Abstract
IMPORTANCE
Current acellular pertussis vaccines may not protect against transmission of Bordetella pertussis.
OBJECTIVE
To assess whether a priming dose of whole-cell pertussis (wP) vaccine is cost-effective at reducing pertussis infection in infants.
DESIGN, SETTING, AND PARTICIPANTS
Mathematical model of pertussis transmission fit to US incidence data in a simulation of the US population. In this simulation study conducted from June 2014 to May 2015, the population was divided into 9 age groups corresponding to the current pertussis vaccination schedule and fit to 2012 pertussis incidence.
INTERVENTIONS
Inclusion of a priming dose of wP vaccine into the current acellular pertussis vaccination schedule.
MAIN OUTCOMES AND MEASURES
Reductions in symptomatic pertussis incidence by age group, increases in wP vaccine–related adverse effects, and quality-adjusted life-years owing to changing vaccine schedule.
RESULTS
Switching to a wP-priming vaccination strategy could reduce whooping cough incidence by up to 95% (95% CI, 91–98), including 96% (95% CI, 92–98) fewer infections in neonates. Although there may be an increase in the number of vaccine adverse effects, we nonetheless estimate a 95% reduction in quality-adjusted life-years lost with a switch to the combined strategy and a cost reduction of 94% (95% CI, 91–97), saving more than $142 million annually.
CONCLUSIONS AND RELEVANCE
Our results suggest that an alternative vaccination schedule including 1 dose of wP vaccine may be highly cost-effective and ethically preferred until next-generation pertussis vaccines become available.
Since the 1990s, the incidence of Bordetella pertussis infection, the primary causative agent of whooping cough, has continued to rise in many industrialized countries,1,2 despite at least 90% vaccination coverage rates in many countries.3,4 In 2012, the United States saw 48 277 reported pertussis cases, the highest number since 1955, which included 16 infant deaths.2 This rise has been widely attributed to the switch from whole-cell pertussis(wP)–derived vaccines to acellular pertussis (aP) vaccines in the mid-1990s. A potential explanatory mechanism that has recently been posited is that aP vaccines protect against whooping cough symptoms, but not against colonization and secondary transmission of the B pertussis bacterium.5,6 This hypothesis implies the existence of a large group of asymptomatically infected transmitters,7 which would account for the documented failure of cocooning, the vaccination of the close contacts of neonatal infants who are too young to be vaccinated.8,9 Despite calls for a more efficacious next-generation B pertussis vaccine,10,11 new vaccines are not likely to be licensed in the near future.12 Here, we consider whether a new interim strategy could minimize B pertussis transmission, lower incidence, and avert infant mortality.
Epidemiological studies have followed pertussis infection in cohorts of children born in the mid-1990s, at the time of the switch from the wP to the aP vaccines, who received their first dose of the B pertussis vaccine schedule as wP and the remainder of their vaccines as aP.13–15 These studies found that those individuals who had been primed with wP vaccine had less than half the incidence of whooping cough than those who received the aP vaccines alone.13–15 Here, we evaluate the effectiveness and cost-effectiveness of priming infants with the wP vaccine, then completing the vaccine series with aP. We developed a dynamic model of B pertussis transmission fit to incidence data on whooping cough to determine the expected number of whooping cough cases and vaccine-related adverse events, comparing the status quo of a vaccination series based entirely on the aP vaccine vs a schedule that combines wP and aP vaccines. Using the results from the dynamic model, in conjunction with literature-derived estimates of the health care costs associated with infant mortality, whooping cough complications, and vaccine-associated adverse events, we conducted a cost-effectiveness analysis for the wP-primed strategy as compared with the current aP strategy. We found that priming with a single dose of the wP vaccine, followed by the current aP schedule, would be associated with a reduction in asymptomatic transmission, thereby averting substantial pertussis-related morbidity and mortality, as well as generating cost-savings sufficient to more than offset a potential increase in adverse vaccine-related events.
Methods
The Model
We assessed 2 vaccination strategies using an age-structured susceptible, infected, removed model for the transmission of B pertussis.7,16 The first strategy is the current US vaccination policy of 5 doses of aP vaccine, given at ages 2 to 4 months, 4 to 6 months, 6 to 8 months, 18 to 24 months, and 4 to 5 years (henceforth referred to as the aP strategy).17 The second strategy consisted of 1 initial priming dose of wP vaccine followed by 4 dosesof aP vaccine at thesamevaccination schedule, combined with a catch-up campaign over 5 years, in which children ages 4 to 5 years are vaccinated with wP vaccine (henceforth referred to as the combined strategy). We also explored scenarios without this catch-up campaign, as are reported in eFigure 1 in the Supplement.
Because this was a simulation study fit to publicly available data, no institutional review board approval was necessary. This study was conducted from June 2014 to May 2015.
The model was run for 600 months using the aP strategy to allow the system to reach dynamical equilibrium. At this point, the model matches the breakdown of symptomatic infections among age groups currently seen in the United States (eFigure 2 in the Supplement).2 These equilibrium values were then used as initial values in the second epoch, in which the 2 vaccine strategies were compared.
Full model description and details on parameterization are given in the eAppendix and eTable 1, eTable 2, eTable 3, eTable 4, and eTable 5 in the Supplement.
Health Outcomes
We considered 2 types of age-stratified health outcomes: disease from B pertussis infection and adverse events in response to wP and aP vaccination. Disease outcomes included moderate infection (including those with paroxysmal episodes, vomiting, exhaustion, and low-grade fever from pertussis who reported their illness to a clinician18) and severe infection (those hospitalized for their infection, experiencing pneumonia, seizures, encephalopathy, or death). We did not account for underreporting or misclassification bias; however, we examined reductions in symptomatic incidence, which, even if misdiagnosed as pertussis, would still be treated and incur societal cost. Adverse events in response to wP and aP vaccination included fever, inconsolable crying, seizures, and encephalopathy. Local reactions and rashes have also been documented but were not included because they are both minor and difficult to quantify. We also excluded events too rare to have reliable incidence rates (ie, permanent brain damage owing to a lack of causal association with wP vaccination17,19,20). Adverse events following aP vaccination are based on data for the diphtheria, tetanus, and acellular pertussis vaccine, which is currently in use in the United States, whereas adverse events following the wP vaccine are based on data for the diphtheria, tetanus, and whole-cell pertussis vaccine, which was replaced by the diphtheria, tetanus, and acellular pertussis vaccine in the 1990s (eTable 3 in the Supplement).
Economic Inputs
Direct and indirect costs for B pertussis infection, complications, and vaccine-related adverse events were parameterized from published and publicly available sources and adjusted to 2012 US dollars (detailed in the eAppendix and eTable 4 in the Supplement). Hospital cost data were obtained from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project’s Nationwide Inpatient Sample database21 and the US Census Bureau. The Nationwide Inpatient Sample is a nationally representative hospital-stratified sample of hospital discharges per year. Patients diagnosed as having pertussis were identified in the Nationwide Inpatient Sample as having International Classification of Diseases, Ninth Revision, Clinical Modification codes 033.0, 033.8, 033.9, or 484.3 as one of the first 15 diagnoses recorded (ie, principal diagnosis and as many as 14 secondary diagnoses). We calculated population-adjusted costs of pertussis hospitalization as the median hospital charge per pertussis hospitalization divided by the cost-to-charge ratio for that hospital obtained from the Healthcare Cost and Utilization Project. Hospitalization costs were adjusted to 2012 US dollars. We did not account for the dollar costs associated with ambulatory visits, which may be substantial, thus our estimates are conservative. We did account for reductions in quality-adjusted life-years (QALYs) lost.
Costs were applied to the corresponding outcomes projected for the different vaccination strategies. For example, the aP rate of hospitalized infants per 100 000 population was multiplied by the cost of hospitalizing an infant owing to B pertussis. This gives the cost of hospitalizing infants per 100 000 population for the aP strategy. This value can then be compared with the cost of hospitalizing infants per 100 000 population for the combined strategy. Costs from all outcomes are summed to give the total cost per 100 000 for each strategy, allowing direct cost comparison of strategies.
Quality-Adjusted Life-Years
To evaluate the health effect of pertussis disease and adverse vaccine-related events, we used QALYs. Disutility values— preference-based weights that measure from 0 to 1 the relative disutility of specific outcomes—were based on published literature (eTable 5 in the Supplement).18,22–24 Individuals with moderate B pertussis symptoms experience disutility for 8 weeks. Individuals with severe B pertussis infection incur severe, hospitalizable symptoms for their duration of hospital stay followed by 6 weeks of moderate pertussis symptoms. This model does not consider mild B pertussis cases because mild cases tend to be unreported. The duration of other B pertussis complications, pneumonia, and seizures correspond to the duration of hospitalization. Because encephalopathy has residual effects, disutility incurs at time of infection and is discounted for the rest of the individual’s lifespan. Because we only considered infant death, we assumed 77 years were lost.25 Vaccine-related seizures and encephalopathy were assumed to have the same disutility and duration as pertussis-induced seizures and encephalopathy.
Results
Base Case
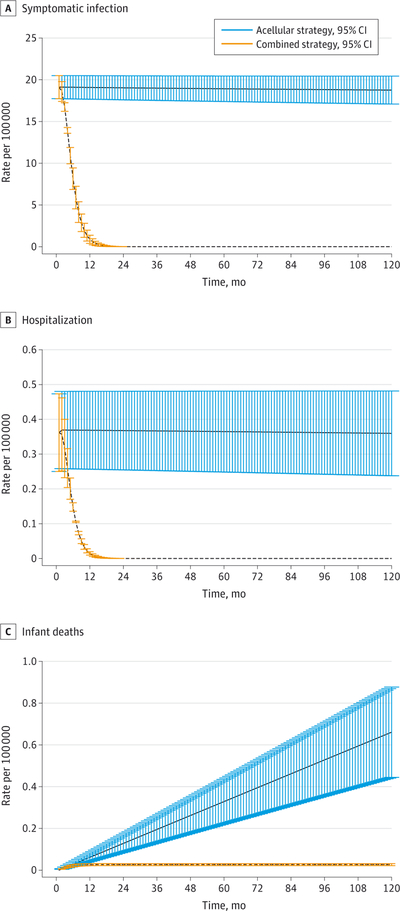
The combined strategy is predicted to be associated with a reduction in the rate of symptomatic pertussis infections, hospitalizations, and infant deaths (Figure 1, Table 1, and eTable 6, eTable 7, and eTable 8 in the Supplement). Compared with the aP strategy, the combined strategy would be predicted to achieve a 95% reduction (95% CI, 91–98) in symptomatic infections, and, importantly, a 96% reduction (95% CI, 92–98) in symptomatic infections in infants (Table 1).
Figure 1. Base Case Results.
Base case estimates for the rates of symptomatic infection (A), hospitalizations (B), and infant deaths (C) per 100 000 total population during the first 10 years in the second epoch. Parameters are given in the Supplement.
Table 1.
Transmission Model Estimates of Pertussis Incidence, Complications, and Vaccine-Related Adverse Effects 10 Years Into the Second Epoch
| Characteristic | Age Group, y | Total | |||
|---|---|---|---|---|---|
| <1 | 1–6 | 7–18 | >18 | ||
| B pertussis incidence, % decrease (95% CI) | |||||
| Infection | 96 (92–98) | 95 (91–98) | 95 (91–98) | 95 (91–98) | 95 (91–98) |
| Rate of hospitalization | 96 (91–97) | 95 (93–96) | 95 (90–98) | 96 (92–99) | 96 (95–99) |
| Infant death rate | 96 (92–98) | NA | NA | NA | NA |
| B pertussis complications, % decrease (95% CI) | |||||
| Rate of pneumonia | 95 (92–99) | 95 (92–99) | 95 (92–98) | 96 (93–99) | 96 (92–99) |
| Rate of seizures | 95 (92–99) | 95 (91–99) | NA | NA | 96 (92–99) |
| Rate of encephalopathy | 96 (92–99) | NA | NA | NA | NA |
| Vaccine-associated adverse effects, % increase (95% CI) | |||||
| Persistent, inconsolable crying | 175 (161–188) | 686 (539–833) | NA | NA | 632 (370–894) |
| Rate of fever | 811 (794–851) | 3150 (2453–3865) | NA | NA | 2917 (1642–4180) |
| Rate of seizures | 63 (53–74) | 261 (212–311) | NA | NA | 240 (152–333) |
| Rate of encephalopathy | 5.78 × 10−4 (5.59–5.87 × 10−4) | NA | NA | NA | NA |
Abbreviations: aP, acelullar pertussis; B pertussis, Bordetella pertussis; NA, not applicable.
The combined strategy was also predicted to generate a shift in the age distribution of asymptomatic infection, with a 69% decrease (95% CI, 69–70) in asymptomatic infections in infants aged 0 to 1 year, a 72% decrease (95% CI, 70–74) in children aged 1 to 6 years, and a 81% increase (95% CI, 76–87) in adolescents (eFigure 3 in the Supplement). Thus, the combined strategy is effective in preventing both symptomatic and asymptomatic infections in children and infants—the groups with the highest contact rates and at greatest risk for severe disease outcomes. Consequently, the combined strategy leads to substantial reductions in serious complications arising from B pertussis infection. Particularly important is the 96% decrease (95% CI, 91–97) in the rates of hospitalization and the 96% decrease (95% CI, 92–98) in deaths among infants younger than 1 year. These rates also saw a reduction in children, adolescents, and adults (Table 1).
Regarding adverse events related to vaccination, the combined strategy’s rates of fever showed a 2917% increase (95% CI, 1642–4180) over the aP strategy and wP rates of seizures showed a 240% increase (95% CI, 152–333) over the aP rates, although the absolute increases were low (10.3 per 100 000 for fever and 0.07 per 100 000 for seizures). Additionally, the combined strategy is predicted to cause a rate of encephalopathy of 5.78 × 10−4 (95% CI, 5.59 × 10−4 to 5.87 × 10−4) per 100 000 total population (Table 1). Despite the higher rates of adverse events, the combined strategy revealed a 96% decrease (95% CI, 95–99) in overall hospitalizations due to either B pertussis infection or vaccine-related adverse events, including pneumonia, seizures, and encephalopathy (Figure 2).
Figure 2. Total Hospitalizable Bordetella pertussis–Related Adverse Events.
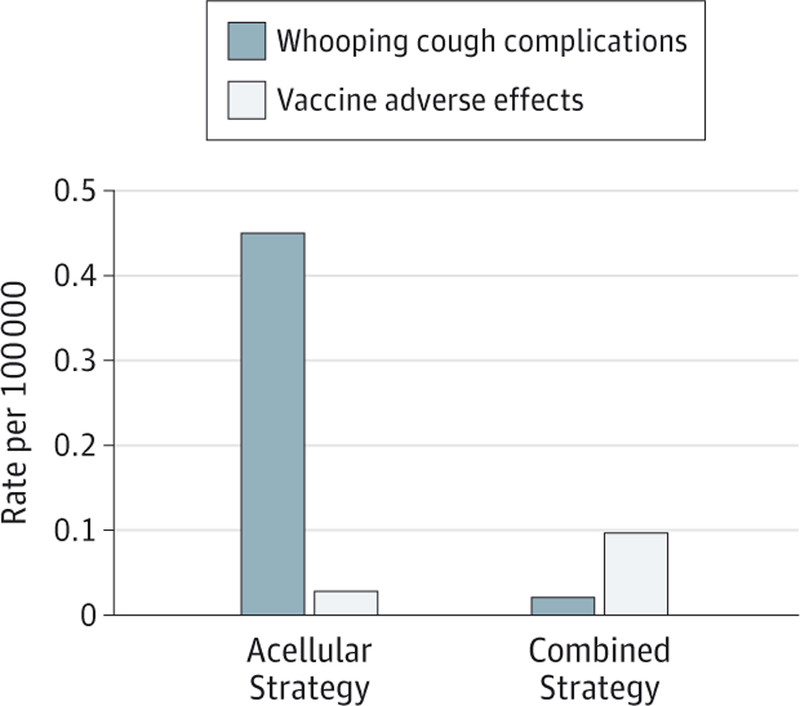
Whooping cough complications include hospitalizations, pneumonia, seizures, and encephalopathy; vaccine adverse effects include seizures and encephalopathy. Parameters are given in the Supplement.
Economic Effect
The combined strategy would reduce disease-related hospitalization costs by 96% (95% CI, 93–98) compared with the aP strategy (Table 2). The economic costs associated with pertussis death would be reduced by 96% (95% CI, 93–99). However, hospital costs for treating vaccine adverse events would more than double (244% increase; 95% CI, 223–264). Considering all 3 components, the aP strategy would overall cost $48 310 per 100 000 population (95% CI, 48 290–48 330), while the combined strategy would overall cost only $2822 per 100 000 population (95% CI, 1395–4248). Consequently, the combined strategy could achieve a 94% cost-savings (95% CI, 91–97) compared with the current strategy. This translates to roughly $142 million per year in the United States.
Table 2.
Cost of Bordetella pertussis Infection Complications and Pertussis Vaccine Adverse Effects
| Variable | Cost per 100 000 (95% CI), $a | ||
|---|---|---|---|
| Acellular Strategy | Combined Strategy | Change, % | |
| Pertussis complications | |||
| Hospitalization | 1584 (1582–1587) | 71.12 (24.69–117.5) | 96 (93–98)b |
| Pneumonia | 194.4 (194.1–194.7) | 8.74 (2.667–14.82) | 94 (92–99)b |
| Seizures | 26.98 (26.94–27.02) | 1.213 (0.3678–2.059) | 96 (92–99)b |
| Encephalopathy | 9.629 (9.615–9.643) | 0.4328 (0.1287–0.7369) | 96 (92–99)b |
| Death | 135.2 (135.1–135.2) | 5.427 (1.251–9.603) | 96 (93–99)b |
| Societal cost | 46 100 (46 080–46 120) | 1850 (426.6–3275) | 96 (93–99)b |
| Total | 48 050 (48 030–48 070) | 1938 (512.7–3363) | 96 (93–99)b |
| Vaccine adverse effects | |||
| Seizures | 256.3 (256.1–256.6) | 874.5 (817.0–932.0) | 241 (218–331)c |
| Encephalopathy | NA | 9.25 (9.02–9.49) | NA |
| Total | 256.3 (256.1–256.6) | 883.8 (826.3–941.3) | 244 (223–264)c |
| Total costs | 48 310 (48 290–48 330) | 2822 (1395–4248) | 94 (91–97)b |
Abbreviation: NA, not applicable.
All costs are adjusted to 2012 US dollars.
Indicates a decrease.
Indicates an increase.
Quality-Adjusted Life-Years
Quality-adjusted life-year loss from pertussis disease would be 0.58 QALYsper 100 000 individuals for the aP strategy and 0.02 QALYs per 100 000 individuals for the combined strategy (Table 3). Quality-adjusted life-year loss due to vaccine-related adverse events is predicted to be 2.6 × 10−4 per 100 000 for the acellular strategy and 9.4 × 10−3 per 100 000 for the combined strategy. On balance, the current acellular strategy incurs a total loss of 0.80 QALYs per 100 000 people, where the combined strategy predicts a total loss of only 0.04 QALYs per 100 000 people. This is a 95% decrease in total loss of QALYs with the combined strategy.
Table 3.
QALYs Lost to Pertussis Infections, Complications, and Vaccine Adverse Effects per 100 000 Total Population
| Variable | Acellular Strategy | Combined Strategy | Change, % |
|---|---|---|---|
| Pertussis infections | 0.58 | 0.02 | 96a |
| Hospitalization | 9.2 × 10−3 | 4.2 × 10−4 | 95a |
| Pertussis complications | 2.1 × 10−2 | 9.4 × 10−4 | 96a |
| Death | 0.33 | 0.01 | 96a |
| Vaccine adverse effects | 2.6 × 10−4 | 9.4 × 10−3 | 3500b |
| Total | 0.80 | 0.04 | 95a |
Abbreviation: QALY, quality-adjusted life-year.
Indicates a decrease.
Indicates an increase.
Sensitivity Analyses
As the probability of symptomatic infection (σ) is unknown, we set the base case to σ = 0.5 and tested the model at σ = 0.25 and σ = 0.75. Our finding that the combined strategy exhibited fewer infections, hospitalizations, adverse events, and deaths due to B pertussis is robust to this variation in the probability of symptomatic infection (eFigure 4, eFigure 5, eFigure 6, and eFigure 7 in the Supplement). We also conducted a sensitivity analysis for the force of infection (β). The model was run for 150 different β values (eFigure 8 in the Supplement). Throughout this variation, the combined strategy was predicted to cause fewer infections, hospitalizations, adverse events, and deaths. We conducted analyses without the catch-up campaign, which averted fewer pertussis incidences, QALYs, and costs than when the combined strategy is supplemented with a catch-up campaign. Finally, we explored a scenario where the rise in wP vaccine adverse events caused the wP vaccination rate to drop (eFigure 1 in the Supplement). This shows a decrease in both asymptomatic and symptomatic infections for all age classes when rates are greater than 50%.
Discussion
We evaluated the cost-effectiveness of an alternative pertussis vaccination schedule that incorporates 1 dose of wP vaccine, a strategy that increases vaccine effectiveness but also increases the risk for vaccine-related adverse events. Our dynamic transmission model was parameterized with empirical data from the United States. Despite the increase in vaccine-related adverse effects, we found a substantial net benefit to wP vaccination through reduced transmission of B pertussis. Specifically, the model predicted that the combined strategy would reduce QALY loss by 95% and reduce costs by 94%. These results suggest that the combined strategy is both an epidemiologically favorable and an economically viable alternative to aP vaccination.
The World Health Organization reports that adverse reactions to wP vaccination increase with age and the number of injections,26 but it has not been determined which of these 2 confounded factors plays the more significant causative role. Because the combined strategy involves the administration of only 1 wP vaccine, the rates of wP adverse events that we assumed are likely overestimates, as our model was parameterized from the empirical estimates for adverse events related to vaccination based on regimens that involved multiple doses of wP vaccine. Consequently, our results with regard to the risk for increased adverse events are conservative. Furthermore, we highlight that despite the increased reactogenicity, the World Health Organization recommends wP vaccines for infant pertussis immunization worldwide.27
There were some limitations to this study. As with any analysis, we made some simplifying assumptions in the absence of empirical data. We assumed that the transmissibility of asymptomatic infection was the same as that for symptomatic infection. Although asymptomatic individuals may shed less bacteria, they would be more likely to be active and expose more people to potential transmission. Additionally, we assumed that the rates of waning natural immunity in adolescents and adults were the same for symptomatic and asymptomatic infections. Both of these assumptions underestimate pertussis disease burden and are, therefore, conservative with regard to our findings. Owing to the nature of asymptomatic infection, studying and determining rates of asymptomatic pertussis infections is challenging without detailed serosurveys and/or immunological studies of household transmission.
We found that the rates of waning immunity, albeit low, are crucial to accurate fitting of the model to epidemiological data on age-specific incidence. Specifically, when waning is not considered, the model predicts almost no cases of pertussis in adolescents and adults. The necessity of incorporating waning immunity to generate an accurate fit to the incidence data underscores its importance to the epidemiological dynamics of pertussis.28,29 Further research on waning immunity is needed to formulate more accurate models.
This study did not consider adolescent and adult boosting doses, as currently recommended by the Advisory Committee on Immunization Practices. Previous work has indicated that children initially vaccinated with aP do not have elevated T-cell responses after aP vaccination or natural boosting. On the other hand, those primed with wP vaccine do.30 This would indicate that the combined wP-aP strategy explored here might be even more effective when considering adolescent and adult boosting, although more study of the Th17 response induced by wP and boosted by aP vaccination is needed.31 We found an 81% increase in adolescent infections under the combined strategy, but inclusion of a booster may lessen this increase.
Finally, this model did not consider the current recommendation of vaccinating pregnant women with the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine.32 The practice gives newborns transient passive protection against B pertussis infection for up to 8 weeks.33 Several studies have verified the safety and effectiveness of maternal vaccination.33–35 As maternal vaccination rates increase and cases in infants drop, the absolute number of pertussis cases averted by a switch from aP to the combined strategy would also be lower. However, as the benefits of maternal vaccination with aP do not have a large population level effect beyond the mother and infant,36 our qualitative insight that a switch from aP to the combined strategy is likely to produce both health and economic benefits would not change.
The ethical implications of our results demand careful consideration. By switching to the combined strategy, the US population could avert 10.5 per 100 000 QALY loss over 10 years and reduce infant mortality from pertussis by 96%. On these grounds alone—and without considering cost-savings—the combined strategy proves to be a much safer alternative than the current vaccine. Of course, rigorous clinical trials for safety and efficacy would be needed before recommendations could be made to support a change in the vaccine schedule. Conducting these studies (or studies for any new pertussis vaccines, for that matter) may be difficult to conduct in the United States where aP vaccines are already recommended for all children. Novel designs or surrogate end points for vaccine efficacy (protection in nonhuman animals, such as baboons; serologic data showing higher and more persistent antibody titers; or human challenge in adults with circulating strains of B pertussis) will likely have to be used to achieve regulatory approval.37 Safety will be the most important consideration, with large enough study sample sizes necessary to observe adverse events. Fortunately, previously licensed wP vaccines with well-known safety profiles could be used in determining study design and in power calculations. Finally, in light of the public concerns regarding vaccine safety, care must be taken when introducing a vaccine schedule that may have increased risks for adverse events. Clear and transparent articulation of the risks and benefits of all recommended vaccine schedules to parents of newborns is essential. In this case, while the individual risk for adverse events may be higher, there are even greater individual- and population-level benefits to be realized by a switch from aP to a combined strategy, in terms of improved herd immunity and a direct immunological benefit.13–15 A drop in coverage may still be expected, despite public education and outreach. We have shown that the combined strategy remains an effective alternative to aP even if vaccination coverage drops to 50%.
Conclusions
Although new pertussis vaccines combining the safety of aP and the efficacy of wP are in early development, such a novel vaccine is still a number of years away from regulatory approval and implementation.12 In the interim, switching to the combined strategy is an effective option for reducing the disease and mortality burdens of B pertussis.
Supplementary Material
Key Points.
Question
Can a priming dose of the adverse effect–prone whole-cell pertussis (wP) vaccine in the current vaccination schedule cost-effectively reduce pertussis incidence?
Findings
This simulation study uses a mathematical model fit to observed pertussis incidence in the United States and found that switching to a wP vaccination strategy could reduce whooping cough incidence by up to 95%, including 96% fewer infections in neonates. While this will be associated with an increase in vaccine-related adverse effects, the model estimates a 95% reduction in quality-adjusted life-years lost with a switch to the combined strategy.
Meaning
Inclusion of a priming dose of wP could substantially reduce pertussis incidence and save more than $142 million annually.
Acknowledgments
Funding/Support: This work was supported by grant ACI-1358567 from the National Science Foundation Research Experience for Undergraduates, MIDAS grant U01 GM087719–06 from the National Institutes of Health, the Omidyar Group, and the Santa Fe Institute.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Contributor Information
Haedi DeAngelis, New Mexico State University, Las Cruces.
Samuel V. Scarpino, Santa Fe Institute, Santa Fe, New Mexico.
Meagan C. Fitzpatrick, Center for Infectious Disease Modeling and Analysis, Yale School of Public Health, New Haven, Connecticut.
Alison P. Galvani, Center for Infectious Disease Modeling and Analysis, Yale School of Public Health, New Haven, Connecticut; Yale Program in Computational Biology and Bioinformatics, Yale University, New Haven, Connecticut.
Benjamin M. Althouse, New Mexico State University, Las Cruces; Santa Fe Institute, Santa Fe, New Mexico.
REFERENCES
- 1.Jackson DW, Rohani P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol Infect. 2014;142(4):672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. http://www.cdc.gov/pertussis/surv-reporting.html. Accessed March 17, 2015.
- 3.Elam-Evans LD, Yankey D, Singleton JA, Kolasa M; Centers for Disease Control and Prevention (CDC). National, state, and selected local area vaccination coverage among children aged 19–35 months: United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(34):741–748. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Global routine vaccination coverage, 2013. Wkly Epidemiol Rec. 2014;21(47):571–578. [Google Scholar]
- 5.Warfel JM, Zimmerman LI, Merkel TJ. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc Natl Acad SciUS A. 2014;111(2): 787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards KM. Unraveling the challenges of pertussis. Proc Natl Acad SciUS A. 2014;111(2): 575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Althouse BM, Scarpino SV. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 2015;13(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castagnini LA, Healy CM, Rench MA, Wootton SH, Munoz FM, Baker CJ. Impact of maternal postpartum tetanus and diphtheria toxoids and acellular pertussis immunization on infant pertussis infection. Clin Infect Dis. 2012;54(1): 78–84. [DOI] [PubMed] [Google Scholar]
- 9.Healy CM, Rench MA, Wootton SH, Castagnini LA. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr Infect Dis J. 2015;34(1):22–26. [DOI] [PubMed] [Google Scholar]
- 10.Meade BD, Plotkin SA, Locht C. Possible options for new pertussis vaccines. J Infect Dis. 2014;209(suppl 1):S24–S27. [DOI] [PubMed] [Google Scholar]
- 11.Thorstensson R, Trollfors B, Al-Tawil N, et al. A phase I clinical study of a live attenuated Bordetella pertussis vaccine, BPZE1: a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. PLoS One. 2014;9(1):e83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locht C, Mielcarek N. Live attenuated vaccines against pertussis. Expert Rev Vaccines. 2014;13(9): 1147–1158. [DOI] [PubMed] [Google Scholar]
- 13.Liko J, Robison SG, Cieslak PR. Priming with whole-cell versus acellular pertussis vaccine. N Engl J Med. 2013;368(6):581–582. [DOI] [PubMed] [Google Scholar]
- 14.Witt MA, Arias L, Katz PH, Truong ET, Witt DJ. Reduced risk of pertussis among persons ever vaccinated with whole cell pertussis vaccine compared to recipients of acellular pertussis vaccines in a large US cohort. Clin Infect Dis. 2013; 56(9):1248–1254. [DOI] [PubMed] [Google Scholar]
- 15.Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA. 2012;308(5):454–456. [DOI] [PubMed] [Google Scholar]
- 16.Hethcote HW. An age-structured model for pertussis transmission. Math Biosci. 1997;145(2): 89–136. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Vaccine information statements (VIS): Diphtheria, Tetanus, and Pertussis (DTaP) VIS. http://www.cdc.gov/vaccines/hcp/vis/vis-statements/dtap.html. Accessed February 2, 2015.
- 18.McGarry LJ, Krishnarajah G, Hill G, et al. Cost-effectiveness of Tdap vaccination of adults aged Š65 years in the prevention of pertussis in the US: a dynamic model of disease transmission. PLoS One. 2014;9(1):e72723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howson CP, Howe CJ, Fineberg HV, eds. Adverse effects of pertussis and rubella vaccines. Washington, DC: National Academies Press; 1991. [PubMed] [Google Scholar]
- 20.Ray P, Hayward J, Michelson D, et al. ; Vaccine Safety Datalink Group. Encephalopathy after whole-cell pertussis or measles vaccination: lack of evidence for a causal association in a retrospective case-control study. Pediatr Infect Dis J. 2006;25(9): 768–773. [DOI] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality. HCUP: overview of the National (Nationwide) Inpatient Sample (NIS). http://www.hcup-us.ahrq.gov/nisoverview.jsp. Published 2012. Accessed June 2, 2014.
- 22.Lee GM, Lett S, Schauer S, et al. ; Massachusetts Pertussis Study Group. Societal costs and morbidity of pertussis in adolescents and adults. Clin Infect Dis. 2004;39(11):1572–1580. [DOI] [PubMed] [Google Scholar]
- 23.Lee GM, Murphy TV, Lett S, et al. Cost effectiveness of pertussis vaccination in adults. Am J Prev Med. 2007;32(3):186–193. [DOI] [PubMed] [Google Scholar]
- 24.Sassi F. Calculating QALYs, comparing QALY and DALY calculations. Health Policy Plan. 2006;21 (5):402–408. [DOI] [PubMed] [Google Scholar]
- 25.Arias E. United states life tables, 2008. Natl Vital Stat Rep. 2012;61(3):1–63. [PubMed] [Google Scholar]
- 26.World Health Organization. Pertussis. http://www.who.int/biologicals/vaccines/pertussis/en/. Accessed May 19, 2015.
- 27.World Health Organization. Pertussis vaccines: WHO position paper. Wkly Epidemiol Rec. 2010;85 (40):385–400. [PubMed] [Google Scholar]
- 28.Wearing HJ, Rohani P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 2009;5(10):e1000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J. 2005;24(5)(suppl):S58–S61. [DOI] [PubMed] [Google Scholar]
- 30.Edwards KM, Berbers GA. Immune responses to pertussis vaccines and disease. J Infect Dis. 2014; 209(suppl 1):S10–S15. [DOI] [PubMed] [Google Scholar]
- 31.Warfel JM, Edwards KM. Pertussis vaccines and the challenge of inducing durable immunity. Curr Opin Immunol. 2015;35:48–54. [DOI] [PubMed] [Google Scholar]
- 32.Williams WW, Lu P-J, O’Halloran A, et al. ; Centers for Disease Control and Prevention (CDC). Noninfluenza vaccination coverage among adults: United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(5):95–102. [PMC free article] [PubMed] [Google Scholar]
- 33.Dabrera G, Amirthalingam G, Andrews N, et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and wales, 2012–2013. Clin Infect Dis. 2015;60(3):333–337. [DOI] [PubMed] [Google Scholar]
- 34.Kharbanda EO, Vazquez-Benitez G, Lipkind HS, et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312(18):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amirthalingam G, Andrews N, Campbell H, et al. Effectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384(9953):1521–1528. [DOI] [PubMed] [Google Scholar]
- 36.Atkins KE, Fitzpatrick MC, Galvani AP, Townsend JP. Cost-effectiveness of pertussis vaccination during pregnancy in the US. Am J Epidemiol. In press. [DOI] [PMC free article] [PubMed]
- 37.Plotkin SA. The pertussis problem. Clin Infect Dis. 2014;58(6):830–833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.