Abstract
Nitric Oxide (NO) and Hydrogen Sulfide (H2S) are components of an “interactome”, which is defined as a redox system involving the interactions of RSS, RNS and ROS. Chemical interaction by these species is common and is characterized by one and two electron oxidation, nitrosylation, nitration and sulfuration/polysulfidation reactions. NO and H2S are gases that penetrate cell membranes, are synthesized by specific enzymes, are ubiquitous, regulate protein activities through post-translational modifications and participate in cell signaling. The two molecules at high concentrations compared to physiological concentrations may result in cellular damage particularly through their interaction with other reactive species. NO and H2S can interact with each other and form a variety of molecular species which may have constructive or destructive behavior depending on the cell type, the cellular environment (ex. oxygen tension, pH, redox state), where the products are produced and in what concentrations. Cross talk exists between NO and H2S, whereby they can influence the generation and signaling behavior of each other. Given the above mentioned properties of NO and H2S and studies in cancer cells and animal models employing NO and H2S donors that generate higher than physiological concentrations of NO and H2S and are effective in killing cancer cells but not normal cells, lend credence to the possibility of the utility of these donors in an approach to the treatment of cancer.
1. Introduction
Nitric oxide (NO) is a gaseous, cell membrane permeable, free radical endowed with a wide range of biological activities. Within normal and tumor cells, NO is generated by the catalyzed oxidation of the amino acid l-arginine by two constitutive and one inducible isoform of the NO synthases (NOS). Most of NO-mediated functions occur through a c-GMP-dependent pathway which is important for vasodilation, neurotransmission, and smooth muscle cell relaxation. Cyclic-GMP-independent pathways involve the reactions of NO with O2, O2−, the transition metal zinc and thiols. Although less frequent, these reactions bear important consequences for cellular signaling [1].
The combination of NO with O2− or with thiols results in nitration and s-nitrosylation of proteins, respectively. S-nitrosylation, but not tyrosine nitration, of cysteine thiols is clearly associated with cell signaling [[2], [3], [4], [5]]. Many intracellular proteins that function in the canonical signaling pathways, such as Ras, Src kinase, EGFR, the ERK1/2 MAP kinases, PI3K, and Akt, have their activities regulated by S-nitrosylation [1,6,7]. NO-mediated S-nitrosylation of canonical signaling pathways either stimulates or inhibits normal or tumor cell proliferation [1,6].
In the last three decades, a growing interest in the carcinogenic and anti-carcinogenic properties of NO has become evident. This dual character is directly related to the NO concentration. At concentrations equal to 200 nM or above, NO acts as an anti-carcinogenic agent; whereas below this threshold, survival and pro-carcinogenic signaling pathways are stimulated [8]. Physiological concentrations of NO such as those generated intracellularly by NOS are carcinogenic [9]. They may facilitate cancer cell proliferation by inhibiting apoptosis, stimulating angiogenesis, and promoting genomic instability [[10], [11], [12], [13]].
Various classes of NO donors have been investigated regarding their anti-carcinogenic actions. These compounds can release NO within a wide range of concentrations and time release [14]. NO is freely diffusible and readily oxidized in the intracellular milieu, an aspect that limits its signaling capacity. A particular class of NO donors, the S-nitrosothiols (SNOs), can overcome this limitation by protecting the NO moiety from oxidation and extending its time of action [1]. The role of S-nitrosylation of signaling proteins and non-physiological concentrations of NO generated by NO donors in anti-carcinogenesis is worth exploring for therapeutic purposes [1,6].
The concept of a Redox Code based on oxidative modifications of specific cysteine residues on proteins (cysteine redox switches) allows for the characterization of other redox-based posttranslational modifications [15]. In addition to S-nitrosylation, sulfhydration/persulfidation also target regulatory cysteine redox switches in proteins. These modifications are mediated by hydrogen sulfide (H2S) and its one-electron oxidation products, the reactive sulfur species (RSS).
H2S is a colorless and smelling gas synthesized in normal and cancer cells by two pyridoxal-5-phosphate-dependent cytosolic enzymes, cystathionine β-synthase (CBS) and cystathionine γ-5-lyase (CSE) [16]. A third enzyme, 3-Mercaptopyruvate sulfur-transferase (3-MST), a pyridoxal-5-phosphate -independent enzyme, acts in combination with another enzyme, cysteine aminotransferase to produce H2S from l-cysteine and α-ketoglutarate. Both enzymes are localized in the cytosol and mitochondria [17]. The three enzymes are constitutively expressed in normal and cancer cells, but only CSE expression is induced by inflammatory mediators [18].
H2S generated by H2S-generating enzymes may play an important role in cancer development [19]. Elevated CSE expression is found in lung adenocarcinomas, various hepatoma cell lines, melanomas, glioblastomas, astrocytomas, and renal carcinoma [20]. CBS levels are suppressed in gastric and colorectal cancer by specific methylation of the enzyme promoter [21]. Suppression of CSE expression is associated with the development and progression of human gliomas [22]. In contrast to CBS and CSE, 3-MST levels are unchanged in human colon cancer cells when compared to the levels measured in normal colon epithelial cells [23]. The expression of the three H2S synthesizing enzymes described for different cancer cell types suggests that the expression of one of them suffices as an endogenous source of H2S.
Like NO, H2S is a gaseous transmitter that also has dual behavior regarding proliferation and cell death. This duality may be explained by the concentrations of H2S and RSS to which normal and cancer cells are exposed. At low concentrations (micromolar range), H2S is cytoprotective and stimulates cell proliferation. At high concentrations (millimolar levels), H2S is cytotoxic, promoting apoptosis and cell death [[24], [25], [26]].
H2S donors have been tested as potential therapeutic agents against hypertension; they provide protection against tissue damage promoted by ischemia-reperfusion and certain types of cancer [24,27]. The use of H2S donors in combination with SNO compounds is effective in inducing vasorelaxation [28]. This synergism may be operative in other clinical settings, such as cancer chemotherapy.
This review article discusses the role of SNOs and H2S donors as anti-carcinogenic agents either as cytotoxic agents themselves or acting in combination through potential synergistic actions in cancer chemotherapy.
2. S-nitrosothiols (SNOs) as anti-carcinogenic agents
A wide range of structurally diverse compounds known as NO donors, release NO and/or reactive nitrogen species at different rates and have been tested in cancer treatment [14].
Cancer cells treated with NO donors undergo apoptotic cell death, whereas normal cells are less affected [14]. This may occur by a number of mechanisms that include invasion suppression, HIF-1α interference and radio-sensitization [29], sensitization to tumor necrosis factor related apoptosis inducing ligand - TRAIL [30], decreasing membrane potential and ATP levels, generating ROS and lowering the mitochondrial permeability transition [31]. Certain NO donors can affect DNA methylation, histone de-acetylation and lysine de-methylation leading to p53 re-activation [32,33].
Among these various types of NO donors, the SNOs are of particular interest. SNOs are derived from the covalent attachment of NO to a sulfur moiety of an organic thiol [1]. They can promote protein and peptide S-nitrosylation through the transfer of a nitroso group from one SNO to a target cysteine thiol, referred to as transnitrosylation [34].
SNOs expand NO signaling capacities by limiting its diffusibility while extending its temporal and spatial actions. For instance, S-nitrosoglutathione (GSNO) at physiological concentrations regulates the activation of Ras and its compartmentalization during the stimulation of cell proliferation [1,35].
Another important aspect of the SNOs chemical biology is their capacity to release NO. SNO ester derivatives of non-steroidal anti-inflammatory drugs, as demonstrated by infrared spectroscopic analysis and theoretical calculations, effectively release NO [36]. Analysis of the conformational and structural aspects of SNOs may provide important information on an NO/SNO-mediated anti-carcinogenic activity of these compounds [36].
The two SNOs that have had their anti-carcinogenic properties extensively explored are: GSNO, which is a physiologically relevant SNO [1], and S-nitroso-N-acetylpenicillamine (SNAP), which is an effective nitrosylating compound [37]. The anti-carcinogenic effects of GSNO have been determined in several experimental settings. Direct effects of supra-physiological concentrations of GSNO inhibit tumor cell proliferation and tumor growth [[38], [39], [40]]. GSNO treatment of MCF-7 breast cancer cells resistant to doxorubicin, reversed drug resistance in these cells [41]. GSNO potentiates the anti-carcinogenic effects of cisplatin and radiation in head and neck squamous cell carcinoma [42].
SNAP also has a dual behavior regarding proliferative as opposed to anti-proliferative effects. Exposure of endothelial cells to concentrations of SNAP that release physiological levels of NO stimulates the Ras-ERK1/2 MAP kinases signaling pathway and cell cycle progression [4,43]. SNAP at concentrations that generate physiological levels of NO maintain vascular tone [44]. A slow and sustained release of NO from SNAP, induces apoptosis in CHP212 neuroblastoma cells [45]. SNAP increases p53 protein levels and induces apoptosis in ovarian cancer cells resistant or not to cisplatin [46]. Radio sensitization promoted by SNAP is observed in a number of cancer cell lines, such as glioma, cervical cancer HeLa cells, and murine mammary adenocarcinoma EMT-6 cells [47].
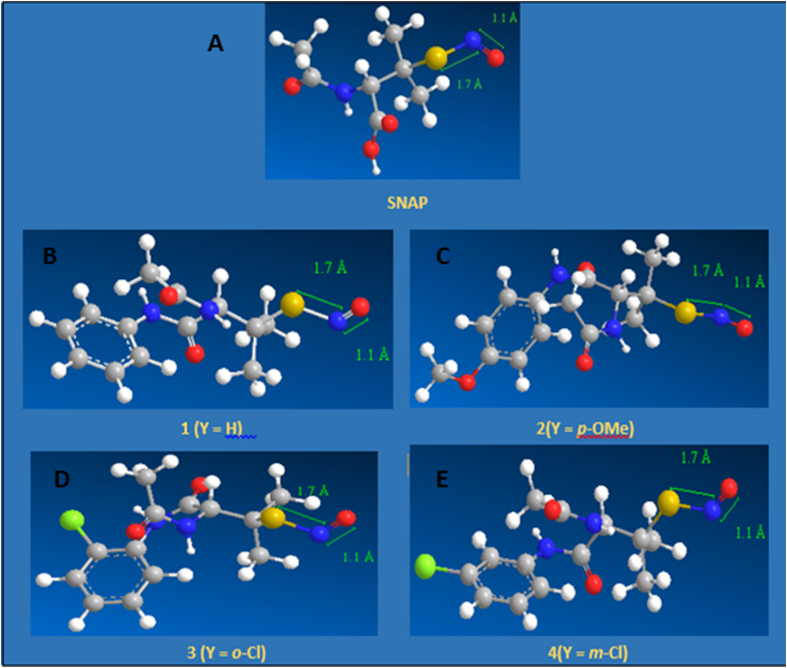
SNAP is a relatively stable and fairly water soluble SNO-derivative of penicillamine [48]. This led us to synthesize and characterize a series of S-nitroso-aryl-butanamides, novel SNO derivatives of penicillamine [49]. S-nitroso-aryl-butanamides are produced by reacting 2-acetamide-3-methyl-3-mercapto-N-aryl-butanamides in acetone and tert-butyl-nitrite [49]. Calculations of the S—N bond length for SNAP and for the S-nitroso-aryl-butanamides yield a value of 1.7 A which gives relative stability, and NO releasing capacity to the compounds [36] (Fig. 1). In vitro studies have revealed that the ortho- and meta chloro derivatives of the S-nitroso-aryl-butanamides are strongly cytotoxic against MCF-7 estrogen receptor positive human breast cancer cells. Human fibroblasts from normal breast tissue are less affected by these compounds [50]. This differential cytotoxicity might be related to the different capacities of normal and tumor cells to handle nitrosative stress conditions.
Fig. 1.
Conformational structures of the S-nitrosothiol-derivatives of penicillamine. A. S-Nitroso-N-Acetylpenicillamine – SNAP. B. S-Nitroso-Aryl-Butanamide – 1(Y = H). C. p-methoxy- S-Nitroso-Aryl-Butanamide – 2(Y = p-OMe). D. o-chloro- S-Nitroso-Aryl-Butanamide – 3(Y = o-Cl). E. m-chloro- S-Nitroso-Aryl-Butanamide – 4(Y = m-Cl). Calculations of the S-N bond length for SNAP and for the S-nitroso-aryl-butanamides yield a value of 1.7 Å, which gives these compounds relative stability and NO releasing capacity.
SNO donors act as anti-carcinogenics by promoting cell death through S-nitrosylation of specific protein targets [51]. S-nitrosylation of Glyceraldehide-3-phosphate-dehydrogenase at Cys 145 located at the active site of the enzyme, promotes cell death [52]. Apoptosis is induced in human acute monocytic leukemia - THP1- cells through the activation of the Ras-ERK1/2 MAP kinases by supra-physiological concentrations of GSNO [38]. Treatment of HEK293 cells with supra-physiological concentrations of GSNO, results in s-nitrosylation of the X-linked inhibitor of apoptosis, and inhibition of its anticaspase-3 and anti-apoptotic functions [53]. S-nitrosylation of specific cysteine residues of the cell death receptors TNF-R1, CD95, TRAIL-R1, and Fas stimulates apoptotic cell death of HepG2, an hepatoblastoma cancer cell line, and SW480 colon cancer cells [54,55]. .
Extracellular GSH and Cys are potential targets to nitroso groups transferred from a SNO compound through transnitrosylation, generating GSNO and CysNO [1]. GSNO and CysNO may function as signal transducers in SNO-mediated signaling events [56]. Extracellular GSNO does not cross the plasma membrane but can transfer its nitroso group to Cys. CysNO utilizes the L-type amino acid transporter on the plasma membrane for cell uptake [57]. Inside the cell, CysNO transfers its nitroso group to cytoplasmic GSH generating increasing concentrations of GSNO; potentially creating nitrosative stress conditions [56].
GSNO reductase and Thioredoxin-1 (Trx-1) have been characterized as denitrosylases in normal and tumor cells [58]. Trx-1 denitrosylase activity is associated with the decomposition of GSNO yielding NO and O2−, leading to the generation of the highly toxic oxidant peroxynitrite (ONOO-) [59,60]. Increasing expression levels of Trx-1 have been directly correlated with tumor progression [60].
Exposure of tumor cells to increasing concentrations of SNO donors might generate high intracellular levels of GSNO through transnitrosylation [56]. High GSNO levels associated with high expression levels of Trx-1 in tumor cells exposed to SNO donors sets up the condition for the establishment of nitrosative/nitrative stress. The high expression levels of Trx-1 in tumor cells may represent an important molecular target to be explored in chemotherapeutic regimens based on SNO donors.
3. H2S donors as anti-carcinogenic agents
H2S, like NO, has dual behavior regarding proliferation and cell death [25,[61], [62], [63]]. This duality may be explained by the concentration of the products of H2S reactivity and the fact that cancer cells have physical and biochemical characteristics that differ from normal cells. Low concentrations of H2S cause cancer cell proliferation, while high concentrations are cytotoxic [27]. Although many H2S donors have been synthesized and have a variety of effects [27], few have been studied for their anti-carcinogenic effect, as compared to NO donors.
Three major events have been consistently associated with H2S-mediated anti-carcinogenic effects: induction of uncontrolled intracellular acidification, induction of cell cycle arrest, and promotion of apoptosis [26].
The increased glucose uptake by cancer cells with the accumulation of lactate is known as the Warburg effect, and is directly associated with tumor growth and metastasis. Acidification derived from the excretion of lactate into the surrounding environment promotes angiogenesis, chemoresistance, and the suppression of the host immune system [64]. Uncontrolled intracellular acidification is stimulated by prolonged exposure of MCF-7 and HepG2 cells, breast and hepatic cancer cell lines respectively, to GYY4137, a slow-releasing H2S donor [65]. A decrease in glycolysis and an increase in lactate production leading to cell death have been observed in a number of cancer cells treated with GYY4137 [66,67], which decreases tumor growth in a xenograph mouse model [66].
Cell cycle inhibition has been consistently observed in, hepatic [66], breast [67], gastric [68] and colon [69] cancer cells exposed to high concentrations of various H2S donors. Various molecular targets have been described.
Apoptosis induced by an H2S donor [70] and an H2S releasing naproxen hybrid donor [71] in melanoma and other tumor cell lines is associated with suppression of NF-κB activity and inhibition of Akt and extracellular signal-regulated kinase pathways [70,71] and the naproxen hybrid decreases tumor development in a mouse melanoma model [71]. H2S donors potentiate green tea polyphenol induced apoptosis in multiple myeloma cells [72]. The precise intermediate by which the H2S donors influence cancer cell death is not fully appreciated.
The organosulfur compound diallyl disulfide (DADS); a natural H2S donor which is present in the oil-soluble fraction of garlic extracts [73], has anti-carcinogenic activities. Like other H2S donors, DADS anti-carcinogenic activities involve the induction of exacerbated intracellular acidification, cell cycle arrest and apoptosis. DADS-induced apoptosis is mediated by increasing ROS production in cells [74]. Ajoene, another organosulfur compound obtained from garlic extracts induces apoptosis mediated by ROS [75]. However, this is not a general anti-carcinogenic mechanism that can be attributed to other classes of H2S donors [27].
4. Interactions between nitric oxide, reactive oxygen species and reactive sulfur species and redox homeostasis
Interactions between NO, ROS, and RSS and their use by the machinery of normal and cancer cells, is necessary for maintenance of a homeostatic condition characterized by the formation, utilization, and elimination of the reactive species and their by-products [76]. This redox homeostasis is maintained by highly efficient detoxification systems, exemplified by the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), and GSH peroxidase, and by the redox systems, GSH, GSNO reductase, and Trx/Trx reductase [76]. In tumor cells, intracellular sources of ROS include the mitochondrial respiratory chain and the enzymes collectively known as the NADPH oxidases (NOXs) [77].
ROS and RSS might have their intracellular levels regulated by the same set of antioxidant enzymes. This is supported by the findings that SOD catalyzes the oxidation of H2S mostly to H2S2, and that CAT eliminates the H2S2 and other polysulfides [78,79].
ROS and RSS may interact with each other in opposite ways, depending on the nature of the donor; RSS can either promote or inhibit ROS generation in cancer cells [61,74,75]. The RSS donor, DADS, induces apoptosis in human leukemia cell lines through activation of NADPH oxidase (NOX) and stimulation of ROS production [80]. The polysulfide Na2S4 inhibits cisplatin-induced NOX activity in non-small-cell-lung cancer cell lines [61].
The interactions between NO and ROS can be exemplified by the very efficient reaction between NO and O2− that generates the highly toxic and potent oxidant ONOO- [81]. Although the reaction is very efficient, production of NO and O2− to generate ONOO-, has to occur at the same place, at the same time, and at the same rate [82]. Normal and cancer cells can avoid the generation of ONOO- by using specific means to prevent the process. SOD lowers the level of O2− through dismutation and generation of H2O2 which is metabolized by CAT. Minimizing NO levels is another way to avoid the reaction. Cancer cells express alternative splicing isoforms of the inducible isoform of NOS [83]. Alternative splicing isoforms form heterodimers with the full-length isoform, lowering the intracellular NO level [84]. Another strategy used by cancer cells to minimize NO levels is the overexpression of arginase [85].
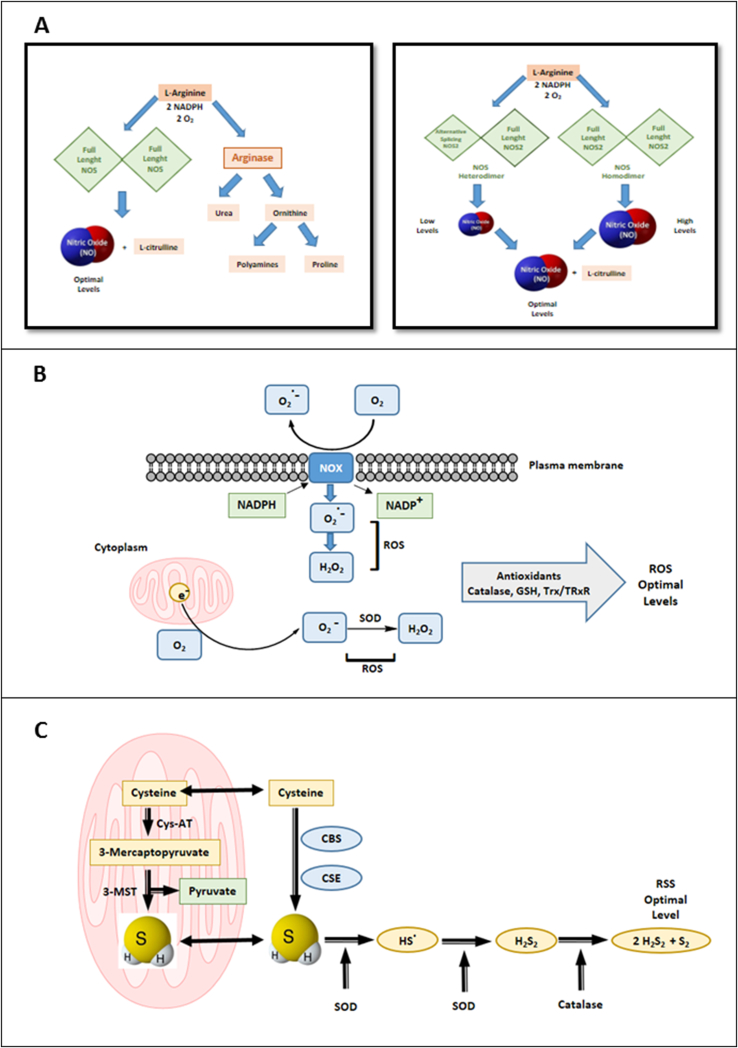
NO interacts with H2S to form intermediates that may include nitrosothiols, nitrosopersulfides, nitroxyl, and nitrous oxide [86]. Effective means of control of this production of a plethora of reactive species arising from interactions between NO, ROS, and RSS are likely to be operative in cancer cells. The putative ways for maintaining intracellular redox homeostasis are summarized in Fig. 2.
Fig. 2.
Biosynthesis of nitric oxide and hydrogen sulfide, generation of reactive oxygen species, and redox homeostasis. A. NO is produced by three nitric oxide synthase (NOS) isoforms: two constitutive isoforms, NOS-1 and NOS-3, and one inducible, NOS-2 catalyzes the oxidation of l-arginine to l-citrulline. NOS-2 is widely expressed in tumor cells and NOS-2 alternative splicing isoforms potentially regulate negatively intracellular NO levels in these cells. Negative regulation of NO production is also achieved through the upregulation of arginase. B. Reactive oxygen species (ROS) are produced through activation of the NADPH oxidase (NOX) enzymes or through leakage of the mitochondrial electron transport chain which releases O2−. O2- is dismutated to H2O2 by Superoxide Dismutases (SOD) and H2O2 is reduced to H2O by Catalase, maintaining intracellular optimal levels of the reactive species. C. H2S is generated from oxidation of l-cysteine and other substrates, including 3-mercaptopyruvate. Two cytoplasmic enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) and two mitochondrial enzymes 3-mercaptopyruvate sulfur-transferase (3-MST) and Cysteine aminotransferase (Cys-AT) are responsible for intracellular generation of H2S. H2S can be converted into polysulfide and other reactive sulfur species (RSS). SOD and Catalase may help in the maintenance of optimal intracellular levels of RSS.
5. Nitric oxide, S-Nitrosothiols and H2S donors: potential synergistic actions against cancer
The fact that NO and H2S can interact to form a variety of intermediate species [86] affecting each other's biochemical behavior, suggests that the combined administration of donors with each of the gaseous transmitters or the sole administration of a single compound with capacity to generate H2S and NO might cause cancer cell apoptosis.
Isomers of NO, H2S-hybrids with acetylsalicylic acid that are capable of releasing NO and H2S, although the causative intermediate(s) have not been characterized nor the extent of release of NO and H2S, cause apoptosis in a number of cancer cell lines [[87], [88], [89]]. One of the compounds, NBS 1120, administered to athymic nude mice bearing a human colon cancer xenograph has significantly reduced the size of the tumor and has functioned as a chemo-preventive agent [90]. When an H2S and an NO-donor have been administered simultaneously as individual agents in treating human colon cancer cells no additive or synergistic behavior in anti-proliferative effects is observed [24].
NaHS releases NO from the SNOs, GSNO and SNAP and from the metal nitrosyl complex nitroprusside under physiological conditions [91]. Transnitrosation reactions between equimolar amounts of Na2S and buffered solutions of GSNO yield thionitrous acid (HSNO) and nitrosopersulfide (SSNO-). HSNO decomposition yields as intermediates, NO and its reduced form, nitroxyl (HNO). The efficiency of HSNO in transnitrosation reactions is much higher compared to two physiologically relevant SNOs, GSNO and CysNO [[92], [93], [94], [95]].
6. Clinical considerations
Substantial progress has been made in understanding the behavior and mechanisms of action of H2S and NO-donors [14,24]. Since the donors in general have the ability to cause cell death in cancer cells but not in normal cells, it has led to the possibility that the donors may be complementarily effective in cancer treatment. As with all therapeutic interventions of chemical agents, awareness is needed of pharmacokinetics, pharmacodistribution, metabolism, drug delivery, bioavailability, tissue specificity, adverse reactions, donor-drug interactions and the physical and chemical properties of the therapeutic agent which in the case of H2S and NO donors are at a very early stage of our knowledge [96].
A number of clinical trials with relatively positive outcomes have been performed involving two classes of NO donors, the organic nitrate glyceryl trinitrate and the compounds derived from dinitroazetidine, RRx-001 and RRx-002 [97,98]. However, SNO donors for use in cancer chemotherapy have not received FDA approval, yet.
Clinical trials for H2S donors and combined H2S and NO donors have not been carried out, but given the cancer cellular and animal model findings [24,[87], [88], [89], [90]], the development of H2S-hybrid donors in clinical trials of osteoarthritis [99] and the protective effect on renal damage induced by cisplatin by an H2S donor [61,100], cautious optimism is in order for moving ahead with clinical trials with H2S and combined H2S and NO donors in patients with cancer.
7. Concluding remarks
H2S and NO as gaseous transmitters have been used therapeutically in the past several decades for specific diseases [24,88,101]. As a greater understanding of the biochemical functions of these gases have been uncovered and especially by employing donors of H2S and NO, it is apparent that donors provide a reasonable approach for assessing their value in treating patients with cancer. The donors of H2S and NO have a variety of chemical structures and conformational isomers that presumably enhance the release of the gaseous transmitters, particularly in the case of NO [36]. Hybrids have been developed that combine a known chemotherapeutic agent capable of releasing H2S or NO, or both gaseous transmitters. These hybrids may be beneficial insofar as providing lower doses of the original chemotherapeutic agent to minimize toxicity of these agents, while releasing the gaseous transmitter and perhaps gaining an additional chemotherapeutic effect. This has been observed for the anti-inflammatory-H2S hybrids used in the treatment of osteoarthritis [99]. Various H2S [26,102] and NO-releasing compounds [24] have been developed that are of relevance to cancer chemotherapy.
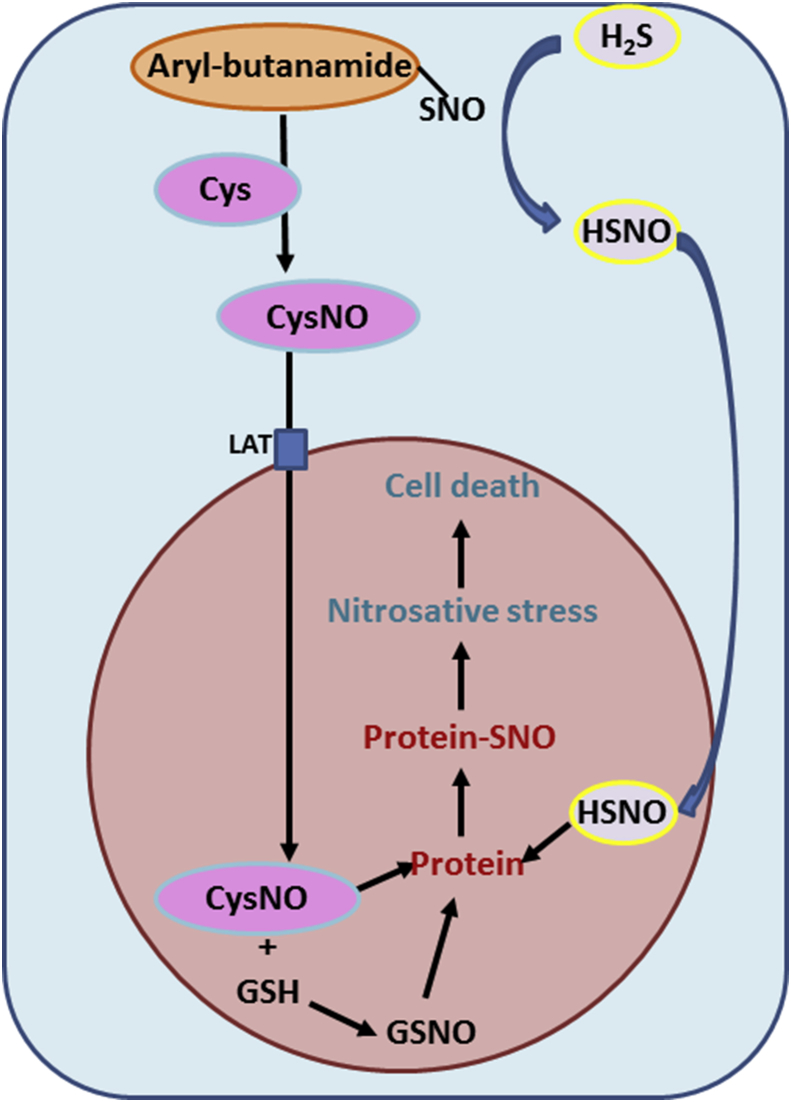
The combination of SNO donors and H2S may prove to be the most efficient, regarding potential chemotherapeutic applications. Formation of CysNO at the external face of the plasma membrane may occur through the reaction of SNO donors and Cysteine. CysNO is membrane impermeable, but is taken up by the cell through the L-type amino acid transporter on the plasma membrane [57]. The reaction between SNO donors and H2S yields HSNO [92] which can diffuse freely through cell membranes. The combined effects of CysNO and HSNO inside the cell may amplify the nitrosating capacity of both compounds. Prolonged exposure of tumor cells to this highly efficient combination of nitrosating species leads to nitrosative stress conditions and consequently to apoptosis (Fig. 3).
Fig. 3.
Proposed mechanism for nitrosative stress in tumor cells exposed to a combination of SNO donors and H2S. The SNO donor SNO-Aryl-butanamide nitrosylates Cys generating CysNO. CysNO directly enters the cell through an amino-acid transporter (LAT). SNO-Aryl-butanamide reacts with H2S generating HSNO which freely diffuses into the cell. CysNO and HSNO nitrosylate GSH and protein thiols promoting nitrosative stress and tumor cell apoptosis.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The authors wish to acknowledge the various students, post-docs, and collaborators whose published reports contributed importantly to this review article. The support from the Brazilian Funding Institutions, FAPESP (Grant Numbers: 2010/51784-3; 2012/10470-1; 2016/06539-7), CNPq (Grant Number: 481154/2013-2), and CAPES, is greatly acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2019.101190.
Contributor Information
Arnold Stern, Email: arnold.stern@nyumc.org.
Hugo Pequeno Monteiro, Email: hugo.monteiro@unifesp.br.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Hess D.T., Matsumoto A., Kim S., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 2.Monteiro H.P. Signal transduction by protein tyrosine nitration: competition or cooperation with tyrosine phosphorylation-dependent signaling events? Free Radic. Biol. Med. 2002;33:765–773. doi: 10.1016/s0891-5849(02)00893-6. [DOI] [PubMed] [Google Scholar]
- 3.Monteiro H.P., Arai R.J., Travassos L.R. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxidants Redox Signal. 2008;10:843–889. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira C.J.R., Curcio M.F., Moraes M.S., Tsujita M., Travassos L.R., Stern A., Monteiro H.P. The low molecular weight S -nitrosothiol , S -nitroso- N -acetylpenicillamine , promotes cell cycle progression in rabbit aortic endothelial cells. Nitric Oxide. 2008;18:241–255. doi: 10.1016/j.niox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Zhou H., Stomberski C.T., Stamler J.S. Cross talk between S-nitrosylation and phosphorylation involving kinases and nitrosylases. Circ. Res. 2018;3:1485–1487. doi: 10.1161/CIRCRESAHA.118.313109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro H.P., Costa P.E., Reis A.K.C.A., Stern A. Nitric oxide: protein tyrosine phosphorylation and protein S -nitrosylation in cancer. Biomed. J. 2015;38:380–388. doi: 10.4103/2319-4170.158624. [DOI] [PubMed] [Google Scholar]
- 7.González R., Molina-Ruiz F.J., Bárcena J.A., Padilla C.A., Muntané J. Regulation of cell survival, apoptosis, and epithelial-to-mesenchymal transition by nitric oxide-dependent post-translational modifications. Antioxidants Redox Signal. 2018;29:1312–1332. doi: 10.1089/ars.2017.7072. [DOI] [PubMed] [Google Scholar]
- 8.Ridnour L.A., Thomas D.D., Switzer C., Flores-santana W., Isenberg J.S., Ambs S., Roberts D.D., Wink D.A. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide. 2008;19:73–76. doi: 10.1016/j.niox.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Oliveira G.A., Cheng R.Y.S., Ridnour L.A., Basudhar D., Somasundaram V., McVicar D.W., Monteiro H.P., Wink D.A. Inducible nitric oxide synthase (NOS2) in the carcinogenesis of gastrointestinal cancers. Antioxidants Redox Signal. 2017;26:1059–1077. doi: 10.1089/ars.2016.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang R., Li Y., Tsung A., Huang H., Du Q., Yang M., Deng M., Xiong S., Wang X., Zhang L., Geller D.A., Cheng B., Billiar T.R. iNOS promotes CD24+CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E10127–E10136. doi: 10.1073/pnas.1722100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wink D.A., Hanbauert I., Krishnat M.C., DeGraff W., Gamson J., Mitchell J.B. Nitric oxide protects against cellular damage and cytotoxicity from reactive. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moraes M.S., Costa P.E., Batista W.L., Paschoalin T., Curcio M.F., Borges R.E., Taha M.O., Fonseca F.V., Stern A., Monteiro H.P. Endothelium-derived nitric oxide ( NO ) activates the NO-epidermal growth factor receptor-mediated signaling pathway in bradykinin- stimulated angiogenesis. Arch. Biochem. Biophys. 2014;558:14–27. doi: 10.1016/j.abb.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Switzer C.H., Glynn S.A., Cheng R.Y., Ridnour L.A., Green J.E., Ambs S., Wink D.A. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol. Canc. Res. 2012:1203–1216. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z., Fu J., Zhang Y. Nitric oxide donor-based cancer Therapy : advances and prospects. J. Med. Chem. 2017:7617–7635. doi: 10.1021/acs.jmedchem.6b01672. [DOI] [PubMed] [Google Scholar]
- 15.Jones D.P., Sies H. The redox code. Antioxidants Redox Signal. 2015;23:734–746. doi: 10.1089/ars.2015.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamoun P. Amino Acids; 2004. Endogenous Production of Hydrogen Sulfide in Mammals; pp. 243–254. [DOI] [PubMed] [Google Scholar]
- 17.Olson K.R. Hydrogen sulfide as an oxygen sensor. Antioxidants Redox Signal. 2015;22:377–397. doi: 10.1089/ars.2014.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paul B.D., Snyder S.H. H2S: a novel gasotransmitter that signals by sulfhydration. Trends Biochem. Sci. 2015;40:687–700. doi: 10.1016/j.tibs.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu D., Si W., Wang M., Lv S., Ji A., Li Y. Hydrogen sulfide in cancer: friend or foe? Nitric Oxide. 2015;50:38–45. doi: 10.1016/j.niox.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Augsburger F., Szabo C. Potential role of the 3-mercaptopyruvate sulfur-transferase (3-MST)—hydrogen sulfide (H2S) pathway in cancer cells. Pharmacol. Res. 2018 doi: 10.1016/j.phrs.2018.11.034. S1043-6618(18)31594-9. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Zhao H., Li Q., Wang J., Su X., Ng K.M., Qiu T., Shan L., Ling Y., Wang L., Cai J., Ying J. Frequent epigenetic silencing of the folate-metabolising gene cystathionine-b-synthase in gastrointestinal cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takano N., Sarfraz Y., Gilkes D.M., Chaturvedi P., Xiang L., Suematsu M., Zagzag D., Semenza G.L. Decreased expression of cystathionine b-synthase promotes glioma tumorigenesis. Mol. Canc. Res. 2014;12:1398–1406. doi: 10.1158/1541-7786.MCR-14-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jurkowska H., Placha W., Nagahara N., Wróbel M. The expression and activity of cystathionine-g-lyase and 3-mercaptopyruvate sulfur-transferase in human neoplastic cell lines. Amino Acids. 2011;41:151–158. doi: 10.1007/s00726-010-0606-3. [DOI] [PubMed] [Google Scholar]
- 24.Szabo C. A timeline of hydrogen sulfide (H2S) research: from environmental toxin to biological mediator. Biochem. Pharmacol. 2018:5–19. doi: 10.1016/j.bcp.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hellmich M.R., Coletta C., Chao C., Szabo C. The therapeutic potential of cystathionine-beta- synthetase/hydrogen sulfide inhibition in cancer. Antioxid. Redox Signal. 2015;22:424–448. doi: 10.1089/ars.2014.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cao X., Ding L., Xie Z., Yang Y., Whiteman M., Moore P.K., Bian J. A review of hydrogen sulfide synthesis , metabolism and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxidants Redox Signal. 2018;0 doi: 10.1089/ars.2017.7058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu D., Hu Q., Zhu Y. Therapeutic application of hydrogen sulfide donors : the potential and challenges. Front. Med. 2015;10:18–27. doi: 10.1007/s11684-015-0427-6. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez J., Maloney R.E., Rassaf T., Bryan N.S., Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc. Natl. Acad. Sci. U. S. A. 2002;100:336–341. doi: 10.1073/pnas.0234600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huerta S., Chilka S., Bonavida B. Nitric oxide donors : novel cancer therapeutics (Review) Int. J. Oncol. 2008;33:909–927. [PubMed] [Google Scholar]
- 30.Yang L., Lan C., Fang Y., Zhang Y., Wang J., Guo J., Wan S., Yang S., Wang R., Fang D. Sodium nitroprusside (SNP) sensitizes human gastric cancer cells to TRAIL-induced apoptosis. Int. Immunopharmacol. 2013;17:383–389. doi: 10.1016/j.intimp.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Pestana C.R., Phelippin D.P.S., Polizello A.C.M., Dorta D.J., Uyemura S.A., Santos A.C., Doro F.G., Rodrigues F.P., Tfouni E., Curti C. Effects on mitochondria of mitochondria-induced nitric oxide release from a ruthenium nitrosyl complex. Nitric Oxide. 2009;20:24–30. doi: 10.1016/j.niox.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Ning S., Bednarski M., Oronsky B., Scicinski J., Saul G., Knox S.J. Dinitroazetidines are a novel class of anticancer agents and hypoxia-activated radiation sensitizers developed from highly energetic materials. Cancer Res. 2012;72:2600–2609. doi: 10.1158/0008-5472.CAN-11-2303. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H., Ning S., Scicinski J., Oronsky B., Knox S.J., Peehl D.M. Epigenetic effects of RRx-001: a possible unifying mechanism of anticancer activity. Oncotarget. 2015;6:43172–43181. doi: 10.18632/oncotarget.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogg N. The biochemistry and physiology of S-nitrosothiols. Annu. Rev. Pharmacol. Toxicol. 2002:585–600. doi: 10.1146/annurev.pharmtox.42.092501.104328. [DOI] [PubMed] [Google Scholar]
- 35.Batista W.L., Ogata F.T., Curcio M.F., Miguel R.B., Arai R.J., Matsuo A.L., Moraes M.S., Stern A., Monteiro H.P. S -nitrosoglutathione and endothelial nitric oxide synthase-derived nitric oxide regulate compartmentalized Ras S-nitrosylation and stimulate cell proliferation. Antioxidants Redox Signal. 2013;18:221–238. doi: 10.1089/ars.2011.4455. [DOI] [PubMed] [Google Scholar]
- 36.Reginato M.M., Paiva D.R., Sensato F.R., Monteiro H.P., Reis A.K.C.A. Conformational study of the electronic interactions and nitric oxide release potential of new S nitrosothiols esters derivatives of ibuprofen , naproxen and phenyl acids substituted (SNO-ESTERS): synthesis, infrared spectroscopy analysis and theoretical c. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019;207:132–142. doi: 10.1016/j.saa.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Wang K., Wen Z., Zhang W., Xian M., Cheng J., Wang P.G. Equilibrium and kinetics studies of transnitrosation between S -nitrosothiols and thiols. Bioorg. Med. Chem. Lett. 2001;11:433–436. doi: 10.1016/s0960-894x(00)00688-0. [DOI] [PubMed] [Google Scholar]
- 38.Tsujita M., Batista W.L., Ogata F.T., Stern A., Monteiro H.P., Arai R.J. The nitric oxide-sensitive p21Ras – ERK pathway mediates S -nitrosoglutathione-induced apoptosis. Biochem. Biophys. Res. Commun. 2008;369:1001–1006. doi: 10.1016/j.bbrc.2008.02.117. [DOI] [PubMed] [Google Scholar]
- 39.Furuhashi S., Sugita H., Takamori H., Horino K.E.I., Nakahara O., Okabe H., Miyake K., Tanaka H., Beppu T., Baba H. NO donor and MEK inhibitor synergistically inhibit proliferation and invasion of cancer cells. Int. J. Oncol. 2012:807–815. doi: 10.3892/ijo.2011.1243. [DOI] [PubMed] [Google Scholar]
- 40.Giri S., Rattan R., Deshpande M., Maguire J.L., Johnson Z., Graham R.P., Shridhar V. Preclinical therapeutic potential of a nitrosylating agent in the treatment of ovarian cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Luca A., Moroni N., Serafino A., Primavera A., Pastore A., Pedersen J.Z., Petruzzelli R., Farrace M.G., Pierimarchi P., Moroni G., Federici G., Vallebona P.S., Bello M.L.O. Treatment of doxorubicin-resistant MCF7/Dx cells with nitric oxide causes histone glutathionylation and reversal of drug resistance. Biochem. J. 2011;183:175–183. doi: 10.1042/BJ20111333. [DOI] [PubMed] [Google Scholar]
- 42.Kaliyaperumal K., Sharma A.K., Mcdonald D.G., Dhindsa J.S., Yount C., Singh A.K., Won J., Singh I. S-nitrosoglutathione-mediated STAT3 regulation in efficacy of radio- therapy and cisplatin therapy in head and neck squamous cell. Redox Biol. 2015;6:41–50. doi: 10.1016/j.redox.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oliveira C.J.R., Schindler F., Ventura A.M., Morais M.S., Arai R.J., Debbas V., Stern A., Monteiro H.P. Nitric oxide and cGMP activate the Ras-MAP kinase pathway-stimulating protein tyrosine phosphorylation in rabbit aortic endothelial cells. Free Radic. Biol. Med. 2003;35:381–396. doi: 10.1016/s0891-5849(03)00311-3. [DOI] [PubMed] [Google Scholar]
- 44.Megson I.L., Morton S., Greig I.R., Mazzei F.A., Field R.A., Butler A.R., Caron G., Gasco A., Fruttero R., Webb D.J. N-substituted analogues of S-nitroso- N -acetyl- D , L -penicillamine : chemical stability and prolonged nitric oxide mediated vasodilatation in isolated rat femoral arteries. Br. J. Pharmacol. 1999;126:639–648. doi: 10.1038/sj.bjp.0702346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terwel D., Nieland L.J.M., Schutte B., Reutelingsperger C.P.M., Ramaekers F.C.S., Steinbusch H.W.M. S -nitroso- N -acetylpenicillamine and nitroprusside induce apoptosis in a neuronal cell line by the production of different reactive molecules. Eur. J. Pharmacol. 2000:19–33. doi: 10.1016/s0014-2999(00)00379-4. [DOI] [PubMed] [Google Scholar]
- 46.Leung E.L., Fraser M., Fiscus R.R., Tsang B.K. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells : involvement in p53 regulation and cisplatin resistance. Br. J. Canc. 2008;98:1803–1809. doi: 10.1038/sj.bjc.6604375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huerta S. Nitric oxide for cancer therapy. Futur. Sci OA. 2015;1 doi: 10.4155/fso.15.44. FSO44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Askew S.C., Butler A.R., Flitney F.W., Kemp D., Megson I.L. Chemical mechanisms underlying the vasodilator and platelet anti-aggregating properties of S-nitroso-N-acetyl-DL- penicillamine and S-nitrosoglutathione. Bioorg. Med. Chem. 1995;3:1–9. doi: 10.1016/0968-0896(94)00139-t. [DOI] [PubMed] [Google Scholar]
- 49.Santana R.G., Paiva D.R., Gomes R. da S., Reis A.K.C.A. 1H and 13C NMR analysis of 2-acetamido-3- mercapto-3-methyl- N -aryl-butanamides and N -aryl-butanamide derivatives. MRC Lett. 2013;51:316–319. doi: 10.1002/mrc.3944. [DOI] [PubMed] [Google Scholar]
- 50.Monteiro H.P., Sartori A., Reis A.K.C.A. Differential loss of cell viability after exposure of MCF-7 breast cancer cells and normal human mammary fibroblast to S-nitroso-arylamides. Abstr. Pap. Am. Chem. Soc. 2013;246 [Google Scholar]
- 51.Wang Y., Chen C., Loake G.J., Chu C. Nitric oxide: promoter or suppressor of programmed cell death? Protein Cell. 2010;1:133–142. doi: 10.1007/s13238-010-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hara M.R., Agrawal N., Kim S.F., Cascio M.B., Fujimuro M., Ozeki Y., Takahashi M., Cheah J.H., Tankou S.K., Hester L.D. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah 1 binding. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- 53.Tsang A.H., Lee Y.I., Ko H.S., Savitt J.M., Pletnikova O., Troncoso J.C., Dawson V.L., Dawson T.M., Chung K.K. S-nitrosylation of XIAP compromises neuronal survival in Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2009;106:4900–4905. doi: 10.1073/pnas.0810595106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Hernández A., Navarro-Villarán E., González R., Pereira S., Soriano-De Castro L.B., Sarrias-Giménez A., Barrera-Pulido L., Álamo-Martínez J.M., Serrablo-Requejo A., Blanco-Fernández G. Regulation of cell death receptor S-nitrosylation and apoptotic signaling by Sorafenib in hepatoblastoma cells. Redox Biol. 2015;6:174–182. doi: 10.1016/j.redox.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leon-Bollotte L., Subramaniam S., Cauvard O., Plenchette-Colas S., Paul C., Godard C., Martinez-Ruiz A., Legembre P., Jeannin J.F., Bettaieb A. S-nitrosylation of the death receptor fas promotes fas ligand-mediated apoptosis in cancer cells. Gastroenterology. 2011;140:2009–2018. doi: 10.1053/j.gastro.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto A., Gow A.J. Membrane transfer of S -nitrosothiols. Nitric Oxide. 2011;25:102–107. doi: 10.1016/j.niox.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Y., Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. U. S. A. 2004;1:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benhar M., Forrester M.T., Stamler J.S. Protein denitrosylation : enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 59.Nikitovic D., Holmgren A. S -nitrosoglutathione is cleaved by the Thioredoxin system with liberation of glutathione and redox regulating nitric oxide. J. Biol. Chem. 1996;271:19180–19185. doi: 10.1074/jbc.271.32.19180. [DOI] [PubMed] [Google Scholar]
- 60.Arai R.J., Ogata F.T., Batista W.L., Masutani H., Yodoi J., Debbas V., Augusto O., Stern A., Monteiro H.P. Thioredoxin-1 promotes survival in cells exposed to S-nitrosoglutathione : correlation with reduction of intracellular levels of nitrosothiols and up-regulation of the ERK1/2 MAP kinases. Toxicol. Appl. Pharmacol. 2008;233:227–237. doi: 10.1016/j.taap.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Cao X., Nie X., Xiong S., Cao L., Wu Z., Moore P.K., Bian J.-S. Renal protective effect of polysulfide in cisplatin-induced nephrotoxicity. Redox Biol. 2018;15:513–521. doi: 10.1016/j.redox.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cai W., Wang M., Ju L., Wang C., Zhu Y. Hydrogen sulfide induces human colon cancer cell proliferation: role of Akt, ERK and p21. Cell Biol. Int. 2010;34:565–572. doi: 10.1042/CBI20090368. [DOI] [PubMed] [Google Scholar]
- 63.Wu D., Li M., Tian W., Wang S., Cui L., Li H., Wang H., Ji A., Li Y. Hydrogen sulfide acts as a double-edged sword in human hepatocellular carcinoma cells through EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci. Rep. 2017;7:1–14. doi: 10.1038/s41598-017-05457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyssiotis C.A., Kimmelman A.C. Metabolic interactions in the tumor microenvironment. Trends Cell Biol. 2017;27:863–875. doi: 10.1016/j.tcb.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee Z.W., Teo X.Y., Tay E.Y., Tan C.H., Hagen T., Moore P.K., Deng L.W. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Br. J. Pharmacol. 2014;171:4322–4336. doi: 10.1111/bph.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu S., Gao Y., Huang X., Wang X. GYY4137, a hydrogen sulfide (H2S) donor, shows potent anti-hepatocellular carcinoma activity through blocking the STAT3 pathway. Int. J. Oncol. 2014;44:1259–1267. doi: 10.3892/ijo.2014.2305. [DOI] [PubMed] [Google Scholar]
- 67.Lee Z.W., Zhou J., Chen C., Zhao Y., Tan C., Li L., Moore P.K., Deng L. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One. 2011;6:1–7. doi: 10.1371/journal.pone.0021077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma K., Liu Y., Zhu Q., Liu C., Duan J., Tan B.K., Zhu Y.Z. H2S donor, S-Propargyl-Cysteine, increases CSE in SGC- 7901 and cancer-induced mice: evidence for a novel anti-cancer effect of endogenous H2S? PLoS One. 2011;6:1–12. doi: 10.1371/journal.pone.0020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Y.C., Wang X.J., Yu L., Chan F.K.L., Cheng A.S.L., Yu J., Sung J.J.Y., Wu W.K.K., Cho C.H. Hydrogen sulfide lowers proliferation and induces protective autophagy in colon epithelial cells. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0037572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panza E., De Cicco P., Armogida C., Scognamiglio G., Gigantino V., Botti G., Germano D., Napolitano M., Papapetropoulos A., Cirino G., Ianaro A. Role of the cystathionine γ lyase/hydrogen sulfide pathway in human melanoma progression. Pigment Cell Melanoma Res. 2015;28:61–72. doi: 10.1111/pcmr.12312. [DOI] [PubMed] [Google Scholar]
- 71.Kodela R., Nath N., Chattopadhyay M., Nesbitt D.e, Martínez C.A.V., Kashfi K. Hydrogen sulfide-releasing naproxen suppresses colon cancer cell growth and inhibits NF-κB signaling. Drug Des. Dev. Ther. 2015;9:4873–4882. doi: 10.2147/DDDT.S91116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bae J., Kumazoe M., Yamashita S., Tachibana H. Hydrogen sulphide donors selectively potentiate a green tea polyphenol EGCG-induced apoptosis of multiple myeloma cells. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-06879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Milner J.A. Preclinical perspectives on garlic and cancer. J. Nutr. 2006;136:827S–831S. doi: 10.1093/jn/136.3.727S. [DOI] [PubMed] [Google Scholar]
- 74.Yi L., Su Q. Molecular mechanisms for the anti-cancer effects of diallyl disulfide. Food Chem. Toxicol. 2013;57:362–370. doi: 10.1016/j.fct.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 75.Dirsch V.M., Gerbes A.L., Vollmar A.M. Ajoene, a compound of garlic, induces apoptosis in human promyeloleukemic cells, accompanied by generation of reactive oxygen species and activation of nuclear factor kB. Mol. Pharmacol. 1998;53:402–407. doi: 10.1124/mol.53.3.402. [DOI] [PubMed] [Google Scholar]
- 76.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., Wink D.A., Fukuto J.M., Jackson A.A., van Goor H., Olson K.R., Feelisch M. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxidants Redox Signal. 2017;27:684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chio I.I.C., Tuveson D.A. ROS in cancer: the burning question. Trends Mol. Med. 2017;23:411–429. doi: 10.1016/j.molmed.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olson K.R., Gao Y., Arif F., Arora K., Patel S., DeLeon E.R., Sutton T.R., Feelisch M., Cortese-Krott M.M., Straub K.D. Metabolism of hydrogen sulfide (H2S) and production of reactive sulfur species (RSS) by superoxide dismutase. Redox Biol. 2018;15:74–85. doi: 10.1016/j.redox.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olson K.R., Gao Y., DeLeon E.R., Arif M., Arif F., Arora N., Straub K.D. Catalase as a sulfide-sulfur oxido-reductase: an ancient (and modern?) regulator of reactive sulfur species (RSS) Redox Biol. 2017;12:325–339. doi: 10.1016/j.redox.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yi L., Ji X.X., Tan H., Lin M., Tang Y., Wen L., Ma Y.H., Su Q. Role of of Ras-related C3 botulinum toxin substrate 2 (Rac 2), NADPH oxidase ande reactive oxygen species in diallyl disulphide-induced apoptosis of human leukaemia HL-60 cells. Clin. Exp. Pharmacol. Physiol. 2010;37:1147–1153. doi: 10.1111/j.1440-1681.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- 81.Szabó C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 82.Jourd'heuil D., Jourd'heuil F.L., Kutchukian P.S., Musah R.A., Wink D.A., Grisham M.B. Reaction of superoxide and nitric oxide with peroxynitrite implications for peroxynitrite-mediated oxidation reactions in vivo. J. Biol. Chem. 2001;276:28799–28805. doi: 10.1074/jbc.M102341200. [DOI] [PubMed] [Google Scholar]
- 83.Eissa N.T., Strauss A.J., Haggerty C.M., Choo E.K., Chu S.C., Moss J. Alternative splicing of human inducible nitric-oxide synthase mRNA: tissue-specific regulation and induction by cytokines. J. Biol. Chem. 1996;271:27184–27187. doi: 10.1074/jbc.271.43.27184. [DOI] [PubMed] [Google Scholar]
- 84.Sousa M.S., Latini F.R., Monteiro H.P., Cerutti J.M. Arginase 2 and nitric oxide synthase: pathways associated with the pathogenesis of thyroid tumors. Free Radic. Biol. Med. 2010;49:997–1007. doi: 10.1016/j.freeradbiomed.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 85.Monteiro H.P., Castro E.D., Rinaldi T., Mathias P.P.M., Stern A. The importance of nitric oxide/inducible nitric oxide synthase in the progression of human colon carcinoma. Nitric Oxide. 2014;42:110. [Google Scholar]
- 86.Cortese-Krott M.M., Fernandez B.O., Kelm M., Butler A.R., Feelisch M. On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide. 2015;46:14–24. doi: 10.1016/j.niox.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 87.Kodela R., Chattopadhyay M., Kashfi K. NOSH-aspirin: a novel nitric oxide − hydrogen sulfide-releasing hybrid: a new class of anti-inflammatory pharmaceuticals. ACS Med. Chem. Lett. 2012;3:257–262. doi: 10.1021/ml300002m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kashfi K., Chattopadhyay M., Kodela R. NOSH-sulindac (AVT-18A) is a novel nitric oxide- and hydrogen sulfide-releasing hybrid that is gastrointestinal safe and has potent anti-inflammatory, analgesic, antipyretic, anti-platelet, and anti-cancer properties. Redox Biol. 2015;6:287–296. doi: 10.1016/j.redox.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chattopadhyay M., Kodela R., Duvalsaint P.L., Kashfi K. Gastrointestinal safety, chemotherapeutic potential, and classic pharmacological profile of NOSH-naproxen (AVT-219 ) a dual NO- and H(2)S-releasing hybrid. Pharmacol. Res. Perspect. 2016;4:1–15. doi: 10.1002/prp2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kodela R., Chattopadhyay M., Velázquez-Martínez C.A., Kashfi K. NOSH-aspirin (NBS-1120), a novel nitric oxide- and hydrogen sulfide-releasing hybrid has enhanced chemo-preventive properties compared to aspirin, is gastrointestinal safe with all the classic therapeutic indications. Biochem. Pharmacol. 2015;98:564–572. doi: 10.1016/j.bcp.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ondrias K., Stasko A., Cacanyiova S., Sulova Z., Krizanova O., Kristek F., Malekova L., Knezl V., Breier A. H(2)S and HS(-) donor NaHS releases nitric oxide from nitrosothiols , metal nitrosyl complex , brain homogenate and murine L1210 leukaemia cells. Pflügers Archiv. 2008;457:271–279. doi: 10.1007/s00424-008-0519-0. [DOI] [PubMed] [Google Scholar]
- 92.Filipovic M.R., Miljkovic J.L., Nauser T., Royzen M., Klos K., Shubina T., Koppenol W.H., Lippard S.J., Ivanovic I.B. Chemical characterization of the smallest S -nitrosothiol, HSNO; cellular cross-talk of H(2)S and S -nitrosothiols. J. Am. Chem. Soc. 2012;134:12016–12027. doi: 10.1021/ja3009693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kolluru G.K., Shen X., Kevil C.G. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol. 2013;1:313–318. doi: 10.1016/j.redox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.King S.B. Potential biological chemistry of hydrogen sulfide (H(2)S) with the nitrogen oxides. Free Radic. Biol. Med. 2013;55:1–7. doi: 10.1016/j.freeradbiomed.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gheibi S., Jeddi S., Kashfi K., Ghasemi A. Regulation of vascular tone homeostasis by NO and H(2)S: implications in hypertension. Biochem. Pharmacol. 2018;149:42–59. doi: 10.1016/j.bcp.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsikas D., Schwedhelm K.S., Surdacki A., Giustarini D., Rossi R., Kukoc-modun L., Kedia G., Ückert S. S -Nitroso- N -acetyl-L-cysteine ethyl ester (SNACET) and N -acetyl-L- cysteine ethyl ester (NACET) – cysteine-based drug candidates with unique pharmacological profiles for oral use as NO, H2S and GSH suppliers and as antioxidants : results and overview. J. Pharm. Anal. 2018;8:1–9. doi: 10.1016/j.jpha.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sukhatme V., Bouche G., Meheus L., Sukhatme V.P., Pantziarka P. Repurposing drugs in oncology (ReDO)— nitroglycerin as an anti-cancer agent. Ecancermedicalscience. 2015;9:1–21. doi: 10.3332/ecancer.2015.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reid T., Oronsky B., Scicinski J., Scribner C.L., Knox S.J., Ning S., Peehl D.M., Korn R., Stirn M., Carter C.A., Oronsky A., Taylor M.J., Fitch W.L., Cabrales P., Kim M.M., Burris H.A., 3rd, Lao C.D., Abrouk N.E.D., Fanger G.R., Infante J.R. Safety and activity of RRx-001 in patients with advanced cancer: a first-in-human, open-label, dose-escalation phase 1 study. Lancet Oncol. 2015;16:1133–1142. doi: 10.1016/S1470-2045(15)00089-3. [DOI] [PubMed] [Google Scholar]
- 99.Wallace J.L., Vaughan D., Dicay M., Macnaughton W.K., De Nucci G. Hydrogen sulfide-releasing therapeutics: translation to the clinic. Antioxidants Redox Signal. 2018;28:1533–1540. doi: 10.1089/ars.2017.7068. [DOI] [PubMed] [Google Scholar]
- 100.Cao X., Xiong S., Zhou Y., Wu Z., Ding L., Zhu Y., Wood M.E., Whiteman M., Moore P.K., Bian J. Renal protective effect of hydrogen sulfide in cisplatin-induced nephrotoxicity. Antioxidants Redox Signal. 2017;29:455–470. doi: 10.1089/ars.2017.7157. [DOI] [PubMed] [Google Scholar]
- 101.Oliveira C., Benfeito S., Fernandes C., Cagide F., Silva T., Borges F. NO and HNO donors, nitrones, and nitroxides: past, present, and future. Med. Res. Rev. 2018;38:1159–1187. doi: 10.1002/med.21461. [DOI] [PubMed] [Google Scholar]
- 102.Kashfi K. The dichotomous role of H(2)S in cancer cell biology ? Déjà vu all over again. Biochem. Pharmacol. 2018:0–1. doi: 10.1016/j.bcp.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.