Abstract
Cellular processes are dictated by the active signaling of proteins relaying messages to regulate cell proliferation, apoptosis, signal transduction and cell communications. An intricate web of protein kinases and phosphatases are critical to the proper transmission of signals across such cascades. By governing 30–50% of all protein dephosphorylation in the cell, with prominent substrate proteins being key regulators of signaling cascades, the phosphatase PP2A has emerged as a celebrated player in various developmental and tumorigenic pathways, thereby posing as an attractive target for therapeutic intervention in various pathologies wherein its activity is deregulated. This review is mainly focused on refreshing our understanding of the structural and functional complexity that cocoons the PP2A phosphatase, and its expression in cancers. Additionally, we focus on its physiological regulation as well as into recent advents and strategies that have shown promise in countering the deregulation of the phosphatase through its targeted reactivation. Finally, we dwell upon one of the key regulators of PP2A in cancer cells-cellular redox status-its multifarious nature, and its integration into the reactome of PP2A, highlighting some of the significant impacts that ROS can inflict on the structural modifications and functional aspect of PP2A.
Keywords: Oncogenesis, Tumor suppressor, ROS, Peroxynitrite, PP2A
1. Protein phosphatases
Post-translational modifications (PTMs) of proteins serve an important function in signaling cascades by limiting the activation and counter-inhibition of moieties in response to appropriate stimuli. In a 2011 study by Khoury et al. phosphorylation modifications were identified as the dominating experimental PTM on proteins [1]. The three putative phosphorylation residues on proteins include serine (S), threonine (T) and tyrosine (Y) wherein phosphoserine has emerged as the predominant site with the highest degree of phosphorylations amongst the three sites, followed by the two latter sites respectively [2]. Usually, the kinases donate the phosphate group, which attaches itself to the hydroxyl (-OH) group on the amino acid residues. The activity of kinases is countered by protein phosphatases which remove the phosphate group on proteins through a nucleophilic reaction in the presence of water [3]. The protein phosphatases fall under three broad categories: the protein serine/threonine phosphatases (PSPs), phosphotyrosine phosphatases (PTPs) and the dual specificity phosphatases (DUSP), which can dephosphorylate all three residues [4]. PSPs are further subclassified into three distinct subcategories: phosphoprotein phosphatases (PPPs), the metal-dependent protein phosphatases (PPMs) and the aspartate-based phosphatases. Subsequent classification of PPPs divides them into subfamilies PP1, PP2A, calcineurin and PP4-PP7, while the PPM family which catalyses Mg2+/Mn2+ dependent dephosphorylations, comprises PP2C and pyruvate dehydrogenase phosphatases. Lastly, the aspartate-based phosphatases distinctly carry an aspartate acid signature (DXDXT/V) and include the TFIIF-associated C-terminal domain phosphatase/small CTD phosphatases and the halo acid dehydrogenase (HAD) enzyme family [5]. Recently, a novel family of phosphatases has been identified that lends itself to the dephosphorylation of phosphohistidine residues [6]. This phosphatase is not well studied yet but seems to play a vital role in some cancers as well as insulin signaling [7], [8], [9]
Compared to the serine/threonine kinases (S/T kinases), of which there exists a vast number making up the kinome, far fewer protein S/T phosphatases exist at a ratio of 30 phosphatases for 420 kinases [3], [10]. However, protein phosphatases compensate their inadequate aggregate through the vast number of regulatory isoform subunits that control their substrate specificity, and thus achieve a measurable functional diversity that is commensurate to antagonise the activity of S/T kinases. Early research on S/T phosphatase function was limited to studies that focused on pharmacological inactivation of the phosphatases through naturally occurring compounds such as the extracts obtained from the marine toxins okadaic acid, calyculin A, tautomycin and microcystins which can strongly inhibit the catalytic activity of PP1 and PP2A [11], [12], [13], [14], [15]. In recent years however, there is a surge in the quest to characterise these phosphatases as they exert their effects on a myriad of proteins that are key intermediates of oncogenic and developmental pathways. This review focuses on the latter of the two phosphatases, PP2A as PP2A has significant precedence in that it is ubiquitously expressed in various cell types, with some of the highest expression reported for its catalytic subunit in cells; and is known to orchestrate cellular events by controlling on average 1% of all protein dephosphorylation events [16]. Therefore, it becomes imperative to characterise the phosphatase PP2A and understand the biology sustaining its functions towards targeting the phosphatase to its myriad of substrates in the various subcellular compartments. (Table 1).
Table 1.
ROS and PP2A interplay: A summary of the different ROS mediated functional impact on PP2A expression, holoenzyme assembly and activity.
| ROS milieu | Targeted PP2A subunit | Functional impact | References |
|---|---|---|---|
| Hydrogen peroxide | PP2A C | Inactivation | [250], [251], [252] |
| Unknown | Activation | [254], [255] | |
| PP2A immunocomplex | Inactivation | [253] | |
| Thiol oxidation | PP2A C/Unknown | Inactivation | [256], [257] |
| Hypoxia | PP2A A | Activation | [258] |
| PP2A B55α | Activation | [259] | |
| PP2A C | Inactivation by loss of expression | [260] | |
| Nitric oxide | PP2A C | Activation | [171], [261], [262], [263] |
| Peroxynitrite | PP2A B56δ | Inactivation | [242] |
2. Serine/threonine phosphatase PP2A
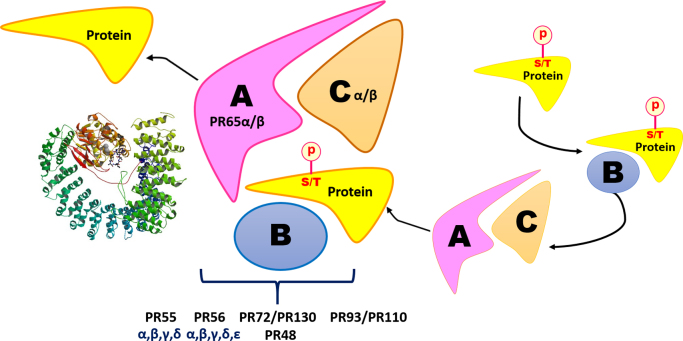
The phosphatase PP2A is a critical mediator of key cellular processes such as glycolytic, fatty acid and lipid metabolism, signal transduction, DNA replication and mitosis, cell proliferation and apoptosis, cytoskeletal organization, protein translation and immune regulation [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]. It is ubiquitously expressed in several subcellular compartments such as the nucleus, plasma membrane and the cytoplasm. PP2A is broadly derived from three structural compartments: the scaffold A subunit (PP2A A), the regulatory B subunit (PP2A B) and the catalytic C subunit [27]. The PP2A A and PP2A C can exist as a dimeric complex and is considered the core enzyme, while the B regulatory subunit can exist singularly. The trimeric complex formed upon the binding of PP2A B to the PP2A AC core dimer comprises the active holoenzyme complex (Fig. 1).
Fig. 1.
PP2A structure and function: Serine/threonine phosphorylated proteins interact with substrate specific B regulatory subunits, which subsequently recruits the substrate protein to the core enzyme. Holoenzyme formation is stabilized by the binding of the phosphorylated substrate to PP2A-B. The catalytic subunit then proceeds to dephosphorylate the substrate. Crystal structure of PP2A (2IAE) adapted from RCSB PDB – Cho and Xu [277].
The catalytic subunit of PP2A (PP2A C) is ubiquitously expressed in mammals. The full length PP2A C subunit comprises 309 amino acids. Two isoforms of PP2A C, Cα and Cβ, are transcribed by two distinct genes, but are highly similar in their sequence with over 97% consensus [28]. However, despite this high degree of sequence conservation, PP2A Cα knock out mice are embryonically lethal, suggesting that the two isoforms are non-redundant and emphasising the importance of the Cα isoform in embryonic development. While the Cα isoform is predominantly detected in the membrane fraction, the Cβ isoform is mostly found in the cytosol or the nuclear fractions [27]. The highly conserved C-terminal amino acids 304TPDYEL309 is essential for the binding of PP2A C to the A scaffold and B regulatory subunits [29], [30], [31], [32].
Correspondingly, the scaffold subunit of PP2A – PP2A A, also exists as two separate isoforms transcribed by two distinct genes PPP2R1A and PPP2R1B as Aα and Aβ subunits, which share 86% sequence homology [33]. Both isoforms are localised in the cytoplasm. The Aα isoform predominates with about 90% of PP2A A expressed as this isoform. The A subunit of PP2A has distinct functions in the dimeric and trimeric enzyme complex. While in the dimeric complex it regulates the catalytic specificity of the enzyme, it mainly serves as a base to aid the interaction between the core dimer, the regulatory subunit and the enzyme substrates in the trimeric holoenzyme complex. It is also structurally disparate in comparison to the other PP2A subunits in comprising HEAT (Huntingtin, Elongation factor 3, PP2A A, and the yeast kinase TOR1) repeats, named after the proteins that contain these tandem repeats. While PP2Ac binds to the domain comprising HEAT repeats 11–15, the regulatory subunits bind repeats 1–10. The AC core dimer binding results in the bending of the A subunit along the loop regions leading to the formation of a pocket that is ideal for the recruitment of the substrate bound B regulatory subunit [32].
The PP2A regulatory subunits are further divided into sub-classes – PP2A B/B55/PR55, PP2A B′/B56/PR56/PR61, PP2A B′′/PR48/PR72/PR130 and the PP2A B′′′/PR93/PR110. The numbers associated with these subfamilies indicate the molecular weights of the isoforms comprising these subunits.
The PP2A B55 subfamily consists of four distinct isoforms, the alpha (α), beta (β), gamma (γ) and delta (δ). The B55 subfamily is distinct from the other regulatory subfamilies in comprising WD40 repeat domains which regulate substrate binding. While the functions of the B55 family members are mostly concentrated on the regulation of cytokinesis and mitotic activity, the B55γ and B55β isoforms are reported to be developmentally regulated and regulate cellular events such as cytoskeletal dynamics and cellular differentiation [34], [35], [36], [37], [38], [39]. Moreover, there also exists a tissue specific distribution of these isoforms, wherein, the B55α and B55δ isoforms are ubiquitously expressed in almost all mammalian tissues; however, the B55β and B55γ are more pronounced in the brain [40], [41]. The B55α isoform has also been known to play a role in cell proliferation and survival through its regulation of one of the focal oncoproteins in the PI3K signaling pathway, AKT/PKB, as well as play an important role in regulating replication stress mediated through the regulation of the DNA repair enzyme RPA2 [42], [43]. In the latter aspect, there is now a growing body of evidence indicating a pivotal role for the B55α subunit in DNA damage response through its regulation of substrates RPA2, Plk-1, Ku-70, ATM and γ-H2AX, which are all key orchestrators of DNA repair [44], [45], [46], [47]. These reports highlight a critical role for the B55-PP2A-AC complex in cell cycle, proliferation and DNA repair, suggesting the paramount significance of the deregulation of this complex in cancers.
The PP2A B56 subfamily has five different isoforms – the alpha (α), beta (β), gamma (γ), delta (δ) and epsilon (ε). Their unique attributes include the feature that this is the only subfamily whose members might themselves be phosphorylated. Studies have indicated the importance of such phosphorylation in regulating the function of PP2A through affecting holoenzyme assembly or other yet unknown mechanisms [48], [49], [50]. They are also structurally distinct in that most members have α helix structures instead of β strand repeats that are noted for the members of the other subfamilies [31], [32]. Their sequences are highly conserved with differences mainly apparent only in the N and C terminal regions which lead to their differential expression in tissues. While B56α, B56β and B56ε have been identified only in the cytoplasm, the B56γ and B56δ isoforms are also localised to the nucleus [27]. The B56 family isoforms have diverse functions in shaping the cancer proteome through their regulation of key cellular processes such as DNA repair, cellular proliferation and differentiation, apoptosis, angiogenesis, invasion and migration [51], [52], [53].
The PR48/72/130 isoforms were first isolated from rabbit skeletal muscle through chromatography and gel filtration. While the PR72 isoform is only expressed in the heart and skeletal muscle tissue, the other isoforms are more ubiquitously expressed [54]. The PP2A B′′ isoforms are poorly studied. The Human Protein Atlas database reports favourable outcome of this regulatory subunit in several cancers, although some cancers such as those of liver, pancreatic and endometrial origin predict an unfavourable outcome of their expression [55]. Their exclusive feature includes the presence of calcium binding motifs which in response to calcium mediated signaling can regulate the ability of this regulatory subunit to bind the AC core dimer [56], [57], [58]. They also, in stark contrast to the B56 subunits activate replication of simian virus 40 (SV40), and have been further reported to play a role in DNA damage response through their regulation of Rb [59], [60], [61].
Finally, the PP2A B′′′ isoforms play a vital role in calcium signaling through their binding of calmodulin (CaM). This subfamily is also critically dependent on ATP and the Mg2+ cation for its activation. This family of enzymes is also reported to regulate the cell cycle as key members of this family include the Striatin and S/G2 nuclear auto antigen (SG2NA) proteins [62].
3. PP2A is a bona-fide tumor suppressor
Evidence suggesting a tumor suppressive role for PP2A originates from the observations that, in a myriad of cancers, the phosphatase PP2A is inactivated through deep-seated genetic mutations as well as post-translational modifications. The COSMIC database identifies the PP2A Aα subunit harbouring the highest number of mutations amongst all the PP2A subunits with a database recognizing an increased propensity for mutations in gynaecological cancers [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73]. The vast majority of the somatic mutations in PP2A Aα are concentrated in the HEAT repeat 5 region, with the two most common mutational hotspots represented by R418W and P179R. The HEAT repeat 5 region holds profound significance as it is the region that has been described on the scaffold subunit to be essential for binding with the B and C subunits of PP2A for holoenzyme assembly. These mutations generally result in holoenzyme disruption as a result of diminished binding to the catalytic C and the regulatory B subunits. Indeed, in-vitro analysis to study the binding competence of the different B regulatory subunits of PP2A verified defective binding of the P179A, R183A, R183E and W257A point mutants [74], [75]. Cancer related mutations in the PP2A Aα subunits usually render the gene product haploinsufficient. For example, point mutations targeting the E64 glutamate residue, E64D and E64G substitutions, result in the inability of the scaffold subunit to bind the regulatory B56α and B56δ subunits of PP2A, respectively [76], [77]. Moreover, these mutations also sensitized mice harbouring the heterozygous E64 mutation to carcinogenic agents like benzopyrene as well as augmented the frequency of developing lung cancer when crossed with a KRAS G12D mutant mouse [76], [78]. Apart from mutations, downregulation of PP2A Aα protein expression has also been observed in a couple of reports in gliomas and breast cancer, although the impact and mechanism of this phenotype is poorly understood [79], [80].
Intriguingly, very few reports highlighting mutations in the PP2A Aβ counterpart have been reported as yet. The first reports discovering mutation in the 11q23 PPP2R1B locus was identified by Wang et al in 1998 in lung and colon carcinomas [81]. Subsequent reports of somatic mutations of this PP2A Aβ isoform emerged in neoplasms ranging from breast, haematopoietic tumors and parathyroid adenomas, albeit suggesting very little impact of these mutations on tumor progression [77], [82], [83]. Conversely, Takagi et al. do report that there is a significant effect of mutations in PP2A Aβ in colorectal cancer, wherein they found diminished phosphatase binding capacity in ~13% of clinical samples tested. However, they have been careful to suggest that the LOH in the 11q23 locus leading to tumor progression may be contributed through mutations in other genes such as ATM, CHK1, MLL1 and DDX10, which have been previously reported to be notably mutated in tumors. The relatively low level of mutation frequency for this isoform of PP2A A is not well elucidated and it is likely that advances in sequencing and mapping of alterations will shine some light on this conundrum in the near future. Loss of protein expression of PP2A Aβ in ~50% cancer cell lines, when matched against their counterpart primary normal human epithelial cells, also suggests an important role for the PP2A Aβ isoform in tumor suppression [84]. Finally, an in-vitro study has also suggested a significant role for PP2A Aβ in promoting transformation of immortal cells through loss of phosphatase activity resulting in activation of the substrate protein RalA GTPase via its phosphorylation at the S183/194 residues [85]. Recently small molecule activators of PP2A that bind to the A scaffold subunit of PP2A have demonstrated great prowess as an attractive chemotherapeutic intervention that is tailored to mediating cancer cell kill via the activation of PP2A phosphatase, further highlighting the pivotal role of the phosphatase in mediating tumor repression [86], [87], [88].
Many of the substrates of the B regulatory subunits of PP2A are key proteins that are involved in key tumor signal transduction pathways. This list includes but is not limited to ERK, AKT, p53, c-Myc, Bcl-2, NF-κB, STAT-3, Rb, E2F1, Cyclins, PLK-1 Chk1 and ATM which are all key regulators of cellular processes in human cancers. Recent studies have helped in elucidating the interactome of phosphatases including PP2A boosting the impact of loss of substrate specific B regulatory subunits in promoting cancer development [45], [52], [89]. Several B55 and B56 regulatory subunits have also been reported to have altered expression or mutations in numerous cancers. These include but are not limited to B55α, B55β, B55γ, B55ε, B56α and B56γ [47], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103]. It is evident that these loss of function mutations of the regulatory subunits have a vast impact on the cancer proteome as it directly impacts the stability and/or activity of its specific substrate.
Complementary regulation of B subunit activity is directly under the purview of the endogenous inhibitor of PP2A - CIP2A - which has been shown to bind the B55 and B56 regulatory isoforms and inhibit substrate specific dephosphorylation by the PP2A phosphatase [104], [105], [106]. It is interesting to note that CIP2A is found commonly overexpressed in a myriad of cancer cells, most strikingly correlating with aggressive disease outcome [106], [107], [108], [109], [110], [111], [112], [113], [114], [115]. The Human Protein Atlas database validates the strong correlation of CIP2A overexpression in cancers with unfavourable prognosis [55]. Serendipitously, targeting this node to treat cancers overexpressing CIP2A has presently emerged as a strong small molecule therapeutic intervention. Indeed, erlotinib derivatives TD-19 and TD-52 have shown promising results in vitro in aggressive breast, lung and hepatocellular carcinomas, and it is likely that their development will be accelerated in the future for treating CIP2A overexpressing cancers [116], [117], [118]. These reports further support the designation of PP2A as a bona-fide tumor suppressor.
Not many mutations have been reported for the catalytic PP2A C subunit. Downregulation of the catalytic subunit of PP2A has been reported in some haematopoietic, gastric and prostate cancers [119], [120], [121], [122], [123], [124]. Sens et al. have reported that PP2A inactivation may also result from the haploinsufficient loss of PPP2R4 (PTPA) gene which enhances PP2A activity by binding with the catalytic subunit of PP2A [125]. Furthermore, overexpression or deregulated activation of the endogenous inhibitor SET/I2PP2A is also frequently observed in cancers [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136]. The SET protein has been reported to directly interact with the catalytic subunit of PP2A, thereby impeding its activity [137]. In-vitro binding and binding sequence mapping of the SET protein to the catalytic subunit has been recently demonstrated [138]. Indeed, reactivation of PP2A through inhibition of SET by the compounds FTY720 and OP-449 is emerging as an attractive therapeutic intervention to combat tumors overexpressing SET [109], [139], [140], [141], [142], [143], [144], [145], [146].
4. Regulation of PP2A activity
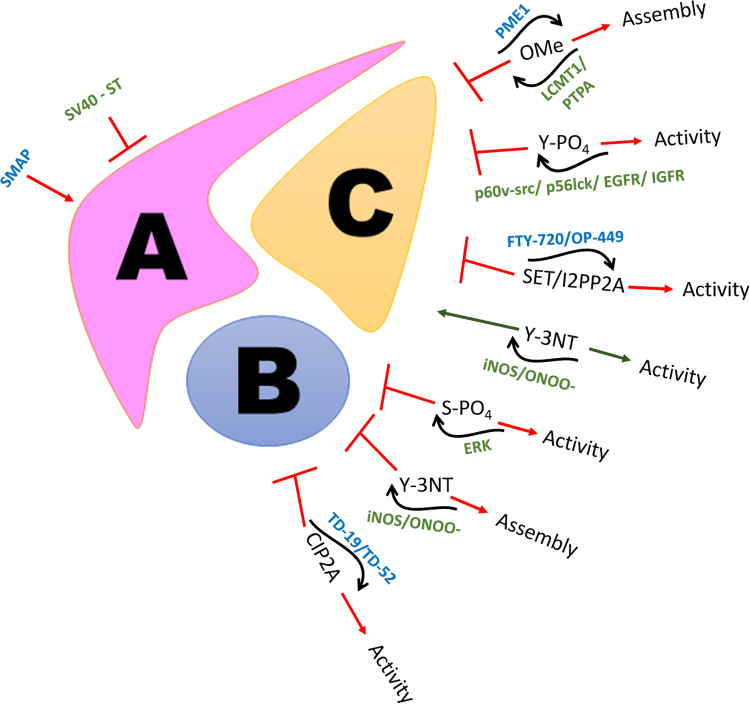
Several post translational modifications have been identified that are key regulators of PP2A activity and thereby critically modify the landscape of the proteome (Fig. 2).
Fig. 2.
Regulation of PP2A: An assortment of post-translational modifications and mutations can intricately affect the activity or assembly of PP2A holoenzyme. Several therapeutic interventions in treating cancers and other pathologies are dependent on the reactivation of the phosphatase PP2A through small molecule compounds targeting the different subunits.
One of the earliest modifications studied was the binding of the SV40 to the scaffold A subunit of PP2A, thereby leading to disruption of the holoenzyme assembly and correspondingly its activity [147], [148], [149], [150]. The small t antigen of SV40 polyoma virus has been demonstrated to bind the Aα subunit of PP2A between HEAT repeats 3–7 displacing the interaction of the core dimer with the substrate bound PP2A B subunit, through both in vitro and in vivo experiments [74], [147], [151], [152], [153]. The SV40 ST antigen itself has two conserved zinc binding motifs that help in the stabilisation of its binding to the PP2A core dimer. However, while the second zinc motif has been elegantly shown to interact with the scaffold subunit, wherein the proline 132 residue binds between the tryptophan 140 and phenylalanine 141 and was determined to be indispensable for A-ST interaction, it is thought that the first zinc motif binds close to the active site of the catalytic subunit of PP2A subunit suggesting that it may alter the binding of PP2A C with the substrate bound regulatory B subunit.
Most post-translational modifications known to alter the activity of PP2A have been identified on the catalytic C subunit of PP2A. Four different types of PTM have been identified to date. These include the carboxymethylation, phosphorylation, nitration and endogenous inhibition of PP2A through SET. The C-terminal region of the catalytic subunit is highly conserved amongst the serine threonine protein phosphatases. This tail region is critically positioned as a wedge between the A and B subunits. The 6 terminal residues 304T-P-D-Y-F-L309 is subject to both phosphorylation and methylation modifications that can regulate the catalytic activity of PP2A [154], [155], [156], [157], [158], [159].
L309 is subject to reversible methylation by the activity of two enzymes LCMT1 (leucine carboxyl methyltransferase) and PME-1 (phosphatase methylesterase) [160], [161], [162], [163], [164]. L309 methylation has been demonstrated to be important for holoenzyme assembly and interaction of the core dimer especially with the B55 regulatory subunit, though some studies do argue that this methylation also enhances the docking affinity of the B56 regulatory subunit to the AC heterodimer. Cho et al. have tried to explain the regulatory mechanism through which methylation aids holoenzyme formation through structural docking studies [32]. They report that the negatively charged carboxyl terminus of the PP2A C subunit usually docks beside the 62EDE64 and E101 amino acid residues of the scaffold subunit which already contributes to a highly negatively charged microenvironment in this region. Therefore, neutralisation of the negative charge on the carboxyl terminus could relieve the negative charge in the microenvironment and promote its docking to the scaffold and B regulatory subunits. In this context, there is also an important role for the Protein Phosphatase 2 Phosphatase Activator (PTPA) in promoting both the catalytic activity of PP2A as well as methylation of the C-terminus of PP2A C by LCMT1. This is achieved by direct binding of PTPA to PP2A C causing the catalytic subunit to undergo a conformational change that helps orient phosphorylated substrate proteins to bind to the active site as well as cooperates with ATP to chelate metal ions that ensures the assignment of the apposite metal ions necessary for the serine/threonine phosphatase activity of PP2A [165]. Intriguingly the association of PTPA with PP2A C also promotes the methylation of the L309 residue of PP2A C through the action of LCMT-1 and it has been suggested that PP2A C bound PTPA interacts with LCMT1 within the same region [166]. Methylation of L309 PP2A C is reversed by the protein phosphatase methylesterase enzyme PME-1. It is important to note that PME-1 which facilitates the demethylation of the PP2A C subunit also directly impacts its catalytic activity by displacing the Mn2+ cations of PP2A C that are essential for its catalytic activity. Moreover, the hydrophobic M335 residue of PME-1 prevents reloading of the Mn2+ ions into the active site of the enzyme [167]. A recent study has also suggested a role for the scaffold A subunit of PP2A to facilitate methylation of PP2A by three mechanisms: (i) alteration of the conformation of the catalytic subunit causing more amenable binding of PP2A C to LCMT-1, (ii) restricting the movement of the catalytic subunit such that its rate of entry within the active binding pocket of LCMT-1 is augmented and (iii) extending weak electrostatic interactions through its positively charged R183 and R258 residues onto the negatively charged LCMT-1 surface facilitating its interaction with PP2A C by acting as a scaffold [168].
Phosphorylation of the C-terminal region of the catalytic subunit of PP2A has been identified on Y307 and T304 residues. The Y307 phosphorylation, in particular, has been demonstrated to inhibit the interaction of PP2A C with the B' regulatory subunit of PP2A by impeding the interaction of the tyrosine residue with V257 residue of the B'γ1 regulatory subunit of PP2A. Moreover, it also seems to have an impact on L309 demethylation by restricting access of the catalytic subunit to the LCMT1 pocket, thereby impairing holoenzyme assembly, and consequently leading to inactivation of PP2A. Secondly, mutagenesis studies have also suggested that T304 phosphorylation of the PP2A C tail impacts the interaction of the core enzyme with the B55 regulatory subunit of PP2A. Such observations, however, need to be confirmed in a physiological setting.
Nitration of the PP2Ac subunit has also been reported to impact its activity in certain situations. However, there is much dissonance on the impact of peroxynitrite on the nature of PP2A activity. While some groups argue that PP2A activity is increased through the tyrosine nitration of its catalytic C subunit through its increased affinity for holoenzyme assembly, one group has reported that iNOS mediated peroxynitrite signaling contributed to nitration in the C-terminal at the Tyr-284 residue of PP2A C which led to the dissociation of the scaffold A subunit, with the p97 associated PP2A catalytic subunit demonstrating an elevated phosphatase activity extending its effect on DUSP1 [169], [170], [171].
Incidentally, post-translational modifications have also been identified on the B regulatory subunits of PP2A, particularly the PR61/B′/B56 subunit. The B56 regulatory subunit of PP2A has been reported to be subjected to phospho-modifications by the MAP kinase protein, ERK which severely impacts the holoenzyme assembly, and hence activity of PP2A [32], [49]. Structural analysis illustrated the impact of ERK on the S337 residue of the B56γ1 subunit, which was earlier delineated by Letourneux et al. to disrupt the ABC heterodimer assembly of PP2A, leading to the inability of the catalytic core in extending its phosphatase activity on its bona-fide substrates. Conversely, Margolis et al. have reported that phosphorylation of the B56δ subunit of PP2A at the N-terminal S37 residue, enhances its activity and promotes the T138 dephosphorylation of Cdc25, extending its impact on the cell cycle [48].
Additionally, our group has recently reported the nitration of a B56 regulatory subunit isoform of PP2A as a novel post-translational modification, which significantly attenuates its activity. Low et al. describe the tyrosine nitration of the Y289 residue of B56δ as a consequence of the intracellular redox milieu in haematopoietic cancers which results in the disruption of the PP2A holoenzyme assembly and the subsequent inability of the phosphatase to extend its activity on the substrate anti-apoptotic protein Bcl-2. These findings have now unfolded a unique role for ROS microenvironment in addressing the fate of the tumor landscape through its regulation of the bona-fide tumor suppressor PP2A.
5. ROS and the regulation of PP2A
Reactive oxygen species (ROS)-as the name lends itself-are radical- or non-radical derivatives of molecular oxygen, which are chemically reactive through either the presence of an unpaired electron in the outermost shell of these radicals which contributes to their reactivity, or through their strong oxidizing capacity. Some of the biologically relevant ROS include superoxide, hydrogen peroxide, peroxynitrite, nitric oxide as well as peroxyl and hydroxyl radicals.
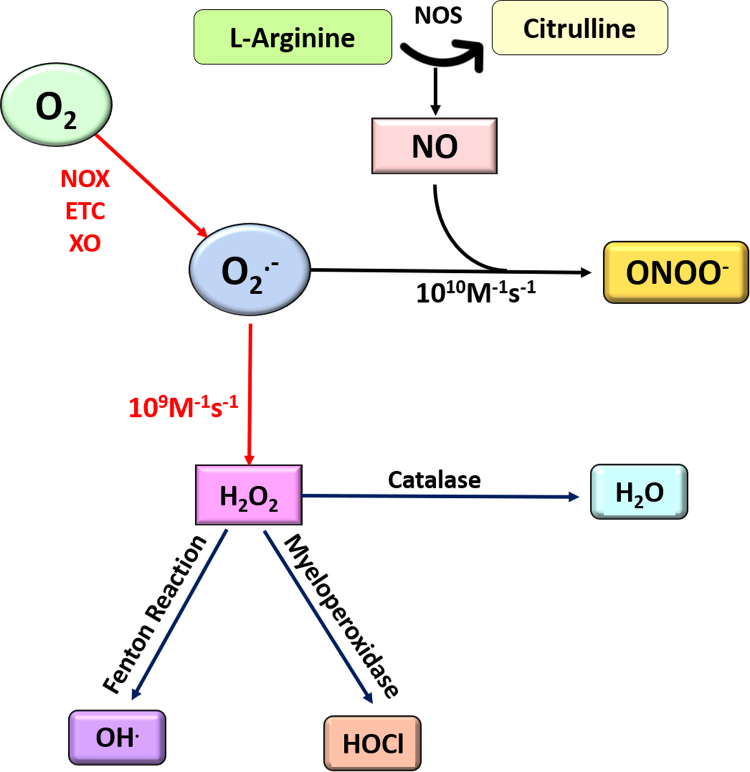
ROS have for long been dogmatically viewed as molecules that are harmful to cellular membranes resulting in tissue injury and death [172], [173], [174]. However, their valuable role in modulating signaling responses in pathways integral to proliferation, growth and development has been palpable [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188]. The two most biologically relevant physiological sources of ROS can be attributed to the NADPH oxidases and Mitochondrial electron transport chain [189], [190], [191], [192], [193]. When a single oxygen molecule in cells gains an unpaired electron, it forms the highly chemically reactive superoxide anion (Fig. 3). Physiologically, superoxide anion has two distinct fates: it is instantaneously either dismutated to hydrogen peroxide through the activity of the enzyme superoxide dismutase (SOD), or to reactive nitrogen species such as peroxynitrite through its reaction with nitric oxide [194], [195], [196]. As is evident, there is a competition between formation of hydrogen peroxide and peroxynitrite in cells that is dictated by the balance between the activities of the two enzymes NOS (nitric oxide synthase) and SOD. By far, hydrogen peroxide and nitric oxide have been considered the most extensively studied ROS species in signal transduction pathways. Physiologically, these two ROS govern the balance between pro-survival and apoptotic signaling in cells and thus are critical mediators that impact the cancer interactome.
Fig. 3.
Reactive oxygen and nitrogen species: Superoxide anion is formed when molecular oxygen gains an electron and gets reduced. The source of electrons can be leakage from the mitochondrial electron transport chain (ETC), NADPH oxidase (NOX) or xanthine oxidase (XO). Superoxide is a short-lived ROS species that is instantaneously either dismutated to hydrogen peroxide through the activity of the enzyme SOD, or to RNS such as peroxynitrite through its reaction with nitric oxide. As is evident, there is a competition between formation of hydrogen peroxide and peroxynitrite in cells that is dictated by the balance between the activities of the two enzymes NOS and SOD. Hydrogen peroxide can further be fated to generate the highly reactive hydroxyl ions as a result of the Haber Weiss reaction in the presence of Ferrous ions or the biologically reactive hypochlorous acid. The activity of the antioxidant catalase on hydrogen peroxide on the other hand detoxifies it to generate water.
To fuel the high bioenergetic needs of cancer cells, they accumulate excessive amounts of ROS by-products, which are capable of building up toxic metabolites, leading to activation of apoptotic or necrotic programs. To counter the redox stress mediated adverse cytotoxic effects, tumor cells step up their antioxidant reserves to maintain redox equilibrium. The impact of ROS on cellular physiology is also governed by the nature and concentration of the ROS species encountered as well as the subcellular compartment where the effect is sustained. In general, a high concentration of cellular ROS causes indiscriminate damage to biomembranes ensued by activation of apoptotic and necrotic programs [174], [197], [198]. Moderate rise in physiological ROS, on the contrary are associated with the modification of pro-apoptotic members of the Bcl-2 family such as Bax or stress response proteins such as JNK [199], [200], [201]. Nevertheless, a mild pro-oxidant physiological state has been associated with the diametric inversion of a favourable microenvironment for signal transduction, growth and development tending to a pro-neoplastic phenotype [202], [203], [204], [205], [206], [207], [208], [209], [210], [211]. Despite strong evidence favoring a correlation between the cellular superoxide concentrations and carcinogenesis, it is discerned that the superoxide anion seldom subsists in its native form to react with biomolecules. It is, in fact dismutated almost instantaneously to hydrogen peroxide at a reaction rate of ~1.6 × 109 M−1 s−1. Indeed, the superoxide anion reacts more readily, by a degree of three orders with nitric oxide. However, this relatively moderate paced dismutation of superoxide into hydrogen peroxide is favoured owing to the large body of SOD enzymes in the different cellular compartments [196].
Hydrogen peroxide can impact signaling pathways through the selective oxidation of cysteine moieties of member proteins constituting these nodes, resulting in conformational changes to their structure and function [212], [213], [214]. Au contraire, hydroxyl radical generated as a result of Fenton reaction in the presence of ferrous or cuprous ions leads to multifarious destruction of nucleic acid, lipid and protein biomolecules [215], [216]. As mentioned earlier, apart from getting dismutated to hydrogen peroxide, the superoxide anion may also react readily with nitric oxide physiologically to generate reactive nitrogen species. One of the most biologically reactive notable species in this regard is the peroxynitrite radical which has been reported to effect the oxidation of fatty acid molecules as well as antioxidant proteins including glutathione, ascorbate (vitamin C) and tocopherol (vitamin E) [217], [218], [219]. Peroxynitrite is also known to induce the breakage of amino acid chains of proteins, DNA strand breaks and lipid peroxidations that contribute to various pathological conditions. [220], [221], [222], [223], [224]. Nonetheless, some of the most impactful biological impact of peroxynitrite is extended through its ability to impose covalent modifications on proteins that include nitration at the ortho position of tyrosine residues on proteins, which have come to be known as nitrotyrosine footprints or S-nitrosylation of cysteine residues. Some notable examples include the tyrosine nitration of the RAS signaling members ERK and AKT in endothelial cells and the cysteine S-nitrosylation of c-Src, PTEN and AKT [225], [226], [227], [228], [229].
As mentioned earlier, ROS is observed to play a biphasic role in cancer progression with respect to the nature of the ROS species, its physiological location and concentration in the cell. While ROS generation through chemotherapeutic agents can be tailored as a therapeutic window in some cancers to induce cell death, most usually by targeting the antioxidant backbone of tumor cells, they can alternatively contribute to the selective survival of cancer cells and contribute to tumor sustenance and progression [230], [231], [232], [233], [234], [235], [236], [237], [238], [239], [240], [241], [242], [243], [244], [245], [246], [247], [248], [249]. In this context, we have reported a novel role for peroxynitrite generated when the balance between the enzymes SOD and iNOS is tilted in favour of iNOS mediated RNS signaling. In this regard, we demonstrated the ability of peroxynitrite to accentuate the anti-apoptotic response mediated by Bcl-2 through its sustained phosphorylation at serine 70 residue, which was a function of tyrosine nitration-mediated inactivation of phosphatase PP2A [242]. It is vital to note that the B56δ regulatory isoform of PP2A, which we demonstrated to be nitrated by peroxynitrite, is not the singular nitration labile arm of PP2A. Indeed, we suspect the analogous tyrosine nitration of other B56 residues of PP2A, opening up a new avenue in the field of the redox regulation of the cancer phosphatase proteome.
As already elucidated, ROS mediated regulation of PP2A, albeit indirect, will have considerable implications on the cancer proteome through its impact on the regulation of PP2A substrates. Serendipitously, other research groups have also identified ROS-mediated modulation of PP2A activity and its impact on its substrates. For instance, it has been proposed that hydrogen peroxide exposure of primary neurons and neuronal cell lines as well as sarcoma cells results in sustained activation of mitogen activated protein kinase (MAPK) via the tyrosine phosphorylation and leucine carboxy-demethylation of PP2A, which subsequently results in neuronal apoptosis and decrease in cell viability of sarcoma cells, respectively [250], [251]. Lending further support to this theory, another research team conscientiously studied PP2A expression, localization and activity in cardiovascular pathologies and described the inhibitory effect of hydrogen peroxide on PP2A activity through the aforementioned post-translational modifications [252]. Hydrogen peroxide has also been demonstrated to impede PP2A phosphatase activity through regulation of the glutathione oxidation which suggests that glutathionylation of PP2A is an important factor in modulating PP2A activity in cells [253]. Paradoxically, it was also reported that hydrogen peroxide could lead to PP2A activation leading to the subsequent dephosphorylation of the pocket proteins pRb, p107 and p130 [254]. In support of PP2A activation through hydrogen peroxide build up in cancer cells, another group has reported that the combination of standard chemotherapeutics sorafenib and vorinostat in gastrointestinal cancer led to upregulation of PP2A activity as a function of ceramide synthesis, a known PP2A activator [255].
There is little studied on the thiol oxidation of PP2A, but the reports do concur that such oxidation potentiates a reversible inhibitory effect on PP2A activity. Thiol oxidation of cysteine residues of proteins lead to the reversible formation of disulphide bonds. In this regard, the catalytic subunit of the phosphatase PP2A is known to contain a number of cysteine residues. Indeed, one such vicinal pair towards the C terminal end at positions 266 and 269 of PP2A C is particularly noteworthy as it comprises the redox sensitive CXXC motif, which is especially susceptible to oxidative modifications leading to the formation of disulphide bonds. Two studies have reported the oxidative modification of PP2A leading to the inhibition of its activity, which can be effectively relieved by mild concentrations of the thiol reducing agent, dithiothreitol (DTT) [256], [257]. However, the exact nature of such modification needs to be studied in greater detail. For instance, the physical formation of disulphide bonds by mass spectrometry, the mapping of disulphide bonds formation to a specific PP2A subunit and pinpointing the residues involved in such redox targeting are urgent to fully understand the nature of thiol oxidation on PP2A conformation and activity.
Reactive nitrogen species have also been reported to definitively impact PP2A holoenzyme assembly and activity. For instance, nitric oxide signaling has been reported by multiple research groups to modulate PP2A activity through post-translational modifications effected on the catalytic subunit C-terminal tail. Contrary to nitric oxide mediated activation of the catalytic subunit [171], [258], [259], [260]. Low et al. further report that peroxynitrite, a key reactive nitrogen species formed through the association of the superoxide radical with nitric oxide, is instead responsible for the nitration mediated inactivation of PP2A by preventing the assembly of the PP2A holoenzyme [242].
Several reports have also suggested an integral role for hypoxic microenvironments in modulating PP2A activity. For example, Heikkinen et al. demonstrate that the hypoxic microenvironment is able to increase PP2A activity through facilitating its interaction with Smad3 and its subsequent dephosphorylation [261]. Correspondingly, Conza et al. mechanistically demonstrated that a hypoxic microenvironment levies restraint on the mTOR signaling pathway rendering p70S6K incapable of inhibiting PP2A, subsequently leading to sustaining PHD2 phosphorylation in colorectal cancer cells [262]. In stark contrast to the above observations, Raghuraman et al. argue that chronic intermittent hypoxia exposure leads to inhibition of PP2A activity alongside a concomitant decrease in PP2A C expression [263]. The above points illustrate the important context of the physiological concentrations and tissue specific biphasic nature of ROS.
6. Concluding remarks
Three decades after the first studies on the recognition of PP2A as a tumor suppressor following the discovery of the marine toxin okadaic acid and its role in tumor progression, we are at the threshold of targeting PP2A regulation as a window into treatment of pathologies where the phosphatase has been effectively demonstrated to regulate key signaling proteins. Small molecules such as FTY720, OP-449 and SMAPs are marking a milestone in the development of therapeutic strategies to target physiological PP2A reactivation in cancers where its expression and activity may be compromised.
At the same time, it is imperative to retain caution when strategizing therapeutic interventions tailored to modulate PP2A activity as the predominant perspective for this phosphatase to be analogous to a tumor suppressor is short-sighted. This hypothesis was first challenged on the basis of the anti-apoptotic role of PP2A in Drosophila melanogaster demonstrated by two independent research groups in the early years of the 21st century [264], [265]. Secondly, some studies have uncovered a positive correlation between PP2A subunits expression and cancer aggressiveness, suggesting a complex dual personality for the PP2A phosphatase as an oncogenic driver in these circumstances [265], [266], [267]. Moreover, several compounds that are potent inhibitors of PP2A activity such as Canthardin, Cytostatin, LB1.2 as well as the small molecule compound LB-100 have shown promising results in alleviating tumor promoting phenotypes with the latter compound LB-100, already endorsed in an active clinical trial against glioblastoma and gliosarcoma (NCT03027388) [268], [269], [270], [271], [272], [273], [274]. Lenalidomide is another therapeutic FDA approved compound that has already been demonstrated to treat 5q del subset of myelodysplastic syndrome mechanistically through its inhibition of PP2A phosphatase. It concurrently, has been reported to activate NK signaling and inhibition of pro-survival cytokines such as IL-6, leading to marked remission of multiple myeloma [275], [276].
Further development and favourable co-treatment strategies of these compounds or tailoring the redox microenvironment to facilitate PP2A modulation alongside the current standards of therapeutic intervention are likely to improve the prognostic states of patients suffering not only from cancers, but other debilitating pathologies where PP2A is a core mediator of the signaling circuitry.
Acknowledgments
The authors wish to acknowledge all authors whose contributions might have been inadvertently overlooked in this review. S.P. is supported by grants from the National Medical Research Council, Singapore (NMRC/CIRG/1433/2015), NMRC/NCIS, Singapore (NR17NMRC329MP) and the Ministry of Education Tier 2, Singapore (MOE2013-T2-2-130).
References
- 1.Khoury G.A., Baliban R.C., Floudas C.A. Proteome-wide post-translational modification statistics: frequency analysis and curation of the swiss-prot database. Sci. Rep. 2011;1:90. doi: 10.1038/srep00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen J.V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127(3):635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139(3):468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Plattner F., Bibb J.A. Chapter 25 - serine and threonine phosphorylation A2 - Brady, Scott T. In: Siegel G.J., Albers R.W., Price D.L., editors. Basic Neurochemistry. eighth ed. Academic Press; New York: 2012. pp. 467–492. [Google Scholar]
- 5.Moorhead Greg B.G., De Wever V., Templeton G., Kerk D. Evolution of protein phosphatases in plants and animals. Biochem. J. 2009;417(2):401. doi: 10.1042/BJ20081986. [DOI] [PubMed] [Google Scholar]
- 6.Ek P., Pettersson G., Ek B., Gong F., Li J.P., Zetterqvist Ö. Identification and characterization of a mammalian 14‐kDa phosphohistidine phosphatase. Eur. J. Biochem. 2002;269(20):5016–5023. doi: 10.1046/j.1432-1033.2002.03206.x. [DOI] [PubMed] [Google Scholar]
- 7.Shen H., Yang P., Liu Q., Tian Y. Nuclear expression and clinical significance of phosphohistidine phosphatase 1 in clear-cell renal cell carcinoma. J. Int. Med. Res. 2015;43(6):747–757. doi: 10.1177/0300060515587576. [DOI] [PubMed] [Google Scholar]
- 8.Xu A., Hao J., Zhang Z., Tian T., Jiang S., Hao J., Liu C., Huang L., Xiao X., He D. 14-kDa phosphohistidine phosphatase and its role in human lung cancer cell migration and invasion. Lung Cancer. 2010;67(1):48–56. doi: 10.1016/j.lungcan.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Kamath V., Kyathanahalli C.N., Jayaram B., Syed I., Olson L.K., Ludwig K., Klumpp S., Krieglstein J., Kowluru A. Regulation of glucose- and mitochondrial fuel-induced insulin secretion by a cytosolic protein histidine phosphatase in pancreatic β-cells. Am. J. Physiol.-Endocrinol. Metab. 2010;299(2):E276–E286. doi: 10.1152/ajpendo.00091.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberti S., Sacco F., Calderone A., Perfetto L., Iannuccelli M., Panni S., Santonico E., Palma A., Nardozza Aurelio P., Castagnoli L., Cesareni G. HuPho: the human phosphatase portal. FEBS J. 2012;280(2):379–387. doi: 10.1111/j.1742-4658.2012.08712.x. [DOI] [PubMed] [Google Scholar]
- 11.MacKintosh C., Beattie Kenneth A., Klumpp S., Cohen P., Codd A. Geoffrey, Cyanobacterial microcystin‐LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 12.Takai A., Bialojan C., Troschka M., Ruegg J.C. Smooth muscle myosin phosphatase inhibition and force enhancement by black sponge toxin. FEBS Lett. 1987;217(1):81–84. doi: 10.1016/0014-5793(87)81247-4. [DOI] [PubMed] [Google Scholar]
- 13.Ishihara H., Martin B.L., Brautigan D.L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem. Biophys. Res. Commun. 1989;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- 14.MacKintosh C., Klumpp S. Tautomycin from the bacterium Streptomyces verticillatus. Another potent and specific inhibitor of protein phosphatases 1 and 2A. FEBS Lett. 1990;277(1–2):137–140. doi: 10.1016/0014-5793(90)80828-7. [DOI] [PubMed] [Google Scholar]
- 15.MacKintosh C., Beattie K.A., Klumpp S., Cohen P., Codd G.A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- 16.Virshup D.M. Protein phosphatase 2A: a panoply of enzymes. Curr. Opin. Cell Biol. 2000;12(2):180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 17.Xiao G., Chan L.N., Klemm L., Braas D., Chen Z., Geng H., Zhang Q.C., Aghajanirefah A., Cosgun K.N., Sadras T., Lee J., Mirzapoiazova T., Salgia R., Ernst T., Hochhaus A., Jumaa H., Jiang X., Weinstock D.M., Graeber T.G., Müschen M. B-cell-specific diversion of glucose carbon utilization reveals a unique vulnerability in B cell malignancies. Cell. 2018;173(2):470–484. doi: 10.1016/j.cell.2018.02.048. (e18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim T.H.Y., Susana A., Philip C. The protein phosphatases involved in cellular regulation. Eur. J. Biochem. 1985;148(2):253–263. doi: 10.1111/j.1432-1033.1985.tb08833.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari E., Bruhn C., Peretti M., Cassani C., Carotenuto W.V., Elgendy M., Shubassi G., Lucca C., Bermejo R., Varasi M., Minucci S., Longhese M.P., Foiani M. PP2A Controls genome integrity by integrating nutrient-sensing and metabolic pathways with the DNA damage response. Mol. Cell. 2017;67(2):266–281. doi: 10.1016/j.molcel.2017.05.027. (e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes Hallett J.E., Luo X., Capaldi A.P. State transitions in the TORC1 signaling pathway and information processing in Saccharomyces cerevisiae. Genetics. 2014;198(2):773. doi: 10.1534/genetics.114.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhury D., Keogh M.-C., Ishii H., Peterson C.L., Buratowski S., Lieberman J. Gamma H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol. Cell. 2005;20(5):801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Lin X.-H., Walter J., Scheidtmann K., Ohst K., Newport J., Walter G. Protein phosphatase 2A is required for the initiation of chromosomal DNA replication. Proc. Natl. Acad. Sci. USA. 1998;95(25):14693–14698. doi: 10.1073/pnas.95.25.14693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasinska L., Domingo-Sananes Maria R., Kapuy O., Parisis N., Harker B., Moorhead G., Rossignol M., Novák B., Fisher D. Protein phosphatase 2A controls the order and dynamics of cell-cycle transitions. Mol. Cell. 2011;44(3):437–450. doi: 10.1016/j.molcel.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Tar K., Csortos C., Czikora I., Olah G., Ma S.-F., Wadgaonkar R., Gergely P., Garcia Joe G.N., Verin Alexander D. Role of protein phosphatase 2A in the regulation of endothelial cell cytoskeleton structure. J. Cell. Biochem. 2006;98(4):931–953. doi: 10.1002/jcb.20829. [DOI] [PubMed] [Google Scholar]
- 25.Crispín J.C., Apostolidis S.A., Finnell M.I., Tsokos G.C. Induction of PP2A Bβ, a regulator of IL-2 deprivation-induced T-cell apoptosis, is deficient in systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA. 2011;108(30):12443–12448. doi: 10.1073/pnas.1103915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baroja M.L., Vijayakrishnan L., Bettelli E., Darlington P.J., Chau T.A., Ling V., Collins M., Carreno B.M., Madrenas J., Kuchroo V.K. Inhibition of CTLA-4 function by the regulatory subunit of serine/threonine phosphatase 2A. J. Immunol. 2002;168(10):5070. doi: 10.4049/jimmunol.168.10.5070. [DOI] [PubMed] [Google Scholar]
- 27.McCright B., Rivers A.M., Audlin S., Virshup D.M. The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem. 1996;271(36):22081–22089. doi: 10.1074/jbc.271.36.22081. [DOI] [PubMed] [Google Scholar]
- 28.Arino J., Woon C.W., Brautigan D.L., Miller T.B., Johnson G.L. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc. Natl. Acad. Sci. USA. 1988;85(12):4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nunbhakdi-Craig V., Schuechner S., Sontag J.-M., Montgomery L., Pallas David C., Juno C., Mudrak I., Ogris E., Sontag E. Expression of protein phosphatase 2A mutants and silencing of the regulatory Bα subunit induce a selective loss of acetylated and detyrosinated microtubules. J. Neurochem. 2007;101(4):959–971. doi: 10.1111/j.1471-4159.2007.04503.x. [DOI] [PubMed] [Google Scholar]
- 30.Janssens V., Longin S., Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem. Sci. 2008;33(3):113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y., Xing Y., Chen Y., Chao Y., Lin Z., Fan E., Yu J.W., Strack S., Jeffrey P.D., Shi Y. Structure of the protein phosphatase 2A Holoenzyme. Cell. 2006;127(6):1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 32.Cho U.S., Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2006;445:53. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 33.Hemmings B.A., Adams-Pearson C., Maurer F., Mueller P., Goris J., Merlevede W., Hofsteenge J., Stone S.R. Alpha.- and .beta.-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990;29(13):3166–3173. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- 34.Manchado E., Guillamot M., de Cárcer G., Eguren M., Trickey M., García-Higuera I., Moreno S., Yamano H., Cañamero M., Malumbres M. Targeting mitotic exit leads to tumor regression In vivo: modulation by Cdk1, Mastl, and the PP2A/B55α,δ phosphatase. Cancer Cell. 2010;18(6):641–654. doi: 10.1016/j.ccr.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Mochida S., Ikeo S., Gannon J., Hunt T. Regulated activity of PP2A–B55δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 2009;28(18):2777–2785. doi: 10.1038/emboj.2009.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolupaeva V., Daempfling L., Basilico C. The B55α regulatory subunit of protein phosphatase 2A mediates fibroblast growth factor-induced p107 Dephosphorylation and growth arrest in chondrocytes. Mol. Cell. Biol. 2013;33(15):2865–2878. doi: 10.1128/MCB.01730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cundell M.J., Hutter L.H., Nunes Bastos R., Poser E., Holder J., Mohammed S., Novak B., Barr F.A. A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J. Cell Biol. 2016;214(5):539–554. doi: 10.1083/jcb.201606033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz M.H.A., Held M., Janssens V., Hutchins J.R.A., Hudecz O., Ivanova E., Goris J., Trinkle-Mulcahy L., Lamond A.I., Poser I., Hyman A.A., Mechtler K., Peters J.-M., Gerlich D.W. Live-cell imaging RNAi screen identifies PP2A–B55α and importin-β1 as key mitotic exit regulators in human cells. Nat. Cell Biol. 2010;12(9) doi: 10.1038/ncb2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turowski P., Myles T., Hemmings B.A., Fernandez A., Lamb N.J.C. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell. 1999;10(6):1997–2015. doi: 10.1091/mbc.10.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strack S., Chang D., Zaucha J.A., Colbran R.J., Wadzinski B.E. Cloning and characterization of Bδ, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett. 1999;460(3):462–466. doi: 10.1016/s0014-5793(99)01377-0. [DOI] [PubMed] [Google Scholar]
- 41.Strack S., Zaucha Julie A., Ebner Ford F., Colbran Roger J., Wadzinski Brian E. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J. Comp. Neurol. 1998;392(4):515–527. [PubMed] [Google Scholar]
- 42.Kuo Y.-C., Huang K.-Y., Yang C.-H., Yang Y.-S., Lee W.-Y., Chiang C.-W. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55α regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J. Biol. Chem. 2008;283(4):1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 43.Wang F., Zhu S., Fisher L.A., Wang W., Oakley G.G., Li C., Peng A. Protein interactomes of protein phosphatase 2A B55 regulatory subunits reveal B55-mediated regulation of replication protein A under replication stress. Sci. Rep. 2018;8:2683. doi: 10.1038/s41598-018-21040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merigliano C., Marzio A., Renda F., Somma M.P., Gatti M., Vernì F., Role A. for the twins protein phosphatase (PP2A-B55) in the maintenance of Drosophila genome integrity. Genetics. 2017;205(3):1151. doi: 10.1534/genetics.116.192781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F., Zhu S., Fisher L.A., Wang W., Oakley G.G., Li C., Peng A. Protein interactomes of protein phosphatase 2A B55 regulatory subunits reveal B55-mediated regulation of replication protein A under replication stress. Sci. Rep. 2018;8(1):2683. doi: 10.1038/s41598-018-21040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L., Guo Q., Fisher L.A., Liu D., Peng A. Regulation of polo-like kinase 1 by DNA damage and PP2A/B55α. Cell Cycle. 2014;14(1):157–166. doi: 10.4161/15384101.2014.986392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalev P., Simicek M., Vazquez I., Munck S., Chen L., Soin T., Danda N., Chen W., Sablina A. Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition. Cancer Res. 2012;72(24):6414. doi: 10.1158/0008-5472.CAN-12-1667. [DOI] [PubMed] [Google Scholar]
- 48.Margolis S.S., Perry J.A., Forester C.M., Nutt L.K., Guo Y., Jardim M.J., Thomenius M.J., Freel C.D., Darbandi R., Ahn J.-H., Arroyo J.D., Wang X.-F., Shenolikar S., Nairn A.C., Dunphy W.G., Hahn W.C., Virshup D.M., Kornbluth S. Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell. 2006;127(4):759–773. doi: 10.1016/j.cell.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Letourneux C., Rocher G., Porteu F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 2006;25(4):727–738. doi: 10.1038/sj.emboj.7600980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahn J.-H., McAvoy T., Rakhilin S.V., Nishi A., Greengard P., Nairn A.C. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56δ subunit. Proc. Natl. Acad. Sci. USA. 2007;104(8):2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hertz Emil Peter T., Kruse T., Davey Norman E., López-Méndez B., Sigurðsson J.ón O., Montoya G., Olsen Jesper V., Nilsson J. A conserved motif provides binding specificity to the PP2A-B56 phosphatase. Mol. Cell. 2016;63(4):686–695. doi: 10.1016/j.molcel.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Wang X., Bajaj R., Bollen M., Peti W., Page R. Expanding the PP2A interactome by defining a B56-Specific SLiM. Structure. 2016;24(12):2174–2181. doi: 10.1016/j.str.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruvolo P.P. The broken “Off” switch in cancer signaling: PP2A as a regulator of tumorigenesis, drug resistance, and immune surveillance. BBA Clin. 2016;6:87–99. doi: 10.1016/j.bbacli.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendrix P., Mayer-Jackel R.E., Cron P., Goris J., Hofsteenge J., Merlevede W., Hemmings B.A. Structure and expression of a 72-kDa regulatory subunit of protein phosphatase 2A. Evidence for different size forms produced by alternative splicing. J. Biol. Chem. 1993;268(20):15267–15276. [PubMed] [Google Scholar]
- 55.Uhlen M., Zhang C., Lee S., Sjöstedt E., Fagerberg L., Bidkhori G., Benfeitas R., Arif M., Liu Z., Edfors F., Sanli K., von Feilitzen K., Oksvold P., Lundberg E., Hober S., Nilsson P., Mattsson J., Schwenk J.M., Brunnström H., Glimelius B., Sjöblom T., Edqvist P.-H., Djureinovic D., Micke P., Lindskog C., Mardinoglu A., Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352) doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 56.Ahn J.-H., Sung J.Y., McAvoy T., Nishi A., Janssens V., Goris J., Greengard P., Nairn A.C. The B″/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc. Natl. Acad. Sci. USA. 2007;104(23):9876–9881. doi: 10.1073/pnas.0703589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssens V., Jordens J., Stevens I., Van Hoof C., Martens E., De Smedt H., Engelborghs Y., Waelkens E., Goris J. Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B"/PR72 subunit of protein phosphatase 2A. J. Biol. Chem. 2003;278(12):10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- 58.Davis A.J., Yan Z., Martinez B., Mumby M.C. protein phosphatase 2A Is targeted to cell division control protein 6 by a calcium-binding regulatory subunit. J. Biol. Chem. 2008;283(23):16104–16114. doi: 10.1074/jbc.M710313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cegielska A., Shaffer S., Derua R., Goris J., Virshup D.M. Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol. Cell. Biol. 1994;14(7):4616–4623. doi: 10.1128/mcb.14.7.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magenta A., Fasanaro P., Romani S., Stefano V. Di, Capogrossi M.C., Martelli F. Protein phosphatase 2A subunit PR70 interacts with pRb and mediates its dephosphorylation. Mol. Cell. Biol. 2008;28(2):873–882. doi: 10.1128/MCB.00480-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Avni D., Yang H., Martelli F., Hofmann F., ElShamy W.M., Ganesan S., Scully R., Livingston D.M. Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Mol. Cell. 2003;12(3):735–746. doi: 10.1016/s1097-2765(03)00355-1. [DOI] [PubMed] [Google Scholar]
- 62.Moreno C.S., Park S., Nelson K., Ashby D., Hubalek F., Lane W.S., Pallas D.C. WD40 Repeat proteins striatin and S/G2 nuclear autoantigen Are members of a novel family of calmodulin-binding proteins that associate with protein phosphatase 2A. J. Biol. Chem. 2000;275(8):5257–5263. doi: 10.1074/jbc.275.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nagendra D.C., Burke J., Maxwell G.L., Risinger J.I. PPP2R1A mutations are common in the serous type of endometrial cancer. Mol. Carcinog. 2011;51(10):826–831. doi: 10.1002/mc.20850. [DOI] [PubMed] [Google Scholar]
- 64.Imielinski M., Berger Alice H., Hammerman Peter S., Hernandez B., Pugh Trevor J., Hodis E., Cho J., Suh J., Capelletti M., Sivachenko A., Sougnez C., Auclair D., Lawrence Michael S., Stojanov P., Cibulskis K., Choi K., de Waal L., Sharifnia T., Brooks A., Greulich H., Banerji S., Zander T., Seidel D., Leenders F., Ansén S., Ludwig C., Engel-Riedel W., Stoelben E., Wolf J., Goparju C., Thompson K., Winckler W., Kwiatkowski D., Johnson Bruce E., Jänne Pasi A., Miller Vincent A., Pao W., Travis William D., Pass Harvey I., Gabriel Stacey B., Lander Eric S., Thomas Roman K., Garraway Levi A., Getz G., Meyerson M. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shih I.-M., Panuganti P.K., Kuo K.-T., Mao T.-L., Kuhn E., Jones S., Velculescu V.E., Kurman R.J., Wang T.-L. Somatic mutations of PPP2R1A in ovarian and uterine carcinomas. Am. J. Pathol. 2011;178(4):1442–1447. doi: 10.1016/j.ajpath.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McConechy M.K., Anglesio M.S., Kalloger S.E., Yang W., Senz J., Chow C., Heravi-Moussavi A., Morin G.B., Mes-Masson A.-M., Carey M.S., McAlpine J.N., Kwon J.S., Prentice L.M., Boyd N., Shah S.P., Gilks C.B., Huntsman D.G. Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J. Pathol. 2011;223(5):567–573. doi: 10.1002/path.2848. [DOI] [PubMed] [Google Scholar]
- 67.Levine D.A., N. The Cancer Genome Atlas Research, Getz G., Gabriel S.B., Cibulskis K., Lander E., Sivachenko A., Sougnez C., Lawrence M., Kandoth C., Dooling D., Fulton R., Fulton L., Kalicki-Veizer J., McLellan M.D., O’Laughlin M., Schmidt H., Wilson R.K., Ye K., Ding L., Mardis E.R., Ally A., Balasundaram M., Birol I., Butterfield Y.S.N., Carlsen R., Carter C., Chu A., Chuah E., Chun H.-J.E., Dhalla N., Guin R., Hirst C., Holt R.A., Jones S.J.M., Lee D., Li H.I., Marra M.A., Mayo M., Moore R.A., Mungall A.J., Plettner P., Schein J.E., Sipahimalani P., Tam A., Varhol R.J., Robertson A. Gordon, Cherniack A.D., Pashtan I., Saksena G., Onofrio R.C., Schumacher S.E., Tabak B., Carter S.L., Hernandez B., Gentry J., Salvesen H.B., Ardlie K., Getz G., Winckler W., Beroukhim R., Gabriel S.B., Meyerson M., Hadjipanayis A., Lee S., Mahadeshwar H.S., Park P., Protopopov A., Ren X., Seth S., Song X., Tang J., Xi R., Yang L., Zeng D., Kucherlapati R., Chin L., Zhang J., Auman J. Todd, Balu S., Bodenheimer T., Buda E., Hayes D. Neil, Hoyle A.P., Jefferys S.R., Jones C.D., Meng S., Mieczkowski P.A., Mose L.E., Parker J.S., Perou C.M., Roach J., Shi Y., Simons J.V., Soloway M.G., Tan D., Topal M.D., Waring S., Wu J., Hoadley K.A., Baylin S.B., Bootwalla M.S., Lai P.H., Triche T.J., Jr, Van Den Berg D.J., Weisenberger D.J., Laird P.W., Shen H., Chin L., Zhang J., Getz G., Cho J., DiCara D., Frazer S., Heiman D., Jing R., Lin P., Mallard W., Stojanov P., Voet D., Zhang H., Zou L., Noble M., Lawrence M., Reynolds S.M., Shmulevich I., Aksoy B. Arman, Antipin Y., Ciriello G., Dresdner G., Gao J., Gross B., Jacobsen A., Ladanyi M., Reva B., Sander C., Sinha R., Sumer S. Onur, Taylor B.S., Cerami E., Weinhold N., Schultz N., Shen R., Benz S., Goldstein T., Haussler D., Ng S., Szeto C., Stuart J., Benz C.C., Yau C., Zhang W., Annala M., Broom B.M., Casasent T.D., Ju Z., Liang H., Liu G., Lu Y., Unruh A.K., Wakefield C., Weinstein J.N., Zhang N., Liu Y., Broaddus R., Akbani R., Mills G.B., Adams C., Barr T., Black A.D., Bowen J., Deardurff J., Frick J., Gastier-Foster J.M., Grossman T., Harper H.A., Hart-Kothari M., Helsel C., Hobensack A., Kuck H., Kneile K., Leraas K.M., Lichtenberg T.M., McAllister C., Pyatt R.E., Ramirez N.C., Tabler T.R., Vanhoose N., White P., Wise L., Zmuda E., Barnabas N., Berry-Green C., Blanc V., Boice L., Button M., Farkas A., Green A., MacKenzie J., Nicholson D., Kalloger S.E., Gilks C. Blake, Karlan B.Y., Lester J., Orsulic S., Borowsky M., Cadungog M., Czerwinski C., Huelsenbeck-Dill L., Iacocca M., Petrelli N., Rabeno B., Witkin G., Nemirovich-Danchenko E., Potapova O., Rotin D., Berchuck A., Birrer M., DiSaia P., Monovich L., Curley E., Gardner J., Mallery D., Penny R., Dowdy S.C., Winterhoff B., Dao L., Gostout B., Meuter A., Teoman A., Dao F., Olvera N., Bogomolniy F., Garg K., Soslow R.A., Levine D.A., Abramov M., Bartlett J.M.S., Kodeeswaran S., Parfitt J., Moiseenko F., Clarke B.A., Goodman M.T., Carney M.E., Matsuno R.K., Fisher J., Huang M., Rathmell W. Kimryn, Thorne L., Le L. Van, Dhir R., Edwards R., Elishaev E., Zorn K., Broaddus R., Goodfellow P.J., Mutch D., Schultz N., Liu Y., Akbani R., Cherniack A.D., Cerami E., Weinhold N., Shen H., Hoadley K.A., Kahn A.B., Bell D.W., Pollock P.M., Wang, Wheeler D.A., Shinbrot E., Karlan B.Y., Berchuck A., Dowdy S.C., Winterhoff B., Goodman M.T., Robertson A. Gordon, Beroukhim R., Pashtan I., Salvesen H.B., Laird P.W., Noble M., Stuart J., Ding L., Kandoth C., Gilks C. Blake, Soslow R.A., Goodfellow P.J., Mutch D., Broaddus R., Zhang W., Mills G.B., Kucherlapati R., Mardis E.R., Levine D.A., Ayala B., Chu A.L., Jensen M.A., Kothiyal P., Pihl T.D., Pontius J., Pot D.A., Snyder E.E., Srinivasan D., Kahn A.B., Shaw K.R. Mills, Sheth M., Davidsen T., Eley Martin G., Ferguson L., Demchok J.A., Yang L., Guyer M.S., Ozenberger B.A., Sofia H.J., Kandoth C., Schultz N., Cherniack A.D., Akbani R., Liu Y., Shen H., Robertson A. Gordon, Pashtan I., Shen R., Benz C.C., Yau C., Laird P.W., Ding L., Zhang W., Mills G.B., Kucherlapati R., Mardis E.R., Levine D.A. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.S. Zhao, M. Choi, J.D. Overton, S. Bellone, D.M. Roque, E. Cocco, F. Guzzo, D.P. English, J. Varughese, S. Gasparrini, I. Bortolomai, N. Buza, P. Hui, M. Abu-Khalaf, A. Ravaggi, E. Bignotti, E. Bandiera, C. Romani, P. Todeschini, R. Tassi, L. Zanotti, L. Carrara, S. Pecorelli, D.-.A. Silasi, E. Ratner, M. Azodi, P.E. Schwartz, T.J. Rutherford, A.L. Stiegler, S. Mane, T.J. Boggon, J. Schlessinger, R.P. Lifton, A.D. Santin, Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma, Proceedings of the National Academy of Sciences, 110(8), 2013, pp. 2916–2921. [DOI] [PMC free article] [PubMed]
- 69.Spaans V.M., Trietsch M.D., Crobach S., Stelloo E., Kremer D., Osse E.M., Haar N.Tt, van Eijk R., Muller S., van Wezel T., Trimbos J.B., Bosse T., Smit V.T.H.B.M., Fleuren G.J. Designing a high-throughput somatic mutation profiling panel specifically for gynaecological cancers. PLoS One. 2014;9(3):e93451. doi: 10.1371/journal.pone.0093451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beltrame L., Di Marino M., Fruscio R., Calura E., Chapman B., Clivio L., Sina F., Mele C., Iatropoulos P., Grassi T., Fotia V., Romualdi C., Martini P., Noris M., Paracchini L., Craparotta I., Petrillo M., Milani R., Perego P., Ravaggi A., Zambelli A., Ronchetti E., D'Incalci M., Marchini S. Profiling cancer gene mutations in longitudinal epithelial ovarian cancer biopsies by targeted next-generation sequencing: a retrospective study. Ann. Oncol. 2015;26(7):1363–1371. doi: 10.1093/annonc/mdv164. [DOI] [PubMed] [Google Scholar]
- 71.Le Gallo M., Rudd M.L., Urick M.E., Hansen N.F., Zhang S., Lozy F., Sgroi D.C., Vidal Bel A., Matias-Guiu X., Broaddus R.R., Lu K.H., Levine D.A., Mutch D.G., Goodfellow P.J., Salvesen H.B., Mullikin J.C., Bell D.W. Somatic mutation profiles of clear cell endometrial tumors revealed by whole exome and targeted gene sequencing. Cancer. 2017;123(17):3261–3268. doi: 10.1002/cncr.30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao J., Chang M.T., Johnsen H.C., Gao S.P., Sylvester B.E., Sumer S.O., Zhang H., Solit D.B., Taylor B.S., Schultz N., Sander C. 3D clusters of somatic mutations in cancer reveal numerous rare mutations as functional targets, genome. Medicine. 2017;9(1):4. doi: 10.1186/s13073-016-0393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bamford S., Dawson E., Forbes S., Clements J., Pettett R., Dogan A., Flanagan A., Teague J., Futreal P.A., Stratton M.R., Wooster R. The COSMIC (catalogue of somatic mutations in cancer) database and website. Br. J. Cancer. 2004;91:355. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ruediger R., Fields K., Walter G. Binding specificity of protein phosphatase 2A core enzyme for regulatory B subunits and T antigens. J. Virol. 1999;73(1):839–842. doi: 10.1128/jvi.73.1.839-842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruediger R., Pham H.T., Walter G. Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the Aα subunit gene. Oncogene. 2001;20:10. doi: 10.1038/sj.onc.1204059. [DOI] [PubMed] [Google Scholar]
- 76.Ruediger R., Ruiz J., Walter G. Human cancer-associated mutations in the Aα subunit of protein phosphatase 2A increase lung cancer incidence in Aα knock-In and knockout mice. Mol. Cell. Biol. 2011;31(18):3832–3844. doi: 10.1128/MCB.05744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calin G.A., di Iasio M.G., Caprini E., Vorechovsky I., Natali P.G., Sozzi G., Croce C.M., Barbanti-Brodano G., Russo G., Negrini M. Low frequency of alterations of the α (PPP2R1A) and β (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene. 2000;19:1191. doi: 10.1038/sj.onc.1203389. [DOI] [PubMed] [Google Scholar]
- 78.Walter G., Ruediger R. Mouse model for probing tumor suppressor activity of protein phosphatase 2A in diverse signaling pathways. Cell Cycle. 2012;11(3):451–459. doi: 10.4161/cc.11.3.19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suzuki K., Takahashi K. Reduced expression of the regulatory A subunit of serine/threonine protein phosphatase 2A in human breast cancer MCF-7 cells. Int. J. Oncol. 2003;23(5):1263–1268. [PubMed] [Google Scholar]
- 80.Colella S., Ohgaki H., Ruediger R., Yang F., Nakamura M., Fujisawa H., Kleihues P., Walter G. Reduced expression of the Aα subunit of protein phosphatase 2A in human gliomas in the absence of mutations in the Aα and Aβ subunit genes. Int. J. Cancer. 2001;93(6):798–804. doi: 10.1002/ijc.1423. [DOI] [PubMed] [Google Scholar]
- 81.Wang S.S., Esplin E.D., Li J.L., Huang L., Gazdar A., Minna J., Evans G.A. Alterations of the PPP2R1B gene in human lung and colon. Cancer, Sci. 1998;282(5387):284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 82.Hemmer S., Wasenius V.-M., Haglund C., Zhu Y., Knuutila S., Franssila K., Joensuu H. Alterations in the suppressor gene PPP2R1B in parathyroid hyperplasias and adenomas. Cancer Genet. Cytogenet. 2002;134(1):13–17. doi: 10.1016/s0165-4608(01)00597-0. [DOI] [PubMed] [Google Scholar]
- 83.Zhu Y., Loukola A., Outimonni, Kuokkanen K., Franssila K., Elonen E., Vilpo J., Joensuu H., Kere J., Aaltonen L., Knuutila S. PPP2R1B gene in chronic lymphocytic Leukemias and mantle cell lymphomas. Leuk. Lymphoma. 2001;41(1–2):177–183. doi: 10.3109/10428190109057968. [DOI] [PubMed] [Google Scholar]
- 84.Zhou J., Pham H.T., Ruediger R., Walter G. Characterization of the Aalpha and Abeta subunit isoforms of protein phosphatase 2A: differences in expression, subunit interaction, and evolution. Biochem. J. 2003;369(Pt 2):387–398. doi: 10.1042/BJ20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sablina A.A., Chen W., Arroyo J.D., Corral L., Hector M., Bulmer S.E., DeCaprio J.A., Hahn W.C. The tumor suppressor PP2A regulates the RalA GTPase. Cell. 2007;129(5):969–982. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sangodkar J., Perl A., Tohme R., Kiselar J., Kastrinsky D.B., Zaware N., Izadmehr S., Mazhar S., Wiredja D.D., O’Connor C.M., Hoon D., Dhawan N.S., Schlatzer D., Yao S., Leonard D., Borczuk A.C., Gokulrangan G., Wang L., Svenson E., Farrington C.C., Yuan E., Avelar R.A., Stachnik A., Smith B., Gidwani V., Giannini H.M., McQuaid D., McClinch K., Wang Z., Levine A.C., Sears R.C., Chen E.Y., Duan Q., Datt M., Haider S., Ma’ayan A., DiFeo A., Sharma N., Galsky M.D., Brautigan D.L., Ioannou Y.A., Xu W., Chance M.R., Ohlmeyer M., Narla G. Activation of tumor suppressor protein PP2A inhibits KRAS-driven tumor growth. J. Clin. Investig. 2017;127(6):2081–2090. doi: 10.1172/JCI89548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kauko O., O’Connor C.M., Kulesskiy E., Sangodkar J., Aakula A., Izadmehr S., Yetukuri L., Yadav B., Padzik A., Laajala T.D., Haapaniemi P., Momeny M., Varila T., Ohlmeyer M., Aittokallio T., Wennerberg K., Narla G., Westermarck J. PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells. Sci. Transl. Med. 2018;10(450) doi: 10.1126/scitranslmed.aaq1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McClinch K., Avelar R.A., Callejas D., Izadmehr S., Wiredja D., Perl A., Sangodkar J., Kastrinsky D.B., Schlatzer D., Cooper M., Kiselar J., Stachnik A., Yao S., Hoon D., McQuaid D., Zaware N., Gong Y., Brautigan D.L., Plymate S.R., Sprenger C.C.T., Oh W.K., Levine A.C., Kirschenbaum A., Sfakianos J.P., Sears R., DiFeo A., Ioannou Y., Ohlmeyer M., Narla G., Galsky M.D. Small-molecule activators of protein phosphatase 2A for the treatment of castration-resistant prostate cancer. Cancer Res. 2018;78(8):2065–2080. doi: 10.1158/0008-5472.CAN-17-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yadav L., Tamene F., Göös H., van Drogen A., Katainen R., Aebersold R., Gstaiger M., Varjosalo M. Systematic analysis of human protein phosphatase interactions and dynamics. Cell Syst. 2017;4(4):430–444. doi: 10.1016/j.cels.2017.02.011. (e5) [DOI] [PubMed] [Google Scholar]
- 90.Cheng Y., Liu W., Kim S.-T., Sun J., Lu L., Sun J., Zheng S.L., Isaacs W.B., Xu J. Evaluation of PPP2R2A as a prostate cancer susceptibility gene: comprehensive germline and somatic study. Cancer Genet. 2011;204(7):375–381. doi: 10.1016/j.cancergen.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curtis C., Shah S.P., Chin S.-F., Turashvili G., Rueda O.M., Dunning M.J., Speed D., Lynch A.G., Samarajiwa S., Yuan Y., Gräf S., Ha G., Haffari G., Bashashati A., Russell R., McKinney S., Group M., Langerød A., Green A., Provenzano E., Wishart G., Pinder S., Watson P., Markowetz F., Murphy L., Ellis I., Purushotham A., Børresen-Dale A.-L., Brenton J.D., Tavaré S., Caldas C., Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mosca L., Musto P., Todoerti K., Barbieri M., Agnelli L., Fabris S., Tuana G., Lionetti M., Bonaparte E., Sirchia S.M., Grieco V., Bianchino G., D'Auria F., Statuto T., Mazzoccoli C., De Luca L., Petrucci M.T., Morabito F., Offidani M., Di Raimondo F., Falcone A., Caravita T., Omedè P., Boccadoro M., Palumbo A., Neri A. Genome-wide analysis of primary plasma cell leukemia identifies recurrent imbalances associated with changes in transcriptional profiles. Am. J. Hematol. 2012;88(1):16–23. doi: 10.1002/ajh.23339. [DOI] [PubMed] [Google Scholar]
- 93.Shouse G.P., de Necochea-Campion R., Mirshahidi S., Liu X., Chen C.-S. Novel AML-associated loss of function mutations in the B55 alpha subunit of PP2A Are associated with increased phosphorylation and activation of AKT and enhanced sensitivity to AKT inhibitor induced cell growth arrest. Blood. 2014;124(21):2206. [Google Scholar]
- 94.Ruvolo P.P., Qui Y., Coombes K.R., Zhang N., Ruvolo V.R., Borthakur G., Konopleva M., Andreeff M., Kornblau S.M. Low expression of PP2A regulatory subunit B55α is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients. Leukemia. 2011;25(11):1711–1717. doi: 10.1038/leu.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Muggerud A.A., Rønneberg J.A., Wärnberg F., Botling J., Busato F., Jovanovic J., Solvang H., Bukholm I., Børresen-Dale A.-L., Kristensen V.N., Sørlie T., Tost J. Frequent aberrant DNA methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res.: BCR. 2010;12(1) doi: 10.1186/bcr2466. (R3-R3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Preuss K.-D., Fadle N., Regitz E., Held G., Pfreundschuh M. Inactivation of protein-phosphatase 2A causing hyperphosphorylation of autoantigenic paraprotein targets in MGUS/MM is due to an exchange of its regulatory subunits. Int. J. Cancer. 2014;135(9):2046–2053. doi: 10.1002/ijc.28864. [DOI] [PubMed] [Google Scholar]
- 97.Bluemn E.G., Spencer E.S., Mecham B., Gordon R.R., Coleman I., Lewinshtein D., Mostaghel E., Zhang X., Annis J., Grandori C., Porter C., Nelson P.S. PPP2R2C loss promotes castration-resistance and is associated with increased prostate cancer-specific mortality. Mol. Cancer Res.: MCR. 2013;11(6):568–578. doi: 10.1158/1541-7786.MCR-12-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fan Y.-l., Chen L., Wang J., Yao Q., Wan J.-q. Over expression of PPP2R2C inhibits human glioma cells growth through the suppression of mTOR pathway. FEBS Lett. 2013;587(24):3892–3897. doi: 10.1016/j.febslet.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 99.Tan J., Lee P.L., Li Z., Jiang X., Lim Y.C., Hooi S.C., Yu Q. B55β-Associated PP2A complex controls PDK1-directed Myc signaling and modulates Rapamycin sensitivity in colorectal cancer. Cancer Cell. 2010;18(5):459–471. doi: 10.1016/j.ccr.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 100.Peng D., Guo Y., Chen H., Zhao S., Washington K., Hu T., Shyr Y., El-Rifai W. Integrated molecular analysis reveals complex interactions between genomic and epigenomic alterations in esophageal adenocarcinomas. Sci. Rep. 2017;7:40729. doi: 10.1038/srep40729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bi D., Ning H., Liu S., Que X., Ding K. miR-1301 promotes prostate cancer proliferation through directly targeting PPP2R2C. Biomed. Pharmacother. 2016;81:25–30. doi: 10.1016/j.biopha.2016.03.043. [DOI] [PubMed] [Google Scholar]
- 102.Grochola L.F., Vazquez A., Bond E.E., Würl P., Taubert H., Müller T.H., Levine A.J., Bond G.L. Recent natural selection identifies a Genetic variant in a regulatory subunit of protein phosphatase 2A that associates with altered cancer risk and survival. Clin. Cancer Res. 2009;15(19):6301. doi: 10.1158/1078-0432.CCR-09-0797. [DOI] [PubMed] [Google Scholar]
- 103.Shouse G.P., Nobumori Y., Liu X. A B56γ mutation in lung cancer disrupts the p53-dependent tumor suppressor function of protein phosphatase 2A. Oncogene. 2010;29(27):3933–3941. doi: 10.1038/onc.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang J., Okkeri J., Pavic K., Wang Z., Kauko O., Halonen T., Sarek G., Ojala P.M., Rao Z., Xu W., Westermarck J. Oncoprotein CIP2A is stabilized via interaction with tumor suppressor PP2A/B56. EMBO Rep. 2017;18(3):437–450. doi: 10.15252/embr.201642788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kauko O., Imanishi S.Y., Kulesskiy E., Laajala T.D., Yetukuri L., Laine A., Jumppanen M., Haapaniemi P., Ruan L., Yadav B., Suni V., Varila T., Corthals G., Reimand J., Wennerberg K., Aittokallio T., Westermarck J. Rules for PP2A-controlled phosphosignalling and drug responses. bioRxiv. 2018 [Google Scholar]
- 106.Junttila M.R., Puustinen P., Niemelä M., Ahola R., Arnold H., Böttzauw T., Ala-aho R., Nielsen C., Ivaska J., Taya Y., Lu S.-L., Lin S., Chan E.K.L., Wang X.-J., Grènman R., Kast J., Kallunki T., Sears R., Kähäri V.-M., Westermarck J. CIP2A Inhibits PP2A in Human Malignancies. Cell. 2007;130(1):51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 107.Lucas C.M., Harris R.J., Giannoudis A., Copland M., Slupsky J.R., Clark R.E. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117(24):6660–6668. doi: 10.1182/blood-2010-08-304477. [DOI] [PubMed] [Google Scholar]
- 108.Liu X., Cao W., Qin S., Zhang T., Zheng J., Dong Y., Ming P., Cheng Q., Lu Z., Guo Y., Zhang B., Liu Y. Overexpression of CIP2A is associated with poor prognosis in multiple myeloma. Signal Transduct. Target. Ther. 2017;2:17013. doi: 10.1038/sigtrans.2017.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Farrell A.S., Allen-Petersen B., Daniel C.J., Wang X., Wang Z., Rodriguez S., Impey S., Oddo J., Vitek M.P., Lopez C., Christensen D.J., Sheppard B., Sears R.C. Targeting inhibitors of the tumor suppressor PP2A for the treatment of pancreatic cancer. Mol. Cancer Res.: MCR. 2014;12(6):924–939. doi: 10.1158/1541-7786.MCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lucas C.M., Scott L.J., Carmell N., Holcroft A.K., Hills R.K., Burnett A.K., Clark R.E. CIP2A- and SETBP1-mediated PP2A inhibition reveals AKT S473 phosphorylation to be a new biomarker in AML. Blood Adv. 2018;2(9):964–968. doi: 10.1182/bloodadvances.2017013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Côme C., Laine A., Chanrion M., Edgren H., Mattila E., Liu X., Jonkers J., Ivaska J., Isola J., Darbon J.-M., Kallioniemi O., Thézenas S., Westermarck J. CIP2A Is associated with human breast cancer aggressivity. Clin. Cancer Res. 2009;15(16):5092–5100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 112.Khanna A., Böckelman C., Hemmes A., Junttila M.R., Wiksten J.-P., Lundin M., Junnila S., Murphy D.J., Evan G.I., Haglund C., Westermarck J., Ristimäki A. MYC-dependent regulation and Prognostic role of CIP2A in gastric cancer. JNCI: J. Natl. Cancer Inst. 2009;101(11):793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 113.Dong Q.-Z., Wang Y., Dong X.-J., Li Z.-X., Tang Z.-P., Cui Q.-Z., Wang E.-H. CIP2A is Overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann. Surg. Oncol. 2011;18(3):857–865. doi: 10.1245/s10434-010-1313-8. [DOI] [PubMed] [Google Scholar]
- 114.Vaarala M.H., Väisänen M.-R., Ristimäki A. CIP2A expression is increased in prostate cancer. J. Exp. Clin. Cancer Res.: CR. 2010;29(1) doi: 10.1186/1756-9966-29-136. (136-136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Laine A., Sihto H., Come C., Rosenfeldt M.T., Zwolinska A., Niemelä M., Khanna A., Chan E.K., Kähäri V.-M., Kellokumpu-Lehtinen P.-L., Sansom O.J., Evan G.I., Junttila M.R., Ryan K.M., Marine J.-C., Joensuu H., Westermarck J. Senescence sensitivity of breast cancer cells Is defined by positive feedback loop between CIP2A and E2F1. Cancer Discov. 2013;3(2):182–197. doi: 10.1158/2159-8290.CD-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu C.-Y., Huang T.-T., Huang C.-T., Hu M.-H., Wang D.-S., Wang W.-L., Tsai W.-C., Lee C.-H., Lau K.-Y., Yang H.-P., Chen M.-H., Shiau C.-W., Tseng L.-M., Chen K.-F. EGFR-independent Elk1/CIP2A signalling mediates apoptotic effect of an erlotinib derivative TD52 in triple-negative breast cancer cells. Eur. J. Cancer. 2017;72:112–123. doi: 10.1016/j.ejca.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 117.Chao T.-T., Wang C.-Y., Lai C.-C., Chen Y.-L., Tsai Y.-T., Chen P.-T., Lin H.-I., Huang Y.-C.T., Shiau C.-W., Yu C.-J., Chen K.-F. TD-19, an Erlotinib derivative, induces Epidermal growth factor receptor wild-type nonsmall-cell lung cancer apoptosis through CIP2A-mediated pathway. J. Pharmacol. Exp. Ther. 2014;351(2):352. doi: 10.1124/jpet.114.215418. [DOI] [PubMed] [Google Scholar]
- 118.Yu H.C., Hung M.H., Chen Y.L., Chu P.Y., Wang C.Y., Chao T.T., Liu C.Y., Shiau C.W., Chen K.F. Erlotinib derivative inhibits hepatocellular carcinoma by targeting CIP2A to reactivate protein phosphatase 2A. Cell Death Dis. 2014;5(7):e1359. doi: 10.1038/cddis.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang T., He K., Mao Y., Zhu M., Yan C., Yu F., Qi Q., Wang T., Wang Y., Du J., Liu L. Genetic variants in PPP2CA are associated with gastric cancer risk in a Chinese population. Sci. Rep. 2017;7:11499. doi: 10.1038/s41598-017-12040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tao M., Liu L., Shen M., Zhi Q., Gong F.-R., Zhou B.P., Wu Y., Liu H., Chen K., Shen B., Wu M.-Y., Shou L.-M., Li W. Inflammatory stimuli promote growth and invasion of pancreatic cancer cells through NF-κB pathway dependent repression of PP2Ac. Cell Cycle. 2016;15(3):381–393. doi: 10.1080/15384101.2015.1127468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singh A.P., Bafna S., Chaudhary K., Venkatraman G., Smith L., Eudy J.D., Johansson S.L., Lin M.-F., Batra S.K. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer Lett. 2008;259(1):28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yuan Q., Li P.D., Li B.H., Yang X.Z., Xu S.B., Liu X.H., Zhou F.X., Zhang W.J. Differential IL-4/Stat6 activities correlate with differential expression of regulatory genes SOCS-1, SHP-1, and PP2A in colon cancer cells. J. Cancer Res. Clin. Oncol. 2009;135(1):131–140. doi: 10.1007/s00432-008-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramaswamy K., Spitzer B., Kentsis A. Therapeutic re-activation of protein phosphatase 2A in acute myeloid leukemia. Front. Oncol. 2015;5:16. doi: 10.3389/fonc.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhardwaj A., Singh S., Srivastava S.K., Arora S., Hyde S.J., Andrews J., Grizzle W.E., Singh A.P. Restoration of PPP2CA expression reverses epithelial-to-mesenchymal transition and suppresses prostate tumour growth and metastasis in an orthotopic mouse model. Br. J. Cancer. 2014;110(8):2000–2010. doi: 10.1038/bjc.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sents W., Meeusen B., Kalev P., Radaelli E., Sagaert X., Miermans E., Haesen D., Lambrecht C., Dewerchin M., Carmeliet P., Westermarck J., Sablina A., Janssens V. PP2A inactivation mediated by PPP2R4 haploinsufficiency promotes cancer development. Cancer Res. 2017;77(24):6825–6837. doi: 10.1158/0008-5472.CAN-16-2911. [DOI] [PubMed] [Google Scholar]
- 126.Liu H., Liu Y., Kong F., Xin W., Li X., Liang H., Jia Y. Elevated levels of set and MYND domain-containing protein 3 Are correlated with overexpression of transforming growth factor-beta1 in gastric cancer. J. Am. Coll. Surg. 2015;221(2):579–590. doi: 10.1016/j.jamcollsurg.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 127.Liu H., Gu Y., Wang H., Yin J., Zheng G., Zhang Z., Lu M., Wang C., He Z. Overexpression of PP2A inhibitor set oncoprotein is associated with tumor progression and poor prognosis in human non-small cell lung cancer. Oncotarget. 2015;6(17):14913–14925. doi: 10.18632/oncotarget.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cristóbal I., Rincón R., Manso R., Caramés C., Zazo S., Madoz-Gúrpide J., Rojo F., García-Foncillas J. Deregulation of the PP2A inhibitor set shows promising therapeutic implications and determines poor clinical outcome in patients with metastatic colorectal cancer. Clin. Cancer Res. 2015;21(2):347–356. doi: 10.1158/1078-0432.CCR-14-0724. [DOI] [PubMed] [Google Scholar]
- 129.Leopoldino A.M., Squarize C.H., Garcia C.B., Almeida L.O., Pestana C.R., Sobral L.M., Uyemura S.A., Tajara E.H., Silvio Gutkind J., Curti C. SET protein accumulates in HNSCC and contributes to cell survival: antioxidant defense, Akt phosphorylation and AVOs acidification. Oral. Oncol. 2012;48(11):1106–1113. doi: 10.1016/j.oraloncology.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 130.Cristóbal I., Garcia-Orti L., Cirauqui C., Cortes-Lavaud X., García-Sánchez M.A., Calasanz M.J., Odero M.D. Overexpression of <em>set</em> is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemia. Haematologica. 2012;97(4):543–550. doi: 10.3324/haematol.2011.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan X., Zhang T., Zheng X., Zhang Y., Feng T., Liu P., Sun Z., Qin S., Liu X., Zhang L., Song J., Liu Y. Overexpression of set oncoprotein is associated with tumor progression and poor prognosis in human gastric cancer. Oncol. Rep. 2017;38(3):1733–1741. doi: 10.3892/or.2017.5788. [DOI] [PubMed] [Google Scholar]
- 132.Kubota D., Yoshida A., Kawai A., Kondo T. Proteomics identified Overexpression of set oncogene product and possible therapeutic utility of protein phosphatase 2A in alveolar soft Part sarcoma. J. Proteome Res. 2014;13(5):2250–2261. doi: 10.1021/pr400929h. [DOI] [PubMed] [Google Scholar]
- 133.Christensen D.J., Chen Y., Oddo J., Matta K.M., Neil J., Davis E.D., Volkheimer A.D., Lanasa M.C., Friedman D.R., Goodman B.K., Gockerman J.P., Diehl L.F., de Castro C.M., Moore J.O., Vitek M.P., Weinberg J.B. SET oncoprotein overexpression in B-cell chronic lymphocytic leukemia and non-Hodgkin lymphoma: a predictor of aggressive disease and a new treatment target. Blood. 2011;118(15):4150–4158. doi: 10.1182/blood-2011-04-351072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cristóbal I., Garcia-Orti L., Cirauqui C., Cortes-Lavaud X., García-Sánchez M.A., Calasanz M.J., Odero M.D. Overexpression of set is a recurrent event associated with poor outcome and contributes to protein phosphatase 2A inhibition in acute myeloid leukemia. Haematologica. 2012;97(4):543–550. doi: 10.3324/haematol.2011.050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang Y.-H., Chu P.-Y., Chen J.-L., Huang C.-T., Lee C.-H., Lau K.-Y., Wang W.-L., Wang Y.-L., Lien P.-J., Tseng L.-M., Liu C.-Y. SET Overexpression is associated with worse recurrence-free survival in patients with primary breast cancer receiving adjuvant Tamoxifen treatment. J. Clin. Med. 2018;7(9):245. doi: 10.3390/jcm7090245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hung M.H., Chen Y.L., Chu P.Y., Shih C.T., Yu H.C., Tai W.T., Shiau C.W., Chen K.F. Upregulation of the oncoprotein SET determines poor clinical outcomes in hepatocellular carcinoma and shows therapeutic potential. Oncogene. 2016;35:4891. doi: 10.1038/onc.2016.21. [DOI] [PubMed] [Google Scholar]
- 137.Arnaud L., Chen S., Liu F., Li B., Khatoon S., Grundke-Iqbal I., Iqbal K. Mechanism of inhibition of PP2A activity and abnormal hyperphosphorylation of tau by I2PP2A/SET. FEBS Lett. 2011;585(17):2653–2659. doi: 10.1016/j.febslet.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tian L., Zhang X., Haesen D., Bravo J., Fominaya J., Choquet S., Zini J.M., Loisel S., Waelkens E., Janssens V., Rebollo A. Identification of PP2A/set binding sites and Design of interacting peptides with potential clinical applications. Int. J. Pept. Res. Ther. 2017 [Google Scholar]
- 139.Richard N.P., Pippa R., Cleary M.M., Puri A., Tibbitts D., Mahmood S., Christensen D.J., Jeng S., McWeeney S., Look A.T., Chang B.H., Tyner J.W., Vitek M.P., Odero M.D., Sears R., Agarwal A. Combined targeting of SET and tyrosine kinases provides an effective therapeutic approach in human T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7(51):84214–84227. doi: 10.18632/oncotarget.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oaks J.J., Santhanam R., Walker C.J., Roof S., Harb J.G., Ferenchak G., Eisfeld A.-K., Van Brocklyn J.R., Briesewitz R., Saddoughi S.A., Nagata K., Bittman R., Caligiuri M.A., Abdel-Wahab O., Levine R., Arlinghaus R.B., Quintas-Cardama A., Goldman J.M., Apperley J., Reid A., Milojkovic D., Ziolo M.T., Marcucci G., Ogretmen B., Neviani P., Perrotti D. Antagonistic activities of the immunomodulator and PP2A-activating drug FTY720 (Fingolimod, Gilenya) in Jak2-driven hematologic malignancies. Blood. 2013;122(11):1923–1934. doi: 10.1182/blood-2013-03-492181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang Y., Huang Q., Lu Y., Li X., Huang S. Reactivating PP2A by FTY720 as a novel therapy for AML with C-KIT tyrosine kinase domain mutation. J. Cell. Biochem. 2011;113(4):1314–1322. doi: 10.1002/jcb.24003. [DOI] [PubMed] [Google Scholar]
- 142.Szymiczek A., Pastorino S., Larson D., Tanji M., Pellegrini L., Xue J., Li S., Giorgi C., Pinton P., Takinishi Y., Pass H.I., Furuya H., Gaudino G., Napolitano A., Carbone M., Yang H. FTY720 inhibits mesothelioma growth in vitro and in a syngeneic mouse model. J. Transl. Med. 2017;15(1):58. doi: 10.1186/s12967-017-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Neviani P., Santhanam R., Oaks J.J., Eiring A.M., Notari M., Blaser B.W., Liu S., Trotta R., Muthusamy N., Gambacorti-Passerini C., Druker B.J., Cortes J., Marcucci G., Chen C.-S., Verrills N.M., Roy D.C., Caligiuri M.A., Bloomfield C.D., Byrd J.C., Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome–positive acute lymphocytic leukemia. J. Clin. Investig. 2007;117(9):2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Agarwal A., MacKenzie R.J., Pippa R., Eide C.A., Oddo J., Tyner J.W., Sears R., Vitek M.P., Odero M.D., Christensen D., Druker B.J. Antagonism of set using OP449 enhances the efficacy of tyrosine kinase inhibitors and overcome drug resistance in myeloid leukemia, clinical cancer research: an official journal of the American Association for. Cancer Res. 2014;20(8):2092–2103. doi: 10.1158/1078-0432.CCR-13-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Janghorban M., Farrell A.S., Allen-Petersen B.L., Pelz C., Daniel C.J., Oddo J., Langer E.M., Christensen D.J., Sears R.C. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc. Natl. Acad. Sci. USA. 2014;111(25):9157–9162. doi: 10.1073/pnas.1317630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gadi S., Zara Z., Irini Markella A., Marilyn S., Aviva T.-H., Michael P.V., Derek L. G. Emily Jane, OP449 inhibits breast cancer growth without adverse metabolic effects. Endocr. -Relat. Cancer. 2017;24(10):519–529. doi: 10.1530/ERC-17-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ruediger R., Roeckel D., Fait J., Bergqvist A., Magnusson G., Walter G. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol. 1992;12(11):4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc. Natl. Acad. Sci. USA. 1990;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sontag E., Fedorov S., Kamibayashi C., Robbins D., Cobb M., Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75(5):887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 150.Hahn W.C., Dessain S.K., Brooks M.W., King J.E., Elenbaas B., Sabatini D.M., DeCaprio J.A., Weinberg R.A. Enumeration of the Simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 2002;22(7):2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ruediger R., Hentz M., Fait J., Mumby M., Walter G. Molecular model of the A subunit of protein phosphatase 2A: interaction with other subunits and tumor antigens. J. Virol. 1994;68(1):123–129. doi: 10.1128/jvi.68.1.123-129.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pallas D.C., Shahrik L.K., Martin B.L., Jaspers S., Miller T.B., Brautigan D.L., Roberts T.M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 153.Chen Y., Xu Y., Bao Q., Xing Y., Li Z., Lin Z., Stock J.B., Jeffrey P.D., Shi Y. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat. Struct. Mol. Biol. 2007;14:527. doi: 10.1038/nsmb1254. [DOI] [PubMed] [Google Scholar]
- 154.Ogris E., Gibson D.M., Pallas D.C. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- 155.Chung H., Nairn A.C., Murata K., Brautigan D.L. Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic Subunit favors association with the α4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry. 1999;38(32):10371–10376. doi: 10.1021/bi990902g. [DOI] [PubMed] [Google Scholar]
- 156.Longin S., Zwaenepoel K., Louis J.V., Dilworth S., Goris J., Janssens V. Selection of protein phosphatase 2A regulatory subunits Is mediated by the C terminus of the catalytic subunit. J. Biol. Chem. 2007;282(37):26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 157.Chen J., Martin B.L., Brautigan D.L. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257(5074):1261. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 158.Favre B., Zolnierowicz S., Turowski P., Hemmings B.A. The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J. Biol. Chem. 1994;269(23):16311–16317. [PubMed] [Google Scholar]
- 159.Tolstykh T., Lee J., Vafai S., Stock J.B. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19(21):5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ogris E., Du X., Nelson K.C., Mak E.K., Yu X.X., Lane W.S., Pallas D.C. A protein phosphatase methylesterase (PME-1) Is One of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J. Biol. Chem. 1999;274(20):14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee J., Chen Y., Tolstykh T., Stock J. A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc. Natl. Acad. Sci. USA. 1996;93(12):6043–6047. doi: 10.1073/pnas.93.12.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee J., Stock J. Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J. Biol. Chem. 1993;268(26):19192–19195. [PubMed] [Google Scholar]
- 163.Xie H., Clarke S. Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J. Biol. Chem. 1994;269(3):1981–1984. [PubMed] [Google Scholar]
- 164.De Baere I., Derua R., Janssens V., Van Hoof C., Waelkens E., Merlevede W., Goris J. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and Cloning of the human homologue. Biochemistry. 1999;38(50):16539–16547. doi: 10.1021/bi991646a. [DOI] [PubMed] [Google Scholar]
- 165.Guo F., Stanevich V., Wlodarchak N., Sengupta R., Jiang L., Satyshur K.A., Xing Y. Structural basis of PP2A activation by PTPA, an ATP-dependent activation chaperone. Cell Res. 2013;24:190. doi: 10.1038/cr.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stanevich V., Jiang L., Satyshur K.A., Li Y., Jeffrey P.D., Li Z., Menden P., Semmelhack M.F., Xing Y. The Structural basis for tight control of PP2A methylation and function by LCMT-1. Mol. Cell. 2011;41(3):331–342. doi: 10.1016/j.molcel.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xing Y., Li Z., Chen Y., Stock J.B., Jeffrey P.D., Shi Y. Structural Mechanism of Demethylation and Inactivation of Protein Phosphatase 2A. Cell. 2008;133(1):154–163. doi: 10.1016/j.cell.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 168.Stanevich V., Zheng A., Guo F., Jiang L., Wlodarchak N., Xing Y. Mechanisms of the Scaffold Subunit in Facilitating Protein Phosphatase 2A Methylation. PLoS One. 2014;9(1):e86955. doi: 10.1371/journal.pone.0086955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wu F., Wilson J.X. Peroxynitrite-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc. Res. 2009;81(1):38–45. doi: 10.1093/cvr/cvn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Deng Y., Guo Y., Liu P., Zeng R., Ning Y., Pei G., Li Y., Chen M., Guo S., Li X., Han M., Xu G. Blocking protein phosphatase 2A signaling prevents endothelial-to-mesenchymal transition and renal fibrosis: a peptide-based drug therapy. Sci. Rep. 2016;6:19821. doi: 10.1038/srep19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohama T., Brautigan D.L. Endotoxin conditioning induces VCP/p97-mediated and inducible Nitric-oxide synthase-dependent Tyr284 Nitration in protein phosphatase 2A. J. Biol. Chem. 2010;285(12):8711–8718. doi: 10.1074/jbc.M109.099788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shigenaga M.K., Ames B.N. Oxidants and mitogenesis as causes of mutation and cancer: the influence of diet. Basic Life Sci. 1993;61:419–436. doi: 10.1007/978-1-4615-2984-2_37. [DOI] [PubMed] [Google Scholar]
- 174.Saito Y., Nishio K., Ogawa Y., Kimata J., Kinumi T., Yoshida Y., Noguchi N., Niki E. Turning point in apoptosis/necrosis induced by hydrogen peroxide. Free Radic. Res. 2006;40(6):619–630. doi: 10.1080/10715760600632552. [DOI] [PubMed] [Google Scholar]
- 175.Sabharwal S.S., Schumacker P.T., Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat. Rev. Cancer. 2014;14(11):709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ushio-Fukai M. Localizing NADPH oxidase-derived ROS. Sci. STKE. 2006;2006(349) doi: 10.1126/stke.3492006re8. (re8) [DOI] [PubMed] [Google Scholar]
- 177.Ushio-Fukai M. Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc. Res. 2006;71(2):226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 178.Larbi A., Kempf J., Pawelec G. Oxidative stress modulation and T cell activation. Exp. Gerontol. 2007;42(9):852–858. doi: 10.1016/j.exger.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 179.Zuo L., Zhou T., Pannell B.K., Ziegler A.C., Best T.M. Biological and physiological role of reactive oxygen species--the good, the bad and the ugly. Acta Physiol. 2015;214(3):329–348. doi: 10.1111/apha.12515. [DOI] [PubMed] [Google Scholar]
- 180.Manea S.A., Constantin A., Manda G., Sasson S., Manea A. Regulation of Nox enzymes expression in vascular pathophysiology: focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015;5:358–366. doi: 10.1016/j.redox.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee M., You H.J., Cho S.H., Woo C.H., Yoo M.H., Joe E.H., Kim J.H. Implication of the small GTPase Rac1 in the generation of reactive oxygen species in response to beta-amyloid in C6 astroglioma cells. Biochem. J. 2002;366(Pt 3):937–943. doi: 10.1042/BJ20020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yellaturu C.R., Bhanoori M., Neeli I., Rao G.N. N-Ethylmaleimide inhibits platelet-derived growth factor BB-stimulated Akt phosphorylation via activation of protein phosphatase 2A. J. Biol. Chem. 2002;277(42):40148–40155. doi: 10.1074/jbc.M206376200. [DOI] [PubMed] [Google Scholar]
- 183.El-Hag A., Clark R.A. Down-regulation of human natural killer activity against tumors by the neutrophil myeloperoxidase system and hydrogen peroxide. J. Immunol. 1984;133(6):3291–3297. [PubMed] [Google Scholar]
- 184.Yi J.W., Park J.Y., Sung J.Y., Kwak S.H., Yu J., Chang J.H., Kim J.H., Ha S.Y., Paik E.K., Lee W.S., Kim S.J., Lee K.E., Kim J.H. Genomic evidence of reactive oxygen species elevation in papillary thyroid carcinoma with Hashimoto thyroiditis. Endocr. J. 2015;62(10):857–877. doi: 10.1507/endocrj.EJ15-0234. [DOI] [PubMed] [Google Scholar]
- 185.Lee G., Won H.S., Lee Y.M., Choi J.W., Oh T.I., Jang J.H., Choi D.K., Lim B.O., Kim Y.J., Park J.W., Puigserver P., Lim J.H. Oxidative Dimerization of PHD2 is responsible for its inactivation and contributes to Metabolic reprogramming via HIF-1alpha activation. Sci. Rep. 2016;6:18928. doi: 10.1038/srep18928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hart P.C., Mao M., de Abreu A.L., Ansenberger-Fricano K., Ekoue D.N., Ganini D., Kajdacsy-Balla A., Diamond A.M., Minshall R.D., Consolaro M.E., Santos J.H., Bonini M.G. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015;6:6053. doi: 10.1038/ncomms7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Park G.B., Kim D. PI3K catalytic isoform alteration promotes the LIMK1-related metastasis Through the PAK1 or ROCK1/2 activation in cigarette smoke-exposed ovarian cancer cells. Anticancer Res. 2017;37(4):1805–1818. doi: 10.21873/anticanres.11515. [DOI] [PubMed] [Google Scholar]
- 188.Ninsontia C., Phiboonchaiyanan P.P., Chanvorachote P. Zinc induces epithelial to mesenchymal transition in human lung cancer H460 cells via superoxide anion-dependent mechanism. Cancer Cell Int. 2016;16:48. doi: 10.1186/s12935-016-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kudin A.P., Debska-Vielhaber G., Kunz W.S. Characterization of superoxide production sites in isolated rat brain and skeletal muscle mitochondria. Biomed. Pharmacother. 2005;59(4):163–168. doi: 10.1016/j.biopha.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 190.Kudin A.P., Malinska D., Kunz W.S. Sites of generation of reactive oxygen species in homogenates of brain tissue determined with the use of respiratory substrates and inhibitors. Biochim. Biophys. Acta. 2008;1777(7–8):689–695. doi: 10.1016/j.bbabio.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 191.Cadenas E., Boveris A., Ragan C.I., Stoppani A.O. Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 1977;180(2):248–257. doi: 10.1016/0003-9861(77)90035-2. [DOI] [PubMed] [Google Scholar]
- 192.Brewer T.F., Garcia F.J., Onak C.S., Carroll K.S., Chang C.J. Chemical approaches to discovery and study of sources and targets of hydrogen peroxide redox signaling through NADPH oxidase proteins. Annu. Rev. Biochem. 2015;84:765–790. doi: 10.1146/annurev-biochem-060614-034018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Goncalves R.L., Quinlan C.L., Perevoshchikova I.V., Hey-Mogensen M., Brand M.D. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J. Biol. Chem. 2015;290(1):209–227. doi: 10.1074/jbc.M114.619072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J. Biol. Chem. 1997;272(30):18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 195.Crow J.P., Beckman J.S. Reactions between nitric oxide, superoxide, and peroxynitrite: footprints of peroxynitrite in vivo. Adv. Pharmacol. 1995;34:17–43. doi: 10.1016/s1054-3589(08)61079-0. [DOI] [PubMed] [Google Scholar]
- 196.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 197.Takeda M., Shirato I., Kobayashi M., Endou H. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron. 1999;81(2):234–238. doi: 10.1159/000045282. [DOI] [PubMed] [Google Scholar]
- 198.Teramoto S., Tomita T., Matsui H., Ohga E., Matsuse T., Ouchi Y. Hydrogen peroxide-induced apoptosis and necrosis in human lung fibroblasts: protective roles of glutathione. Jpn. J. Pharmacol. 1999;79(1):33–40. doi: 10.1254/jjp.79.33. [DOI] [PubMed] [Google Scholar]
- 199.Ahmad K.A., Iskandar K.B., Hirpara J.L., Clement M.V., Pervaiz S. Hydrogen peroxide-mediated cytosolic acidification is a signal for mitochondrial translocation of Bax during drug-induced apoptosis of tumor cells. Cancer Res. 2004;64(21):7867–7878. doi: 10.1158/0008-5472.CAN-04-0648. [DOI] [PubMed] [Google Scholar]
- 200.Inoshita S., Takeda K., Hatai T., Terada Y., Sano M., Hata J., Umezawa A., Ichijo H. Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J. Biol. Chem. 2002;277(46):43730–43734. doi: 10.1074/jbc.M207951200. [DOI] [PubMed] [Google Scholar]
- 201.Schroeter H., Boyd C.S., Ahmed R., Spencer J.P., Duncan R.F., Rice-Evans C., Cadenas E. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem. J. 2003;372(Pt 2):359–369. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Naughton R., Quiney C., Turner S.D., Cotter T.G. Bcr-Abl-mediated redox regulation of the PI3K/AKT pathway. Leukemia. 2009;23(8):1432–1440. doi: 10.1038/leu.2009.49. [DOI] [PubMed] [Google Scholar]
- 203.Leslie N.R., Bennett D., Lindsay Y.E., Stewart H., Gray A., Downes C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003;22(20):5501–5510. doi: 10.1093/emboj/cdg513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pervaiz S., Clement M.V. Superoxide anion: oncogenic reactive oxygen species? Int. J. Biochem. Cell Biol. 2007;39(7–8):1297–1304. doi: 10.1016/j.biocel.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 205.Hornsveld M., Dansen T.B. The Hallmarks of cancer from a Redox perspective. Antioxid. Redox Signal. 2016;25(6):300–325. doi: 10.1089/ars.2015.6580. [DOI] [PubMed] [Google Scholar]
- 206.Xanthoudakis S., Miao G., Wang F., Pan Y.C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Clement M.V., Pervaiz S. Intracellular superoxide and hydrogen peroxide concentrations: a critical balance that determines survival or death. Redox Rep. 2001;6(4):211–214. doi: 10.1179/135100001101536346. [DOI] [PubMed] [Google Scholar]
- 208.Ahmad K.A., Clement M.V., Pervaiz S. Pro-oxidant activity of low doses of resveratrol inhibits hydrogen peroxide-induced apoptosis. Ann. N. Y. Acad. Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 209.Clement M.V., Sivarajah S., Pervaiz S. Production of intracellular superoxide mediates dithiothreitol-dependent inhibition of apoptotic cell death. Antioxid. Redox Signal. 2005;7(3–4):456–464. doi: 10.1089/ars.2005.7.456. [DOI] [PubMed] [Google Scholar]
- 210.Clement M.V., Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J. 1996;15(2):216–225. [PMC free article] [PubMed] [Google Scholar]
- 211.Pervaiz S., Ramalingam J.K., Hirpara J.L., Clement M.V. Superoxide anion inhibits drug-induced tumor cell death. FEBS Lett. 1999;459(3):343–348. doi: 10.1016/s0014-5793(99)01258-2. [DOI] [PubMed] [Google Scholar]
- 212.D'Autreaux B., Toledano M.B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 213.Gorrini C., Baniasadi P.S., Harris I.S., Silvester J., Inoue S., Snow B., Joshi P.A., Wakeham A., Molyneux S.D., Martin B., Bouwman P., Cescon D.W., Elia A.J., Winterton-Perks Z., Cruickshank J., Brenner D., Tseng A., Musgrave M., Berman H.K., Khokha R., Jonkers J., Mak T.W., Gauthier M.L. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 2013;210(8):1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Salmeen A., Andersen J.N., Myers M.P., Meng T.C., Hinks J.A., Tonks N.K., Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423(6941):769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 215.Lloyd R.V., Hanna P.M., Mason R.P. The origin of the Hydroxyl Radical oxygen in the Fenton reaction. Free Radic. Biol. Med. 1997;22(5):885–888. doi: 10.1016/s0891-5849(96)00432-7. [DOI] [PubMed] [Google Scholar]
- 216.Halliwell B., Gutteridge J.M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Koppenol W.H., Moreno J.J., Pryor W.A., Ischiropoulos H., Beckman J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992;5(6):834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 218.Bartlett D., Church D.F., Bounds P.L., Koppenol W.H. The kinetics of the oxidation of L-ascorbic acid by peroxynitrite. Free Radic. Biol. Med. 1995;18(1):85–92. doi: 10.1016/0891-5849(94)e0133-4. [DOI] [PubMed] [Google Scholar]
- 219.Hogg N., Joseph J., Kalyanaraman B. The oxidation of alpha-tocopherol and trolox by peroxynitrite. Arch. Biochem. Biophys. 1994;314(1):153–158. doi: 10.1006/abbi.1994.1423. [DOI] [PubMed] [Google Scholar]
- 220.Cuzzocrea S., Caputi A.P., Zingarelli B., Peroxynitrite-mediated DNA strand breakage activates poly (ADP-ribose) synthetase and causes cellular energy depletion in carrageenan-induced pleurisy. Immunology. 1998;93(1):96–101. doi: 10.1046/j.1365-2567.1998.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 221.Epe B., Ballmaier D., Roussyn I., Briviba K., Sies H. DNA damage by peroxynitrite characterized with DNA repair enzymes. Nucleic Acids Res. 1996;24(21):4105–4110. doi: 10.1093/nar/24.21.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ferrante R.J., Shinobu L.A., Schulz J.B., Matthews R.T., Thomas C.E., Kowall N.W., Gurney M.E., Beal M.F. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann. Neurol. 1997;42(3):326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 223.Smith M.A., Richey Harris P.L., Sayre L.M., Beckman J.S., Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 1997;17(8):2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kooy N.W., Royall J.A., Ye Y.Z., Kelly D.R., Beckman J.S. Evidence for in vivo peroxynitrite production in human acute lung injury. Am. J. Respir. Crit. Care Med. 1995;151(4):1250–1254. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 225.Pinzar E., Wang T., Garrido M.R., Xu W., Levy P., Bottari S.P. Angiotensin II induces tyrosine nitration and activation of ERK1/2 in vascular smooth muscle cells. FEBS Lett. 2005;579(22):5100–5104. doi: 10.1016/j.febslet.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 226.Rahman M.A., Senga T., Ito S., Hyodo T., Hasegawa H., Hamaguchi M. S-Nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J. Biol. Chem. 2010;285(6):3806–3814. doi: 10.1074/jbc.M109.059782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pei D.-S., Sun Y.-F., Song Y.-J. S-nitrosylation of PTEN invovled in ischemic brain injury in rat hippocampal CA1 region. Neurochem. Res. 2009;34(8):1507. doi: 10.1007/s11064-009-9938-3. [DOI] [PubMed] [Google Scholar]
- 228.Shinozaki S., Choi C.S., Shimizu N., Yamada M., Kim M., Zhang T., Dong H.H., Kim Y.-B., Kaneki M. Liver-specific inducible nitric-oxide synthase expression is sufficient to cause hepatic insulin resistance and mild hyperglycemia in mice. J. Biol. Chem. 2011;286(40):34959–34975. doi: 10.1074/jbc.M110.187666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Csibi A., Communi D., Muller N., Bottari S.P. Angiotensin II inhibits insulin-stimulated GLUT4 translocation and Akt activation through tyrosine nitration-dependent mechanisms. PLoS One. 2010;5(4):e10070. doi: 10.1371/journal.pone.0010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Tagde A., Singh H., Kang M.H., Reynolds C.P. The glutathione synthesis inhibitor buthionine sulfoximine synergistically enhanced melphalan activity against preclinical models of multiple myeloma, blood. Cancer J. 2014;4:e229. doi: 10.1038/bcj.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P.J., Achanta G., Arlinghaus R.B., Liu J., Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by β-phenylethyl isothiocyanate. Cancer Cell. 2006;10(3):241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 232.Lo M., Ling V., Low C., Wang Y.Z., Gout P.W. Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Curr. Oncol. 2010;17(3):9–16. doi: 10.3747/co.v17i3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.M.-z. Ma, G. Chen, P. Wang, W.-h. Lu, C.-f. Zhu, M. Song, J. Yang, S. Wen, R.-h. Xu, Y. Hu, P. Huang, Xc-inhibitor sulfasalazine sensitizes colorectal cancer to cisplatin by a GSH-dependent mechanism, Cancer Lett., 368(1), pp. 88–96. [DOI] [PubMed]
- 234.Gumireddy K., Li A., Cao L., Yan J., Liu L., Xu X., Pazoles C., Huang Q., NOV-002, Glutathione Disulfide A. Mimetic, suppresses tumor cell invasion and metastasis. J. Carcinog. Mutagen. 2013;2013:S7–002. doi: 10.4172/2157-2518.S7-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Marzano C., Gandin V., Folda A., Scutari G., Bindoli A., Rigobello M.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in cisplatin-resistant human ovarian cancer cells. Free Radic. Biol. Med. 2007;42(6):872–881. doi: 10.1016/j.freeradbiomed.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 236.Liu J.J., Liu Q., Wei H.L., Yi J., Zhao H.S., Gao L.P. Inhibition of thioredoxin reductase by auranofin induces apoptosis in adriamycin-resistant human K562 chronic myeloid leukemia cells. Pharmazie. 2011;66(6):440–444. [PubMed] [Google Scholar]
- 237.Li G.-Z., Liang H.-F., Liao B., Zhang L., Ni Y.-A., Zhou H.-H., Zhang E.-L., Zhang B.-X., Chen X.-P. PX-12 inhibits the growth of hepatocelluar carcinoma by inducing S-phase arrest, ROS-dependent apoptosis and enhances 5-FU cytotoxicity. Am. J. Transl. Res. 2015;7(9):1528–1540. [PMC free article] [PubMed] [Google Scholar]
- 238.Glasauer A., Sena L.A., Diebold L.P., Mazar A.P., Chandel N.S. Targeting SOD1 reduces experimental non–small-cell lung cancer. J. Clin. Investig. 2014;124(1):117–128. doi: 10.1172/JCI71714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Dachert J., Schoeneberger H., Rohde K., Fulda S. RSL3 and Erastin differentially regulate redox signaling to promote Smac mimetic-induced cell death. Oncotarget. 2016;7(39):63779–63792. doi: 10.18632/oncotarget.11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Shaw A.T., Winslow M.M., Magendantz M., Ouyang C., Dowdle J., Subramanian A., Lewis T.A., Maglathin R.L., Tolliday N., Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc. Natl. Acad. Sci. USA. 2011;108(21):8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Azad N., Iyer A.K.V., Manosroi A., Wang L., Rojanasakul Y. Superoxide-mediated proteasomal degradation of Bcl-2 determines cell susceptibility to Cr(VI)-induced apoptosis. Carcinogenesis. 2008;29(8):1538–1545. doi: 10.1093/carcin/bgn137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Low I.C., Loh T., Huang Y., Virshup D.M., Pervaiz S. Ser70 phosphorylation of Bcl-2 by selective tyrosine nitration of PP2A-B56delta stabilizes its antiapoptotic activity. Blood. 2014;124(14):2223–2234. doi: 10.1182/blood-2014-03-563296. [DOI] [PubMed] [Google Scholar]
- 243.Doublier S., Belisario D.C., Polimeni M., Annaratone L., Riganti C., Allia E., Ghigo D., Bosia A., Sapino A. HIF-1 activation induces doxorubicin resistance in MCF7 3-D spheroids via P-glycoprotein expression: a potential model of the chemo-resistance of invasive micropapillary carcinoma of the breast. BMC Cancer. 2012;12(1):4. doi: 10.1186/1471-2407-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thews O., Nowak M., Sauvant C., Gekle M. Hypoxia-induced extracellular acidosis increases p-glycoprotein activity and chemoresistance in tumors in vivo via p38 signaling pathway. In: LaManna J.C., Puchowicz M.A., Xu K., Harrison D.K., Bruley D.F., editors. Oxygen Transport to Tissue XXXII. Springer; US, Boston, MA: 2011. pp. 115–122. [DOI] [PubMed] [Google Scholar]
- 245.Selvendiran K., Bratasz A., Kuppusamy M.L., Tazi M.F., Rivera B.K., Kuppusamy P. Hypoxia induces chemoresistance in ovarian cancer cells by activation of signal transducer and activator of transcription 3, International journal of cancer. J. Int. du Cancer. 2009;125(9):2198–2204. doi: 10.1002/ijc.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rohwer N., Dame C., Haugstetter A., Wiedenmann B., Detjen K., Schmitt C.A., Cramer T. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One. 2010;5(8):e12038. doi: 10.1371/journal.pone.0012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Switzer C.H., Glynn S.A., Cheng R.Y., Ridnour L.A., Green J.E., Ambs S., Wink D.A. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol. Cancer Res. 2012;10(9):1203–1215. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Muerkoster S.S., Lust J., Arlt A., Hasler R., Witt M., Sebens T., Schreiber S., Folsch U.R., Schafer H. Acquired chemoresistance in pancreatic carcinoma cells: induced secretion of IL-1[beta] and NO lead to inactivation of caspases. Oncogene. 2006;25(28):3973–3981. doi: 10.1038/sj.onc.1209423. [DOI] [PubMed] [Google Scholar]
- 249.Jin Z., Wang W., Jiang N., Zhang L., Li Y., Xu X., Cai S., Wei L., Liu X., Chen G., Zhou Y., Liu C., Li Z., Jin F., Chen B. Clinical implications of iNOS levels in triple-negative breast cancer responding to Neoadjuvant chemotherapy. PLoS One. 2015;10(7):e0130286. doi: 10.1371/journal.pone.0130286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Han X., Xu B., Beevers C.S., Odaka Y., Chen L., Liu L., Luo Y., Zhou H., Chen W., Shen T., Huang S. Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Carcinogenesis. 2012;33(4):868–875. doi: 10.1093/carcin/bgs029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen L., Liu L., Yin J., Luo Y., Huang S. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int. J. Biochem. Cell Biol. 2009;41(6):1284–1295. doi: 10.1016/j.biocel.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 252.DeGrande S.T., Little S.C., Nixon D.J., Wright P., Snyder J., Dun W., Murphy N., Kilic A., Higgins R., Binkley P.F., Boyden P.A., Carnes C.A., Anderson M.E., Hund T.J., Mohler P.J. Molecular mechanisms underlying cardiac protein phosphatase 2A regulation in heart. J. Biol. Chem. 2013;288(2):1032–1046. doi: 10.1074/jbc.M112.426957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rao R.K., Clayton L.W. Regulation of protein phosphatase 2A by hydrogen peroxide and glutathionylation. Biochem. Biophys. Res. Commun. 2002;293(1):610–616. doi: 10.1016/S0006-291X(02)00268-1. [DOI] [PubMed] [Google Scholar]
- 254.Cicchillitti L., Fasanaro P., Biglioli P., Capogrossi M.C., Martelli F. Oxidative stress induces protein phosphatase 2A-dependent dephosphorylation of the pocket proteins pRb, p107, and p130. J. Biol. Chem. 2003;278(21):19509–19517. doi: 10.1074/jbc.M300511200. [DOI] [PubMed] [Google Scholar]
- 255.Park M.A., Mitchell C., Zhang G., Yacoub A., Allegood J., Häussinger D., Reinehr R., Larner A., Spiegel S., Fisher P.B., Voelkel-Johnson C., Ogretmen B., Grant S., Dent P. Vorinostat and sorafenib increase CD95 activation in gastrointestinal tumor cells through a Ca2+ - de novo ceramide - PP2A - ROS dependent signaling pathway. Cancer Res. 2010;70(15):6313–6324. doi: 10.1158/0008-5472.CAN-10-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Foley T.D., Petro L.A., Stredny C.M., Coppa T.M. Oxidative inhibition of protein phosphatase 2A activity: role of catalytic subunit disulfides. Neurochem. Res. 2007;32(11):1957–1964. doi: 10.1007/s11064-007-9394-x. [DOI] [PubMed] [Google Scholar]
- 257.Sommer D., Coleman S., Swanson S.A., Stemmer P.M. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch. Biochem. Biophys. 2002;404(2):271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 258.Yakovlev V.A. Nitric oxide–dependent downregulation of BRCA1 expression promotes genetic instability. Cancer Res. 2013;73(2):706–715. doi: 10.1158/0008-5472.CAN-12-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sripathi S.R., He W., Um J.-Y., Moser T., Dehnbostel S., Kindt K., Goldman J., Frost M.C., Jahng W.J. Nitric oxide leads to cytoskeletal reorganization in the retinal pigment epithelium under oxidative stress. Adv. Biosci. Biotechnol. 2012;03(08):12. doi: 10.4236/abb.2012.38143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.An X.Z., Zhao Z.G., Luo Y.X., Zhang R., Tang X.Q., Hao D., Zhao X., Lv X., Liu D. Netrin-1 suppresses the MEK/ERK pathway and ITGB4 in pancreatic cancer. Oncotarget. 2016;7(17):24719–24733. doi: 10.18632/oncotarget.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Heikkinen P.T., Nummela M., Leivonen S.-K., Westermarck J., Hill C.S., Kähäri V.-M., Jaakkola P.M. Hypoxia-activated Smad3-specific Dephosphorylation by PP2A. J. Biol. Chem. 2010;285(6):3740–3749. doi: 10.1074/jbc.M109.042978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Di Conza G., Trusso Cafarello S., Loroch S., Mennerich D., Deschoemaeker S., Di Matteo M., Ehling M., Gevaert K., Prenen H., Zahedi R.P., Sickmann A., Kietzmann T., Moretti F., Mazzone M. The mTOR and PP2A pathways regulate PHD2 phosphorylation to fine-tune HIF1α levels and colorectal cancer cell survival under Hypoxia. Cell Rep. 2017;18(7):1699–1712. doi: 10.1016/j.celrep.2017.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Raghuraman G., Rai V., Peng Y.J., Prabhakar N.R., Kumar G.K. Pattern-specific sustained activation of tyrosine hydroxylase by intermittent hypoxia: role of reactive oxygen species-dependent downregulation of protein phosphatase 2A and upregulation of protein kinases. Antioxid. Redox Signal. 2009;11(8):1777–1789. doi: 10.1089/ars.2008.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Li X., Scuderi A., Letsou A., Virshup D.M. B56-Associated protein phosphatase 2A Is required For survival and protects from apoptosis in Drosophila melanogaster. Mol. Cell. Biol. 2002;22(11):3674. doi: 10.1128/MCB.22.11.3674-3684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Silverstein A.M., Barrow C.A., Davis A.J., Mumby M.C. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc. Natl. Acad. Sci. USA. 2002;99(7):4221–4226. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yu S., Li L., Wu Q., Dou N., Li Y., Gao Y. PPP2R2D, a regulatory subunit of protein phosphatase 2A, promotes gastric cancer growth and metastasis via mechanistic target of rapamycin activation. Int. J. Oncol. 2018;52(6):2011–2020. doi: 10.3892/ijo.2018.4329. [DOI] [PubMed] [Google Scholar]
- 267.Duong F.H.T., Dill M.T., Matter M.S., Makowska Z., Calabrese D., Dietsche T., Ketterer S., Terracciano L., Heim M.H. Protein phosphatase 2A promotes hepatocellular carcinogenesis in the diethylnitrosamine mouse model through inhibition ofp53. Carcinogenesis. 2014;35(1):114–122. doi: 10.1093/carcin/bgt258. [DOI] [PubMed] [Google Scholar]
- 268.Li W., Xie L., Chen Z., Zhu Y., Sun Y., Miao Y., Xu Z., Han X. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Sci. 2010;101(5):1226–1233. doi: 10.1111/j.1349-7006.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Lu J., Kovach J.S., Johnson F., Chiang J., Hodes R., Lonser R., Zhuang Z. Inhibition of serine/threonine phosphatase PP2A enhances cancer chemotherapy by blocking DNA damage induced defense mechanisms. Proc. Natl. Acad. Sci. USA. 2009;106(28):11697–11702. doi: 10.1073/pnas.0905930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Conlon N., McDermott M., Browne B., Roche S., O'Neill F., Meiller J., Eustace A.J., Collins D., O'Donovan N., Crown J. Pre-clinical evaluation of the PP2A inhibitor LB-100 for the treatment of lapatinib resistant HER2-positive breast cancer. J. Clin. Oncol. 2018;36(15_suppl) (e13021-e13021) [Google Scholar]
- 271.Chung V., Mansfield A.S., Braiteh F., Richards D., Durivage H., Ungerleider R.S., Johnson F., Kovach J.S. Safety, tolerability, and preliminary activity of LB-100, an inhibitor of protein phosphatase 2A, in patients with relapsed solid tumors: an open-label, dose escalation, first-in-human, phase I trial. Clin. Cancer Res. 2017;23(13):3277. doi: 10.1158/1078-0432.CCR-16-2299. [DOI] [PubMed] [Google Scholar]
- 272.Kawada M., Amemiya M., Ishizuka M., Takeuchi T. Cytostatin, an inhibitor of cell adhesion to extracellular matrix, selectively inhibits protein phosphatase 2A. Biochim. Biophys. Acta (BBA) - Mol. Cell Res. 1999;1452(2):209–217. doi: 10.1016/s0167-4889(99)00126-3. [DOI] [PubMed] [Google Scholar]
- 273.Kawada M., Kawatsu M., Masuda T., Ohba S.-I., Amemiya M., Kohama T., Ishizuka M., Takeuchi T. Specific inhibitors of protein phosphatase 2A inhibit tumor metastasis through augmentation of natural killer cells. Int. Immunopharmacol. 2003;3(2):179–188. doi: 10.1016/s1567-5769(02)00231-x. [DOI] [PubMed] [Google Scholar]
- 274.Gordon I.K., Lu J., Graves C.A., Huntoon K., Frerich J.M., Hanson R.H., Wang X., Hong C.S., Ho W., Feldman M.J., Ikejiri B., Bisht K., Chen X.S., Tandle A., Yang C., Arscott W.T., Ye D., Heiss J.D., Lonser R.R., Camphausen K., Zhuang Z. Protein phosphatase 2A inhibition with LB100 enhances radiation-induced mitotic catastrophe and tumor growth delay in glioblastoma. Mol. Cancer Ther. 2015;14(7):1540–1547. doi: 10.1158/1535-7163.MCT-14-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Wang H., Chen X., Eksioglu E.A., Zhou J., Fortenbery N.R., Djeu J., List A., Wei S. Lenalidomide and arsenic trioxide have independent non-interfering effects when used in combination on myeloma cell lines in vitro. J. Cancer Ther. 2013;40(03):10. [Google Scholar]
- 276.Sborov D.W., Benson D.M., Williams N., Huang Y., Bowers M.A., Humphries K., Efebera Y., Devine S., Hofmeister C.C. Lenalidomide and vorinostat maintenance after autologous transplant in multiple myeloma. Br. J. Haematol. 2015;171(1):74–83. doi: 10.1111/bjh.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cho U.S., Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445(7123):53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]