Abstract
In sickle cell disease (SCD), recurrent painful vasoocclusive crisis are likely caused by repeated episodes of hypoxia and reoxygenation. The sickle erythrocyte (SSRBC) adhesion plays an active role in vasoocclusion. However, the effect of prolonged reoxygenation after hypoxic stress on the molecular mechanisms in SSRBCs involved in onset of episodic vasoocclusion remain unclear. Exposure of human SSRBCs to hypoxia followed by 2 h reoxygenation, increased reactive oxygen species (ROS) production. Using specific pharmacological inhibitors, we show that excess ROS production in both reticulocytes and mature SSRBCs is regulated by NADPH oxidases (NOXs), the mitogen-activated protein kinase (ERK1/2), and G-protein coupled-receptor kinase 2 (GRK2). Consequently, SSRBC ROS create an intracellular positive feedback loop with ERK1/2 and GRK2 to mediate SSRBC adhesion to endothelium in vitro, and vasoocclusion in a mouse model of vasoocclusion in vivo. Importantly, reducing ROS levels in SSRBCs with redox-active manganese (Mn) porphyrins, commonly known as mimics of superoxide dismutase (SOD), disrupted the cycle created by ROS by affecting NOX and GRK2 activities and ERK1/2 phosphorylation, thus abrogating RBC-endothelial interactions. Inhibition adhesion assays show that LW (ICAM-4, CD242) blood group glycoprotein and CD44 are the RBC adhesion molecules mediating endothelial binding. Conversely, hypoxia/reoxygenation of normal RBCs failed to activate this feedback loop, and adhesion. These findings provide novel insights into the pathophysiological significance of the deleterious cycle created by NOX-dependent ROS, GRK2 and ERK1/2 within SSRBCs activated by hypoxia/reoxygenation, and involved in SSRBC adhesion and vasoocclusion. Thus, this loop in SSRBCs, which can be disrupted by Mn porphyrins, likely drives the profound SCD vasculopathy, and may point to new therapeutic targets to prevent chronic vasoocclusive events.
Keywords: Sickle red blood cell, Adhesion, NADPH oxidases, Extracellular signal-regulated kinase-1/2 (ERK1/2), G protein-coupled receptor kinase 2 (GRK2), Manganese (Mn) porphyrins, Hypoxia/reoxygenation
1. Introduction
Sickle cell disease (SCD) is a monogenetic genetic disorder due to a single mutation that occurs in the globin β-chain, resulting in the formation of hemoglobin S (HbS). In SCD, vascular occlusion, and its main clinical consequence, the painful vasoocclusive crises, are the cause of complex manifestations that include damage to many vital organs, and chronic function impairment, followed by organ failure [1], [2], [3], [4]. Repeated events of painful vasoocclusive crisis appear to be caused by recurrent episodes of hypoxia, involving polymerization of deoxygenated HbS and subsequent red blood cell (RBC) sickling under hypoxic conditions [5], [6]. RBCs in SCD patients are heterogeneous, with a variable number of unusually dense cells, including irreversibly sickled cells (ISCs) [7]. Dense cells were thought to initiate vasoocclusion by being trapped in small blood vessels due to their rigidity when oxygenated, and increased tendency to sickle at low oxygen tension [8], [9]. Yet, experimental and clinical evidence shows no correlation between the disease severity and the percentage of circulating dense cells [10]. In fact, sickle RBC (SSRBC) adhesion to endothelium has been postulated to be a major candidate in the initiation of vasoocclusion, prolonging SSRBC transit times in the microcirculation, which leads to HbS polymerization and sickling, thus depriving the cells from traversing narrow capillaries [3], [11], [12].
Recent in vitro studies under hypoxic conditions, have demonstrated the simultaneous and synergistic effects of adhesion and polymerization of deoxygenated HbS in the human SSRBCs on the mechanisms underlying vasoocclusive pain crisis [13]. However, abnormal adherence of SSRBCs with the endothelium can occur even under normoxic conditions and for different RBC shapes [3], and under various flow conditions [14]. In addition, RBC sickling has been shown to be completely reversed after the first 50 min reoxygenation period [15]. Therefore, the question remains of the possible molecular mechanisms in SSRBCs precipitating the next vasoocclusive episode, when deoxygenated RBCs trapped in occlusions are being re-oxygenated during blood restoration in vivo.
It is well established that HbS has the ability to undergo autoxidation in the presence of oxygen to produce reactive oxygen species (ROS), including superoxide (O2•−) and hydroxyl radicals (•OH) within SSRBCs [16], [17], at a rate higher than that produced by normal hemoglobin (HbA) [16], [18]. As such, the ensuing oxidative stress in SSRBCs can lead to RBC membrane rigidity and mechanical instability that may contribute to hemolysis [19]. Studies have also evidenced the existence of an enzymatic source, the NADPH oxidases (NOXs), of ROS production in SSRBCs, and shown that NOX activity can be triggered by endothelin-1 and tumor growth factor (TGF)-β1 [20]. Nevertheless, the contribution of NOX enzymes in SSRBCs exposed to hypoxia followed by reoxygenation, to endothelial adhesion and recurrent vasoocclusion [21], [22], [23], [24] may be at play, and deserve further exploration. The present studies were designed to elucidate the role of NOX enzymes and the exact signaling pathways activated by NOXs within human SSRBCs both under normoxia (ambient air) and after prolonged (2 h) reoxygenation of deoxygenated cells, a time during which sickling is well reversed [15], and highlight the processes underlying the onset of occlusion. Identification of various molecular factors in SSRBCs that contribute to episodic vasoocclusion may lead to identification of critical mechanisms unraveling SCD pathophysiology. This study may also trigger new investigations into novel therapeutics to prevent/treat recurrent vasoocclusive events in SCD.
2. Materials and methods
2.1. Endothelial cells
Human dermal microvascular ECs (HMVECs-d) (Lonza, Walkersville, MD) were grown as monolayers in EBM2 medium (Clonetics, Walkersville, MD) supplemented with EGM2 (Clonetics) as previously described [22].
2.2. Antibodies
Antibodies (abs) used were purified immunoglobulin [Ig] unless otherwise noted. Monoclonal anti-human Abs included the following: anti-glycophorin C and A produced in our laboratory [25]; anti-LW (BS46, generously provided by Dr. Jean-Pierre Cartron, INSERM Unité 665, Paris, France) [26]; anti-CD47 (6H9)[27]; anti-CD44 (Sigma-Aldrich, St. Louis, MO); anti-3-nitrotyrosine (Santa Cruz biotechnology, CA). Polyclonal anti-human Abs included the following: anti-mitogen-activated protein kinase (MAPK) ERK1/2 (Upstate, Charlottesville, VA); anti-phospho-ERK1/2 (Cell Signaling Technology, Danvers, MA); and anti-G protein-coupled receptor kinase 2 (GRK2) (Santa Cruz Biotechnology). In all studies, Abs were used at saturating dilutions unless otherwise indicated.
2.3. Blood collection, RBC preparation and treatment
Blood samples obtained from human participants has been approved by Duke University's Institutional Review Board (IRB), and written informed consent has been obtained from the participants. Blood samples were collected from adult SCD patients homozygous for HbS, and from healthy adult donors. All SCD patients had not been transfused for at least three months and had not experienced acute vasoocclusive crises for three weeks, and 98% of the patients tested were on hydroxyurea. Blood samples were collected into citrate tubes. All RBCs were washed in PBS with Ca2+ and Mg2+ with removal of the plasma and buffy coat. Packed RBCs were analyzed for leukocyte and platelet contaminations using an Automated Hematology Analyzer K-1000 (Sysmex Corporation, Kobe, Japan).
Packed RBCs were exposed to ambient air (normoxia) or transient hypoxia at 8% oxygen (O2) for 2 h, followed by reoxygenation for 2 h at 37 °C by return to ambient air [Hypoxia/reoxygenation (H/R)], with one of the following reagents added during the reoxygenation period: 50 μM the specific NOX inhibitor apocynin (Sigma-Aldrich), 50 μM the NOX inhibitor diphenyleneiodonium (DPI) (a pan NOX inhibitor) (Sigma-Aldrich); 1 and 10 μM H2O2; 5 μM U0126 specific inhibitor for the mitogen-activated protein kinase kinase MEK1/2 (MEKI) (Calbiochem, La Jolla, CA). Sham-treated RBCs were incubated with the same buffer (PBS with Ca2+ and Mg2+) and vehicle, but without the active agent. Cells were then washed 5 times in PBS with Ca2+ and Mg2+ before different assays. Prior to adhesion studies, washed treated RBCs were fluorescence-labeled as described previously in detail [22], [23], [28].
Manganese (Mn) porphyrin-based potent superoxide dismutase (SOD) mimics, MnTE-2-PyP5+ (MnE, BMX-010, AEOL10113) and MnTnBuOE-2-PyP5+ (MnBuOE, BMX-001) were synthesized as reported [29], [30]. Aqueous solutions were used at 0.5 and 1 μM to treated RBCs during reoxygenation for in vitro and in vivo studies.
2.4. Mature SSRBC and SS reticulocyte separation
Separation of mature SSRBCs from SS reticulocytes was accomplished using anti-transferrin receptor mAb 5E9 and goat anti-mouse IgG-coated micro-bead affinity columns (MACS, Miltenyi Biotec, Inc, Auburn, CA), following the manufacturer's instructions.
2.5. Flow cytometric analysis
Transferrin receptor expression on SSRBC subpopulations was tested by flow cytometric analysis as previously described [31].
2.6. RBC ROS detection
To detect ROS, treated RBCs were resuspended at 0.125% hematocrit in PBS and incubated with CM-H2-DCFDA (Invitrogen, Carlsbad, CA) at 8 μM for 30 min at 37 °C. Samples were then washed once with warm PBS and analyze for mean fluorescence intensity of 2′,7′-dichlorofluorescein using a FACSCalibur flow cytometer (Becton–Dickinson). For each sample, a minimum of 100 000 RBCs were acquired and the data were analyzed using CellQuest software (Becton–Dickinson).
Additionally, H2O2 was determined. The treated RBCs were incubated with fluorimetric horseradish peroxidase-catalyzed oxidation of Amplex Red using Amplex Red H2O2/Peroxidase Assay Kit (Molecular Probes, Grand Island, NY) according to the manufacturer's instructions. Absorbance was determined at 560 nm at multiple time points to follow the kinetics of the reactions.
2.7. Western blot
Treated RBCs were lysed with hypotonic buffer (5 mM Na2HPO4 + 1 mM EDTA + 0.1% NaN3, pH 8) containing 2 mM phenylmethylsulphonyl fluoride (PMSF), phosphatase inhibitor cocktail (Sigma-Aldrich) and protease inhibitor cocktail (Sigma-Aldrich). Protein separation by polyacrylamide gel electrophoresis used 50 μg RBC membrane ghost proteins per lane. Western blots [32] using the appropriate Ab were performed. Bands were analyzed densitometrically using ImageJ software downloaded from the NIH website. Phospho-kinase data were normalized according to total protein expression and are presented as fold change in kinase phosphorylation.
2.8. In vitro adhesion assays
Slides coated with normal HMVECs-d were fitted into a variable height flow chamber and tested for their ability to support adhesion of activated RBCs as described in “Supplemental Material and Methods”.
For adhesion inhibition assays, washed treated SSRBCs were incubated for 30 min with 20 μg/ml RBC-reactive IgG, or 40 μg/ml annexin V before additional washing, followed by adhesion assays.
2.9. Phosphatidylserine exposure
Phosphatidylserine (PS) exposure on treated SSRBCs was measured using annexin V binding by flow cytometry. Cells were adjusted to 1 × 106/ml in a final volume of 500 μl binding buffer, then incubated for 30 min at room temperature in the dark with 5 μl of GFP-conjugated annexin V. One hundred thousand events per sample were acquired and tested by flow cytometric analysis.
Mice. Animal work was approved by the Institutional Animal Care and Use Committee (IACUC) at Duke University. All animal experiments were carried out in strict accordance with and following the National Institutes of Health (NIH) guidelines and recommendations for the Care and Use of Laboratory Animals. The protocol was approved by the Committee on the Ethics of Animal Experiments of Duke University. All surgery was performed under anesthesia by intra-peritoneal injection of 100 mg/kg of ketamine (Abbott Laboratory, Chicago, IL) and 10 mg/kg of xylazine (Bayer, Shawnee Mission, KS), and all efforts were made to minimize suffering. Male and Female athymic homozygous nude (nu-/nu-) mice (Foxn1nu, formerly Hfh11nu), 8–12 weeks of age, were bred and housed at Duke University.
2.10. Window chamber surgery, RBC infusion and intravital microscopy
Dorsal skin-fold window chamber surgery and treated human RBC infusion were performed on anesthetized nude mice as described in detail in “Supplemental Material and Methods [23], [33], [34], [35], [36]. Briefly, washed, hypoxia/air exposed human RBCs were treated ex vivo with vehicle, 1 μM MnE, 1 μM MnBuOE, or 50 μM apocynin (300 μl, hematocrit [Hct] 50% in saline), then fluorescence-labeled with Dil. Cells were washed gain, then infused to anesthetized nude mice as described in detail in ”Supplemental Material and Methods.” Human RBC adhesion and blood flow dynamics were observed in subdermal vessels and recorded for at least 30 min using 10× and 20× magnifications.
Human RBC adhesion was quantified by measuring the fluorescence intensity [fluorescence unit (FU)] of adherent fluorescence-labeled RBCs on still images (20×) using ImageJ software downloaded from the National Institute of Health (NIH) website. A color, single-channel fluorescent image (using equal size images for each and for comparison of treatment conditions) was converted to “monochrome” for full control of how primary colors were converted correctly to grayscale. The image was then converted to a true 8-bit grayscale image suitable for fluorescence intensity quantification. Fluorescence intensity was quantified after two consecutive channel selections, in order to completely subtract both gray non-fluorescent areas and moving, dimly fluorescent RBCs, which appear less bright than adherent cells on still images. The values obtained from vessel segments analyzed in the accompanying videos were averaged among groups of animals (n = 5) to obtain mean FU and for statistical analysis. The percentages of vessels with normal blood flow, slow blood flow and no blood flow were also calculated by dividing of number of vessels (both small and large) with normal blood flow, slow blood flow, and no blood flow (occluded vessels) by the total number of vessels recorded, respectively, then averaged among groups of animals (n = 5).
2.11. Statistical analysis
Results using sham and treated RBCs were compared only with results using the same donor sample, because SSRBC adhesion varies among SCD patients [22]. Data were compared using parametric analyses (GraphPad Prism 5 Software, San Diego, CA), including repeated and non-repeated measures of analysis of variance (ANOVA). One-way ANOVA analyses were followed by Bonferroni corrections for multiple comparisons (multiplying the p value by the number of comparisons). A p value < 0.05 was considered significant.
3. Results
3.1. SSRBC NOX-dependent ROS production is regulated by ERK1/2 activation, a mechanism stimulated by hypoxia/reoxygenation
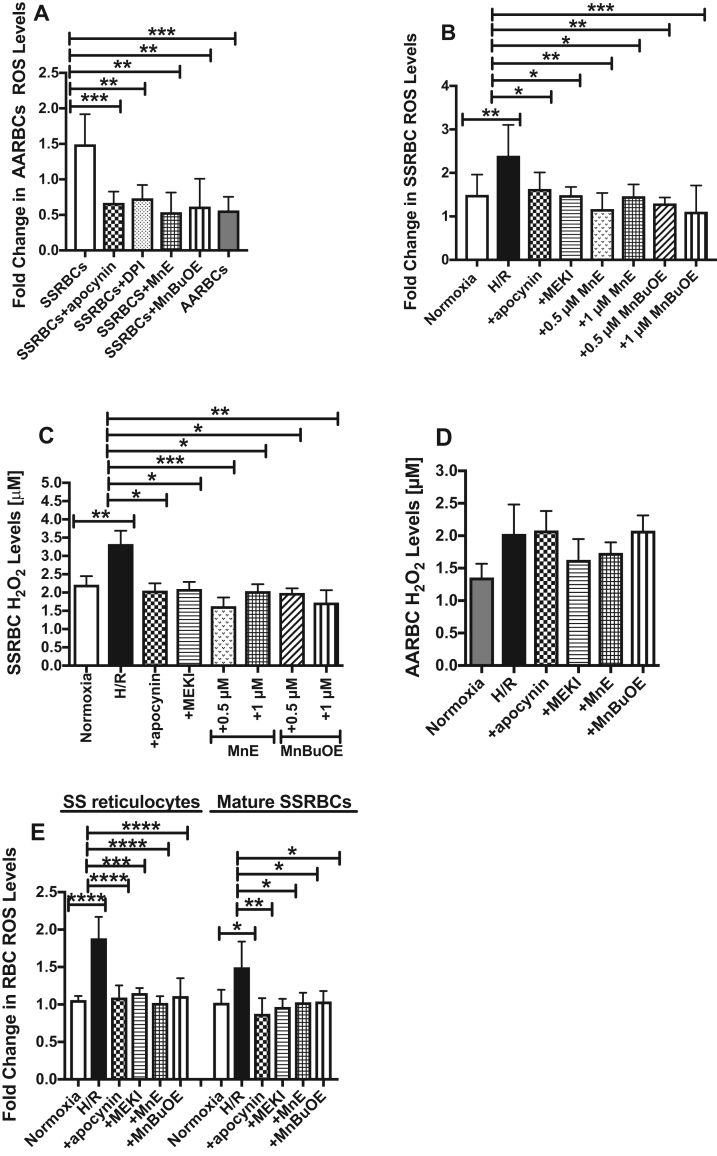
In SSRBCs, in addition to unstable HbS oxidation driving the excessive production of ROS [16], an enzymatic source of ROS production in SSRBCs involving the NOX enzymes also exist [20]. We first analyzed the levels of RBC ROS generated by NOX enzymes. SSRBCs have a distinctly elevated profile of ROS generation compared to normal RBCs (AARBCs) (p < 0.001; Fig. 1A). Pretreatment of SSRBCs with the NOX inhibitors, apocynin and DPI, significantly reduced ROS production to levels comparable to AARBC's levels (p < 0.01 for apocynin and DPI treatments; Fig. 1A), further confirming that at least a significant portion of ROS production in SSRBCs is due to NOX activity [20]. We have used Mn porphyrins commonly known as SOD mimics, MnE and MnBuOE as mechanistic tools. It has been reported that these compounds reduce the levels of ROS either directly by affecting NOX enzymes, or more likely via impacting cellular transcriptional activity and in turn the activity of NOXs [37], [38]. MnE and MnBuOE decreased SSRBC ROS levels to AARBC baseline levels (p < 0.01 for MnE and MnBuOE treatments; Fig. 1A), suggesting that SSRBC NOX activity may also be suppressed by these compounds. Our SSRBC preparations contained no platelets, and very low levels of contamination with leukocytes (0.2 ± 0.06 × 103/μl), present in only 10% of the samples tested. When similar numbers of isolated SCD patient leukocytes were examined for ROS generation, ROS was not detectable, demonstrating that the elevated ROS levels were in fact derived from SSRBCs.
Fig. 1.
H/R increased NOX-dependent ROS production in SSRBCs via the ERK1/2 signaling pathway. A. Comparison of ROS levels produced by NOX in SSRBCs vs. AARBCs. Compared with AARBCs, SSRBCs manifest higher levels of ROS measurements of DCFDA-derived signal. Treatment of SSRBCs (n = 9) with 50 μM the NOX inhibitors apocynin and DPI, and 1 μM the redox-active Mn porphyrins, MnE and MnBuOE, reduced ROS generation to levels similar to AARBC baseline levels (n = 4). p < 0.01 compared to untreated SSRBCs. B-E. H/R stimulates ROS production in all SSRBC populations, unseparated, SS reticulocytes, and mature SSRBCs, dependently of NOX and ERK1/2. SSRBCs (B and C), AARBCs (D), SS reticulocytes (E) and mature SSRBCs (E) were exposed to normoxia (B-E), H/R (B-E), or hypoxia then treated during reoxygenation period with 50 μM apocynin (B-E), 5 μM MEKI (B-E), 0.5 (B-E) or 1 μM MnE (B and C), or 0.5 (B-E) or 1 μM (B and C) MnBuOE. Cells were then washed and tested for the levels of ROS, and/or H2O2 using Amplex Red Hydrogen Peroxide/Peroxidase Assay kit. Prolonged H/R increased both ROS and H2O2 production in SSRBCs (n = 7), and ROS generation in SS reticulocytes (n = 4) and mature SSRBCs (n = 4) via activation of NOX and ERK1/2. In contrast, prolonged H/R failed to induce changes in H2O2 levels in AARBCs (n = 9). Error bars show SEM. p < 0.01 for SSRBCs exposed to H/R vs. normoxia; p < 0.05 compared to SSRBCs exposed to H/R.
HbS autoxidation leads to increased ROS generation inside the SSRBC in vitro [16], [39]. We next tested whether exposure of SSRBCs to hypoxia followed by the prolonged reoxygenation period, affects RBC NOX activity. We found that prolonged H/R increased SSRBC ROS production by 1.5–fold compared to cells exposed to normoxia (p < 0.01; Fig. 1B). ROS levels in H/R exposed SSRBCs were significantly reduced with the NOX inhibitor apocynin (p < 0.05), and U0126 MEKI (p < 0.05) (Fig. 1B), suggesting that H/R-induced excess SSRBC ROS are produced via activation of NOXs, and MEK1/2 and its downstream effector ERK1/2. Additionally, MnE and MnBuOE at 0.5 and 1 μM suppressed the effect of H/R from up-regulating SSRBC ROS production (p < 0.05 for MnE and MnBuOE; Fig. 1B). We also quantified the levels of H2O2 (derived primarily from NOXs) as affected by different treatments. The NOX inhibitor apocynin (p < 0.05), and U0126 MEKI (p < 0.05) were both able to decrease the levels of H2O2 in H/R exposed SSRBCs (Fig. 1C). MnE and MnBuOE at 0.5 and 1 μM also appear to directly negatively affect NOX activity in SSRBCs, as reflected by a decrease in SSRBC H2O2 levels (p < 0.05 for MnE and MnBuOE; Fig. 1C). In contrast, there was no significant change in AARBC H2O2 profiles after H/R exposure followed or not by the different pharmacological agents used to treat SSRBCs (Fig. 1D).
SS reticulocytes, 95% of which expressed the transferrin receptor, and mature SSRBCs both separated from packed SSRBCs, showed not statistically difference in ROS levels (Fig. 1E). H/R markedly increased ROS generation in both cell populations (p < 0.05 for SS reticulocytes, and p < 0.0001 for mature SSRBCs). H/R-induced increased ROS levels in SS reticulocytes and mature SSRBCs were reduced to baseline levels by the inhibitors of NOXs and MEK1/2, apocynin and U0126, respectively, and the Mn porphyrins, MnE and MnBuOE (p < 0.05 for all treatments; Fig. 1E). Together, these data suggest that H/R stimulates NOX activation in both immature and mature SSRBCs to generate ROS through ERK1/2 activation, a NOX stimulatory pathway deactivated by Mn porphyrins.
3.2. Hypoxia/reoxygenation-induced SSRBC NOX-dependent ROS generation acts in a positive feedback loop regulating ERK1/2 signaling
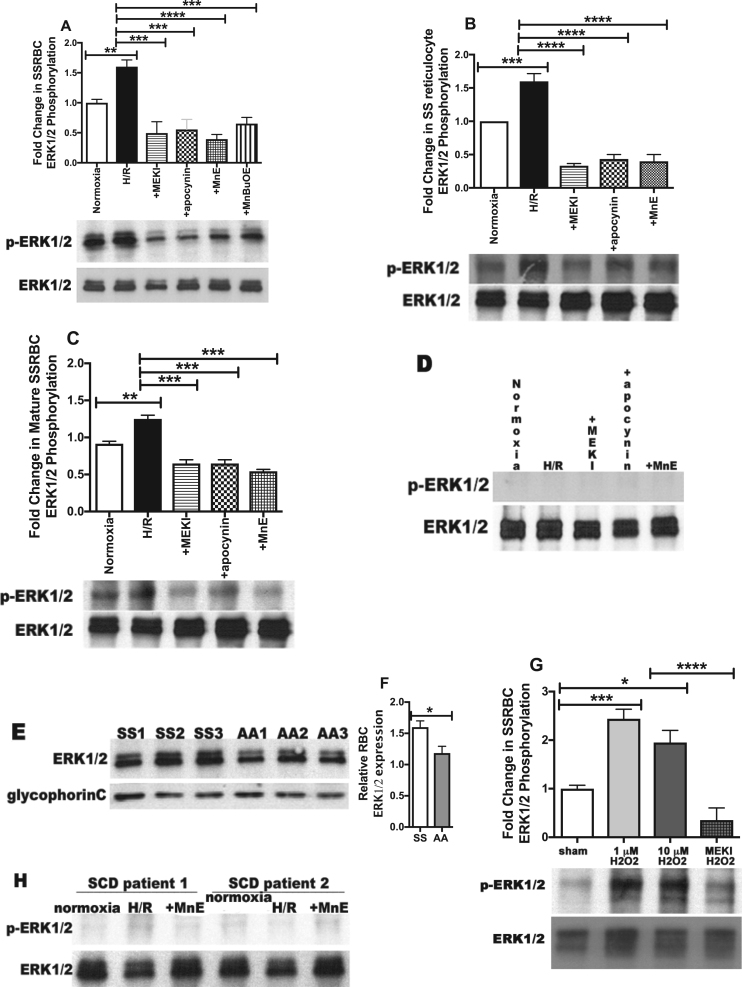
ERK1/2 signaling can up-regulate NOX activity in SSRBCs (Fig. 1). ROS can also activate signaling pathways in many cell types [40], [41]. Because ERK1/2 is activated at baseline in SSRBCs, but not in normal RBCs, and since activation of this kinase is required for adhesion of these sickle cells to endothelium, promoting vasoocclusion [21], [24], we hypothesized that increased SSRBC NOX-dependent ROS production by H/R can subsequently activate the ERK1/2 signaling cascade. SSRBC ERK1/2 is phosphorylated at baseline (Fig. 2A) [24]. H/R up-regulated SSRBC ERK1/2 phosphorylation by 1.62 ± 0.18-fold (p < 0.01). As expected, incubation of SSRBCs with the MEK1/2 inhibitor U0126, specifically inhibiting ERK1/2, repressed the effect of H/R on ERK1/2 phosphorylation, which decreased to levels below baseline levels (p < 0.001) (Fig. 2A). Interestingly, the NOX inhibitor apocynin also inhibited ERK1/2 phosphorylation enhanced by H/R (p < 0.001). Mn porphyrins, MnE and MnBuOE, had a significant and similar inhibitory effect on ERK1/2 phosphorylation to the effect of apocynin (p < 0.001) (Fig. 2A), as they actually in addition to deactivation of NOXs, also suppress phosphorylated ERK1/2 signaling [42]. Overall, this suggests that SSRBC NOX activation exert a positive feedback loop, consequently activating ERK1/2 signaling pathway, a loop stimulated by H/R, and disrupted with Mn porphyrins, likely by deactivating NOXs, and consequently ERK1/2, or both NOXs and ERK1/2.
Fig. 2.
H/R-induced increased endogenous NOX-dependent ROS levels acts in a positive feedback loop involving ERK1/2 activation in SSRBCs. A-G. SSRBCs [panels A (n = 4), and E-G (n = 3)], SS reticulocytes (n = 3; panel B) and mature SSRBCs (n = 3; panel C), AARBCs [panels D (n = 4) were exposed to normoxia (A-D), H/R (A-D), or hypoxia then incubated during reoxygenation with 5 μM the MEK inhibitor U0126 (MEKI) (A-D), 50 μM apocynin (A-D), 0.5 μM MnE (A-D), or 0.5 μM MnBuOE (A). E-G. SSRBCs [panels E-G, (n = 3)] and AARBCs [panels E-F (n = 3)] were sham-treated (E-G), or treated with 1 or 10 μM H2O2 (G), or 5 μM MEKI and 1 μM H2O2 (G). Fifty μg of membrane protein ghosts prepared from RBCs were used per lane. Western blots of protein ghosts were stained with antibodies against ERK1/2, and phosphorylated ERK1/2 (p-ERK1/2) under non-reducing conditions. Quantitative analysis of the data is presented as fold change in SSRBC ERK1/2 phosphorylation normalized according to total ERK1/2 expression; quantitation is given under “Material and Methods.” A-C. ERK1/2 is phosphorylated at baseline in SSRBCs, SS reticulocytes and mature SSRBCs, and undergoes increased phosphorylation by H/R via ROS-dependent NOX activation. p < 0.01 for SSRBCs exposed to H/R vs. cells exposed to normoxia; p < 0.001 compared to cells exposed to H/R. D. ERK1/2 in AARBCs is not phosphorylated and failed to undergo increased phosphorylation after H/R exposure. E-F. Quantitative analysis of the data presented as relative RBC ERK1/2 expression compared to AARBCs (panel F). ERK1/2 is expressed at higher levels in SSRBCs (SS1, SS2, SS3) compared to AARBCs (AA1, AA2, AA3) (p < 0.05 for SS vs AARBCs). G. Exogenous H2O2 up-regulates SSRBC ERK1/2 phosphorylation. p < 0.05 compared to sham-treated; p < 0.001 compared to cells treated with 1 μM H2O2. H. ERK1/2 activity in leukocytes contaminating SSRBC sample preparations. Leukocytes isolated from blood from SCD patients (n = 2) at numbers similar to those contaminating SSRBC samples were exposed to normoxia, H/R, or treated during reoxygenation period with 0.5 μM MnE. There was no detectable ERK1/2 activity in leukocyte preparations.
Furthermore, prolonged H/R enhanced ERK1/2 phosphorylation in both SS reticulocytes and mature SSRBCs (p < 0.01 for either cell type) (Figs. 2B and 2C). Up-regulation of ERK1/2 phosphorylation was significantly inhibited by the inhibitors of NOXs and MEK1/2, apocynin and U0126, respectively, and the Mn porphyrin MnE (p < 0.001 for both cell types). In contrast, ERK1/2 was not found to be phosphorylated in the AARBC samples examined, and failed to undergo phosphorylation after cell exposure to H/R (Fig. 2D). These normal cells expressed lower levels of ERK1/2 compared to the levels of ERK1/2 expression in SSRBC samples tested (p < 0.05, Figs. 2E and 2F) [24]. These data suggest that in contrast to AARBCs, ERK1/2 activity in SSRBCs is preserved regardless of cell age and is regulated by NOXs in both cell populations: immature and mature cells, and the levels of RBC ERK1/2 expression could at least in part affect kinase activity.
Furthermore, to determine the contribution of external ROS that may likely be generated by autoxidation of free HbS, released by hemolyzed cells, to SSRBC ERK1/2 phosphorylation, SSRBCs were incubated with H2O2 then washed prior to membrane ghost preparation. H2O2 at 1 and 10 μM increased SSRBC ERK1/2 phosphorylation (p < 0.01); an effect inhibited with U0126 MEK inhibitor (p < 0.001) (Fig. 2G). To exclude the contribution of contaminating leukocytes to SSRBC ERK1/2 activity, leukocytes isolated from SCD patients at numbers similar to those contaminating our SSRBC samples were tested, and showed no detectable ERK1/2 activity signal (Fig. 2H).
3.3. Increased SSRBC NOX-dependent ROS production affects activation of GRK2
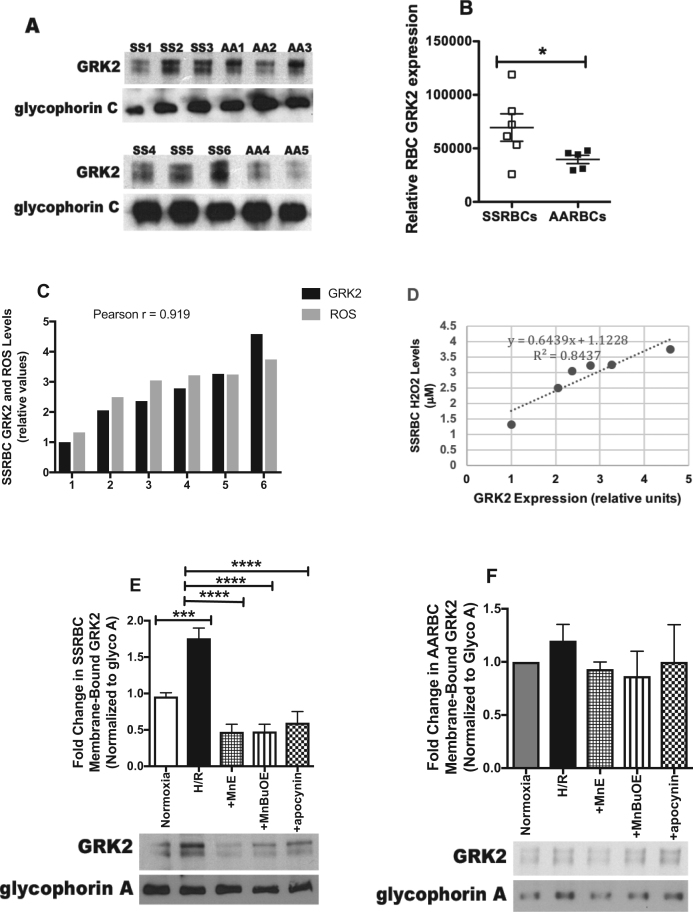
GRK2 was first identified as a G protein-coupled receptor (GPCR) regulator [43]. Given the emerging role of GRK2 in altering non-GPCR-related molecules contributing to a variety of cellular functions and pathology, including ischemia/reperfusion [44], we tested the hypothesis that GRK2 is present in RBCs, and is involved in ROS-induced signaling [45] activated by H/R. GRK2 is present in RBCs (Fig. 3A). The levels of GRK2-bound to the cytoplasmic membrane varied among SSRBC samples, and the mean level was significantly higher in SSRBCs (n = 6) vs. AARBCs (n = 5) (p < 0.05; Figs. 3A and 3B). To determine the relationship between the levels of GRK2 bound to the SSRBC membrane and SSRBC ROS levels, we quantified RBC H2O2 amounts. The levels of GRK2 bound to SSRBC membrane correlated with H2O2 amounts (coefficient correlation (r) = 0.919; coefficient of determination (r2) = 0.8437; Figs. 3C and 3D). Exposure of SSRBCs to prolonged H/R increased GRK2 membrane translocation by 1.84 ± 0.1-fold compared to SSRBCs exposed to normoxia (p < 0.01) (Fig. 3E). The effect of H/R was completely blocked with MnE (p < 0.0001), MnBuOE (p < 0.0001), and apocynin (p < 0.0001) (Fig. 3E). In contrast, in AARBCs, H/R alone or in combination with MnE, MnBuOE, or apocynin failed to trigger changes in GRK2 membrane recruitment to the cytoplasmic membrane (p > 0.05; Fig. 3F). These data suggest that prolonged H/R stimulates SSRBC GRK2 activation as indicated by kinase membrane translocation, and that this kinase participates in the regulatory feedback loop created by ROS and NOXs, and can be inhibited by Mn porphyrins.
Fig. 3.
Increased NOX-dependent ROS production in SSRBCs by H/R induces GRK2 membrane-translocation. RBCs were non-treated (A-D), exposed to normoxia or H/R (E-F), or treated during the reoxygenation with 0.5 μM MnE, 0.5 μM MnBuOE, or 50 μM apocynin (E-F). Fifty μg of membrane protein ghosts was used per lane. RBC proteins were blotted with antibodies against GRK2, and glycophorin A as a loading control under non-reducing conditions. Quantitative analysis of the blots representing GRK2-bound to the RBC plasma membrane, and is presented as Relative RBC GRK2 expression (B), fold change in SSRBC or AARBC membrane-bound GRK2 normalized according to glycophorin A (glyco A) expression (D and E). A-B. GRK2 is expressed in RBCs. GRK2 is present in RBCs, and is bound at higher levels to the membrane of SSRBCs (SS1, SS2, SS3, SS4, SS5 and SS6) than AARBCs (AA1, AA2, AA3. AA4 and AA5). p < 0.05 compared to AARBCs. C-D. Correlation between GRK2 bound to the RBC membrane and endogenous ROS levels. The levels of GRK2 bound to SSRBC membranes correlate with the levels of SSRBC H2O2 (r = 0.919; r2 =0.8437). E-F. GRK2 bound to SSRBC membrane increased by H/R, and kinase membrane-translocation is regulated by NOX-induced ROS generation. GRK2 bound to the SSRBC membrane increased following cell exposure to H/R. This effect was inhibited by MnE, MnBuOE, and apocynin (n = 4; panel E). In AARBCs, GRK2 binding to the membrane was not affected by H/R (n = 4; panel F). Error bars show SEM. P = 0.0002 for SSRBCs exposed to H/R vs. cells exposed to normoxia; p < 0.0001 compared to cells exposed to H/R.
3.4. Hypoxia/reoxygenation up-regulates SSRBC adhesion to vascular endothelial cells in vitro via NOX-dependent ROS generation and ERK1/2
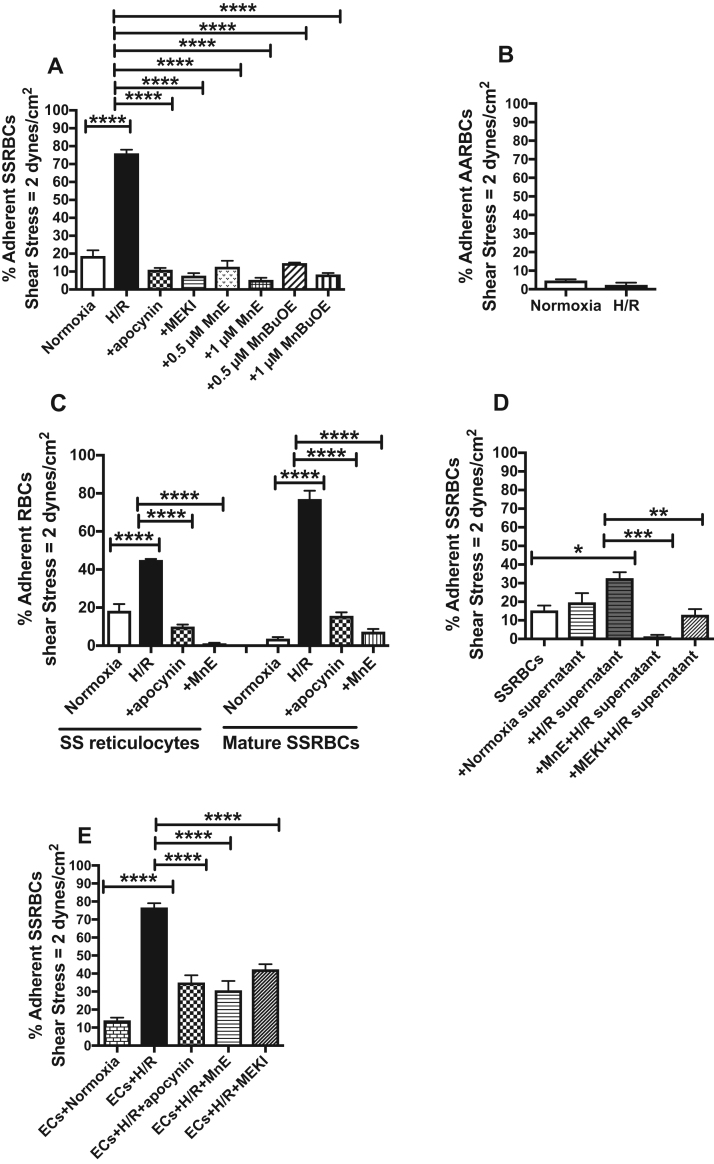
Exposure of SSRBCs to prolonged H/R up-regulated adhesion of these sickle cells to the microvascular ECs, HMVECs-d, by 76 ± 3.9% at a shear stress of 2 dynes/cm2 in intermittent flow condition assays (p < 0.0001) (Fig. 4A). However, SSRBC treatments with the NOX inhibitor apocynin markedly reduced by 86 ± 0.88% adhesion to HMVECs-d of SSRBCs exposed to H/R (p < 0.0001) (Fig. 4A). The MEK inhibitor also almost completely inhibited the effect of H/R (90 ± 2.1% inhibition) on SSRBC adhesion [21], [24], [46] (p < 0.0001). Similar inhibitory adhesive effects were observed when the Mn porphyrins, MnE and MnBuOE, were used at either 0.5 or 1 μM, which decreased H/R-stimulated SSRBC adhesion by 83 ± 4.5%, 93 ± 1.5%, 81 ± 0%, and 89 ± 1.2%, respectively (p < 0.0001). In contrast, prolonged H/R exposure of normal RBCs failed to significantly stimulate adhesion of these normal cells to HMVECs-d (p > 0.05; Fig. 4B).
Fig. 4.
Increased NOX-dependent ROS levels in SSRBCs by H/R mediates adhesion to vascular endothelial cellsin vitro. A-C. SSRBCs, AARBCs, SS reticulocytes, and mature SSRBCs were exposed to normoxia, H/R, or hypoxia then incubated during reoxygenation with 50 μM apocynin (A and C), 5 μM MEKI (A), 0.5 (A) or 1 μM (A and C) MnE, or 0.5 or 1 μM MnBuOE (A). D. SSRBCs were incubated with supernatants collected from SSRBC samples exposed to normoxia, H/R, or supernatant collected from SSRBCs exposed to H/R and 1 μM MnE or 5 μM MEKI. Adhesion of RBCs to HMVECs-d was tested in intermittent flow condition assays. Results are presented as % adherent SSRBCs or AARBCs at a shear stress of 2 dynes/cm2. Error bars show SEM of five different experiments. A.p < 0.0001 compared to SSRBCs exposed to H/R. C.p < 0.0001 compared to SSRBCs exposed to H/R. D.p < 0.05 compared to SSRBCs; p < 0.01 compared to cells exposed to supernatants collected from SSRBCs exposed to H/R. E. Adhesion of SSRBCs to HMVECs-d exposed to H/R. HMVECs-d were exposed to normoxia, H/R, or hypoxia then treated during reoxygenation with 50 μM apocynin, 1 μM MnE, or 5 μM MEKI. Treated HMVECs-d was tested for their ability to support adhesion of non-treated SSRBCs. Error bars show SEM of three different experiments. p < 0.0001 for SSRBC adhesion to ECs exposed to H/R vs. SSRBC adhesion to ECs exposed to normoxia; p < 0.0001 compared to SSRBC adhesion to ECs exposed to H/R.
To define the cell population, which responded to H/R and its downstream mechanism, SS reticulocytes and mature SSRBCs were analyzed for adhesion response following RBC exposure to H/R. Exposure of each cell population to H/R enhanced the adhesion to HMVECs-d of both SS reticulocytes (p < 0.0001) and mature SSRBCs (p < 0.0001) (Fig. 4C). Increased adhesion of these two cell populations was decreased by apocynin (p < 0.0001) and MnE (p < 0.0001) to below baseline levels (Fig. 4C). These data demonstrate that H/R stimulation of NOX-dependent ROS generation and its positive feedback loop in both immature and mature SSRBCs mediates adhesion to endothelium, a mechanism that can be suppressed by Mn porphyrins.
We also determined whether ROS that could be generated by free HbS autoxidation contribute to SSRBC binding to endothelium. We incubated SSRBCs with supernatants collected from SSRBCs that have been exposed to normoxia or H/R. SSRBCs treated with the supernatants were then washed prior to adhesion assays. Incubation of SSRBCs with supernatant collected from SSRBCs exposed to H/R increased SSRBC adhesion to endothelium (p = 0.0113) (Fig. 4D). This effect was abrogated with MnE (p = 0.00002), and MEKI (p = 0.0051). In contrast, supernatant collected from SSRBCs exposed to normoxia was not successful in increasing SSRBC adhesion (Fig. 4D). These data suggest that exogenous radicals generated by free oxidized HbS and SSRBC NOX enzymes act synergistically to mediate SSRBC adhesion via the ERK1/2 pathway.
We next examined the effect of H/R on ECs and the mechanism involved. H/R enhanced by 82 ± 2.8% the percentage of ECs supporting adhesion of non-treated SSRBCs (Fig. 4E). Apocynin, MnE, and MEKI were able to significantly subdued adhesion of non-treated SSRBCs to H/R-activated HMVECs-d by 54 ± 4% (p < 0.0001), 60 ± 5.5% (p < 0.0001), and 45 ± 2.3% (p = 0.0001), respectively (Fig. 4E). These data underscore the relevance of NOXs and its downstream effectors MEK1/2 and ERK1/2, in mediating SSRBC endothelial adhesion whether the SSRBC or EC is exposed to H/R, a signaling mechanism that can be interrupted through inhibition of NOX activation and/or ERK1/2 signaling with Mn porphyrins.
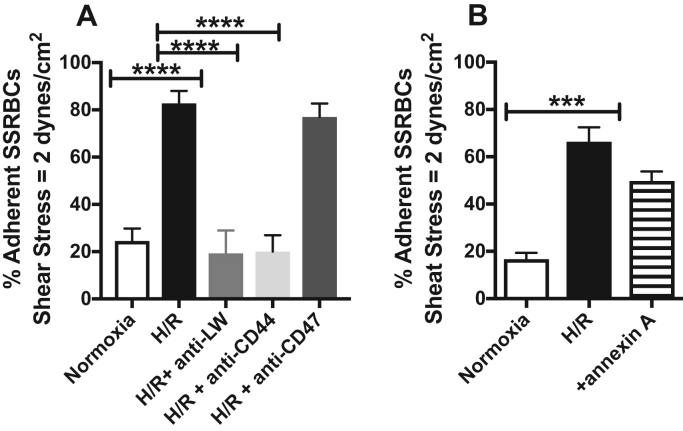
3.5. Adhesion of SSRBCs exposed to hypoxia/reoxygenation to vascular endothelial cells is mediated by the adhesion molecules LW and CD44
Inhibition adhesion assays were performed using antibodies against adhesion molecules expressed by both mature and immature RBCs. In vitro antibody blocking assays showed that antibodies to both the RBC LW (20 μg/ml) and the RBC CD44 adhesion molecules successfully blocked endothelial adhesion of H/R-exposed SSRBCs by 77 ± 6.4% (p < 0.0001) and 76 ± 4.6% (p < 0.0001), respectively (Fig. 5A). In contrast, antibody to the RBC CD47 thrombospondin receptor (20 μg/ml) (Fig. 5A) failed to inhibit the effect of H/R on SSRBC adhesion to HMVECs-d in vitro.
Fig. 5.
Increased NOX-dependent ROS generation by H/R mediates SSRBC-endothelial binding via RBC LW and CD44 adhesion moleculesin vitro. A and B. SSRBCs were exposed to normoxia, or H/R, then washed. Treated SSRBCs exposed to H/R were incubated with 20 μg/ml anti-LW, 20 μg/ml anti-CD44, 100 μg/ml anti-CD47 mAb IgG, or 40 μg/ml annexin V. Cells were then washed again before in vitro adhesion assays. Error bars show SEM of four different experiments. p < 0.0001 compared to SSRBCs exposed to H/R.
PS exposure is elevated in SSRBCs of SCD patients, and PS was found to mediate endothelial adhesion of SSRBCs [47]. When SSRBCs were exposed to H/R, then treated with annexin V to cloak PS, H/R-induced up-regulated adhesion of these sickle cells to ECs was not significantly inhibited by annexin V (p = 0.0703; Fig. 5B). We also determined the levels of PS exposure on our SSRBC samples. Exposure of SSRBCs to H/R failed to enhance the percentage of annexin V positive SSRBCs [< 3% annexin V positive SSRBCs were detected (n = 10)]. These data demonstrate that SSRBC endothelial adhesion is mediated via LW and CD44 adhesion molecules, receptors activated by H/R through NOXs and ERK1/2 signaling pathway [24], and exclude the contribution of PS on SSRBCs to adhesion.
3.6. Hypoxia/reoxygenation exposure of SSRBCs mediates adhesion to vascular endothelial cells and vasoocclusion in vivo via RBC NOX-dependent ROS
In vivo studies of the pathophysiological significance of prolonged H/R on NOX-dependent ROS generation in SSRBCs and the contribution of this mechanism to in vivo adhesion to the vascular endothelium and vasoocclusion were also carried out. Vasoocclusion experiments were designed exclusively to study the effect of H/R on regulating NOX-mediated human SSRBC adhesion in an intact vasculature in the presence of physiological blood flow and shear stresses, in the absence of potentially confounding non-RBC signals, such as those derived from H/R stimulation of murine endothelium, leukocytes and platelets, and in the context of whole blood where human RBCs interact with other cells and a myriad of plasma proteins in vivo. In these studies, the quantity of human SSRBCs infused to animals never exceeded 10% of the total circulating RBCs, assuming that the mouse blood volume is 1.5 ml, thereby minimizing any possible rheological effects attributable to increased hematocrit [48]. RBCs in these small concentrations should also not influence O2 delivery.
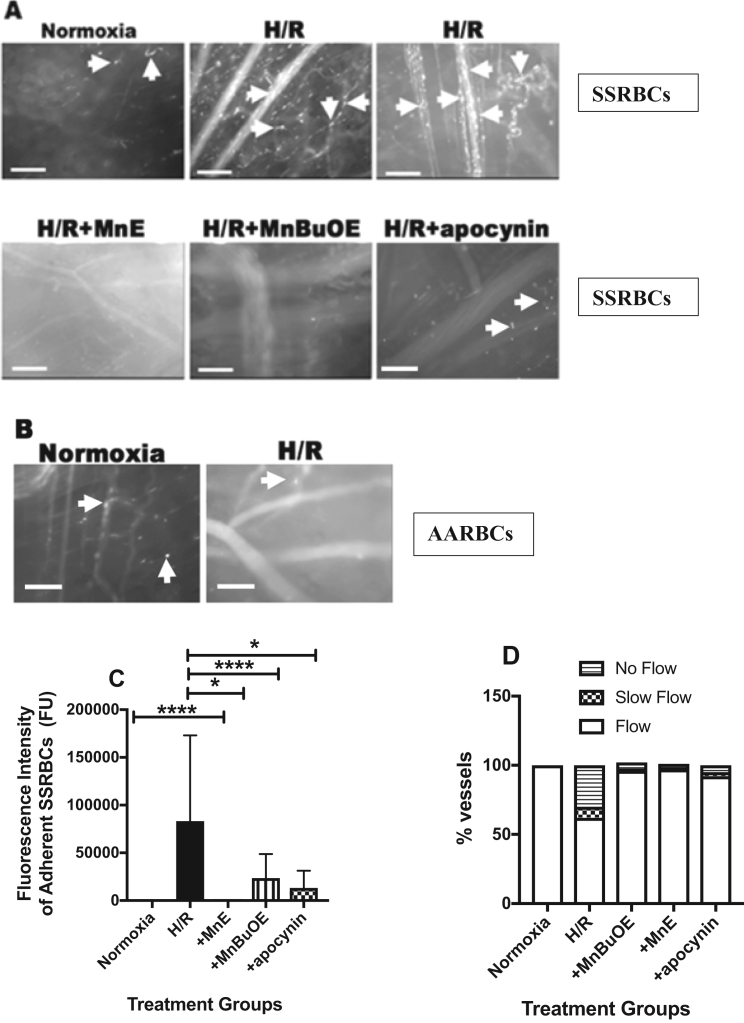
To examine the pathological relevance of both exposure of SSRBCs to hypoxia, followed by a prolonged period of reoxygenation and the effect of H/R on NOX-mediated human SSRBC adhesion in vivo, and exclude the potential effect of H/R on non-human RBCs, human SSRBCs were exposed to normoxia or transient hypoxia at 8% O2 for 2 h, followed by reoxygenation for 2 h with or without the Mn porphyrins, MnE or MnBuOE, or apocynin ex vivo, then washed extensively prior to adoptive transfer into nude mice. Intravital microscopy observation of venules and arterioles visible through the dorsal skin-fold window chamber, showed very little adhesion of normoxia-exposed SSRBCs. SSRBCs exposed to normoxia adhered only very occasionally to small postcapillary venules ≤ 25 µm in diameter (Fig. 6A, Supplemental Movie 1). In sharp contrast, infusion of H/R-exposed SSRBCs resulted in marked adhesion in vessels of 8–25 µm diameter (Fig. 6A, Supplemental Movie 2). Adhesion of H/R exposed SSRBCs was also observed in vessels with diameters larger than 25 µm. Treated SSRBC adhesion took place within the first 5 min following SSRBC infusion, causing progressively intermittent or permanent blockage of some vessel segments. Vasoocclusion occurred frequently where vessels curved and at junctions, although it was also observed in straight non-junctional venular segments (Fig. 6A, Supplemental Movie 2). However, treatment of H/R-exposed SSRBCs with the Mn porphyrins, MnE and MnBuOE at 1 μM, thus disturbing the positive regulatory feedback loop created by NOX-dependent ROS, MEK1/2 and ERK1/2, had anti-SSRBC adhesive benefits. These two compounds, MnE and MnBuOE, significantly abrogated the outcome of H/R on adhesion of the sickle cells to the vascular endothelium, and reduced vasoocclusion, which was observed periodically only in very small vessels with a diameter less than 12 µm (Fig. 6A, Supplemental Movie 3). Inhibition of SSRBC NOXs with apocynin at 50 μM had a similar inhibitory effect to Mn porphyrins, and almost completely blocked the effect of H/R on SSRBC adhesion and vasoocclusion (Fig. 6A, Supplemental Movie 4). These sickle cells also demonstrated sporadic adhesion, which occurred in vessels with mean diameter no larger than 18 µm. The involvement of H/R in activation of NOXs in normal RBCs to mediate adhesion in vivo was next tested. Exposure of normal human RBCs to H/R ex vivo failed to promote adhesion of these cells to vessels in nude mice in vivo (Fig. 6B), further confirming that H/R has no real consequences on normal RBC adhesive function.
Fig. 6.
Increased SSRBC NOX-dependent ROS generation by H/R mediates adhesionandvasoocclusionin vivo. A-D. In in vivo studies, anesthetized nude mice implanted with dorsal skin-fold window chambers were infused with washed, fluorescence-labeled human SSRBCs [panels A, C and D (n = 5)] or AARBCs [panel B (n = 3)] exposed to normoxia (A-D), H/R (A-D), or hypoxia then incubated during reoxygenation with 1 μM MnE (A, C and D), 1 μM MnBuOE (A, C and D), or 50 μM apocynin (A, C and D). Intravital microscopic imaging of post-capillary venules and arterioles using 10X and 20X magnifications was conducted through the window chamber immediately after infusion of washed, fluorescence labeled treated RBCs. A-B. Representative images of post-capillary venules (10X and 20X magnifications) are shown. Vessels without adherent cells appear gray, due to the rapid movement of fluorescence labeled human RBCs. Adhesion of human RBCs in vessels and vasoocclusion are indicated with arrows. Scale bar = 50 µm. Human SSRBCs exposed to normoxia showed very little to no adhesion to vessel walls. However, human SSRBCs exposed to H/R showed marked adhesion to normal vascular endothelial cells with intermittent vasoocclusion; effects inhibited with MnE, MnBuOE, and apocynin. In contrast, H/R completely failed to increase adhesion of AARBCs in venules. C. Video frames showing vessel and arteriole segments were used to quantify adhesion of fluorescence-labeled treated human SSRBCs presented as adherent SSRBCs (FU). The values of at least 30 segments of vessels and arterioles were analyzed and averaged among groups of nude mice. Error bars show SEM of 5 different experiments for each treatment condition. p < 0.05 compared to SSRBCs exposed to H/R regardless of the vessel diameter within the ranges specified. D. Represents percentage of vessels without blood flow, percentage of vessels with slow blood flow; and percentage of normal flowing vessels.
Supplementary material related to this article can be found online at doi:10.1016/j.redox.2019.101097.
The following is the Supplementary material related to this article Movie 1, Movie 2, Movie 3, Movie 4.
Adhesion of normoxia-exposed human SSRBCsin vivo. Exposure to normoxia of SSRBCs showed no real adhesion in post-capillary vessels and arterioles.
Adhesion of H/R-exposed human SSRBCsin vivo. Exposure of SSRBCs to H/R ex vivo followed by infusion to animals showed extensive adhesion in small post-capillary vessels and arterioles and progression of vasoocclusion. This led to permanent blockade of some venules both at junctions and straight segments causing blood flow stasis.
Mn porphyrin treatment of human SSRBCs reduced the effect of H/R on adhesionin vivo. Ex vivo treatment with 1 μM MnE of Human SSRBC exposed to H/R, followed by washing prior to infusion to nude mice, abrogated adhesion of these sickle cells in vessels and arterioles, reduced vasoocclusion, and restored blood flow.
Inhibition of NOX enzymes reduced the effect of H/R on human SSRBC adhesionin vivo. Apocynin treatment of human SSRBC exposed to H/R ex vivo, washed, then infused to nude mice, reduced adhesion of these sickle cells to the vessel and arteriole walls, and prevented vasoocclusion.
Quantitative analysis of cell adhesion indicated that SSRBC adhesion increased by 99.9% after exposure to H/R (n = 5; p < 0.0001) (Fig. 6C). MnE and MnBuOE reduced H/R-exposed SSRBC adhesion in vessels and arterioles recorded by 99.6% (p < 0.0001) and 72% (p = 0.0115), respectively. Similarly, NOX inhibition with apocynin also inhibited the effect of H/R on SSRBCs adhesion by 84% (n = 5; p = 0.0218) (Fig. 6C). As a result of disrupting SSRBC NOX-dependent ROS generation and its vicious cycle with MnE and MnBuOE in SSRBCs exposed to H/R, SSRBC circulatory behavior was improved, and blood flow was preserved in 97% and 96% of vessels and arterioles, respectively, as opposed to 62% of vessels with normal blood flow in mice infused with SSRBCs exposed to H/R (Fig. 6D). SSRBC NOX inhibition, led, likewise, to normal blood flow, which was well-kept in 92% of the vessels and arterioles recorded (Fig. 6D). These data strongly argue that SSRBC adhesion in the vasculature and vasoocclusion are stimulated by reoxygenation of deoxygenated sickle cells, even after prolonged exposure to oxygen. This adhesive effect can be prevented by interfering with the vicious cycle created at least in part by activation of NOX enzymes, GRK2, and ERK1/2 within SSRBCs.
3.7. Increased SSRBC NOX-dependent ROS generation by hypoxia/reoxygenation leads to protein tyrosine nitration
Oxidative stress can lead to an important oxidative modification. Superoxide can react with nitric oxide (•NO) to form peroxynitrite (ONOO−) [49]. ONOO− nitrates tyrosine residues of proteins giving rise to nitrotyrosine, thereby altering protein structure and functionality. Because 3-nitrotyrosine is considered a marker of ONOO− action, and since RBCs contain •NO, we next measured the 3-nitrotyrosine level in SSRBCs, and determined whether this level is affected by H/R. Tyrosine residues of SSRBC membrane proteins are nitrated to some extent, and H/R exposure upregulated the nitrotyrosine levels (p < 0.01); an effect reduced by apocynin (p < 0.01), and MnE (p < 0.001) (Supplemental Fig. 1). These data suggest that increased NOX-dependent reactive species generation in response to H/R can induce SSRBC membrane protein tyrosine nitration, a marker of protein structural and functional alteration and/or damage. This modification is regulated by NOXs, and appears to be prevented by Mn porphyrins-induced deactivation of NOXs, GRK2, and/or ERK1/2.
4. Discussion
SCD involves repeated transient ischemic episodes, leading to multifocal microvascular occlusions. Here, we have simulated this process in vitro by subjecting human SSRBCs to hypoxia, a time during which SSRBCs are trapped in a hypoxic environment, followed by prolonged (2 h) reoxygenation, a time during which vasoocclusion is resolved, blood flow is restored, and SSRBCs are reoxygenated, and defined the exact molecular mechanisms within these human SSRBCs contributing to the onset of the next vasoocclusive event. We now describe a critical unprecedented mechanism in human SSRBCs stimulated by H/R, consisting of a positive feedback loop created by a signaling complex, NOX-dependent ROS, GRK2, MEK1/2 and ERK1/2, which can be disrupted by Mn porphyrins-based redox-active drugs commonly known as SOD mimics. This mechanism is directly involved in mediating human SSRBC adhesion to the vascular endothelium both in vitro and in vivo, promoting vasoocclusion.
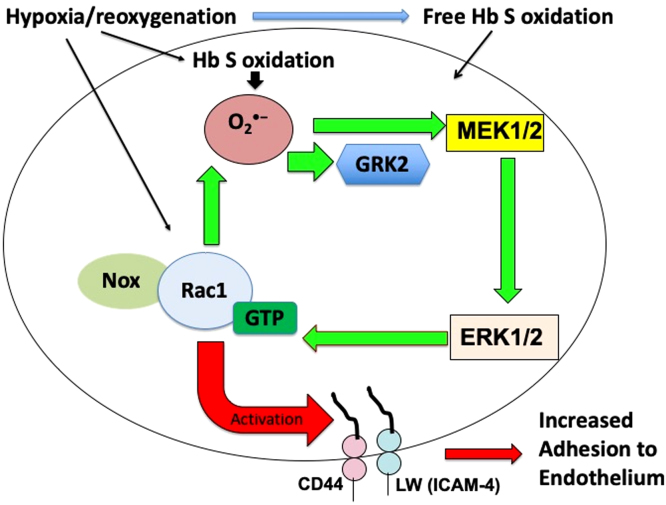
We show that excessive SSRBC NOX-dependent ROS production stimulated by H/R mediates SSRBC adhesive interactions with the vascular endothelium. Increased NOX activity was detected in both immature and mature SSRBCs, and was dependent upon ERK1/2 activation. Our studies also suggest that enhanced SSRBC NOX-dependent ROS production by H/R creates a positive feedback loop to subsequently regulate ERK1/2 and GRK2, as reflected by increased ERK1/2 phosphorylation and GRK2 membrane-translocation. Previous studies have revealed that ERK1/2 signaling is exclusively active in SSRBCs, but not in normal RBCs, and this kinase directly contributes to adhesion of these sickle cells to endothelial αvβ3 integrin via activation of the RBC adhesion molecule LW [21], [24]. GRK2 was also described earlier to regulate signaling at levels downstream of receptors, through its interactions with various non-receptor substrates and binding partners [50]. The true complexity of GRK2-mediated regulation of cellular signaling other than G protein-coupled receptors (GPCRs) is only recently being explored and suggests that GRK2 involvement may in some disease states not be due simply to dysregulation of GPCR signaling. Changes in expression levels and/or activity of GRK2 were described to be deleterious and associated with a number of different diseases, including hypertension [51] and heart failure [52]. Furthermore, excessive NOX-dependent ROS production in SSRBCs by H/R caused increased protein tyrosine nitration, which likely exert structural and functional effects with various consequences such as inhibition or activation of biological activities in SSRBCs [53]. An overview of the demonstrated mechanism of increased endogenous NOX-dependent ROS production and their positive feedback loop in SSRBCs, is shown in Fig. 7.
Fig. 7.
Proposed model for the signaling mechanism of excessive NOX-dependent ROS production and its deleterious cycle in SSRBCs exposed to H/R. In SSRBCs, activation of NOX triggered by H/R is involved in excessive production of endogenous ROS. Increased SSRBC ROS generation activates both the MAPK cascade and GRK2. Subsequently, SSRBC NOX-dependent ROS production, MEK1/2, ERK1/2 and GRK2 exert a positive feedback loop. This deleterious feedback loop created by ROS is a key regulator of SSRBC adhesion to the vascular endothelium. Such endothelial adhesion is mediated by the RBC adhesion molecules CD44 and LW. Furthermore, ROS produced by HbS oxidation appears to participate in activation of this signaling mechanism possibly exacerbating downstream events. Thus, this critical new molecular mechanism in SSRBCs stimulated by H/R, and could be disrupted with redox-active Mn porphyrins, may drive the profound pathophysiology of SCD.
ROS levels were higher in SSRBCS than in AARBCs [20], and despite the presence of MEK1/2, ERK1/2, and GRK2 in AARBCs [24], H/R failed to significantly elicit GRK2 and ERK1/2 activation, and enhance NOX-dependent ROS generation. Studies have described RBCs undergoing maturation-related loss of multiple protein kinase activities, including PKA, PKC, and casein kinases [24], [54]. The overall youth of SSRBCs (unseparated, reticulocyte-enriched and mature) in circulation relative to AARBCs, and the higher levels of expression of both ERK1/2 and GRK2 in SSRBCs compared to AARBCs, likely contribute to the preserved and/or increased SSRBC NOX, ERK1/2 and GRK2 activities both basally and upon induction. Additionally, excessive ROS produced by HbS autoxidation within SSRBCs likely participate in the maintenance and/or increased GRK2 and ERK1/2 activities and the downstream mechanisms of sickle cell adhesion. Based on our data, exogenous ROS also seems to act synergistically with endogenous ROS to feasibly further exacerbate these signaling events within the SSRBC.
Mechanism of action of Mn porphyrins, which undergo intricate interactions with numerous redox-sensitive pathways, has been extensively explored [38]. While potent scavengers of superoxide and peroxynitrite, recent studies have suggested that Mn porphyrins are involved in thiol signaling [38], [55], [56]. Thus, Mn porphyrins were shown to catalytically oxidize protein cysteines in a glutathione peroxidase (GPx) fashion [38], [57]. Numerous proteins were found to be oxidized, among those NF-кB seems to be a major target of Mn porphyrins both in injuries of normal tissue and in cancer [38], [55], [56], [58], [59]. Additionally, several MAPKs, including ERK1/2, AKT, c-Jun N-terminal kinase (JNK), and p38, seem to be oxidized by Mn porphyrins, and subsequently inactivated [38], [42], [60], [61]. Evidence was also provided that MnE suppresses NOX4 upregulation (presumably via NF-кB pathway) due to radiation-induced increase in ROS levels [37]. Finally evidence was provided that MnBuOE can activate Nrf2 presumably via oxidizing Keap1; subsequently up-regulating the endogenous antioxidative defenses [62]. Inhibition of NF-кB (and in turn NOXs), MAPKs, and Nrf2 by Mn porphyrins would result in decreased ROS levels. Indeed, in this work we have demonstrated that Mn porphyrins suppress NOX activity with subsequent reduction in ROS levels. We have also shown the suppressed activities of different kinases: ERK1/2, and GRK2 by these Mn porphyrins.
SSRBCs contain no nucleus; it is thus an ideal system that allows us to distinguish the impact of Mn porphyrins directly on NOXs and kinases, from the effect of these compounds at transcriptional level. In other words NOXs were not downregulated via NF-кB. The reduction of ROS levels is therefore not the consequence of the inhibition of NF-кB and activation of Nrf2. Consequently, our data for the first time suggest that Mn porphyrins act directly: (i) on NOXs by deactivating these enzymes, which in turn inactivate ERK1/2, (ii) or on ERK1/2 and GRK2 by inactivating the kinases, which in turn inactivate NOXs, (iii) or by affecting all three enzymes at once. Such action/s of Mn porphyrins likely occurs through a mechanism independent of their SOD-like activities. Literature data support such reasoning. It was previously reported that oxidation of cysteine and methionine residues of calcium-binding domain of NOX5 could be implicated in its inactivation [63]. Mutation of one cysteine in one of the subunits of NOXs, p47PHOX, also affected oxidase activity [64]. Because cysteines seem to be involved in redox regulation of both ERK1/2 and oxidase activities, and since NOX1, NOX2, NOX4, and NOX5 isoforms were all detectable in both normal RBCs and SSRBCs [20], we can safely postulate that Mn porphyrins may directly deactivate at least both NOX enzymes and phosphorylated ERK1/2, and possibly GRK2 in SSRBCs via oxidation of their cysteines. While Mn porphyrins suppress the activities of NOXs and different kinases which results in reduced ROS levels, further work on oxidation and/or S-glutathionylation of NOXs and kinases is needed to clarify events at the molecular level.
Oxidative stress in SCD has been linked to increased adhesiveness of SSRBCs. The considerable complexities of oxidative mechanisms in SSRBCs due to iron decompartmentalization and HbS autoxidation in particular [65], have been implicated in RBC membrane pathobiology, including protein oxidation of sickle membranes and lipid peroxidation, which lead to phospholipid destabilization. Conversely, it remains unproven whether these defects of the sickle membrane are actually responsible for the increased SSRBC adhesion, with the exception of the involvement of deoxygenation-induced dehydration and sickling of dense cells [13], [66], and HbS polymerization in sickle reticulocytes and mature sickle cells [13]. Our data show that prolonged reoxygenation of deoxygenated unseparated, and separated mature SSRBCs and SS reticulocytes, stimulated the adhesive function of these different cell populations, due to up-regulation of activation at least in part of NOXs, triggering ERK1/2 and GRK2 signaling pathways, and vise-versa; signaling and adhesive phenotypes disrupted by Mn porphyrins. Nonetheless, HbS autoxidation in SSRBCs likely provide an additional stimulating factor of ERK1/2 and GRK2 activation by further generated ROS within the SSRBC. Furthermore, while we did not examine the shape of the sickle cells once reoxygenated for 2 h, sickling of these cells may not play part in activation of SSRBC adhesive function, since RBC sickling has been previously shown to be completely reversed after only 50 min reoxygenation period at ambient air [15].
In SCD, it is now clear that in SSRBCs, enhanced NOX activation stimulated by cell exposure to H/R, is an inducer of endothelial adhesive interactions and episodic vasoocclusive pathology. SSRBC endothelial adhesive events up-regulated by exposure to H/R, were mediated by the RBC adhesion molecules LW and CD44. Previous studies have shown that deoxygenation-induced sickling can destabilize membrane phospholipid asymmetry, and has been postulated to enhance PS availability on the surface of SSRBC membrane [67]. However, prolonged SSRBC exposure to H/R failed to significantly increase PS externalization in non-hemolyzed SSRBCs, supporting the exclusion of PS from being involved in the SSRBC adhesive interactions with endothelium. Furthermore, our data suggest that exogenous free radicals generated by autoxidized HbS released by homolyzed cells by H/R, can contribute to activation of the intracellular SSRBC feedback loop through activation of ERK1/2, leading to anomalous SSRBC adhesion, and possibly episodic vasoocclusive crises. Thus, in addition to the endogenous positive feedback loop within SSRBCs, the recurrent SSRBC adhesive events collectively with hemolysis and progressive oxidative endothelial damage create an extracellular feedback cycle that drives the complex manifestations and severe outcomes in SCD. Moreover, it may be anticipated that abnormal rheologic properties would participate in these frequent vasoocclusive crises as well.
Finally, our data suggest that episodic vasoocclusive events might be lessened and/or prevented by disturbing the SSRBC NOXs, GRK2, MEK1/2, and ERK1/2 positive feedback loop with Mn porphyrins. Thus, components of this SSRBC ROS deleterious cycle could represent potential therapeutic targets for curtailing recurrent vasoocclusive events and minimizing SSRBC oxidative stress. In addition to their ability to reduce SSRBC ROS (Fig. 1), we have indeed, previously shown that MEK1/2 inhibitors, which directly target the MEK1/2 signaling pathway, prevented sickle cell adhesion and vasoocclusion, and reversed ongoing vasoocclusion in two different animal models of SCD [21], [46]. Several studies have also demonstrated the efficacy of hydroxyurea, the only FDA-approved treatment for SCD, in reducing painful vasoocclusive crises in addition to its ability to increase fetal hemoglobin (HbF) levels [68], [69]. Increased HbF might be associated with reduced systemic oxidative stress in human [70], [71]. Oral administration of ORY-3001, the inhibitor of LSD1, a component of corepressor complexes that repress γ-globin gene expression, has recently been proposed to increase HbF as well in sickle mice and baboons [72]. The pharmacological agents, Mn porphyrins, capable of disrupting this deleterious cycle by deactivating one or more of its components, might therefore be particularly valuable therapy alone, or as a multimodal strategy with MEK inhibitors, hydroxyurea or ORY-3001, or agents targeting other pathological pathways, acting in synergy to curtail/prevent both sickle RBC-induced recurrent painful vasoocclusive crises and SSRBC oxidative damage, the hallmarks of SCD.
Acknowledgments
This work was supported by the Grant 1R01HL137930-01 to RZ from National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH).
Acknowledgments
Authorship contributions
A.M. and E.W. investigated and validated the in vitro part of the study, and analyzed the data; I.S. and I.B-H synthesized the Mn porphyrins; I.B.H. helped with the methodology of the studies related to Mn porphyrins, and reviewed and edited the manuscript; R.Z. conceptualized the study, designed and supervised all of the research study, investigated the in vivo part of the study, curated, analyzed and validated the data, wrote the original draft, reviewed and edited the manuscript.
Conflict-of-interest disclosure
The authors declare no competing financial interests.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2019.101097.
Appendix A. Supplementary material
Supplementary material
References
- 1.Kaul D.K., Tsai H.M., Liu X.D., Nakada M.T., Nagel R.L., Coller B.S. Monoclonal antibodies to alphaVbeta3 (7E3 and LM609) inhibit sickle red blood cell-endothelium interactions induced by platelet-activating factor. Blood. 2000;95:368–374. [PubMed] [Google Scholar]
- 2.Bernaudin F., Verlhac S., Chevret S., Torres M., Coic L., Arnaud C. G6PD deficiency, absence of alpha-thalassemia, and hemolytic rate at baseline are significant independent risk factors for abnormally high cerebral velocities in patients with sickle cell anemia. Blood. 2008;112:4314–4317. doi: 10.1182/blood-2008-03-143891. [DOI] [PubMed] [Google Scholar]
- 3.Hebbel R.P., Boogaerts M.A., Eaton J.W., Steinberg M.H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N. Engl. J. Med. 1980;302:992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- 4.Morris C.R., Kato G.J., Poljakovic M., Wang X., Blackwelder W.C., Sachdev V. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA: J. Am. Med. Assoc. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brittenham G.M., Schechter A.N., Noguchi C.T. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985;65:183–189. [PubMed] [Google Scholar]
- 6.Hebbel R.P. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. [PubMed] [Google Scholar]
- 7.Bertles J.F., Milner P.F. Irreversibly sickled erythrocytes: a consequence of the heterogeneous distribution of hemoglobin types in sickle-cell anemia. J. Clin. Investig. 1968;47:1731–1741. doi: 10.1172/JCI105863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baez S., Kaul D.K., Nagel R.L. Microvascular determinants of blood flow behavior and HbSS erythrocyte plugging in microcirculation. Blood Cells. 1982;8:127–137. [PubMed] [Google Scholar]
- 9.Kaul D.K., Fabry M.E., Nagel R.L. Vaso-occlusion by sickle cells: evidence for selective trapping of dense red cells. Blood. 1986;68:1162–1166. [PubMed] [Google Scholar]
- 10.Ballas S.K. Sickle cell anemia with few painful crises is characterized by decreased red cell deformability and increased number of dense cells. Am. J. Hematol. 1991;36:122–130. doi: 10.1002/ajh.2830360211. [DOI] [PubMed] [Google Scholar]
- 11.Mohandas N., Evans E. Sickle erythrocyte adherence to vascular endothelium. Morphologic correlates and the requirement for divalent cations and collagen-binding plasma proteins. J. Clin. Investig. 1985;76:1605–1612. doi: 10.1172/JCI112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaul D.K., Chen D., Zhan J. Adhesion of sickle cells to vascular endothelium is critically dependent on changes in density and shape of the cells. Blood. 1994;83:3006–3017. [PubMed] [Google Scholar]
- 13.Papageorgiou D.P., Abidi S.Z., Chang H.Y., Li X., Kato G.J., Karniadakis G.E. Simultaneous polymerization and adhesion under hypoxia in sickle cell disease. Proc. Natl. Acad. Sci. USA. 2018;115:9473–9478. doi: 10.1073/pnas.1807405115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaul D.K., Fabry M.E., Nagel R.L. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proc. Natl. Acad. Sci. USA. 1989;86:3356–3360. doi: 10.1073/pnas.86.9.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osarogiagbon U.R., Choong S., Belcher J.D., Vercellotti G.M., Paller M.S., Hebbel R.P. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- 16.Hebbel R.P., Morgan W.T., Eaton J.W., Hedlund B.E. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Natl. Acad. Sci. USA. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebbel R.P., Eaton J.W., Balasingam M., Steinberg M.H. Spontaneous oxygen radical generation by sickle erythrocytes. J. Clin. Investig. 1982;70:1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng K., Shariff M., Hebbel R.P. Comparative oxidation of hemoglobins A and S. Blood. 1998;91:3467–3470. [PubMed] [Google Scholar]
- 19.Kuypers F.A., Scott M.D., Schott M.A., Lubin B., Chiu D.T.Y. Use of Ektacytometry to determine red-cell susceptibility to oxidative stress. J. Lab. Clin. Med. 1990;116:535–545. [PubMed] [Google Scholar]
- 20.George A., Pushkaran S., Konstantinidis D.G., Koochaki S., Malik P., Mohandas N. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121:2099–2107. doi: 10.1182/blood-2012-07-441188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zennadi R. MEK inhibitors, novel anti-adhesive molecules, reduce sickle red blood cell adhesion in vitro and in vivo, and vasoocclusion in vivo. PLoS One. 2014;9:e110306. doi: 10.1371/journal.pone.0110306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zennadi R., Hines P.C., De Castro L.M., Cartron J.P., Parise L.V., Telen M.J. Epinephrine acts through erythroid signaling pathways to activate sickle cell adhesion to endothelium via LW-alphavbeta3 interactions. Blood. 2004;104:3774–3781. doi: 10.1182/blood-2004-01-0042. [DOI] [PubMed] [Google Scholar]
- 23.Zennadi R., Moeller B.J., Whalen E.J., Batchvarova M., Xu K., Shan S. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110:2708–2717. doi: 10.1182/blood-2006-11-056101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zennadi R., Whalen E.J., Soderblom E.J., Alexander S.C., Thompson J.W., Dubois L.G. Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium. Blood. 2012;119:1217–1227. doi: 10.1182/blood-2011-03-344440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telen M.J., Scearce R.M., Haynes B.F. Human erythrocyte antigens. III. characterization of a panel of murine monoclonal antibodies that react with human erythrocyte and erythroid precursor membranes. Vox Sang. 1987;52:236–243. doi: 10.1111/j.1423-0410.1987.tb03035.x. [DOI] [PubMed] [Google Scholar]
- 26.Bloy C., Blanchard D., Hermand P., Kordowicz M., Sonneborn H.H., Cartron J.P. Properties of the blood group LW glycoprotein and preliminary comparison with Rh proteins. Mol. Immunol. 1989;26:1013–1019. doi: 10.1016/0161-5890(89)90065-5. [DOI] [PubMed] [Google Scholar]
- 27.Lindberg F.P., Lublin D.M., Telen M.J., Veile R.A., Miller Y.E., Donis-Keller H. Rh-related antigen CD47 is the signal-transducer integrin-associated protein. J. Biol. Chem. 1994;269:1567–1570. [PubMed] [Google Scholar]
- 28.Unthank J.L., Lash J.M., Nixon J.C., Sidner R.A., Bohlen H.G. Evaluation of carbocyanine-labeled erythrocytes for microvascular measurements. Microvasc. Res. 1993;45:193–210. doi: 10.1006/mvre.1993.1018. [DOI] [PubMed] [Google Scholar]
- 29.Batinic-Haberle I., Benov L., Fridovich I. An anionic impurity in preparations of cytochrome c interferes with assays of cationic catalysts of the dismutation of the superoxide anion radical. Anal. Biochem. 1999;275:267. doi: 10.1006/abio.1999.4318. [DOI] [PubMed] [Google Scholar]
- 30.Rajic Z., Tovmasyan A., Spasojevic I., Sheng H., Lu M., Li A.M. A new SOD mimic, Mn(III) ortho N-butoxyethylpyridylporphyrin, combines superb potency and lipophilicity with low toxicity. Free Radic. Biol. Med. 2012;52:1828–1834. doi: 10.1016/j.freeradbiomed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udani M., Zen Q., Cottman M., Leonard N., Jefferson S., Daymont C. Basal cell adhesion molecule/lutheran protein. The receptor critical for sickle cell adhesion to laminin. J. Clin. Investig. 1998;101:2550–2558. doi: 10.1172/JCI1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Algire G.H., Legallais F.Y. Recent developments in the transparent-chamber technique as adapted to the mouse. J. Natl. Cancer Inst. 1949;10:225–253. (incl 8 pl) [PubMed] [Google Scholar]
- 34.Kalambur V.S., Mahaseth H., Bischof J.C., Kielbik M.C., Welch T.E., Vilback A. Microvascular blood flow and stasis in transgenic sickle mice: utility of a dorsal skin fold chamber for intravital microscopy. Am. J. Hematol. 2004;77:117–125. doi: 10.1002/ajh.20143. [DOI] [PubMed] [Google Scholar]
- 35.Dewhirst M.W., Shan S., Cao Y., Moeller B., Yuan F., Li C.Y. Intravital fluorescence facilitates measurement of multiple physiologic functions and gene expression in tumors of live animals. Dis. Markers. 2002;18:293–311. doi: 10.1155/2002/820102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Castro L.M., Zennadi R., Jonassaint J.C., Batchvarova M., Telen M.J. Effect of propranolol as antiadhesive therapy in sickle cell disease. Clin. Transl. Sci. 2012;5:437–444. doi: 10.1111/cts.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pazhanisamy S.K., Li H., Wang Y., Batinic-Haberle I., Zhou D. NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability. Mutagenesis. 2011;26:431–435. doi: 10.1093/mutage/ger001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batinic-Haberle I., Tovmasyan A., Spasojevic I. Mn porphyrin-based redox-active drugs: differential effects as cancer therapeutics and protectors of normal tissue Against oxidative injury. Antioxid. Redox Signal. 2018;29:1691–1724. doi: 10.1089/ars.2017.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Misra H.P., Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J. Biol. Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- 40.Lander H.M., Milbank A.J., Tauras J.M., Hajjar D.P., Hempstead B.L., Schwartz G.D. Redox regulation of cell signalling. Nature. 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 41.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans M.K., Tovmasyan A., Batinic-Haberle I., Devi G.R. Mn porphyrin in combination with ascorbate acts as a pro-oxidant and mediates caspase-independent cancer cell death. Free Radic. Biol. Med. 2014;68:302–314. doi: 10.1016/j.freeradbiomed.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J., Ahn S., Ren X.R., Whalen E.J., Reiter E., Wei H. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan Q., Chen M., Zuo L., Shang X., Huang M.Z., Ciccarelli M. Myocardial Ablation of G protein-coupled receptor kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. PloS One. 2013;8:e66234. doi: 10.1371/journal.pone.0066234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakami M., Hattori M., Ohashi W., Fujimori T., Hattori K., Takebe M. Role of G protein-coupled receptor kinase 2 in oxidative and nitrosative stress-related neurohistopathological changes in a mouse model of sepsis-associated encephalopathy. J. Neurochem. 2018;145:474–488. doi: 10.1111/jnc.14329. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y., Schwartz E.A., Palmer G.M., Zennadi R. MEK1/2 inhibitors reverse acute vascular occlusion in mouse models of sickle cell disease. FASEB J. 2016;30:1171–1186. doi: 10.1096/fj.15-278481. [DOI] [PubMed] [Google Scholar]
- 47.Setty B.N., Kulkarni S., Stuart M.J. Role of erythrocyte phosphatidylserine in sickle red cell-endothelial adhesion. Blood. 2002;99:1564–1571. doi: 10.1182/blood.v99.5.1564. [DOI] [PubMed] [Google Scholar]
- 48.King M.R., Bansal D., Kim M.B., Sarelius I.H. The effect of hematocrit and leukocyte adherence on flow direction in the microcirculation. Ann. Biomed. Eng. 2004;32:803–814. doi: 10.1023/b:abme.0000030256.37022.02. [DOI] [PubMed] [Google Scholar]
- 49.Koppenol W.H., Moreno J.J., Pryor W.A., Ischiropoulos H., Beckman J.S. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem. Res. Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 50.Penela P., Murga C., Ribas C., Lafarga V., Mayor F., Jr. The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br. J. Pharmacol. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris D.M., Cohn H.I., Pesant S., Eckhart A.D. GPCR signalling in hypertension: role of GRKs. Clin. Sci. 2008;115:79–89. doi: 10.1042/CS20070442. [DOI] [PubMed] [Google Scholar]
- 52.Dorn G.W., 2nd GRK mythology: g-protein receptor kinases in cardiovascular disease. J. Mol. Med. 2009;87:455–463. doi: 10.1007/s00109-009-0450-7. [DOI] [PubMed] [Google Scholar]
- 53.Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Jindal H.K., Ai Z., Gascard P., Horton C., Cohen C.M. Specific loss of protein kinase activities in senescent erythrocytes. Blood. 1996;88:1479–1487. [PubMed] [Google Scholar]
- 55.Jaramillo M.C., Briehl M.M., Batinic-Haberle I., Tome M.E. Manganese (III) meso-tetrakis N-ethylpyridinium-2-yl porphyrin acts as a pro-oxidant to inhibit electron transport chain proteins, modulate bioenergetics, and enhance the response to chemotherapy in lymphoma cells. Free Radic. Biol. Med. 2015;83:89–100. doi: 10.1016/j.freeradbiomed.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jaramillo M.C., Briehl M.M., Crapo J.D., Batinic-Haberle I., Tome M.E. Manganese porphyrin, MnTE-2-PyP5+, acts as a pro-oxidant to potentiate glucocorticoid-induced apoptosis in lymphoma cells. Free Radic. Biol. Med. 2012;52:1272–1284. doi: 10.1016/j.freeradbiomed.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tovmasyan A., Sampaio R.S., Boss M.K., Bueno-Janice J.C., Bader B.H., Thomas M. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Biol. Med. 2015;89:1231–1247. doi: 10.1016/j.freeradbiomed.2015.10.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheng H., Yang W., Fukuda S., Tse H.M., Paschen W., Johnson K. Long-term neuroprotection from a potent redox-modulating metalloporphyrin in the rat. Free Radic. Biol. Med. 2009;47:917–923. doi: 10.1016/j.freeradbiomed.2009.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sheng H., Spasojevic I., Tse H.M., Jung J.Y., Hong J., Zhang Z. Neuroprotective efficacy from a lipophilic redox-modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: rodent models of ischemic stroke and subarachnoid hemorrhage. J. Pharmacol. Exp. Ther. 2011;338:906–916. doi: 10.1124/jpet.110.176701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shin S.W., Choi C., Lee G.H., Son A., Kim S.H., Park H.C. Mechanism of the antitumor and radiosensitizing effects of a manganese porphyrin, MnHex-2-PyP. Antioxid. Redox Signal. 2017;27:1067–1082. doi: 10.1089/ars.2016.6889. [DOI] [PubMed] [Google Scholar]
- 61.Tovmasyan A., Bueno-Janice J.C., Jaramillo M.C., Sampaio R.S., Reboucas J.S., Kyui N. Radiation-mediated tumor growth inhibition is significantly enhanced with redox-active compounds that cycle with ascorbate. Antioxid. Redox Signal. 2018;29:1196–1214. doi: 10.1089/ars.2017.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y., Carroll D.W., You Y., Chaiswing L., Wen R., Batinic-Haberle I. A novel redox regulator, MnTnBuOE-2-PyP(5+), enhances normal hematopoietic stem/progenitor cell function. Redox Biol. 2017;12:129–138. doi: 10.1016/j.redox.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrushanko I.Y., Lobachev V.M., Kononikhin A.S., Makarov A.A., Devred F., Kovacic H. Oxidation of capital ES, Cyrillicsmall a, Cyrillic2+-binding domain of NADPH Oxidase 5 (NOX5): toward understanding the mechanism of inactivation of NOX5 by ROS. PLoS One. 2016;11:e0158726. doi: 10.1371/journal.pone.0158726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inanami O., Johnson J.L., Babior B.M. The leukocyte NADPH oxidase subunit p47PHOX: the role of the cysteine residues. Arch. Biochem. Biophys. 1998;350:36–40. doi: 10.1006/abbi.1997.0484. [DOI] [PubMed] [Google Scholar]
- 65.Hebbel R.P. The sickle erythrocyte in double jeopardy: autoxidation and iron decompartmentalization. Semin. Hematol. 1990;27:51–69. [PubMed] [Google Scholar]
- 66.Stone P.C., Stuart J., Nash G.B. Effects of density and of dehydration of sickle cells on their adhesion to cultured endothelial cells. Am. J. Hematol. 1996;52:135–143. doi: 10.1002/(SICI)1096-8652(199607)52:3<135::AID-AJH2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 67.Cytlak U.M., Hannemann A., Rees D.C., Gibson J.S. Identification of the Ca(2)(+) entry pathway involved in deoxygenation-induced phosphatidylserine exposure in red blood cells from patients with sickle cell disease. Pflug. Arch.: Eur. J. Physiol. 2013;465:1651–1660. doi: 10.1007/s00424-013-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Youssry I., Abdel-Salam A., Ismail R., Bou-Fakhredin R., Mohamed Samy R., Ezz El-Deen F. Enhancing effect of hydroxyurea on Hb F in sickle cell disease: ten-year egyptian experience. Hemoglobin. 2017;41:267–273. doi: 10.1080/03630269.2017.1408646. [DOI] [PubMed] [Google Scholar]
- 69.Moreira J.A., Machado R.P., Laurentino M.R., Lemes R.P., Barbosa M.C., Santos T.E. Influence of betaS-globin haplotypes and Hydroxyurea on Arginase I levels in sickle cell disease. Dis. Markers. 2016;2016:9172726. doi: 10.1155/2016/9172726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho C.S., Kato G.J., Yang S.H., Bae S.W., Lee J.S., Gladwin M.T. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid. Redox Signal. 2010;13:1–11. doi: 10.1089/ars.2009.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silva D.G., Belini Junior E., Torres Lde S., Ricci Junior O., Lobo Cde C., Bonini-Domingos C.R. Relationship between oxidative stress, glutathione S-transferase polymorphisms and hydroxyurea treatment in sickle cell anemia. Blood Cells Mol. Dis. 2011;47:23–28. doi: 10.1016/j.bcmd.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Rivers A., Vaitkus K., Jagadeeswaran R., Ruiz M.A., Ibanez V., Ciceri F. Oral administration of the LSD1 inhibitor ORY-3001 increases fetal hemoglobin in sickle cell mice and baboons. Exp. Hematol. 2018;67 doi: 10.1016/j.exphem.2018.08.003. (60-4 e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Adhesion of normoxia-exposed human SSRBCsin vivo. Exposure to normoxia of SSRBCs showed no real adhesion in post-capillary vessels and arterioles.
Adhesion of H/R-exposed human SSRBCsin vivo. Exposure of SSRBCs to H/R ex vivo followed by infusion to animals showed extensive adhesion in small post-capillary vessels and arterioles and progression of vasoocclusion. This led to permanent blockade of some venules both at junctions and straight segments causing blood flow stasis.
Mn porphyrin treatment of human SSRBCs reduced the effect of H/R on adhesionin vivo. Ex vivo treatment with 1 μM MnE of Human SSRBC exposed to H/R, followed by washing prior to infusion to nude mice, abrogated adhesion of these sickle cells in vessels and arterioles, reduced vasoocclusion, and restored blood flow.
Inhibition of NOX enzymes reduced the effect of H/R on human SSRBC adhesionin vivo. Apocynin treatment of human SSRBC exposed to H/R ex vivo, washed, then infused to nude mice, reduced adhesion of these sickle cells to the vessel and arteriole walls, and prevented vasoocclusion.
Supplementary material