Beginning in 2012, [18F]- and [68Ga]-labeled inhibitors of prostate-specific membrane antigen (PSMA) entered early clinical development for positron emission tomography (PET) imaging of prostate cancer (PCa) and showed immediate promise for sensitive and specific identification of local and distant sites of disease [1,2]. In the years since then, PSMA-targeted PET imaging has become widely utilized, with more than 300 publications now indexed on PubMed [3].
As with any imaging test, PSMA-targeted PET imaging of PCa has potential interpretive pitfalls and equivocal findings [4]. In other areas of radiology, these issues have been addressed in part with the adoption of so-called reporting and data systems (RADS) that aim to standardize the interpretation and reporting of findings from a specific imaging modality. Urologists are already familiar with the PI-RADS system for prostate magnetic resonance imaging (MRI) [5], but may be less aware that a number of other RADS have been developed, including BI-RADS for breast imaging [6] and LI-RADS for liver imaging [7]. Inspired by the successful clinical implementation of these various RADS, our group recently developed the first systematic approach to the interpretation and reporting of PSMA-targeted PET imaging studies known as PSMA-RADS version 1.0 [8].
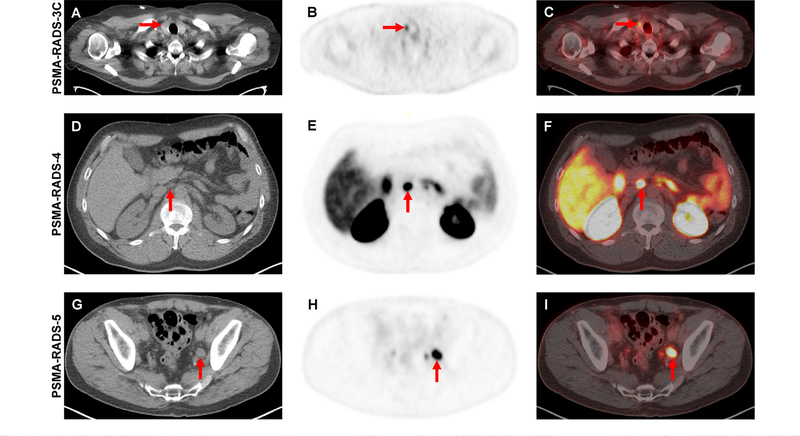
In the simplest terms, PSMA-RADS is a framework for classifying PSMA-targeted PET scans and individual findings on these studies into categories that reflect the likelihood of the presence of PCa. The system is optimized for findings outside of the prostate and is not intended to replace PI-RADS for categorizing findings on prostate MRI. PSMA-RADS version 1.0 is organized around a 5-point scale, with higher numbers indicating a greater probability of PCa (Table 1). At the lower end of the scale, PSMA-RADS-1 and PSMA-RADS-2 scans/lesions are either certainly or almost certainly benign, respectively. By contrast, PSMA-RADS-4 indicates a high likelihood that PCa is present and PSMA-RADS-5 scans/ lesions almost certainly represent PCa (Fig. 1).
Table 1 –
The PSMA-RADS version 1.0 classification schema (adapted from [8])
| Definitively benign/likely benign |
| PSMA-RADS-1 |
| PSMA-RADS-1A: Lesions without radiotracer uptake that are definitively benign |
| PSMA-RADS-1B: Lesions with radiotracer uptake that are definitively benign |
| PSMA-RADS-2 |
| Low level radiotracer uptake in bone or soft tissue sites that would be atypical for metastatic PCa |
| Equivocal |
| PSMA-RADS-3 |
| PSMA-RADS-3A: Equivocal radiotracer uptake in soft tissue lesions such as lymph nodes in a distribution typical for PCa |
| PSMA-RADS-3B: Equivocal radiotracer uptake in bone lesions that are not clearly benign |
| PSMA-RADS-3C: Lesions that would be atypical for PCa but have high levels of uptake and may represent a non-prostate malignancy |
| PSMA-RADS-3D: Lesions that are concerning for the presence of PCa or a non-prostate malignancy but lack radiotracer uptake |
| Many of the findings in the PSMA-RADS-3 category will require further work-up to definitively classify, with the nature of the work-up depending on the type of lesion [8] |
| Definitively cancer/likely cancer |
| PSMA-RADS-4 |
| Lesions with high radiotracer uptake that would be typical for PCa but lack a definitive anatomic abnormality |
| PSMA-RADS-5 |
| Lesions with high levels of radiotracer uptake and corresponding anatomic findings that are indicative of the presence of PCa |
PCa = prostate cancer.
Fig. 1 –
(A–C) PSMA-RADS-3C lesion. (A) Axial attenuation-corrected CT image, (B) axial [18F]-DCFPyL PET image, and (C) axial fused [18F]-DCFPyL PET/CT image demonstrating focal radiotracer uptake in a thyroid nodule (red arrows). While this is almost certainly not a site of PCa, in the proper clinical setting this finding should be further evaluated to rule out the presence of well-differentiated thyroid cancer. (D–F) PSMA-RADS-4 lesion. (D) Axial attenuation-corrected CT image, (E) axial [18F]-DCFPyL PET image, and (F) axial fused [18F]-DCFPyL PET/CT image in a patient with recurrent PCa and a 0.6-cm lymph node (ie, not pathologically enlarged) with intense radiotracer uptake (red arrows). (G-I) PSMA-RADS-5 lesion. (G) Axial attenuation-corrected CT image, (H) axial [18F]-DCFPyL PET image, and (I) axial fused [18F]-DCFPyL PET/CT image in a patient with recurrent PCa and a 1.5-cm left pelvic lymph node (ie, pathologically enlarged) with intense radiotracer uptake (red arrows). CT = computed tomography; PET = positron emission tomography; PCa = prostate cancer; [18F]-DCFPyL = 2-(3-(1-carboxy-5-[(6-[18F]-fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid.
The most complex of the PSMA-RADS version 1.0 categories is PSMA-RADS-3, which is further divided into four subcategories that reflect either uncertainty as to whether a given scan/lesion is compatible with PCa (PSMA-RADS-3A, PSMA-RADS-3B, and some PSMA-RADS-3D findings) or suggest the presence of another malignancy (PSMA-RADS-3C and some PSMA-RADS-3D findings). Of these subcategories, PSMA-RADS-3C (representing a scan or lesion in which radiotracer uptake is present but for which the pattern of uptake would be unlikely to represent PCa; Fig. 1) and PSMA-RADS-3D (representing a scan or lesion in which a finding is suspicious for cancer but lacks radiotracer uptake) are of particular importance. In regards to PSMA- RADS-3C, a number of non-prostate malignancies are known to be avid for PSMA-targeted radiotracers [9] and further work-up of these lesions is of critical importance. Similarly, some non-avid lesions in the PSMA-RADS-3D category will be aggressive tumors such as neuroendocrine differentiated PCa [10].
Given that the PSMA-RADS version 1.0 categories apply to both the scan and individual lesions, we advocate including both in the interpretation of each PSMA-targeted PET imaging study. In our clinical practice, we generally provide the overall scan category at the start of the “impression” portion of the dictation. The overall scan category will generally correspond to the highest PSMA-RADS category assigned to an individual lesion on the scan, although multiple scan scores will sometimes need to be given if a patient has one or more PSMA-RADS-3 findings that could indicate the presence of a non-prostate malignancy. Following the overall scan score, we then include individual scores for up to five lesions. To date, the clinicians at our institution have found this to be a clear-cut method of communicating salient scan findings.
Beyond providing for clear clinical communication of the findings on a PSMA-targeted PET scan, we believe that PSMA-RADS has additional advantages. First, the launching of PSMA-RADS is in keeping with the quality movement in diagnostic imaging, whereby the aim of structured reporting is to decrease interpretive variance. Notably, such quality measures are beginning to be incorporated into reimbursement schemes for medical practice [11]. Second, the systematic manner in which findings must be categorized will allow for easier harmonization of data from participating sites in multicenter studies. Related to this, findings between individual studies can be more easily compared when a standardized reporting system is used. Finally, as PSMA-RADS interpretations become part of the electronic medical record and are entered into various clinical databases, retrospective analyses looking at particular findings will be easier to perform. This would also facilitate the identification of candidates for clinical trials that require certain imaging findings for eligibility.
Despite the apparent advantages of this proposed system, we would be remiss not to emphasize that the field of PSMA-targeted PET imaging is still rapidly developing. In the coming years, we are certain to learn a great deal more regarding the most appropriate means of interpreting many of the findings on PSMA-targeted PET scans. In conjunction with increased knowledge regarding outcomes for patients who have undergone PSMA-targeted PET and the emergence of further data on the role of focal therapy for recurrent/metastatic PCa, we will incorporate additional nuance into the PSMA-RADS categories. Whether these will be minor changes (PSMA-RADS version 1.1) or a ground-up rethinking (PSMA-RADS version 2.0), PSMA-RADS should be viewed as a dynamic system that will take into account new discoveries in order to remain relevant as a tool for the delivery of precision medicine based on PSMA-targeted PET scan results.
Footnotes
Conflicts of interest: Creation of the images in this manuscript involved the administration of 18F-DCFPyL, a PSMA-targeted PET radiotracer that is being investigated under a US Food and Drug Administration investigational new drug application (IND 121064). Martin G. Pomper is a co-inventor on a US patent covering 18F-DCFPyL, and is thus entitled to a portion of any licensing fees and royalties generated by this technology. Michael A. Gorin has served as a consultant to Progenics Pharmaceuticals, the licensee of 18F-DCFPyL. Steven P. Rowe, Kenneth J. Pienta, Martin G. Pomper, and Michael A. Gorin have received research support from Progenics Pharmaceuticals. have received research support from Progenics Pharmaceuticals.
References
- [1].Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med 2012;53:1883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging 2012;39:1085–6. [DOI] [PubMed] [Google Scholar]
- [3].Murphy DG, Sweeney CJ, Tombal B. “Gotta catch ‘em all”, or do we? Pokemet approach to metastatic prostate cancer. Eur Urol 2017;72:1–3. [DOI] [PubMed] [Google Scholar]
- [4].Sheikhbahaei S, Afshar-Oromieh A, Eiber M, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging 2017;44:2117–36. [DOI] [PubMed] [Google Scholar]
- [5].Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging-Reporting and Data System: 2015, version 2. Eur Urol 2016;69:16–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Orel SG, Kay N, Reynolds C, Sullivan DC. BI-RADS categorization as a predictor of malignancy. Radiology 1999;211:845–50. [DOI] [PubMed] [Google Scholar]
- [7].Purysko AS, Remer EM, Coppa CP, et al. LI-RADS: a case-based review of the new categorization of liver findings in patients with end-stage liver disease. Radiographics 2012;32:1977–95. [DOI] [PubMed] [Google Scholar]
- [8].Rowe SP, Pienta KJ, Pomper MG, Gorin MA. Proposal of a structured reporting system for prostate-specific membrane antigen (PSMA)-targeted PET imaging: PSMA-RADS version 1.0. J Nucl Med In press. 10.2967/jnumed.117.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rowe SP, Gorin MA, Hammers HJ, et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med 2015;29:877–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tosoian JJ, Gorin MA, Rowe SP, et al. Correlation of PSMA-targeted 18F-DCFPyL PET/CT findings with immunohistochemical and genomic data in a patient with metastatic neuroendocrine prostate cancer. Clin Genitourin Cancer 2017;15:e65–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rosenkrantz AB, Hirsch JA, Silva E 3rd, Nicola GN. Radiologists may be accountable for containing Medicare costs and spending under MACRA. J Am Coll Radiol 2017;14:1298–300. [DOI] [PubMed] [Google Scholar]