Abstract
Up to 15% of male infertility has an immunological origin, either due to repetitive infections or to autoimmune responses mainly affecting the epididymis, prostate, and testis. Clinical observations and epidemiological data clearly contradict the idea that the testis confers immune protection to the whole male genital tract. As a consequence, the epididymis, in which posttesticular spermatozoa mature and are stored, has raised some interest in recent years when it comes to its immune mechanisms. Indeed, sperm cells are produced at puberty, long after the establishment of self-tolerance, and they possess unique surface proteins that cannot be recognized as self. These are potential targets of the immune system, with the risk of inducing autoantibodies and consequently male infertility. Epididymal immunity is based on a finely tuned equilibrium between efficient immune responses to pathogens and strong tolerance to sperm cells. These processes rely on incompletely described molecules and cell types. This review compiles recent studies focusing on the immune cell types populating the epididymis, and proposes hypothetical models of the organization of epididymal immunity with a special emphasis on the immune response, while also discussing important aspects of the epididymal immune regulation such as tolerance and tumour control.
Keywords: epididymis, immune response, lymphocytes, mononuclear phagocytes, tolerance
INTRODUCTION
About 15% of couples do not achieve pregnancy within 1 year of unprotected intercourse, and male causes for infertility are found in half of these couples.1 About 15% of known male infertility originates from autoimmune disorders or from inflammation caused by urogenital infections,1 and this rate is likely to be underestimated, as 30% of male infertility cases are idiopathic.2 Contrary to orchitis, epididymitis is a frequent condition, and bacterial infections are common causes of epididymitis, especially when involving sexually transmitted Chlamydia trachomatis and Neisseria gonorrhoeae in young men, and enteric Escherichia coli and Pseudomonas spp. in older men.3,4 Even if successfully treated, these infections have been shown to induce stenosis in the epididymal duct, reduction of sperm counts, and azoospermia in up to 40% of patients.5,6,7,8,9 These clinical data imply a crucial need for an efficient but finely controlled immune response to pathogens in the epididymis.
On the other hand, spermatozoa are produced at puberty, long after the setting of tolerance to self-antigens, and therefore sperm-specific antigens are unknown to the immune system.10 These antigens are thus potential targets of the male immune system and are at risk of being destroyed. Therefore, there is a special need for tolerance to sperm cells all along their testicular production and epididymal maturation, combined with the need for efficient immune response to pathogens. While the testis relies on a very well-sealed seminiferous epithelium restricting entry of immune cells and molecules into the luminal compartment, the epididymis has evolved different strategies.
New data have emerged in recent years on the immunity existing in the epididymis. This review proposes three figures to illustrate our hypothetical vision of the murine epididymal immune cell organization. These propositions are based both on the available epididymal literature and on the canonical functions known for the cells only recently identified in the murine epididymis. Our purpose will particularly focus on the immune response to pathogens (Figure 1), but it will also address less described aspects of the immune regulation that are the control of cancer cells in the tissue (Figure 2) and the tolerance to sperm cells (Figure 3).
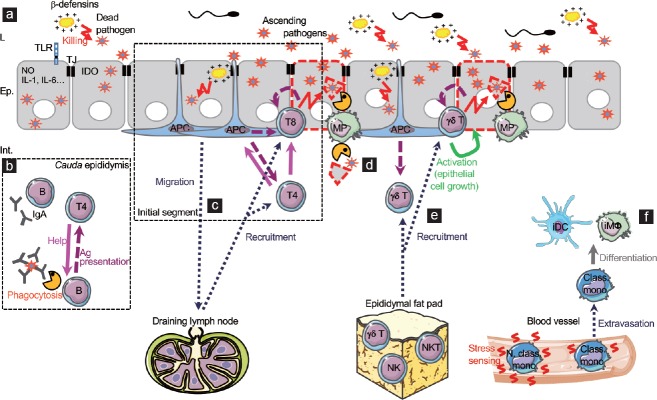
Figure 1.
Model of innate and adaptive responses to urogenital tract ascending pathogens. (a) Epididymal epithelial cells have evolved innate mechanisms that fight pathogens, of which expression of various TLRs, antimicrobial molecules (nitric oxide, IDO, β-defensins, etc.), and pro-inflammatory cytokines (IL-1, IL-6, etc.). (b) In the cauda epididymidis, interstitial B cells may be responsible for the secretion of local IgAs or may phagocytose antibody-coated bacteria and limit their dissemination in the tissue and induce specific effector T cells. Activated B cells may then classically activate helper T cells. (c) In the initial segment, the high proportion of MPs/APCs and canonical effector T cells in the tissue suggest that classical immune responses are set towards pathogens. APCs could sample circulating foreign antigens and present them to effector CD4+ and CD8+ T cells, inducing cytotoxicity of infected cells while helper T cells sustain the reaction. Depending on the population of APCs, some cells could migrate to the draining lymph node to elicit the recruitment of effector cells to the infected epididymis. (d) In case of epithelial damage due to a severe infection, some CX3CR1+CD11c+ MPs may participate in the elimination of cellular or pathogen debris. (e) The newly identified epididymal gd T cells could be activated either by APCs or directly by infected cells. Once activated, they could become cytotoxic thus participating in bacterial clearance. They are also expected to promote epithelial cell maintenance in case of cell injury following severe infections. The epididymal fat pad is suggested as a potential immune reservoir of cytotoxic cells before being recruited during infections. (f) The few monocytes identified in the epididymis are expected to sense local stress signal that induces their extravasation into the tissue and their subsequent differentiation into inflammatory MPs (iDCs and iMΦ) that temporarily sustain the immune response. Ep: epithelium; L: lumen; Int.: interstitium; TJ: tight junction; TLR: Toll-like receptor; NO: nitric oxide; IDO: indoleamine 2,3-dioxygenase; IL: interleukin; Ig: immunoglobulin; Ag: antigen; APC: antigen-presenting cell; MP: mononuclear phagocyte; NK: natural killer cell; NKT: natural killer T cell; iDC: inflammatory dendritic cell; iMf: inflammatory macrophage; Class. mono: classical monocyte; N. class. mono: nonclassical monocyte. Note: parts of the Figure 1–3 use illustrations modified from Servier Medical Art, licensed under the Creative Commons Attribution 3.0 unported license. To view a copy of this license, visit http://creativecommons.org/licenses/by/3.0/ .
Figure 2.
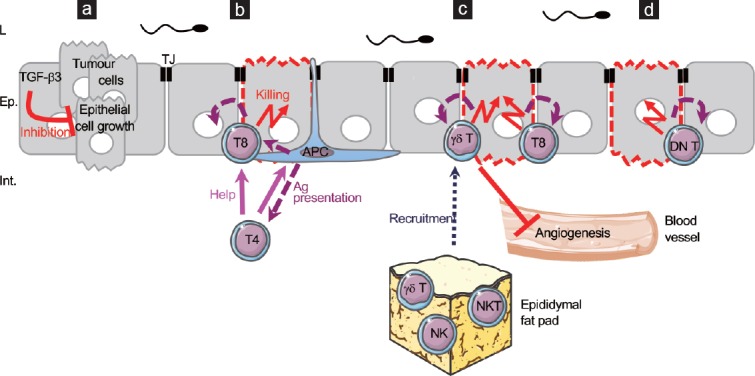
Hypothetical complementary mechanisms of early immune surveillance of cancer cells. (a) TGF-β3 has been proposed as an autocrine inhibitor of epididymal epithelial cell growth to limit uncontrolled proliferation. (b) A classical immune response may be set for tumour cells. Modified antigens could be sampled by APCs and presented to effector T cells, inducing a cytotoxic response to epithelial tumour cells. (c) γδ T cells can be directly activated by tumour cells to become cytotoxic and can induce indirect activation of cytotoxic CD8+ T cells by upregulating stimulating molecules on the tumour cell surface. γδ T cells can also inhibit angiogenesis. As suggested for the immune response, the epididymal fat pad could participate in the early elimination of cancer cells by the release of such cytotoxic cells as γδ T cells, NK cells and NKT cells. (d) DN T cells can selectively recognize transforming cells and become cytotoxic and suppress them. Ep: epithelium; L: lumen; Int.: interstitium; TJ: tight junction; TGF-β: transforming growth factor-beta; APC: antigen-presenting cell; Ag: antigen; NK: natural killer cell; NKT: natural killer T cell; DNT: double-negative T cell.
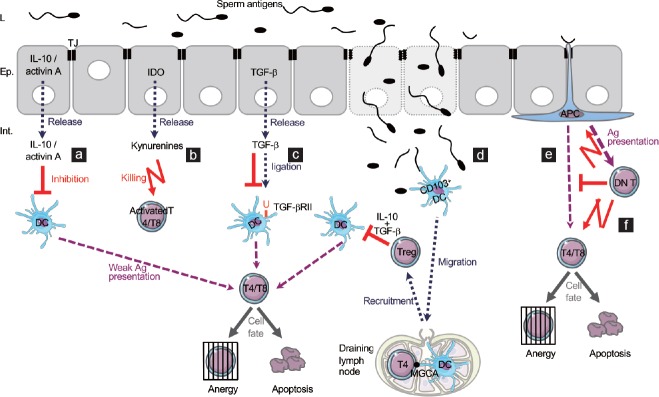
Figure 3.
Model of immune tolerance to sperm cells at steady state or following an interstitial leak of sperm antigens. (a–c) The epididymal epithelial cells are known to express several molecules implicated in the inhibition of activation ([a] IL-10, activin A, [c] TGF-β) or in the killing of activated T cells ([b]IDO). This list may not be exhaustive. (d) In particular cases such as severe tissue injuries leading to a leak of sperm antigens into the interstitium, tissue CD103+ DCs may migrate to the draining lymph node where they present some MGCAs to T cells. The activation of lymph node T cells could then lead to the epididymal recruitment of Treg cells able to inhibit effector T cells activation thanks to their secretion of IL-10 and TGF-β. (e) A classical way to induce a tolerogenic response is the weak activation of effector cells by local APCs in an immunosuppressive microenvironment, leading either to their anergy or to their apoptosis. (f) A new immunosuppressive population of DN T cells has been described in the mouse epididymis. As in other organs, they are proposed to become activated by local APCs and become cytotoxic to activated T cells and APCs. Ep: epithelium; L: lumen; Int.: interstitium; TJ: tight junction; IL: interleukin; TGF-β: transforming growth factor-beta; IDO: indoleamine 2,3-dioxygenase; APC: antigen-presenting cell; Ag: antigen; DN T: double negative T cell; Treg: regulatory T cell; DC: dendritic cell; MGCAs: meiotic germ cell antigens.
The epididymal epithelium: a first line of defence
The study of the epididymal immune system has long been neglected compared with that of the testis. Indeed, the anatomical continuity that exists between the two organs led to the idea that the testis conferred its immunity to the whole genital tract. However, clinical data seriously challenge this idea. First, the epididymal tumour incidence is about 50 times less than that of the testis and 80% of epididymal cancers are not malignant.11 Second, epididymitis, the inflammation of epididymis caused by the immune response to pathogens, is the most common intrascrotal inflammation,12 while isolated orchitis is rare and generally associated with mumps infection in young boys.13 Thus, as cancers arise as a consequence of a defective immune surveillance, and inflammations arise as a response to efficient immune responses, it can be assumed that the organization of the immune system greatly differs between the epididymis and the testis. Experimental data reinforce this assumption. Indeed, the survival of parathyroid grafts in the interstitial tissue has been assessed in both the testis and epididymis of rats. Most intratesticular parathyroid allografts survive for at least twice as long as control grafts in nonprivileged organs, i.e., around 41 days, and one-third of the grafts are still functional at 100 days.14 The intratesticular environment is thus prone to immune tolerance, probably with the help of local testosterone, as the suppression of its secretion by Leydig cells via oestrogen pretreatment leads to rapid graft rejections.15 In sharp contrast, intra-epididymal allogeneic parathyroid grafts, preceded by orchiectomy to exclude any possible influence of intratesticular factors, are rejected as soon as 7–12 days posttransplantation.16 Thus, if the testis is considered an immunologically privileged site,14 this cannot be the case for the epididymis.
The first line of defence of this organ is the blood–epididymis barrier (BEB). The BEB is a complex structure comprising a combination of three components that are anatomical, physiological, and immunological barriers.17 The anatomical barrier is formed by the tight junctions and the basolateral and apical membranes of the epithelial cells.18 It limits the entry and the exit of molecules and cells from and to the lumen. The physiological barrier comprises transporters that control the movement of substances in or out of the lumen to create a specialized microenvironment that is thought to play a role in the maturation of sperm cells.19,20 Finally, the immunological barrier is composed of nonimmune cells, i.e., epithelial cells that have developed defence systems, and immune cells that have known and suggested roles that will be exposed in the subsequent sections of this review.
Concerning the epithelial cells, several mechanisms of defence have been described. Among them is the expression of Toll-like receptors (TLRs), which are evolutionarily conserved innate receptors that sense pathogen-associated molecular patterns (PAMPs) derived from viruses, bacteria, fungi, and protozoa (Figure 1a). TLRs are localized to the epithelial and interstitial cells in the rat epididymis at steady state, some being more abundant than others. Moreover, their expression pattern varies. Indeed, TLRs1–4 are detected in principal cells throughout the epididymal epithelium in all regions of the duct, whereas clear cells of the cauda epididymidis do not express TLRs5–7 or TLR11.21,22 This may reflect the specificity of action of the different cell types populating the duct. Moreover, a study has demonstrated that an in vitro Staphylococcus aureus infection induces the upregulation of TLR2 but not of TLR4 by epididymal epithelial cells (EECs), and this is concomitant with their secretion of pro-inflammatory cytokines and nitric oxide.23 This is supported by the in vivo lipopolysaccharide (LPS) treatment of rats that showed no impact on the expression of TLR4; however, that study did not describe the TLR2 expression.24 In mice, steady state primary cultures of EECs show an abundant production of TLR4 and TLR5 in principal cells, basal cells, and some interstitial cells, while TLR11 is not detected.25 This study also showed that TLR4 and TLR5 cooperatively mediate pro-inflammatory cytokine production by EECs, while TLR4 alone mediates the expression of interferon (IFN)-α and IFN-β, after uropathogenic E. coli infection of mice. TLRs are also detected in the rooster where 9 of the 10 known chicken TLRs are expressed in the epididymis.26,27 LPS injection does not affect the level of TLR4 in the epididymis of roosters,26 whereas Salmonella enteritidis infection results in a significant induction of some TLR genes of mature roosters compared with that in healthy birds of the same age.27 Finally, TLR3, specific to double stranded RNA, has been shown to be constitutively expressed by mouse and human EECs in combination with other viral sensors. These EECs are then able to produce type I IFNs in response to viral ligands.28,29 Whatever the species, given the diversity of the TLRs expressed by the EECs, these cells appear well armed to fight bacteria (TLRs1, 2, 4–6, 9) and viruses (TLRs3, 7–9) that can be found in the epididymis.
β-defensins are an important class of antimicrobial agents found in the EECs, and more generally in male and female reproductive tracts (Figure 1a). They are small cationic peptides with a broad-spectrum action against bacteria, fungi, and enveloped viruses. A great number of β-defensins have been identified so far in the human, mouse, and rat epididymis, some of them being specific to this organ while others are shared with the testis.30,31,32 They are of particular interest as, besides their ability to kill microorganisms rapidly through membrane permeabilization,33,34 they are chemotactic for immature dendritic cells and T lymphocytes to sites of microbial invasion in man.35
The innate defence of EECs also relies on many other antimicrobial proteins, such as cathelicidins, mucins, protease inhibitors, lactoferrin, lysozyme, LPS-binding proteins, and chemokines well described in previous articles.30,36,37,38
An interesting enzyme, indoleamine 2,3-dioxygenase or IDO, has also been described in the epithelial cells of the murine epididymis (Figure 1a).39,40 Contrary to other organs where it is induced by inflammation, IDO is constitutively expressed in the epididymis,41 suggesting a crucial role for this enzyme. Even if it is generally considered for its tolerogenic properties, IDO is a well-described antimicrobial effector through its catabolic activity which deprives the environment of tryptophan. Even if the in vivo effect of IDO, antimicrobial or immunoregulatory, has been generally shown to be dependent on the intracellular pathogen,42 it is reported to act efficiently in vitro against the measles virus, and against bacterial tryptophan auxotrophs, of which the Chlamydiae species.43,44,45
The epididymal immune response to pathogens: innate and adaptive immune cells
In addition to nonimmune cells, the response to pathogens relies on immune cells, whatever the organ. The innate immune cells are the first cells to interact with pathogens and induce nonspecific responses rapidly that limit the spread of pathogens. Thereafter, innate antigen-presenting cells (APCs, mostly macrophages and dendritic cells) initiate the adaptive response, which is late but pathogen-specific, and which triggers memory cells that will be able to fight the pathogen rapidly during a second encounter.
As early as 1983, lymphocytes and macrophages were described in the human epididymis at steady state.46 These first observations were completed by the observation that most intraepithelial lymphocytes were T cells, and mainly CD8+ T cells, contrasting with the predominance of the CD4+ T cell subset in the interstitial tissue.47 Moreover, the CD8+ intraepithelial T cells were prone to cytotoxicity as judged by their granzyme B-positive staining.48 In rats, CD4+ (helper) T cells, CD8+ (cytotoxic) T cells, and macrophages have been identified along the length of the epididymis, with a preferential presence in the interstitium compared with the intraepithelial compartment.49,50 Intraepithelial, CD4+ T cells, and macrophages were mostly found close to the basement membrane, whereas CD8+ T cells were observed at various heights between epithelial cells.51 B cells, although rarely seen in the epididymal epithelium, were often located close to the base of epithelial cells, and represented 1% to 5% of the immune cells of the tissue, depending on the age of the animals. None of the immune cells identified in the rat epididymis were found in the lumen.51 Similar to the rat and human epididymis, the mouse organ was shown to contain macrophages, helper CD4+ T cells, and cytotoxic CD8+ T cells.52 Macrophages were the most frequent leukocytes and mainly located in the peritubular layer. The few T cells were essentially observed in the interstitium, and helper CD4+ T cells and cytotoxic CD8+ T cells showed similar regional and histological distribution patterns. The authors did not notice any distortion in the helper/cytotoxic cells ratio. Finally, there was no invasion of leukocytes into the murine epididymal lumen.52 Although studies describing epididymal immune cells are scarce and seem restricted by technical limitations, recent works have shed light on new immune populations, thanks to innovative tools such as transgenic animal models and flow cytometry. Such studies were necessary owing to the rapidly growing knowledge on the immune cell populations and ontogeny.53 The first work reported a newly identified dense network of dendritic cells (eDCs) at the base of the epididymal epithelium in mice expressing CD11c-EYFP and CX3 CR1-GFP reporters.54 From their differential expression of surface markers, the eDCS could be separated into two populations, the CD11c+ CD103+ cells and the CD11c+ CD103− cells. eDCs were shown to project dendrites between epithelial cells and to be able to cross the tight junctions to reach the lumen in the initial segment. In more distal segments, the eDCs morphology differed, as they lost their dendrites to become flat cells in the cauda epididymidis. Moreover, these eDCs were shown to be functional, at least in vitro. Indeed, when cocultured with chicken ovalbumin-specific CD4+ and CD8+ T cells in the presence of ovalbumin, eDCs were able to induce the proliferation of both populations of T cells, showing their ability to present the antigen and to activate helper CD4+ and cytotoxic CD8+ T cells efficiently. However, these cells were referred to as mononuclear phagocytes (MPs) instead of dendritic cells in subsequent work from the same team, as the authors could not exclude that they were macrophages.55,56 Indeed, the use of a unique marker to characterise a MP population appears to be insufficient.53 This led our team to undertake a screening of the various MP and lymphocyte populations in the murine epididymis.57 Using flow cytometry and a wide set of markers, we identified and quantified the innate populations of monocytes, macrophages and dendritic cells subtypes. This study also revealed the presence of B lymphocytes, γδ T cells, and double-negative T cells (T cells devoid of the CD4 and CD8 markers), whose potential roles will be discussed in subsequent sections. All lymphocytes proved to be distributed along the organ, in the interstitium, and to a lesser extent in the epithelium.
Although all the above studies describe the steady state, they give clues concerning the organization of the immune system in the epididymis. A model of epididymal immunity to a pathogen infection is presented in Figure 1 with mentions of special region-specific responses when necessary.
Epididymitis is mostly caused by pathogens entering the tissue via the cauda epididymidis, where B cells are abundant.57 From the two functions of B cells (antigen presentation and immunoglobulin secretion), two complementary hypotheses could explain the higher presence of B cells in the cauda compartment when compared with the caput epididymidis (Figure 1b). First, these cells could secrete the high amount of local immunoglobulins A (IgAs) described in the tissue,58 immunoglobulins typical of mucosa that need to be associated with a secretory component to be secreted in the mucosal lumen. As no secretory component was described in association with these IgAs in the rat,58 they must be confined to the interstitium where most B cells reside.51,57 Second, B cells could limit the systemic spread of pathogens in case of an epithelial breach. Indeed, several reproductive organs such as the placenta and testis, as well as splenic B cells, have been shown to express a receptor specific for IgA/IgM, the Fca/mR.59 Thus, if epididymal B cells were to express this receptor, which has still to be proven, they could phagocytose antibody-coated bacteria, process and present the pathogenic antigens to local helper CD4+ T lymphocytes, highly represented in the cauda epididymidis,57 leading to the elimination of the bacteria.
The caput epididymidis, as the last region before ascending pathogens reach the testis, is a crucial compartment for the avoidance of any alteration to sperm production. The typical immune response relies on pathogenic antigen presentation by APCs to T cells that become activated effector cells capable of eliminating the pathogen. Interestingly, most of the MP/APC populations are more numerous in the caput than in the cauda epididymidis, and canonical effectors cells, i.e., CD4+ and CD8+ T lymphocytes, are highly represented in this region.57 The existence of classical immune responses can thus be questioned in the caput epididymidis, especially in the initial segment where the basal APCs could sample luminal antigens, as they are shown to project dendrites through the apical tight junctions (Figure 1c).54 Epithelial MPs/APCs could then process the antigens to present them to the T cells,54 which could finally contribute to pathogen elimination, either by a direct cytotoxic action of CD8+ T cells48 or by indirectly sustaining cytotoxicity when considering the helper CD4+ T cells (Figure 1c). This potential immune response is supported by experimental infections where APCs or lymphocytes or both increase in the interstitium of inflamed epididymides.60,61,62,63 Moreover, in chronic human epididymitis, elevated numbers of dendritic cells, macrophages, and helper T cells are also observed.64,65
In more distal segments, MPs lose these projections but intraepithelial CX3 CR1+ CD11c+ MPs have the ability to engulf apoptotic cells and debris after efferent duct ligation, before returning to their steady state appearance at the end of the apoptotic wave.56 Such characteristics could contribute to the resolution of a severe infection leading to a partial destruction of the BEB and to a local release of cell and pathogen debris (Figure 1d).
In the steady state epididymis, several populations of APCs/MPs have been described on the basis of their expression of various surface markers, including CD11c, CD103, and CX3 CR1.54 As such, they resemble APC populations of the gut, where CX3 CR1+ cells sample the luminal content and CD103+ cells then migrate to the draining lymph node to induce the activation of T cells into effector cells.66,67 Thus functional characterisation of epididymal DCs and macrophages subsets should be considered for future experiments.
The γδ T cells, typical mucosal cells, have recently been identified in the interstitial and intraepithelial compartments of the murine epididymis.57 Many tissue-resident γδ T cells show a limited TCR diversity, and only one subtype (Vg6d1+ T cells) has been identified so far in the mouse reproductive tract including the uterus, placenta, and testis. Depending on the context, this subtype is able to produce a broad range of cytokines, including IL-17, IL-22, IFN-g, and TGF-β, to have cytotoxic activity, and to promote bacterial clearance.68 Moreover, these cells are also found in lungs where they participate in epithelial maintenance after injury,69 suggesting a similar potential role for them in case of a breach in the epididymal epithelium following severe infections (Figure 1e). Given the diversity of these cells, it appears of primary importance to establish the epididymal γδ T cells phenotype, in order to have solid clues regarding their actual role in the organ.
Finally, whereas few monocytes have been identified in the epididymis at steady state, these cells are expected to rise in number after infection, as they mostly sense the stress signature that allows their recruitment and local differentiation into inflammatory DCs or macrophages (Figure 1f).70
This model surely underestimates the role of such innate immune cells as polymorphonuclear cells (PMNs), owing to the scarcity of studies describing these cells,60,61,71 even though they are crucial during the early steps of infections for inducing pathogen-specific immunity and limiting pathogen dissemination. Moreover, the role of the EECs and defence molecules should not be forgotten when considering the overall response to ascending pathogens (Figure 1a). In the future, accurate work will be needed to assess the functional implication of each immune cell type described in the epididymis, especially in the context of experimental bacterial infections. On the other hand, the origin and phenotypes of effector cells found in the epididymis are interesting points to decipher, as they might influence the kinetics and efficiency of the immune response. We suggest that they could originate from the tissue or from the draining lymph node, but another potential source of effector cells has been described close to the epididymis, the epididymal fat pad. Indeed, at steady state, this adipose tissue was shown to possess up to 70% of γδ T cells, natural killer (NK), and natural killer T (NKT) cells among all its lymphocytes, which is quite unusual. In comparison, the subcutaneous inguinal fat pad presented a more conventional profile with high levels of ab T cells (i.e., CD4+ or CD8+ lymphocytes) and B cells.72 Moreover, the epididymal fat pad of normal mice show physiologically activated NKT cells expressing high levels of intracellular IFN-g.73 Even if all these cell types regulate fat pad functions, as suggested by the authors, it is tempting to consider the epididymal fat pad as a reservoir of immune cells, especially in inflammatory conditions. Indeed, infection of the epididymis must create a local inflammation, not limited to the tissue, and the dense lymphatic and blood networks that link the two contiguous organs are well defined.74,75 Overall, one can suggest that the effector cells (and particularly the γδ T cells here) naturally present in the epididymal fat pad, but absent from the steady state epididymis, might be recruited to the site of infection to support the local response (Figure 1e). This hypothesis will definitely have to be tested. Finally, a recent study elegantly demonstrated that antigen-experienced, i.e., memory CD8+ T cells, were able to migrate through the peritoneal cavity to the infected epididymis by directly crossing the capsule, thus not utilising the classical vascular entry route into nonlymphoid organs. This work also proved that, at the end of the infection, some functional resident memory CD8+ T cells remain in the tissue.76
The particular case of epididymal cancers
As mentioned above, epididymal tumours are rare. Indeed, they account for 0.03% of all male cancers, whereas the most common prostate cancer represents 20% of male cancers in Western countries.11 Most epididymal tumours are benign and primary, and have an epithelial origin.11,77 A hypothetical model of the antitumoural defences at work in the epididymis is illustrated in Figure 2.
Some nonimmune mechanisms have been suggested that could prevent the initiation of epididymal tumour development, such as strong antioxidant mechanisms, active tumour suppressors and inactive oncogene products that render the cells less likely to become cancerous.11 A probable absence of stem cells has been evoked as another potential anticancerous mechanism,11 but a recent study found that the rat basal cells can act as adult stem cells, thus challenging this concept.78 The presence of persistent tight junctions and a combination of anti-angiogenic factors and misplaced pro-angiogenic factors also contribute to prevent metaplasia.11 For example, transforming growth factor-beta3 (TGF-β3), which is highly expressed in the rat epididymis and which is present in its active form in the secretory epithelium of the corpus region, has been proposed to act as an autocrine inhibitor of epithelial cell growth in limiting uncontrolled proliferation (Figure 2a).79
Furthermore, senescence and immune cells play a crucial role in the early elimination of transforming cells. It has been shown that both innate cells (DCs, macrophages, NK, and NKT cells) and adaptive cells (helper CD4+ and cytotoxic CD8+ T cells, γδ T cells) are required to protect the host against developing tumours.80 As evoked in the previous section, both APC populations and adaptive cells are found in the epididymis, suggesting that this early immune surveillance can be effective in the organ (Figure 2b). This proposition is supported by the fact that the caput epididymidis, where most APCs and numerous effector T cells reside,57 is less affected by tumours than the cauda compartment.11 In addition to the classical immune responses (APC/effector T cells), γδ T cells can be directly activated by tumour cells to become cytotoxic and release perforin and granzymes in some experimental conditions. They also have the possibility of expressing ligands that engage death receptors, and they can secrete IFN-γ, thus inhibiting angiogenesis while at the same time enhancing major histocompatibility complex (MHC) class I expression by tumour cells. Consequently, the expression of “modified self”, i.e., antigens from tumour cells, on the MHC class I molecules promotes cytotoxic CD8+ T cell responses (Figure 2c).81 Moreover, some effector cells, such as γδ T cells, NK, and NKT cells, may come from the contiguous epididymal fat pad to support the local response, either to pathogens as proposed earlier, or to tumour cells, as NK and NKT cells are powerful and well-described antitumoural effector cells (Figure 2c).
Nevertheless, NK and NKT cells have never been described in the epididymis. We propose that their absence could be overcome, at least in part, by the presence of the newly described epididymal double-negative (DN) T cells.57 Indeed, these cells are highly cytotoxic towards leukemia cells from patients, as well as cell lines derived from leukemias and lymphomas, but not towards normal cells. They selectively recognize these tumour cells via activator receptors also expressed by NK cells, Natural Killer receptor Group 2, member D (NKG2D), and DNAX accessory molecule-1 (DNAM-1, also known as CD226), further supporting the view that DN T cells could replace NK cells in the epididymis.82 In another model, they are able to suppress lymphoma cells both in vivo and in vitro by apoptosis via the Fas-FasL mechanism (Figure 2d).83
When the elimination phase fails at eradicating every early transforming cells, some tumour cells may enter an equilibrium phase called dormancy, which can last for the animal's lifetime.80 A mouse model of primary chemical carcinogenesis has been used to demonstrate that equilibrium is mechanistically distinct from elimination and escape phases of cancers,84 as dormancy is only maintained by adaptive immunity, helper CD4+ and cytotoxic CD8+ T cells. It also proved that neoplastic cells in equilibrium are transformed but proliferate poorly in vivo.84 Given the low incidence of cancer in the epididymis and the high proportion of CD4+ and CD8+ T cells,57 such a mechanism of immune control can be suggested to exist in the tissue and complete the immune surveillance. To investigate the antitumoural mechanisms that really occur in the epididymis, reliable mouse models are lacking; even though many models are available, they induce hyperplasia, hypertrophy, or dysplasia, but fail to reach malignancy, proving a tight control of cell transformation.85,86,87,88,89,90,91,92,93 These models have only targeted one oncogene, regulator, or growth factor at a time, except the study by Frew and colleagues93 that combined the deletion of two tumour suppressor genes. To improve animal models, future work will certainly have to aim for well-chosen multiple targets.
Immune tolerance to sperm cells
On the other side of the epididymal immune balance is the peripheral tolerance that must be set and maintained towards spermatozoa, as presented in Figure 3.
The first level of protection, again, is the BEB, as one of its functions is to protect the spermatozoa from the immune system, either by preventing sperm antigens from escaping the duct or by impeding immune cells from infiltrating into the lumen. Under physiological conditions, all lymphocytes and APCs are localised in the epididymal epithelium and interstitium but not in the lumen, demonstrating an effective role of the BEB.46,47,49,50,51,52,54,57
It has also been suggested that EECs could play a role in the tolerogenic state of the epididymis, as do Sertoli cells in the testis, thanks to their expression of immunosuppressive molecules known to inhibit T cell activation or survival, namely IL-10, IDO, TGF-β, and activin A.94
Indeed, IL-10 is localised in the principal cells of the murine epididymal epithelium, suggesting that this cytokine can be secreted into the interstitial tissue and tubular lumen and can keep the T cells migrating to the organ quiet and inactive (Figure 3a).95
Activin A, a member of the TGF-β superfamily, is another well-known immunomodulatory molecule. Activin A is found expressed in the mouse epididymis,96 as well as in the epithelial cells of the human caput and cauda epididymidis.97
Activin A could act together with the IL-10 in diverting local immune responses towards an anti-inflammatory profile, suppressing effector T cell activation and proliferation by downregulating the expression of costimulatory receptors on the DC surface (Figure 3a).98,99 Activin B, which is emerging for its similar functions to activin A,99 has also been described in the mouse and human epididymis at the protein level and in the rhesus monkey and human epididymis at the mRNA level.97,100,101
As mentioned earlier, IDO is constitutively expressed by murine epididymal epithelial cells, where it could play a dual role as innate effector to ascending pathogens and inducer of tolerance to sperm cells.39,40,41 Moreover, IDO activity induces the release of high amounts of tryptophan metabolites, kynurenines, in the epididymis.40 In an in vitro model, kynurenines are able to kill activated T cells without affecting DCs and show additive suppressing effects on T cell responses (Figure 3b).102 Kynurenines have also been shown to be implicated in the fetal tolerance during pregnancy.103
TGF-β is found in the epididymis of various species. TGF-β1 proteins are present in the rat epididymis in a latent form, whereas active TGF-β3 protein is detected in the corpus epididymidis. Active TGF-β2 is not found, whatever the epididymal region considered.79 Another study has described TGF-β1 and one of its receptors, TGF-βRII, in the epididymis of the marmoset monkey.104 Whereas TGF-β1 is found in the apical cells of the caput and corpus epididymidis, its receptor is found in the principal cells of the same regions, suggesting a paracrine process.104 The mouse epididymis also presents with high Tgf- β1 mRNA levels in the caput and corpus regions,105 and we have recently observed that TGF-β isoforms and receptors are present in murine epididymal cells, with differential patterns of expression in the three regions of the organ (Voisin, unpublished results). Finally, some studies have brought light on the potential mechanisms used by TGF-β that create and maintain a pro-tolerogenic environment towards sperm cells. Indeed, a recent study has revealed that epididymal tolerance to sperm cells partly relies on TGF-β signalling in DCs, as transgenic mice with TGF-βRII-deficient DCs develop epididymal leukocytosis and antisperm antibodies (Figure 3c).106
The second level of protection against immune responses to sperm cells relies on the immune cells populating the tissue.
As regulatory T cells (Treg cells) are the canonical tolerance-inducing cells in peripheral organs, they may be key players in a context of severe tissue injury. Indeed, vasectomy, which is used as a model for investigating immune responses to self-antigens after a leak of sperm antigens into the epididymal interstitium, induces a sperm-specific systemic tolerance, which is abrogated by a simultaneous depletion of the Treg cells.107 The tolerance state is suggested to rely on the presentation of meiotic germ cell antigens (MGCA) to antigen-specific T cells in the draining epididymal lymph node, leading to a rapid expansion of Treg cell activity (Figure 3d).108
Once activated, Treg cells may promote a local epididymal tolerogenic environment to sperm cells, as a study has shown significant increases in Il-10 and Tgf-β mRNA levels in the epididymis following vasectomy.105 These two immunosuppressive molecules secreted by the Treg cells are known to inhibit immune responses, particularly T cell proliferation and differentiation into effector cells (Figure 3d).109 Thus, considering these data and the work by Pierucci-Alves et al.,106 it may be suggested that Tgf-β produced by the newly recruited Treg cells after severe tissue injury may signal, through the DCs, a maintenance of a tolerogenic environment and a limitation of epididymal damage.
Another argument in favour of a possible recruitment of Treg cells in the epididymis is the presence of CD103+ DCs in this organ.54,57 Indeed, gut CD103+ DCs are reported to migrate to the draining lymph nodes where they induce tolerance by promoting the differentiation of naïve T cells into immunosuppressive Treg cells (Figure 3d).66 However, the capacity of gut DCs to induce Treg cells is associated with their expression of the immunosuppressive enzyme IDO.110 Yet, despite IDO being highly expressed in the murine epididymis,39 epithelial cells, but not DCs, from the caput epididymidis were found to express it.40 This apparent paradox will have to be examined.
Another intriguing fact is that there are no or few Treg cells in the steady state epididymis.57,106 This is quite surprising, as transcriptomic data strongly suggest the presence of Treg cells in the murine epididymis.111 This discrepancy may be explained by many regulatory T cell populations having been described, independently of Foxp3 expression, as are Th3 and Tr1 cells.112 This means that there may be unconventional populations of regulatory T cells at steady state, which could play their canonical immunosuppressive roles in helping maintain the tolerogenic state of the murine epididymis.
Altogether these results suggest that Treg cells are not present at steady state, while being massively recruited in the tissue from the draining lymph node after a severe injury leading to a leak of sperm antigens in the epididymal interstitium.
The previous hypothesis implies that distinct mechanisms exist at steady state that maintain the tolerance to sperm cells in the epididymis. One possibility may be the weak activation of tissue effector T cells by local DCs in an immunosuppressive environment, leading to anergy or apoptosis of T cells (Figure 3e).
We were the first to describe an unexpected antigen-specific regulatory T cell population, the epididymal DN T cells, that are significantly present in the caput epididymidis.57 These cells have been mostly described in the female genital tract, the kidney, and the intestine113 where they act as immunosuppressive cells, suggesting that they could play a similar role in the tolerance to spermatozoa. Indeed, as described in the mouse spleen, DN T cells could acquire sperm antigens from local DCs by trogocytosis, a mechanism of membrane transfer between APCs and lymphocytes, to become presenting cells themselves before killing autoreactive CD8+ T cells.114 In fact, they are able to prevent autoimmune responses efficiently by targeting a broad panel of immune cells including cytotoxic CD8+ T cells, helper CD4+ T cells, B cells, NK cells, macrophages, and DCs (Figure 3f).115,116 However, other work on human DN T cells has pointed to mechanisms of action distinct from those of murine DN T cells, supporting the need for further studies on these promising cells.117
CONCLUSIONS
This review has highlighted the wealth of immune mechanisms, proven or suspected, at work in the epididymis that maintain an efficient balance between immune responses to ascending pathogens and tolerance to sperm cells. As often happens in the consideration of immunity, many cells and molecules are suggested to play multiple roles in the epididymis, and their actual implication in the regulation of epididymal immune balance will need to be demonstrated in further studies. For instance, TGF- b isoforms appear to affect (i) BEB maintenance, through the control of epithelial cell growth;79 (ii) BEB function, through the induction of tight junction loss and consequent epithelial permeability to MP migration;118 and (iii) tolerance to sperm cells, through its inhibition of T cell activation.94 Another important focus should also be on the so-far underestimated epididymal fat pad, which seems to be a promising reservoir of immune cells potentially implicated in both sides of the immune balance, although not all species have this fat pad. Concerning this last point, there is a crucial need for animal models as useful tools to decipher the mechanisms underlying the extreme epididymal resistance to tumourigenesis, which could lead to new therapeutic strategies to cure certain cancers. Finally, as human data are dramatically lacking in the field, the transposition of ever-growing animal data to human remains limited, as well as do the opportunities for treating human pathologies associated with epididymal immune defaults.
AUTHOR CONTRIBUTIONS
AV and RG drafted the manuscript. FS and JRD revised the article. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
AV was supported by the French Ministry of Higher Education and Research (MESR). FS, JRD and RG received funds from INSERM, CNRS, and MESR.
REFERENCES
- 1.Dohle GR, Colpi GM, Hargreave TB, Papp GK, Jungwirth A, et al. EAU guidelines on male infertility. Eur Urol. 2005;48:703–11. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Jungwirth A, Giwercman A, Tournaye H, Diemer T, Kopa Z, et al. European Association of Urology guidelines on male infertility: the 2012 update. Eur Urol. 2012;62:324–32. doi: 10.1016/j.eururo.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Harnisch JP, Berger RE, Alexander ER, Monda G, Holmes KK. Aetiology of acute epididymitis. Lancet. 1977;1:819–21. doi: 10.1016/s0140-6736(77)92773-8. [DOI] [PubMed] [Google Scholar]
- 4.Berger RE, Alexander ER, Harnisch JP, Paulsen CA, Monda GD, et al. Etiology, manifestations and therapy of acute epididymitis: prospective study of 50 cases. J Urol. 1979;121:750–4. doi: 10.1016/s0022-5347(17)56978-5. [DOI] [PubMed] [Google Scholar]
- 5.Tozzo PJ. Semen analysis in unilateral epididymitis. N Y State J Med. 1968;68:2769–70. [PubMed] [Google Scholar]
- 6.Ludwig G, Haselberger J. [Epididymitis and fertility. Treatment results in acute unspecific epididymitis] Fortschr Med. 1977;95:397–9. Article in German. [PubMed] [Google Scholar]
- 7.Weidner W, Garbe C, Weissbach L, Harbrecht J, Kleinschmidt K, et al. Initial therapy of acute unilateral epididymitis using ofloxacin. II. Andrological findings] Urologe A. 1990;29:277–80. Article in German. [PubMed] [Google Scholar]
- 8.Dietz O. The change in the degree of fertility during the course of acute nonspecific epididymitis. (Contribution to the pathogenesis of primary inhibition of spermiogenesis) Arch Med Infant. 1960;211:160–6. Article in German. [PubMed] [Google Scholar]
- 9.Osegbe DN. Testicular function after unilateral bacterial epididymo-orchitis. Eur Urol. 1991;19:204–8. doi: 10.1159/000473620. [DOI] [PubMed] [Google Scholar]
- 10.Witkin SS, Jeremias J, Bongiovanni AM, Munoz MG. Immune regulation in the male genital tract. Infect Dis Obstet Gynecol. 1996;4:131–5. doi: 10.1155/S1064744996000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung CH, Wang K, Cooper TG. Why are epididymal tumours so rare? Asian J Androl. 2012;14:465–75. doi: 10.1038/aja.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore CA, Lockett BL, Lennox KW, McAminch JW, Wurster JC, et al. Prednisone in the treatment of acute epididymitis: a cooperative study. J Urol. 1971;106:578–80. doi: 10.1016/s0022-5347(17)61345-4. [DOI] [PubMed] [Google Scholar]
- 13.Trojian TH, Lishnak TS, Heiman D. Epididymitis and orchitis: an overview. Am Fam Physician. 2009;79:583–7. [PubMed] [Google Scholar]
- 14.Head JR, Neaves WB, Billingham RE. Immune privilege in the testis. I. Basic parameters of allograft survival. Transplantation. 1983;36:423–31. doi: 10.1097/00007890-198310000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Head JR, Billingham RE. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation. 1985;40:269–75. doi: 10.1097/00007890-198509000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Kazeem AA. A critical consideration of the rat epididymis as an immunologically privileged site. Scand J Immunol. 1988;27:149–56. doi: 10.1111/j.1365-3083.1988.tb02333.x. [DOI] [PubMed] [Google Scholar]
- 17.Mital P, Hinton BT, Dufour JM. The blood-testis and blood-epididymis barriers are more than just their tight junctions. Biol Reprod. 2011;84:851–8. doi: 10.1095/biolreprod.110.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffer AP, Hinton BT. Morphological evidence for a blood-epididymis barrier and the effects of gossypol on its integrity. Biol Reprod. 1984;30:991–1004. doi: 10.1095/biolreprod30.4.991. [DOI] [PubMed] [Google Scholar]
- 19.Cooper TG, Waites GM. Investigation by luminal perfusion of the transfer of compounds into the epididymis of the anaesthetized rat. J Reprod Fertil. 1979;56:159–64. doi: 10.1530/jrf.0.0560159. [DOI] [PubMed] [Google Scholar]
- 20.Hinton BT, Howards SS. Permeability characteristics of the epithelium in the rat caput epididymidis. J Reprod Fertil. 1981;63:95–9. doi: 10.1530/jrf.0.0630095. [DOI] [PubMed] [Google Scholar]
- 21.Palladino MA, Johnson TA, Gupta R, Chapman JL, Ojha P. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod. 2007;76:958–64. doi: 10.1095/biolreprod.106.059410. [DOI] [PubMed] [Google Scholar]
- 22.Palladino MA, Savarese MA, Chapman JL, Dughi MK, Plaska D. Localization of Toll-like receptors on epididymal epithelial cells and spermatozoa. Am J Reprod Immunol. 2008;60:541–55. doi: 10.1111/j.1600-0897.2008.00654.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhao YT, Guo JH, Wu ZL, Xiong Y, Zhou WL. Innate immune responses of epididymal epithelial cells to Staphylococcus aureus infection. Immunol Lett. 2008;119:84–90. doi: 10.1016/j.imlet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues A, Queiróz DB, Honda L, Silva EJ, Hall SH, et al. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia coli. Biol Reprod. 2008;79:1135–47. doi: 10.1095/biolreprod.108.069930. [DOI] [PubMed] [Google Scholar]
- 25.Cheng L, Chen Q, Zhu W, Wu H, Wang Q, et al. Toll-like receptors 4 and 5 cooperatively initiate the innate immune responses to uropathogenic Escherichia coli infection in mouse epididymal epithelial cells. Biol Reprod. 2016;94:58. doi: 10.1095/biolreprod.115.136580. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Nii T, Isobe N, Yoshimura Y. Expression of toll-like receptors and effects of lipopolysaccharide on the expression of proinflammatory cytokines and chemokine in the testis and epididymis of roosters. Poult Sci. 2012;91:1997–2003. doi: 10.3382/ps.2012-02236. [DOI] [PubMed] [Google Scholar]
- 27.Anastasiadou M, Avdi M, Michailidis G. Expression of avian β-defensins and Toll-like receptor genes in the rooster epididymis during growth and Salmonella infection. Anim Reprod Sci. 2013;140:224–31. doi: 10.1016/j.anireprosci.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhu W, Zhao S, Liu Z, Cheng L, Wang Q, et al. Pattern recognition receptor-initiated innate antiviral responses in mouse epididymal epithelial cells. J Immunol. 2015;194:4825–35. doi: 10.4049/jimmunol.1402706. [DOI] [PubMed] [Google Scholar]
- 29.Browne JA, Leir SH, Eggener SE, Harris A. Region-specific innate antiviral responses of the human epididymis. Mol Cell Endocrinol. 2018;473:72–8. doi: 10.1016/j.mce.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall SH, Hamil KG, French FS. Host defense proteins of the male reproductive tract. J Androl. 2002;23:585–97. [PubMed] [Google Scholar]
- 31.Com E, Bourgeon F, Evrard B, Ganz T, Colleu D, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro CM, Silva EJ, Hinton BT, Avellar MC. β-defensins and the epididymis: contrasting influences of prenatal, postnatal, and adult scenarios. Asian J Androl. 2016;18:323–8. doi: 10.4103/1008-682X.168791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, et al. Interaction of human defensins with Escherichia coli.Mechanism of bactericidal activity. J Clin Invest. 1989;84:553–61. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yenugu S, Hamil KG, Radhakrishnan Y, French FS, Hall SH. The androgen-regulated epididymal sperm-binding protein, human beta-defensin 118 (DEFB118) (formerly ESC42), is an antimicrobial beta-defensin. Endocrinology. 2004;145:3165–73. doi: 10.1210/en.2003-1698. [DOI] [PubMed] [Google Scholar]
- 35.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 36.Malm J, Nordahl EA, Bjartell A, Sørensen OE, Frohm B, et al. Lipopolysaccharide-binding protein is produced in the epididymis and associated with spermatozoa and prostasomes. J Reprod Immunol. 2005;66:33–43. doi: 10.1016/j.jri.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Collin M, Linge HM, Bjartell A, Giwercman A, Malm J, et al. Constitutive expression of the antibacterial CXC chemokine GCP-2/CXCL6 by epithelial cells of the male reproductive tract. J Reprod Immunol. 2008;79:37–43. doi: 10.1016/j.jri.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Linge HM, Collin M, Giwercman A, Malm J, Bjartell A, et al. The antibacterial chemokine MIG/CXCL9 is constitutively expressed in epithelial cells of the male urogenital tract and is present in seminal plasma. J Interferon Cytokine Res. 2008;28:191–6. doi: 10.1089/jir.2007.0100. [DOI] [PubMed] [Google Scholar]
- 39.Britan A, Maffre V, Tone S, Drevet JR. Quantitative and spatial differences in the expression of tryptophan-metabolizing enzymes in mouse epididymis. Cell Tissue Res. 2006;324:301–10. doi: 10.1007/s00441-005-0151-7. [DOI] [PubMed] [Google Scholar]
- 40.Jrad-Lamine A, Henry-Berger J, Gourbeyre P, Damon-Soubeyrand C, Lenoir A, et al. Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J Biol Chem. 2011;286:8030–42. doi: 10.1074/jbc.M110.172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takikawa O, Tagawa Y, Iwakura Y, Yoshida R, Truscott RJ. Interferon-gamma-dependent/independent expression of indoleamine 2,3-dioxygenase. Studies with interferon-gamma-knockout mice. Adv Exp Med Biol. 1999;467:553–7. doi: 10.1007/978-1-4615-4709-9_68. [DOI] [PubMed] [Google Scholar]
- 42.Divanovic S, Sawtell NM, Trompette A, Warning JI, Dias A, et al. Opposing biological functions of tryptophan catabolizing enzymes during intracellular infection. J Infect Dis. 2012;205:152–61. doi: 10.1093/infdis/jir621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlin JM, Borden EC, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity inhibits Chlamydia psittaci replication in human macrophages. J Interferon Res. 1989;9:329–37. doi: 10.1089/jir.1989.9.329. [DOI] [PubMed] [Google Scholar]
- 44.Pantoja LG, Miller RD, Ramirez JA, Molestina RE, Summersgill JT. Inhibition of Chlamydia pneumoniae replication in human aortic smooth muscle cells by gamma interferon-induced indoleamine 2, 3-dioxygenase activity. Infect Immun. 2000;68:6478–81. doi: 10.1128/iai.68.11.6478-6481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obojes K, Andres O, Kim KS, Däubener W, Schneider-Schaulies J. Indoleamine 2,3-dioxygenase mediates cell type-specific anti-measles virus activity of gamma interferon. J Virol. 2005;79:7768–76. doi: 10.1128/JVI.79.12.7768-7776.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang YF, Holstein AF. Intraepithelial lymphocytes and macrophages in the human epididymis. Cell Tissue Res. 1983;233:517–21. doi: 10.1007/BF00212221. [DOI] [PubMed] [Google Scholar]
- 47.Ritchie AW, Hargreave TB, James K, Chisholm GD. Intra-epithelial lymphocytes in the normal epididymis. A mechanism for tolerance to sperm auto-antigens? Br J Urol. 1984;56:79–83. doi: 10.1111/j.1464-410x.1984.tb07169.x. [DOI] [PubMed] [Google Scholar]
- 48.Yakirevich E, Yanai O, Sova Y, Sabo E, Stein A, et al. Cytotoxic phenotype of intra-epithelial lymphocytes in normal and cryptorchid human testicular excurrent ducts. Hum Reprod. 2002;17:275–83. doi: 10.1093/humrep/17.2.275. [DOI] [PubMed] [Google Scholar]
- 49.Hooper P, Smythe E, Richards RC, Howard CV, Lynch RV, et al. Total number of immunocompetent cells in the normal rat epididymis and after vasectomy. J Reprod Fertil. 1995;104:193–8. doi: 10.1530/jrf.0.1040193. [DOI] [PubMed] [Google Scholar]
- 50.Flickinger CJ, Bush LA, Howards SS, Herr JC. Distribution of leukocytes in the epithelium and interstitium of four regions of the Lewis rat epididymis. Anat Rec. 1997;248:380–90. doi: 10.1002/(SICI)1097-0185(199707)248:3<380::AID-AR11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 51.Serre V, Robaire B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol Reprod. 1999;61:705–14. doi: 10.1095/biolreprod61.3.705. [DOI] [PubMed] [Google Scholar]
- 52.Nashan D, Malorny U, Sorg C, Cooper T, Nieschlag E. Immuno-competent cells in the murine epididymis. Int J Androl. 1989;12:85–94. doi: 10.1111/j.1365-2605.1989.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 53.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, et al. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–8. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Da Silva N, Cortez-Retamozo V, Reinecker HC, Wildgruber M, Hill E, et al. A dense network of dendritic cells populates the murine epididymis. Reproduction. 2011;141:653–63. doi: 10.1530/REP-10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, et al. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod. 2014;90:90. doi: 10.1095/biolreprod.113.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith TB, Cortez-Retamozo V, Grigoryeva LS, Hill E, Pittet MJ, et al. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology. 2014;2:755–62. doi: 10.1111/j.2047-2927.2014.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voisin A, Whitfield M, Damon-Soubeyrand C, Goubely C, Henry-Berger J, et al. Comprehensive overview of murine epididymal mononuclear phagocytes and lymphocytes: unexpected populations arise. J Reprod Immunol. 2018;126:11–7. doi: 10.1016/j.jri.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Stern JE, Gardner S, Quirk D, Wira CR. Secretory immune system of the male reproductive tract: effects of dihydrotestosterone and estradiol on IgA and secretory component levels. J Reprod Immunol. 1992;22:73–85. doi: 10.1016/0165-0378(92)90007-q. [DOI] [PubMed] [Google Scholar]
- 59.Shibuya A, Sakamoto N, Shimizu Y, Shibuya K, Osawa M, et al. Fcα/μ receptor mediates endocytosis of IgM-coated microbes. Nat Immunol. 2000;1:441–6. doi: 10.1038/80886. [DOI] [PubMed] [Google Scholar]
- 60.Kuzan FB, Patton DL, Allen SM, Kuo CC. A proposed mouse model for acute epididymitis provoked by genital serovar E, Chlamydia trachomatis. Biol Reprod. 1989;40:165–72. doi: 10.1095/biolreprod40.1.165. [DOI] [PubMed] [Google Scholar]
- 61.Jantos C, Baumgärtner W, Durchfeld B, Schiefer HG. Experimental epididymitis due to Chlamydia trachomatis in rats. Infect Immun. 1992;60:2324–8. doi: 10.1128/iai.60.6.2324-2328.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nashan D, Jantos C, Ahlers D, Bergmann M, Schiefer HG, et al. Immuno-competent cells in the murine epididymis following infection with Escherichia coli. Int J Androl. 1993;16:47–52. doi: 10.1111/j.1365-2605.1993.tb01152.x. [DOI] [PubMed] [Google Scholar]
- 63.Ludwig M, Johannes S, Bergmann M, Failing K, Schiefer HG, et al. Experimental Escherichia coli epididymitis in rats: a model to assess the outcome of antibiotic treatment. BJU Int. 2002;90:933–8. doi: 10.1046/j.1464-410x.2002.03029.x. [DOI] [PubMed] [Google Scholar]
- 64.Duan YG, Wang P, Zheng W, Zhang Q, Huang W, et al. Characterisation of dendritic cell subsets in chronically inflamed human epididymis. Andrologia. 2016;48:431–40. doi: 10.1111/and.12463. [DOI] [PubMed] [Google Scholar]
- 65.Zheng W, Wang Y, Chen J, Zhu Y, Li M, et al. Identification and function analysis of macrophages subsets in chronically inflamed human epididymis. Int J Clin Exp Pathol. 2017;10:2461–77. [Google Scholar]
- 66.Niess JH, Brand S, Gu X, Landsman L, Jung S, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 67.Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, et al. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–14. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 69.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, et al. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation. 2008;31:167–79. doi: 10.1007/s10753-008-9062-6. [DOI] [PubMed] [Google Scholar]
- 70.Mildner A, Marinkovic G, Jung S. Murine monocytes: origins, subsets, fates, and functions. Microbiol Spectr. 2016;4:MCHD. doi: 10.1128/microbiolspec.MCHD-0033-2016. [DOI] [PubMed] [Google Scholar]
- 71.Turner TT, Mammen T, Kavoussi P, Lysiak JJ, Costabile RA. Cytokine responses to E.coli-induced epididymitis in the rat: blockade by vasectomy. Urology. 2011;77:1507.e9–14. doi: 10.1016/j.urology.2011.02.037. [DOI] [PubMed] [Google Scholar]
- 72.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579:3487–92. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 73.Kondo T, Toyoshima Y, Ishii Y, Kyuwa S. Natural killer T cells in adipose tissue are activated in lean mice. Exp Anim. 2013;62:319–28. doi: 10.1538/expanim.62.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pérez-Clavier R, Harrison RG, Macmillian EW. The pattern of the lymphatic drainage of the rat epididymis. J Anat. 1982;134:667–75. [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki F. Microvasculature of the mouse testis and excurrent duct system. Am J Anat. 1982;163:309–25. doi: 10.1002/aja.1001630404. [DOI] [PubMed] [Google Scholar]
- 76.Steinert EM, Thompson EA, Beura LK, Adam OA, Mitchell JS, et al. Cutting edge: evidence for nonvascular route of visceral organ immunosurveillance by T cells. J Immunol. 2018;201:337–42. doi: 10.4049/jimmunol.1800279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beccia DJ, Krane RJ, Olsson CA. Clinical management of non-testicular intrascrotal tumors. J Urol. 1976;116:476–9. doi: 10.1016/s0022-5347(17)58867-9. [DOI] [PubMed] [Google Scholar]
- 78.Mandon M, Hermo L, Cyr DG. Isolated rat epididymal basal cells share common properties with adult stem cells. Biol Reprod. 2015;93:115. doi: 10.1095/biolreprod.115.133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desai KV, Flanders KC, Kondaiah P. Expression of transforming growth factor-beta isoforms in the rat male accessory sex organs and epididymis. Cell Tissue Res. 1998;294:271–7. doi: 10.1007/s004410051177. [DOI] [PubMed] [Google Scholar]
- 80.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 81.Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15:683–91. doi: 10.1038/nri3904. [DOI] [PubMed] [Google Scholar]
- 82.Lee J, Minden MD, Chen WC, Streck E, Chen B, et al. Allogeneic human double negative T cells as a novel immunotherapy for acute myeloid leukemia and its underlying mechanisms. Clin Cancer Res. 2018;24:370–82. doi: 10.1158/1078-0432.CCR-17-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Young KJ, Kay LS, Phillips MJ, Zhang L. Antitumor activity mediated by double-negative T cells. Cancer Res. 2003;63:8014–21. [PubMed] [Google Scholar]
- 84.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 85.Jhappan C, Gallahan D, Stahle C, Chu E, Smith GH, et al. Expression of an activated Notch-related int-3 transgene interferes with cell differentiation and induces neoplastic transformation in mammary and salivary glands. Genes Dev. 1992;6:345–55. doi: 10.1101/gad.6.3.345. [DOI] [PubMed] [Google Scholar]
- 86.Stöcklin E, Botteri F, Groner B. An activated allele of the c-erbB-2 oncogene impairs kidney and lung function and causes early death of transgenic mice. J Cell Biol. 1993;122:199–208. doi: 10.1083/jcb.122.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guy CT, Cardiff RD, Muller WJ. Activated neu induces rapid tumor progression. J Biol Chem. 1996;271:7673–8. doi: 10.1074/jbc.271.13.7673. [DOI] [PubMed] [Google Scholar]
- 88.Gilbert E, Morel A, Tulliez M, Maunoury R, Terzi F, et al. In vivo effects of activated H-ras oncogene expressed in the liver and in urogenital tissues. Int J Cancer. 1997;73:749–56. doi: 10.1002/(sici)1097-0215(19971127)73:5<749::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 89.Korpelainen EI, Karkkainen MJ, Tenhunen A, Lakso M, Rauvala H, et al. Overexpression of VEGF in testis and epididymis causes infertility in transgenic mice: evidence for nonendothelial targets for VEGF. J Cell Biol. 1998;143:1705–12. doi: 10.1083/jcb.143.6.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.La Perle KM, Blomme EA, Sagartz JE, Capen CC. Epididymal cribriform hyperplasia with nuclear atypia in p53 homozygous knockout mice on a mixed 129/Sv-FVB/N background. Comp Med. 2002;52:568–71. [PubMed] [Google Scholar]
- 91.Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, et al. Activation of β-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–87. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- 92.Sipilä P, Shariatmadari R, Huhtaniemi IT, Poutanen M. Immortalization of epididymal epithelium in transgenic mice expressing simian virus 40 T antigen: characterization of cell lines and regulation of the polyoma enhancer activator 3. Endocrinology. 2004;145:437–46. doi: 10.1210/en.2003-0831. [DOI] [PubMed] [Google Scholar]
- 93.Frew IJ, Minola A, Georgiev S, Hitz M, Moch H, et al. Combined Vhlh and Pten mutation causes genital tract cystadenoma and squamous metaplasia. Mol Cell Biol. 2008;28:4536–48. doi: 10.1128/MCB.02132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hedger MP. Immunophysiology and pathology of inflammation in the testis and epididymis. J Androl. 2011;32:625–40. doi: 10.2164/jandrol.111.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Veräjänkorva E, Pöllänen P, Hänninen A, Martikainen M, Sundström J, et al. Il-10 is highly expressed in the cryptorchid cryptepididymal epithelium: a probable mechanism preventing immune responses against autoantigenic spermatozoa in the epididymal tubule. Int J Androl. 2002;25:129–33. doi: 10.1046/j.1365-2605.2002.00336.x. [DOI] [PubMed] [Google Scholar]
- 96.Winnall WR, Wu H, Sarraj MA, Rogers PA, de Kretser DM, et al. Expression patterns of activin, inhibin and follistatin variants in the adult male mouse reproductive tract suggest important roles in the epididymis and vas deferens. Reprod Fertil Dev. 2013;25:570–80. doi: 10.1071/RD11287. [DOI] [PubMed] [Google Scholar]
- 97.Bahathiq AO, Stewart RL, Baxter L, Wells M, Moore HD, et al. Tissue immunoexpression and messenger ribonucleic acid localization of inhibin/activin subunit in human epididymis. Fertil Steril. 2005;83:78–85. doi: 10.1016/j.fertnstert.2004.05.104. [DOI] [PubMed] [Google Scholar]
- 98.Segerer SE, Müller N, van den Brandt J, Kapp M, Dietl J, et al. The glycoprotein-hormones activin A and inhibin A interfere with dendritic cell maturation. Reprod Biol Endocrinol. 2008;6:17. doi: 10.1186/1477-7827-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hedger MP, Winnall WR, Phillips DJ, de Kretser DM. The regulation and functions of activin and follistatin in inflammation and immunity. Vitam Horm. 2011;85:255–97. doi: 10.1016/B978-0-12-385961-7.00013-5. [DOI] [PubMed] [Google Scholar]
- 100.Wijayarathna R, de Kretser DM, Sreenivasan R, Ludlow H, Middendorff R, et al. Comparative analysis of activins A and B in the adult mouse epididymis and vas deferens. Reproduction. 2018;155:15–23. doi: 10.1530/REP-17-0485. [DOI] [PubMed] [Google Scholar]
- 101.Zhang T, Guo CX, Hu ZY, Liu YX. Localization of plasminogen activator and inhibitor, LH and androgen receptors and inhibin subunits in monkey epididymis. Mol Hum Reprod. 1997;3:945–52. doi: 10.1093/molehr/3.11.945. [DOI] [PubMed] [Google Scholar]
- 102.Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med. 2002;196:447–57. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu BT. Development of selective immune tolerance towards the allogeneic fetus during pregnancy: role of tryptophan catabolites. Int J Mol Med. 2010;25:831–5. doi: 10.3892/ijmm_00000411. [DOI] [PubMed] [Google Scholar]
- 104.Bomgardner D, Wehrenberg U, Rune GM. Tgf-β could be involved in paracrine actions in the epididymis of the marmoset monkey (Callithrix jacchus) J Androl. 1999;20:375–83. [PubMed] [Google Scholar]
- 105.Oh YS, Na EJ, Gye MC. Effects of bilateral vasectomy on the interleukin 1 system in mouse epididymis. Am J Reprod Immunol. 2016;76:235–42. doi: 10.1111/aji.12548. [DOI] [PubMed] [Google Scholar]
- 106.Pierucci-Alves F, Midura-Kiela MT, Fleming SD, Schultz BD, Kiela PR. Transforming growth factor beta signaling in dendritic cells is required for immunotolerance to sperm in the epididymis. Front Immunol. 2018;9:1882. doi: 10.3389/fimmu.2018.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wheeler K, Tardif S, Rival C, Luu B, Bui E, et al. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc Natl Acad Sci U S A. 2011;108:7511–6. doi: 10.1073/pnas.1017615108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rival C, Wheeler K, Jeffrey S, Qiao H, Luu B, et al. Regulatory T cells and vasectomy. J Reprod Immunol. 2013;100:66–75. doi: 10.1016/j.jri.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Toda A, Piccirillo CA. Development and function of naturally occurring CD4+ CD25+ regulatory T cells. J Leukoc Biol. 2006;80:458–70. doi: 10.1189/jlb.0206095. [DOI] [PubMed] [Google Scholar]
- 110.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, et al. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 111.Johnston DS, Jelinsky SA, Bang HJ, DiCandeloro P, Wilson E, et al. The mouse epididymal transcriptome: transcriptional profiling of segmental gene expression in the epididymis. Biol Reprod. 2005;73:404–13. doi: 10.1095/biolreprod.105.039719. [DOI] [PubMed] [Google Scholar]
- 112.Shevach EM, DiPaolo RA, Andersson J, Zhao DM, Stephens GL, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 113.Martina MN, Noel S, Saxena A, Rabb H, Hamad AR. Double negative (DN) αβ T cells: misperception and overdue recognition. Immunol Cell Biol. 2015;93:305–10. doi: 10.1038/icb.2014.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang ZX, Yang L, Young KJ, DuTemple B, Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000;6:782–9. doi: 10.1038/77513. [DOI] [PubMed] [Google Scholar]
- 115.Gao JF, McIntyre MS, Juvet SC, Diao J, Li X, et al. Regulation of antigen-expressing dendritic cells by double negative regulatory T cells. Eur J Immunol. 2011;41:2699–708. doi: 10.1002/eji.201141428. [DOI] [PubMed] [Google Scholar]
- 116.Hillhouse EE, Lesage S. A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. J Autoimmun. 2013;40:58–65. doi: 10.1016/j.jaut.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 117.Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-αβ+ CD4- CD8- double-negative T cells. Eur J Immunol. 2011;41:739–48. doi: 10.1002/eji.201040982. [DOI] [PubMed] [Google Scholar]
- 118.Stammler A, Müller D, Tabuchi Y, Konrad L, Middendorff R. TGFβs modulate permeability of the blood-epididymis barrier in an in vitro model. PLoS One. 2013;8:e80611. doi: 10.1371/journal.pone.0080611. [DOI] [PMC free article] [PubMed] [Google Scholar]