Abstract
Polyunsaturated fatty acids present in plant membranes react with reactive oxygen species through so-called lipid oxidation events. They generate great diversity of highly-reactive lipid-derived chemical species, which may be further degraded enzymatically or non-enzymatically originating new components like Reactive Carbonyl Species (RCS). Such RCS are able to selectively react with proteins frequently producing loss of function through lipoxidation reactions. Although a basal concentration of lipoxidation products exists in plants (likely involved in signaling), their concentration and variability growth exponentially when plants are subjected to biotic/abiotic stresses. Such conditions typically increase the presence of ROS and the expression of antioxidant enzymes, together with RCS and also metabolites resulting from their reaction with proteins (advanced lipoxidation endproducts, ALE), in those plants susceptible to stress. On the contrary, plants designed as resistant may or may not display enhanced levels of ROS and antioxidant enzymes, whereas levels of lipid oxidation markers as malondialdehyde (MDA) are typically reduced. Great efforts have been made in order to develop methods to identify and quantify RCS, ALE, and other adducts with high sensitivity. Many of these methods are applied to the analysis of plant physiology and stress resistance, although their use has been extended to the control of the processing and conservation parameters of foodstuffs derived from plants. These foods may accumulate either lipid oxidation/lipoxidation products, or antioxidants like polyphenols, which are sometimes critical for their organoleptic properties, nutritional value, and health-promoting or detrimental characteristics. Future directions of research on different topics involving these chemical changes are also discussed.
Abbreviations: AER, NADPH-dependent Alkenal Reductase; AGE, Advanced Glycosylation Product; ALE, Advanced Lipoxidation Product; ALH, NADPH:2-alkenal α,β-hydrogenase; AOR, NADPH-dependent Alkenal/one Oxidoreductase; EVOO, Extra-Virgin Olive Oil; HHE, 4-Hydroxy-2-HExenal; HNE, 4-Hydroxy-2-NonEnal; iTRAQ, isobaric Tag for Relative and Absolute Quantitation; JA, Jasmonic acid; LOOH, Lipid peroxide; MDA, MalonDiAldehyde; MS, Mass Spectrometry; NO2-FAs, Nitro-fatty acids; PUFA, PolyUnsaturated Fatty Acid; RCS, Reactive Carbonyl Species; RNS, Reactive Nitrogen Species; ROS, Reactive Oxygen Species; RSLV, Reactive Short-chain Leaf Volatiles; TBARS, ThioBarbituric Acid Reactive Substances
Keywords: Advanced lipoxidation endproducts, Food, Lipid peroxides, Reactive carbonyl species, Reactive oxygen species, Stress
Graphical abstract
Highlights
-
•
Lipid (per)oxidation occurs in plants as a signaling mechanism and after stress.
-
•
Electrophylic mediators are widely used to assess plant physiology.
-
•
Few lypoxidation targets have been identified in plants, mainly related to stress.
-
•
Lipoxidation frequently inactivates or highly affects enzyme activity in plants.
-
•
Lipid oxidation/lipoxidation affect the quality and healthy properties of plant foods.
1. Lipid oxidation biochemistry. How does it affect plant function?
1.1. Fatty acid peroxidation in plants
Reactive Oxygen Species (ROS) are inevitably generated in plants as a consequence of aerobic metabolism. ROS levels in cells and tissues are tightly regulated by the action of a plethora of antioxidant defenses both of enzymatic and non-enzymatic type, which maintain ROS balanced within physiological conditions. Moreover, evidence is rising regarding the paramount role of ROS in signaling events concerned in plant functions like the response to stimuli, growth, reproduction, nutrition, photosynthesis, adaptation to changes in the temperature, salinity and many others [29], [62]. ROS may also regulate the activity of numerous enzymes and key molecules through key PTMs, and even modulate gene expression [90].
Antioxidant systems can be sometimes surpassed as the consequence of events like the presence of adverse environmental conditions (intense light, wounding, senescence, drought, heat and cold, presence of heavy metals, salt, herbicides…) or biological attacks to the plant (by insects, bacteria, viruses…), then occurring an imbalance in the levels of ROS, named oxidative stress. Due to their sessile nature, plants are often exposed to such stressing surroundings. Under these conditions, ROS accumulate and their toxicity can be exerted through their interaction with biomolecules like lipid, proteins and nucleic acids, resulting sometimes in cell death, and in consequence, to a limitation in biomass and yield production [20].
Polyunsaturated fatty acids (PUFAs) are lipid components commonly and easily oxidized by unbalanced ROS (mainly hydroxyl radical due to its indiscriminative reactive character). As described by Vistoli et al. [92], the reaction of PUFAs with ROS triggers sequential lipid peroxidation reactions, starting with the extraction of an allylic hydrogen atom from PUFAs, which results in the formation of a lipid radical. This lipid radical reacts with molecular oxygen to form lipid peroxyl and lipid alkoxyl radicals, both causing radical chain oxidation to lipid peroxidation by reacting with neighboring lipid molecules. PUFAs are unevenly present in plant tissues, organelles and cell compartments. Cell plasma membranes and chloroplasts are exposed to the generation of ROS [5], [6], and are at the same time cell structures rich in PUFA. Therefore, they are exposed to a high risk of lipid peroxidation. As the result of the described chain modifications, lipids are degraded and fluidity and integrity of membranes may be compromised, resulting sometimes in cell damage and loss of the function. Lipid peroxidation reactions in plants can be produced through three different pathways, namely lipoxigenase activity, singlet oxygen generation and radical-catalyzed mechanisms, which quantitatively diverge between green tissues and underground tissues, as per differences in the nature of ROS generated (reviewed by [26]).
Lipid-rich tissues as those of the seeds and fruits, particularly in oleaginous plant species, are also particularly prone to lipid peroxidation under stress situations. Moreover, many of these tissues are used in the preparation of vegetal oils and foodstuffs, as it will be discussed later on in this review.
1.2. Generation of Reactive Carbonyl Species (RCS) in plants
Lipid hydroperoxides (LOOH) are frequently the subject of fragmentation either by enzymes or by non-enzymatic mechanisms, and a wide variety of short-chain oxidation products are generated as the result of these actions. Chemistry of these products is highly complex, and include aldehydes, alkanes and alkenes (see review by [76]). From these molecules, the reactive carbonyl species (RCS): malondialdehyde (MDA), 4-hydroxy-(E)-2-nonenal (HNE) and acrolein are the players of a large set of studies, some of which have been carried out in plant species. This so-called “second phase of lipid peroxidation” has been widely reviewed in plants by Farmer and Mueller [26], who describe the structure of most electrophilic species resulting from lipid fragmentation in plants.
RCS production in plants is highly dependent on the degree of fatty acid desaturation present among their lipids. Thus, pioneer analyses carried out in Arabidopsis thaliana by Mano et al. [45] showed that fad7fad8 mutants defective in fatty acid desaturases (able to convert 16:2 and 18:2 fatty acids into 16:3 and 18:3 fatty acids at the chloroplast) showed a lower level of lipid-derived RCSs like acrolein, (E)-2-pentenal and MDA in their leaves compared with wild-type plants [45].
RCSs are also detoxified in plants by different mechanisms including the enzymatic reduction of the aldehyde groups to alcohol groups by aldo-keto reductase (AKR) and aldose/aldehyde reductase (ALR), through the use of NAD(P)H [68], [102]. Other detoxifying enzymes (aldehyde dehydrogenase, ALDH) transform the aldehyde groups into carboxylic acid groups using NAD(P)+ [39], [63], [68]. Finally, alkenal reductase (AER), alkenal hydrogenase (ALH) (P1-ZCr) and alkenal/one reductase (AOR) have been described to detoxify the highly toxic, lipid peroxide-derived α,β-unsaturated carbonyls through NAD(P)H-dependent reduction [48], [102].
1.3. Plant protein modifications caused by RCS and lipoxidation
RCSs resulting from lipid oxidation can react with specific amino acids on proteins and form adducts called “advanced lipoxidation endproducts” (ALEP) [57]. Reactions can take place by reaction of the carbonyl moieties with Lys to form Schiff bases. Moreover, addition of carbonyls to protein may also occur after direct attack of oxidants like HOCl to amino acid side chains, causing oxidative deamination, or by Michael addition of α,β-unsaturated aldehydes to specific amino acids, like amino groups of Lys and Arg, which are often target of this reaction, and also histidyl or cysteinyl residues. Domingues et al. [24] widely review the different pathways for the formation of protein covalent adducts with electrophilic lipids including fatty-acid aldehydes, phospholipids, prostaglandins and other fatty-acid derivatives, and analyze the occurrence and biological significance of both Schiff and Michael adducts. They also compile and provide diverse examples of the methods to detect both adduct types. Since Cys, His and Lys residues usually take part of the active center of many enzymes; the most common effect of protein lipoxidation is enzyme inactivation [24], [87]. Although some of these post-transcriptional modifications have been described as irreversible in some instances [57], they can be reverted in most cases. Therefore, RCS (and ROS) detoxification is vital in the prevention of cell damage.
Numerous key plant enzymes have been described to become inactivated as the consequence of protein lipoxidation (Table 1). Many of these RCS-specifically targeted proteins have been identified and characterized after the induction of different types of stress or direct treatment with RCS of the plants assayed. Thus, Yamauchi et al. [99], Yamauchi and Sugimoto [100] describe damages and dysfunctions of photosystem II after MDA treatment, due to modifications of OEC33, CP47 and CP43 components in spinach leaves. Mano et al. [45] identified 39 types of protein as sensitive ROS and RCS targets in Arabidopsis plants subjected to salt stress. Such proteins included chloroplast, cytosol, apoplast, mitochondrion, nucleus, plasma membrane ribosome and peroxisome proteins (Table 1), confirming that lipid peroxidation products can diffuse across membranes, allowing the reactive aldehyde-containing lipids to covalently modify proteins localized throughout the cell and relatively far away from initial site of ROS formation [87], [88].
Table 1.
Examples of protein targets of lipoxidation identified in plants.
| PROTEIN | Plant | Effect | Reference |
|---|---|---|---|
| 2-oxoglutarate dehydrogenase (OGDC) | Potato tuber | Inhibition (80%) | Millar and Leaver [56] |
| Pyruvate dehydrogenase complex (PDC) | Potato tuber | Inhibition (80%) | Millar and Leaver [56] |
| NAD-malic enzyme | Potato tuber | Inhibition (50%) | Millar and Leaver [56] |
| Oxygen-evolving complex 33 kDa protein (OEC33) | Spinach | Increased presence in stressed plants vs non-stressed controls. | Yamauchi and Sugimoto [100] |
| Light-harvesting complex protein (LHCP) | Arabidopsis thaliana | No relevant change in photosynthetic activity | Yamauchi and Sugimoto [100] |
| Aldo-keto reductase family AKR4C | Arabidopsis thaliana | Inactivation at different degrees | Saito et al. [68] |
| Germin-like protein subfamily 3 member 1 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Luminal-binding protein 2 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Nitrile-specifier protein 5 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Peroxidase 34 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Triosephosphate isomerase, cytosolic | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Putative 2,3-bisphosphoglycerate-independent phosphoglycerate mutase | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Heat shock cognate 70 kDa protein 3 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Isoform 2 of glycine-rich RNA-binding protein 7 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Nitrilase 1 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| 40 S ribosomal protein Sa- 1 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Peptidyl-prolyl cis-trans isomerase (Cyclophilin 20–3) | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| 4-Coumarate-CoA ligase-like protein | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Nucleoside diphosphate kinase | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Probable phosphoglucomutase, cytoplasmic 1 | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Putative leucine aminopeptidase | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| Cysteine synthase | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
| L-Ascorbate peroxidase | Arabidopsis thaliana | Increased presence, modification with HNE and greater presence of carbonyls in stressed plants (>2.0 ×) vs non-stressed controls. | Mano et al. [46] |
Protein lipoxidations do not occur randomly. Several studies carried out preferentially in animal and human systems indicate the concurrence of factors involving microenvironment around the potential amino acids involved, which may include low pH in the vicinity of the adduct, steric factors, surface accessibility, nucleophilicity… [3]. Therefore, protein lipoxidation may affect defined amino acids in certain proteins and not in others, and with different potency. This is the case of differential selectivity of protein modification by cyclopentenone prostaglandins described by Gayarre et al. [30] and reviewed by Oeste and Pérez-Sala [64]. The presence of antioxidant molecules like glutathione, glutathione S-transferases, catalase, etc., may additionally modulate the extent of lipoxidation [30], [64]. Also, the presence of proteins highly susceptible to lipoxidation may restrain lipoxidation of other proteins present in complex fluids like plasma, acting as carbonyl scavengers (reviewed by [3]). Because the proteins affected, and particularly the Cys involved in lipoxidation frequently exert critical biological effects, differential sensitivity to lipoxidation may represent a crucial event of signaling and enzyme regulation, also affecting gene expression (review by [26]), sometimes via differential selectivity of transcription factors, which might be exploited for example to the design of therapeutical agents. Additional examples of lipoxidation regulatory events include Ras proteins and the cytoskeletal protein vimentin, which behave as sensors of electrophilic species [3].
Although the list of plant proteins prone to lipoxidation is increasing, additional proteins potentially targeted by lipoxidation have yet to be identified in plants. For this purpose, the methods described next in this review are proposed. Alternatively, some primary candidates of lipoxidation can be identified as plant orthologous of well-determined proteins present in animals, which have been previously acknowledged as lipoxidation targets. As an example, methylglyoxal was shown to inactivate in a time and dose dependent manner the bovine glutathione peroxidase (GPX), a major antioxidant enzyme, by irreversible modifying the arginine residues located in the glutathione binding site of GPX (Park et al., 2003). Plant GPXs contain quite conserved sequences, which could be also prone to lipoxidation ([65]; Patón, personal communication). Other proteins described as targets for lipoxidation in animals like Cu, Zn-SOD, Mn-SOD, glutamine synthase, enolase, peroxiredoxin 1… (review by [24]) are also present in plants in a relatively well-conserved manner. Moreover, at least 67 different proteins from yeast lisates were detected to contain amino acid sites of HNE modification. They include stress proteins, pyruvate kinase, enolase, 3-phosphoglycerate kinase, fructose 1,6-biphosphate aldolase, ribosomal proteins, translational elongation factor, etc. Roe et al. [66], which likely merit for lipoxidation assessment in their plants orthologous, as per the high conservation between plant and yeast proteins.
2. Detection and analysis of RCS, lipid oxidation and lipoxidation products in plants
Detection and analysis of RCS is extremely challenging, due to the high reactivity of these compounds, which are in many cases present in the form of covalent adducts with DNA, proteins or other biomolecules.
As a general assessment of the extent of oxidative lipid damage in plants, the widely used thiobarbituric acid-reactive substances (TBARS) assay, based on the use of thiobarbituric acid as a reagent, is able to collectively measure the presence of aldehydes, alkenals and hydroxyalkenals including the cytotoxic compounds MDA and HNE [56], [79], [99]. The accuracy and tendency to overestimate MDA content of TBARS was improved by Hodges et al. [36] for use in plant material containing anthocyanins and/or other interfering compounds. TBARS assay is also commonly used to assess lipid oxidation rate and the effects of processing on foodstuff like almond-based products [84]. An alternative method of high simplicity is the use of luminol/EuT-sensitized chemiluminescence (CL) [72], [105], which is also used to assess the presence or early products of lipid oxidation in vivo and in vitro, and that has been also used for the evaluation of almonds and almond-based products during processing and storage [84], [85].
Advanced analytical methods to identify HNE and other prominent carbonyl-containing lipid oxidation compounds have been thoroughly described by Yan and Forster [104], and Sousa et al. [76]. Many of them have been developed and assayed to identify a broad range of aldehydes and carbonyls on proteins of biomedical interest in human samples. As they include complex mixtures, chromatographic separation is required, with either pre-column or post-column derivatization, as well as combination to specific probes. Numerous new methods to detect and quantify MDA and HNE and other compounds by means of GC-MS, LC-MS/LC-MSMS are arising [1], [76]. In many cases, set up of these procedures is performed by using well-known model recombinant or purified proteins like bovine serum albumin (BSA), Rubisco, ß-lactoglobulin, cytochrome c, RNase A, ubiquitin, lysozyme or human serum albumin (HSA), which are treated with also model RCS like MDA, acrolein, pentanal and others [1], [99], [107].
In plants, comprehensive descriptions of the presence of carbonyl-containing lipid oxidation compounds are not widespread. Methods have been developed to identify most common carbonyl species. As an example, Matsui et al. [52] described two methods based in HPLC analyses of short-chain aldehydes after derivatization with 2,4-dinitrophenyl-hydrazine (DNPH), and solid phase microextraction fiber to collect volatile compounds, desorption with dichloromethane and subsequent GC-MS analysis. Mano et al. [47], [46] describe diverse observations through a metabolomic approach the spectrum of carbonyls present in A. thaliana after extraction, derivatization with 2,4-dinitrophenylhydrazine and separation with a reverse-phase HPLC equipped with a photodiode array detector and a Fourier transform ion cyclotron resonance mass spectrometer. Thus, they first describe [47] changes in the levels of the 2-alkenals: (E)-2-pentenal, acrolein and (E)-2-hexenal, in leaves of transgenic tobacco overexpressing 2-alkenal reductase (AER) under photoinhibitory illumination, as a part of 40 carbonyl species detected by this method. Further, Mano et al. [45] describe through a metabolomic approach the spectrum of carbonyls present in A. thaliana rosette leaves obtained after extraction, derivatization with 2,4-dinitrophenylhydrazine and separation with a reverse-phase HPLC equipped with a photodiode array detector and a Fourier transform ion cyclotron resonance mass spectrometer. After using these methods, they managed to detect 33 distinct carbonyl-containing compounds, which were compared between lines of Arabidopsis displaying differential fatty acid composition (wild type and the fad7fad8 double mutant lacking trienoic fatty acids in the plastid). Main differences were centered in the concentration of MDA, acrolein, (E)-2-pentenal, acetone, 3-pentanone, and n-hexanal.
The adducts formed by MDA, HNE and other aldehydes with proteins have been used as immunogens to raise a number of highly valuable antibodies, which can be used in a broad panel of methods, including Western blotting, ELISA, immunocytochemistry/immunohistochemistry, immunoprecipitation and other immunoassays, leading to the identification of oxidized lipid adducts with proteins. Many of these antibodies have medical and clinical interest [25], [89]. Production of these antibodies is frequently mediated by using keyhole limpet hemocyanin as a carrier, and multiple types of antibodies have been generated, including polyclonal and monoclonal, some of the commercially available [76], [77].
In plants, immunological detection methods to detect RCS have been used as well. Monoclonal antibodies that recognize MDA binding to proteins were used by Yamauchi et al. [99] as tools to detect the formation of adducts between MDA and model proteins like BSA and Rubisco. Also, spinach protein extracts obtained from control and heat-stressed plants were scrutinized for MDA-modified proteins by using the same antibody in Western blotting experiments. Mano et al. [45] used commercial polyclonal antibodies to acrolein, monoclonal antibodies to crotonaldehyde and HHE, and antisera to HNE and MDA to reveal and quantify RCS-modified proteins accumulated in leaves of Arabidopsis with the progress of salt treatment by means of Western blotting approaches. Moreover, these authors used a highly sophisticated strategy to increase the specificity of the identified lipoxidation targets. Such strategy included the use of a affinity-purified polyclonal antibody (anti-HNE, previously assessed in their consistency to detect RCS-sensitive proteins). After affinity-trapping and then elution of the proteins of the stressed and non-stressed plants, the authors used quantitative proteomics to compare both eluates, by means of isobaric tag for relative and absolute quantitation (iTRAQ method) [108]. This method is based in applying reduction, alkalization, trypsin digestion of both protein populations to be analyzed, followed by N-terminus labeling with two distinct but isobaric compounds (a pair of commercial iTRAQ tags). The labeled proteins from both populations are mixed, and then peptides are separated by using chromatographic methods and subjected to subsequent MS/MS analysis. According to the authors, the basis of the analysis consists in that “one type of protein fragment labeled with two isobaric tags (corresponding to two different samples) is cleaved to different masses to give a heavier fragment and a lighter fragment, so that the fragments of the same type from the two protein populations are determined separately”. The difference in the signal intensity of a given peptide in the two different populations represents the difference in the affinity-trapped amounts of the same type of protein in both samples. After using this method, Mano et al. [46] managed to identify 34 types of HNE-modified proteins. Further assays were performed in this case to identify “oxidized” proteins by using the same iTRAQ protocol; however in this case, the eluates of the two samples used (stressed and non-stressed plants) were trapped in a streptavidin affinity column after biotin labeling of the protein carbonyls with a aldehyde-reactive probe (ARP; [15]). This further analysis resulted in 23 types of oxidized target proteins. Comparison of HNE-labeled and oxidized proteins resulted in 17 selected types of proteins, representing lipoxidation targets with a high probability (see Table 1).
Plant cell localization of RCS has been described in the literature in a very limited manner. Mène-Saffrané et al. [55] depict the fluorescence detection of aldehyde-TBA adducts by fluorescence microscopy using specifically-constructed filters. Farmer and Mueller [26] describe putative MDA pools in zones of cell proliferation in Arabidopsis also by means of fluorescence microscopy, based in thermoluminescence and autoluminiscence detection to image events like spontaneous decomposition of lipid peroxides.
3. Contribution of RCS, lipid oxidation and lipoxidation to plant growth and development
Enhanced RCS and lipoxidation adduct levels have been detected in samples corresponding to human diseases and in response to stress conditions, therefore contributing to their overall consideration as deleterious substances for cell physiology. However, recent evidence is showing that lipid oxidation products are present in minute concentrations in cells under physiological conditions. In plants, Mène-Saffrané et al. [55] and Mano et al. [45], [47] have described that several types of RCS as well as saturated carbonyls are contained in leaves and roots at micromolar levels under non-stressed, relaxed conditions. It is under environmental stresses (i.e. high light conditions or excessive aluminum concentrations - Yin et al. [111]) when RCS levels highly rise as the result of the environmental change.
Farmer and Mueller [26] review ROS-mediated lipid peroxidation and Reactive Electrophile Species (RES) signaling in plants under an innovative point of view consisting in to consider that the machinery designed to detoxify non-enzymatically modified lipids, has evolved in plants to manage other different chemicals like herbicides and xenobiotics. Also, interesting considerations of these authors include the suggestion of oxidation-prone fatty acids and carotenoids acting as protective scavengers to 1O2. Thus, they propose for example that PUFAs present in the thylakoid membranes might be considered as structural, supramolecular antioxidants.
Some recent physiological analyses also support the beneficial contribution of RCS metabolism on growth. Thus, Takagi et al. [83] evaluated the physiological importance of a chloroplast-localized alkenal/one oxidoreductase (AtAOR) for the detoxification of lipid-derived RCS in Arabidopsis thaliana. The aor mutants showed smaller plant sizes than wild-type plants when they were grown under day/night cycle conditions, which was attributable to the decrease in carbon utilization during the night. Therefore, they conclude that detoxification by AtAOR in chloroplasts contributes to the protection of dark respiration and supports plant growth during the night.
Lipid-derived RCS have been shown also to be able of reprogramming transcriptional patterns in plants, affecting important cellular processes, including detoxification, heat stress, cell division, auxin signaling, and programmed cell death [12], [26], [83]. Therefore, they play an important role in signaling processes, and the production of lipid-derived RCS should be strictly regulated in plants cells. A detailed example of such effects was described by Yamauchi et al. [103], who reported that exposure to lipid-derived RCS of the RSLV type (reactive short-chain leaf volatiles) modifies the expression of transcriptional factors in plant cells, with the modification of gene expression networks providing tolerance to heat stress. Such RSLV signaling is transmitted via both HSFA1-dependent and-independent pathways (HSFA1 is a heat stress response master regulator), and the chemical signaling is accompanied by oxidative stress. Whether transcriptional reprogramming brought about by RCS is also mediated by protein lipoxidation, is presently unknown. At this regard, Farmer and Mueller [26] hypothesized that RCS-induced expression of chaperones could insulate proteins from modifications by oxidized lipids, which might result in changes in their function or even lead them to degradation. Therefore, the potential and mutual relationships between lipoxidation and transcription reprogramming should be further investigated.
4. RCS, lipid oxidation and lipoxidation in a context of plant biotic stress
Fatty acid-based signaling systems in plants have focused mainly on the hormonally active compound, jasmonic acid (JA) [37]. Its synthesis and subsequent action coordinates plant defense responses against insect herbivores and other pathogens [14], [19], [37], [69]. Jasmonic acid (JA) is a final product of octadecanoid pathway, initiated by lipoxigenase by adding molecular oxygen to linoleic acid (reviewed by [28]). The jasmonate family of compounds is composed of biologically active cyclopentenones and cyclopentanones (like JA), with related structure and biosynthetic origin [37], which fulfill important aspects of plant defense. Thus, plant treatments with JA upregulate defense-related genes, whereas infected/infested plants usually display enhanced levels of JA. Also, mutants involved in JA generation or perception are substantively susceptible to pathogen attacks. Candidate molecules to these effects other than JA are cyclopentenones like OPDA (12-oxo-phytodienoic acid) [37], [80] and other oxylipins [53]. The panel of these compounds has been identified as part of the sensing and signaling systems of higher plants, termed oxylipin signature [37], [98] and represents a complex mix of signals supporting the defense system of plants. Among the functions of cyclopentenone-based compounds in plant cells, they have been described to regulate the expression of genes related to detoxification processes, like those involved in the cytochrome-P450 system and heat shock proteins like HSP70 [44], [59]. Moreover, they are able to induce the expression of glutathione-S-transferase 1, involved in the detoxification of lipid peroxidation products, therefore contributing to protection against lipid oxidation and lipoxidation ([86], review by [26]).
In the case of bacterial infections, a good example of the changes in lipid profiles was provided by Zoeller et al. [109], who performed systematic lipidomic analysis of Arabidopsis after Pseudomonas syringae infection. Lipid peroxidation was in this case predominantly confined to plastid lipids comprising galactolipid and triacylglyceride species and preceded programmed cell death. Parallel analysis of lox2 mutants carried out by the authors, revealed that LOX2 is essential for enzymatic membrane peroxidation but not for the pathogen-induced free jasmonate production. These authors also described an accumulation of fragmented lipids, and therefore provided direct evidence of the reasons of radical amplification and formation of electrophile signals such as phytoprostanes, MDA, and hexenal in plastids.
Plant infections by fungi also elicit major changes in lipid peroxidation products, as repeatedly reported by different authors, who describe infections by numerous species of fungi in different host species, many of them of paramount agronomic interest. Some examples include the infection by Blast, a major disease of wheat caused by the fungus Pyricularia oryzae. At this respect, Debona et al. [21], [22] described the occurring changes in a broad panel of antioxidant enzymes like superoxide dismutase, catalase, peroxidase glutathione-S-transferase, ascorbate peroxidase, glutathione reductase, and glutathione peroxidase, and the concentrations of superoxide, hydrogen peroxide and MDA in both a susceptible and resistant plants and between inoculated and non-inoculated plants, and made a model of the involvement of the resistance character on the observed changes. Higher MDA content and lower cell membrane integrity were also the results of infection of potato tubers with differently virulent strains of Fusarium sulphureum and Fusarium sambucinum. These changes were also accompanied by differences in the activity of many antioxidant enzymes [7]. Fusarium (Fusarium proliferatum, F. subglutinans) and Aspergillus flavus are the infectious agents studied by Lanubile et al. [41] in the developing kernels of resistant and susceptible maize genotypes. These authors investigated the expression of pathogenesis-related genes, and protective genes against oxidative stress (peroxidase, catalase, superoxide dismutase and ascorbate peroxidase) after inoculation. Also, they analyzed the hydrogen peroxide and MDA content, to evaluate the oxidation level. Another disease: mango wilt, caused by Ceratocystis fimbriata is one of the most important diseases affecting mango production. The activities of a range of enzymes, metabolites and some markers for oxidative stress (e.g. hydrogen peroxide and MDA equivalents), were comparatively evaluated in different cultivars of mango after inoculation with C. fimbriata. Noticeable differences were also described for this disease [11]. Necrotic lesions developed in tomato plants due to treatment with toxins from Alternaria alternate (almost similar as those produced by the pathogen and by the pathogen itself), produced oxidative burst reaction, increased MDA content and reduced chlorophyll. The activities of antioxidative defense enzymes increased in both the plants infected with the pathogen and in the toxin-treated samples [54]. In an opposite manner, experimental Infection of leaves of Arabidopsis thaliana with conidial suspensions of the necrotrophic pathogen Botrytis cinerea [58], resulted in a large decrease in the level of ascorbic acid. The aldehydic product of lipid peroxidation, 4-hydroxy-2-nonenal (4-HNE) was not observed, and in the case of MDA, the levels observed in the infected plants were appreciably lower than in the healthy controls. As stated by these authors, these findings are surprising and demonstrate a difference in the response of A. thaliana to infection with B. cinerea compared with tissues from other plant families studied previously.
Virus infections like Mal de Rio Cuarto virus (MRCV) produce a wheat disease, which is physiologically characterized by a remarkable increase in soluble sugar, starch and soluble protein contents, decrease in chlorophyll content, and increase in MDA, which could indicate oxidative damage associated to biotic stress in these plants [23]. The effects of another viral disease, leaf curl disease, caused by Cotton Leaf Curl Burewala virus (CLCuBuV), were studied in leaves of two susceptible and resistant cotton genotypes [74], who analyzed phenolic compounds, total soluble proteins, MDA and the activities of phenylalanine ammonia-lyase, peroxidase, catalase, proteases, superoxide dismutase, and polyphenol oxidase.
Viruses infections are frequently resulting from the combined action of the virus and their corresponding vectors. Viruses and insects cause severe symptomatology in different genotypes. An example of this interaction is Delphacodes kuscheli, an insect acting as the vector for the already mentioned Mal de Rio Cuarto virus (MRCV) infection [23].
War et al. [94], [95], [96], [97] analyzed the effects of infestation with different vectors like Helicoverpa armigera (a leaf defoliator), Aphis craccivora (a sap-sucking insect), Spodoptera litura, and leafhoppers (Empoasca kerri) in groundnut genotypes distinctly resistant to the infestation, by analyzing the activity of oxidative enzymes, the amounts of other host plant defense components (including MDA as a marker of lipid oxidation). Moreover, the effects of spraying jasmonic acid (JA) (a signal molecule to induce resistance in plants against herbivores/insect damage) followed or jointly by/with the infestation were assessed.
Wang et al. [93] investigated the population dynamics of the soybean aphid Aphis glycines, an important pest, using field surveying, as well as the physiological responses of soybean plants to its feeding. Biochemical assays indicated that the amount of MDA and the activities of four defense-related enzymes in soybean leaves significantly changed between 0 day and 7 days of aphid infestation. Activities of defense-related enzymes, and concentrations of MDA and soluble protein in leaves were measured in rice plants with or without leaf folder infestation (Cnaphalocrocis medinalis) and with or without Silicon amendment (which can confer enhanced resistance to herbivores). In this study, carried out by Han et al. [34], Silicon addition alone did not change activities of defense-related enzymes and MDA concentration in rice leaves, whereas with leaf folder infestation, activities of the defense-related enzymes increased and MDA concentration decreased in plants amended with Si. MDA content and activity of five antioxidant enzymes change in cucumber plants as a response to biotic stress due to infestation with the aphid Aphis gossypii Glover in a resistant and a susceptible cucumber line [43]. The results indicated that MDA content in seedlings infected by aphid were always higher than in control plants of both lines. MDA content increased initially after aphid infestation and then declined in both two lines. Therefore, these results indicated that the MDA content is likely to be involved in aphid-resistance in cucumber. In all these analyses involving infestation by different types of insects, it is also common the assessment of insect survival as an additional parameter.
5. Lipid oxidation and lipoxidation in a context of plant abiotic stress
As stated in Section 3, the contributions of MDA and other RCS on healthy, non-stressed plant functions are not yet fully understood. What we clearly know is that RCS cause adverse effects in plants, due to their chemical attachment to biomolecules, especially proteins. Thus, free MDA, HNE and other RCS have been determined in various sources as oxidative stress markers in stressed plants [48]. Therefore, research carried out in order to understand the plant responses and mechanisms to cope with abiotic stress has been frequently designed by experimentally inducing abiotic stress conditions, and then assessing plant and enzymatic functions with the intermediate analysis of both ROS, and RCS as markers of potential damage, with related and sequential, yet different acting forms.
As some examples, mulberry leaves have been found to accumulate three different phytoalexins: moracin C, moracin N, as well as chalcomoracin and MDA when treated with UV-C. The three were capable of scavenge the superoxide anion generated by a xanthine-xanthine oxidase system, and also lipid peroxidation [71]. Yamauchi et al. [99] used heat-stressed spinach plants and detected modification of OEC33 (oxygen-evolving complex 33 kDa protein) by MDA. Mano et al. [46] investigated the protein carbonyls formed by ROS or by the lipid peroxide-derived α,β-unsaturated aldehydes and ketones (i.e. reactive carbonyl species, or RCS) in the leaves of Arabidopsis thaliana under salt stress. Also, the effect of varying salicylic acid supply on growth, mineral uptake, membrane permeability, lipid peroxidation, H2O2 concentration, UV-absorbing substances, chlorophyll and carotenoid concentrations of salt stressed NaCl maize plants was investigated by Gunes et al. [32]. Salicylic acid has been proposed as a signal molecule responsible for inducing abiotic stress tolerance in plants. The effect of toxic concentrations of aluminum was investigated on contents of protein-thiols, non-protein and total thiols, protein carbonyl formation and protease activity in the seedlings of Al-sensitive and Al-tolerant cultivars of rice by Bhoomika et al. [10]. The effects of cultivation of rice seedlings with high concentration of Cd in the medium consisted in elevated levels of lipid peroxides, increase in superoxide anion generation and a concomitant rise in the activities of guaiacol peroxidase and superoxide dismutase [70].
6. Health and alimentary engagements of lipid oxidation and lipoxidation in plants
Both lipid oxidation and lipoxidation occur in plant derived dietary products, particularly in lipid-rich foodstuffs (nuts, oilseeds, purified oils, fruits…), sometimes in a similar way as described above. However, its effects are sometimes enhanced by the use of “extreme” treatments when compared with more “natural” environments of plants, like the use of high temperatures during cooking procedures, or low temperatures associated with conservation and storage of foods. In addition, food processing involves sometimes other factors like presence of oxygen, changes of pH, addition of antioxidants and other additives, light treatments, drying, supplementation of lipids, and even enzyme treatments. As the result of mainly lipid oxidation, changes in organoleptic properties, the appearance and even the nutritional value of food may occur. Therefore, monitorization of these chemical events by means of analytical methods is a requirement for the food processing industry. Methods to analyze lipid oxidation have been widely developed and optimized for particular food materials of high production and multiple uses like almond [84], [85] and walnut [42]. They include measurements of peroxides, spectrophotometry, detection of TBARS, measurement of enzyme-related activities (i.e. lipoxygenase, lysosomal enzyme…) and chemiluminescence, among others.
Hidalgo and Zamora [35] have recently reviewed the chemical changes produced in foods during processing, describing the carbonyl-amine reactions initiated by carbohydrates (the Maillard reaction), the carbonyl-amine reactions initiated by lipids, the technological problems of lipid oxidation and its control by antioxidants, the formation of carbonyl-amine adducts (AGEs and ALEs), the antioxidant properties of carbonyl-amine adducts, and their production as an additional benefit of food processing. However, carbonyl-amine adducts are usually determined “in whole” in food, and targets of lipoxidation are not usually analyzed in specific manner.
AGE and ALE affect the physiology and development of chronic diseases, while continuous intake of both types of chemicals contributes to the excessive accumulation of these products into body tissues, which in turn negatively influences the innate immune system, inflammatory responses, and resistance to diseases [4], [8], [31]. The formation of AGE and ALE can be counteracted and their degradation can be promoted by using pharmaceuticals and natural compounds, as reviewed by Aldini et al. [4]. These authors describe inhibitors of their formation, cross-link breakers, ALE/AGE elimination by enzymes and proteolytic systems, receptors for advanced glycation products (RAGEs), and blockade of the ligand-RAGE axis. RAGE have a key role as master switches regulating the development of diseases like allergic and autoimmune diseases, Alzheimer disease and other degenerative disorders, cataracts, atherosclerosis, cancer, and diabetes mellitus type 2, as well as a number of endocrine, gastrointestinal, skeleton-muscle, and urogenital alterations. (reviewed by [31]).
Plant tissues and plant-derived foodstuffs also contain protective components with beneficial effects, which may counteract deleterious effects of AGE and ALE, or even prevent their formation. Numerous examples are constantly provided in the literature [9], [75], [81]. Some classical examples include polyphenols present in vegetable, fruits, olive oil and wine, which constitute the basis of the Mediterranean diet.
As mentioned above, fatty acids-derived compounds through at least one step of oxidation (oxylipins) are volatile compounds formed in plants usually from C18 and C16 fatty acids, through the action of fatty acid hydroperoxide lyase (HPL), and further modification of several hydroperoxides generated from linoleic and linolenic acids into aldehydes, which can be further reduced to short-chain alcohols or their acetates [51], [52]. These arrays of oxylipins are important flavor compounds in many food materials of plant origin (i.e. vegetable oils, nuts and seeds, wines, macroalgae and foodstuffs) [52], [53], providing properties like green leaf-like and other flavors. Moreover, their structural similarities with prostanoids and isoprostanes is causing a high expectation, related with their bioavailability and their biological activity, related in humans with anti-inflammatory, anti-tumor, immunomodulatory and other capacities [53].
7. Future perspectives
One of the main conclusions extracted from the literature, is that MDA (and other increasing amount of RCS) are becoming useful markers, widely used to assess plant stresses of both biotic and abiotic type. They are also widely used in medicine uses as diagnosis tools. These markers are normally included among the panel of antioxidant enzymes and metabolites used to assess the use of different treatments, and the effects of physiological conditions in plants (including critical mutants).
In spite of the growing number of methods available to identify and analyze carbonyl-containing and reactive lipid oxidation and lipoxidation products, many of these components are still underinvestigated in plants [76], particularly the later. As an example, whereas markers for lipid oxidation are widely used, evidence and specific data regarding protein lipoxidation is scarce in several of the conditions described here (i.e. during infections by fungi and viruses and through plant infestations), and it is even more limited in the case of foods. The identification and assessment of potential targets for lipoxidation, particularly under plant pathophysiological conditions will surely increase our knowledge on these topics and will provide tools for diagnosis and stress and pathology relief, in a parallel manner with human and animal models [3], and furthermore, to increase food quality. New methods (also new antibodies to aldehyde-protein adducts) have yet to be developed. Sousa et al. [76] also focus the attention of researchers to the fact that reactions of RCS and proteins are selective, and some proteins are differentially prone to react with a particular RCS, thus depending from numerous factors. This is highly connected with the signaling role of these chemicals. The analysis of the diversity of RCS and lipid oxidation products should be fostered not only in plants, but also in plant-derived foods, as they may take active part in the prevention and also the development of different diseases.
Also, increasing awareness is arising as regard to the potential of lipid oxidation, lipoxidation, protein carbonyl formation, and formation of ALE and AGE inhibitors in the progress of plant stress situations, as well as the potential of the overexpression of antioxidant and carbonyl scavenging systems and the definition of their action mechanisms. Thus, even new in vitro high-resolution mass spectrometry methods are being set-up specifically to test the ability of compounds, mixtures and extracts to inhibit protein lipoxidation induced by reactive carbonyl species [16].
Different transgenic plants have been successfully constructed in order to overexpress key enzymes for the detoxification of lipid-derived RCS (reviewed by [83]). Alternatively, some of these key enzymes have been disrupted in their action for comparison purposes by means of the construction of DNA-tagged knockout, or RNA interference mutants. Some of these key enzymes include aldehyde dehydrogenases [40], [67], [73], [78], [101], [110], Alkenal Oxidoreductase (AOR) [83], aldo-keto-reductase [87], [88], [91] and myo-inositol-O-methyltransferase (Imt1) [106], among others. Although most of these studies have been performed in Arabidopsis thaliana, these assays are being extended to plants of further agronomical interest, as initially performed in groundnut [60] by the creation of transgenic plants co-expressing aldo-keto reductase (AKR1) and protein L-isoaspartyl methyltransferase (PIMT2), or in rice and tobacco plants overexpressing AKR1 [61]. Such promising constructs enhance seed viability, seedling vigor and/or overall abiotic stress tolerance in the modified plants.
Studies of lipoxidation and lipid oxidation parameters in the context of biotic stress will be beneficial in determining the seasonal occurrence of the pests involved, and selecting insect-resistant varieties, and thus in developing a theoretical framework for appropriate management strategies [93]. All these strategies will also be helpful in breeding programs focused on resistance to pathogens and grain safety and in the generation and selection of plant of high resistance to biotic stresses. Evidence obtained after gas chromatography analysis of volatiles extracted from leaves and flowers of plant species coupled to electroantennograme recordings show that some chemical products (including lipoxidation products) attracted tsetse flies in the wind tunnel [82]. This information could help to design strategies of biological fight in the case of biotic stress due to insects.
Still many topics involved in plant lipid oxidation and lipoxidation are understudied. This is the case of the cellular and subcellular localization of lipoxidation, accumulation of RCS, ALE and AGE, and the use of cell localization methods to better understand these processes. Different fluorophores, currently used for chromatography and MS identification of these products could be adapted to the cellular analysis by microscopy techniques, thus helping to identify and even quantify their production. Moreover, the recently developed antibodies to RCS-protein adducts could be extender in their use to immunocytochemical localizations.
Also, no much information is available as regard to the involvement of RCS in physiological events like programmed cell death (PCD), with a few exceptions [12], [13].
Likewise, and as discussed by Farmer and Mueller [26], peroxynitrite radical (one of the most highly reactive nitrogen species or RNS [17]), may become a relevant source of other secondary radicals, which have been described to be able of generating lipid peroxidation. Thus, hydroxyl radical can be generated through peroxynitrite lysis (either spontaneously or by acid-catalyzed lysis), and carbonate radicals can be generated through the reaction of peroxynitrite with carbon dioxide. Nitro fatty acids have been recognized as new signaling molecules, playing important biological roles as anti-inflammatory and anti-hypertensive reagents in mammalian tissues (reviewed by [24]), in spite of their micromolar concentrations. They are responsible for post-transcriptional modifications sharing some of the protein targets with other electrophilic lipids already described here. In plants, the presence of free and protein-adducted nitro-fatty acids (NO2-FAs) has been reported in Arabidopsis and in fresh olive fruits and extra-virgin olive oil (EVOO) [27], [49], [50]. NO2-FAs have been proposed by these authors to be involved in plant-defense response against different abiotic stress and also in the beneficial effects of EVOO on human health. Their capacity to release NO in vivo and in vitro, also accounts for their signaling potential, with important implications in plant physiology.
The assessment of these lipid modifications caused by RNS in plants would be an interesting field of study, particularly in those plant tissues and organelles where intense generation of NO has been described, also with signaling functions, as it is the case of pollen grains and pollen tubes [38], photosynthetic tissues and fruits over ripening [18]. Regulatory character of NO could even act in a differential way, by mitigating salt stress by regulating levels of osmolytes and antioxidant enzymes, as described in chickpea [2].
8. Concluding remarks
Lipid (per)oxidation widely occurs in plants, as consequence of high PUFA content, ROS generation through metabolism (including photosynthesis), sessile nature and susceptibility to stresses. Markers for lipid oxidation are becoming extensively used to assess plant physiology and plant adaptations to biotic and abiotic stresses. For this purpose, methods for detection, analysis and quantification of electrophilic mediators have been developed and/or adapted to plant materials. As it happens for ROS and RNS, not only deleterious, but signaling/regulatory effects are exerted by electrophilic mediators resulting from lipid oxidation. Lipoxidation processes in plants are just beginning to be revealed, and nalysis of protein lipoxidation adducts is still a developing field in plants. Only few protein lipoxidation targets have been identified in plants to date, which have been related with stress conditions. Lipoxidation often produces significant effects on these identified plant enzyme activities and even in gene expression. Both lipid oxidation and lipoxidation also occur in foodstuffs derived from plants, in a highly depending manner from their plant origin and processing procedures. Lipoxidation targets in foods are little studied, however lipid oxidation markers are commonly used to control food quality. Lipid oxidation and lipoxidation products (i,e carbonyl-amine adducts), as well as antioxidants like polyphenols, are key components present in food which may generate positive or detrimental effects on human health, including pathologies encompassing oxidative unbalances.
Funding
This work was supported by H2020-MSCA-ITN-2014 International Training Network (European Commission) and the European Regional Development Fund (ERDF) co-funded projects: BFU-2016-77243-P (Ministerio de Economía y Competitividad, Spain, -MINECO-), RTC-2017-6654-2 (Ministerio de Ciencia, Innovación y Universidades, Spain, -MCIU-), and P2011-CVI-7487 (Junta de Andalucía, Spain).
Competing interests
The author has no competing interests to declare.
Foreword
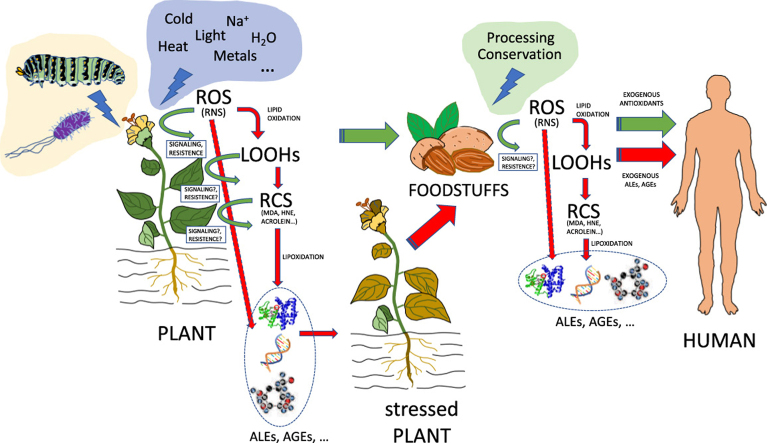
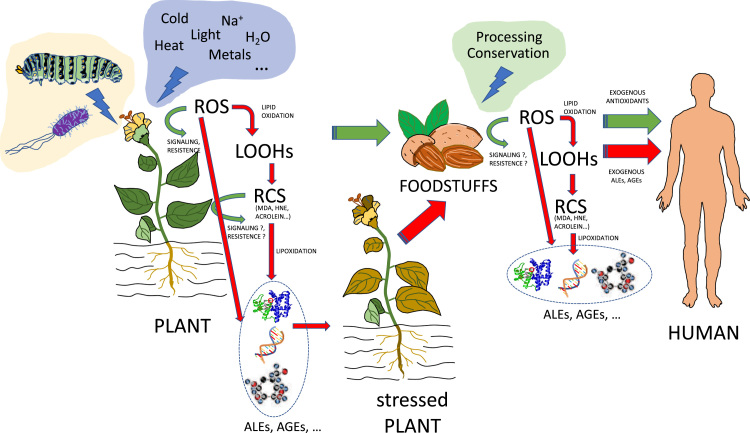
Oxidative processes inevitably occur in plants as a consequence of aerobic breakdown [33], and are enhanced by stress situations because of plant sessile nature. This short review focuses on the occurrence of oxidation phenomena in plant lipid molecules (lipid oxidation), which are frequently followed by modifications in proteins (protein lipoxidation), caused by the highly-reactive oxidized lipids generated through lipid oxidation. Both types of chemical reactions are frequent in plants, although have been much more documented in the former. The present review investigates the biochemistry of both processes in plants, with particular emphasis in the generation of derived carbonyl-containing fragments of fatty acids and their effects on proteins. The methods to chemically analyze these oxidized compounds are also reviewed here, together with their contribution to plant growth and development, as they may play important roles in signaling, gene regulation, generation of stress resistance, etc. Because lipid oxidation and protein lipoxidation are concomitantly enhanced with biotic and abiotic stresses, these topics have been specifically addressed in specific sections of this article. Finally, since plants are primarily used as raw material by food industry, one section was devoted to analyze the involvements of lipid oxidation and protein lipoxidation in food quality, as well as in health benefits and drawbacks (Fig. 1 and graphical abstract).
Fig. 1.
An overview of RCS generation, lipid oxidation and lipoxidation in Higher Plants and foodstuffs. Oxidative processes occur in plants and are enhanced by stress situations. Oxidation in plant lipid molecules (lipid oxidation) by ROS is frequently followed by modifications in proteins (protein lipoxidation), caused by the highly-reactive derived carbonyl-containing fragments of fatty acids and other lipid components. RCS may also play important roles in signaling, gene regulation, generation of stress resistance, etc. Lipid oxidation and protein lipoxidation are enhanced with biotic and abiotic stresses. Plants are primarily used as raw material by food industry, and foodstuffs may contain RCS and ROS, with levels sometimes enhanced by food processing procedures, greatly affecting food quality. Health benefits and drawbacks can be attributed to these components.
Acknowledgements
The author would like to acknowledge collaborative effort and encouragement of Dr. Dolores Pérez-Sala (CNB-CSIC, Madrid, Spain) for the preparation of the present review.
References
- 1.Afonso C.B., Sousa B.C., Pitt A.R., Spickett C.M. A mass spectrometry approach for the identification and localization of small aldehyde modifications of proteins. Arch. Biochem. Biophys. 2018;646:38–45. doi: 10.1016/j.abb.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad P., Latef A.A.A., Hashem A., Abd Allah E.F., Gucel S., Tran L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016;7:347. doi: 10.3389/fpls.2016.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldini G., Domingues M.R., Spickett C.M., Domingues P., Altomare A., Sánchez-Gomez F.J., Oeste C.L., Pérez-Sala M.D. Protein lipoxidation: detection strategies and challenges. Redox Biol. 2015;5:254–266. doi: 10.1016/j.redox.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldini G., Vistoli G., Stefek M., Chondrogianni N., Grune T., Sereikaite J., Sadowska-Bartosz I., Bartosz G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Rad. Res. 2013;47(Suppl 1):93–137. doi: 10.3109/10715762.2013.792926. [DOI] [PubMed] [Google Scholar]
- 5.Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asada K., Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle D.J., editor. Vol. 9. Elsevier; 1987. pp. 227–287. (Photoinhibition (Topics on Photosynthesis)). [Google Scholar]
- 7.Bao G.H., Bi Y., Li Y.C., Kou Z.H., Hu L.G., Ge Y.H., Wang Y., Wang D. Overproduction of reactive oxygen species involved in pathogenicity of Fusarium in potato tubers. Physiol. Mol. Plant Pathol. 2014;86:35–42. [Google Scholar]
- 8.Bengmark S. Advanced glycation and lipoxidation end products–amplifiers of inflammation: the role of food. J. Parenter. Enter. Nutr. 2007;31:430–440. doi: 10.1177/0148607107031005430. [DOI] [PubMed] [Google Scholar]
- 9.Bengmark S., Mesa M.D., Gil A. Plant-derived health - the effects of turmeric and curcuminoids. Nutr. Hosp. 2009;24(2009):273–281. [PubMed] [Google Scholar]
- 10.Bhoomika K., Pyngrope S., Dubey R.S. Effect of aluminum on protein oxidation, non-protein thiols and protease activity in seedlings of rice cultivars differing in aluminum tolerance. J. Plant Physiol. 2014;171:497–508. doi: 10.1016/j.jplph.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Bispo W.M.S., Araujo L., Bermudez-Cardona M.B., Cacique I.S., DaMatta F.M., Rodrigues F.A. Ceratocystis fimbriata-induced changes in the antioxidative system of mango cultivars. Plant Pathol. 2015;64:627–637. [Google Scholar]
- 12.Biswas M.S., Mano J. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide-induced and salt-induced programmed cell death in plants. Plant Physiol. 2015;168:885–898. doi: 10.1104/pp.115.256834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas M.S., Mano J. Reactive carbonyl species activate Caspase-3-Like protease to initiate programmed cell death in plants. Plant Cell Physiol. 2016;57:1432–1442. doi: 10.1093/pcp/pcw053. [DOI] [PubMed] [Google Scholar]
- 14.Browse J. Jasmonate passes muster: a receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009;60:183–205. doi: 10.1146/annurev.arplant.043008.092007. [DOI] [PubMed] [Google Scholar]
- 15.Chavez J., Wu J., Han B., Chung W.-G., Maier C.S. New role for an old probe: affinity labeling of oxylipid protein conjugates by N′-aminooxymethylcarbonylhydrazino D-biotin. Anal. Chem. 2006;78:6847–6854. doi: 10.1021/ac0607257. [DOI] [PubMed] [Google Scholar]
- 16.Colzani M., Criscuolo A., De Maddis D., Garzon D., Yeum K.J., Vistoli G., Carini M., Aldini G. A novel high resolution MS approach for the screening of 4-hydroxy-trans-2-nonenal sequestering agents. J. Pharm. Biomed. Anal. 2014;91:108–118. doi: 10.1016/j.jpba.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Corpas F.J., Barroso J.B. Peroxynitrite (ONOO−) is endogenously produced in Arabidopsis peroxisomes and is overproduced under cadmium stress. Ann. Bot. 2014;113:87–96. doi: 10.1093/aob/mct260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corpas F.J., Freschi L., Rodríguez-Ruiz M., Mioto P.T., González-Gordo S., Palma J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018;69:3449–3463. doi: 10.1093/jxb/erx453. [DOI] [PubMed] [Google Scholar]
- 19.Creelman R.A., Mullet J.E. Biosynthesis and action of jasmonates in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:355–381. doi: 10.1146/annurev.arplant.48.1.355. [DOI] [PubMed] [Google Scholar]
- 20.Czarnocka W., Karpiński S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018;122:4–20. doi: 10.1016/j.freeradbiomed.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Debona D., Rodrigues F.A., Rios J.A., Nascimento K.J.T. Biochemical changes in the leaves of wheat plants infected by Pyricularia oryzae. Phytopathology. 2012;102 doi: 10.1094/PHYTO-06-12-0125-R. [DOI] [PubMed] [Google Scholar]
- 22.Debona D., Rodrigues F.A., Rios J.A., Nascimento K.J.T., Silva L.C. The effect of silicon on antioxidant metabolism of wheat leaves infected by Pyricularia oryzae. Plant Pathol. 2014;63:581–589. [Google Scholar]
- 23.Di Feo L.D., Laguna I.G., Biderbost E.B. Physiological alterations associated to the Mal de Rio Cuarto virus (MRCV) infection and to vector (Delphacodes kuscheli Fennah) phytotoxicity in wheat. Trop. Plant Pathol. 2010;35:79–87. [Google Scholar]
- 24.Domingues R.M., Domingues P., Melo T., Pérez-Salas D., Reis A., Spickett C.M. Lipoxidation adducts with peptides and proteins: deleterious modifications or signaling mechanisms? J. Prot. 2013;92:110–131. doi: 10.1016/j.jprot.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Domínguez M., de Oliveira E., Odena M.A., Portero M., Pamplona R., Ferrer I. Redox proteomic profiling of neuroketal-adducted proteins in human brain: regional vulnerability at middle age increases in the elderly. Free Radic. Biol. Med. 2016;95:1–15. doi: 10.1016/j.freeradbiomed.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 26.Farmer E.E., Mueller M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant Biol. 2013;64:429–450. doi: 10.1146/annurev-arplant-050312-120132. [DOI] [PubMed] [Google Scholar]
- 27.Fazzari M., Trostchansky A., Schopfer F.J., Salvatore S.R., Sánchez-Calvo B., Vitturi D., Valderrama R., Barroso J.B., Radi R., Freeman B.A., Rubbo H. Olives and olive oil are sources of electrophilic fatty acid nitroalkenes. PLoS One. 2014;9:e84884. doi: 10.1371/journal.pone.0084884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feussner I., Wasternack C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002;53:275–297. doi: 10.1146/annurev.arplant.53.100301.135248. [DOI] [PubMed] [Google Scholar]
- 29.Foyer Ch. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environm. Exp. Bot. 2018;154:134–142. doi: 10.1016/j.envexpbot.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gayarre J., Stamatakis K., Renedo M., Pérez-Sala M.D. Differential selectivity of protein modification by the cyclopentenone prostaglandins PGA1 and 15-deoxy-Δ12,14-PGJ2: role of glutathione. FEBS Let. 2005;579:5803–5808. doi: 10.1016/j.febslet.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 31.Gil A., Bengmark S. Advanced glycation and lipoxidation end products--amplifiers of inflammation: the role of food. Nutr. Hosp. 2007;22:625–640. [PubMed] [Google Scholar]
- 32.Gunes A., Inal A., Alpaslan M., Eraslan F., Bagci E.G., Cicek N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007;164:728–736. doi: 10.1016/j.jplph.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 33.Gupta D.K., Palma J.M., Corpas F.J., editors. Preface. Redox state as a central regulator of plant-cell stress responses. Springer; 2016. p. 386. [Google Scholar]
- 34.Han Y.Q., Li P., Gong S.L., Yang L., Wen L.Z., Hou M.L. Defense Responses in Rice Induced by Silicon Amendment against Infestation by the Leaf Folder Cnaphalocrocis medinalis. PLOS ONE. 2016;11:e0153918. doi: 10.1371/journal.pone.0153918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hidalgo F.J., Zamora R. Food Processing Antioxidants. Food Nutr. Res. 2017;81:31–64. doi: 10.1016/bs.afnr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Hodges D.M., DeLong J.M., Forney C.F., Prange R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s00425-017-2699-3. [DOI] [PubMed] [Google Scholar]
- 37.Howe G.A. Cyclopentenone signals for plant defense: remodeling the jasmonic acid response. PNAS. 2001;98:12317–12319. doi: 10.1073/pnas.231480898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez-Quesada M.J., Carmona R., Lima-Cabello E., Traverso J.A., Castro A.J., Claros M.G., Alché J.D. Generation of nitric oxide by olive (Olea europaea L.) pollen during in vitro germination and assessment of the S-nitroso- and nitro-proteomes by computational predictive methods. Nitric Oxide. 2016;68:23–37. doi: 10.1016/j.niox.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Kirch H.H., Bartels D., Wei Y., Schnable P.S., Wood A.J. The ALDH gene superfamily of Arabidopsis. Trends Plant Sci. 2004;9:371–377. doi: 10.1016/j.tplants.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kotchoni S.O., Kuhns C., Ditzer A., Kirch H.H., Bartels D. Overexpression of different aldehyde dehydrogenase genes in Arabidopsis thaliana confers tolerance to abiotic stress and protects plants against lipid peroxidation and oxidative stress. Plant Cell Environ. 2006;29:1033–1048. doi: 10.1111/j.1365-3040.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 41.Lanubile A., Maschietto V., De Leonardis S., Battilani O., Paciolla C., Marocco A. Defense responses to Mycotoxin-producing Fungi Fusarium proliferatum, F. subglutinans, and Aspergillus flavus in Kernels of Susceptible and Resistant Maize Genotypes. Mol. Plant Micr. Inter. 2015;28:546–557. doi: 10.1094/MPMI-09-14-0269-R. [DOI] [PubMed] [Google Scholar]
- 42.Li W.J., Gao H.Y., Fang X.J., Tao F., Chen H.J., Mu H.L., Jiang Y.M. Accumulation of lipofuscin-like pigments of walnuts (Carya cathayensis) during storage: potential roles of lipid oxidation and non-enzymatic glycosylation. J. Sci. Food Agric. 2014;94:2505–2513. doi: 10.1002/jsfa.6587. [DOI] [PubMed] [Google Scholar]
- 43.Liang D.N., Cao L., Song L.L., Qi X.H., Chen X.H. Effects of stress from Aphis gossypii Glover on malondialdehyde content and protective enzymes activities in cucumber. Acta Hortic. 2016;1129:27–33. [Google Scholar]
- 44.Loeffler C., Berger S., Guy A., Durand T., Bringmann G., Dreyer M., von Rad U., Durner J., Mueller M.J. B(1)-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol. 2005;137:328–340. doi: 10.1104/pp.104.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mano J., Khorobrykh S., Matsui K., Iijima Y., Sakurai N., Suzuki H., Shibata D. Acrolein is formed from trienoic fatty acids in chloroplast: a targeted metabolomics approach. Plant Biotech. 2014;31:535–543. [Google Scholar]
- 46.Mano J., Nagata M., Okamura S., Shiraya T., Mitsui T. Identification of oxidatively modified proteins in salt-stressed arabidopsis: a carbonyl-targeted proteomics approach. Plant Cell Physiol. 2014;55:1233–1244. doi: 10.1093/pcp/pcu072. [DOI] [PubMed] [Google Scholar]
- 47.Mano J., Tokushige K., Mizoguchi H., Fujii H., Khorobrykh S. Accumulation of lipid peroxide-derived, toxic a,b-unsaturated aldehydes (E)-2-pentenal, acrolein and (E)-2-hexenal in leaves under photoinhibitory illumination. Plant Biotechnol. 2010;27:193–197. [Google Scholar]
- 48.Mano J., Torii Y., Hayashi S., Takimoto K., Matsui K., Nakamura K., Inzé D., Babiychuk E., Kushnir S., Asada K. The NADPH: quinone oxidoreductase P1-z-crystallin in Arabidopsis catalyzes the a,b-hydrogenation of 2-alkenals: detoxication of the lipid peroxide-derived reactive aldehydes. Plant Cell Physiol. 2002;43:1445–1455. doi: 10.1093/pcp/pcf187. [DOI] [PubMed] [Google Scholar]
- 49.Mata-Pérez C., Sánchez-Calvo B., Padilla M.N., Begara-Morales J.C., Luque F., Melguizo M., Jiménez-Ruiz J., Fierro-Risco J., Peñas-Sanjuán A., Valderrama R., Corpas F.J., Barroso J.B. Nitro-Fatty Acids in Plant Signaling: nitro-linolenic Acid Induces the Molecular Chaperone Network in Arabidopsis. Plant Physiol. 2016;170:686–701. doi: 10.1104/pp.15.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mata-Pérez C., Sánchez-Calvo B., Padilla M.N., Begara-Morales J.C., Valderrama R., Corpas F.J., Barroso J.B. Nitro-fatty acids in plant signaling: new key mediators of nitric oxide metabolism. Redox Biol. 2017;11:554–561. doi: 10.1016/j.redox.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsui K. Green leaf volatiles: hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006;9(2006):274–280. doi: 10.1016/j.pbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Matsui K., Sugimoto K., Kakumyan P., Khorobrykh S.A., Mano J. Volatile oxylipins and related compounds formed under stress in plants. In: Armstrong D., editor. Vol. 580. Humana Press; 2009. (Lipidomics. Methods in Molecular Biology™ (Methods and Protocols)). [DOI] [PubMed] [Google Scholar]
- 53.Medina S., Gil-Izquierdo A., Durand, T. Ferreres F., Domínguez-Perles R. Structural/functional matches and divergences of phytoprostanes and phytofurans with bioactive human oxylipins. Antioxidants. 2018;7:165. doi: 10.3390/antiox7110165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meena M., Zehra A., Dubey M.K., Aamir M., Gupta V.K., Upadhyay R.S. Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by alternaria alternata and its toxic metabolites (TeA, AOH, and AME) Front. Plant Sci. 2016;7:1408. doi: 10.3389/fpls.2016.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mène-Saffrané L., Davoine C., Stolz S., Majcherczyk P., Farmer E.E. Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant. J. Biol. Chem. 2007;282:33749–33756. doi: 10.1074/jbc.M706838200. [DOI] [PubMed] [Google Scholar]
- 56.Millar A.H., Leaver C.J. The cytotoxic lipid peroxidation product, 4-hydroxy-2-nonenal, specifically inhibits decarboxylating dehydrogenases in the matrix of plant mitochondria. FEBS Lett. 2000;484:117–121. doi: 10.1016/s0014-5793(00)01976-1. [DOI] [PubMed] [Google Scholar]
- 57.Mock H.P., Dietz K.J. Redox proteomics for the assessment of redox-related posrttranslational regulation in plants. Biochim. Biophys. Acta. 1864;2016:967–973. doi: 10.1016/j.bbapap.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Muckenschnabel I., Goodman B.A., Williamson B., Lyon G.D., Deighton N. Infection of leaves of Arabidopsis thaliana by Botrytis cinerea: changes in ascorbic acid, free radicals and lipid peroxidation products. J. Exp. Bot. 2002;53:207–214. doi: 10.1093/jexbot/53.367.207. [DOI] [PubMed] [Google Scholar]
- 59.Mueller S., Hilbert B., Dueckershoff K., Roitsch T., Krischke M., Mueller M.J., Berger S. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell. 2008;20:768–785. doi: 10.1105/tpc.107.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Namratha M.R. Univ. Agric. Sci.; Bengaluru: 2015. Co-expression of aldo-keto reductase (AKR1) and protein l-isoaspartyl methyltransferase (PIMT2) to enhance seed viability, seedling vigour and abiotic stress tolerance in groundnut transgenics (Arachis hypogaea L.) (Master Thesis) [Google Scholar]
- 61.Nisarga K.N., Vemanna R.S., Kodekallu Chandrashekar B., Rao H., Vennapusa A.R., Narasimaha A., Makarla U., Basavaiah M.R. Aldo-ketoreductase 1 (AKR1) improves seed longevity in tobacco and rice by detoxifying reactive cytotoxic compounds generated during ageing. Rice (N.Y.) 2017;10:11. doi: 10.1186/s12284-017-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noctor G., Reichheld J.P., Foyer C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018;80:3–12. doi: 10.1016/j.semcdb.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 63.Oberschall A., Deák M., Török K., Sass L., Vass I., Kovács I., Fehér A., Dudits D., Horváth G.V. A novel aldose/aldehyde reductase protects transgenic plants against lipid peroxidation under chemical and drought stresses. Plant J. 2000;24:437–446. doi: 10.1046/j.1365-313x.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 64.Oeste C., Pérez-Sala M.D. Modification of cysteine residues by cyclopentenone prostaglandins: interplay with redox regulation of protein function. Mass Spect. Rev. 2014;33:110–125. doi: 10.1002/mas.21383. [DOI] [PubMed] [Google Scholar]
- 65.Patón M.M. University of Granada; 2017. Identificación y análisis in silico de los transcritos de glutatión peroxidase en la semilla del olivo y caracterización enzimática (Master Thesis) p. 38. [Google Scholar]
- 66.Roe M.R., Xie H., Bandhakavi S., Griffin T.J. Proteomic mapping of 4-hydroxynonenal protein modification sites by solid-phase hydrazide chemistry and mass spectrometry. Anal. Chem. 2007;79:3747–3756. doi: 10.1021/ac0617971. [DOI] [PubMed] [Google Scholar]
- 67.Rodrigues S.M., Andrade M.O., Gomes A.P.S., Damatta F.M., Baracat-Pereira M.C., Fontes E.P. Arabidopsis and tobacco plants ectopically expressing the soybean antiquing-like ALDH7 gene display enhanced tolerance to drought, salinity, and oxidative stress. J. Exp. Bot. 2006;57:1909–1918. doi: 10.1093/jxb/erj132. [DOI] [PubMed] [Google Scholar]
- 68.Saito R., Shimakawa G., Nishi A., Iwamoto T., Sakamoto K., Yamamoto H., Amako K., Makino A., Miyake C. Functional analysis of the AKR4C subfamily of Arabidopsis thaliana: model structures, substrate specificity, acrolein toxicity, and responses to light and [CO2] Biosci. Biotechnol. Biochem. 2013;77:2038–2045. doi: 10.1271/bbb.130353. [DOI] [PubMed] [Google Scholar]
- 69.Sembdner G., Parthier B. The biochemistry and the physiological and molecular actions of jasmonates. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993;44:568–569. [Google Scholar]
- 70.Shah K., Kumar R.G., Verma S., Dubey R.S. Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci. 2001;161:1135–1144. [Google Scholar]
- 71.Sharma R., Sharma A., Shono T., Takasugi M., Shirata A., Fujimura T., Machii H. Mulberry Moracins: scavengers of UV Stress-generated Free Radicals. Biosc. Biotech. Biochem. 2001;65:1402–1405. doi: 10.1271/bbb.65.1402. [DOI] [PubMed] [Google Scholar]
- 72.Sharov V.S., Kasamanov V.A., Vladimirov Y.A. Selective sensitisation of chemiluminescence resulted from lipid and oxygen radical reactions. Free Rad. Biol. Med. 1989;7:237–242. doi: 10.1016/0891-5849(89)90130-5. [DOI] [PubMed] [Google Scholar]
- 73.Shin J.H., Kim S.R., An G. Rice aldehydedehydrogenase7 is needed for seed maturation and viability. Plant Physiol. 2009;149:905–915. doi: 10.1104/pp.108.130716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siddique Z., Akhtar K.P., Hameed A., Sarwar N., Imran-Ul-Haq, Khan S.A. Biochemical alterations in leaves of resistant and susceptible cotton genotypes infected systemically by cotton leaf curl Burewala virus. J. Plant Inter. 2014;9:702–711. [Google Scholar]
- 75.Soler-Cantero A., Jové M., Cacabelos D., Boada J., Naudí A., Romero M.P., Cassanyé A., Serrano J.C.E., Arola L., Valls J., Bellmunt M.J., Prat J., Pamplona R., Portero-Otin M., Motilva J.J. Plant-derived phenolics inhibit the accrual of structurally characterised protein and lipid oxidative modifications. PLoS ONE. 2012;7:e43308. doi: 10.1371/journal.pone.0043308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sousa B.C., Pitt A.R., Spickett C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Rad. Biol. Med. 2017;111:294–308. doi: 10.1016/j.freeradbiomed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 77.Spickett C.M. The lipid peroxidation product 4-hydroxy-2-nonenal: advances in chemistry and analysis. Redox Biol. 2013;1:145–152. doi: 10.1016/j.redox.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stiti N., Missihoun T.D., Kotchoni S.O., Kirch H.H., Bartels D. Aldehyde dehydrogenases in Arabidopsis thaliana: biochemical requirements, metabolic pathways, and functional analysis. Front. Plant Sci. 2011;2:65. doi: 10.3389/fpls.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steward R.C., Bewley J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980;65:245–248. doi: 10.1104/pp.65.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stintzi A., Weber H., Reymond Ph, Browse J., Farmer E.E. Plant defense in the absence of jasmonic acid: the role of cyclopentenones. PNAS. 2001;98:12837–12842. doi: 10.1073/pnas.211311098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Surh Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 82.Syed Z., Guerin P.M. Tsetse flies are attracted to the invasive plant Lantana camara. J. Insect Physiol. 2004;50:43–50. doi: 10.1016/j.jinsphys.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 83.Takagi D., Ifuku K., Ikeda K., Inoue K.I., Park P., Tamoi M., Inoue H., Sakamoto K., Saito R., Miyake C. Suppression of chloroplastic alkenal/one oxidoreductase represses the carbon catabolic pathway in arabidopsis leaves during night. Plant Physiol. 2016;170:2024–2039. doi: 10.1104/pp.15.01572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tazi S., Plantevin F., Di Falco Ch, Puigserver A., Ajandouza E.H. Effects of light, temperature and water activity on the kinetics of lipoxidation in almond-based products. Food Chem. 2009;115:958–964. [Google Scholar]
- 85.Tazi S., Puigserver A., Ajandouz E.H. A novel, fast and accurate chemiluminescence method for measuring lipoxidation in almonds and almond-based products during processing and storage. Food Chem. 2009;116:999–1004. [Google Scholar]
- 86.Thoma I., Loeffler C., Sinha A.K., Gupta M., Krischke M., Steffan B., Roitsch T., Mueller M.J. Cyclopentenone isoprostanes induced by reactive oxygen species trigger defense gene activation and phytoalexin accumulation in plants. Plant J. Cell Mol. Biol. 2003;34:363–375. doi: 10.1046/j.1365-313x.2003.01730.x. [DOI] [PubMed] [Google Scholar]
- 87.Turóczy Z. Hungarian Academy of Sciences; 2011. The role of rice aldo/keto reductases in the detoxification of reactive carbonyls and their use to create stress resistant transgenic plants (Ph.D. Thesis) p. 109. [Google Scholar]
- 88.Turóczy Z., Kis P., Török K., Cserháti M., Lendvai A., Dudits D., Horváth G.V. Overproduction of a rice aldo-keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol. Biol. 2011;75:399–412. doi: 10.1007/s11103-011-9735-7. [DOI] [PubMed] [Google Scholar]
- 89.Salomon R.G., Kaur K., Podrez E., Hoff H.F., Krushinsky A.V., Sayre L.M. HNE-Derived 2-Pentylpyrroles Are Generated during Oxidation of LDL, Are More Prevalent in Blood Plasma from Patients with Renal Disease or Atherosclerosis, and Are Present in Atherosclerotic Plaques. Chem. Res. Toxicol. 2000;13:557–564. doi: 10.1021/tx000007u. [DOI] [PubMed] [Google Scholar]
- 90.Van Ruyskensvelde V., Van Breusegem F., Van Der Kelen K. Post-transcriptional regulation of the oxidative stress response in plants. Free Rad. Biol. Med. 2018;122:181–192. doi: 10.1016/j.freeradbiomed.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 91.Vemanna R.S., Babitha K.C., Solanki J.K., Amarnatha Reddy V., Sarangi S.K., Udayakumar M. Aldo-keto reductase-1 (AKR1) protect cellular enzymes from salt stress by detoxifying reactive cytotoxic compounds. Plant Physiol. Biochem. 2017;113:177–186. doi: 10.1016/j.plaphy.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 2013;47(Suppl 1):3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 93.Wang X.Y., Zhou L.H., Xu B.A., Xing X., Xu G.Q. Seasonal occurrence of Aphis glycines and physiological responses of soybean plants to its feeding. Insect Sci. 2014;21:342–351. doi: 10.1111/1744-7917.12099. [DOI] [PubMed] [Google Scholar]
- 94.War A.R., Munghate R.S., Sharma H.C. Expression of different mechanisms of resistance to insects in groundnut under field conditions. Phytoparasitica. 2015;43:669–677. [Google Scholar]
- 95.War A.R., Paulraj M.G., Ignacimuthu S., Sharma H.C. Defensive responses in groundnut against chewing and sap-sucking. Insects J. Plant Growtg Reg. 2013;32:259–272. [Google Scholar]
- 96.War A.R., Paulraj M.G., War M.Y., Ignacimuthu S. Jasmonic acid-mediated-induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner) (Lepidoptera: noctuidae) J. Plant Growth Reg. 2011;30:512–523. [Google Scholar]
- 97.War A.R., Paulraj M.G., War M.Y., Ignacimuthu S. Differential defensive response of groundnut germplasms to Helicoverpa armigera (Hubner) (Lepidoptera: noctuidae) J. Plant Inter. 2012;7 [Google Scholar]
- 98.Weber H., Vick B.A., Farmer E.E. Dinor-oxo-phytodienoic acid: a new hexadecanoid signal in the jasmonate family. PNAS. 1997;94:10473–10478. doi: 10.1073/pnas.94.19.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamauchi Y., Furutera A., Seki K., Toyoda Y., Tanaka K., Sugimoto Y. Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol. Biochem. 2008;46:786–793. doi: 10.1016/j.plaphy.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 100.Yamauchi Y., Sugimoto Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta. 2010;231:1077–1088. doi: 10.1007/s00425-010-1112-2. [DOI] [PubMed] [Google Scholar]
- 101.Yamauchi Y., Hasegawa A., Mizutani M., Sugimoto Y. Chloroplastic NADPH-dependent alkenal/one oxidoreductase contributes to the detoxification of reactive carbonyls produced under oxidative stress. FEBS Lett. 2012;586:1208–1213. doi: 10.1016/j.febslet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Yamauchi Y., Hasegawa A., Taninaka A., Mizutani M., Sugimoto Y. NADPH-dependent reductases involved in the detoxification of reactive carbonyls in plants. J. Biol. Chem. 2011;286:6999–7009. doi: 10.1074/jbc.M110.202226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamauchi Y., Kunishima M., Mizutani M., Sugimoto Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015;5:8030. doi: 10.1038/srep08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yan L.J., Forster M.J. Chemical probes for analysis of ted proteins: a review. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011;879:1308–1315. doi: 10.1016/j.jchromb.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yasuda M., Narita S. Simultaneous determination of phospholipids hydroperoxides and cholesteryl ester hydroperoxides in human plasma by high-performance liquid chromatography with chemiluminescence detection. J. Chromat. B: Biomed. Sci. Applic. 1997;693:211–217. doi: 10.1016/s0378-4347(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 106.Zhu B., Peng R.H., Xiong A.S., Xu J., Fu X.Y., Zhao W., Jin X.F., Meng X.R., Gao J.J., Cai R., Yao H. Transformation with a gene for myo-inositol O-methyltransferase enhances the cold tolerance of Arabidopsis thaliana. Biol. Plant. 2012;56:135–139. [Google Scholar]
- 107.Zhu X., Tang X., Zhang J., Tochtrop G.P., Anderson V.E., Sayre L.M. Mass spectrometric evidence for the existence of distinct modifications of different proteins by 2(E),4(E)-decadienal. Chem. Res. Toxicol. 2010;23:467–473. doi: 10.1021/tx900379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zieske L.R. A perspective on the use of iTRAQ reagent technology for protein complex and profiling studies. J. Exp. Bot. 2006;57:1501–1508. doi: 10.1093/jxb/erj168. [DOI] [PubMed] [Google Scholar]
- 109.Zoeller M., Stingl N., Krischke M., Fekete A., Waller F., Berger S., Mueller M.J. Lipid profiling of the arabidopsis hypersensitive response reveals specific lipid peroxidation and fragmentation processes: biogenesis of Pimelic and Azelaic Acid. Plant Physiol. 2012;160:365–378. doi: 10.1104/pp.112.202846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sunkar R., Bartels D., Kirch H.H. Overexpression of a stress‐inducible aldehyde dehydrogenase gene from Arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J. 2003;35:452–464. doi: 10.1046/j.1365-313x.2003.01819.x. [DOI] [PubMed] [Google Scholar]
- 111.Yin L., Mano J., Wang Sh., Tsuji W., Tanaka K. The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol. 2010;152:1406–1417. doi: 10.1104/pp.109.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]