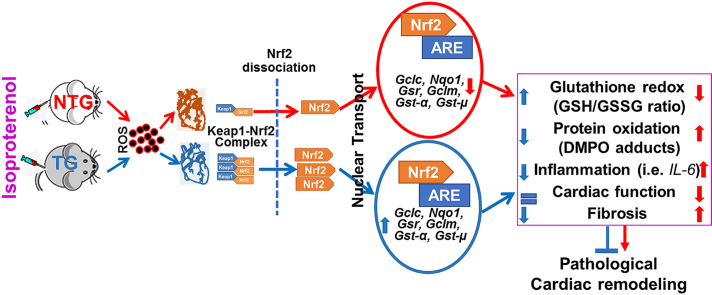
Abstract
Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2/Nrf2) is an inducible transcription factor that is essential for maintenance of redox signaling in response to stress. This suggests that if Nrf2 expression response could be enhanced for a defined physiological pro-oxidant stress then it would be protective. This has important implications for the therapeutic manipulation of the Keap1/Nrf2 signaling pathway which is now gaining a lot of attention. We tested this hypothesis through the generation of Nrf2 transgene expression mouse model with and without isoproterenol-induced cardiac stress. Cardiac-specific mouse Nrf2 transgenic (mNrf2-TG) and non-transgenic (NTG) mice were subjected to isoproterenol (ISO) treatment and assessed for myocardial structure, function (echocardiography and electrocardiography), and glutathione redox state. Myocardial infarction and fibrosis along with increased inflammation leading to myocardial dysfunction was noted in NTG mice exposed to ISO, while mNrf2-TG hearts were resistant to the ISO insult. Preservation of myocardial structure and function in the mNrf2-TG mice was associated with the enhanced Nrf2 expression displayed in these hearts with an increased basal and post-treatment expression of redox modulatory genes and an overall enhanced antioxidant status. Of note, myocardium of ISO-treated TG mice displayed significantly increased stabilization of the KEAP1-NRF2 complex and enhanced release of NRF2 to the nucleus resulting in overall decreased pro-oxidant markers. Taken together, we suggest that a basal enhanced Nrf2 expression in mouse heart results in maintenance of redox homeostasis and counteracts ISO-induced oxidative stress, and suppresses pathological remodeling. These data suggest that an alternative therapeutic approach to enhance the efficacy of the Keap1-Nrf2 system is to stimulate basal expression of Nrf2.
Keywords: Nrf2, Isoproterenol, Antioxidants, Cardiac remodeling, Echocardiography
Graphical abstract
Highlights
-
•
Isoproterenol induces oxidative/inflammatory stresses and leading to myocardial remodeling.
-
•
Cardiac specific expression of Nrf2 augments Keap1-Nrf2 association, thereby timely responds to isoproterenol-induced stress.
-
•
Augmented levels of Keap1-Nrf2 signaling is crucial to combat isoproterenol toxicity in the heart.
-
•
Enhanced Nrf2-dependent antioxidant defense suppresses oxidative stress and prevents pathological cardiac remodeling.
-
•
A controlled activation of global antioxidant signaling is vital for myocardial protection in stress conditions.
1. Introduction
Myocardial infarction (MI) and pathological cardiac hypertrophy are among the common leading causes of cardiovascular deaths across the globe [[1], [2], [3], [4]]. In response to MI or hypertrophy, cardiomyocytes are irreversibly damaged eventually leading to the development of heart failure (HF) [2,3,[5], [6], [7]]. Post-mitotic cardiomyocytes have limited ability to regenerate, therefore the repair of damaged hearts is a challenging task. On the other hand, cytoprotective signaling in post MI or hypertrophic hearts is closely regulated by the intracellular redox milieu [[8], [9], [10], [11], [12]]. MI and reperfusion injury have been shown to be associated with impaired redox signaling by shifting the redox milieu towards a more oxidative state [[13], [14], [15]]. However, mitigation of oxidative stress using exogenous antioxidant supplements seems to be ineffective in the MI hearts [12,[16], [17], [18], [19]].
Keap1-Nrf2 is a redox-sensitive signaling pathway that facilitates transcriptional regulation of a family of redox modulatory and cytoprotective genes that facilitate the defense against oxidative stress [[20], [21], [22]]. Therefore, it is important to investigate whether an imbalance in Keap1 vs. Nrf2 levels in the myocardium is contributing to MI-dependent and/or pathological hypertrophy. Triggering Nrf2 antioxidant signaling provides defense against ischemia-reperfusion injury [23,24] and pressure-overload hypertrophy in the mouse myocardium [25,26]. In our previous studies, we showed that mice with an Nrf2 gene deletion are susceptible to high-intensity exercise stress-induced cardiac hypertrophy on aging [27]. However, the potential prophylactic role of Nrf2 signaling in preventing MI and pathological cardiac remodeling remains of interest. To address this important question, we have taken a genetic approach to enhance the master transcriptional regulator of antioxidants, Nrf2 in the heart by transgene expression. Importantly, this construct is inducible and lacks the Nrf2 promotor of the endogenous gene allowing its levels to be regulated with less-dependence on the basal redox signaling in the unchallenged heart.
Cardiac specific mouse Nrf2 transgenic (mNrf2-TG) and non-transgenic (NTG) litter mates in the C57/BL6 background were subjected to isoproterenol (ISO) treatment (50 mg/kg bw/day, for 7 days), and were assessed for the myocardial structure, function (Echocardiography), electrophysiology and Nrf2-dependent defense mechanisms. We found that the NTG mice exposed to ISO exhibited severe pathological remodeling with increased inflammation leading to myocardial dysfunction, but mNrf2-TG hearts were protected from ISO insult.
2. Materials and methods
2.1. Mouse Nrf2 (mNrf2) plasmid designing and generation of heart-specific transgenic mice
A recombinant plasmid construct containing a mouse Nrf2 (mNrf2) cDNA was inserted downstream of a mouse alpha-myosin heavy chain (α-MHC) promoter to facilitate cardiomyocyte specific expression. Restriction digestion and subsequent sequencing analysis confirmed orientation and integrity of the inserts. The plasmid backbone was then removed using SalI and the α-MHC-mNrf2 fragment was gel purified. Transgenic mice were generated by pronuclear injection; founders were back-crossed with C57/BL6 background for at least 6 generations. Transgenic animals were genotyped and confirmed with the presence of a ∼350bp PCR product. Based on the expression of mNrf2 transgene normalized to endogenous Nrf2, determined by quantitative RT-PCR using genotyping primers for TG (mNrf2) and endogenous Nrf2 specific primers (see Table 1), transgenic founders #M1517 and #M9876 were confirmed and M1517 line was further expanded for production of transgenic mice. Mice were housed under a controlled temperature and humidity, a 12 h light/dark cycle, and fed with a standard rodent diet and water ad libitum. The Institutional Animal Care and Use Committee (IACUC) at the University of Alabama at Birmingham and the University of Utah, Salt Lake City, Utah approved all animal experiments, in accordance with the standards established by the US Animal Welfare Act.
Table-1.
List of Real-Time qPCR Primer Sequences.
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Tg(mNrf2) | ACTTTACATGGAGTCCTGGTGGGA | TCTCCTGTTCCTTCTGGAGTTGCT |
| Nrf2 | CTGAACTCCTGGACGGGACTA | CGGTGGGTCTCCGTAAATGG |
| Gclc | GGACAAACCCCAACCATCC | GTTGAACTCAGACATCGTTCCT |
| Nqo1 | AGGATGGGAGGTACTCGAATC | TGCTAGAGATGACTCGGAAGG |
| Gsr | CACGGCTATGCAACATTCGC | GTGTGGAGCGGTAAACTTTTTC |
| Gclm | CTTCGCCTCCGATTGAAGATG | AAAGGCAGTCAAATCTGGTGG |
| Il6 | TCTATACCACTTCACAAGTCGGA | GAATTGCCATTGCACAACTCTTT |
| Keap1 | TGCCCCTGTGGTCAAAGTG | GGTTCGGTTACCGTCCTGC |
| Gapdh | TGACCTCAACTACATGGTCTACA | CTTCCCATTCTCGGCCTTG |
| Arbp1 | TGAGATTCGGGATATGCTGTTGG | CGGGTCCTAGACCAGTGTTCT |
2.2. Isoproterenol (ISO) treatment and autopsy
NTG and mNrf2-TG mice at the age of ∼6 months were treated with subcutaneous injection of ISO (50 mg/kg bw) dissolved in PBS once daily for seven consecutive days [[28], [29], [30]]. Another group of NTG mice received PBS and served as the experimental control. Echocardiography was performed before starting the ISO treatment and on the day of sacrifice (after 7 days of ISO treatment; after 24 hrs of last injection), to measure ISO-induced changes in cardiac structure and function. Mice were anesthetized using isoflurane and euthanized by cervical dislocation. Hearts were immediately excised, perfused with ice-cold phosphate buffered saline and appropriately stored for RNA, protein, biochemical and histological analyses. Tissues stored in RNAlater were used for RNA isolation and for proteins; tissues were immediately flash frozen in liquid nitrogen. A portion of the heart tissue (∼20 mg) was immediately processed for glutathione redox (GSH and GSSG) assay. For histological measurements, middle region of myocardial tissues were embedded in paraffin and sections (10 μm) were stained with hematoxylin and eosin to determine the cardiac damage and picrosirius stain (PSR) for assessing collagen accumulation.
Supplemental information: Detailed methods for protein isolation, immunoblotting, detection of ROS by DMPO radical-protein adducts, immunofluorescent analysis, RNA isolation and real-time qPCR analysis, Nrf2-ARE binding activity are described in the supplementary information section.
3. Results
3.1. Generation and characterization of cardiomyocyte specific mNrf2 transgenic mice
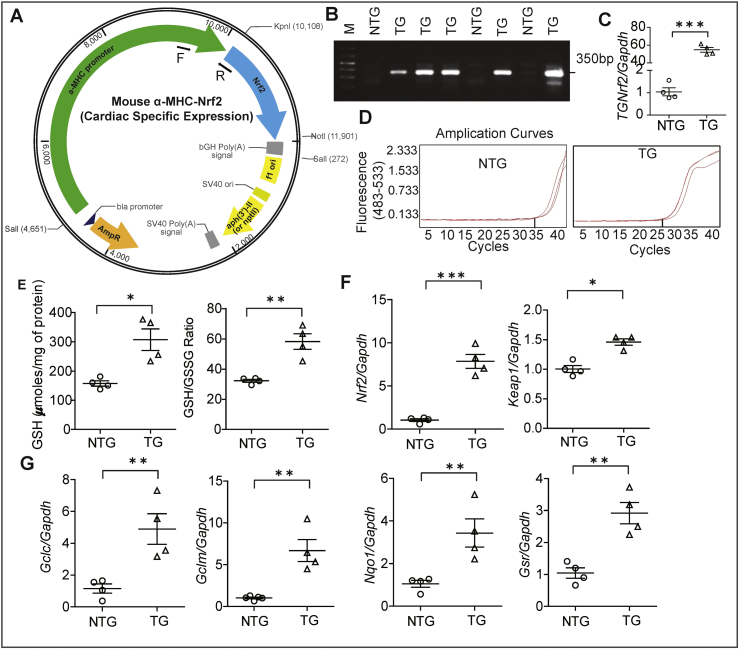
To generate cardiac specific transgenic mouse, full-length mouse Nrf2 cDNA was inserted downstream of mouse alpha myosin heavy chain (α-MHC) promoter (Fig. 1A). Germline transmission of the transgene was confirmed by genotyping using PCR (Fig. 1B); transgene expression was also quantified by qPCR using the primers that recognize the transgene only (Fig. 1C–D). The TG hearts showed significantly elevated levels of GSH (∼1.6 Fold) and its redox ratio (∼2.0 fold) when compared to NTG littermates (Fig. 1E). To determine the endogenous levels of Nrf2, we performed a qPCR using a primer that recognizes both wild-type and transgene versions of Nrf2. As shown, when compared to the endogenous Nrf2 levels in NTG hearts, the TG mice had ~6.0-fold higher levels of Nrf2 (Fig. 1F). Interestingly, the Keap1 mRNA expression also increased 1.5-fold in TG when compared to NTG mice consistent with an enhanced activity of the Keap1/Nrf2 signaling pathway (Fig. 1F). Furthermore, the key targets for Nrf2 genes which involve glutathione metabolism i.e Gclc, Gclm, Nqo1 and Gsr were also significantly upregulated in TG mice myocardium (Fig. 1G).
Fig. 1.
Generation of a cardiac specific mNrf2-TG mouse line. (A) Cloning strategy for generating the cardiac specific mNrf2-TG mouse line. (B) Genotyping by PCR shows presence of mNrf2 transgene in the progeny of founder animals. (C) Quantitative PCR validation of the mNrf2 transgene expression vs Gapdh and (D) qRT-PCR amplification curves of mNrf2 transgene in NTG and TG mice hearts samples. (E) Myocardial reduced glutathione, and GSH/GSSG redox (GSH/GSSG ratio) levels, (F) Endogenous mNrf2 and Keap1 gene expression in the NTG and TG mouse heart, (n = 4/group). (G) Antioxidant gene transcript levels (Key targets of Nrf2 and genes involved in GSH metabolism) by real time qPCR (n = 4/group); relative gene expression was analyzed by normalizing with levels of Gapdh/Arbp1 in NTG mice. Values are presented as mean ± SEM. Significance: *, p<0.05; **, p<0.01; ***, p < 0.001.
3.2. Augmentation of Nrf2 signaling prevents ISO-induced myocardial infarction and inflammation
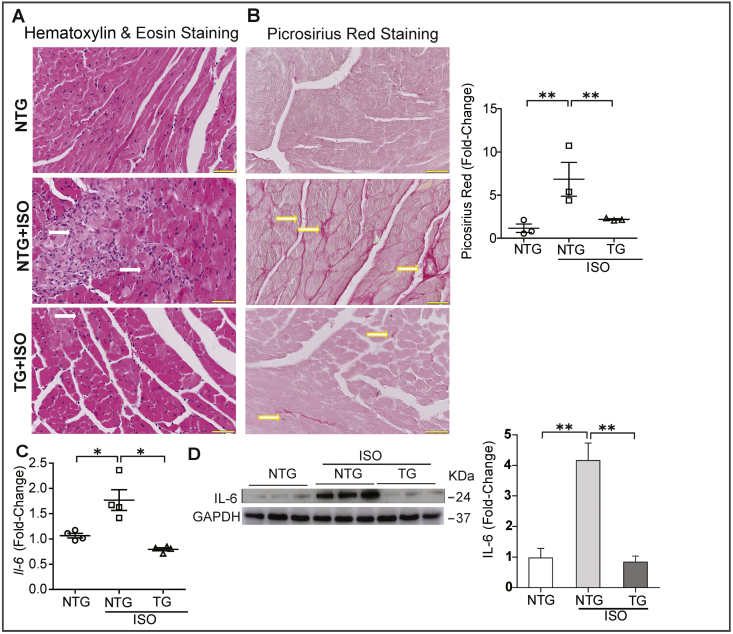
Histological analyses of heart tissues from NTG mice treated with ISO revealed myocardial damage evidenced by widespread myocardial necrosis and leukocyte infiltration (Fig. 2A) when compared to NTG control group. However, TG mice appeared to be normal or displayed a minimal necrosis/infiltration compared to the NTG-PBS group (Fig. 2A). While NTG mice treated with ISO showed increased collagen deposition (PSR staining) in the myocardium, the TG mice showed a markedly reduced amount of fibrotic tissue (Fig. 2B). This observation suggests that Nrf2 transgene expression protects the myocardium from ISO induced remodeling. Furthermore, the expression of cardiac inflammatory cytokine was examined by qPCR and western blotting. Treatment with ISO in NTG littermates for 7 days significantly elevated Il-6 (1.8 fold) gene expression compared to controls, and IL-6 protein levels (Fig. 2C–D), whereas the enhanced availability of Nrf2 suppressed the inflammatory responses in mNrf2-TG mice receiving ISO.
Fig. 2.
Activation of Nrf2 signaling protects the myocardium from ISO-induced injury and inflammation. (A) Representative images of Hematoxylin & Eosin staining for hearts from NTG, as well as NTG and TG mice after 7 days of ISO treatment. Prominent inflammation and tissue damage (indicated by white arrows) in ISO-treated NTG mice hearts were suppressed in TG mice received the same dose of ISO. (B) Representative images from Picrosirius Red (PSR) staining of heart sections obtained from the three experimental groups. Upregulated collagen deposition (indicated by yellow arrows) was observed in the ISO-treated NTG mouse hearts (n = 3/group). (C) Quantitative real time RT-PCR analysis of Il-6 gene level (indicator for inflammation, n = 4/group). (D) Immunoblot images for cardiac IL-6 protein expression in NTG, as well as NTG and TG mice after 7 days of ISO treatment (n = 3/group). Values are presented as mean ± SEM. Significance: *, p < 0.05; **, p < 0.01.
3.3. Activation of Nrf2 signaling retains the electrocardiographic signatures in ISO induced mice
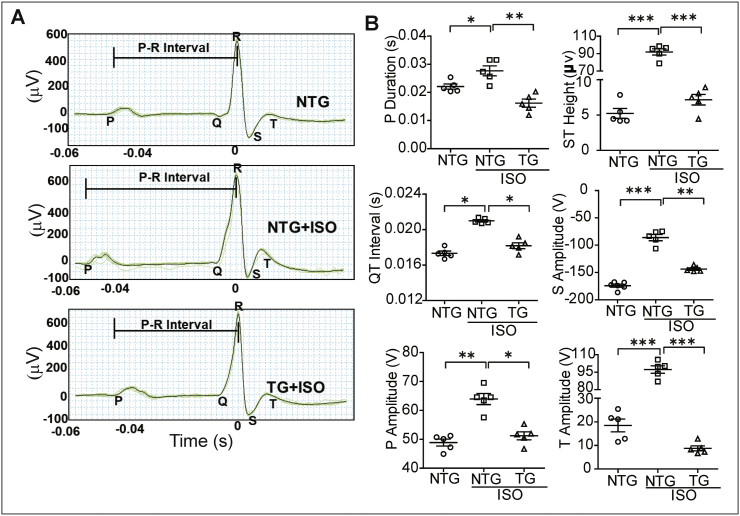
Next, we determined whether the electrocardiogram (ECG) signatures were altered due to ISO in TG mice. ISO administration significantly impaired the P wave duration, QT interval, ST elevation, P, S and T amplitudes in NTG mice (Fig. 3A–B), suggesting a collective defect in electrophysiological signals that depicts an infarcted myocardial pattern. Notably, the TG mice that received ISO exhibited a relatively normal ECG signature as compared to PBS-receiving NTG mice, indicating that enhanced Nrf2 could preserve the electrophysiological signals in response to ISO insult (Fig. 3A–B).
Fig. 3.
Activation of Nrf2 signaling retains the electrocardiographic signatures in mouse receiving ISO. All mice from three different groups were subjected to anesthesia and electrocardiography was performed before sacrifice using Animal BioAmp. (A) Representative electrocardiographic pattern from all three experimental groups. (B) Quantitative measurement of changes in P duration, QT interval, ST Height, P, S and T amplitude in captured ECG tracing and analyzed using Labchart 8 software (n = 5/group). Values are presented as mean ± SEM. Significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
3.4. Cardiac-restricted mNrf2 expression preserved the myocardial function in ISO administered mice
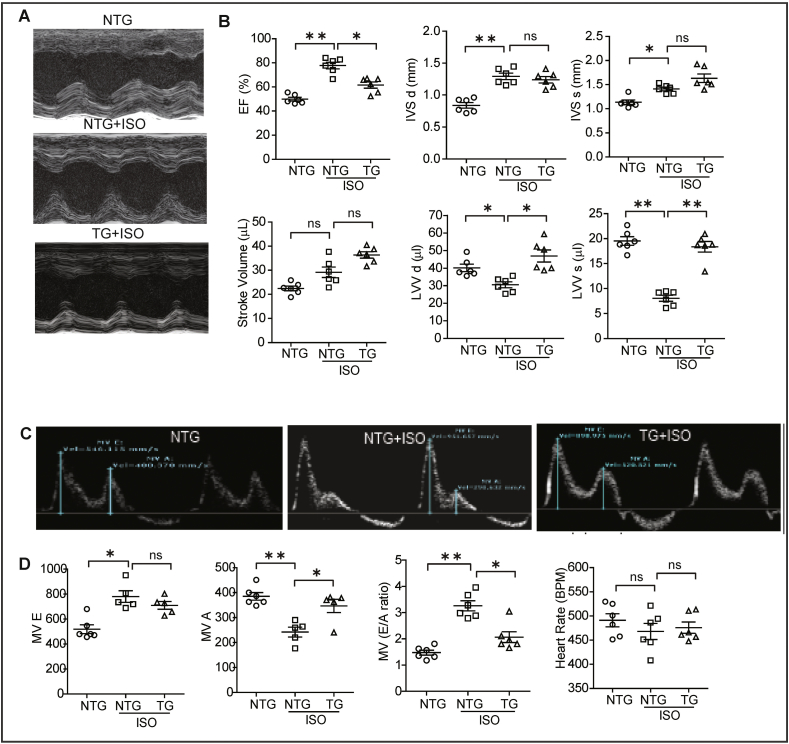
As myocardial structure was majorly affected by ISO injection, we measured cardiac function by echocardiography [28,30]. Representative parasternal short axis M-mode images displayed an abnormal cardiac structure and function in ISO infused mice (Fig. 4A). While ejection fraction (EF) was significantly increased along with significant decrease in end-systolic and diastolic ventricular volume in ISO treated NTG littermates, the TG mice administered with ISO did not show these responses (Fig. 4A–B). Further, the analysis of interventricular septal (IVS) dimensions revealed that IVSd and IVSs were significantly elevated in NTG-ISO treated mice compared to control mice. Of note, these structural changes were absent in TG mice that received ISO supporting the potential of enhancing Nrf2 dependent antioxidant signaling prior to stress.
Fig. 4.
Activation of Nrf2-antioxidant signaling preserves systolic and diastolic functions in mouse receiving ISO. Echocardiography was performed on animals from all the three groups on the day of sacrifice using Vevo2100 high resolution (38 MHz) imaging system. (A) Echocardiograms (M-Mode) for NTG (PBS control), as well as NTG mice and TG mice after 7 days of ISO treatment. (B) Cardiac functions were analyzed using LAX B-mode and SAX M-mode images; graphs showing changes in cardiac systolic functions for ISO-treated NTG and TG mice normalized with measurements from untreated/control NTG mice (n = 6/group). (C) The mitral valve flow pattern was recorded by pulse wave Doppler mode using Vevo2100 high-resolution echocardiography for NTG (PBS), as well as NTG mice and TG mice after 7 days of ISO treatment. (D) Mitral valve filling velocities (MV E, A and E/A) showing changes in diastolic function were measured using mitral valve images represented as dot plot, (n = 6/group). Values are presented as mean ± SEM. Significance: *, p < 0.05; **, p < 0.01; ns, nonsignificant.
3.5. Cardiac Nrf2 expression prevents ISO mediated diastolic dysfunction
Isoproterenol treatment in NTG mice showed an unaltered early left ventricular filling wave (MV E) and significantly reduced peak late left ventricular filling wave (MV A) which led to an increase in the atrial filling wave velocity (E/A) ratio. These results are consistent with significant diastolic dysfunction in this group compared to NTG (PBS) control mice. However, mice expressing Nrf2 in the heart received ISO significantly increased MV A waves which leads to a stabilized atrial filling wave velocity (E/A) ratio (Fig. 4C–D). These results indicate that cardiac specific Nrf2 expression prevented the structural and functional remodeling induced by ISO administration.
3.6. Transgenic expression of mNrf2 augments KEAP1-NRF2 association and preserves antioxidants and prevents oxidative damage caused by ISO
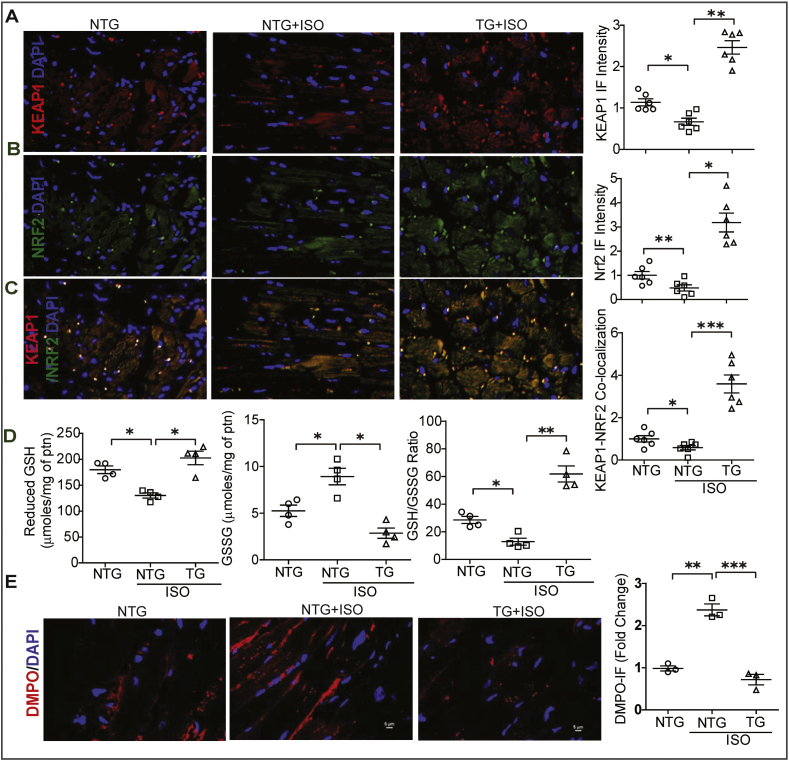
In order to define whether the enhanced Nrf2-dependent antioxidant function in the TG hearts is related to the levels of Keap1, we analyzed the interactions between the Keap1 and Nrf2 proteins by immunofluorescence. Interestingly, ISO administration reduces both KEAP1 and NRF2 levels in NTG mice treated with ISO and this was positively reflected in their association (individually and co-localization) in NTG mice (Fig. 5A–C). Interestingly, the association between these proteins significantly increased in TG when compared to NTG mice receiving ISO. These results suggest that the association of KEAP1-NRF2 may be an important contribution to the cytoprotective effects in TG hearts during stress condition. In addition, excess Nrf2 from the transgenic hearts will escape from Keap1 association and proteasomal degradation, directly translocate into the nucleus and result in transactivation of the target genes.
Fig. 5.
Cardiac specific expression of mNrf2 augments Keap1-Nrf2 association and prevents ISO-induced oxidative stress. (A) Representative fluorescence images of KEAP1, (B) NRF2 fluorescence images in heart tissues of the three experimental groups, and co-localized images of KEAP1-NRF2 (C). The fold-changes of fluorescence intensity for specific antibodies in heart tissue sections obtained from the three experimental groups. (D) Myocardial reduced glutathione, oxidized glutathione (GSSG) and GSH redox levels (GSH/GSSG ratio) levels in NTG, as well as NTG and TG mouse after 7 days of ISO treatment using enzymatic recycling assay (n = 4/group). (E) Representative fluorescence images of DMPO and DAPI staining for heart tissue sections obtained from NTG, as well as NTG and TG mice after 7 days of ISO treatment. DMPO staining used to detect radical protein adduct in these tissue sections and DAPI nuclear-staining used for standard fluorescence intensity. The fold-changes of DMPO fluorescence intensity in heart tissue sections obtained from the three experimental groups (n = 3/group). Values are presented as mean ± SEM. Significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001, ns-non-significant.
Next, we investigated whether increased KEAP1-NRF2 complex in TG mice preserves glutathione and its redox levels in ISO treated hearts. Enzymatic recycling assay for GSH showed a significant decline in reduced glutathione and its redox ratio (GSH/GSSG ratio) with an increase in oxidized glutathione (GSSG) in ISO treated NTG hearts compared to NTG control mice (Fig. 5D). However, TG mice treated with ISO showed significant increases in reduced glutathione and its redox ratio (GSH/GSSG) with decreased GSSG levels compared to NTG-ISO treated mice suggesting that basal enhanced Nrf2 expression in the mouse hearts preserves the glutathione levels and protects from the ISO induced injury (Fig. 5D). Next we investigated whether basal enhanced Nrf2 expression prevents ISO-mediated ROS induction and protein radical adduct formation, we injected DMPO to all groups of mice 2 h before sacrifice. Immunofluorescence detection of DMPO in NTG mice injected with ISO showed an increased accumulation of ROS production with formation of oxidative protein adducts. But mNrf2-TG mice that received ISO showed a significant reduction in oxidation of proteins as evidenced by the lower DMPO reactivity (Fig. 5E).
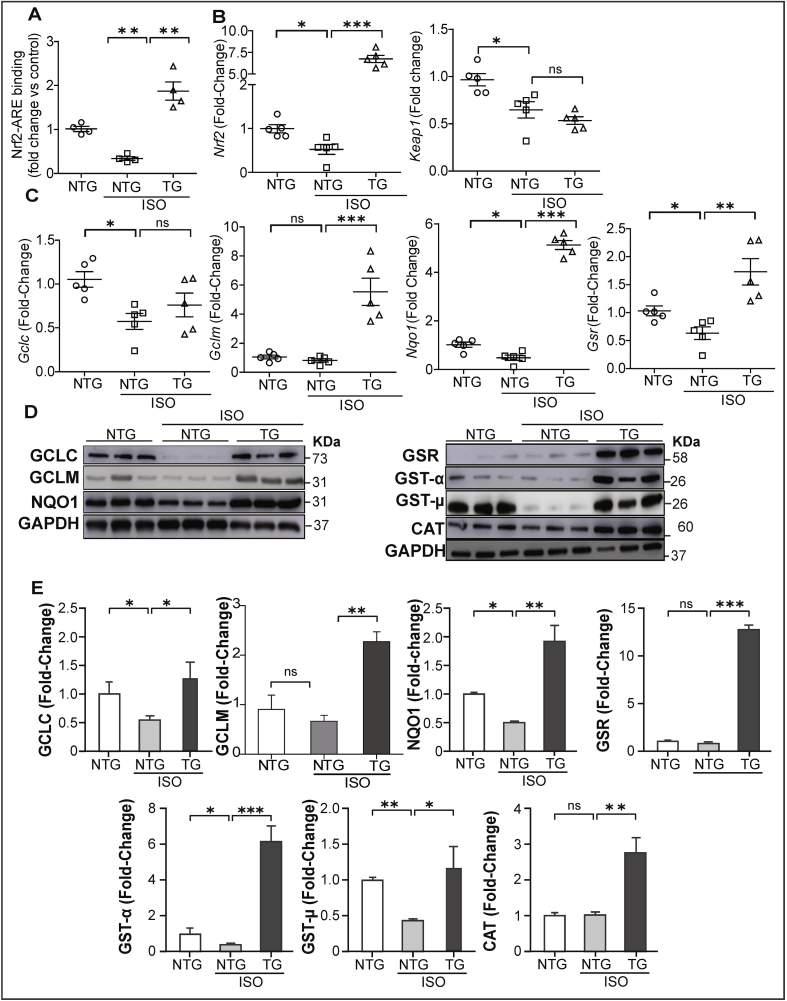
Next, we assessed Nrf2/antioxidant response element (ARE) DNA-binding ability to determine whether mNrf2-TG stabilizes the Nrf2 transcriptional activity in ISO-induced hearts using the Trans-AM-ELISA. NTG littermates that received ISO showed decreased Nrf2 binding activity, whereas the TG mice that received ISO displayed an increased Nrf2 activity compared to control and ISO-treated NTG mice (Fig. 6A). Nuclear extracts were incubated with either wildtype or mutated Nrf2 oligonucleotides to confirm the specificity of binding; incubation with normal rabbit IgG was used as a control (data not shown). Next, we analyzed the transcriptome level of Nrf2 and its repressor gene Keap1 to determine whether ISO administration alters these genes. ISO administration decreased Nrf2 and Keap1 gene levels in NTG mice while TG mice showed augmented Nrf2 levels; moreover, the Keap1 gene levels further decreased in TG vs. NTG mice, suggesting downregulation of Keap1 transcription in response to ISO insult (Fig. 6B). To confirm Nrf2 mediated transactivation of antioxidant genes, we measured their respective mRNA levels by qPCR. NTG littermates receiving ISO showed a significant decrease in expression of glutamate-cysteine ligase, catalytic subunit (Gclc) and NAD(P)H dehydrogenase quinone 1 (Nqo1) genes (Fig. 6C). mNrf2-TG mice receiving ISO displayed a clear upregulation of these genes including Gclc, Nqo1, glutamate-cysteine ligase, modifier subunit (Gclm) and glutathione reductase (Gsr).
Fig. 6.
Activation of Nrf2 signaling promotes transactivation of Nrf2-antioxidant genes/proteins in ISO-challenged mouse hearts. (A) Nrf2-ARE binding activities in nuclear lysates of heart obtained from NTG (PBS vehicle), as well as ISO treated NTG and TG mice for 7 days (n = 4/group). (B) Quantification of Nrf2 and Keap1 gene expression levels in NTG-PBS, NTG and TG mice received ISO (n = 5/group), (C) Quantification of antioxidant gene transcript levels by real time qPCR (n = 5/group); relative gene expression was analyzed by normalizing with levels of Gapdh/Arbp1 in NTG mice (PBS). (D) Representative immunoblots of cytosolic proteins from hearts of NTG (control), as well as NTG and TG mice treated with ISO for 7 days (n = 3/group). (E) Densitometry analysis of representative antioxidant enzymes in all the experimental groups (n = 3/group). GAPDH was used as control; the fold-changes of protein expression in NTG and TG under ISO treatment were normalized to NTG mouse (PBS as sham). Values are presented as mean ± SEM. Significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, nonsignificant.
Subsequent to the down regulation of antioxidant genes, administration of ISO in NTG littermates significantly decreased the levels of some antioxidant proteins namely GCLC, GCLM, NQO1, GST-α and GST-μ, whereas catalase and GSR were unchanged compared to control groups. mNrf2-TG expression protected the myocardium from ISO-mediated downregulation of antioxidants by significantly increasing these proteins (Fig. 6D-E). These results indicate that other factors such as redox dependent post-translational events may influence the levels of antioxidant proteins.
4. Discussion
We have previously shown that Nrf2 is a key player in myocardial cytoprotective defense under stress or aging conditions [20,31,32] and in protein aggregation cardiac disease [33,34]. Here, we demonstrate that augmentation of Nrf2/antioxidant signaling by transgenic expression of Nrf2 (within the heart) can prevent ISO induced oxidative stress, thereby protecting the myocardium from pathological remodeling. Furthermore, we identified that (a) moderate levels of Nrf2 preserves transcription and translation of antioxidants, (b) augments intracellular glutathione levels, (c) suppresses protein oxidation, and (d) prevents myocardial necrosis and hypertrophy in response to an ISO challenge.
While Nrf2 is not required for the basal regulation, it is mandatory for the transactivation of several antioxidants in response to stress. It has been shown that Nrf2 function was up-regulated in the heart after 2 weeks in an Ang II-induced hypertension [35,36] and in diabetes-induced oxidative damage and cardiomyopathy [[37], [38], [39], [40]]. On the other hand, downregulation of Nrf2 function is found to be responsible for redox-sensitive vascular dysfunction under hypertension condition [41,42]. Although previous reports have demonstrated that the effects of loss-of-function for Nrf2 in the heart including TAC models, the potential of enhancing the Nrf2 signaling under basal conditions independent of the endogenous redox control and then assessing the response to stress has not been studied. In this study, we address this issue using a novel transgenic mouse model for Nrf2 regulation in the context of an isoproterenol-induced myocardial remodeling. Moreover, we show that up-regulation of antioxidant enzymes, most of which are regulated by the enhanced Keap1-Nrf2 signaling; prevent ISO-induced oxidative stress and cardiac remodeling. Earlier reports indicate that sulforaphane-based activation of Nrf2 protects the myocardium from Ang-II toxicity [43]. Our novel model of cardiac specific mNrf2-TG suggests a significant change in myocardial glutathione in relation to NTG littermates, suggesting a pre-existing reductive-redox milieu in the TG hearts. In particular, our approach was to determine whether establishing a more dynamic basal Keap1-Nrf2 signaling axis in the myocardium could prevent the ISO-induced infarction and/or pathological remodeling.
In line with the previous reports, ISO treatment resulted in severe myocardial infarction along with significant structural remodeling (i.e. hypertrophy) in the NTG mice [[28], [29], [30]]. Interestingly, we noticed an increased ejection fraction (EF) and interventricular septum (IVS) dimensions at both diastole and systole along with profound infarction and fibrosis in the NTG mice, which suggests an adaptive remodeling of the myocardium in response to the ISO insult. However, a significant decrease in LV volume at both diastole and systole in the ISO-treated NTG hearts indicate a progressive pathological remodeling of the myocardium. Importantly, while prolonged QT interval might affect the contraction patterns, an elevated ST segment could be a result of significant myocardial infarction [44,45] and necrosis in response to ISO-administration leading to pathological remodeling in NTG mice. Taken together, the pathological remodeling (functional and structural) is coupled with increased oxidation of proteins as evidenced by the DMPO-based measurements and downregulation of antioxidants at both the transcriptional and translational levels in ISO-treated NTG mice. These data are consistent with pro-oxidant mechanism combined with a sub-optimal Keap1-Nrf2 pathway contributes to the pathogenesis.
As anticipated, the damage to hearts in response to ISO was significantly suppressed as evidenced from lesser infarction and fibrosis in the TG mice. Furthermore, enhanced availability of Nrf2 resulted in up-regulation of the key antioxidant genes and proteins in TG hearts and efficiently prevented the oxidation of proteins and suppressed the inflammatory response to ISO insult. While a clear rescue of ECG patterns, ejection fraction and LV volume in TG mice receiving ISO was apparent, IVS dimensions remain unchanged. However, it is expected that there could be physiological adaptations to ISO-induced arrhythmogenic changes (ECG) in the TG hearts and it might require a longer time for a complete recovery. Of note, lesser infarction in TG hearts might be attributed to reduced myocardial damage and increased LV volume than the ISO-treated NTG mice.
Stimulated Nrf2-antioxidant signaling provides defense against ischemia-reperfusion injury [24] and pressure-overload hypertrophy in the myocardium [26]. While activation of Nrf2 using sulforaphane curbed Akt2 ablation-mediated benefits, its inhibition promoted the Akt2 ablation-induced beneficial effect against paraquat challenge [46]. We previously reported that abrogation of Nrf2 increases the susceptibility to age-associated cardiac hypertrophy in response to high-intensity exercise stress [27] and a decrease in Nrf2 signaling prevents reductive stress in the protein aggregation cardiomyopathy caused by hR120GCryAB in mice [33]. Recently, we reported that constitutive activation of Nrf2 (caNrf2) directly promotes a reductive and hyper-reductive conditions in the myocardium [47], which is likely to be detrimental under a chronic setting. Therefore, here we used a novel genetic model displaying modulation of the Keap1-Nrf2 signaling axis in the myocardium that protects it from ISO-induced oxidative stress and pathological remodeling. Our results support the notion that manipulation of myocardial Nrf2 signaling and antioxidant defense under stress conditions would be highly beneficial as a pre-emptive measure to protect the myocardium.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Acknowledgments
This study was supported by funding from NHLBI (HL118067&2HL118067), NIA (AG042860), the AHA (BGIA 0865015F), University of Utah Center for Aging Pilot grant (2009), the Division of Cardiovascular Medicine/Department of Medicine, University of Utah and the start-up funds (for NSR) by Department of Pathology, the University of Alabama at Birmingham, AL, UABAMC21 grant NSR and VDU by the University of Alabama at Birmingham, AL and the UAB Nathan Shock Center P30 G050886 (VDU). Authors thank Miss. Snekha N. Rajasekaran and Miss. Melissa D. Blair for their English editorial assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101212.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Shih H., Lee B., Lee R.J., Boyle A.J. The aging heart and post-infarction left ventricular remodeling. J. Am. Coll. Cardiol. 2010;57(1):9–17. doi: 10.1016/j.jacc.2010.08.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchis-Gomar F., Perez-Quilis C., Leischik R., Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016;4(13):256. doi: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiong M., Wang Z.V., Pedrozo Z., Cao D.J., Troncoso R., Ibacache M., Criollo A., Nemchenko A., Hill J.A., Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Disease. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi M., Shimizu W., Albert C.M. The spectrum of epidemiology underlying sudden cardiac death. Circ. Res. 2015;116(12):1887–1906. doi: 10.1161/CIRCRESAHA.116.304521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piek A., de Boer R.A., Silljé H.H.W. The fibrosis-cell death axis in heart failure. Heart Fail. Rev. 2016;21:199–211. doi: 10.1007/s10741-016-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Empel V.P.M., Bertrand A.T.A., Hofstra L., Crijns H.J., Doevendans P.A., De Windt L.J. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005;67(1):21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Schirone L., Forte M., Palmerio S., Yee D., Nocella C., Angelini F., Pagano F., Schiavon S., Bordin A., Carrizzo A., Vecchione C., Valenti V., Chimenti I., De Falco E., Sciarretta S., Frati G. A review of the molecular mechanisms underlying the development and progression of cardiac remodeling. Oxid. Med. Cell. Longev. 2017;2017:16. doi: 10.1155/2017/3920195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sag C.M., Santos C.X.C., Shah A.M. Redox regulation of cardiac hypertrophy. J. Mol. Cell. Cardiol. 2014;73:103–111. doi: 10.1016/j.yjmcc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Shao D., Oka S.-i., Brady C.D., Haendeler J., Eaton P., Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J. Mol. Cell. Cardiol. 2012;52(3):550–558. doi: 10.1016/j.yjmcc.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare J.M., Stamler J.S. NO/redox disequilibrium in the failing heart and cardiovascular system. J. Clin. Investig. 2005;115(3):509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura W., Muralidhar S., Canseco D.C., Puente B., Zhang C.C., Xiao F., Abderrahman Y.H., Sadek H.A. Redox signaling in cardiac renewal. Antioxidants Redox Signal. 2014;21(11):1660–1673. doi: 10.1089/ars.2014.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgoyne J.R., Mongue-Din H., Eaton P., Shah A.M. Redox signaling in cardiac physiology and pathology. Circ. Res. 2012;111(8):1091–1106. doi: 10.1161/CIRCRESAHA.111.255216. [DOI] [PubMed] [Google Scholar]
- 13.Kurian G.A., Rajagopal R., Vedantham S., Rajesh M. The role of oxidative stress in myocardial ischemia and reperfusion injury and remodeling: revisited. Oxid. Med. Cell. Longev. 2016;2016:14. doi: 10.1155/2016/1656450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: the evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kris-Etherton P.M., Lichtenstein A.H., Howard B.V., Steinberg D., Witztum J.L. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110(5):637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 17.Myung S.K., Ju W., Cho B., Oh S.W., Park S.M., Koo B.K., Park B.J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ (Clinical Res. ed.) 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivekananthan D.P., Penn M.S., Sapp S.K., Hsu A., Topol E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet (London, England) 2003;361(9374):2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 19.Ye Y., Li J., Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthusamy V.R., Kannan S., Sadhaasivam K., Gounder S.S., Davidson C.J., Boeheme C., Hoidal J.R., Wang L., Rajasekaran N.S. Acute exercise stress activates Nrf2/ARE signaling and promotes antioxidant mechanisms in the myocardium. Free Radic. Biol. Med. 2012;52(2):366–376. doi: 10.1016/j.freeradbiomed.2011.10.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palliyaguru D.L., Chartoumpekis D.V., Wakabayashi N., Skoko J.J., Yagishita Y., Singh S.V., Kensler T.W. Withaferin A induces Nrf2-dependent protection against liver injury: role of Keap1-independent mechanisms. Free Radic. Biol. Med. 2016;101:116–128. doi: 10.1016/j.freeradbiomed.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo E., Chhunchha B., Singh P., Sasaki H., Singh D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017;7:14130. doi: 10.1038/s41598-017-14520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan J., Guan Y., Mu F., Guo C., Zhang E., Yin Y., Wei G., Zhu Y., Cui J., Cao J., Weng Y., Wang Y., Xi M., Wen A. Protective effect of butin against ischemia/reperfusion-induced myocardial injury in diabetic mice: involvement of the AMPK/GSK-3β/Nrf2 signaling pathway. Sci. Rep. 2017;7:41491. doi: 10.1038/srep41491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J., Wang L., Akinyi M., Li Y., Duan Z., Zhu Y., Fan G. Danshensu protects isolated heart against ischemia reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling. Int. J. Clin. Exp. Med. 2015;8(9):14793–14804. [PMC free article] [PubMed] [Google Scholar]
- 25.Li J., Ichikawa T., Villacorta L., Janicki J.S., Brower G.L., Yamamoto M., Cui T. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1843–1850. doi: 10.1161/ATVBAHA.109.189480. [DOI] [PubMed] [Google Scholar]
- 26.Zhou S., Sun W., Zhang Z., Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid. Med. Cell. Longev. 2014;2014:260429. doi: 10.1155/2014/260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanmugam G., Narasimhan M., Conley R.L., Sairam T., Kumar A., Mason R.P., Sankaran R., Hoidal J.R., Rajasekaran N.S. Chronic endurance exercise impairs cardiac structure and function in middle-aged mice with impaired Nrf2 signaling. Front. Physiol. 2017;8(268) doi: 10.3389/fphys.2017.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouet S., Di Pietrantonio L., Daskalopoulos E.P., Esfahani H., Horckmans M., Vanorle M., Lemaire A., Balligand J.L., Beauloye C., Boeynaems J.M., Communi D. Loss of mouse P2Y6 nucleotide receptor is associated with physiological macrocardia and amplified pathological cardiac hypertrophy. J. Biol. Chem. 2016;291(30):15841–15852. doi: 10.1074/jbc.M115.684118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivey M.J., Kuwabara J.T., Pai J.T., Moore R.E., Sun Z., Tallquist M.D. Resident fibroblast expansion during cardiac growth and remodeling. J. Mol. Cell. Cardiol. 2018;114:161–174. doi: 10.1016/j.yjmcc.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L., Jia Z., Yang L., Zhu M., Zhang J., Liu J., Wu P., Tian W., Li J., Qi Z., Tang X. Exercise protects against chronic β-adrenergic remodeling of the heart by activation of endothelial nitric oxide synthase. PLoS One. 2014;9(5) doi: 10.1371/journal.pone.0096892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gounder S.S., Kannan S., Devadoss D., Miller C.J., Whitehead K.J., Odelberg S.J., Firpo M.A., Paine R., 3rd, Hoidal J.R., Abel E.D., Rajasekaran N.S. Impaired transcriptional activity of Nrf2 in age-related myocardial oxidative stress is reversible by moderate exercise training. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0045697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vomund S., Schäfer A., Parnham M.J., Brüne B., von Knethen A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017;18(12):2772. doi: 10.3390/ijms18122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannan S., Muthusamy V.R., Whitehead K.J., Wang L., Gomes A.V., Litwin S.E., Kensler T.W., Abel E.D., Hoidal J.R., Rajasekaran N.S. Nrf2 deficiency prevents reductive stress-induced hypertrophic cardiomyopathy. Cardiovasc. Res. 2013;100(1):63–73. doi: 10.1093/cvr/cvt150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajasekaran N.S., Varadharaj S., Khanderao G.D., Davidson C.J., Kannan S., Firpo M.A., Zweier J.L., Benjamin I.J. Sustained activation of nuclear erythroid 2-related factor 2/antioxidant response element signaling promotes reductive stress in the human mutant protein aggregation cardiomyopathy in mice. Antioxid. Redox Signal. 2011;14(6):957–971. doi: 10.1089/ars.2010.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C., Luo Z., Carter G., Wellstein A., Jose P.A., Tomlinson J., Leiper J., Welch W.J., Wilcox C.S., Wang D. NRF2 prevents hypertension, increased ADMA, microvascular oxidative stress, and dysfunction in mice with two weeks of ANG II infusion. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;314(3):R399–R406. doi: 10.1152/ajpregu.00122.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satta S., Mahmoud A.M., Wilkinson F.L., Yvonne Alexander M., White S.J. The role of Nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev. 2017;2017:9237263. doi: 10.1155/2017/9237263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z., Wang S., Ji H., Zhang Z., Chen J., Tan Y., Wintergerst K., Zheng Y., Sun J., Cai L. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Sci. Rep. 2016;6:30252. doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen J., Zhang Z., Cai L. Diabetic cardiomyopathy and its prevention by Nrf2: current status. Diabetes Metab. J. 2014;38(5):337–345. doi: 10.4093/dmj.2014.38.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David J.A., Rifkin W.J., Rabbani P.S., Ceradini D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017;2017:4826724. doi: 10.1155/2017/4826724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu J., Cheng Y., Wu H., Kong L., Wang S., Xu Z., Zhang Z., Tan Y., Keller B.B., Zhou H., Wang Y., Xu Z., Cai L. Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66(2):529–542. doi: 10.2337/db15-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopes R.A., Neves K.B., Tostes R.C., Montezano A.C., Touyz R.M. Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension. Hypertension (Dallas, Tex. : 1979) 2015;66(6):1240–1250. doi: 10.1161/HYPERTENSIONAHA.115.06163. [DOI] [PubMed] [Google Scholar]
- 42.Majzunova M., Dovinova I., Barancik M., Chan J.Y.H. Redox signaling in pathophysiology of hypertension. J. Biomed. Sci. 2013;20(1):69. doi: 10.1186/1423-0127-20-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin Y., Bai Y., Jiang X., Zhou S., Wang Y., Wintergerst K.A., Cui T., Ji H., Tan Y., Cai L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 2018;15:405–417. doi: 10.1016/j.redox.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khorrami A., Hammami M., Garjani M., Maleki-Dizaji N., Garjani A. Tacrolimus ameliorates functional disturbances and oxidative stress in isoproterenol-induced myocardial infarction. DARU J. Pharm. Sci. 2014;22(1):68. doi: 10.1186/s40199-014-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panda S., Kar A., Biswas S. Preventive effect of Agnucastoside C against Isoproterenol-induced myocardial injury. Sci. Rep. 2017;7(1):16146. doi: 10.1038/s41598-017-16075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S., Zhu X., Xiong L., Ren J. Ablation of Akt2 prevents paraquat-induced myocardial mitochondrial injury and contractile dysfunction: role of Nrf2. Toxicol. Lett. 2017;269:1–14. doi: 10.1016/j.toxlet.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Shanmugam G., Narasimhan M., Tamowski S., Darley-Usmar V., Rajasekaran N.S. Constitutive activation of Nrf2 induces a stable reductive state in the mouse myocardium. Redox Biol. 2017;12:937–945. doi: 10.1016/j.redox.2017.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.