Abstract
Background
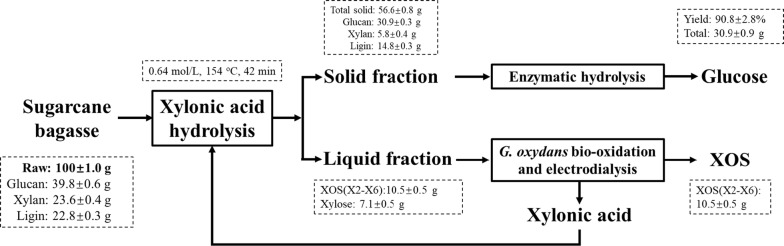
Obtaining high-value products from lignocellulosic biomass is central for the realization of industrial biorefinery. Acid pretreatment has been reported to yield xylooligosaccharides (XOS) and improve enzymatic hydrolysis. Moreover, xylose, an inevitable byproduct, can be upgraded to xylonic acid (XA). The aim of this study was to valorize sugarcane bagasse (SB) by starting with XA pretreatment for XOS and glucose production within a multi-product biorefinery framework.
Results
SB was primarily subjected to XA pretreatment to maximize the XOS yield by the response surface method (RSM). A maximum XOS yield of 44.5% was achieved by acid pretreatment using 0.64 M XA for 42 min at 154 °C. Furthermore, XA pretreatment can efficiently improve enzymatic digestibility, and achieved a 90.8% cellulose conversion. In addition, xylose, the inevitable byproduct of the acid-hydrolysis of xylan, can be completely converted to XA via bio-oxidation of Gluconobacter oxydans (G. oxydans). Subsequently, XA and XOS can be simultaneously separated by electrodialysis.
Conclusions
XA pretreatment was explored and exhibited a promising ability to depolymerize xylan into XOS. Mass balance analysis showed that the maximum XOS and fermentable sugars yields reached 10.5 g and 30.9 g per 100 g raw SB, respectively. In summary, by concurrently producing XOS and fermentable sugars with high yields, SB was thus valorized as a promising feedstock of lignocellulosic biorefinery for value-added products.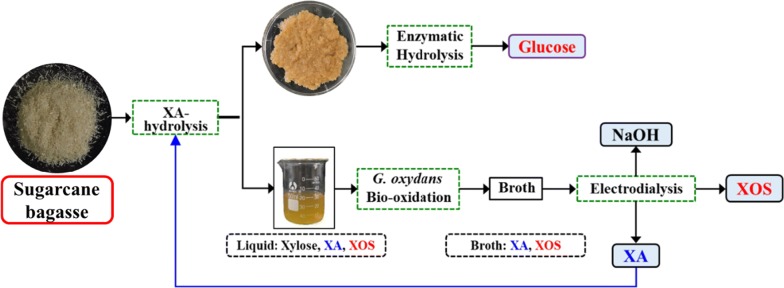
Keywords: Xylooligosaccharides, Xylonic acid, Xylose, Sugarcane bagasse, Enzymatic hydrolysis
Background
The global overconsumption of fossil fuels has driven the development of the lignocellulosic biorefinery concept, an industrial process that converts sustainably sourced lignocellulosic biomass into energy, chemicals, and fuels [1, 2]. A suitable candidate for such processing is sugarcane bagasse (SB), which is one of the most abundant types of lignocellulosic biomass in China and in other parts of the world [3]. SB is comprised of all biomass refuse after sugarcane harvest, and is usually discarded or burned at the field [4]. Both of these ongoing practices represent environmentally imprudent or even harmful usage of a readily available biomass resource [3–5]. The results of previous studies on the biorefining of SB indicated SB as well-suited for the production of value-added products especially due to its high polysaccharide content. Specifically, several sources of SB have been shown to consist of 40–50% cellulose and 20–30% hemicelluloses based on their dry mass [6, 7]. However, the natural structure of SB (and in general, of all lignocellulosic biomass) is strongly recalcitrant to enzymatic hydrolysis of polysaccharides (cellulose in particular). This characteristic thus necessitates the implementation of economical pretreatment that deconstructs the lignocellulosic entanglement without inducing damaging its constituents. To date, the pretreatment technologies that have received the most attention include hydrothermal, steam explosion, diluted acid, and diluted alkaline pretreatment [5, 8, 9].
Of particular note is that diluted acid pretreatment is not only beneficial for enzymatic hydrolysis, but it is also an effective mean to obtain desirable xylooligosaccharide (XOS) products from SB [10, 11]. This is because SB hemicelluloses are predominately composed of xylan. Here, XOS, from degradation of xylan, are short-chain oligosaccharides that are composed of 2–7 xylose molecules, tethered by β-(1,4) glycosidic linkages [12]. The most attractive biological feature of XOS is that it cannot be digested or absorbed by the human digestive system when consumed. Furthermore, it promotes the growth of beneficial intestinal bacteria. Therefore, XOS with a low degree of polymerization (DP), can be considered as key ingredients toward the improvement of gut health. Ingestion leads to a boost in calcium absorption, lowers cholesterol, improves the immune system, and reduces the risk of colon cancer [13–16]. In addition, the rapid growth of the functional food/feed in markets has boosted the demand for high-quality XOS, which the current market prices reflect as much as $22–50/kg [17–19]. Thus, it is beneficial to implement diluted acid pretreatment in a biorefinery process to optimize XOS production. Doing so would add an additional profit-generating product to the product portfolio of a biorefinery with the potential to achieve a high price.
Pretreatments with diluted acid use mineral or organic acid reagents, both of which have been reported to be capable of yielding XOS [20–22]. However, it has been suggested that mineral acids promote an increased extent of xylose and furfural production, which directly translates to lower XOS yields [20]. In contrast, organic acids tend to favor XOS generation, while also yielding additional benefits such as little furfural yield, lower corrosiveness and decreased generation of enzymatic hydrolysis inhibitors [11, 23, 24]. Various organic acids, such as acetic acid, oxalic acid, and gluconic acid, have already been explored as reagents for XOS production during acid pretreatment [25, 26]. Lin et al. [23] developed a microwave-induced hydrolysis of beechwood xylan with oxalic acid strategy to produce XOS, which achieved a yield of 39.31%. Zhang et al. [11] developed an acetic acid pretreatment method to produce XOS and improve enzymatic hydrolysis efficiency from corncob, and the acetic acid hydrolysis achieved XOS yield of 45.9%. In addition, Zhou et al. [24] found that gluconic acid exhibited a good ability to degrade SB xylan into XOS with a yield of 53.2%. It is important to note that this pretreatment technology effectively reduces biomass’ recalcitrance to enzymatic digestion [11, 27]. The key concern during diluted acid pretreatment for XOS production is to control how much xylose will be generated, which is an inevitable chemical reaction given the lability of glycosidic bonds in acidic media. Interestingly, previous reports suggested and successfully demonstrated that this xylose could be upgraded to xylonic acid (XA) and recovered as an additional product stream via electrodialysis [28, 29]. XA, with a structure similar to gluconic and derived from the oxidation of xylose, can also release H+ to depolymerize xylan [30]. In addition, it has been demonstrated that acidic pretreatment can efficiently weaken the hydrolyzed glycosidic bond of hemicellulose and lignin–hemicellulose bonds, which leads to sugar dissolution in the hemicellulose and to an increased porosity of the plant cell wall [6]. Overall, it is feasible to assume that XA can be used as an acid catalyst during diluted acid pretreatment. This study aspired to further optimize the acid pretreatment for obtain high value-added XOS that utilizes XA as key reagent. Thus, the influences of XA concentration, hydrolysis duration, and temperature on the composition of obtained hydrolysates were primarily assessed to maximize the XOS yield. Furthermore, the enzymatic hydrolysis efficiency of XA pretreated SB solids was also investigated. In summary, this work demonstrates an integrated biorefinery process that utilizes a reagent derived from biomass itself to produce XOS, a substrate that remains easily digestible by cellulolytic enzyme systems.
Results and discussion
Response surface method optimization of XOS yields
The main purpose of this study aspired to maximize the XOS yield due to the high value and the proportion of the soluble compounds (XOS) generally depends on the operation conditions. Here, temperature, acid concentration, and reaction time are crucial parameters in the hydrolysis of hemicelluloses. This is because each of these factors affects both hydrolysis rate and selectivity. Consequently, each of these three parameters was primarily optimized to achieve the highest XOS yield. RSM is an effective statistical procedure that uses a minimum set of experiments to determine the coefficients of a mathematical model as well as the optimum conditions. Thus, in the present study, SB samples were pretreated at different temperatures (130–170 °C) over a time range of 15–75 min with 3.0 g of SB and 30 mL XA solutions with different XA concentrations. Values of independent process variables were studied and the responses obtained from 13 different combinations of reaction conditions are shown in Table 1.
Table 1.
Independent variables of the central composite design and results of response surface analysis [xylooligosaccharide (XOS) yields]
| Variables | Responses | ||
|---|---|---|---|
| Reaction temperature (°C) | Reaction time (min) | XA concentration (mol/L) | XOS yields (%) |
| 130 | 15 | 0.25 | 1.03 |
| 130 | 15 | 1 | 1.31 |
| 130 | 75 | 0.25 | 16.99 |
| 130 | 75 | 1 | 11.68 |
| 150 | 45 | 0.625 | 42.61 |
| 150 | 45 | 0 | 0.88 |
| 150 | 45 | 1 | 32.50 |
| 150 | 95 | 0.625 | 40.18 |
| 170 | 15 | 0.25 | 15.95 |
| 170 | 15 | 1 | 31.22 |
| 170 | 75 | 0.25 | 18.96 |
| 170 | 75 | 1 | 8.75 |
| 183 | 45 | 0.625 | 17.97 |
After the XA hydrolysis of SB hemicelluloses was completed, the hydrolysis products in the hyrolysate of each sample were analyzed. Although XOS with DP larger than 6 could be generated, each sample reached up to DP 6, due to the lack of > DP6 of the standards, such as X7 and X8. Thus, X2–X6 were calculated for XOS yield, which is listed in Table 1. In addition, the relative contents of X2–X6, xylose, and furfural are shown in Fig. 1. According to the fit summary reports, the following regression equation represented the XOS yield from the experimental responses:
Fig. 1.
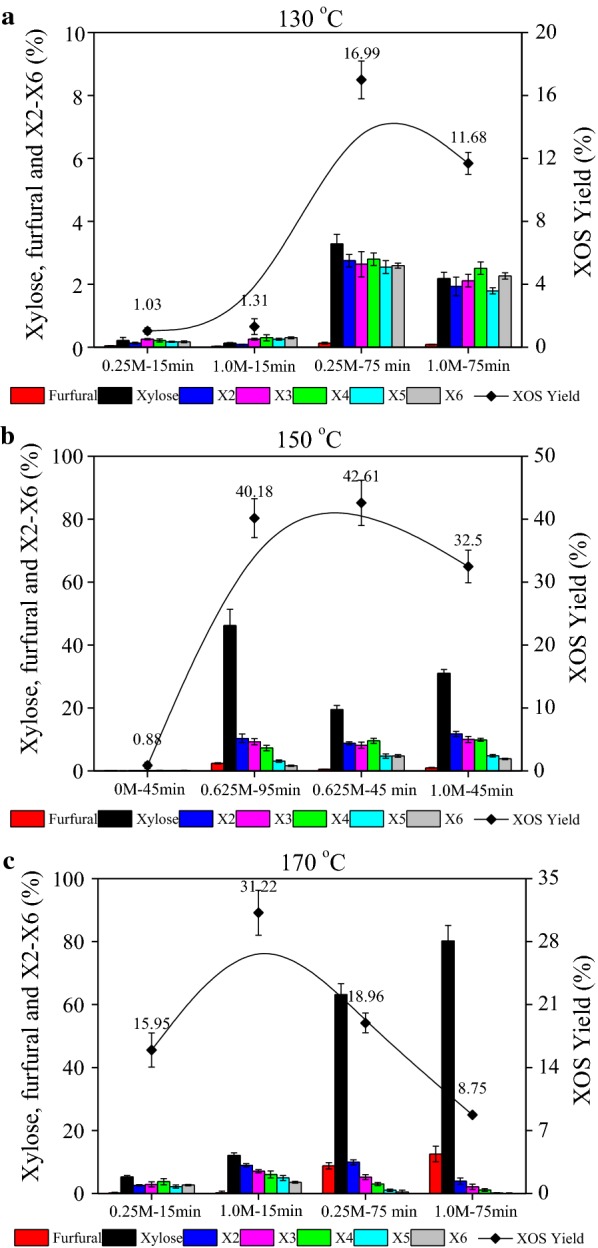
Yields of furfural, xylose, X2, X3, X4, X5, and X6 in hydrolysate produced from SB with different acid concentrations and times at a 130 °C, b 150 °C, and c 170 °C
In the formula, a1, a2, and a3 represent temperature, hydrolysis time, and XA concentration, respectively. The determination coefficient R2 was used as correlation measure to test the goodness-of-fit of the regression equation, which is defined as the ratio of the explained variation to the total variation, and demonstrates the agreement between the observed and predicted results [31]. In the present study, the R2 for XOS yield was 0.974. The relatively high R2 values of the models indicate close agreement between the experimental results and the predicted values provided by the model. This similarity can also be verified by high correlations between the observed and predicted values (curves are supplied in Additional file 1). The results from analysis of variance yield a model’s F-value of 12.48 and a P-value of 0.031, indicating that the model’s quadratic terms accurately fitted the experimental data. Analysis of XOS yield showed that the P-values of a1, a1a2, a21, and a23 were < 0.05, indicating that the independent variable a1 and the quadratic terms of a1a2, a21, and a23 exerted significant effects on XOS yield. Two-dimensional contour plots and three-dimensional response surface plots were generated by Design-Expert software and is showed in Fig. 2a–c. The analysis results also demonstrated that the effects of the reaction temperature was more significant for XOS yield than that of hydrolysis time and acid concentration.
Fig. 2.
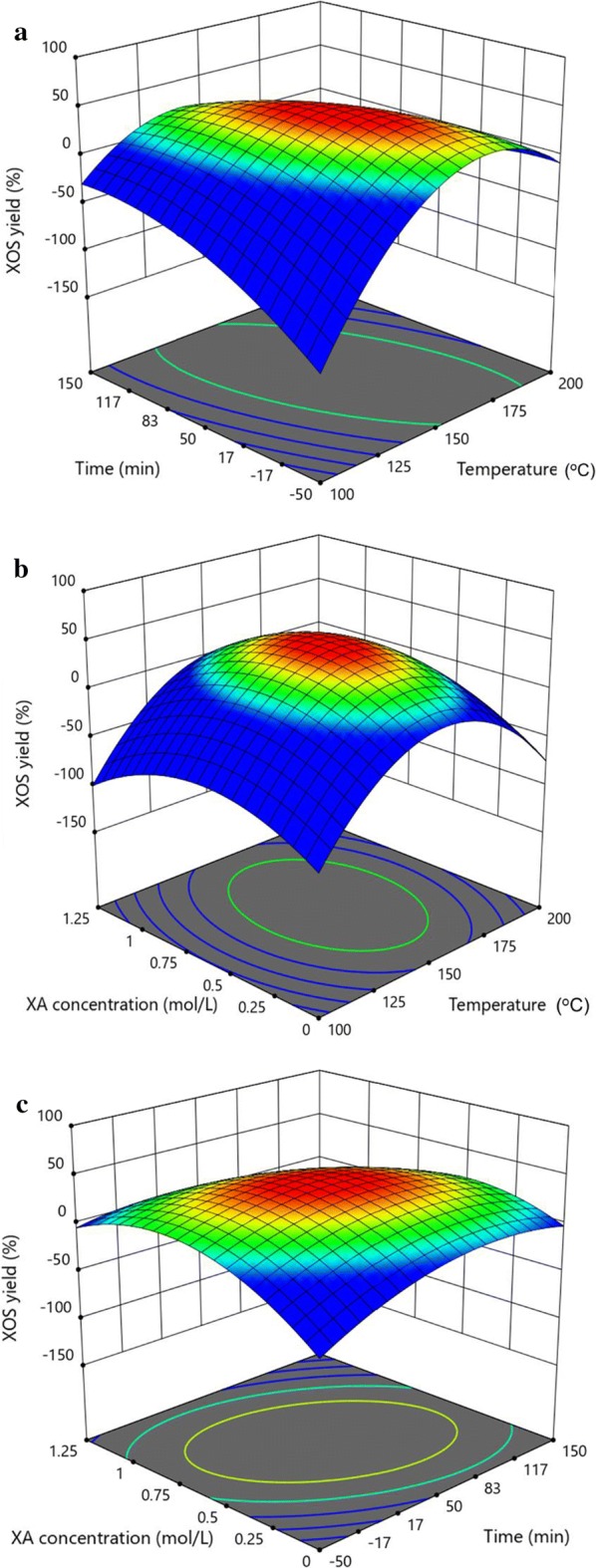
Response surface showing the effects of independent variables on XOS yields. a Reaction temperature (°C) and time (min); b XA concentration (mol/L) and reaction temperature (°C); c XA concentration (mol/L) and reaction time (min)
A relatively low temperature and short treatment time resulted in very low xylose and XOS yields. This is due to the time it takes to hydrolyze hemicelluloses into oligosaccharides. However, the XOS yield increased strongly from 1.3% (at 130 °C, 15 min, and 1.0 M) to 31.2% at elevated temperature (170 °C, 15 min, and 1.0 M). This was because the higher temperature accelerated the degradation of xylan-type hemicelluloses. Since the DP decreased with increasing acid concentration and hydrolysis time, the formation of monomers in the hydrolysate was inevitable. The results indicated that higher temperature and longer reaction times were conducive to the further degradation of XOS. With increased reaction temperature and acid concentration, X5, X6, and > X6 continued to be hydrolyzed into smaller oligosaccharides such as X2, X3, and X4. A higher level of X2 and X3 concentrations and lower amounts of X5 and X6 were observed at higher temperature with long reaction time. Although higher XOS yield (> 40%) also could be achieved by higher XA concentration with longer reaction time, more xylose and furfural were generated (150 °C, 95 min, and 0.625 M). As shown in Fig. 1a–c, the results indicated that the levels of both xylose and furfural increased during hydrolysis with increasing temperature and retention time.
The red zone in Fig. 2a–c shows the optimal condition for the production of XOS. The hydrolytic depolymerization of xylan-rich hemicelluloses proceeded most efficiently at 154 °C and 42 min with 0.64 M XA, with a predicted optimum XOS yield of 45.2%. The real contents of X2–X6 and xylose in hydrolysate by the optimized condition pretreatment were 3.22 g/L, 2.53 g/L, 2.37 g/L, 1.38 g/L, 0.96 g/L, and 7.11 g/L. Namely, the experimentally obtained XOS yield under this optimized condition was 44.5%. Clearly, the experimental values for XOS yields were found to be close to the predicted values obtained by the fitted model. The predicted optimum exactly matches the experimental optimum, thus verifying the accuracy of the established response surface. In addition, 0.28 g/L furfural was very low with this condition. Thus, condition using 154 °C and 42 min with 0.64 M XA is feasible for squeezing the maximum quantity of profit-generating products.
Xylonic acid fermentation and separation
XOS are the prior target of this study since these are higher value-added products. The results presented above indicated that acid hydrolysis at high temperature can effectively depolymerize xylan-type hemicelluloses into XOS and achieve high yield. The results also proved useful to demonstrate XA as an effective catalyst for XOS production. However, xylose was also simultaneously generated during acid pretreatment. Under the optimum conditions for XOS production, 10.5 g/(100 g SB) XOS could be produced, and 7.1 g/(100 g SB) xylose was released into the hydrolysate. Moreover, approximately 102.1 g/L XA (as catalyst) still remained in the hydrolysate. In general, a commercial XOS product mainly consists of DP 2–6 with a purity of 70–95% [17]. Thus, both xylose and the catalyst (XA) need to be removed to produce XOS with sufficient purity. Previously, Cao et al. successfully purified sodium xylonate (XA·Na) broth via electrodialysis; however, the broth still contains residual sugar (xylose). Their results showed that XA, xylose, and NaOH could be separated simultaneously [29]. In addition, xylose can be converted into bio-oxidized XA by G. oxydans with a yield close to 100% [28, 32]. Overall, xylose can be converted to XA, which can then be separated by electrodialysis.
After XA pretreatment, the pH of pre-hydrolysate was adjusted to 6.0 using NaOH. pH adjustment is a requirement because a low pH inhibits the activity of G. oxydans cells. Next, the pre-hydrolysate was directly subjected to G. oxydans for the conversion of xylose into XA. During XA fermentation, NaOH was used to maintain the fermentation pH so that XA·Na remained in the hydrolysate. After 24 h of fermentation, approximately 109.1 g/L XA accumulated in the broth, with a corresponding yield of 95.1% (Fig. 3). Xylose could be completely and efficiently converted to XA by G. oxydans, even without addition of any further nutrients or inorganic salts to the hydrolysate. These observations also suggested that xylose was exclusively oxidized to XA without further catabolism. In addition, X2–X6 curves (Fig. 3) and chromatograms (Fig. 4a, b) confirmed that xylose was bio-converted into XA and XOS was not utilized by G. oxydans. This enabled the preservation of XOS and XA·Na for their downstream purification/separation. Thus, the XA-pre-hydrolysate was subjected to electrodialysis for XOS and XA separation and recovery. Electrodialysis was performed at a current density of 50 mA/cm2 and for about 30 min; 96.8% XA of the fermentation broth was recycled and close to 100% XOS were retained in the broth. In summary, electrodialysis with bipolar membranes is a very promising and prospective method for the simultaneous separation of ionic salts and for the recycling of sugar compounds for other uses.
Fig. 3.
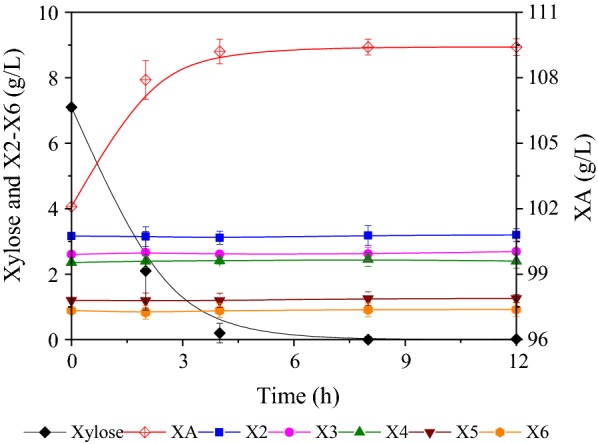
Xylose bioconversion by Gluconobacter oxydans
Fig. 4.
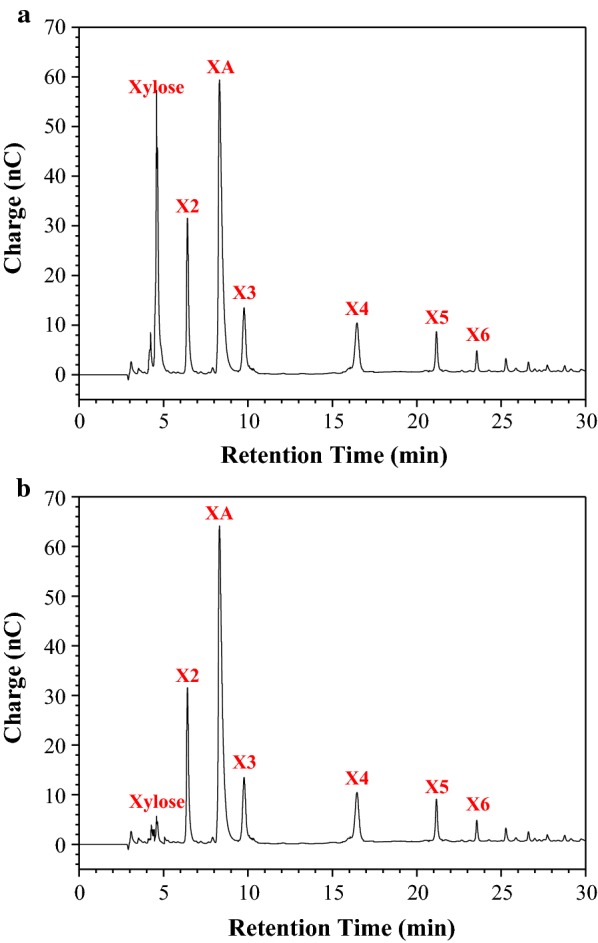
Chromatogram of high-performance anion exchange chromatography (HPAEC) analysis: a hydrolysate from xylonic acid (XA) pretreatment of sugarcane bagasse (SB); b hydrolysate after xylose bio-oxidation by G. oxydans
Enzymatic hydrolysis of pretreated solids
In the present study, XA (as an organic acid) was introduced to produce XOS, which also greatly changed the composition of SB. After XA pretreatment at RSM optimized conditions, the pretreated solids contained 54.6% glucan, 10.3% xylan, and 26.3% lignin. The hemicellulose fraction was mostly hydrolyzed during acid pretreatment, and higher cellulose and acid-insoluble lignin contents were recovered in the acid-pretreated solid residue. Furthermore, to investigate whether XA pretreatment can improve enzymatic hydrolysis, both raw and pretreated SB were subjected to enzymatic hydrolysis for 48 h at 5% w/v of the solid. Enzymatic hydrolysis was performed at 50 °C for 48 h and the enzymatic hydrolysis yield was expressed as the yield of glucose and cellobiose released into the enzymatic hydrolysate. Figure 5 showed that the XA pretreatment solid achieved a higher enzymatic hydrolysis yield. The enzymatic hydrolysis yield improved to 90.8% compared with the control without pretreatment (22.4%). It is noted that dilute acid pretreatments normally are capable of removing hemicellulosic fraction, but not lignin [33, 34]. Although the lignin cannot be effectively removed or degraded, another process occurring during acid pretreatment is lignin depolymerization and repolymerization through the formation of a common carbocation intermediate [35]. It has been proved that redistribution of lignin and degradation of hemicellulose jointly results in greatly altering the pore size distribution and the accessible surface area [36, 37]. As shown in Fig. 6, XA-pretreated SB featured a particularly higher quantity of newly exposed surfaces and generally rougher surface. All evidences indicated that XA pretreatment effectively improved the efficiency of enzymatic hydrolysis compared with the raw material.
Fig. 5.
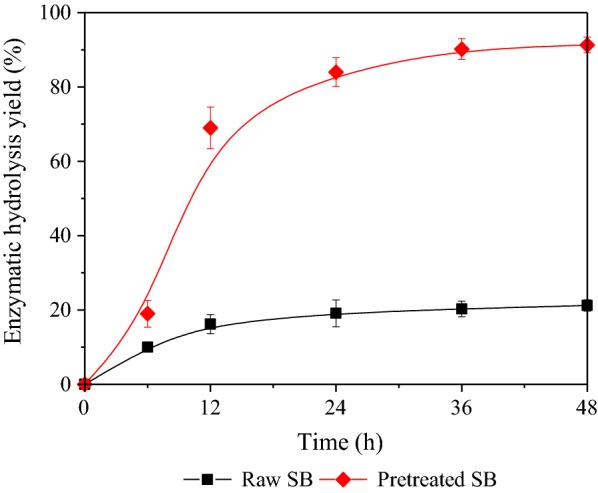
Yield of enzymatic hydrolysis after XA pretreatment
Fig. 6.

Scanning electron micrographs of treated and untreated SB
The mass balances were systematically analyzed during the co-production of glucose and XOS (Fig. 7). Using pretreated SB after XOS extraction as substrate and starting from 100 g of raw and dry SB, enzymatic hydrolysis obtained approximately 30.9 g of glucose and 10.5 g of XOS. The glucan and xylan recovery rates were 77.6% and 44.5%, respectively. These results clearly indicated pretreatment with XA as a promising option for the concurrent maximization of the economic value of SB by waste upgrading and the promotion of the current XOS and fermentable sugars co-production.
Fig. 7.
Mass balance for co-production of XOS and fermentable sugars
Conclusions
This study used an agricultural waste (SB) as biorefinery feedstock for the production of high-value XOS and fermentable sugars. XA pretreatment was explored and exhibited great potential for the depolymerization of xylan into XOS. Mass balance analysis showed that the maximum yields of XOS and fermentable sugars reached 10.5 g and 30.9 g per 100 g of raw SB. In addition, xylose as byproduct from xylan hydrolysis can be converted into XA, which is able to be separated and recycled for running new acid pretreatment process. In summary, by concurrently producing XOS and glucose with XA pretreatment, SB was valorized as a promising feedstock for lignocellulosic biorefinery for the generation of value-added products.
Methods
Raw material and chemical composition analysis
SB was collected in Hainan Province, China, in the summer of 2019. In the laboratory, the material was broken into particles through 40 mesh. These particles were then oven-dried at 105 °C for 24 h until a constant weight was reached. The chemical composition (wt%) of SB was determined as follows: glucan 39.8%, xylan 23.6%, and lignin 22.8%.
Experimental design for xylonic acid pre-hydrolysis
The hydrolysis parameters were optimized using RSM. A central composite design with three factors and three replicates at the center point was employed. Temperature (a1), hydrolysis time (a2), and XA concentration (a3) were chosen as independent variables. The ranges of these three independent variables and the center point values are listed in Table 1. In total, 13 experimental runs were conducted in three replicates with five center points, according to the Box–Behnken Design matrix. XOS yields were determined as the response variable (Y). The relationship between the independent variables and the response variable was calculated by a quadratic polynomial equation:
Here, Y represents the predicted XOS yields (%), a0 represents a constant term, a (ai and aj) represent independent variables, and ai, aii, and aij represent coefficients of linear, quadratic, and interaction parameters, respectively. The statistical software Design-Expert (Version 11.0) was used for a regression analysis of the experimental data and for response surface plots. Analysis of variance was used to estimate statistical parameters.
Xylonic acid pretreatment of sugarcane bagasse
SB (3.0 ± 0.1 g) was mixed with 30 mL of different concentrations of XA solution (prepared via xylose bio-oxidation) in a 50-mL 316 stainless steel tube reactor (inner height × diameter is 80.0 × 28.0 mm, 5.0 mm wall) which also capped with 316 stainless steel cap. After loading, the sealed stainless steel tube reactor was immersed into preheated oil baths (dimethyl silicon oil) at different desired temperature, where it remained for varying durations depending on the utilized experimental design. The temperature of oil bath was controlled by the Parr PID controller. When the reaction finished, the stainless steel tube reactor immediately removed from the heater and cooled down using cold water, and then opened it. The resultant solid and liquid fractions were then separated and harvested by filtration. The separated liquid fraction was used to determine the XOS yields, while the solid fraction was subjected to component analysis and enzymatic hydrolysis.
Xylose bioconversion
Gluconobacter oxydans (ATCC 621H) was used to convert xylose into the XA and maintained on plates (sorbitol 50 g/L, yeast extract 5 g/L, and agar 20 g/L) at 4 °C [29, 38]. The seed medium was prepared in a 500 mL Erlenmeyer flask, containing 100 mL medium (sorbitol 100 g/L and yeast extract 10 g/L), where it was cultured for 24 h at 220 rpm and 30 °C. Cell pellets of G. oxydans were harvested by centrifugation at 6000 rpm for 5 min. The pH of hydrolysate was adjusted to 6.0 by NaOH and then it was filtered by a 0.45-μm Millipore filter prior to inoculation. Fermentation assay was performed at 220 rpm and 30 °C with 1000 mL Erlenmeyer flask, containing 200 mL hydrolysate and 2 g/L G. oxydans cells (calculate as dry cell weight).
Electrodialysis and separation of the fermented broth
A bipolar membrane electrodialyzer (GJBMED-90 × 210-5) from Xinke Co. (Liaoning, China) was used in this study and it consisted of a control unit (adjustable outputs of voltage 60 V and current 10 A). The parameters of electrodialysis are as follows. Single membrane effective area: 90 × 210 mm2; number of membrane stack repeat units: 15 pairs; electrode material: titanium-coated electrode plate; membrane material: polyphenylene oxide. [29]. In addition, electrodialysis equipment was composed of four chambers: acid, alkali, salt, and electrode chamber, and the working solution was online transported across the bipolar membrane to the acid chamber [39]. The electrodialysis stack was connected to a peristaltic pump. When starting the electrodialysis, sodium xylonate (Na·XA) in salt chamber was directly pumped into bipolar membrane electrodialysis stack. As a result, Na·XA was converted to XA with the protons produced from the water dissociation, which were transported into the compartment, while the Na+ was removed from the compartment. After ion exchange, XA accumulated in the acidic chamber, while the formed NaOH accumulated in the alkaline chamber. Finally, the aqueous solution (containing XOS) could be retained in salt chamber (The schematic of bipolar membrane electrodialysis was showed in Additional file 1) [29]. In addition, in the electrode chambers, 0.3 mol/L sodium sulfate solution was added to improve the conductivity and to reduce the resistance of the membrane stack.
Enzymatic hydrolysis of pretreated sugarcane bagasse
Prior to enzymatic hydrolysis, solid residues from the XA pretreatment were washed with deionized water and were dried at room temperature until constant weight. Enzymatic hydrolysis was conducted in 150 mL screw-capped bottles at 50 °C, pH 4.8 (0.1 mol/L sodium acetate buffer), and 150 rpm with 5% solid loading and constant cellulases concentration (Cellic CTec2, Novozymes, NA, Franklinton, USA) of 15 FPU/g glucan. After enzymatic hydrolysis, the rendered enzymatic hydrolysate was collected by centrifugation.
Analytical methods
The chemical composition of SB (cellulose, hemicelluloses, and lignin) was determined using a standard protocol provided by the National Renewable Energy Laboratory [40]. Carbohydrate contents (glucan and xylan) and lignin (acid-insoluble lignin and acid-soluble lignin) were analyzed for the raw material and the pretreated samples following two-step sulfuric acid pretreatment. Briefly, milled samples (20–80 meshes) were first hydrolyzed by 72% (w/w) sulfuric acid at 30 °C water bath for 1 h with frequent mixing. Then, the slurry was diluted to a concentration of 4% (w/w) sulfuric acid by adding deionized water and hydrolyzed at 121 °C for 1 h. The autoclaved slurry was cooled and filtered by filtering crucibles. The separated liquid fraction was used for analysis carbohydrates and acid-soluble lignin. The carbohydrates were analyzed by high performance liquid chromatography (HPLC) (Agilent 1260, USA) equipped with an Aminex Bio-Rad HPX-87H column (Bio-Rad Laboratories, USA). HPLC was performed at 50 °C, with 0.005 M sulfuric acid as eluent at a flow rate of 0.6 mL/min. The acid-soluble lignin was determined at 240 nm by UV spectrophotometer (UV-1800, Shimadzu, Japan). The separated solid fraction was dried at 105 °C oven until constant weight. After record the weight, the dry solid was heated at 575 °C muffle oven for 4 h to calculate the insoluble lignin.
Microscopic images of the raw and pretreated SB were captured by a scanning electron microscope (FEI Quanta 400, Hitachi, Japan), which was operated at a voltage of 15 kV. Prior to observation, all samples were sputter-coated with gold. SEM photomicrographs were taken at a magnification of 500×.
XA, xylose, xylobiose (X2), xylotriose (X3), xylotetraose (X4), xylopentaose (X5), and xylohexaose (X6) were analyzed by high-performance anion exchange chromatography (HPAEC) (Dionex ICS-5000, ThermoFisher, USA) coupled with a CarboPac™ PA200 column (ThermoFisher, USA) [41]. Glucose, cellobiose, and furfural were measured according to the HPLC method described above.
XOS, enzymatic hydrolysis [42], and XA yield were calculated as follows:
Supplementary information
Additional file 1. Actual vs. predicted xylooligosaccharide (XOS) yields from XA hydrolysis of SB. Schematic of bipolar membrane electrodialysis.
Acknowledgements
The authors acknowledge the financial support from National Natural Science Foundation of China and National Key R&D Program of China. The research would also like to acknowledge the Scientific Research Start-up Funds of Nanjing Forestry University.
Abbreviations
- XOS
Xylooligosaccharides
- XA
Xylonic acid
- SB
Sugarcane bagasse
- XA·Na
Sodium xylonate
- DP
Degrees of polymerization
- X2
Xylobiose
- X3
Xylotriose
- X4
Xylotetraose
- X5
Xylopentaose
- X6
Xylohexose
- X7
Xyloheptaose
- X8
Xylooctaose
- RSM
Response surface method
- HPLC
High-performance liquid chromatography
- HPAEC
High-performance anion exchange chromatography
Authors’ contributions
XZ developed the idea for the study, performed the research and data analysis, and prepared the manuscript. YX helped to analyze data and revise the manuscript. Both authors read and approved the final manuscript.
Funding
This study was funded by the National Key R&D Program of China (2017YFD0601001), the National Natural Science Foundation of China (31901270), and the Scientific Research Start-up Funds of Nanjing Forestry University, China (163030127).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13068-019-1614-5.
References
- 1.Lancefield CS, Panovic I, Deuss PJ, Barta K, Westwood NJ. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: towards complete biomass valorisation. Green Chem. 2017;19:202–214. doi: 10.1039/C6GC02739C. [DOI] [Google Scholar]
- 2.Khazraie T, Zhang Y, Tarasov D, Gao W, Price J, Demartini N, Hupa L, Fatehi P. A process for producing lignin and volatile compounds from hydrolysis liquor. Biotechnol Biofuels. 2017;10:47. doi: 10.1186/s13068-017-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas R, Uellendahl H, Ahring BK. Wet explosion pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Biomass Bioenerg. 2014;61:104–113. doi: 10.1016/j.biombioe.2013.11.027. [DOI] [Google Scholar]
- 4.Vargas Betancur GJ, Pereira N., Jr Sugarcane bagasse as feedstock for second generation ethanol production: part I: diluted acid pretreatment optimization. Electron J Biotechnol. 2011;13:1–9. [Google Scholar]
- 5.Tang S, Liu R, Sun FF, Dong C, Wang R, Gao Z, Zhang Z, Xiao Z, Li C, Li H. Bioprocessing of tea oil fruit hull with acetic acid organosolv pretreatment in combination with alkaline H2O2. Biotechnol Biofuels. 2017;10:86. doi: 10.1186/s13068-017-0777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang LQ, Fang Z, Li XK, Luo J, Fan SP. Combination of dilute acid and ionic liquid pretreatments of sugarcane bagasse for glucose by enzymatic hydrolysis. Process Biochem. 2013;48:1942–1946. doi: 10.1016/j.procbio.2013.09.012. [DOI] [Google Scholar]
- 7.Pandey A, Soccol CR, Nigam P, Soccol VT. Biotechnological potential of agro-industrial residues. I: sugarcane bagasse. Bioresoure Technol. 2000;74:69–80. doi: 10.1016/S0960-8524(99)00142-X. [DOI] [Google Scholar]
- 8.Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: a review. Bioresour Technol. 2010;101:4851–4861. doi: 10.1016/j.biortech.2009.11.093. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Wyman CE. Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuel Bioprod Bioresour. 2010;2:26–40. doi: 10.1002/bbb.49. [DOI] [Google Scholar]
- 10.Zhao X, Morikawa Y, Feng Q, Jing Z, Liu D. A novel kinetic model for polysaccharide dissolution during atmospheric acetic acid pretreatment of sugarcane bagasse. Bioresour Technol. 2014;151:128–136. doi: 10.1016/j.biortech.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Xu Y, Yu S. Co-production of functional xylooligosaccharides and fermentable sugars from corncob with effective acetic acid prehydrolysis. Bioresour Technol. 2017;234:343–349. doi: 10.1016/j.biortech.2017.02.094. [DOI] [PubMed] [Google Scholar]
- 12.Otieno DO, Ahring BK. The potential for oligosaccharide production from the hemicellulose fraction of biomasses through pretreatment processes: xylo-oligosaccharides (XOS), arabino-oligosaccharides (AOS), and manno-oligosaccharides (MOS) Carbohydr Res. 2012;360:84–92. doi: 10.1016/j.carres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Vázquez MJ, Alonso JL, DomíNguez H, Parajó JC. Xylooligosaccharides: manufacture and applications. Trends Food Sci Tech. 2000;11:387–393. doi: 10.1016/S0924-2244(01)00031-0. [DOI] [Google Scholar]
- 14.Katrien S, Courtin CM, Delcour JA. Non-digestible oligosaccharides with prebiotic properties. Crit Rev Food Sci. 2006;46:459–471. doi: 10.1080/10408390500215746. [DOI] [PubMed] [Google Scholar]
- 15.Singh RD, Banerjee J, Sasmal S, Muir J, Arora A. High xylan recovery using two stage alkali pre-treatment process from high lignin biomass and its valorisation to xylooligosaccharides of low degree of polymerisation. Bioresour Technol. 2018;256:110–117. doi: 10.1016/j.biortech.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Huang C, Wang X, Liang C, Jiang X, Yang G, Xu J, Yong Q. A sustainable process for procuring biologically active fractions of high-purity xylooligosaccharides and water-soluble lignin from Moso bamboo prehydrolyzate. Biotechnol Biofuels. 2019;12:189. doi: 10.1186/s13068-019-1527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moure A, Dominguez GH, Parajo JC. Advances in the manufacture, purification and applications of xylo-oligosaccharides as food additives and nutraceuticals. Process Biochem. 2006;41:1913–1923. doi: 10.1016/j.procbio.2006.05.011. [DOI] [Google Scholar]
- 18.Jain I, Kumar V, Satyanarayana T. Xylooligosaccharides: an economical prebiotic from agroresidues and their health benefits. Indian J Exp Biol. 2015;53:131–142. [PubMed] [Google Scholar]
- 19.Lai C, Jia Y, Wang J, Wang R, Zhang Q, Chen L, Shi H, Huang C, Li X, Yong Q. Co-production of xylooligosaccharides and fermentable sugars from poplar through acetic acid pretreatment followed by poly (ethylene glycol) ether assisted alkali treatment. Bioresour Technol. 2019;288:121569. doi: 10.1016/j.biortech.2019.121569. [DOI] [PubMed] [Google Scholar]
- 20.Kootstra AMJ, Beeftink HH, Scott EL. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem Eng J. 2009;46:126–131. doi: 10.1016/j.bej.2009.04.020. [DOI] [Google Scholar]
- 21.Akpinar O, Erdogan K, Bostanci S. Production of xylooligosaccharides by controlled acid hydrolysis of lignocellulosic materials. Carbohydr Res. 2009;344:660–666. doi: 10.1016/j.carres.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Bian J, Peng P, Peng F, Xiao X, Xu F, Sun RC. Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food Chem. 2014;156:7–13. doi: 10.1016/j.foodchem.2014.01.112. [DOI] [PubMed] [Google Scholar]
- 23.Lin Q, Li H, Ren J, Deng A, Li W, Liu C, Sun R. Production of xylooligosaccharides by microwave-induced, organic acid-catalyzed hydrolysis of different xylan-type hemicelluloses: optimization by response surface methodology. Carbohydr Polym. 2017;157:214–225. doi: 10.1016/j.carbpol.2016.09.091. [DOI] [PubMed] [Google Scholar]
- 24.Zhou X, Zhao J, Zhang X, Xu Y. An eco-friendly biorefinery strategy for xylooligosaccharides production from sugarcane bagasse using cellulosic derived gluconic acid as efficient catalyst. Bioresour Technol. 2019;289:121755. doi: 10.1016/j.biortech.2019.121755. [DOI] [PubMed] [Google Scholar]
- 25.Qin L, Liu ZH, Li BZ, Dale BE, Yuan YJ. Mass balance and transformation of corn stover by pretreatment with different dilute organic acids. Bioresour Technol. 2012;112:319–326. doi: 10.1016/j.biortech.2012.02.134. [DOI] [PubMed] [Google Scholar]
- 26.Deng A, Ren J, Wang W, Li H, Lin Q, Yan Y, Sun R, Liu G. Production of xylo-sugars from corncob by oxalic acid-assisted ball milling and microwave-induced hydrothermal treatments. Ind Crop Prod. 2016;79:137–145. doi: 10.1016/j.indcrop.2015.11.032. [DOI] [Google Scholar]
- 27.Amnuaycheewa P, Hengaroonprasan R, Rattanaporn K, Kirdponpattara S, Cheenkachorn K, Sriariyanun M. Enhancing enzymatic hydrolysis and biogas production from rice straw by pretreatment with organic acids. Ind Crop Prod. 2016;87:247–254. doi: 10.1016/j.indcrop.2016.04.069. [DOI] [Google Scholar]
- 28.Zhou X, Zhou X, Xu Y. Improvement of fermentation performance of Gluconobacter oxydans by combination of enhanced oxygen mass transfer in compressed-oxygen-supplied sealed system and cell-recycle technique. Bioresour Technol. 2017;244:1137–1141. doi: 10.1016/j.biortech.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 29.Cao R, Xu Y. Efficient preparation of xylonic acid from xylonate fermentation broth by bipolar membrane electrodialysis. Appl Biochem Biotech. 2019;187:196–406. doi: 10.1007/s12010-018-2827-y. [DOI] [PubMed] [Google Scholar]
- 30.Strobel BW. Influence of vegetation on low-molecular-weight carboxylic acids in soil solution: a review. Geoderma. 2001;99:169–198. doi: 10.1016/S0016-7061(00)00102-6. [DOI] [Google Scholar]
- 31.Nath A, Chattopadhyay PK. Optimization of oven toasting for improving crispness and other quality attributes of ready to eat potato-soy snack using response surface methodology. J Food Eng. 2007;80:1282–1292. doi: 10.1016/j.jfoodeng.2006.09.023. [DOI] [Google Scholar]
- 32.Zhou X, Xin Z, Liu G, Yong X, Balan V. Integrated production of gluconic acid and xylonic acid using dilute acid pretreated corn stover by two-stage fermentation. Biochem Eng J. 2018;137:18–22. doi: 10.1016/j.bej.2018.05.005. [DOI] [Google Scholar]
- 33.Foston M, Ragauskas AJ. Changes in the structure of the cellulose fiber wall during dilute acid pretreatment in populus studied by 1H and 2H NMR. Energy Fuel. 2010;24:5677–5685. doi: 10.1021/ef100882t. [DOI] [Google Scholar]
- 34.Samuel R, Foston M, Jiang N, Allison L, Ragauskas AJ. Structural changes in switchgrass lignin and hemicelluloses during pretreatments by NMR analysis. Polym Degrad Stabil. 2011;96:2002–2009. doi: 10.1016/j.polymdegradstab.2011.08.015. [DOI] [Google Scholar]
- 35.Sannigrahi P, Ragauskas AJ, Miller SJ. Effects of two-stage dilute acid pretreatment on the structure and composition of lignin and cellulose in loblolly pine. Bioenergy Res. 2008;1:205–214. doi: 10.1007/s12155-008-9021-y. [DOI] [Google Scholar]
- 36.Rollin JA, Zhu Z, Sathitsuksanoh N, Zhang YHP. Increasing cellulose accessibility is more important than removing lignin: a comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol Bioeng. 2011;108:22–30. doi: 10.1002/bit.22919. [DOI] [PubMed] [Google Scholar]
- 37.Vinícius L, Gurgel A, Marabezi K, Arcia M, Zanbom D, Aprigio A, Curvelo S. Dilute acid hydrolysis of sugar cane bagasse at high temperatures: a kinetic study of cellulose saccharification and glucose decomposition. Part I: sulfuric acid as the catalyst. Ind Eng Chem Res. 2012;51:1173–1185. doi: 10.1021/ie2025739. [DOI] [Google Scholar]
- 38.Zhou X, Xu Y. Integrative process for sugarcane bagasse biorefinery to co-produce xylooligosaccharides and gluconic acid. Bioresour Technol. 2019;282:82–87. doi: 10.1016/j.biortech.2019.02.129. [DOI] [PubMed] [Google Scholar]
- 39.Prochaska K, Staszak K, Woźniak-Budych MJ, Regel-Rosocka M, Adamczak M, Wiśniewski M, Staniewski J. Nanofiltration, bipolar electrodialysis and reactive extraction hybrid system for separation of fumaric acid from fermentation broth. Bioresour Technol. 2014;167:219–225. doi: 10.1016/j.biortech.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Sluiter AD, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton DW, Crocker D. Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced. 2012;1617:1–16. [Google Scholar]
- 41.Yong X, Li F, Xing W, Qiang Y, Yu SY. Simultaneous separation and quantification of linear xylo- and cello-oligosaccharides mixtures in lignocellulosics processing products on high-performance anion-exchange chromatography coupled with pulsed amperometric detection. BioResources. 2013;8:3247–3259. [Google Scholar]
- 42.Bhagia S, Dhir R, Kumar R, Wyman CE. Deactivation of cellulase at the air-liquid interface is the main cause of incomplete cellulose conversion at low enzyme loadings. Sci Rep. 2018;8:1350. doi: 10.1038/s41598-018-19848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Actual vs. predicted xylooligosaccharide (XOS) yields from XA hydrolysis of SB. Schematic of bipolar membrane electrodialysis.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.