recent studies have revealed that immune cells possess distinct metabolic characteristics that influence their immunological functions [127]. For example, macrophage polarization is related to distinct metabolic characteristics pertaining to lipid metabolism, among others [128]. In this sense, it has been found that genes involved in glycolysis and phospholipid metabolism, differentially expressed between M1 and M2 macrophages, are major distinguishing features of inflammatory (M1) macrophages [128].
Clinically manifest neoplasms can develop when tumour cells are able to escape immunosurveillance [129], [130]. In addition, the efficacy of most chemotherapeutic and radiotherapeutic agents commonly employed in the clinic, critically depends on the activation or reactivation of tumour-targeting immune responses [131], [132], [133].
Tumour-infiltrating leukocyte subsets can play strikingly antagonistic functions. One of the key features of inflammation is the functional phenotype of macrophages that depend on the activating stimuli in their microenvironment. Macrophages are prototypical O2.-, H2O2, and NO producing cells, and oxidants represent one of the most potent weapons of activated macrophages in the fight against cancer cells [134], [135]. In addition, it is known that the increase of oxidant is associated with higher formation of lipid peroxidation products and, therefore, this could lead to a higher presence of lipoxidation adducts [136]. Moreover, it has been reported that macrophages, when stimulated, can produce HNE through COX-2 [124]. The inhibition of COX-2 in murine macrophages was associated with a decrease in HNE production following E. faecalis infection (P < 0.001). In the same study, using IL-10-knockout mice colonized by E. faecalis, it was observed increased levels of COX-2 expression in colonic macrophages in association with HNE-protein lipoxidation adducts [124].
Natural killer (NK) cells and CD8+ cytotoxic T lymphocytes (CTLs) provide highly complementary anti-tumour strategies. Oxidants have a dual role in the regulation of CTLs and NK cell function. It has been observed that the most potent caspase inhibitor, X-linked inhibitor of apoptosis protein (XIAP), confers resistance to antibody-dependent cellular cytotoxicity (ADCC). Thus, XIAP is a critical modulator of ADCC responsiveness [137]. In this sense, strategies have been proposed to reduce the oxidative stress to enhance the ability of CTLs to kill tumour cells. However, activated CTLs may partly adapt to the oxidative stress in the tumour microenvironment by upregulating antioxidant proteins as demonstrated with IL-2-activated NK cells [138] and as was described above.
On the other hand, Th17 cells have been associated with poor prognosis in some type of cancers and its pro-tumour functions have been tightly linked to angiogenesis and promotion of tumour vascularization. Nevertheless, the role of Th17 cells is much more controversial due to its association with better overall survival in ovarian cancer and in esophageal squamous cell carcinoma [10]. In this sense, lipid peroxidation products may also have an influence since it has been reported that aldehydes, such as MDA, transcriptionally upregulate the expression of IL-17E in lymphocytes and alter lymphocyte differentiation towards the pathogenic Th17 subset [68]. Finally, Foxp3+ regulatory T (Treg) cells accumulation in the tumour microenvironment is considered a bad prognosis factor [10]. This population can also be influenced by lipoxidation effects, as it was observed in atherosclerotic lesions of a mice model, in which there was an inhibition in the generation of Treg cells induced by MDA-laminin adduct [126].
In sum, the modulation of immune components in the tumour microenvironment has a very relevant effect over the development of tumours as well as over the type of patient's response to a specific treatment, and lipoxidation products may have a very important role in this modulation. In this regard, the combination of conventional therapeutics with ROS modulators may increase specific tumour cytotoxicity.
3.4. Molecular targets and signalling properties of lipoxidation
Lipoxidation adducts may alter progressively the structure and function of circulating and tissular proteins, with consequences on the inflammatory status, cell proliferation and viability, thus influencing cancer development [5]. Studies of proteins modified by reactive aldehydes indicated hundreds of molecular targets [8], [139], [140], therefore, we will highlight in this section targeted protein involved in cell proliferation, apoptosis, and some protein kinases.
3.4.1. Modification of tyrosine kinase receptors
It has been previously reported that HNE present in oxLDLs or exogenously added induces both modification and dysfunction of tyrosine kinase receptors (TKRs), such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR), involving lipoxidation adducts, which triggers TKR autophosphorylation and the activation of the downstream signalling pathways, extracellular signal-regulated kinase (ERK)1/2 phosphorylation and cell cycle progression [141], [142]. However, high concentrations of HNE inhibit cell proliferation mediated by EGFR and PDGFR involving the formation of HNE and ACR adducts with PDGFRβ [64], [143]. Thus, it has been suggested that HNE and others electrophilic lipids may potentially disturb PDGFR-mediated responses such as proliferation and cell migration [144].
3.4.2. Apoptosis signalling and other protein kinases
In human myeloid HL-60 cells, HNE adducts were shown to be correlated with the induction of apoptosis, the activation of c-Jun N-terminal kinase (JNK) and caspase 3, and they have been associated with the activation of caspases 3, 8, and 9 in embryonic fibroblasts isolated from mice [145], [146]. Moreover, HNE induce the expression of antioxidant genes such as heme-oxygenase and thioredoxine-1 via the activation of the mitogen-activated protein kinase (MAPK) pathway and the transcription factor Nrf2 [147], [148]. Thioredoxin and thioredoxin reductase are involved in the maintenance of various proteins in a reduced state required for their normal function, and they are also targets of lipoxidation by 15d-PGJ2, what results in their inactivation [149]. Modified thioredoxin reductase may mediate the conformational disruption of p53 and PG-induced apoptosis via activation of caspase 3 [150]. Moreover, in Jurkat cells, it was reported that both Fas and Daxx proteins are targets of lipoxidation by HNE. Fas adducts promote proapoptotic signalling via ASK1, JNK, and caspase 3. While Daxx lipoxidation promotes its export from the nucleus to the cytosol, where it interacts with Fas to self-limit the extent of apoptosis by inhibiting the downstream proapoptotic signalling [151]. In addition, the proapoptotic protein BAX is a direct target of lipoxidation by PGA2, triggering a conformational change that leads to BAX activation and induction of apoptosis [152]. Different studies reported the direct modification and inactivation of the phosphoinositide-3-phosphatase and tumour suppressor PTEN by several reactive aldehydes and ketones, such as ACR, HNE and α,β-enones such as PGA2, Δ12-PGJ2 and 15d-PGJ2, with ensuing activation of PKB/Akt kinase, phosphorylation of Akt substrates, increased cell proliferation, and increased nuclear β-catenin signalling [153], [154], [155]. This combined and sustained inactivation of tumour suppressors could contribute significantly to inflammation-associated tumorigenesis [153]. Additionally, it has been observed that cyPG and cyclopentenone isoprostanes target the oncogenic H-Ras proteins. Whereas 15d-PGJ2 and Δ12-PGJ2 preferentially target the C-terminal region, PGA1 and 8-iso-PGA1 bind mainly to cysteine 118, located in the GTP-binding motif what has been correlated with H-Ras activation [156]. In human hepatic stellate cells, the p46 and p54 isoforms of JNKs were identified as HNE targets and were activated by this aldehyde. This leads to JNK nuclear translocation as well as to c-jun and AP-1 induction [157]. Furthermore, it has been shown that 15d-PGJ2 can covalently modify c-Jun at cysteine 269, which is located in the c-Jun DNA binding domain, and directly inhibit the DNA binding activity of AP-1, both in vitro and in intact cells [59], [158].
4. Concluding remarks and future perspectives
Many of the previously described studies provide emerging molecular evidence of the importance of lipoxidation in carcinogenesis, where inflammation represents one of the fundamental links. There is a great complexity in the possible roles of lipoxidation products in cancer pathology. It has been reported contradictory results in which lipoxidation products seem to be toxic for tumour cells [159] but also, other studies report an association with the increase of the level of malignancy in tumours [31]. Therefore, lipoxidation products can have a crucial role not only in carcinogenesis but also in the host defence against cancer, through their effects in tumour cells and through their interactions with immune components.
Future studies will be necessary to distinguish physiologic and pathologic roles of lipoxidation processes occurring during carcinogenesis, with particular attention to the pro-oxidant anticancer agents and the drug-resistant mechanisms, which could be modulated to obtain a better response to cancer therapy [34].
Acknowledgements
We acknowledge the European Commission's Horizon 2020 research and innovation programme for the Marie Sklodowska-Curie grant agreement number 675132 (MSCA-ITN-ETN MASSTRPLAN) to University of Aveiro and to Centro Hospitalar e Universitário de Coimbra. Thanks are due to the University of Aveiro, FCT/MEC, European Union, QREN, COMPETE for the financial support to the QOPNA (FCT UID/QUI/00062/2013) and CESAM (UID/AMB/50017 - POCI-01–0145-FEDER-007638), through national funds and where applicable co-financed by the FEDER, within the PT2020 Partnership Agreement, to the Portuguese Mass Spectrometry Network (LISBOA-01–0145-FEDER-402–022125).
Acknowledgments
Conflicts of interest
The authors have no competing financial interests to declare.
References
- 1.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Zarkovic K., Jakovcevic A., Zarkovic N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic. Biol. Med. 2017;111:110–126. doi: 10.1016/j.freeradbiomed.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Aldini G., Domingues M.R., Spickett C.M., Domingues P., Altomare A., Sánchez-Gómez F.J., Oeste C.L., Pérez-Sala D. Protein lipoxidation: detection strategies and challenges. Redox Biol. 2015;5:253–266. doi: 10.1016/j.redox.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegedűs C., Kovács K., Polgár Z., Regdon Z., Szabó É., Robaszkiewicz A., Forman H.J., Martner A., Virág L. Redox control of cancer cell destruction. Redox Biol. 2018;16:59–74. doi: 10.1016/j.redox.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen D.R., Doorn J.A. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic. Biol. Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Chavez J.D., Wu J., Bisson W., Maier C.S. Site-specific proteomic analysis of lipoxidation adducts in cardiac mitochondria reveals chemical diversity of 2-alkenal adduction. J. Proteom. 2011;74:2417–2429. doi: 10.1016/j.jprot.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karin M., Lawrence T., Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Kurien B.T., Hensley K., Bachmann M., Scofield R.H. Oxidatively modified autoantigens in autoimmune diseases. Free Radic. Biol. Med. 2006;41:549–556. doi: 10.1016/j.freeradbiomed.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Castro J.P., Jung T., Grune T., Siems W. 4-Hydroxynonenal (HNE) modified proteins in metabolic diseases. Free Radic. Biol. Med. 2017;111:309–315. doi: 10.1016/j.freeradbiomed.2016.10.497. [DOI] [PubMed] [Google Scholar]
- 10.Lança T., Silva-Santos B. The split nature of tumor-infiltrating leukocytes: implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–725. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reis A. Oxidative Phospholipidomics in health and disease: achievements, challenges and hopes. Free Radic. Biol. Med. 2017;111:25–37. doi: 10.1016/j.freeradbiomed.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizzimenti S., Toaldo C., Pettazzoni P., Dianzani M.U., Barrera G. The "two-faced" effects of reactive oxygen species and the lipid peroxidation product 4-hydroxynonenal in the hallmarks of cancer. Cancers. 2010;2:338–363. doi: 10.3390/cancers2020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrera G., Pizzimenti S., Ciamporcero E.S., Daga M., Ullio C., Arcaro A., Cetrangolo G.P., Ferretti C., Dianzani C., Lepore A., Gentile F. Role of 4-hydroxynonenal-protein adducts in human diseases. Antioxid. Redox Signal. 2015;22:1681–1702. doi: 10.1089/ars.2014.6166. [DOI] [PubMed] [Google Scholar]
- 15.Thomas J.P., Maiorino M., Ursini F., Girotti A.W. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J. Biol. Chem. 1990;265:454–461. [PubMed] [Google Scholar]
- 16.Singhal S.S., Singh S.P., Singhal P., Horne D., Singhal J., Awasthi S. Antioxidant role of glutathione S-transferases: 4-hydroxynonenal, a key molecule in stress-mediated signaling. Toxicol. Appl. Pharmacol. 2015;289:361–370. doi: 10.1016/j.taap.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spite M., Baba S.P., Ahmed Y., Barski O.A., Nijhawan K., Petrash J.M., Bhatnagar A., Srivastava S. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes. Biochem. J. 2007;405:95–105. doi: 10.1042/BJ20061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Méndez D., Hernáez M.L., Diez A., Puyet A., Bautista J.M. Combined proteomic approaches for the identification of specific amino acid residues modified by 4-hydroxy-2-nonenal under physiological conditions. J. Proteome Res. 2010;9:5770–5781. doi: 10.1021/pr100555v. [DOI] [PubMed] [Google Scholar]
- 19.Madian A.G., Regnier F.E. Proteomic identification of carbonylated proteins and their oxidation sites. J. Proteome Res. 2010;9:3766–3780. doi: 10.1021/pr1002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levonen A.-L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G., Morrow J.D., Darley-Usmar V.M. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prakash J., Bansal R., Post E., de Jager-Krikken A., Lub-de Hooge M.N., Poelstra K. Albumin-binding and tumor vasculature determine the antitumor effect of 15-deoxy-Δ12,14-prostaglandin-J2in vivo. Neoplasia. 2009;11:1348–1358. doi: 10.1593/neo.91188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sánchez-Gómez F.J., Díez-Dacal B., Pajares M.A., Llorca O., Pérez-Sala D. Cyclopentenone prostaglandins with dienone structure promote cross-linking of the chemoresistance-inducing enzyme glutathione transferase P1-1. Mol. Pharmacol. 2010;78:723–733. doi: 10.1124/mol.110.065391. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.E., Park Y.S. Role of lipid peroxidation-derived α, β-unsaturated aldehydes in vascular dysfunction. Oxid. Med. Cell. Longev. 2013;2013:629028. doi: 10.1155/2013/629028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDowell R.E., McGeown J.G., Stitt A.W., Curtis T.M. Therapeutic potential of targeting lipid aldehydes and lipoxidation end-products in the treatment of ocular disease. Future Med. Chem. 2013;5:189–211. doi: 10.4155/fmc.12.202. [DOI] [PubMed] [Google Scholar]
- 25.Aldini G., Orioli M., Carini M. Protein modification by acrolein: relevance to pathological conditions and inhibition by aldehyde sequestering agents. Mol. Nutr. Food Res. 2011;55:1301–1319. doi: 10.1002/mnfr.201100182. [DOI] [PubMed] [Google Scholar]
- 26.Pamplona R. Advanced lipoxidation end-products. Chem. Biol. Interact. 2011;192:14–20. doi: 10.1016/j.cbi.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 27.Guéraud F. 4-Hydroxynonenal metabolites and adducts in pre-carcinogenic conditions and cancer. Free Radic. Biol. Med. 2017;111:196–208. doi: 10.1016/j.freeradbiomed.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Negre-Salvayre A., Auge N., Ayala V., Basaga H., Boada J., Brenke R., Chapple S., Cohen G., Feher J., Grune T., Lengyel G., Mann G.E., Pamplona R., Poli G., Portero-Otin M., Riahi Y., Salvayre R., Sasson S., Serrano J., Shamni O., Siems W., Siow R.C.M., Wiswedel I., Zarkovic K., Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 29.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 30.Shoeb M., Ansari N.H., Srivastava S.K., Ramana K.V. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr. Med. Chem. 2014;21:230–237. doi: 10.2174/09298673113209990181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarkovic K., Uchida K., Kolenc D., Hlupic L., Zarkovic N. Tissue distribution of lipid peroxidation product acrolein in human colon carcinogenesis. Free Radic. Res. 2006;40:543–552. doi: 10.1080/10715760500370048. [DOI] [PubMed] [Google Scholar]
- 32.Custovic Z., Zarkovic K., Cindric M., Cipak A., Jurkovic I., Sonicki Z., Uchida K., Zarkovic N. Lipid peroxidation product acrolein as a predictive biomarker of prostate carcinoma relapse after radical surgery. Free Radic. Res. 2010;44:497–504. doi: 10.3109/10715761003636831. [DOI] [PubMed] [Google Scholar]
- 33.Gęgotek A., Nikliński J., Žarković N., Žarković K., Waeg G., Łuczaj W., Charkiewicz R., Skrzydlewska E. Lipid mediators involved in the oxidative stress and antioxidant defence of human lung cancer cells. Redox Biol. 2016;9:210–219. doi: 10.1016/j.redox.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Csala M., Kardon T., Legeza B., Lizák B., Mandl J., Margittai É., Puskás F., Száraz P., Szelényi P., Bánhegyi G. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta - Mol. Basis Dis. 2015;1852:826–838. doi: 10.1016/j.bbadis.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Barrera G., Pizzimenti S., Dianzani M.U. Lipid peroxidation: control of cell proliferation, cell differentiation and cell death. Mol. Asp. Med. 2008;29:1–8. doi: 10.1016/j.mam.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Bur H., Haapasaari K.-M., Turpeenniemi-Hujanen T., Kuittinen O., Auvinen P., Marin K., Koivunen P., Sormunen R., Soini Y., Karihtala P. Oxidative stress markers and mitochondrial antioxidant enzyme expression are increased in aggressive Hodgkin lymphomas. Histopathology. 2014;65:319–327. doi: 10.1111/his.12389. [DOI] [PubMed] [Google Scholar]
- 37.Karihtala P., Kauppila S., Puistola U., Jukkola-Vuorinen A. Divergent behaviour of oxidative stress markers 8-hydroxydeoxyguanosine (8-OHdG) and 4-hydroxy-2-nonenal (HNE) in breast carcinogenesis. Histopathology. 2011;58:854–862. doi: 10.1111/j.1365-2559.2011.03835.x. [DOI] [PubMed] [Google Scholar]
- 38.Young O., Crotty T., O’Connell R., O’Sullivan J., Curran A.J. Levels of oxidative damage and lipid peroxidation in thyroid neoplasia. Head Neck. 2009 doi: 10.1002/hed.21247. [DOI] [PubMed] [Google Scholar]
- 39.Skrzydlewska E., Stankiewicz A., Sulkowska M., Sulkowski S., Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J. Toxicol. Environ. Heal. Part A. 2001;64:213–222. doi: 10.1080/15287390152543690. [DOI] [PubMed] [Google Scholar]
- 40.Milkovic L., Cipak Gasparovic A., Zarkovic N. Overview on major lipid peroxidation bioactive factor 4-hydroxynonenal as pluripotent growth-regulating factor. Free Radic. Res. 2015;49:850–860. doi: 10.3109/10715762.2014.999056. [DOI] [PubMed] [Google Scholar]
- 41.Tjalkens R.B., Cook L.W., Petersen D.R. Formation and export of the glutathione conjugate of 4-hydroxy-2,3-e-nonenal (4-HNE) in hepatoma cells. Arch. Biochem. Biophys. 1999;361:113–119. doi: 10.1006/abbi.1998.0946. [DOI] [PubMed] [Google Scholar]
- 42.G. Juric-Sekhar, K. Zarkovic, G. Waeg, A. Cipak, N. Zarkovic, Distribution of 4-hydroxynonenal-protein conjugates as a marker of lipid peroxidation and parameter of malignancy in astrocytic and ependymal tumors of the brain, Tumori. 95 (n.d.) 762–8. [DOI] [PubMed]
- 43.Dianzani M.U. Lipid peroxidation and cancer: a critical reconsideration. Tumor. J. 1989;75:351–357. doi: 10.1177/030089168907500410. [DOI] [PubMed] [Google Scholar]
- 44.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., Scrimieri F., Winter J.M., Hruban R.H., Iacobuzio-Donahue C., Kern S.E., Blair I.A., Tuveson D.A. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leinonen H.M., Kansanen E., Pölönen P., Heinäniemi M., Levonen A.-L. Role of the Keap1–Nrf2 pathway in cancer. Adv. Cancer Res. 2014;122:281–320. doi: 10.1016/B978-0-12-420117-0.00008-6. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y., Li W., Kong A.-N. Anti-oxidative stress regulator NF-E2-related factor 2 mediates the adaptive induction of antioxidant and detoxifying enzymes by lipid peroxidation metabolite 4-hydroxynonenal. Cell Biosci. 2012;2:40. doi: 10.1186/2045-3701-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanito M., Agbaga M.-P., Anderson R.E. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic. Biol. Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Player T.J., Mills D.J., Horton A.A. Lipid peroxidation of the microsomal fraction and extracted microsomal lipids from DAB-induced hepatomas. Br. J. Cancer. 1979;39:773–778. doi: 10.1038/bjc.1979.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammer A., Ferro M., Tillian H.M., Tatzber F., Zollner H., Schauenstein E., Schaur R.J. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative activity in hepatomas of different degrees of differentiation. Free Radic. Biol. Med. 1997;23:26–33. doi: 10.1016/s0891-5849(96)00630-2. [DOI] [PubMed] [Google Scholar]
- 50.Canuto R.A., Muzio G., Maggiora M., Biocca M.E., Dianzani M.U. Glutathione-S-transferase, alcohol dehydrogenase and aldehyde reductase activities during diethylnitrosamine-carcinogenesis in rat liver. Cancer Lett. 1993;68:177–183. doi: 10.1016/0304-3835(93)90144-x. [DOI] [PubMed] [Google Scholar]
- 51.Petrash J.M. All in the family: aldose reductase and closely related aldo-keto reductases. Cell. Mol. Life Sci. 2004;61:737–749. doi: 10.1007/s00018-003-3402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan R., Zu X., Ma J., Liu Z., Adeyanju M., Cao D. Aldo–keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: implication for cancer intervention. Int. J. Cancer. 2007;121:2301–2306. doi: 10.1002/ijc.22933. [DOI] [PubMed] [Google Scholar]
- 53.Martin H.-J., Breyer-Pfaff U., Wsol V., Venz S., Block S., Maser E. Purification and characterization of AKR1B10 from human liver: role in carbonyl reduction of xenobiotics. Drug Metab. Dispos. 2006;34:464–470. doi: 10.1124/dmd.105.007971. [DOI] [PubMed] [Google Scholar]
- 54.Díez-Dacal B., Gayarre J., Gharbi S., Timms J.F., Coderch C., Gago F., Pérez-Sala D. Identification of aldo-keto reductase AKR1B10 as a selective target for modification and inhibition by prostaglandin A1: implications for antitumoral activity. Cancer Res. 2011;71:4161–4171. doi: 10.1158/0008-5472.CAN-10-3816. [DOI] [PubMed] [Google Scholar]
- 55.Díez-Dacal B., Pérez-Sala D. A-class prostaglandins: early findings and new perspectives for overcoming tumor chemoresistance. Cancer Lett. 2012;320:150–157. doi: 10.1016/j.canlet.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Cagen L.M., Pisano J.J., Ketley J.N., Habig W.H., Jakoby W.B. The conjugation of prostaglandin A1 and glutathione catalyzed by homogeneous glutathione s-transferases from human and rat liver. Biochim. Biophys. Acta. 1975;398:205–208. doi: 10.1016/0005-2760(75)90184-8. [DOI] [PubMed] [Google Scholar]
- 57.van Iersel M.L., Cnubben N.H., Smink N., Koeman J.H., van Bladeren P.J. Interactions of prostaglandin A2 with the glutathione-mediated biotransformation system. Biochem. Pharmacol. 1999;57:1383–1390. doi: 10.1016/s0006-2952(99)00048-9. [DOI] [PubMed] [Google Scholar]
- 58.Sultana R., Butterfield D.A. Oxidatively modified GST and MRP1 in Alzheimer's disease brain: implications for accumulation of reactive lipid peroxidation products. Neurochem. Res. 2004;29:2215–2220. doi: 10.1007/s11064-004-7028-0. [DOI] [PubMed] [Google Scholar]
- 59.Gayarre J., Isabel Avellano M., Sanchez-Gomez F.J., Carrasco M.J., Canada F.J., Perez-Sala D. Modification of proteins by cyclopentenone prostaglandins is differentially modulated by GSH in vitro. Ann. N. Y. Acad. Sci. 2007;1096:78–85. doi: 10.1196/annals.1397.072. [DOI] [PubMed] [Google Scholar]
- 60.Sánchez-Gómez F.J., Gayarre J., Avellano M.I., Pérez-Sala D. Direct evidence for the covalent modification of glutathione-S-transferase P1-1 by electrophilic prostaglandins: implications for enzyme inactivation and cell survival. Arch. Biochem. Biophys. 2007;457:150–159. doi: 10.1016/j.abb.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 61.Su F., Hu X., Jia W., Gong C., Song E., Hamar P. Glutathion S transferase pi indicates chemotherapy resistance in breast cancer. J. Surg. Res. 2003;113:102–108. doi: 10.1016/s0022-4804(03)00200-2. [DOI] [PubMed] [Google Scholar]
- 62.Oberley T.D., Toyokuni S., Szweda L.I. Localization of hydroxynonenal protein adducts in normal human kidney and selected human kidney cancers. Free Radic. Biol. Med. 1999;27:695–703. doi: 10.1016/s0891-5849(99)00117-3. [DOI] [PubMed] [Google Scholar]
- 63.Jung K.-A., Kwak M.-K. Enhanced 4-hydroxynonenal resistance in KEAP1 silenced human colon cancer cells. Oxid. Med. Cell. Longev. 2013;2013:423965. doi: 10.1155/2013/423965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu W., Akhand A.A., Kato M., Yokoyama I., Miyata T., Kurokawa K., Uchida K., Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J. Cell Sci. 1999;112(Pt 14):2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 65.Andronicos N.M., Ranson M., Bognacki J., Baker M.S. The human ENO1 gene product (recombinant human alpha-enolase) displays characteristics required for a plasminogen binding protein. Biochim. Biophys. Acta. 1997;1337:27–39. doi: 10.1016/s0167-4838(96)00146-x. [DOI] [PubMed] [Google Scholar]
- 66.Aluise C.D., Rose K., Boiani M., Reyzer M.L., Manna J.D., Tallman K., Porter N.A., Marnett L.J. Peptidyl-prolyl cis / trans -isomerase A1 (Pin1) Is a target for modification by lipid electrophiles. Chem. Res. Toxicol. 2013;26:270–279. doi: 10.1021/tx300449g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner T.M., Mullally J.E., Fitzpatrick F.A. Reactive lipid species from cyclooxygenase-2 inactivate tumor suppressor LKB1/STK11. J. Biol. Chem. 2006;281:2598–2604. doi: 10.1074/jbc.M509723200. [DOI] [PubMed] [Google Scholar]
- 68.Pizzimenti S., Laurora S., Briatore F., Ferretti C., Dianzani M.U., Barrera G. Synergistic effect of 4-hydroxynonenal and PPAR ligands in controlling human leukemic cell growth and differentiation. Free Radic. Biol. Med. 2002;32:233–245. doi: 10.1016/s0891-5849(01)00798-5. [DOI] [PubMed] [Google Scholar]
- 69.Backos D.S., Fritz K.S., Roede J.R., Petersen D.R., Franklin C.C. Posttranslational modification and regulation of glutamate-cysteine ligase by the α,β-unsaturated aldehyde 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2011;50:14–26. doi: 10.1016/j.freeradbiomed.2010.10.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gentile F., Pizzimenti S., Arcaro A., Pettazzoni P., Minelli R., D’Angelo D., Mamone G., Ferranti P., Toaldo C., Cetrangolo G., Formisano S., Dianzani M.U., Uchida K., Dianzani C., Barrera G. Exposure of HL-60 human leukaemic cells to 4-hydroxynonenal promotes the formation of adduct(s) with α-enolase devoid of plasminogen binding activity. Biochem. J. 2009;422:285–294. doi: 10.1042/BJ20090564. [DOI] [PubMed] [Google Scholar]
- 71.Ji C., Kozak K.R., Marnett L.J. IκB kinase, a Molecular target for Inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 72.Okada K., Wangpoengtrakul C., Osawa T., Toyokuni S., Tanaka K., Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J. Biol. Chem. 1999;274:23787–23793. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- 73.Schrader E.K., Harstad K.G., Matouschek A. Targeting proteins for degradation. Nat. Chem. Biol. 2009;5:815–822. doi: 10.1038/nchembio.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grune T., Davies K.J.A. The proteasomal system and HNE-modified proteins. Mol. Asp. Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 75.Imajoh-Ohmi S., Kawaguchi T., Sugiyama S., Tanaka K., Omura S., Kikuchi H. Lactacystin, a specific inhibitor of the proteasome, induces apoptosis in human monoblast U937 cells. Biochem. Biophys. Res. Commun. 1995;217:1070–1077. doi: 10.1006/bbrc.1995.2878. [DOI] [PubMed] [Google Scholar]
- 76.Shinohara K., Tomioka M., Nakano H., Toné S., Ito H., Kawashima S. Apoptosis induction resulting from proteasome inhibition. Biochem. J. 1996;317(Pt 2):385–388. doi: 10.1042/bj3170385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Drexler H.C. Activation of the cell death program by inhibition of proteasome function. Proc. Natl. Acad. Sci. USA. 1997;94:855–860. doi: 10.1073/pnas.94.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 79.Pettazzoni P., Ciamporcero E., Medana C., Pizzimenti S., Dal Bello F., Minero V.G., Toaldo C., Minelli R., Uchida K., Dianzani M.U., Pili R., Barrera G. Nuclear factor erythroid 2-related factor-2 activity controls 4-hydroxynonenal metabolism and activity in prostate cancer cells. Free Radic. Biol. Med. 2011;51:1610–1618. doi: 10.1016/j.freeradbiomed.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 80.Michalik L., Wahli W. PPARs mediate lipid signaling in inflammation and cancer. PPAR Res. 2008;2008:134059. doi: 10.1155/2008/134059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michalik L., Desvergne B., Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat. Rev. Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 82.Peters J.M., Shah Y.M., Gonzalez F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer. 2012;12:181–195. doi: 10.1038/nrc3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang X.Y., Wang L.H., Mihalic K., Xiao W., Chen T., Li P., Wahl L.M., Farrar W.L. Interleukin (IL)-4 indirectly suppresses IL-2 production by human T lymphocytes via peroxisome proliferator-activated receptor γ activated by macrophage-derived 12/15-lipoxygenase ligands. J. Biol. Chem. 2002;277:3973–3978. doi: 10.1074/jbc.M105619200. [DOI] [PubMed] [Google Scholar]
- 84.Wang L.H., Yang X.Y., Zhang X., Huang J., Hou J., Li J., Xiong H., Mihalic K., Zhu H., Xiao W., Farrar W.L. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity. 2004;20:205–218. doi: 10.1016/s1074-7613(04)00030-5. [DOI] [PubMed] [Google Scholar]
- 85.Kostadinova R., Wahli W., Michalik L. PPARs in diseases: control mechanisms of inflammation. Curr. Med. Chem. 2005;12:2995–3009. doi: 10.2174/092986705774462905. [DOI] [PubMed] [Google Scholar]
- 86.Forman B.M., Tontonoz P., Chen J., Brun R.P., Spiegelman B.M., Evans R.M. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 87.Shiraki T., Kamiya N., Shiki S., Kodama T.S., Kakizuka A., Jingami H. α,β-Unsaturated ketone is a core moiety of natural ligands for covalent binding to peroxisome proliferator-activated receptor γ. J. Biol. Chem. 2005;280:14145–14153. doi: 10.1074/jbc.M500901200. [DOI] [PubMed] [Google Scholar]
- 88.Soares A.F., Nosjean O., Cozzone D., D’Orazio D., Becchi M., Guichardant M., Ferry G., Boutin J.A., Lagarde M., Géloën A. Covalent binding of 15-deoxy-delta12,14-prostaglandin J2 to PPARγ. Biochem. Biophys. Res. Commun. 2005;337:521–525. doi: 10.1016/j.bbrc.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 89.Reddy A.T., Lakshmi S.P., Banno A., Reddy R.C. Identification and molecular characterization of peroxisome proliferator-activated receptor δ as a novel target for covalent modification by 15-deoxy-Δ 12,14 -prostaglandin J 2. ACS Chem. Biol. 2018 doi: 10.1021/acschembio.8b00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Coleman J.D., Prabhu K.S., Thompson J.T., Reddy P.S., Peters J.M., Peterson B.R., Reddy C.C., Vanden Heuvel J.P. The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) Free Radic. Biol. Med. 2007;42:1155–1164. doi: 10.1016/j.freeradbiomed.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aldini G., Vistoli G., Regazzoni L., Gamberoni L., Facino R.M., Yamaguchi S., Uchida K., Carini M. Albumin is the main nucleophilic target of human plasma: a protective role against pro-atherogenic electrophilic reactive carbonyl species? Chem. Res. Toxicol. 2008;21:824–835. doi: 10.1021/tx700349r. [DOI] [PubMed] [Google Scholar]
- 92.Baraibar M.A., Friguet B. Oxidative proteome modifications target specific cellular pathways during oxidative stress, cellular senescence and aging. Exp. Gerontol. 2013;48:620–625. doi: 10.1016/j.exger.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Waku T., Shiraki T., Oyama T., Morikawa K. Atomic structure of mutant PPARγ LBD complexed with 15d-PGJ 2: novel modulation mechanism of PPARγ/RXRα function by covalently bound ligands. FEBS Lett. 2009:320–324. doi: 10.1016/j.febslet.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 94.Stemmer U., Hermetter A. Protein modification by aldehydophospholipids and its functional consequences. Biochim. Biophys. Acta. 1818;2012:2436–2445. doi: 10.1016/j.bbamem.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Garzón B., Gayarre J., Gharbi S., Díez-Dacal B., Sánchez-Gómez F.J., Timms J.F., Pérez-Sala D. A biotinylated analog of the anti-proliferative prostaglandin A1 allows assessment of PPAR-independent effects and identification of novel cellular targets for covalent modification. Chem. Biol. Interact. 2010;183:212–221. doi: 10.1016/j.cbi.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 96.Zorrilla S., Garzón B., Pérez-Sala D. Selective binding of the fluorescent dye 1-anilinonaphthalene-8-sulfonic acid to peroxisome proliferator-activated receptor γ allows ligand identification and characterization. Anal. Biochem. 2010;399:84–92. doi: 10.1016/j.ab.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 97.Díez-Dacal B., Pérez-Sala D. Anti-inflammatory prostanoids: focus on the interactions between electrophile signaling and resolution of inflammation. Sci. World J. 2010;10:655–675. doi: 10.1100/tsw.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Konturek P.C., Konturek S.J., Brzozowski T. Gastric cancer and Helicobacter pylori infection. J. Physiol. Pharmacol. 2006;57(Suppl 3):51–65. [PubMed] [Google Scholar]
- 99.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat. Rev. Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 100.Bartsch H., Nair J. Accumulation of lipid peroxidation-derived DNA lesions: potential lead markers for chemoprevention of inflammation-driven malignancies. Mutat. Res. Mol. Mech. Mutagen. 2005;591:34–44. doi: 10.1016/j.mrfmmm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 101.Lee J.Y., Je J.H., Jung K.J., Yu B.P., Chung H.Y. Induction of endothelial iNOS by 4-hydroxyhexenal through NF-κB activation. Free Radic. Biol. Med. 2004;37:539–548. doi: 10.1016/j.freeradbiomed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 102.Kumagai T., Matsukawa N., Kaneko Y., Kusumi Y., Mitsumata M., Uchida K., Lipid Peroxidation-derived A. Inflammatory mediator. J. Biol. Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 103.Wang G., Ansari G.A.S., Khan M.F. Involvement of lipid peroxidation-derived aldehyde–protein adducts in autoimmunity mediated by trichloroethene. J. Toxicol. Environ. Heal. Part A. 2007;70:1977–1985. doi: 10.1080/15287390701550888. [DOI] [PubMed] [Google Scholar]
- 104.Nitti M., Domenicotti C., d’Abramo C., Assereto S., Cottalasso D., Melloni E., Poli G., Biasi F., Marinari U., Pronzato M. Activation of PKC-β isoforms mediates HNE-induced MCP-1 release by macrophages. Biochem. Biophys. Res. Commun. 2002;294:547–552. doi: 10.1016/S0006-291X(02)00512-0. [DOI] [PubMed] [Google Scholar]
- 105.Page S., Fischer C., Baumgartner B., Haas M., Kreusel U., Loidl G., Hayn M., Ziegler-Heitbrock H.W., Neumeier D., Brand K. 4-Hydroxynonenal prevents NF-kappaB activation and tumor necrosis factor expression by inhibiting IkappaB phosphorylation and subsequent proteolysis. J. Biol. Chem. 1999;274:11611–11618. doi: 10.1074/jbc.274.17.11611. [DOI] [PubMed] [Google Scholar]
- 106.Donath B., Fischer C., Page S., Prebeck S., Jilg N., Weber M., da Costa C., Neumeier D., Miethke T., Brand K. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 107.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 108.Je J.H., Lee J.Y., Jung K.J., Sung B., Go E.K., Yu B.P., Chung H.Y. NF-κB activation mechanism of 4-hydroxyhexenal via NIK/IKK and p38 MAPK pathway. FEBS Lett. 2004;566:183–189. doi: 10.1016/j.febslet.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 109.Straus D.S., Pascual G., Li M., Welch J.S., Ricote M., Hsiang C.H., Sengchanthalangsy L.L., Ghosh G., Glass C.K. 15-deoxy-Delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc. Natl. Acad. Sci. USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cernuda-Morollón E., Pineda-Molina E., Cañada F.J., Pérez-Sala D. 15-Deoxy-Δ 12,14 -prostaglandin J 2 inhibition of NF-κB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 111.Trivedi S.G., Newson J., Rajakariar R., Jacques T.S., Hannon R., Kanaoka Y., Eguchi N., Colville-Nash P., Gilroy D.W. Essential role for hematopoietic prostaglandin D2 synthase in the control of delayed type hypersensitivity. Proc. Natl. Acad. Sci. USA. 2006;103:5179–5184. doi: 10.1073/pnas.0507175103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rajakariar R., Hilliard M., Lawrence T., Trivedi S., Colville-Nash P., Bellingan G., Fitzgerald D., Yaqoob M.M., Gilroy D.W. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyΔ12–14 PGJ2. Proc. Natl. Acad. Sci. USA. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xia Y., Shen S., Verma I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014;2:823–830. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim Y.S., Park Z.Y., Kim S.Y., Jeong E., Lee J.Y. Alteration of Toll-like receptor 4 activation by 4-hydroxy-2-nonenal mediated by the suppression of receptor homodimerization. Chem. Biol. Interact. 2009;182:59–66. doi: 10.1016/j.cbi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Wang G., König R., Ansari G.A.S., Khan M.F. Lipid peroxidation-derived aldehyde–protein adducts contribute to trichloroethene-mediated autoimmunity via activation of CD4+ T cells. Free Radic. Biol. Med. 2008;44:1475–1482. doi: 10.1016/j.freeradbiomed.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Knutson K.L., Disis M.L. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol. Immunother. 2005;54:721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shanmugam N., Figarola J.L., Li Y., Swiderski P.M., Rahbar S., Natarajan R. Proinflammatory effects of advanced lipoxidation end products in monocytes. Diabetes. 2008;57:879–888. doi: 10.2337/db07-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chacko B.K., Wall S.B., Kramer P.A., Ravi S., Mitchell T., Johnson M.S., Wilson L., Barnes S., Landar A., Darley-Usmar V.M. Pleiotropic effects of 4-hydroxynonenal on oxidative burst and phagocytosis in neutrophils. Redox Biol. 2016;9:57–66. doi: 10.1016/j.redox.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zarkovic N., Ilic Z., Jurin M., Schaur R.J., Puhl H., Esterbauer H. Stimulation of HeLa cell growth by physiological concentrations of 4‐hydroxynonenal. Cell Biochem. Funct. 1993;11:279–286. doi: 10.1002/cbf.290110409. [DOI] [PubMed] [Google Scholar]
- 121.Masotti L., Casali E., Galeoti T. Lipid peroxidation in tumour cells. Free Radic. Biol. Med. 1988;4:377–386. doi: 10.1016/0891-5849(88)90089-5. [DOI] [PubMed] [Google Scholar]
- 122.Burton S.T., Ingold G.W., Cheeseman K.U. KH, application of deuterated alpha-tocopherols to the biokinetics and bioavailability of vitamin E. Free Radic. Res. Commun. 1990;11:99–107. doi: 10.3109/10715769009109672. [DOI] [PubMed] [Google Scholar]
- 123.Kreuzer T., Grube R., Wutte A., Zarkovic N., Schaur R.J. 4-Hydroxynonenal modifies the effects of serum growth factors on the expression of the c-fos proto-oncogene and the proliferation of HeLa carcinoma cells. Free Radic. Biol. Med. 1998;25:42–49. doi: 10.1016/s0891-5849(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 124.Wang X., Allen T.D., Yang Y., Moore D.R., Huycke M.M. Cyclooxygenase-2 generates the endogenous mutagen trans-4-hydroxy-2-nonenal in Enterococcus faecalis-infected macrophages. Cancer Prev. Res. 2013;6:206–216. doi: 10.1158/1940-6207.CAPR-12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Natarajan K., Mathialagan G.D., Raghavan S., Shanmugam N. The advanced lipoxidation end product precursor malondialdehyde induces IL-17E expression and skews lymphocytes to the Th17 subset. Cell. Mol. Biol. Lett. 2015;20:647–662. doi: 10.1515/cmble-2015-0038. [DOI] [PubMed] [Google Scholar]
- 126.Dunér P., To F., Berg K., Alm R., Björkbacka H., Engelbertsen D., Fredrikson G.N., Nilsson J., Bengtsson E. Immune responses against aldehyde-modified laminin accelerate atherosclerosis in Apoe−/− mice. Atherosclerosis. 2010;212:457–465. doi: 10.1016/j.atherosclerosis.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 127.Biswas S.K. Metabolic reprogramming of immune cells in cancer progression. Immunity. 2015;43:435–449. doi: 10.1016/j.immuni.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 128.Jha A.K., Huang S.C.-C., Sergushichev A., Lampropoulou V., Ivanova Y., Loginicheva E., Chmielewski K., Stewart K.M., Ashall J., Everts B., Pearce E.J., Driggers E.M., Artyomov M.N. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 129.Senovilla L., Galluzzi L., Zitvogel L., Kroemer G. Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol. 2013;34:471–481. doi: 10.1016/j.it.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 130.Zitvogel L., Tesniere A., Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 131.Galluzzi L., Senovilla L., Zitvogel L., Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat. Rev. Drug Discov. 2012;11:215–233. doi: 10.1038/nrd3626. [DOI] [PubMed] [Google Scholar]
- 132.Zitvogel L., Galluzzi L., Smyth M.J., Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39:74–88. doi: 10.1016/j.immuni.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 133.Fucikova J., Moserova I., Urbanova L., Bezu L., Kepp O., Cremer I., Salek C., Strnad P., Kroemer G., Galluzzi L., Spisek R. Prognostic and predictive value of DAMPs and DAMP-associated processes in cancer. Front. Immunol. 2015;6:402. doi: 10.3389/fimmu.2015.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin C.-Y., Wang W.-H., Chen S.-H., Chang Y.-W., Hung L.-C., Chen C.-Y., Chen Y.-H. Lipopolysaccharide-induced nitric oxide, prostaglandin E2, and cytokine production of mouse and human macrophages are suppressed by pheophytin-b. Int. J. Mol. Sci. 2017;18:2637. doi: 10.3390/ijms18122637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cohen M.S., Ryan J.L., Root R.K. The oxidative metabolism of thioglycollate-elicited mouse peritoneal macrophages: the relationship between oxygen, superoxide and hydrogen peroxide and the effect of monolayer formation. J. Immunol. 1981;127:1007–1011. [PubMed] [Google Scholar]
- 136.Zarkovic N., Cipak A., Jaganjac M., Borovic S., Zarkovic K. Pathophysiological relevance of aldehydic protein modifications. J. Proteom. 2013;92:239–247. doi: 10.1016/j.jprot.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 137.Evans M.K., Sauer S.J., Nath S., Robinson T.J., Morse M.A., Devi G.R. X-linked inhibitor of apoptosis protein mediates tumor cell resistance to antibody-dependent cellular cytotoxicity. Cell Death Dis. 2016;7:e2073. doi: 10.1038/cddis.2015.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mimura K., Kua L.-F., Shimasaki N., Shiraishi K., Nakajima S., Siang L.K., Shabbir A., So J., Yong W.-P., Kono K. Upregulation of thioredoxin-1 in activated human NK cells confers increased tolerance to oxidative stress. Cancer Immunol. Immunother. 2017;66:605–613. doi: 10.1007/s00262-017-1969-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Grune T., Michel P., Sitte N., Eggert W., Albrecht-Nebe H., Esterbauer H., Siems W.G. Increased levels of 4-hydroxynonenal modified proteins in plasma of children with autoimmune diseases. Free Radic. Biol. Med. 1997;23:357–360. doi: 10.1016/s0891-5849(96)00586-2. [DOI] [PubMed] [Google Scholar]
- 140.Vila A., Tallman K.A., Jacobs A.T., Liebler D.C., Porter N.A., Marnett L.J. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem. Res. Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suc I., Escargueil-Blanc I., Troly M., Salvayre R., Nègre-Salvayre A. HDL and ApoA prevent cell death of endothelial cells induced by oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 1997;17:2158–2166. doi: 10.1161/01.atv.17.10.2158. [DOI] [PubMed] [Google Scholar]
- 142.Escargueil-Blanc I., Salvayre R., Vacaresse N., Jürgens G., Darblade B., Arnal J.F., Parthasarathy S., Nègre-Salvayre A. Mildly oxidized LDL induces activation of platelet-derived growth factor beta-receptor pathway. Circulation. 2001;104:1814–1821. doi: 10.1161/hc4001.097179. [DOI] [PubMed] [Google Scholar]
- 143.Vindis C., Escargueil-Blanc I., Elbaz M., Marcheix B., Grazide M.-H., Uchida K., Salvayre R., Nègre-Salvayre A. Desensitization of platelet-derived growth factor receptor-beta by oxidized lipids in vascular cells and atherosclerotic lesions: prevention by aldehyde scavengers. Circ. Res. 2006;98:785–792. doi: 10.1161/01.RES.0000216288.93234.c3. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Z., Ren X., Lu X., Wang D., Hu X., Zheng Y., Song L., Pang H., Yu R., Ding K. GZD856, a novel potent PDGFRα/β inhibitor, suppresses the growth and migration of lung cancer cells in vitro and in vivo. Cancer Lett. 2016;375:172–178. doi: 10.1016/j.canlet.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 145.Cheng J.-Z., Singhal S.S., Sharma A., Saini M., Yang Y., Awasthi S., Zimniak P., Awasthi Y.C. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch. Biochem. Biophys. 2001;392:197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- 146.McElhanon K.E., Bose C., Sharma R., Wu L., Awasthi Y.C., Singh S.P. Gsta4 null mouse embryonic fibroblasts exhibit enhanced sensitivity to oxidants: role of 4-hydroxynonenal in oxidant toxicity. Open J. Apoptosis. 2013;02:1–11. doi: 10.4236/ojapo.2013.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chen Z.-H., Saito Y., Yoshida Y., Sekine A., Noguchi N., Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J. Biol. Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 148.Siow R.C.M., Ishii T., Mann G.E. Modulation of antioxidant gene expression by 4-hydroxynonenal: atheroprotective role of the Nrf2/ARE transcription pathway. Redox Rep. 2007;12:11–15. doi: 10.1179/135100007X162167. [DOI] [PubMed] [Google Scholar]
- 149.Shibata T., Yamada T., Ishii T., Kumazawa S., Nakamura H., Masutani H., Yodoi J., Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J. Biol. Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- 150.Cassidy P.B., Edes K., Nelson C.C., Parsawar K., Fitzpatrick F.A., Moos P.J. Thioredoxin reductase is required for the inactivation of tumor suppressor p53 and for apoptosis induced by endogenous electrophiles. Carcinogenesis. 2006;27:2538–2549. doi: 10.1093/carcin/bgl111. [DOI] [PubMed] [Google Scholar]
- 151.Sharma R., Sharma A., Dwivedi S., Zimniak P., Awasthi S., Awasthi Y.C. 4-Hydroxynonenal self-limits fas-mediated DISC-independent apoptosis by promoting export of Daxx from the nucleus to the cytosol and its binding to fas †. Biochemistry. 2008;47:143–156. doi: 10.1021/bi701559f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lalier L., Cartron P.-F., Olivier C., Logé C., Bougras G., Robert J.-M., Oliver L., Vallette F.M. Prostaglandins antagonistically control Bax activation during apoptosis. Cell Death Differ. 2011;18:528–537. doi: 10.1038/cdd.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Covey T.M., Edes K., Coombs G.S., Virshup D.M., Fitzpatrick F.A. Alkylation of the tumor suppressor PTEN activates Akt and β-catenin signaling: a mechanism linking inflammation and oxidative stress with cancer. PLoS One. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shearn C.T., Fritz K.S., Reigan P., Petersen D.R. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50:3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 155.Shearn C.T., Smathers R.L., Backos D.S., Reigan P., Orlicky D.J., Petersen D.R. Increased carbonylation of the lipid phosphatase PTEN contributes to Akt2 activation in a murine model of early alcohol-induced steatosis. Free Radic. Biol. Med. 2013;65:680–692. doi: 10.1016/j.freeradbiomed.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Renedo M., Gayarre J., García-Domínguez C.A., Pérez-Rodríguez A., Prieto A., Cañada F.J., Rojas J.M., Pérez-Sala D. Modification and activation of ras proteins by electrophilic prostanoids with different structure are site-selective †. Biochemistry. 2007;46:6607–6616. doi: 10.1021/bi602389p. [DOI] [PubMed] [Google Scholar]
- 157.Parola M., Robino G., Marra F., Pinzani M., Bellomo G., Leonarduzzi G., Chiarugi P., Camandola S., Poli G., Waeg G., Gentilini P., Dianzani M.U. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J. Clin. Investig. 1998;102:1942–1950. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pérez-Sala D., Cernuda-Morollón E., Cañada F.J. Molecular basis for the direct inhibition of AP-1 DNA binding by 15-deoxy-delta 12,14-prostaglandin J2. J. Biol. Chem. 2003;278:51251–51260. doi: 10.1074/jbc.M309409200. [DOI] [PubMed] [Google Scholar]
- 159.Bauer G., Zarkovic N. Revealing mechanisms of selective, concentration-dependent potentials of 4-hydroxy-2-nonenal to induce apoptosis in cancer cells through inactivation of membrane-associated catalase. Free Radic. Biol. Med. 2015;81:128–144. doi: 10.1016/j.freeradbiomed.2015.01.010. [DOI] [PubMed] [Google Scholar]