Abstract
Motile cilia on airway cells are necessary for clearance of mucus-trapped particles out of the lung. Ciliated airway epithelial cells are uniquely exposed to oxidants through trapping of particles, debris and pathogens in mucus and the direct exposure to inhaled oxidant gases. Dynein ATPases, the motors driving ciliary motility, are sensitive to the local redox environment within each cilium. Several redox-sensitive cilia-localized proteins modulate dynein activity and include Protein Kinase A, Protein Kinase C, and Protein Phosphatase 1. Moreover, cilia are rich in known redox regulatory proteins and thioredoxin domain-containing proteins that are critical in maintaining a balanced redox environment. Importantly, a nonsense mutation in TXNDC3, which contains a thioredoxin motif, has recently been identified as disease-causing in Primary Ciliary Dyskinesia, a hereditary motile cilia disease resulting in impaired mucociliary clearance. Here we review current understanding of the role(s) oxidant species play in modifying airway ciliary function. We focus on oxidants generated in the airways, cilia redox targets that modulate ciliary beating and imbalances in redox state that impact health and disease. Finally, we review disease models such as smoking, asthma, alcohol drinking, and infections as well as the direct application of oxidants that implicate redox balance as a modulator of cilia motility.
Keywords: S-nitrosation, Alcohol, Cilia, Redox regulation, Hydrogen peroxide, Superoxide, Nitric oxide
Abbreviations: AICD, alcohol-induced ciliary dysfunction; ADP, adenosine diphosphate; ATP, adenosine triphosphate; CAT, catalase; CBF, ciliary beat frequency; DC, docking complex; DTT, dithiothreitol; DTNB, 5,5′-dithio-bis-[2-nitrobenzoic acid]; DUOX, dual oxidase; FiO2, fraction of inhaled oxygen; GSH, glutathione; HA, hyaluranon; HC, heavy chain; Hsp90, heat shock protein 90; HX, hypoxanthine; IDA, inner dynein arm; LC, light chain; l-NAME, N-Nitroarginine methyl ester; NOS, nitric oxide synthase; NOX, nicatinomide adenine dinucleotide phosphate-oxidase; Nrf2, Nuclear factor (erythroid-derived 2)-like 2; ODA, outer dynein arm; PCD, primary ciliary dyskinesia; PKA, protein kinase A; PKC, protein kinase C; PP1, protein phosphatase 1; PP2A, protein phosphatase 2A; Prx6, peroxiredoxin 6; PA, Pseudomonas Aeruginosa; redox, reduction/oxidation; RHAMM, receptor for hyaluronic acid-mediated motility; RONS, reactive oxygen/nitrogen species; RSH, reduced thiol; RSNO, nitrosothiol; RSOH, sulfenic acid; RSO2H, sulfinic acid; RSO3H, sulfonic acid; RSSR, disulfide; RSV, respiratory syncytial virus; sGC, soluble guanylyl cyclase; SOD, superoxide dismutase; Trx1, thioredoxin 1; TrxR2, thioredoxin reductase 2; TXNDC, thioredoxin domain-containing; XDH/XO, xanthine dehydrogenase/xanthine oxidase
1. Introduction
Functional motile cilia are key components of human biology. The collection of cytoskeletal structure and motility components that make up cilia is referred to as the axoneme. The axoneme is mostly conserved throughout all instances in human biology including the tail of the sperm, the oviducts, the ventricular system of the brain and spinal cord, the embryonic node and the airways. Additionally, instances of non-motile, primary cilia occur on nearly every cell type and have become increasingly recognized as central hubs for many signaling cascades related to proliferation and differentiation, and to determine left-right asymmetry [1,2]. Left-right asymmetry is associated with motile cilia at the embryonic node and potentially with non-motile cilia surrounding the node. While cilia are present in many locations throughout the body, this review is focused on oxidant regulation of airway-localized cilia and therefore is not intended be a comprehensive review of all cilia types. The motile cilia that line the epithelium of the airways are uniquely exposed to a variety of oxidants through inhalation and deposition of gases, particles and debris. Mucociliary clearance, driven by airway lining cilia, is a necessary defense system to remove pathogens from the lungs and out of the airways to be swallowed or expectorated.
Housed within the cilium are all of the necessary components for cilia bending. Upon addition of exogenous substrates and cofactors (nucleotides, ATP, metals), isolated demembranated cilia can beat independently of the cell [[3], [4], [5]]. These proteins include those of the cytoskeletal structure, regulatory motor proteins (such as specialized dyneins), ATP/ADP-generating enzymes (Nucleoside diphosphate kinases) and many phosphatases (PP1 and PP2A) and kinases (PKA and PKC) that regulate cilia motility [6,7]. Moreover, cilia contain or are in close proximity to oxidant-generating systems such as nitric oxide synthases (NOS) or NADPH oxidases (NOX/DUOX) and mitochondria, respectively [8,9] (Fig. 1).
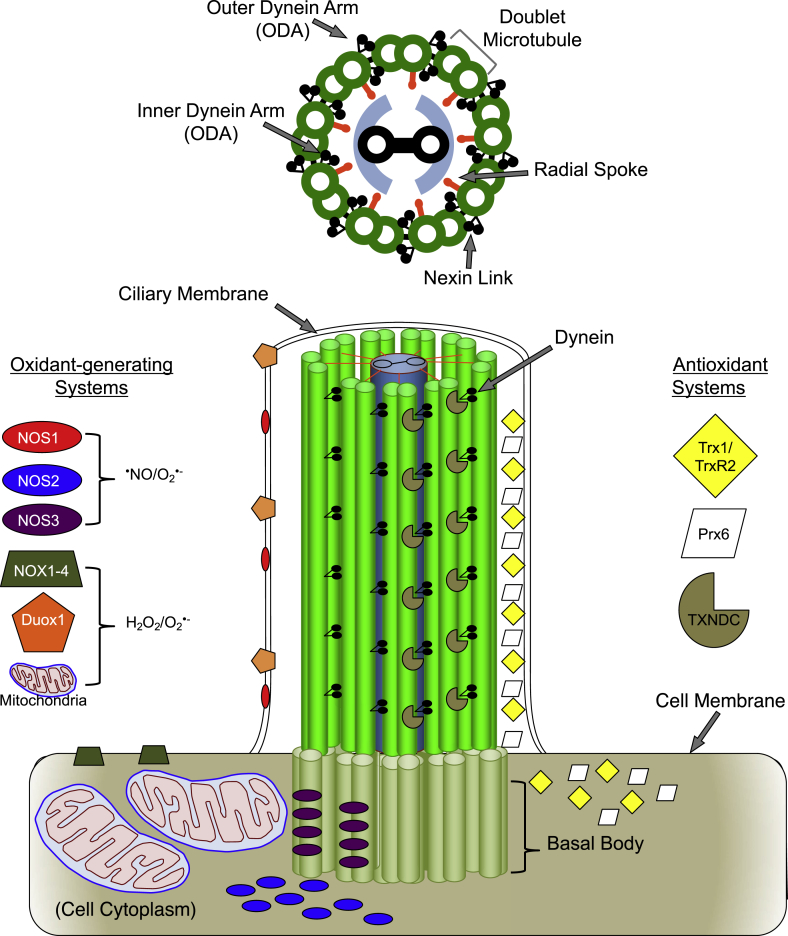
Fig. 1.
Cilia Structure and regulatory redox systems. Upper: Graphical representation of a cross section of an individual cilium depicting “9+2” arrangement with inner and outer dynein arms, radial spokes and nexin links making up the axoneme. Lower left: Localization of known oxidant-generating systems. Nitric oxide synthase (NOS) 1 and dual oxidase 1 (DUOX1) localize along the length of the ciliary membrane. NOS2 localizes to the cytoplasm and NOS3 localizes to the basal body. The NOS enzymes produce nitric oxide (•NO) or superoxide (O2•-). The NADPH oxidase (NOX) 1–4 enzymes localize to the apical surface of the cell membrane. The apical portion of ciliated cells is packed with mitochondria near basal bodies. NOX and DUOX enzymes and mitochondria generate hydrogen peroxide (H2O2) and O2•-. Lower right: Localization of antioxidant systems. Both the ciliary matrix and cytoplasm of airway ciliated cells are densely packed with thioredoxin 1 (Trx1), thioredoxin reductase 2 (TrxR2) peroxiredoxin 6 (Prx6). Additionally, several thioredoxin domain-containing (TXNDC) proteins are anchored along the length of the axoneme in close proximity to dynein. *Note – Outer Arm Dynein (ODA) is represented with two heavy chains as in mammalian species (other species such as Chlamydomonas reinhardtii have three heavy chains).
Changes in reduction/oxidation (redox) state are gaining increased appreciation as signaling mechanisms for many cellular processes including proliferation, senescence, differentiation, transcription factor activation, apoptosis and motility [10]. The redox state within the cytoplasm is normally kept in a reduced state by an abundance of thiol-based enzymatic systems such as the thioredoxin and glutathione families. Local regulation of protein thiol-oxidation, however, can have a profound transient or irreversible impact on tertiary and quaternary structure, protein stability, protein-protein interaction and enzymatic activity [11]. Motile cilia are rich in thiol-dense and thiol-regulatory proteins [6,7], of which the local redox environment governs function. Redox species are often short-lived due to their reactive nature, and thus proximity of production of redox species to the moieties with which they react is a common characteristic of redox-regulated events.
2. Reduction/oxidation (redox) signaling and stress
Ambient air comprises 78% nitrogen and 21% oxygen, where oxygen exists as a diatomic molecule of two atoms of oxygen covalently bound (O2). The electron configuration of oxygen is such that each oxygen atom of O2 holds a single unpaired electron (free radical). This electron configuration gives oxygen a special reactivity, resulting in other atoms or molecules gaining or losing electrons in the presence of oxygen. These reactions are generally referred to as oxidation/reduction or redox reactions.
Redox signaling refers to the shift of electrons, or change in oxidation states from one atomic, ionic or molecular species to another. Specifically, an oxidation consists of an electrophilic species (oxidant) acquiring electrons from a nucleophile (reductant), leaving the nucleophile in a more oxidized state. The complementary reaction, or donation of an electron from a nucleophile to an electrophile is a “reduction”. In both cases, one species is oxidized (loses an electron) and the other species is reduced (gains an electron). One or two electrons can be transferred resulting in one of three scenarios: 1) A single electron is transferred, and one or both reactants are left with an unpaired electron (free radical); 2) a two electron oxidation (predominant), which results in an oxidized nucleophile plus a neutralized electrophile or; 3) an addition reaction in which a covalent bond is formed (adduct) between the nucleophile and an electrophile (example: disulfide, RSSR; or nitrosothiol, RSNO; Fig. 2).
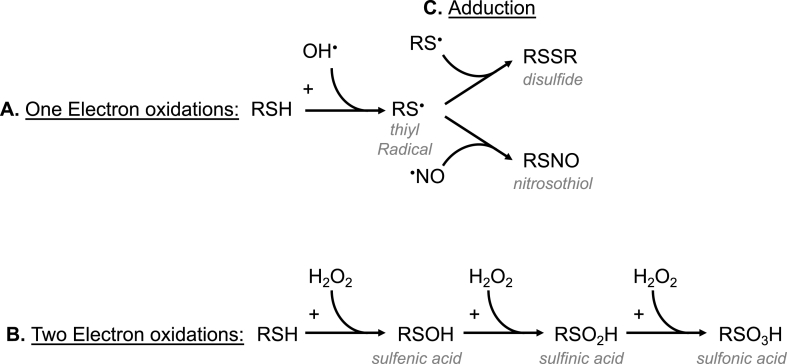
Fig. 2.
Common thiol redox signaling reactions. A) One electron oxidation of a protein thiol (RSH) by hydroxyl radical (OH•) to form a thiyl radical (RS•) B) A two electron oxidation of RSH by hydrogen peroxide (H2O2) to sulfenic (RSOH), sulfinic (RSO2H) or sulfonic (RSO3H) acids. C) Adduction of a thiyl radical with a thiyl radical or nitric oxide (•NO) to form a disulfide (RSSR) or nitrosothiol (RSNO).
2.1. Oxidants in biological systems
The major oxidant species in biology are reactive oxygen and nitrogen species (RONS), which are molecules derived from oxygen alone, from nitrogen or from a combination of nitrogen and oxygen. Free radicals as described above consist of any atom or molecule with an unpaired electron, which include some species of RONS. Indeed, O2 having two unpaired electrons is a free radical, and serves a crucial function as the final electron acceptor for electron transport during mitochondrial respiration and numerous other reactions in biology. Not all RONS, however, are free radicals. Common examples of RONS that are not free radicals include but are not limited to hydrogen peroxide (H2O2) and peroxynitrite (ONOO−).
In general, RONS come from endogenous oxygen metabolizing enzymes such as NADPH oxidases (NOX/DUOX), nitric oxide synthases (NOS1-3), cytochrome P450 enzymes, xanthine dehydrogenase/oxidase (XDH/XO), or exogenous sources such as radiation/UV, products of combustion (i.e. smoking), air pollutants and metals.
Thiol redox status is perhaps the best-described mechanism of protein regulation by oxidant signaling. Thiols, compared to other side chains of amino acids, have the potential to be more reactive due to a relatively low dissociation constant (pKa). Specifically, when a thiol residue (pKa < 6.5) is in proximity to a basic amino acid, a metal center or aromatic amino acid, the sulfur moiety becomes increasingly susceptible to biologically relevant oxidation [11]. A reduced thiol (RSH) can be oxidized to sulfenic (RSOH), sulfinic (RSO2H) or sulfonic (RSO3H) acids or form a disulfide (RSSR; Fig. 2) by reactive oxygen species, or to a nitrosothiol (RSNO) by a reactive nitrogen species. Oxidation of a key thiol (or thiols) results in gain or loss of function due to post-translational modifications such as conformational changes or protein-protein interactions. In some cases a thiol can be irreversibly oxidized, resulting in permanent functional changes to a protein [12].
2.1.1. Motile cilia: organelle structure
The basic cytoskeletal structure of a cilium (or axoneme) as observed by electron microscopy is a circular arrangement of 9 doublet microtubules. In motile airway cilia there is a 10th pair of microtubules positioned at the center of the 9-doublet ring. This conformation is referred to as the “9 + 2” arrangement, where 9 refers to the ring of doublet microtubules and 2 refers to the two microtubules in the center. There are two distinct densities seen projecting from the outer microtubules toward the inner microtubules and projecting from one outer microtubule pair to the next; referred to as “radial spokes” and “nexin links”, respectively (Fig. 1).
Positioned along the length of the axoneme on the outer doublets are the complexes of molecular motors, dynein ATPases. These dynein complexes, which consist of combinations of multiple heavy and light chain dynein molecules, repeat along the long axis of the microtubules at a periodicity of 96-nm. Two primary complexes of dynein function as the motors for ciliary motility: inner dynein arms (IDA) and outer dynein arms (ODA). IDAs are implicated in regulating waveform, while ODAs drive beat frequency [13]. Initial understanding of the precise mechanisms by which dyneins take these configurations and how they are integrated into a functional regulated system with other structural and regulatory components has only recently been elucidated. For a detailed comprehensive review of motile cilia structure refer to recent review by Ishikawa [14]. Many components of cilia structure and regulation rely on a specific redox state to maintain the function and integrity of the cilium as an organelle.
3. Cilia-localized redox-sensitive thiol-regulatory proteins
3.1. Direct evidence of redox regulation of ciliary motility
Several cilia regulatory components have been suggested by experimental evidence to be sensitive to redox regulation.
3.1.1. Dynein
Perhaps the best-studied redox-sensitive component key to cilia motility is dynein. Within the ODAs and IDAs are multiple heavy and light chains (HC and LC). A significant portion of published work has been focused on the ODAs of the model organism Chlamydomonas reinhardtii. Within the ODA, the HCs contain the ATPase motors and LCs are generally thought to be regulatory molecules.
3.1.1.1. Thioredoxin motifs within dynein subunits
Three subunits of the ODA (i.e. DC3, LC3 and LC5) contain thioredoxin redox motifs [15,16]. Two light-chains (LC) of Chlamydomonas ODA, LC3 and LC5, have full copies of the thioredoxin active site redox sensitive motif, which contains two reactive cysteine moieties (i.e. WCGPCK). This special orientation of amino acids results in low pKa and high reactivity of the flanking cysteines.
Wakabayashi and King found in Chlamydomonas that detergent-extracted reactivated cell models of motility showed a clear decrease in beat frequency upon increased presence of oxidants, and conversely, an increase in beat frequency with reducing conditions depending on the light adapted state. Importantly, mutants lacking LC5 were resistant to oxidant-mediated slowing, but also did not demonstrate increased beating with reducing agents [15]. Oxidation experiments coupled with two-dimensional gel electrophoresis and mass spectrometry revealed that the oxidations that occur within the LCs create transient disulfides to other thiol containing protein in close proximity. Specifically, these experiments revealed LC interaction with two thioredoxin proteins and two uncharacterized flagellar proteins [15]. The importance of these thioredoxins is clear since disruption of the mammalian/human orthologues of LC3 and LC5, TXNDC3 (NM23-H8) and TXNDC6 (TXL-2/NME9) are associated with lung diseases [17,18]. Indeed, recent evidence has identified that a common variant of TXNDC3 causes a genetic disease of dysfunctional mucociliary clearance known as Primary Ciliary Dyskinesia (PCD) [19].
3.1.1.2. DC3
For ODAs to be positioned on the correct site on microtubules, they must come in to contact with a specialized group of interacting proteins, called the ODA-docking complex (ODA-DC). The ODA-DC consists of three subunits, DC1, DC2 and DC3. Loss of DC1 or DC2 results in the complete loss of ODAs leading to erratic and slow swimming in Chlamydomonas. Loss of DC3 results in a similar, but less pronounced phenotype with only partial loss of ODAs. Mutants lacking DC3 are unable to swim backward upon photoshock [20]. The ODA-DC likely serves in part as a calcium sensor because calcium is a key regulator of cilia motility. Of the ODA-DC subunits, DC1 and DC2 likely serve as regulatory proteins to modulate calcium binding of DC3, which contains four EF-hand domains (EF1-4) [20]. Within the four EF-hand domains of DC3, EF2 contains three cysteines, two of which are located in the calcium-binding loop [21]. Moreover, when studied in vitro, DC3 only binds calcium in the presence of dithiothreitol (a strong reducing agent), indicating redox-sensitivity. Despite this, upon reconstitution of DC3–lacking mutants with DC3 having altered EF-domains, Chlamydomonas swimming was apparently restored to normal by all means tested. This suggests that the calcium-binding activity of DC3 may not play a role in vivo, despite clear evidence that DC3 binds calcium in vitro. Additionally, while DC3 shows a stronger affinity for calcium, it also shows affinity for magnesium. Given the relative concentrations of calcium and magnesium in cilia, it is possible that DC3 is normally magnesium-loaded and that shifts in calcium concentration displace magnesium as a component of regulation. Further work is needed to determine the role of DC3 as a redox-sensitive calcium sensor to transmit a signal to the ODA via the thioredoxin-like light chains of the ODA.
3.1.1.3. Gamma heavy chain (HC)
Early evidence in sea urchin sperm and tetrahymena demonstrated the redox sensitivity of the dynein ATPases. These experiments demonstrated enhanced outer dynein arm activity in the presence of irreversible oxidizing agents such as N-ethylamide, p-hydroxymercury benzoate and p- sulfonate suggesting a role for reduced thiols to govern dynein activity [22]. Experimental evidence from Chlamydomonas also suggests that dynein is directly regulated by oxidation. Direct redox regulation of the gamma heavy chain (HC) dynein ATPase has been demonstrated to be biphasic. In isolated axonemes from Chlamydomonas, treatment with increasing amounts of up to 10 μM DTNB, revealed a 50% increase in ATPase activity. Further increasing DTNB up to 10 mM resulted in a decline in ATPase activity to 10% of the rate in the absence of DTNB [23]. In Chlamydomonas mutants lacking the ODAs, but not IDAs, ATPase activity was not stimulated at lower concentrations of DTNB but still showed inhibition upon high doses. In mutants lacking the LC5 and alpha HC domain the magnitude of activation is blunted. However, this limitation disappears upon removal of the beta HC motor domain, suggesting that multiple intra dynein protein-protein interactions are involved in modulating ATPase activation of the gamma HC [23].
3.1.2. Phosphoregulatory systems
Anchored within the cytoskeletal structure of the axoneme and localized in close proximity to the dyneins associated with radial spokes are several phosphoregulatory enzymes; the kinases and phosphatases [24]. Several of these phosphoregulatory enzymes have been shown to be redox-sensitive in purified form or in contexts other than cilia, and there is direct evidence for a redox regulatory component of two enzymes, protein phosphatase 1 (PP1) and protein kinase C PKC.
3.1.2.1. Protein phosphatase 1
The PP1 catalytic subunit (PP1c) predominately localizes to the central pair in Chlamydomonas with a small fraction localizing near ODAs [25]. There are at least two putative redox sensitive elements of PP1: 1) PP1 contains metals (likely Fe2+ and Zn2+; the metals in the active site vary based on whether the enzyme is recombinant or native) in its native active site [26]; and 2) PP1 contains a putative oxidoreductase active site [27]. Recombinant PP1 (rPP1) activity can be enhanced or suppressed by H2O2. Sommer et al. reported an approximately 2-fold increase in PP1 activity after incubation with an oxidant generating system (Xanthine/Xanthine Oxidase) [28]. Importantly, stimulation of rPP1 activity was blocked by catalase, but not superoxide dismutase, implicating H2O2 as the activating oxidant [28]. The precise mechanism of PP1 activation by H2O2 has not been further reported. NADPH oxidase 4 (NOX4) generated H2O2 has been demonstrated to inhibit recombinant PP1 by one electron oxidation within a dinuclear metal center [29]. Additionally, PP1 inactivation coincides with oxidation of residues Cys62 and Cys105 to sulfenic, sulfinic, or irreversibly to sulfonic acid [30]. Results obtained studying recombinant PP1, however, may not reflect the in vivo redox sensitivity of PP1, as the metals contained in recombinant PP1 are likely different and subject to different oxidation energies [26]. In this context, the precise mechanisms by which PP1 can be redox regulated can only be speculated. Oxidation of PP1 could alter binding with any of the over 250 identified binding partners of PP1directly or alter PP1 catalytic activity directly [31]. Recently, our laboratory identified S-nitrosation of Cys155 that correlated with activation of PP1, and subsequent cilia dysfunction (discussed later) [32].
3.1.2.2. Protein kinase C
Several studies demonstrate Protein Kinase C (PKC) activation decreases CBF in mammalian cells [[33], [34], [35]]. Salathe and colleagues found that PKC dependent phosphorylation of a single membrane-bound 37-kDa target was associated with decreased CBF in cilia from sheep tracheal rings [36]. Furthermore, Kobyashi demonstrated that H2O2 could slow cilia, and blocking PKC activity could reverse this cilia inhibition [37]. Several PKC isoforms exist and have varying functions within the cell. Wyatt et al. demonstrated localization of the ε isoform in bovine isolated axonemes [35]. PKC is implicated in cilia slowing related to cigarette smoking [35,[38], [39], [40]], aging [34], H2O2 [37] and fungal aflatoxin exposure [41].
PKC contains cysteine residues that are susceptible to posttranslational redox modifications in both the C-terminal catalytic domain and the N-terminal regulatory domain [42]. Reversible activation or inactivation can occur by disulfide formation within the regulatory domain or catalytic domain, respectively [43]. Redox modification of the C-terminus results in phorbol ester-independent activation of PKC activity, resulting in activation of PKC without a need for translocation of the enzyme to membrane. In some cases, H2O2 activates PKC in correlation with a reverse, membrane to cytosol, distribution [42,44].
3.2. Putative redox sensitive ciliary motility regulatory components
Several other axoneme-localized proteins that have been shown to regulate ciliary motility also have been shown to be redox-sensitive in other biologic systems. The redox-sensitivity of these proteins has yet to be formally examined in the context of cilia regulation. These include but are not limited to protein phosphatase 2A (PP2A) [45], soluble guanylyl cyclase (sGC) [8], protein kinase A (PKA) [7,8,46] and heat shock protein 90 (Hsp90) [47].
3.3. Antioxidant enzymes and oxidant generating systems
3.3.1. Antioxidant proteins
Several canonical antioxidant proteins are present in cilia. In addition to the dynein subunits described previously, tracheal and lung tissue show enhanced staining for the redoxin family proteins TrxR2, Prx6, Trx1 from both mice and humans under baseline conditions [48,49]. Additionally, specialized nucleoside diphosphate kinases contain multiple copies of thioredoxin domains, but have yet been demonstrated to function in redox reactions governing cilia function (Fig. 1) [19].
3.3.2. Oxidant generating systems
There are several RONS sources in close proximity to the axoneme. RONS are often generated from enzymes that metabolize oxygen creating more reactive products including superoxide O2•-, H2O2, and •NO.
3.3.2.1. Nitric oxide synthases
•NO is a key regulatory molecule for airway ciliary motility. •NO is a highly reactive molecule first characterized as the signaling molecule originally called endothelial derived relaxing factor (EDRF) [50]. Biological •NO comes primarily from the three isoforms of the homodimeric •NO synthase (NOS) enzymes: 1) neuronal NOS (nNOS or NOS1); 2) inducible NOS (iNOS or NOS2); and 3) endothelial NOS (eNOS or NOS3) [[51], [52], [53]]. NOS1 and NOS3 are constitutively expressed and posttranslationally activated, while NOS2 is transcriptionally regulated and constitutively active. The NOS enzymes share the common mechanism of catalyzing the reaction of oxygen, NADPH, tetrahydrobiopterin (BH4) and l-Arginine to the free radical NO• and l-citrulline [51]. •NO then acts as a signaling intermediate by autocrine or paracrine mechanisms [54]. A well-characterized “canonical” example of this is the covalent binding of •NO to the heme of guanylyl cyclase to stimulate the production of cGMP, which activates PKG [51,54]. In contrast, signaling properties of aberrant or non-canonical •NO production are poorly characterized. In the absence of NADPH, BH4 or l-Arginine, the passing of electrons between the domains of the NOS enzyme becomes uncoupled, resulting in the additional production of the reactive free radical anion O2•- [55]. The reaction of O2•- with •NO, one the fastest known biological chemical reactions, results in the formation of the highly reactive anion peroxynitrite (ONOO−) [55,56]. •NO and ONOO−, as RONS, can catalyze the reversible nitrogen oxidation adduction of protein thiols, termed S-nitrosation or oxidation of tyrosine residues, termed nitration [57]. Our group found that alcohol exposure of isolated axonemes rapidly and robustly increased •NO production and CBF. By adding the stereo-specific NOS inhibitor l-NAME (NOS isoform non-specific pan-NOS inhibitor), both NOS production and CBF responsiveness to alcohol exposure were blocked [58]. Upon addition of the •NO donor, sodium nitroprusside, CBF responsiveness was restored. These data suggest both •NO producing capacity localized to the axoneme, and a •NO responsive element of the axoneme. Additionally, •NO is key to many receptor-mediated increases in CBF such as bitter taste receptors and adrenergic receptors. All three NOS isoforms have been differentially localized in ciliated airway epithelial cells and reports of localization of NOS in ciliated airway epithelial cells are varied (Fig. 1).
3.3.2.2. NOS1
NADPH diaphorase staining of NOS1 knockout mice and immunohistochemistry for NOS1 from human bronchioles revealed strong localization of NOS1 to airway epithelium [59,60]. Recently, NOS1 has been localized directly and specifically to cilia of airway epithelial cells [9]. In this study, NOS1 was found at the proximal portion of the axoneme in human explants as well as primary cells cultured at an air-liquid interface [9]. Despite these data, no published reports demonstrate a specific role for NOS1 to regulate cilia function.
3.3.2.3. NOS2
NOS2 enzyme has great affinity for calcium and therefore does not require a burst of calcium to produce •NO resulting in abundant production of •NO far greater than that of NOS1 or NOS3 [61]. From a redox perspective, the levels of •NO radicals generated by NOS2 result in oxidizing conditions and can quickly deplete antioxidant stores. In vivo NOS2 is constitutively expressed in airway epithelial cells, but this expression is lost in vitro [62]. Cytosolic expression of NOS2 in cultured ciliated cells can be maintained upon addition of cytokines IL-4 and Interferon γ [62]. In sinus explants treated with TNFα, rapid and abundant expression of NOS2 is associated with a decrease in CBF [63].
3.3.2.4. NOS3
In rat and bovine bronchial ciliated epithelial cells NOS3 and PKG-1β localize near the basal body of the axoneme, suggesting a role in the function of cilia [8,64,65]. Additionally, in response to noxious stimuli, NOS3 has been found to translocate from the basal body region to more distal in the axoneme in bovine cells [47].
3.3.2.5. Nox Family of NADPH oxidases
The NADPH oxidase (Nox) includes Nox1-4 and dual oxidase (Duox) 1 and 2. This family of enzymes exhibit several conserved properties: 1) six conserved transmembrane domains; 2) four highly conserved and heme-binding histidines, and 3) and an electron flow from bound NADPH to FAD and through the heme domains [66]. These conserved properties ultimately result in the reduction of O2 to O2•- through the heme domains [66]. Additionally, moieties that regulate each enzyme's calcium sensitivity or the potential to generate H2O2, differentiate the isoforms. Bedard and Krause provide a thorough review of the Nox Family enzymes [67].
The primary Nox family members found in ciliated airway epithelial cells are Duox1 [68], Duox2 [69,70], Nox4 [71] and Nox2 [72,73]. In these cells these enzymes localize to the apical surface of the cell, with staining for Duox1 extending into the cilia (Fig. 1) [74]. Strikingly, Duox1/2 and Nox4 have been associated with predominance toward H2O2 versus canonical production of O2•- [70,75]. It is likely that these enzymes produce O2•- that rapidly dismutates to H2O2. While these Nox enzymes clearly play a role in airway diseases associated with dysfunctional mucociliary clearance, Nox4 is the only enzyme that has been directly associated with cilia dysfunction [76].
3.3.2.6. Mitochondria
In airway epithelial cells mitochondria are densely localized to the apical surface of the cell near the basal body of the axoneme, presumably to provide a source of ATP to meet the demands of ciliary motility (Fig. 1). With close proximity to cilia, mitochondria are a likely source of oxidant signaling and stress to motile cilia. Both Murphy [77] and Mailloux [78] have written extensive detailed reviews of the mechanisms by which mitochondria generate and are regulated by redox signaling and stress.
3.4. Direct effects of oxidants and oxidant generating systems
Numerous studies have reported the effects of relatively stable oxidants such as O2 or H2O2, or the direct effect of oxidant generating systems to study the role of transient oxidants such as O2•- or hydroxyl radicals.
3.4.1. Oxygen stress
Increasing the fraction of inhaled oxygen (FiO2) is a common form of cellular hyperoxia encountered clinically in the context of supplemental oxygen therapy. Unlike tissues that are exposed to this increased oxygen through the circulatory system, the airways are in direct contact with the highest levels of oxygen as an inhaled gas. Despite its therapeutic role to increase hemoglobin saturation and restore tissue oxygenation, the effect of oxygen on ciliated cells varies based on the length of exposure. With short exposure to increased FiO2, Stanek et al. found an increase in CBF in cultured human nasal epithelia [79]. In contrast, prolonged exposure to high and prolonged FiO2 decreases the number of cilia in rat, bovine and human cultured cells and explants [80,81]. Al-Shmangi et al. found that co-incubation with vitamin E and/or vitamin C partially mitigated oxygen-induced cilia loss [82]. Additional reports of the mechanistic nature behind acute or chronic oxygen exposure to cilia do not currently exist in published literature.
3.4.2. Xanthine/xanthine oxidase – opposing roles for O2•- and H2O2
Purified xanthine oxidase (XO) in combination with xanthine (X) or hypoxanthine (HX) is a commonly used oxidant generating system. This system generates abundant quantities of O2•- and H2O2 depending on the culture conditions and substrate levels. Addition of superoxide dismutase (SOD) and or catalase (CAT) allow for the differentiation of O2•- or H2O2 as the acting oxidant. The effects of XO systems on CBF are varied.
Direct exposure to X/XO or H2O2 results in ciliary slowing [[83], [84], [85], [86]]. Interestingly, slowing by X/XO further declines upon preincubation with SOD. Surprisingly, in the presence of CAT, X/XO stimulates CBF and with the combination of SOD + CAT CBF is similar to conditions in the absence of X/XO [83,84]. As SOD generates H2O2 and this further slows cilia, these data suggest that H2O2 slows cilia since CAT selectively consumes H2O2 and there is no slowing with the presence of CAT. Slowing of CBF by X/XO seems to be a H2O2 and DNA damage-dependent process. Feldman et al. and Min et al., found that inhibition of DNA repair by 3-aminobenzamide blocks both X/XO or direct H2O2-mediated CBF slowing [83,85].
Since CAT stimulates CBF in the presence of X/XO, which is attenuated by SOD, this suggests that O2•- stimulates CBF. Manzanares et al., found that inhibition of hyaluronon (HA) synthesis prevents stimulation of CBF by X/XO and that addition of exogenous HA amplifies the stimulatory response [87]. Additionally, CBF stimulation by X/XO was reduced upon blocking the receptor for hyaluronic acid-mediated motility (RHAMM), which signals through recepteur d'origine nantais (RON), a tyrosine kinase at the apical surface of the cell, to activate a CBF stimulation pathway [87,88]. These experiments suggest that oxidatively modified HA can activate RHAMM. In combination, the above findings utilizing the X/XO system highlight the importance of identifying the oxidant species generated to affect cilia function.
4. Redox associated acquired ciliopathies
Continuous and dynamic beating of airway cilia is necessary to clear mucus and mucous-trapped pathogens, particles and debris upon inhalation as a protective mechanism for the lung. This is evident in cases of inherited genetic mutations in motile cilia specific proteins that result in disordered or immotile cilia function. These genetic diseases, known as primary ciliary dyskinesia (PCD), result in chronic mucus plugging, pneumonias and bronchiectasis. Knowles provides a detailed review of PCD [89]. In addition to heritable genetics causing cilia dysfunction, extrinsic elements such as exposure to pollutants, microbes or lifestyle factors can impact ciliary motility. Many of these exposure-associated, or “acquired ciliopathies” are driven directly or indirectly by perturbations of redox balance (Table 1). These exposures change redox balance by containing or altering oxidizing elements, generating intracellular or extracellular RONS by activating local cellular defense systems, or by driving recruitment of inflammatory cells with powerful oxidant producing capacity. Tilley et al. has provided a broad overview of secondary ciliary dysfunction or acquired ciliopathies [90].
Table 1.
Redox associated acquired ciliopathies.
| Insult | Redox Species | Source | Effect on Ciliary Motility |
|---|---|---|---|
| Hyperoxia | O2 | • Inspired O2 > 21% | Low/brief  CBF CBFHigh/prolonged  CBF CBF |
| Exogenous Xanthine Oxidase | H2O2 O2•- |
• Enzymatic product of XO activity |
 CBF with XO alone or in the presence of superoxide dismutase CBF with XO alone or in the presence of superoxide dismutase CBF in the presence of catalase CBF in the presence of catalase |
| Asthma | H2O2 Increase or decrease •NO |
• Nox/Duox |
 CBF CBF |
| Respiratory Syncytial Virus | H2O2 | • Decreased Nrf2 • unknown |
 CBF CBFLoss of ciliated cells |
| Pseudomonas Aeruginosa | H2O2 | • Cell-free supernatant • Pyocyanin • Pseudomonas • Quinolone Signal • Increased Duox |
 CBF CBF |
| Streptococcus pneumoniae | H2O2 | • H2O2 secretion via pyruvate oxidase |
 CBF CBF |
| Tobacco Smoke | Aldehyde adducts H2O2 |
• Direct effect of combustion products • Aldehyde metabolism Duox1 |
 CBF CBF |
| Alcohol Exposure | •NO | • NOS3 | Transient  CBF, CBF,Prolonged desensitization to β-agonists |
4.1. Asthma
Asthma is characterized by hyperreactivity and inflammation of the airways. This chronic inflammation leads to mucus cell metaplasia and mucus plugging [91]. In addition to increased mucus production asthma is associated with an observed slowing of CBF. Bonser et al. reported decreased CBF in bronchial rings collected from severe asthmatics that was reversible ex vivo by removal of apically tethered MUC5AC. The conclusion made by the authors was that tethering of this mucin was causative of CBF slowing, based on the observation that upon MUC5AC removal, CBF was restored [91]. While it was demonstrated that removal of MUC5AC correlated with CBF slowing, the method of removal of the mucin required a strong cell-permeable reducing agent dithiothreitol (DTT). Thus an alternative conclusion can be drawn; that oxidants are responsible for CBF slowing. Consistent with this, based on a recent study reported by Wan et al., ciliary slowing was correlated to a redox event exclusive to neutrophilic asthma [76]. In a study of ciliary function in bronchial strips collected from 11 patients with neutrophilic asthma and 10 patients with nonneutrophilic asthma, CBF was only slow in those patients with neutrophilic asthma and correlated well with the percent sputum neutrophils (r = −0.70; P < .001) and did not correlate with sputum eosinophils. Furthermore, in bronchial strips from the same patients, Nox4 levels and ROS generation were increased in samples from neutrophilic asthmatic and Nox4 inhibition by GKT137831 improved CBF in these strips. Interestingly, when primary cells were cultured and differentiated from the same donors, Nox4 expression remained high but there was no detectable difference in cilia function. In contrast, in an ovalbumin mouse model of asthma, which results in a neutrophilic asthma pattern, CBF and the percent ciliated cells in tracheal rings was decreased in ovalbumin challenged mice compared to controls. Importantly, oral administration of the Nox4 inhibitor GKT137831 prevented cilia slowing and loss, despite the persistence of neutrophil infiltration. These data suggest that RONS generated by NOX4 in combination with neutrophil infiltration drive cilia dysfunction [76].
4.2. Respiratory syncytial virus
Viruses are a common pathogen in the airways. Of these, Respiratory Syncytial Virus (RSV) specifically targets airway epithelial cells via the CX3CR1 receptor [92,93] receptor and results in cilia loss [94]. In vitro RSV infection rapidly drives ciliary dyskinesia [95], loss of cilia [96] and ballooning and detachment of ciliated cells from the airway epithelium [97].
In airway epithelial cells RSV drives a robust increase in H2O2 and downregulates the antioxidant defenses [[98], [99], [100]]. Induction of Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in a mouse model is protective against RSV replication and RSV-driven pulmonary inflammation [101]. Nrf2 is a transcription factor controlling the expression of several antioxidant enzymes including thioredoxin reductase 1 (TXNRD1), glutathione-cysteine ligase (GCLC), glutathione S-transferase (GST) and heme oxygenase-1 (HO-1). Mata et al., reported that pre-incubation with N-acetyl-l-cysteine, a precursor to the antioxidant molecule glutathione (GSH), prevented viral replication and loss of ciliogenesis in vitro in human cells [102].
4.3. Pseuodomonas aeruginosa
A common pathogenic organism in Cystic Fibrosis is Pseuodomonas aeruginosa (PA). This organism produces several redox-active virulence factors. In a study of sheep tracheal explants, a cell free supernatant derived from PA resulted in ciliary slowing of sheep tracheal explants that was blocked by co-incubation with catalase, suggesting H2O2 as the insulting redox species [103]. Pyocyanin, a redox-active phenazine pigment, is found in the sputa of colonized individuals near 27 μg/ml and associated with epithelial cell disruption and cilia slowing [104,105]. Interestingly, an array of antioxidants including catalase, SOD and N-acetyl-l-cysteine did not prevent cilia slowing by pyocyanin [106]. These data suggest either that ciliary slowing by pyocyanin is not a redox-dependent event, or that the antioxidants used were unable to target the redox-sensitive components involved. In addition to pyocyanin, Pseudomonas Quinolone Signal (PQS) is another virulence factor source of oxidative stress in airway epithelial cells, resulting in increased apoptosis and decreased antioxidant capacity by driving down Nrf-2 and Heme Oxygenase 1 [107]. Finally, the type-three secretion system of Pseuodomonas activates Duox in airway epithelial cells in a calcium-dependent and ATP-independent manner [108]. A specific role of Duox-dependent cilia dysfunction with Pseuodomonas is in need of further study.
4.4. Streptococcus pneumoniae
Streptococcus pneumoniae (S. pneumoniae) is the most common community acquired bacterial cause of pneumonia [109]. In addition to the pore forming toxin, pneumolysin, S. pneumoniae secretes H2O2 via pyruvate oxidase enzymes [110]. Although the virulence of pneumolysin-deficient S. pneumoniae to cause pneumonia is diminished compared to their pneumolysin-positive counterparts [111], it is apparent that this H2O2 is a strong virulence factor since equivalent levels of H2O2 induce cilia slowing and pneumolysin deficient pneumococci are cytotoxic to rat airway epithelial cells [112,113].
4.5. Smoke
Smoking is one of the strongest risk factors for bronchitis, chronic obstructive pulmonary disease (COPD) and increased mortality from pneumonia [114]. Combustion of tobacco smoke produces one of the highest biologically relevant sources of acetaldehyde (nearly 1 mg per cigarette) in addition to several other oxidant species [115,116]. Smoking can push reversible signaling reactions toward irreversibly oxidized moieties [117]. For over half a century cigarette smoking has been associated with cilia slowing and cilia shortening or loss [39,118,119].
We have shown in mice exposed to whole-body smoke from cigarettes that PKCε was slightly increased by 6-weeks and increased 10-fold by 12 weeks, which was mirrored by slowing of CBF in tracheal rings compared to mice exposed to air alone [38]. Additionally, cell cultures of mouse tracheal epithelium exposed to both smoke and alcohol demonstrate increased PKCε activity compared to control or to either smoke or alcohol individually. Importantly, slowing does not occur in smoke-exposed PKCε−/− mice [35]. While the precise mechanism of PKCε activation by cigarette smoke is not fully understood, it has recently been reported that smoke increases Duox1 and H2O2 and IL-8 release in an immortalized bronchial epithelial cell-line and in smoke-exposed mice. Inhibition of Duox1 with a non-specific Nox inhibitor DPI blocked IL-8 release [120]. In a separate bovine bronchial epithelial cell model, exogenous IL-8 caused cilia dysfunction [121]. Moreover, as described previously, PKCε can be activated by H2O2 and other oxidants. Smoking may act on PKCε through an IL-8 or H2O2 driving mechanism.
4.6. Alcohol
Alcohol drinking is associated with increased prevalence of treatment-resistant pneumonia [122,123] and is associated with pro-oxidant environment in the lung and airways [[124], [125], [126], [127]]. Alcohol has different effects on ciliary motility dependent on both dose and length of exposure. Alcohol modifies the •NO-driven kinase-dependent mechanism of airway ciliary regulation in two ways: 1) brief alcohol exposure transiently stimulates airway CBF; and 2) prolonged alcohol exposure desensitizes cilia to subsequent stimulation of motility [128,129]. For example up to 6 h alcohol exposure (up to 100 mM) to cultured airway epithelial cells stimulates CBF. In contrast, after 24 h of alcohol exposure, CBF returns to baseline and is unresponsive to a β-agonist (desensitization), termed alcohol-induced ciliary dysfunction (AICD).
Ethanol rapidly increases the activity of adenylyl cyclase (AC) to produce cyclic adenosine monophosphate (cAMP), activating PKA and subsequently increasing CBF [130,131]. In parallel, alcohol exposure increases •NO production by activating endothelial nitric oxide synthase (eNOS or NOS3). •NO then stimulates soluble guanylyl cyclase (GC) to produce cyclic guanosine monophosphate (cGMP); activating PKG. CBF stimulation by alcohol depends upon sequential activation of PKG and PKA [129]. In conditions of prolonged stimulation by alcohol exposure, ciliary beating returns to baseline and becomes unresponsive to other stimulation such as β-agonists. Alcohol-induced blunting of CBF stimulation occurs through the desensitization of both PKA and PKG to cyclic-nucleotide stimulus [128]. This dual kinase desensitization is a central molecular component of AICD [128]. We recently expanded the ciliary metabolon to include protein phosphatase 1 (PP1) [132]. In conditions of prolonged alcohol exposure, protein phosphatase activity is increased at the level of the cilia organelle. PP1 inhibition can reverse AICD, restoring CBF and cyclic nucleotide-dependent kinase responsiveness [132]. However, the mechanism of alcohol-driven phosphatase activation is unknown.
Interestingly, mice drinking alcohol with concomitant feeding of antioxidants (N-acetyl cysteine or procysteine) do not develop AICD [133]. This suggests that PP1 is activated by alcohol-driven oxidative stress. Three cysteine residues, Cys62, Cys105 and Cys155 (see above subheading Protein Phosphatase 1) have been reported to have a potential redox regulatory role to govern PP1. Our lab has found that alcohol drives S-nitrosation of Cys155, reverses S-nitrosation of Cys62 and has no effect on the redox status of Cys105. These data are consistent with other published literature that oxidation of Cys62 inactivates PP1 and oxidation of Cys155 activates PP1. In this context, the precise mechanism by which S-nitrosation stimulates PP1 activity can only be speculated. S-nitrosation of the oxidoreductase site could directly alter PP1 catalytic activity, or alter binding with any of the over 250 identified binding partners of PP1 [31]. Our data suggest direct S-nitrosation of PP1 as a novel mechanism of activation in bovine isolated airway axonemes.
Additionally, Cho et al. reported an alcohol triggered increase in CBF in mouse nasal epithelial cells but, in contrast to tracheal epithelial cells, this increase in CBF was not •NO-dependent [134]. Attempts to understand the effects of alcohol on motile cilia from human sources have been conflicting. Smith et al. reported no significant increase in CBF from nasal epithelial cells over a range of ethanol concentration (0.1%, 0.5% and 1%) [135]. Despite these inconsistencies in vitro, there is increased prevalence of pneumonia amongst populations of individuals with alcohol use disorder and alcohol use is associated with a redox imbalance in the airways [126,136].
5. Conclusion and future directions
It is becoming increasingly clear that redox signaling contributes to the regulation of many functions within the cell. Similarly, the recognition of the complexity of redox systems has increased. Having a unique exposure to the outside world, motile airway cilia contain many components known to be sensitive to oxidation state. The redox state within the ciliary compartment housing the axoneme is likely distinct from that of the cytoplasm of the cell body. While small molecules such as oxidants can likely freely travel between the cytoplasm of the cell body and cilia (through the transition zone), the proximity of where oxidants are produced or reduced is likely a key component to the local redox state in the axoneme. Motile cilia are enriched with both oxidant generating and antioxidant systems, which likely do not freely pass through the transition zone, and these systems are perturbed in multiple diseases associated with dysfunctional mucociliary clearance.
The airway epithelium lends to a unique target of inhaled therapies as drugs can be inhaled to come in direct contact with the epithelium and cilia. Many inhaled oxidant targeted therapies have not resulted in functional improvements in lung function or infections in clinical trials; this is likely due to an incomplete understanding of the redox dynamics of cilia function. Only a handful of studies have directly assessed intrinsic control of cilia regulation be redox mechanisms and have identified that the dynein motors and regulatory complexes are directly redox sensitive. Future work should be focused on delineating the function of these cilia regulatory proteins in the context of an oxidant stressor. Moreover, functional clearance studies are lacking. More research will likely uncover novel therapeutic redox targets, and effective therapeutics will need to target a specific redox species within a specific compartment.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable.
Conflicts of interest
Both authors declare that they have no competing interests.
Authors' contributions
The manuscript was conceived and written collaboratively by the two authors. Both authors read and approved the final manuscript.
Author information
MEP and JHS: Department of Internal Medicine, Pulmonary, Critical Care, Sleep & Allergy Division, University of Nebraska Medical Center, Omaha, NE, USA.
MEP: Department of Cellular and Integrative Physiology, University of Nebraska Medical Center, Omaha, NE, USA.
Funding
This work was supported by NIH NIAAA F30AA024676 to MEP and NIH NIAAA R01AA08769 to JHS.
Acknowledgments
The authors would like to thank Dr. Adam Case for reading draft manuscripts and providing valuable comments.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101146.
Contributor Information
Michael E. Price, Email: michael.price@unmc.edu.
Joseph H. Sisson, Email: jsisson@unmc.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bangs F., Anderson K.V. Primary cilia and mammalian hedgehog signaling. Cold Spring Harb. Perspect. Biol. 2017;9(5) doi: 10.1101/cshperspect.a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shinohara K., Hamada H. Cilia in left-right symmetry breaking. Cold Spring Harb. Perspect. Biol. 2017;9(10) doi: 10.1101/cshperspect.a028282. pii: a028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrowski L.E. Cell Biology, Four-Volume Set. Anonymous Elsevier Inc.; 2006. Preparation of cilia from human airway epithelial cells. [Google Scholar]
- 4.Hastie A.T., Dicker D.T., Hingley S.T., Kueppers F., Higgins M.L., Weinbaum G. Isolation of cilia from porcine tracheal epithelium and extraction of dynein arms. Cell Motil. Cytoskelet. 1986;6(1):25–34. doi: 10.1002/cm.970060105. [DOI] [PubMed] [Google Scholar]
- 5.Weaver A., Hard R. Newt lung ciliated cell models: effect of MgATP on beat frequency and waveforms. Cell Motil. 1985;5(5):377–392. doi: 10.1002/cm.970050503. [DOI] [PubMed] [Google Scholar]
- 6.Blackburn K., Bustamante-Marin X., Yin W., Goshe M.B., Ostrowski L.E. Quantitative proteomic analysis of human airway cilia identifies previously uncharacterized proteins of high abundance. J. Proteome Res. 2017;16(4):1579–1592. doi: 10.1021/acs.jproteome.6b00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowski L.E., Blackburn K., Radde K.M., Moyer M.B., Schlatzer D.M., Moseley A., Boucher R.C. A proteomic analysis of human cilia: identification of novel components. Mol. Cell. Proteomics. 2002;1(6):451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- 8.Stout S.L., Wyatt T.A., Adams J.J., Sisson J.H. Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J. Histochem. Cytochem. 2007;55(5):433–442. doi: 10.1369/jhc.6A7089.2007. [DOI] [PubMed] [Google Scholar]
- 9.Jackson C.L., Lucas J.S., Walker W.T., Owen H., Premadeva I., Lackie P.M. Neuronal NOS localises to human airway cilia. Nitric Oxide. 2015;44:3–7. doi: 10.1016/j.niox.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poole L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015;80:148–157. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schilter D. Thiol oxidation: a slippery slope. Nat. Rev. Chem. 2017;1:0013. [Google Scholar]
- 13.Brokaw C.J., Kamiya R. Bending patterns of Chlamydomonas flagella: IV. Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil. Cytoskelet. 1987;8(1):68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa T. Axoneme structure from motile cilia. Cold Spring Harb. Perspect. Biol. 2017;9(1) doi: 10.1101/cshperspect.a028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakabayashi K., King S.M. Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. J. Cell Biol. 2006;173(5):743–754. doi: 10.1083/jcb.200603019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carvalho A.P., Fernandes P.A., Ramos M.J. Similarities and differences in the thioredoxin superfamily. Prog. Biophys. Mol. Biol. 2006;91(3):229–248. doi: 10.1016/j.pbiomolbio.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Pazour G.J., Agrin N., Walker B.L., Witman G.B. Identification of predicted human outer dynein arm genes: candidates for primary ciliary dyskinesia genes. J. Med. Genet. 2006;43(1):62–73. doi: 10.1136/jmg.2005.033001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadek C.M., Jimenez A., Damdimopoulos A.E., Kieselbach T., Nord M., Gustafsson J.A., Spyrou G., Davis E.C., Oko R., van der Hoorn F.A., Miranda-Vizuete A. Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelium and spermatid manchette and axoneme. J. Biol. Chem. 2003;278(15):13133–13142. doi: 10.1074/jbc.M300369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duriez B., Duquesnoy P., Escudier E., Bridoux A.M., Escalier D., Rayet I., Marcos E., Vojtek A.M., Bercher J.F., Amselem S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl. Acad. Sci. U. S. A. 2007;104(9):3336–3341. doi: 10.1073/pnas.0611405104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey D.M., Inaba K., Pazour G.J., Takada S., Wakabayashi K., Wilkerson C.G., Kamiya R., Witman G.B. DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol. Biol. Cell. 2003;14(9):3650–3663. doi: 10.1091/mbc.E03-01-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casey D.M., Yagi T., Kamiya R., Witman G.B. DC3, the smallest subunit of the Chlamydomonas flagellar outer dynein arm-docking complex, is a redox-sensitive calcium-binding protein. J. Biol. Chem. 2003;278(43):42652–42659. doi: 10.1074/jbc.M303064200. [DOI] [PubMed] [Google Scholar]
- 22.King S.M. Academic Press; 2017. Dyneins: the Biology of Dynein Motors. [Google Scholar]
- 23.Harrison A., Sakato M., Tedford H.W., Benashski S.E., Patel-King R.S., King S.M. Redox-based control of the gamma heavy chain ATPase from Chlamydomonas outer arm dynein. Cell Motil. Cytoskelet. 2002;52(3):131–143. doi: 10.1002/cm.10044. [DOI] [PubMed] [Google Scholar]
- 24.Wirschell M., Yamamoto R., Alford L., Gokhale A., Gaillard A., Sale W.S. Regulation of ciliary motility: conserved protein kinases and phosphatases are targeted and anchored in the ciliary axoneme. Arch. Biochem. Biophys. 2011;510(2):93–100. doi: 10.1016/j.abb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang P., Fox L., Colbran R.J., Sale W.S. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J. Cell Sci. 2000;113(Pt 1):91–102. doi: 10.1242/jcs.113.1.91. (Pt 1) [DOI] [PubMed] [Google Scholar]
- 26.Heroes E., Rip J., Beullens M., Van Meervelt L., De Gendt S., Bollen M. Metals in the active site of native protein phosphatase-1. J. Inorg. Biochem. 2015;149:1–5. doi: 10.1016/j.jinorgbio.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Fetrow J.S., Siew N., Skolnick J. Structure-based functional motif identifies a potential disulfide oxidoreductase active site in the serine/threonine protein phosphatase-1 subfamily. FASEB J. 1999;13(13):1866–1874. doi: 10.1096/fasebj.13.13.1866. [DOI] [PubMed] [Google Scholar]
- 28.Sommer D., Coleman S., Swanson S.A., Stemmer P.M. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch. Biochem. Biophys. 2002;404(2):271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 29.Santos C.X., Hafstad A.D., Beretta M., Zhang M., Molenaar C., Kopec J., Fotinou D., Murray T.V., Cobb A.M., Martin D., Zeh Silva M., Anilkumar N., Schroder K., Shanahan C.M., Brewer A.C., Brandes R.P., Blanc E., Parsons M., Belousov V., Cammack R., Hider R.C., Steiner R.A., Shah A.M. Targeted redox inhibition of protein phosphatase 1 by Nox4 regulates eIF2alpha-mediated stress signaling. EMBO J. 2016;35(3):319–334. doi: 10.15252/embj.201592394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H.S., Song M.C., Kwak I.H., Park T.J., Lim I.K. Constitutive induction of p-Erk1/2 accompanied by reduced activities of protein phosphatases 1 and 2A and MKP3 due to reactive oxygen species during cellular senescence. J. Biol. Chem. 2003;278(39):37497–37510. doi: 10.1074/jbc.M211739200. [DOI] [PubMed] [Google Scholar]
- 31.Cohen P.T. Protein phosphatase 1--targeted in many directions. J. Cell Sci. 2002;115(Pt 2):241–256. doi: 10.1242/jcs.115.2.241. [DOI] [PubMed] [Google Scholar]
- 32.Price M.E., Pavlik J.A., Liu M., Ding S.J., Wyatt T.A., Sisson J.H. Alcohol drives S-nitrosylation and redox activation of protein phosphatase 1, causing bovine airway cilia dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;312(3):L432–L439. doi: 10.1152/ajplung.00513.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong L.B., Park C.L., Yeates D.B. Neuropeptide Y inhibits ciliary beat frequency in human ciliated cells via nPKC, independently of PKA. Am. J. Physiol. 1998;275(2 Pt 1):C440–C448. doi: 10.1152/ajpcell.1998.275.2.C440. [DOI] [PubMed] [Google Scholar]
- 34.Bailey K.L., Bonasera S.J., Wilderdyke M., Hanisch B.W., Pavlik J.A., DeVasure J., Robinson J.E., Sisson J.H., Wyatt T.A. Aging causes a slowing in ciliary beat frequency, mediated by PKCepsilon. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306(6):L584–L589. doi: 10.1152/ajplung.00175.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyatt T.A., Sisson J.H., Allen-Gipson D.S., McCaskill M.L., Boten J.A., DeVasure J.M., Bailey K.L., Poole J.A. Co-exposure to cigarette smoke and alcohol decreases airway epithelial cell cilia beating in a protein kinase Cepsilon-dependent manner. Am. J. Pathol. 2012;181(2):431–440. doi: 10.1016/j.ajpath.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salathe M., Pratt M.M., Wanner A. Protein kinase C-dependent phosphorylation of a ciliary membrane protein and inhibition of ciliary beating. J. Cell Sci. 1993;106(Pt 4):1211–1220. doi: 10.1242/jcs.106.4.1211. (Pt 4) [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi K., Salathe M., Pratt M.M., Cartagena N.J., Soloni F., Seybold Z.V., Wanner A. Mechanism of hydrogen peroxide-induced inhibition of sheep airway cilia. Am. J. Respir. Cell Mol. Biol. 1992;6(6):667–673. doi: 10.1165/ajrcmb/6.6.667. [DOI] [PubMed] [Google Scholar]
- 38.Simet S.M., Sisson J.H., Pavlik J.A., Devasure J.M., Boyer C., Liu X., Kawasaki S., Sharp J.G., Rennard S.I., Wyatt T.A. Long-term cigarette smoke exposure in a mouse model of ciliated epithelial cell function. Am. J. Respir. Cell Mol. Biol. 2010;43(6):635–640. doi: 10.1165/rcmb.2009-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sisson J.H., Papi A., Beckmann J.D., Leise K.L., Wisecarver J., Brodersen B.W., Kelling C.L., Spurzem J.R., Rennard S.I. Smoke and viral infection cause cilia loss detectable by bronchoalveolar lavage cytology and dynein ELISA. Am. J. Respir. Crit. Care Med. 1994;149(1):205–213. doi: 10.1164/ajrccm.149.1.8111584. [DOI] [PubMed] [Google Scholar]
- 40.Wyatt T.A., Gentry-Nielsen M.J., Pavlik J.A., Sisson J.H. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin. Exp. Res. 2004;28(7):998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee R.J., Workman A.D., Carey R.M., Chen B., Rosen P.L., Doghramji L., Adappa N.D., Palmer J.N., Kennedy D.W., Cohen N.A. Fungal aflatoxins reduce respiratory mucosal ciliary function. Sci. Rep. 2016;6:33221. doi: 10.1038/srep33221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberg S.F. Mechanisms for redox-regulation of protein kinase C. Front. Pharmacol. 2015;6:128. doi: 10.3389/fphar.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopalakrishna R., Anderson W.B. Reversible oxidative activation and inactivation of protein kinase C by the mitogen/tumor promoter periodate. Arch. Biochem. Biophys. 1991;285(2):382–387. doi: 10.1016/0003-9861(91)90377-u. [DOI] [PubMed] [Google Scholar]
- 44.Ohmori S., Shirai Y., Sakai N., Fujii M., Konishi H., Kikkawa U., Saito N. Three distinct mechanisms for translocation and activation of the delta subspecies of protein kinase C. Mol. Cell Biol. 1998;18(9):5263–5271. doi: 10.1128/mcb.18.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elam C.A., Wirschell M., Yamamoto R., Fox L.A., York K., Kamiya R., Dutcher S.K., Sale W.S. An axonemal PP2A B-subunit is required for PP2A localization and flagellar motility. Cytoskeleton (Hoboken) 2011;68(7):363–372. doi: 10.1002/cm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kultgen P.L., Byrd S.K., Ostrowski L.E., Milgram S.L. Characterization of an A-kinase anchoring protein in human ciliary axonemes. Mol. Biol. Cell. 2002;13(12):4156–4166. doi: 10.1091/mbc.E02-07-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simet S.M., Pavlik J.A., Sisson J.H. Proteomic analysis of bovine axonemes exposed to acute alcohol: role of endothelial nitric oxide synthase and heat shock protein 90 in cilia stimulation. Alcohol Clin. Exp. Res. 2013;37(4):609–615. doi: 10.1111/acer.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Godoy J.R., Funke M., Ackermann W., Haunhorst P., Oesteritz S., Capani F., Elsasser H.P., Lillig C.H. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim. Biophys. Acta. 2011;1810(1):2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Dammeyer P., Arner E.S. Human Protein Atlas of redox systems - what can be learnt? Biochim. Biophys. Acta. 2011;1810(1):111–138. doi: 10.1016/j.bbagen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marletta M.A. Nitric oxide synthase: function and mechanism. Adv. Exp. Med. Biol. 1993;338:281–284. [PubMed] [Google Scholar]
- 52.Munakata M. Pulmonary nitric oxide synthase isoform expression and their functional significance. Nihon Rinsho. 1996;54(2):358–363. [PubMed] [Google Scholar]
- 53.Nathan C., Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 54.Lane P., Gross S.S. Cell signaling by nitric oxide. Semin. Nephrol. 1999;19(3):215–229. [PubMed] [Google Scholar]
- 55.Kuzkaya N., Weissmann N., Harrison D.G., Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J. Biol. Chem. 2003;278(25):22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 56.Botti H., Moller M.N., Steinmann D., Nauser T., Koppenol W.H., Denicola A., Radi R. Distance-dependent diffusion-controlled reaction of *NO and O2*- at chemical equilibrium with ONOO- J. Phys. Chem. B. 2010;114(49):16584–16593. doi: 10.1021/jp105606b. [DOI] [PubMed] [Google Scholar]
- 57.Koppenol W.H. NO nomenclature? Nitric Oxide. 2002;6(1):96–98. doi: 10.1006/niox.2001.0406. [DOI] [PubMed] [Google Scholar]
- 58.Sisson J.H. Ethanol stimulates apparent nitric oxide-dependent ciliary beat frequency in bovine airway epithelial cells. Am. J. Physiol. 1995;268(4 Pt 1):L596–L600. doi: 10.1152/ajplung.1995.268.4.L596. [DOI] [PubMed] [Google Scholar]
- 59.Shan J., Carbonara P., Karp N., Tulic M., Hamid Q., Eidelman D.H. Localization and distribution of NOS1 in murine airways. Nitric Oxide. 2007;17(1):25–32. doi: 10.1016/j.niox.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Ricciardolo F.L., Timmers M.C., Geppetti P., van Schadewijk A., Brahim J.J., Sont J.K., de Gouw H.W., Hiemstra P.S., van Krieken J.H., Sterk P.J. Allergen-induced impairment of bronchoprotective nitric oxide synthesis in asthma. J. Allergy Clin. Immunol. 2001;108(2):198–204. doi: 10.1067/mai.2001.116572. [DOI] [PubMed] [Google Scholar]
- 61.Forstermann U., Schmidt H.H., Kohlhaas K.L., Murad F. Induced RAW 264.7 macrophages express soluble and particulate nitric oxide synthase: inhibition by transforming growth factor-beta. Eur. J. Pharmacol. 1992;225(2):161–165. doi: 10.1016/0922-4106(92)90096-e. [DOI] [PubMed] [Google Scholar]
- 62.Guo F.H., Erzurum S.C. Characterization of inducible nitric oxide synthase expression in human airway epithelium. Environ. Health Perspect. 1998;106(Suppl 5):1119–1124. doi: 10.1289/ehp.98106s51119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J.H., Takeno S., Osada R., Ueda T., Yajin K. Modulation of ciliary activity by tumor necrosis factor-alpha in cultured sinus epithelial cells. Possible roles of nitric oxide. Hiroshima J. Med. Sci. 2000;49(1):49–55. [PubMed] [Google Scholar]
- 64.Xue C., Botkin S.J., Johns R.A. Localization of endothelial NOS at the basal microtubule membrane in ciliated epithelium of rat lung. J. Histochem. Cytochem. 1996;44(5):463–471. doi: 10.1177/44.5.8627003. [DOI] [PubMed] [Google Scholar]
- 65.Zhan X., Li D., Johns R.A. Immunohistochemical evidence for the NO cGMP signaling pathway in respiratory ciliated epithelia of rat. J. Histochem. Cytochem. 1999;47(11):1369–1374. doi: 10.1177/002215549904701103. [DOI] [PubMed] [Google Scholar]
- 66.Vignais P.V. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell. Mol. Life Sci. 2002;59(9):1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 68.Shao M.X., Nadel J.A. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 2005;102(3):767–772. doi: 10.1073/pnas.0408932102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joo J.H., Ryu J.H., Kim C.H., Kim H.J., Suh M.S., Kim J.O., Chung S.Y., Lee S.N., Kim H.M., Bae Y.S., Yoon J.H. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxidants Redox Signal. 2012;16(1):57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- 70.Krick S., Wang J., St-Pierre M., Gonzalez C., Dahl G., Salathe M. Dual oxidase 2 (Duox2) regulates pannexin 1-mediated ATP release in primary human airway epithelial cells via changes in intracellular pH and not H2O2 production. J. Biol. Chem. 2016;291(12):6423–6432. doi: 10.1074/jbc.M115.664854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim H.J., Park Y.D., Moon U.Y., Kim J.H., Jeon J.H., Lee J.G., Bae Y.S., Yoon J.H. The role of Nox4 in oxidative stress-induced MUC5AC overexpression in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2008;39(5):598–609. doi: 10.1165/rcmb.2007-0262OC. [DOI] [PubMed] [Google Scholar]
- 72.Fink K., Duval A., Martel A., Soucy-Faulkner A., Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-kappaB in airway epithelial cells. J. Immunol. 2008;180(10):6911–6922. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- 73.Heppner D.E., Hristova M., Dustin C.M., Danyal K., Habibovic A., van der Vliet A. The NADPH oxidases DUOX1 and NOX2 play distinct roles in redox regulation of epidermal growth factor receptor signaling. J. Biol. Chem. 2016;291(44):23282–23293. doi: 10.1074/jbc.M116.749028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwarzer C., Machen T.E., Illek B., Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J. Biol. Chem. 2004;279(35):36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 75.Forteza R., Salathe M., Miot F., Forteza R., Conner G.E. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2005;32(5):462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 76.Wan W.Y., Hollins F., Haste L., Woodman L., Hirst R.A., Bolton S., Gomez E., Sutcliffe A., Desai D., Chachi L., Mistry V., Szyndralewiez C., Wardlaw A., Saunders R., O'Callaghan C., Andrew P.W., Brightling C.E. NADPH oxidase-4 overexpression is associated with epithelial ciliary dysfunction in neutrophilic asthma. Chest. 2016;149(6):1445–1459. doi: 10.1016/j.chest.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murphy M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mailloux R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. doi: 10.1016/j.redox.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stanek A., Brambrink A.M., Latorre F., Bender B., Kleemann P.P. Effects of normobaric oxygen on ciliary beat frequency of human respiratory epithelium. Br. J. Anaesth. 1998;80(5):660–664. doi: 10.1093/bja/80.5.660. [DOI] [PubMed] [Google Scholar]
- 80.Rankin H.V., Moody A.J., Moate R.M., Macnaughton P.D., Rahamim J., Smith M.E., Sneyd J.R. Elevated oxygen fraction reduces cilial abundance in explanted human bronchial tissue. Ultrastruct. Pathol. 2007;31(5):339–346. doi: 10.1080/01913120701643686. [DOI] [PubMed] [Google Scholar]
- 81.Kay R.J., Moate R.M., Bray I., Sneyd J.R., Langton J.A. Cultured rat trachea as a model for the study of ciliary abundance: the effect of oxygen. Anesthesiology. 2002;97(1):275–277. doi: 10.1097/00000542-200207000-00039. [DOI] [PubMed] [Google Scholar]
- 82.Al-Shmgani H.S., Moate R.M., Sneyd J.R., Macnaughton P.D., Moody A.J. Hyperoxia-induced ciliary loss and oxidative damage in an in vitro bovine model: the protective role of antioxidant vitamins E and C. Biochem. Biophys. Res. Commun. 2012;429(3–4):191–196. doi: 10.1016/j.bbrc.2012.10.113. [DOI] [PubMed] [Google Scholar]
- 83.Feldman C., Anderson R., Kanthakumar K., Vargas A., Cole P.J., Wilson R. Oxidant-mediated ciliary dysfunction in human respiratory epithelium. Free Radic. Biol. Med. 1994;17(1):1–10. doi: 10.1016/0891-5849(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 84.Yoshitsugu M., Matsunaga S., Hanamure Y., Rautiainen M., Ueno K., Miyanohara T., Furuta S., Fukuda K., Ohyama M. Effects of oxygen radicals on ciliary motility in cultured human respiratory epithelial cells. Auris Nasus Larynx. 1995;22(3):178–185. doi: 10.1016/s0385-8146(12)80056-3. [DOI] [PubMed] [Google Scholar]
- 85.Min Y.G., Ohyama M., Lee K.S., Rhee C.S., Oh S.H., Sung M.W., Yun J.B., Jung I.H. Effects of free radicals on ciliary movement in the human nasal epithelial cells. Auris Nasus Larynx. 1999;26(2):159–163. doi: 10.1016/s0385-8146(98)00073-x. [DOI] [PubMed] [Google Scholar]
- 86.Honda A., Murayama R., Matsuda Y., Tsuji K., Sawahara T., Fukushima W., Hayashi T., Shimada A., Takano H. Effects of hydrogen peroxide on mucociliary transport in human airway epithelial cells. Toxicol. Mech. Methods. 2014;24(3):191–195. doi: 10.3109/15376516.2013.876136. [DOI] [PubMed] [Google Scholar]
- 87.Manzanares D., Monzon M.E., Savani R.C., Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am. J. Respir. Cell Mol. Biol. 2007;37(2):160–168. doi: 10.1165/rcmb.2006-0413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakamoto O., Iwama A., Amitani R., Takehara T., Yamaguchi N., Yamamoto T., Masuyama K., Yamanaka T., Ando M., Suda T. Role of macrophage-stimulating protein and its receptor, RON tyrosine kinase, in ciliary motility. J. Clin. Invest. 1997;99(4):701–709. doi: 10.1172/JCI119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knowles M.R., Zariwala M., Leigh M. Primary ciliary dyskinesia. Clin. Chest Med. 2016;37(3):449–461. doi: 10.1016/j.ccm.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tilley A.E., Walters M.S., Shaykhiev R., Crystal R.G. Cilia dysfunction in lung disease. Annu. Rev. Physiol. 2015;77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bonser L.R., Zlock L., Finkbeiner W., Erle D.J. Epithelial tethering of MUC5AC-rich mucus impairs mucociliary transport in asthma. J. Clin. Invest. 2016;126(6):2367–2371. doi: 10.1172/JCI84910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johnson S.M., McNally B.A., Ioannidis I., Flano E., Teng M.N., Oomens A.G., Walsh E.E., Peeples M.E. Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures. PLoS Pathog. 2015;11(12) doi: 10.1371/journal.ppat.1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeong K.I., Piepenhagen P.A., Kishko M., DiNapoli J.M., Groppo R.P., Zhang L., Almond J., Kleanthous H., Delagrave S., Parrington M. CX3CR1 Is Expressed in Differentiated Human Ciliated Airway Cells and Co-Localizes with Respiratory Syncytial Virus on Cilia in a G Protein-Dependent Manner. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang L., Peeples M.E., Boucher R.C., Collins P.L., Pickles R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002;76(11):5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smith C.M., Kulkarni H., Radhakrishnan P., Rutman A., Bankart M.J., Williams G., Hirst R.A., Easton A.J., Andrew P.W., O'Callaghan C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur. Respir. J. 2014;43(2):485–496. doi: 10.1183/09031936.00205312. [DOI] [PubMed] [Google Scholar]
- 96.Tristram D.A., Hicks W., Jr., Hard R. Respiratory syncytial virus and human bronchial epithelium. Arch. Otolaryngol. Head Neck Surg. 1998;124(7):777–783. doi: 10.1001/archotol.124.7.777. [DOI] [PubMed] [Google Scholar]
- 97.Henderson F.W., Hu S.C., Collier A.M. Pathogenesis of respiratory syncytial virus infection in ferret and fetal human tracheas in organ culture. Am. Rev. Respir. Dis. 1978;118(1):29–37. doi: 10.1164/arrd.1978.118.1.29. [DOI] [PubMed] [Google Scholar]
- 98.Hosakote Y.M., Jantzi P.D., Esham D.L., Spratt H., Kurosky A., Casola A., Garofalo R.P. Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am. J. Respir. Crit. Care Med. 2011;183(11):1550–1560. doi: 10.1164/rccm.201010-1755OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Komaravelli N., Tian B., Ivanciuc T., Mautemps N., Brasier A.R., Garofalo R.P., Casola A. Respiratory syncytial virus infection down-regulates antioxidant enzyme expression by triggering deacetylation-proteasomal degradation of Nrf2. Free Radic. Biol. Med. 2015;88(Pt B):391–403. doi: 10.1016/j.freeradbiomed.2015.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hosakote Y.M., Liu T., Castro S.M., Garofalo R.P., Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009;41(3):348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cho H.Y., Imani F., Miller-DeGraff L., Walters D., Melendi G.A., Yamamoto M., Polack F.P., Kleeberger S.R. Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 2009;179(2):138–150. doi: 10.1164/rccm.200804-535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mata M., Sarrion I., Armengot M., Carda C., Martinez I., Melero J.A., Cortijo J. Respiratory syncytial virus inhibits ciliagenesis in differentiated normal human bronchial epithelial cells: effectiveness of N-acetylcysteine. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0048037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jackowski J.T., Szepfalusi Z., Wanner D.A., Seybold Z., Sielczak M.W., Lauredo I.T., Adams T., Abraham W.M., Wanner A. Effects of P. aeruginosa-derived bacterial products on tracheal ciliary function: role of O2 radicals. Am. J. Physiol. 1991;260(2 Pt 1):L61–L67. doi: 10.1152/ajplung.1991.260.2.L61. [DOI] [PubMed] [Google Scholar]
- 104.Wilson R., Sykes D.A., Watson D., Rutman A., Taylor G.W., Cole P.J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988;56(9):2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilson R., Roberts D., Cole P. Effect of bacterial products on human ciliary function in vitro. Thorax. 1985;40(2):125–131. doi: 10.1136/thx.40.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanthakumar K., Taylor G., Tsang K.W., Cundell D.R., Rutman A., Smith S., Jeffery P.K., Cole P.J., Wilson R. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect. Immun. 1993;61(7):2848–2853. doi: 10.1128/iai.61.7.2848-2853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdalla M.Y., Hoke T., Seravalli J., Switzer B.L., Bavitz M., Fliege J.D., Murphy P.J., Britigan B.E. Pseudomonas Quinolone signal (PQS) induces oxidative stress and inhibits heme oxygenase-1 expression in lung epithelial cells. Infect. Immun. 2017;85(9) doi: 10.1128/IAI.00176-17. pii: e00176-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rada B., Leto T.L. Characterization of hydrogen peroxide production by Duox in bronchial epithelial cells exposed to Pseudomonas aeruginosa. FEBS Lett. 2010;584(5):917–922. doi: 10.1016/j.febslet.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Restrepo M.I., Mortensen E.M., Velez J.A., Frei C., Anzueto A. A comparative study of community-acquired pneumonia patients admitted to the ward and the ICU. Chest. 2008;133(3):610–617. doi: 10.1378/chest.07-1456. [DOI] [PubMed] [Google Scholar]
- 110.Spellerberg B., Cundell D.R., Sandros J., Pearce B.J., Idanpaan-Heikkila I., Rosenow C., Masure H.R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 1996;19(4):803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 111.Berry A.M., Yother J., Briles D.E., Hansman D., Paton J.C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 1989;57(7):2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hirst R.A., Sikand K.S., Rutman A., Mitchell T.J., Andrew P.W., O'Callaghan C. Relative roles of pneumolysin and hydrogen peroxide from Streptococcus pneumoniae in inhibition of ependymal ciliary beat frequency. Infect. Immun. 2000;68(3):1557–1562. doi: 10.1128/iai.68.3.1557-1562.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feldman C., Anderson R., Cockeran R., Mitchell T., Cole P., Wilson R. The effects of pneumolysin and hydrogen peroxide, alone and in combination, on human ciliated epithelium in vitro. Respir. Med. 2002;96(8):580–585. doi: 10.1053/rmed.2002.1316. [DOI] [PubMed] [Google Scholar]
- 114.Bello S., Menendez R., Antoni T., Reyes S., Zalacain R., Capelastegui A., Aspa J., Borderias L., Martin-Villasclaras J.J., Alfageme I., Rodriguez de Castro F., Rello J., Luis M., Ruiz-Manzano J. Tobacco smoking increases the risk for death from pneumococcal pneumonia. Chest. 2014;146(4):1029–1037. doi: 10.1378/chest.13-2853. [DOI] [PubMed] [Google Scholar]
- 115.Seeman J.I., Dixon M., Haussmann H.J. Acetaldehyde in mainstream tobacco smoke: formation and occurrence in smoke and bioavailability in the smoker. Chem. Res. Toxicol. 2002;15(11):1331–1350. doi: 10.1021/tx020069f. [DOI] [PubMed] [Google Scholar]
- 116.Chepiga T.A., Morton M.J., Murphy P.A., Avalos J.T., Bombick B.R., Doolittle D.J., Borgerding M.F., Swauger J.E. A comparison of the mainstream smoke chemistry and mutagenicity of a representative sample of the US cigarette market with two Kentucky reference cigarettes (K1R4F and K1R5F) Food Chem. Toxicol. 2000;38(10):949–962. doi: 10.1016/s0278-6915(00)00086-7. [DOI] [PubMed] [Google Scholar]
- 117.Kuipers I., Bracke K.R., Brusselle G.G., Wouters E.F., Reynaert N.L. Smoke decreases reversible oxidations S-glutathionylation and S-nitrosylation in mice. Free Radic. Res. 2012;46(2):164–173. doi: 10.3109/10715762.2011.647011. [DOI] [PubMed] [Google Scholar]
- 118.CHANG S.C. Microscopic properties of whole mounts and sections of human bronchial epithelium of smokers and nonsmokers. Cancer. 1957;10(6):1246–1262. doi: 10.1002/1097-0142(195711/12)10:6<1246::aid-cncr2820100623>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 119.Ballenger J.J. Experimental effect of cigarette smoke on human respiratory cilia. N. Engl. J. Med. 1960;263:832–835. doi: 10.1056/NEJM196010272631704. [DOI] [PubMed] [Google Scholar]
- 120.Tian Z., Zhang H., Dixon J., Traphagen N., Wyatt T.A., Kharbanda K., Simet Chadwick S., Kolliputi N., Allen-Gipson D.S. Cigarette smoke impairs A2A adenosine receptor mediated wound repair through up-regulation of duox-1 expression. Sci. Rep. 2017;7:44405. doi: 10.1038/srep44405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Allen-Gipson D.S., Romberger D.J., Forget M.A., May K.L., Sisson J.H., Wyatt T.A. IL-8 inhibits isoproterenol-stimulated ciliary beat frequency in bovine bronchial epithelial cells. J. Aerosol Med. 2004;17(2):107–115. doi: 10.1089/0894268041457138. [DOI] [PubMed] [Google Scholar]
- 122.Yeligar S.M., Chen M.M., Kovacs E.J., Sisson J.H., Burnham E.L., Brown L.A.S. Alcohol and lung injury and immunity. Alcohol. 2016;55:51–59. doi: 10.1016/j.alcohol.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boe D.M., Vandivier R.W., Burnham E.L., Moss M. Alcohol abuse and pulmonary disease. J. Leukoc. Biol. 2009;86(5):1097–1104. doi: 10.1189/jlb.0209087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Guidot D.M., Brown L.A. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin. Exp. Res. 2000;24(7):1070–1076. [PubMed] [Google Scholar]
- 125.Lois M., Brown L.A., Moss I.M., Roman J., Guidot D.M. Ethanol ingestion increases activation of matrix metalloproteinases in rat lungs during acute endotoxemia. Am. J. Respir. Crit. Care Med. 1999;160(4):1354–1360. doi: 10.1164/ajrccm.160.4.9811060. [DOI] [PubMed] [Google Scholar]
- 126.Polikandriotis J.A., Rupnow H.L., Elms S.C., Clempus R.E., Campbell D.J., Sutliff R.L., Brown L.A., Guidot D.M., Hart C.M. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am. J. Respir. Cell Mol. Biol. 2006;34(3):314–319. doi: 10.1165/rcmb.2005-0320OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yeh M.Y., Burnham E.L., Moss M., Brown L.A. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am. J. Respir. Crit. Care Med. 2007;176(3):270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wyatt T.A., Sisson J.H. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281(3):L575–L581. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
- 129.Wyatt T.A., Forget M.A., Sisson J.H. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am. J. Pathol. 2003;163(3):1157–1166. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoshimura M., Tabakoff B. Selective effects of ethanol on the generation of cAMP by particular members of the adenylyl cyclase family. Alcohol Clin. Exp. Res. 1995;19(6):1435–1440. doi: 10.1111/j.1530-0277.1995.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 131.Sisson J.H., Pavlik J.A., Wyatt T.A. Alcohol stimulates ciliary motility of isolated airway axonemes through a nitric oxide, cyclase, and cyclic nucleotide-dependent kinase mechanism. Alcohol Clin. Exp. Res. 2009;33(4):610–616. doi: 10.1111/j.1530-0277.2008.00875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Price M.E., Pavlik J.A., Sisson J.H., Wyatt T.A. Inhibition of protein phosphatase 1 reverses alcohol-induced ciliary dysfunction. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;308(6):L577–L585. doi: 10.1152/ajplung.00336.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simet S.M., Pavlik J.A., Sisson J.H. Dietary antioxidants prevent alcohol-induced ciliary dysfunction. Alcohol. 2013;47(8):629–635. doi: 10.1016/j.alcohol.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cho D.Y., Skinner D., Zhang S., Fortenberry J., Sorscher E.J., Dean N.R., Woodworth B.A. Cystic fibrosis transmembrane conductance regulator activation by the solvent ethanol: implications for topical drug delivery. Int. Forum Allergy Rhinol. 2016;6(2):178–184. doi: 10.1002/alr.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith C.M., Radhakrishnan P., Sikand K., O'Callaghan C. The effect of ethanol and acetaldehyde on brain ependymal and respiratory ciliary beat frequency. Cilia. 2013;2(1) doi: 10.1186/2046-2530-2-5. 5-2530-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kershaw C.D., Guidot D.M. Alcoholic lung disease. Alcohol Res. Health. 2008;31(1):66–75. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.