Abstract
Pubertal development may be altered in boys with cryptorchidism and hypospadias, but existing knowledge is inconsistent. Therefore, we investigated the association between cryptorchidism and hypospadias and pubertal development in a large cohort study. Boys in the Puberty Cohort, a cohort nested within the Danish National Birth Cohort, were included in this study. Information on cryptorchidism and hypospadias was retrieved from the Danish National Patient Register. From 11 years until 18 years or full pubertal development, information on physical markers of pubertal development was provided biannually, including Tanner stages, axillary hair, acne, voice break, and first ejaculation. In multivariate regression models for interval censored data, the mean (95% confidence intervals [CIs]) differences in months in obtaining the pubertal markers between boys with and without the anomalies were estimated. Among 7698 boys, 196 (2.5%) had cryptorchidism and 60 (0.8%) had hypospadias. Boys with hypospadias experienced first ejaculation and voice break 7.7 (95% CI: 2.5–13.0) months and 4.5 (95% CI: 0.3–8.7) months later than boys without hypospadias. The age at attaining the Tanner stages for gonadal and pubic hair growth was also higher, though not statistically significant. Pubertal development seemed unaffected in boys with mild as well as severe cryptorchidism. In conclusion, hypospadias may be associated with delayed pubertal development, but pubertal development seems unaffected by cryptorchidism. The relation between hypospadias and later pubertal development may be due to the underlying shared in utero risk or genetic factors.
Keywords: congenital abnormalities, cryptorchidism, hypospadias, prenatal exposure delayed effects, puberty, Tanner stages
INTRODUCTION
The congenital abnormalities, cryptorchidism and hypospadias, are the two most common pediatric disorders related to the male genitalia.1,2 Cryptorchidism, or undescended testis, is the absence of testes in the scrotum and results from failed testicular descent during fetal life. Hypospadias is a malformation with displacement of the external urethral meatus along the ventral side of the penis, scrotum or perineum which occurs due to an incomplete urethral fold fusion. Although conflicting results exist, the genital anomalies are associated with an increased risk of testicular malignancy3,4,5,6,7,8 and impaired fertility in adulthood.9,10 Still, less is known about other aspects of reproductive health, including pubertal development.
Puberty marks the biological transition from childhood to adolescence and adulthood and involves a complex sequence of hormonal events, marked by the reactivation of the hypothalamic-pituitary-gonadal (HPG)-axis.11 The HPG-axis is established in the first trimester of fetal life and is active during three important developmental phases of male reproductive life. During fetal development, the placental hormone human chorionic gonadotropin (hCG) and later the HPG-axis are responsible for stimulating the fetal androgen production which is essential for the organogenesis of the male genital organs and testicular descent.12,13 In the first 6–9 months of life, there is increased activity of the HPG-axis, a phenomenon known as “mini-puberty.”14 The resulting postnatal gonadotropin surge is considered important for the male genital development, as the subsequent increase in androgens stimulates penile and testicular growth. After “mini-puberty,” the HPG-axis remains quiescent throughout childhood in a “juvenile phase” until the prepubertal period, where it reemerges and initiates pubertal development. In boys, this results in the gradual maturation of the gametogenesis, enlargement of the testes, development of secondary sexual characteristics and ultimately reproductive capacity.15
Only a few studies have investigated puberty in boys with cryptorchidism or hypospadias,16,17,18,19,20 and results are inconclusive. One study found that cryptorchid boys had a slightly higher age at first ejaculation,17 whereas the others did not.18,20 Concerning hypospadias, one study found delayed pubertal onset,19 and another found similar pubertal development as the background population.16 Thus, the literature in this field is sparse, and well-conducted large studies are needed.
Sufficient androgen action is essential for both male genital formation and normal pubertal development, and we hypothesized that boys with genital anomalies experience delayed pubertal development compared to unaffected boys. We investigated pubertal development in boys with cryptorchidism or hypospadias using data from a large Danish cohort.
PARTICIPANTS AND METHODS
Data source and study population
This study was based on the Puberty Cohort, established in Denmark in 2012, to investigate risk factors for altered pubertal timing. The Puberty Cohort is nested within the Danish National Birth Cohort, including 91 661 pregnant women between 1996 and 2003,21 recruited at the first antenatal care visit at the general practitioner from gestational weeks 6–12. About 50% of all general practitioners took part in recruitment, and around 60% of those invited agreed to participate (30% of all pregnant women during the recruitment period). Participants were asked to complete four computer-assisted telephone interviews: two during pregnancy (gestational weeks 17 and 32) and two postpartum (6 and 18 months).
When the children were 7 and 11 years old, they were invited to participate in two follow-up web-based questionnaires, the latter included questions on pubertal development. The data collection for the Puberty Cohort was initiated shortly after the questionnaire at 11 years. All singletons born from 2000 to 2003, who were alive and had not withdrawn their consent before 2012, were eligible to participate, in total 56 641 children. It was economically unfeasible to invite all and 27 subgroups were identified based on 12 main exposures of interest before recruitment. To ensure statistical efficiency, children were oversampled within these groups, and a randomly selected reference group of 8000 children, resulting in 22 439 invited children. This sampling procedure was handled statistically, as explained below.
Genital anomalies
The Danish National Patient Register22 holds data on all in- and out-patient hospital contacts. We obtained information on hospital discharge diagnoses of congenital abnormalities from the date of birth until February 10, 2016. Ascertainment of cryptorchidism was based on the International Classification of Diseases, Tenth Revision (ICD-10) codes: Q53, Q531, Q531A, Q532, Q532A, or Q539. Cryptorchidism was further subdivided into mild (unilateral and palpable undescended testes, ICD-10: Q53, Q531, Q539) and severe cryptorchidism (bilateral or impalpable undescended testes, ICD-10: Q531A, Q532, Q532A). Ascertainment of hypospadias was based on Q540, Q541, Q542, Q543, Q548, and Q549.
Pubertal development
The outcome of interest was self-reported age at manifestation of various physical markers of pubertal development, gathered using a translated version of the questionnaire developed by the British Avon Longitudinal Study of Parents and Children (ALSPAC; available at www.dnbc.dk [last accessed on February 1, 2019]). For boys, the questionnaires included sexual maturity staging by use of the Tanner rating scales capturing the visible development of pubic hair and gonadal growth ranging from 1 (prepubertal stage) to 5 (adult appearance).22,23 The questions were guided by explanatory texts and illustrations for all Tanner stages. Further, they reported whether they had achieved the following pubertal milestones; first ejaculation (not differentiated between nocturnal emission or masturbation), voice break, axillary hair growth, and acne.
From the age of 11.5 years, they were asked to complete these questionnaires every 6 months until they reached full sexual maturation, defined as Tanner stage 5 for genital and pubic hair development or at the age of 18 years, whichever came first. These detailed data were used in combination with information on pubertal development from the questionnaire forwarded when the children were 11 years old.
Covariates
Information on parental health and lifestyle factors was available from the interviews. Further, the Puberty Cohort is consecutively linked with register-based data from Statistics Denmark, including information on socioeconomic status, classified according to the International Standard Class of Occupation and Education codes (ISCO-88 and ISCED) and information on parity obtained from the Medical Birth Register.25 A priori, decisions were made on which covariates to be included in the statistical models based on the existing literature and by use of Directed Acyclic Graphs:26 maternal age at menarche (earlier than peers, same time as peers or later than peers), cigarette smoking in the first trimester (nonsmoker, smoker), nulliparity (yes, no) and parental highest social class (high-grade professional, low-grade professional, skilled worker, unskilled worker).
Statistical analyses
There were few missing data on covariates (<1%) (Table 1) and complete-case analyses were applied. As participants provided information on their current pubertal stage biannually, data on age at attaining pubertal milestones were interval censored (if the pubertal milestone was attained between two questionnaires), left censored (if attained before the first questionnaire), or right censored (if attained after the last completed questionnaire). Thus, we analyzed data with a regression model for interval censored, normally distributed time-to-event data using the Stata 13.1 MP software (Stata Corporation, College Station, TX, USA) intreg package. The model takes the censoring into account assuming that the underlying distribution of age at the various pubertal milestones follows a normal distribution. This assumption was assessed and considered fulfilled by comparing a nonparametric stepwise cumulative incidence function of the residuals based on the Turnbull estimator with the parametric cumulative incidence function of the residuals based on the normal distribution in the icenreg package in R (x64 3.3.1). The plots were further divided by levels of included covariates to assess the assumption of constant variance.
Table 1.
Parental and child characteristics according to genital anomalies among 7698 boys in the puberty cohort
| Characteristics | Male genital anomalies | Missing, n (%) | |||
|---|---|---|---|---|---|
| No cryptorchidism | With cryptorchidism | No hypospadias | With hypospadias | ||
| Total, n (%) | 7502 (97.5) | 196 (2.5) | 7638 (99.2) | 60 (0.8) | 0 |
| Maternal prepregnancy BMI, n (%) | 101 (1.3) | ||||
| <18.5 kg m−2 | 500 (6.8) | 13 (6.8) | 510 (6.8) | 3 (5.0) | |
| 18.5 to <25 kg m−2 | 4651 (62.8) | 100 (52.1) | 4715 (62.6) | 36 (60.0) | |
| ≥25 kg m−2 | 2254 (30.4) | 79 (41.1) | 2312 (30.7) | 21 (35.0) | |
| Maternal cigarette smoking in first trimester, n (%) | 22 (0.3) | ||||
| Nonsmoker | 5450 (72.8) | 122 (62.9) | 5528 (72.6) | 44 (73.3) | |
| Smoker | 2032 (27.2) | 72 (37.1) | 2088 (27.4) | 16 (26.7) | |
| Maternal alcohol consumption in first trimester, n (%) | 11 (0.1) | ||||
| 0 unit per week | 3814 (50.9) | 104 (53.3) | 3887 (51.0) | 31 (51.7) | |
| 1 unit per week | 2364 (31.6) | 58 (29.7) | 2404 (31.5) | 18 (30.0) | |
| >1 unit per week | 1314 (17.5) | 33 (16.9) | 1336 (17.5) | 11 (18.3) | |
| Maternal age (year), mean (s.d.) | 30.6 (4.4) | 29.8 (4.3) | 30.6 (4.4) | 30.8 (3.5) | 4 (0.05) |
| Highest socioeconomic status of parents, n (%) | 21 (0.3) | ||||
| High grade professional | 1926 (25.7) | 55 (28.1) | 1962 (25.8) | 19 (31.7) | |
| Low grade professional | 2451 (32.8) | 55 (28.1) | 2486 (32.6) | 20 (33.3) | |
| Skilled worker | 2062 (27.6) | 56 (28.6) | 2105 (27.6) | 13 (21.7) | |
| Unskilled worker | 1042 (13.9) | 30 (15.3) | 1064 (14.0) | 8 (13.3) | |
| Maternal age at menarche, n (%) | 63 (0.8) | ||||
| Earlier than peers | 1867 (25.1) | 60 (30.8) | 1915 (25.3) | 12 (20.3) | |
| Same time as peers | 4286 (57.6) | 106 (54.4) | 4357 (57.5) | 35 (59.3) | |
| Later than peers | 1287 (17.3) | 29 (14.9) | 1304 (17.2) | 12 (20.3) | |
| Parity, n (%) | 0 | ||||
| First child | 3805 (50.7) | 115 (58.7) | 3886 (50.9) | 34 (56.7) | |
| Second or more child | 3697 (49.3) | 81 (41.3) | 3752 (49.1) | 26 (43.3) | |
| Birth weight (g), mean (s.d.) | 3594 (611) | 3375 (742) | 3590 (615) | 3311 (650) | 30 (0.4) |
| Gestational age (week), n (%) | 46 (0.6) | ||||
| <37 | 571 (7.7) | 30 (15.3) | 591 (7.8) | 10 (16.9) | |
| ≥37 | 6885 (92.3) | 166 (84.7) | 7002 (92.2) | 49 (83.1) | |
BMI: body mass index; s.d.: standard deviation
We estimated the crude and adjusted mean differences in months with 95% confidence intervals (CIs) at attaining the pubertal milestones between boys with the two genital anomalies and those without. All models were fitted with robust standard errors to account for clustering of siblings (n = 113). Further, the models also accounted for the sampling procedure described above, with sample weights calculated as the inverse probability of being sampled for each individual according to the sampling frames.
In a secondary analysis, we considered the severity of cryptorchidism. Further, preterm birth and low birth weight may act as confounders of the association between cryptorchidism and pubertal development, but in the main analysis, we did not condition on birth weight and gestational age as it may cause collider-stratification bias.27 However, in sub-analyses, we (1) adjusted for birth weight z-scores28 and (2) stratified on children born small-for-gestational-age, appropriate-for-gestational age, and large-for-gestational-age. Finally, we performed sub-analyses also adjusting for maternal body mass index (BMI; second order polynomial to allow for any departure from linearity).
Ethical approvals
The Danish National Birth Cohort was approved by The Committee for Biomedical Research Ethics in Denmark ([KF] 01-471/94). All women provided written informed consent for herself and her child up until the age of 18 years. The present study was approved by the Danish Data Protection Agency (2016-051-000001) and the steering committee of the Danish National Birth Cohort (2017–2031).
RESULTS
In total, 22 439 children, among them 11 437 boys, were invited to participate in the Puberty Cohort and 15 822 (70.5%) children, including 7698 (67.3%) boys participated by returning at least one and up to 11 questionnaires until March 2017. A total of 196 boys (2.5%) were diagnosed with cryptorchidism (152 mild and 44 severe) and 60 (0.8%) boys were diagnosed with hypospadias. Table 1 shows maternal and infant characteristics. Compared to mothers of unaffected boys, mothers of cryptorchid boys were on an average younger, had higher prepregnancy BMI, were more often smokers, had lower parity and had more often experienced age at menarche earlier than their peers. Mothers of boys with hypospadias had higher prepregnancy BMI, lower parity, higher socioeconomic status and had more often experienced age at menarche later than their peers. Overall, affected boys were more often born preterm and had a lower birth weight than unaffected boys.
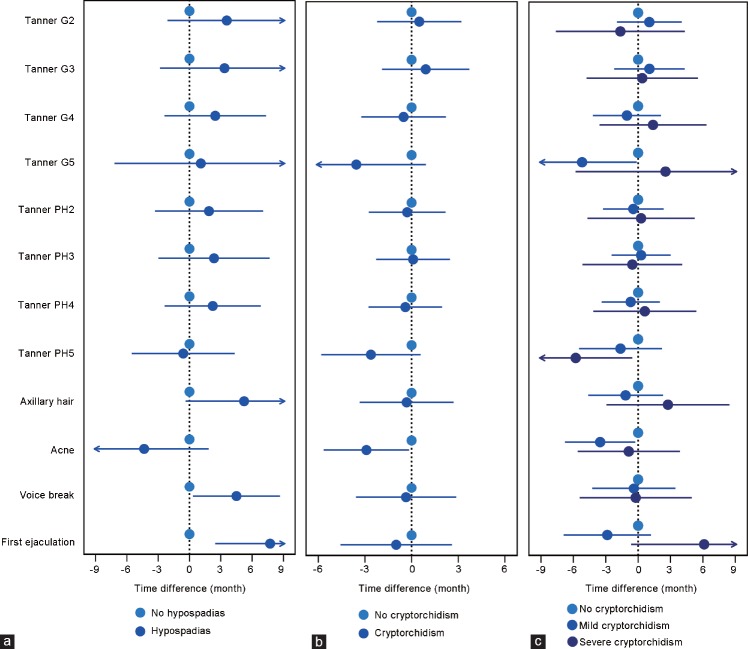
Figure 1a and Supplementary Table 1 present the mean differences in months with 95% CIs for attaining the various pubertal milestones in boys with hypospadias compared to boys without. An overall tendency toward a slightly higher age at pubertal development was seen for Tanner stages of pubic hair and gonadal growth, though CIs were wide. The tendency was most pronounced for age at first ejaculation (7.7 [95 % CI: 2.5–13.0] months later), age at voice break (4.5 [95 % CI: 0.3–8.7] months later], and axillary hair growth (5.2 [95 % CI: -0.4–10.8] months later). Figure 1b and Supplementary Table 2 show the mean differences in months with 95% CIs for attaining the pubertal milestones in boys with cryptorchidism compared to unaffected boys. No difference in pubertal development among boys with cryptorchidism was observed. Results persisted when considering the severity of cryptorchidism; thus, there was no difference in pubertal development among boys with severe cryptorchidism compared to boys without cryptorchidism (Figure 1c). Further, the analyses for birth weight z-scores and stratification on small-for-gestational age, appropriate-for-gestational age and large-for-gestational age did not show any association between cryptorchidism and pubertal development (data not shown). Adjusting for maternal BMI did not change the main results (data not shown).
Figure 1.
Adjusted mean differences in age at pubertal development according to (a) hypospadias, (b) cryptorchidism (any), and (c) cryptorchidism (mild or severe) among 7698 boys in the puberty cohort. G: gonads; PH: pubic hair.
Supplementary Table 1.
Crude and Adjusted Mean Differences in Age at Pubertal Development According to Hypospadias among 7,698 Boys in the Puberty Cohort
| Puberty outcomes | No hypospadias (reference) Mean age (years) |
Any hypospadias N = 60 Mean difference in months |
||
|---|---|---|---|---|
| Crude | Adjusteda (95% CI) | |||
| Tanner stages | ||||
| Gonads | 2 | 10.9 | 3.2 | 3.6 (-2.1; 9.2) |
| 3 | 12.5 | 3.1 | 3.3 (-2.8; 9.5) | |
| 4 | 13.7 | 2.1 | 2.5 (-2.4; 7.3) | |
| 5 | 15.6 | 2.0 | 1.1 (-7.2; 9.4) | |
| Pubic hair | 2 | 11.3 | 2.0 | 1.9 (-3.3; 7.1) |
| 3 | 12.8 | 1.9 | 2.4 (-3.0; 7.7) | |
| 4 | 13.5 | 2.0 | 2.2 (-2.4; 6.8) | |
| 5 | 14.9 | -1.1 | -0.6 (-5.5; 4.3) | |
| Axillary hair | 13.3 | 4.7 | 5.2 (-0.4; 10.8) | |
| Acne | 12.3 | -4.3 | -4.3 (-10.5; 1.8) | |
| Voice break | 13.1 | 4.2 | 4.5 (0.3; 8.7) | |
| 1st ejaculation | 13.3 | 7.9 | 7.7 (2.5; 13.0) | |
Supplementary Table 2.
Crude and Adjusted Mean Differences in Age at Pubertal Development According to Cryptorchidism among 7,698 Boys in the Puberty Cohort
| Puberty outcomes |
No cryptorchidism (reference) Mean age (years) |
Any cryptorchidism N = 196 Mean difference, months |
Mild cryptorchidism N = 152 Mean difference, months |
Severe cryptorchidism N = 44 Mean difference, months |
||||
|---|---|---|---|---|---|---|---|---|
| Crude | Adjusteda
(95% CI) |
Crude | Adjusteda
(95% CI) |
Crude | Adjusteda
(95% CI) |
|||
| Tanner stages | ||||||||
| stages | ||||||||
| Gonads | 2 | 10.9 | 0.4 | 0.5 (-2.2; 3.2) | 0.9 | 1.0 (-2.0; 4.0) | -1.6 | -1.6 (-7.6; 4.3) |
| 3 | 12.5 | 0.7 | 0.9 (-1.9; 3.7) | 0.7 | 1.1 (-2.2; 4.3) | 0.6 | 0.4 (-4.8; 5.5) | |
| 4 | 13.7 | -0.7 | -0.5 (-3.2; 2.2) | -1.3 | -1.0 (-4.2; 2.1) | 1.5 | 1.4 (-3.6; 6.3) | |
| 5 | 15.6 | -3.6 | -3.6 (-8.0; 0.9) | -5.3 | -5.2 (-10.3; - | 2.6 | 2.5 (-5.8; 10.9) | |
| 0.1) | ||||||||
| Pubic | 2 | 11.3 | -0.4 | -0.3 (-2.8; 2.2) | -0.6 | -0.5 (-3.3; 2.4) | 0.1 | 0.3 (-4.7; 5.2) |
| hair | ||||||||
| 3 | 12.8 | -0.1 | 0.1 (-2.3; 2.5) | -0.0 | 0.3 (-2.5; 3.0) | -0.3 | -0.5 (-5.2; 4.1) | |
| 4 | 13.5 | -0.6 | -0.4 (-2.8; 2.0) | -0.9 | -0.7 (-3.4; 2.0) | 0.7 | 0.6 (-4.2; 5.4) | |
| 5 | 14.9 | -2.7 | -2.6 (-5.8; 0.6) | -1.7 | -1.6 (-5.5; 2.2) | -5.6 | -5.8 (-11.0; -0.5) | |
| Axillary hair | 13.4 | -0.7 | -0.3 (-3.3; 2.7) | -1.8 | -1.2 (-4.6; 2.3) | 3.1 | 2.8 (-2.9; 8.5) | |
| Acne | 12.3 | -3.6 | -2.9 (-5.7; -0.2) | -4.2 | -3.5 (-6.8; -0.3) | -1.7 | -0.9 (-5.6; 3.9) | |
| Voice break | 13.2 | -0.9 | -0.4 (-3.6; 2.9) | -0.8 | -0.4 (-4.3; 3.5) | -1.3 | -0.2 (-5.4; 5.0) | |
| 1st ejaculation | 13.4 | -0.9 | -1.0 (-4.6; 2.6) | -2.8 | -2.9 (-6.9; 1.2) | 6.2 | 6.1 (-0.6; 12.9) | |
aAdjusted for maternal age at birth (earlier than peers, same time as peers, or later than peers), maternal cigarette smoking in first trimester (non-smoker or smoker), parity (1st child or ≥ 2nd child) and parental socio-economic status (high grade professional, low grade professional, skilled worker or unskilled worker).
DISCUSSION
Main findings
In this population-based cohort study, we observed that boys with hypospadias had a slightly higher age when attaining the various markers of pubertal development. We observed no association between cryptorchidism and timing of pubertal development.
Current knowledge
The present study investigated indicators of the onset of puberty and not progression of puberty. Whether genital anomalies may affect the progression of puberty remains unknown. Pubertal development in boys with cryptorchidism or hypospadias has only been sparsely investigated and previous studies have focused on indicators of the onset of pubertal development and not progression of puberty. In 1976, Avellán16 published a case-series study of 127 boys with hypospadias followed annually from 10 to 18 years assessing Tanner stages, testicular growth, and first ejaculation. There was no comparison group, the boys were compared to a reference material published in 1970. Avellán16 concluded that the pubertal development resembled the normal reference curve of the general population – there was no difference in age at first ejaculation, but the mean age at reaching each stage of pubic hair development was slightly lower and the ages at reaching each stage of genital development was slightly older among boys with hypospadias. Recently, Raĭgorodskaia et al.19 (written in Russian) published a case series of 39 boys with hypospadias aged 10–15 years and found later pubarche.
Three studies have explored pubertal development and testicular growth in cryptorchid boys.17,18,20 In a cohort study by Taskinen et al.17 including 76 cryptorchid boys, the mean age of the first ejaculation was 13.7 years among the cryptorchid boys, slightly older than 13.0 years in the reference group. Crespo Chozas et al.18 (written in Spanish) published a case series of 20 postpubertal cryptorchid boys aged 15–21 years. They concluded that the onset and development of puberty were within the normal range, but there was no comparison group of unaffected boys. Recently, a Finnish cohort study by Sadov et al.20 was published on the onset of testicular growth in 51 boys with cryptorchidism. When comparing cryptorchid boys with unaffected boys, they found similar age at onset of testicular growth. Yet, testicular growth differed, as boys with cryptorchidism attained a smaller final testicular size.
Overall, current knowledge in this field is characterized by inconsistency. Previous studies are small, and some are case-series with no comparison group, and none of the studies had the ability to adjust for potential confounders. Further, some were prone to recall bias due to self-reported information obtained years after the completion of puberty. To directly compare our findings with those of prior studies is therefore challenging. We found that boys with hypospadias experienced puberty later than unaffected boys, in line with the findings by Raĭgorodskaia et al.19 While the potential mechanism remains speculative, such relation could be due to shared genetic or intrauterine risk factors. Fetal life is a highly sensitive period and both genital organogenesis and pubertal development may be programmed in utero through an affected endocrine system. The organogenesis of the male reproductive organs as well as the process of testicular migration to the scrotum depends on a normally functioning HPG-axis and androgen action. Insufficient androgen levels in early fetal life may lead to cryptorchidism and hypospadias.12,13 It may also affect the sertoli cell differentiation in “mini-puberty,”29 and reduce the number of Leydig cells, thus further affecting the capacity to produce androgens.30,31 In prepubertal boys with hypospadias, these hormonal disparities may persist33,34,35,36 and could well induce a later reactivation of the HPG-axis and thus delay pubertal onset.
Methodological considerations
While this study has several strengths, important methodological considerations and limitations should also be discussed. This study is the largest of its kind, and a major strength of the Puberty Cohort was the high participation rate (70.5%). Despite high participation rates both among boys born with genital anomalies and unaffected boys, we cannot exclude that selective mechanisms have influenced our results. With such a large study population, we had the ability to study both mild and severe cryptorchidism, and all estimates were close to the null, indicating that cryptorchidism does not affect pubertal development. Hypospadias is less frequent and confidence intervals were wider. Although only a few of the pubertal markers reached statistical significance, all estimates, except for onset of acne, were consistently shifted toward attaining pubertal markers at a higher age.
Information on the onset of pubertal markers was self-reported and the boys did not undergo clinical examinations, which is a limitation of this study. A major strength was, however, the longitudinal design with detailed information on markers of pubertal development throughout puberty. Every 6 months, the participants were asked to state their current Tanner stage in a self-administered questionnaire with explanatory text and illustrations to improve reliability. Previous results on the reliability of self-reported Tanner stages have been diverging.37,38,39,40 Recently, a validation study on a subset of the Puberty Cohort including 197 children, compared the self-reported information with a clinical examination. The study showed some degree of misclassification as boys tended to slightly underestimate the genitalia staging. However, the misclassification was evenly distributed across socioeconomic status and most likely, it does not differ between boys with genital anomalies and boys without. Regarding information on genital anomalies, we also expected a high accuracy based on previous validation studies showing a positive predictive value (PPV) above 80% for cryptorchidism41 and a PPV above 98% for hypospadias.42 Further, due to the detailed information on parental and child characteristics, we were able to adjust for several potential confounders. We did not adjust for the use of assisted reproductive technologies, which could have influenced our results. In sub-analyses, we further accounted for gestational age at birth, and all results were similar to the main results. We consider our findings robust, but residual or unmeasured confounding cannot be excluded.
Clinical perspectives
The concern of the normalcy of pubertal development is frequent in pediatrics, and disentangling the normal variation from precocious or delayed puberty remains a clinical challenge.48 In boys, delayed puberty is clinically defined as the absence of testicular enlargement of 2–2.5 standard deviations above the population mean (traditionally 14 years).49 In most cases, delayed puberty is due to the constitutional delay of growth and puberty (CDGP) rather than overt pathology, but the clinical distinction remains challenging.50 There is a large variability in age at onset of pubertal development,43 but some conditions that may require treatment are important to identify timely to avoid potential adverse effects on growth, reproductive health, and psychosocial development.51 Inherent factors are ascribed to account for half of the variation in pubertal development.44 Yet, besides ethnic disparities45 and influence of adiposity and metabolic disorders,46,47 very little is known about the causes of altered pubertal timing. Our results indicate that boys with hypospadias are at increased risk of delayed pubertal onset, which may be caused by underlying shared in utero risk factors or genetic factors.
CONCLUSIONS
In summary, our findings indicated that boys with hypospadias might experience delayed pubertal development, whereas cryptorchidism did not seem to affect the timing of puberty much. Further research on risk factors of altered pubertal development is important and improving our knowledge on this subject may improve the counseling of patients with pubertal delay.
AUTHOR CONTRIBUTIONS
LHA conceptualized and designed the study, planned the study, contributed to the statistical analyses, interpreted the results, and drafted the initial manuscript. CHRH and JO conceptualized and designed the study, were responsible for the data collection and contributed to the interpretation of the results. AE conceptualized and designed the study, performed the statistical analyses and contributed to the interpretation of the results. NB and LLBL contributed to the interpretation of the results. All authors reviewed and revised the manuscript, approved the final version of the manuscript and have agreed to be accountable for all aspects of the work.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the Danish Council for Independent Research (DFF4183-00152). The Danish National Birth Cohort was established with a significant grant from the Danish National Research Foundation. Additional support was obtained from the Danish Regional Committees, the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation, the Health Foundation and other minor grants. The DNBC Biobank has been supported by the Novo Nordisk Foundation and the Lundbeck Foundation. Follow-up of mothers and children have been supported by the Danish Medical Research Council (SSVF 0646, 271-08-0839/06-066023, O602-01042B, 0602-02738B), the Lundbeck Foundation (195/04, R100-A9193), The Innovation Fund Denmark (0603-00294B (09-067124)), the Nordea Foundation (02-2013-2014), Aarhus Ideas (AU R9-A959-13-S804), University of Copenhagen Strategic Grant (IFSV 2012), and the Danish Council for Independent Research (DFF – 4183-00594).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Sijstermans K, Hack WW, Meijer RW, van der Voort-Doedens LM. The frequency of undescended testis from birth to adulthood: a review. Int J Androl. 2008;31:1–11. doi: 10.1111/j.1365-2605.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Springer A, van den Heijkant M, Baumann S. Worldwide prevalence of hypospadias. J Paediatr Urol. 2016;12:152.e1–7. doi: 10.1016/j.jpurol.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Chilvers C, Dudley NE, Gough MH, Jackson MB, Pike MC. Undescended testis: the effect of treatment on subsequent risk of subfertility and malignancy. J Paediatr Surg. 1986;21:691–6. doi: 10.1016/s0022-3468(86)80389-x. [DOI] [PubMed] [Google Scholar]
- 4.Giwercman A, Grindsted J, Hansen B, Jensen OM, Skakkebaek NE. Testicular cancer risk in boys with maldescended testis: a cohort study. J Urol. 1987;138:1214–6. doi: 10.1016/s0022-5347(17)43553-1. [DOI] [PubMed] [Google Scholar]
- 5.Swerdlow AJ, Higgins CD, Pike MC. Risk of testicular cancer in cohort of boys with cryptorchidism. BMJ. 1997;314:1507–11. doi: 10.1136/bmj.314.7093.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieckmann KP, Pichlmeier U. Clinical epidemiology of testicular germ cell tumors. World J Urol. 2004;22:2–14. doi: 10.1007/s00345-004-0398-8. [DOI] [PubMed] [Google Scholar]
- 7.Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104:1329–33. doi: 10.1111/j.1464-410X.2009.08854.x. [DOI] [PubMed] [Google Scholar]
- 8.Cook MB, Akre O, Forman D, Madigan MP, Richiardi L, et al. A systematic review and meta-analysis of perinatal variables in relation to the risk of testicular cancer--experiences of the son. Int J Epidemiol. 2010;39:1605–18. doi: 10.1093/ije/dyq120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KD, Coughlin MT, Lee PA. Fertility after unilateral cryptorchidism.Paternity, time to conception, pretreatment testicular location and size, hormone and sperm parameters. Horm Res. 2001;55:249–53. doi: 10.1159/000050005. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen HE, Bjerknes R, Cortes D, Jørgensen N, Rajpert-De Meyts E, et al. Cryptorchidism: classification, prevalence and long-term consequences. Acta Paediatr. 2007;96:611–6. doi: 10.1111/j.1651-2227.2007.00241.x. [DOI] [PubMed] [Google Scholar]
- 11.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57(Suppl 2):2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 12.Welsh M, Saunders PT, Fisken M, Scott HM, Hutchison GR, et al. Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism. J Clin Invest. 2008;118:1479–90. doi: 10.1172/JCI34241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macleod DJ, Sharpe RM, Welsh M, Fisken M, Scott HM, et al. Androgen action in the masculinization programming window and development of male reproductive organs. Int J Androl. 2010;33:279–87. doi: 10.1111/j.1365-2605.2009.01005.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuiri-Hanninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Horm Res Paediatr. 2014;82:73–80. doi: 10.1159/000362414. [DOI] [PubMed] [Google Scholar]
- 15.Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4:254–64. doi: 10.1016/S2213-8587(15)00418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avellán L. The development of puberty, the sexual debut and sexual function in hypospadiacs. Scand J Plast Reconstr Surg. 1976;10:29–44. doi: 10.1080/02844317609169744. [DOI] [PubMed] [Google Scholar]
- 17.Taskinen S, Hovatta O, Wikstrom S. Sexual development in patients treated for cryptorchidism. Scand J Urol Nephrol. 1997;31:361–4. doi: 10.3109/00365599709030620. [DOI] [PubMed] [Google Scholar]
- 18.Crespo Chozas D, Alonso Blanco M, Yturriaga Matarranz R, Barrio Castellanos R. The evaluation of gonadal function in postpubertal patients treated for cryptorchism in childhood. An Esp Pediatr. 1999;50:33–8. Article in Spanish. [PubMed] [Google Scholar]
- 19.Raĭgorodskaia N, Bolotova NV, Morozov DA, Sedova LN, Zakharova NB, et al. Sexual development of boys with hypospadias. Urologiia. 2013;2:88–92. Article in Russian. [PubMed] [Google Scholar]
- 20.Sadov S, Koskenniemi JJ, Virtanen HE, Perheentupa A, Petersen JH, et al. Testicular Growth during puberty in boys with and without a history of congenital cryptorchidism. J Clin Endocrinol Metab. 2016;101:2570–7. doi: 10.1210/jc.2015-3329. [DOI] [PubMed] [Google Scholar]
- 21.Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, et al. The Danish National Birth Cohort – Its background, structure and aim. Scand J Public Health. 2001;29:300–7. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976;51:170–9. doi: 10.1136/adc.51.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen LB, Olsen J. The Danish medical birth registry. Dan Med Bull. 1998;45:320–3. [PubMed] [Google Scholar]
- 26.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 27.Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–8. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, et al. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996;85:843–8. doi: 10.1111/j.1651-2227.1996.tb14164.x. [DOI] [PubMed] [Google Scholar]
- 29.Zivkovic D, Hadziselimovic F. Development of sertoli cells during mini-puberty in normal and cryptorchid testes. Urol Int. 2009;82:89–91. doi: 10.1159/000176032. [DOI] [PubMed] [Google Scholar]
- 30.Lee PA, Coughlin MT. Leydig cell function after cryptorchidism: evidence of the beneficial result of early surgery. J Urol. 2002;167:1824–7. [PubMed] [Google Scholar]
- 31.Liu Y, Li X. Molecular basis of cryptorchidism-induced infertility. Sci China Life Sci. 2010;53:1274–83. doi: 10.1007/s11427-010-4072-7. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Gong C, Qin M, Liu Y, Tian Y. Clinical and genetic features of 64 young male paediatric patients with congenital hypogonadotropic hypogonadism. Clin Endocrinol. 2017;87:757–66. doi: 10.1111/cen.13451. [DOI] [PubMed] [Google Scholar]
- 33.Nonomura K, Fujieda K, Sakakibara N, Terasawa K, Matsuno T, et al. Pituitary and gonadal function in prepubertal boys with hypospadias. J Urol. 1984;132:595–8. doi: 10.1016/s0022-5347(17)49755-2. [DOI] [PubMed] [Google Scholar]
- 34.Moriya K, Mitsui T, Tanaka H, Nakamura M, Nonomura K. Long-term outcome of pituitary-gonadal axis and gonadal growth in patients with hypospadias at puberty. J Urol. 2010;184(4 Suppl):1610–4. doi: 10.1016/j.juro.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Okuyama A, Namiki M, Koide T, Itatani H, Nishimoto N, et al. Pituitary and gonadal function in prepubertal and pubertal boys with hypospadias. Acta Endocrinol. 1981;98:464–9. doi: 10.1530/acta.0.0980464. [DOI] [PubMed] [Google Scholar]
- 36.Ratan SK, Aggarwal S, Mishra TK, Saxena A, Yadav S, et al. Children with isolated hypospadias have different hormonal profile compared to those with associated anomalies. J Pediatr Endocrinol Metab. 2012;25:111–9. doi: 10.1515/jpem.2011.421. [DOI] [PubMed] [Google Scholar]
- 37.Rockett JC, Lynch CD, Buck GM. Biomarkers for assessing reproductive development and health: part 1-Pubertal development. Environ Health Perspect. 2004;112:105–12. doi: 10.1289/ehp.6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rasmussen AR, Wohlfahrt-Veje C, Tefre de Renzy-Martin K, Hagen CP, Tinggaard J, et al. Validity of self-assessment of pubertal maturation. Pediatrics. 2015;135:86–93. doi: 10.1542/peds.2014-0793. [DOI] [PubMed] [Google Scholar]
- 39.Jaruratanasirikul S, Kreetapirom P, Tassanakijpanich N, Sriplung H. Reliability of pubertal maturation self-assessment in a school-based survey. J Pediatr Endocrinol Metab. 2015;28:367–74. doi: 10.1515/jpem-2014-0053. [DOI] [PubMed] [Google Scholar]
- 40.Desmangles JC, Lappe JM, Lipaczewski G, Haynatzki G. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol Metab. 2006;19:213–21. doi: 10.1515/jpem.2006.19.3.213. [DOI] [PubMed] [Google Scholar]
- 41.Jensen MS, Snerum TM, Olsen LH, Thulstruo AM, Bonde JP, et al. Accuracy of cryptorchidism diagnoses and corrective surgical treatment registration in the Danish National Patient Registry. J Urol. 2012;188:1324–9. doi: 10.1016/j.juro.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 42.Arendt LH, Ernst A, Lindhard MS, Jønsson AA, Henriksen TB, et al. Accuracy of the hypospadias diagnoses and surgical treatment registrations in the Danish National Patient Register. Clin Epidemiol. 2017;9:483–9. doi: 10.2147/CLEP.S143118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123:84–8. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 44.Gajdos ZK, Henderson KD, Hirschhorn JN, Palmert MR. Genetic determinants of pubertal timing in the general population. Mol Cell Endocrinol. 2010;324:21–9. doi: 10.1016/j.mce.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramnitz MS, Lodish MB. Racial disparities in pubertal development. Semin Reprod Med. 2013;31:333–9. doi: 10.1055/s-0033-1348891. [DOI] [PubMed] [Google Scholar]
- 46.Wagner IV, Sabin MA, Pfaffle RW, Hiemisch A, Sergeyev E, et al. Effects of obesity on human sexual development. Nat Rev Endocrinol. 2012;8:246–54. doi: 10.1038/nrendo.2011.241. [DOI] [PubMed] [Google Scholar]
- 47.Biro FM, Kiess W. Contemporary trends in onset and completion of puberty, gain in height and adiposity. Endocr Dev. 2016;29:122–33. doi: 10.1159/000438881. [DOI] [PubMed] [Google Scholar]
- 48.Pitteloud N. Managing delayed or altered puberty in boys. BMJ. 2012;345:e7913. doi: 10.1136/bmj.e7913. [DOI] [PubMed] [Google Scholar]
- 49.Palmert MR, Dunkel L. Clinical practice. Delayed puberty. N Engl J Med. 2012;366:443–53. doi: 10.1056/NEJMcp1109290. [DOI] [PubMed] [Google Scholar]
- 50.Ambler GR. Androgen therapy for delayed male puberty. Curr Opin Endocrinol Diabetes Obes. 2009;16:232–9. doi: 10.1097/med.0b013e32832b20a8. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Chan YM. Adult consequences of self-limited delayed puberty. Pediatrics. 2017;139:e20163177. doi: 10.1542/peds.2016-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]