Abstract
Recommendations for managing clinically localized prostate cancer are structured around clinical risk criteria, with prostate biopsy (PB) Gleason score (GS) being the most important factor. Biopsy to radical prostatectomy (RP) specimen upgrading/downgrading is well described, and is often the rationale for costly imaging or genomic studies. We present simple, no-cost analyses of clinical parameters to predict which GS 6 and GS 8 patients will change to GS 7 at prostatectomy. From May 2006 to December 2012, 1590 patients underwent robot-assisted radical prostatectomy (RARP). After exclusions, we identified a GS 6 cohort of 374 patients and a GS 8 cohort of 91 patients. During this era, >1000 additional patients were enrolled in an active surveillance (AS) program. For GS 6, 265 (70.9%) of 374 patients were upgraded, and the cohort included 183 (48.9%) patients eligible for AS by the Prostate Cancer Research International Active Surveillance Study (PRIAS) standards, of which 57.9% were upgraded. PB features that predicted a >90% chance of upgrading included ≥7 cores positive, maximum foci length ≥8 mm in any core, and total tumor involvement ≥30%. For GS 8, downgrading occurred in 46 (50.5%), which was significantly higher for single core versus multiple cores (80.4% vs 19.6%, P = 0.011). Biochemical recurrence (BCR) occurred in 3.4% of GS 6 upgraded versus 0% nonupgraded, and in GS 8, 19.6% downgraded versus 42.2% nondowngraded. In counseling men with clinically localized prostate cancer, the odds of GS change should be presented, and certain men with high-volume GS 6 or low-volume GS 8 can be counseled with GS 7-based recommendations.
Keywords: biopsy, downgrade, Gleason score, prostate cancer, upgrade
INTRODUCTION
Decision-making in newly diagnosed, clinically localized prostate cancer (PCa) is often organized by risk classification, which is a combination of Gleason score (GS), prostate-specific antigen (PSA), and clinical stage.1,2 Of those three, the GS tends to have the strongest prognostic power for predicting pathologic stage and disease recurrence.1,2 An early decision is whether or not a patient is best suited for active surveillance (AS), active intervention, or perhaps combination therapy – the latter possibly on a clinical trial.3 In simplified thinking, low-risk disease may have AS, intermediate-risk disease may have monotherapy (surgery or radiation), and high-risk disease may have multimodality therapy/clinical trials. However, with full case details, patients will certainly crossover, i.e., some intermediate risk to AS and some high risk to monotherapy. Such details can include the number of positive cores and length of tumor foci (or percentage cancer in a core).4 Recent studies have refined risk classifications: intermediate-risk disease can be classified into favorable versus unfavorable,5 and high-risk disease can be high-versus very high-risk disease.4,6 Additional prognostic information can be gained from magnetic resonance imaging (MRI) staging and/or genomic testing,7 but these come at an additional cost.
We have observed that certain patterns of low-risk disease are commonly upgraded to intermediate grade at RP and should possibly treated as intermediate-risk disease in clinical decision-making. At the same time, we have observed high-risk disease that is downgraded to intermediate-risk disease at surgery and should be managed as such. The observations are similar to the statistical term “regression to the mean” which states that extreme values often merge back to the mean when re-measured. In this article, we sought to explore the observation whereby simple biopsy patterns can be analyzed for prognostic power that would change clinical risk classification.
PATIENTS AND METHODS
We performed a retrospective analysis of prospectively entered data from a single tertiary cancer hospital (MD Anderson Cancer Center, Houston, TX, USA) involving multiple surgeons. Institutional Review Board's approval was obtained from MD Anderson Cancer Center, DR 10-0554; patients signed general clinical data use consent and the protocol included a waiver of study specific consent. From May 2006 to December 2012, 1590 patients underwent robot-assisted radical prostatectomy (RARP). In this same era, >1000 additional patients were enrolled in an AS program. Key inclusion criteria were for two cohorts: biopsy GS (3+3) undergoing RARP (n = 396) and biopsy GS (4+4) undergoing RARP (n = 145). Exclusion criteria included neoadjuvant hormones or chemotherapy (n = 67), diagnosed with transurethral prostate resection (n = 9), or biopsy Gleason pattern 5. After exclusions, 374 patients in the GS (3+3) cohort and 91 patients in the GS (4+4) were eligible for final analysis. All outside biopsies were re-reviewed by a small core of genitourinary pathologists, and all RP specimens were read by a single experienced genitourinary pathologist (ISUP 2005 criteria8). Data abstraction included demographics, patient and disease characteristics, and the Prostate Cancer Research International Active Surveillance Study (PRIAS) Active Surveillance criteria (T1c–T2, PSA ≤10 ng ml−1, one or two positive cores, prostate-specific antigen density [PSAD] <0.2 ng ml−1).9 We noted biopsy features such as high-grade prostatic intraepithelial neoplasia (HGPIN) and/or atypical small acinar proliferation (ASAP) status. We measured all possible ratios of biopsy core and tumor foci metrics, i.e., number of positive cores, percent of positive cores, maximum positive core length, percent of max positive core length in the core, total positive core length, and percentage of PCa in the specimen (total length of the positive core/total core length). In addition, associated presence of GS 6–7 score was evaluated in GS (4+4) patients (as opposed to all cores GS [4+4]). Postoperative evaluation included GS changes, pathological stage, and biochemical recurrence (BCR). Patients with a PSA level >0.2 ng ml−1 were considered as BCR after RARP during the follow-up period.
Of note, this cohort is predominately in the perfusion biopsy era, and most patients would have had only 10–12 core systematic biopsies performed in or out of the institution, with all cases having outside pathology re-reviewed.
Statistical analyses
Statistical analysis was performed using STATA/SE version 14.1 statistical software (Stata Corp., LP, College Station, TX, USA). Descriptive statistics were used to summarize the study population. Student' s t-test (or Wilcoxon's rank sum) and Person's Chi-square test (or Fisher's exact test) were used to find association between groups. Univariate logistic regression methods and receiver operating characteristics (ROC) were used to determine the best cutoff points for continuous variables. Kaplan–Meier methods were used to determine BCR-free survival rates in each group. Statistical significance was considered as P < 0.05.
RESULTS
Gleason score 6 (3+3) cohort
Patients' characteristics for GS 6 (3+3) and upgrade status are shown in Table 1. The overall incidence of biopsy GS 6 upgrading was 265 (70.9%) of 374 patients, of which most were to GS (3+4) (90.9%, n = 241) followed by GS (4+3) (7.2%, n = 19) and GS > (4+3) (1.9%, n = 5). In the era of this cohort, patients with low-risk disease were recommended to consider AS on a protocol or per clinical guidelines. However, some chose RARP, and 183 (48.9%) of the 374 cases were eligible for AS by PRIAS standards, and 57.9% of these patients were also upgraded. Higher age, PSA, PSAD, clinical and pathological stage, number of positive cores, percent of positive cores, maximum positive core length, percentage of tumor in maximum positive core, total positive core length, percentage of PCa in total specimen, and lower number of biopsy cores were associated with GS upgrade in univariate analysis. Upgrading was detected in a significantly lower percentage of Hispanics (Table 1).
Table 1.
Patients’ characteristics of Gleason score (3+3) and upgrade status
| Characteristics | Total | Gleason score upgrade | ||
|---|---|---|---|---|
| No | Yes | P | ||
| Patients, n (%) | 374 (100) | 109 (29.1) | 265 (70.9) | |
| Age (year), median (IQR) | 58 (53–63) | 56 (52–61) | 59 (54–64) | 0.001* |
| Ethnicity, n (%) | 0.010* | |||
| Caucasian | 301 (80.5) | 81 (26.9) | 220 (73.1) | |
| African-American | 36 (9.6) | 8 (22.2) | 28 (77.8) | |
| Hispanic | 31 (8.3) | 16 (51.6) | 15 (48.4) | |
| Other | 6 (1.6) | 4 (66.7) | 2 (33.3) | |
| PSA (ng ml−1), median (IQR) | 4.8 (3.6–6.4) | 4.2 (3.0–5.4) | 5.1 (3.9–6.6) | 0.001* |
| Prostate volume (ml), median (IQR) | 35 (26–46) | 37 (30–45) | 33 (25–46) | 0.064 |
| PSAD (ng ml−1), median (IQR) | 0.13 (0.09–0.20) | 0.11 (0.08–0.15) | 0.15 (0.10–0.20) | <0.001* |
| BMI (kg m−2), median (IQR) | 28 (25–31) | 27 (26–30) | 28 (25–31) | 0.231 |
| Clinical stage, n (%) | 0.014* | |||
| cT1 | 325 (86.9) | 102 (31.4) | 223 (68.6) | |
| cT2 | 49 (13.1) | 7 (14.3) | 42 (85.7) | |
| Number of biopsy cores, median (IQR) | 12 (10–12) | 12 (11–12) | 12 (10–12) | 0.022* |
| Number of positive cores, median (IQR) | 2 (1–3) | 1 (1–2) | 2 (1–3) | <0.001* |
| Number of positive cores/total biopsy cores (%), median (IQR) | 17 (8–29) | 11 (8–17) | 17 (10–33) | <0.001* |
| Max positive core length (mm), median (IQR) | 2 (1–4) | 2 (1–3) | 3 (2–5) | <0.001* |
| Maximum positive core length/total core length (%), median (IQR) | 15 (8–29) | 11 (7–20) | 19 (10–31) | <0.001* |
| Total positive core length (mm), median (IQR) | 3 (2–7) | 2 (1–4) | 4 (2–8) | <0.001* |
| Percentage of PCa (total positive cores length/total cores length), median (IQR) | 4 (1–10) | 3 (1–7) | 5 (2–13) | <0.001* |
| ASAP/HGPIN status, n (%) | <0.001* | |||
| None | 203 (54.3) | 69 (34.0) | 134 (66.0) | |
| HGPIN and/or ASAP | 171 (45.7) | 40 (23.4) | 131 (76.6) | |
| Patients meet PRIAS criteria, n (%) | <0.001* | |||
| Eligible | 183 (48.9) | 77 (42.1) | 106 (57.9) | |
| Noneligible | 191 (51.1) | 32 (16.8) | 159 (83.2) | |
| Pathological stage, n (%) | 0.007* | |||
| pT2 | 352 (94.1) | 108 (30.7) | 244 (69.3) | |
| pT3 | 22 (5.9) | 1 (4.5) | 21 (95.5) | |
*P<0.05 was considered statistically significant. ASAP: atypical small acinar proliferation; BMI: body mass index; HGPIN: high-grade prostatic intraepithelial neoplasia; IQR: interquartile range; PCa: prostate cancer; PRIAS: Prostate Cancer Research International Active Surveillance Study; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density
Sensitivity and specificity analyses for the best cutoff points were performed. The estimated cutoff values were age ≥60 years (sensitivity 46.4%, specificity 71.6%, P = 0.001), PSA ≥4.3 ng ml−1 (sensitivity 67.2%, specificity 52.3%, P < 0.001), PSAD ≥0.13 ng ml−1 (sensitivity 62.4%, specificity 63.8%, P < 0.001), total number of biopsy cores ≥12 (sensitivity 62.5%, specificity 28.4%, P = 0.095), number of positive cores ≥2 (sensitivity 59.6%, specificity 57.8%, P = 0.002), maximum core positive length ≥2 mm (sensitivity 67.6%, specificity 56.0%, P < 0.001), percent of tumor in maximum positive core ≥13% (sensitivity 67.2%, specificity 53.1%, P < 0.001), and percent of PCa in total specimen ≥4% (sensitivity 57.9%, specificity 57.8%, P = 0.006) (Table 2). In multivariate logistic regression analyses, higher age and clinical stage, PSAD ≥0.13 ng ml-1, and presence of HGPIN and/or ASAP predicted upgrade from GS (3+3) to higher GS at RP. Hispanic ethnicity seems to be protective for GS 6 upgrade (Table 3). The correlation between number of core+ and maximum tumor length in any foci was also evaluated and shown in Table 4.
Table 2.
Sensitivity and specificity analyses for Gleason score (3+3) upgrade
| Characteristics | No upgrade, n (%) | Upgraded, n (%) | P | Sensitivity (%) | Specificity (%) | Correctly classify (%) | ROC area |
|---|---|---|---|---|---|---|---|
| Core+ ≥2 | 0.002* | 59.6 | 57.8 | 59.1 | 0.59 | ||
| No | 63 (37.1) | 107 (62.9) | |||||
| Yes | 46 (22.5) | 158 (77.5) | |||||
| Maximum core+ length ≥2 mm | <0.001* | 67.6 | 56.0 | 64.1 | 0.62 | ||
| No | 61 (42.1) | 84 (57.9) | |||||
| Yes | 48 (21.5) | 175 (78.5) | |||||
| Percentage of tumor in maximum core+ ≥13% | <0.001* | 67.2 | 53.1 | 63.1 | 0.60 | ||
| No | 58 (40.0) | 87 (60.0) | |||||
| Yes | 51 (22.3) | 178 (77.7) | |||||
| Percentage of PCa in total specimen ≥4% | 0.006* | 57.9 | 57.8 | 57.9 | 0.58 | ||
| No | 63 (37.1) | 107 (62.9) | |||||
| Yes | 46 (23.8) | 147 (76.2) | |||||
| Age ≥60 years | 0.001* | 46.4 | 71.6 | 53.7 | 0.59 | ||
| No | 78 (35.5) | 142 (64.5) | |||||
| Yes | 31 (20.1) | 123 (79.9) | |||||
| PSA ≥4.3 ng ml−1 | <0.001* | 67.2 | 52.3 | 62.8 | 0.60 | ||
| No | 57 (39.6) | 87 (60.4) | |||||
| Yes | 52 (22.6) | 178 (77.4) | |||||
| PSAD ≥0.13 ng ml−1 | <0.001* | 62.4 | 63.8 | 62.8 | 0.63 | ||
| No | 60 (40.5) | 88 (59.5) | |||||
| Yes | 34 (18.9) | 146 (81.1) | |||||
| Total number of biopsy cores ≥12 | 0.095 | 62.5 | 28.4 | 52.6 | 0.45 | ||
| No | 31 (23.8) | 99 (76.2) | |||||
| Yes | 78 (32.1) | 165 (67.9) |
*P<0.05 was considered statistically significant. PCa: prostate cancer; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; ROC: receiver operating characteristic
Table 3.
Multivariate logistic regression models for Gleason score (3+3) upgrade
| Characteristics | OR | 95% CI | P |
|---|---|---|---|
| Age ³60 years (yes vs no) | 2.06 | 1.13–3.78 | 0.019* |
| Ethnicity | |||
| Caucasian | Reference | ||
| African American | 1.63 | 0.60–4.47 | 0.341 |
| Hispanic | 0.41 | 0.16–1.01 | 0.052 |
| PSA ³4.3 ng ml−1 (yes vs no) | 1.67 | 0.89–3.15 | 0.111 |
| PSAD ³0.13 ng ml−1 (yes vs no) | 2.53 | 1.33–4.82 | 0.005* |
| Clinical stage (cT2 vs cT1) | 3.31 | 1.14–9.58 | 0.027* |
| Total number of biopsy cores ³12 (yes vs no) | 0.57 | 0.31–1.04 | 0.068 |
| Core+ ³2 (yes vs no) | 1.44 | 0.77–2.68 | 0.252 |
| ASAP/HGPIN status (HGPIN and/or ASAP vs None) |
1.90 | 1.06–3.40 | 0.031* |
| Maximum core+ length ³2 mm (yes vs no) | 1.54 | 0.71–3.33 | 0.272 |
| Percentage of tumor in maximum positive core ³13% (yes vs no) | 1.73 | 0.79–3.80 | 0.172 |
| Percentage of PCa in total specimen ³4% (yes vs no) | 1.10 | 0.60–1.99 | 0.764 |
*P<0.05 was considered statistically significant. ASAP: atypical small acinar proliferation; CI: confidence interval; HGPIN: high-grade prostatic intraepithelial neoplasia; OR: odds ratio; PCa: prostate cancer; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density
Table 4.
Upgrade status from Gleason score (3+3) to > Gleason score 6 according to the core+ number and tumor length
| Number of core+ | Maximum length of tumor foci | |||
|---|---|---|---|---|
| <3 mm, n/total (%) | 3–6 mm, n/total (%) | >6 mm, n/total (%) | Total, n/total (%) | |
| 1 core+ | 82/138 (59.4) | 21/28 (75.0) | 4/4 (100.0) | 107/170 (62.9) |
| 2 cores+ | 38/60 (63.3) | 20/28 (71.4) | 1/1 (100.0) | 59/89 (66.3) |
| 3 cores+ | 17/20 (85.0) | 19/23 (82.6) | 4/5 (80.0) | 40/48 (83.3) |
| 4 cores+ | 6/6 (100.0) | 13/15 (86.6) | 7/9 (77.7) | 26/30 (86.6) |
| 5 cores+ | 1/1 (100.0) | 10/11 (90.9) | 3/3 (100.0) | 14/15 (93.3) |
| 6 cores+ | – | 5/6 (83.3) | 3/4 (75.0) | 8/10 (80.0) |
| 7 cores+ | – | 3/3 (100.0) | – | 3/3 (100.0) |
| ³8 cores+ | – | 2/2 (100.0) | 6/7 (85.7) | 8/9 (88.9) |
| Total | 144/225 (64.0) | 93/116 (80.2) | 28/33 (84.8) | 265/374 (70.9) |
–: no patient with these parameters for analysis
BCR occurred in 9 (2.4%) patients in a median (interquartile range [IQR]) follow-up of 63 (46–82) months. All of these cases were in the upgraded group. There was no difference between the upgraded and nonupgraded patients in terms of follow-up time (61 vs 63 months, P = 0.335). According to the Kaplan–Meier survival analysis, the 5-year BCR-free survival rate was statistically significantly lower in the upgraded group compared to the nonupgraded group (100.0% vs 96.7%, P = 0.047, Figure 1a).
Figure 1.
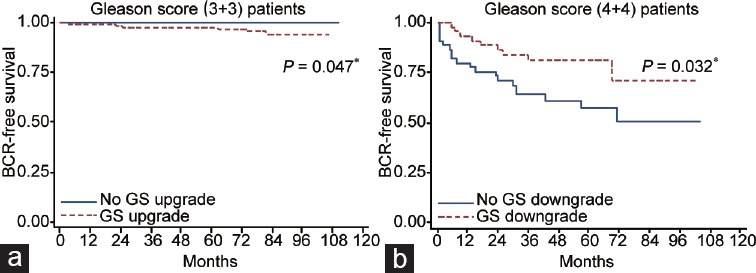
Kaplan–Meier analyses of BCR-free survival rates. (a) Comparison of upgraded and nonupgraded patients in Gleason score (3+3) cohort. The 5-year BCR-free survival rate was statistically significantly lower in the upgraded group (100.0% vs 96.7%, P = 0.047). (b) Comparison of downgraded and nondowngraded patients in Gleason score (4+4) cohort. The 5-year BCR-free survival rate was detected significantly higher in the downgraded group (71.1% vs 50.8%, P = 0.032). *P < 0.05 was considered statistically significant. BCR: biochemical recurrence.
Gleason score 8 (4+4) cohort
Patients' characteristics for GS 8 (4+4) and downgrade status are shown in Table 5. For GS 8, 79.1% of patients also had GS 6−GS 7 in other biopsies. Downgrading occurred in 46 biopsies (50.5%). Lower number of positive cores with GS (4+4) was the sole parameter associated with downgrade in univariate analyses (Table 5).
Table 5.
Patients’ characteristics of Gleason score (4+4) and downgrade status
| Characteristics | Total | Gleason score downgrade | ||
|---|---|---|---|---|
| No | Yes | P | ||
| Patients, n (%) | 91 (100.0) | 45 (49.5) | 46 (50.5) | 0.921 |
| Age (year), median (IQR) | 61 (56–66) | 63 (57–66) | 61 (56–67) | |
| Ethnicity, n (%) | 0.358 | |||
| Caucasian | 70 (76.9) | 33 (47.1) | 37 (52.9) | |
| African American | 9 (9.9) | 6 (66.7) | 3 (33.3) | |
| Hispanic | 7 (7.7) | 5 (71.4) | 2 (28.6) | |
| Asian | 2 (2.2) | 0 (0.0) | 2 (100.0) | |
| Other | 3 (3.3) | 1 (33.3) | 2 (66.7) | |
| PSA (ng ml−1), median (IQR) | 6.4 (4.9–9.2) | 7.0 (5.2–9.4) | 6.0 (4.6–8.5) | 0.130 |
| Prostate volume (ml), median (IQR) | 33 (25–40) | 35 (27–40) | 32 (22–44) | 0.522 |
| PSAD (ng ml−1), median (IQR) | 0.19 (0.15–0.31) | 0.20 (0.15–0.32) | 0.19 (0.13–0.30) | 0.395 |
| BMI (kg m−2), median (IQR) | 29 (26–33) | 30 (27–32) | 28 (26–33) | 0.290 |
| Clinical stage, n (%) | 0.999 | |||
| cT1 | 52 (57.1) | 26 (50.0) | 26 (50.0) | |
| cT2 | 37 (40.7) | 18 (48.6) | 19 (51.4) | |
| cT3 | 2 (2.2) | 1 (50.0) | 1 (50.0) | |
| Number of biopsy cores, median (IQR) | 12 (10–12) | 12 (10–12) | 12 (10–12) | 0.464 |
| Number of Gleason (4+4) positive cores, median (range) | 1 (1–6) | 1 (1–6) | 1 (1–6) | 0.014* |
| Total number of positive cores, median (IQR) | 3 (2–6) | 3 (2–5) | 3 (2–6) | 0.847 |
| Number of positive cores/total biopsy cores, % median (IQR) | 33 (20–50) | 33 (20–50) | 33 (20–50) | 0.937 |
| Biopsy that includes Gleason 6–7, n (%) | 72 (79.1) | 32 (44.4) | 40 (55.6) | 0.063 |
| Gleason (4+4) maximum positive core length (mm), median (IQR) | 4 (1–14) | 4 (2–7) | 3 (1–5) | 0.190 |
| Total positive core length (mm), median (IQR) | 12 (6–22) | 12 (6–22) | 13 (8–20) | 0.833 |
| Percentage of PCa (total positive cores length/total cores length), median (IQR) | 9 (5–21) | 7 (5–27) | 11 (5–18) | 0.959 |
| ASAP/HGPIN status, n (%) | 0.872 | |||
| None | 64 (70.3) | 32 (50.0) | 32 (50.0) | |
| HGPIN and/or ASAP | 27 (29.7) | 13 (48.1) | 14 (51.9) | |
*P<0.05 was considered statistically significant. ASAP: atypical small acinar proliferation; BMI: body mass index; HGPIN: highgrade prostatic intraepithelial neoplasia; IQR: interquartile range; PCa: prostate cancer; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density
Sensitivity and specificity analyses for the best cutoff points were performed. The estimated cutoff values were age ≤65 years (sensitivity 41.3%, specificity 62.2%, P = 0.731), PSA ≤6.05 ng ml−1 (sensitivity 52.2%, specificity 57.8%, P = 0.342), PSAD ≤0.20 ng ml−1 (sensitivity 63.2%, specificity 50.0%, P = 0.241), total number of biopsy cores ≤6 (sensitivity 15.2%, specificity 84.4%, P = 0.964), number of positive cores <2 (sensitivity 80.4%, specificity 44.4%, P = 0.011), maximum core positive length ≤5 mm (sensitivity 78.3%, specificity 35.6%, P = 0.145), percentage of tumor in maximum positive core ≤16.7% (sensitivity 84.8%, specificity 40.0%, P = 0.008), and percentage of PCa in total specimen ≤12% (sensitivity 59.2%, specificity 43.2%, P = 0.799) (Table 6). In multivariate logistic regression analyses, none of these parameters were associated with GS downgrade (All P > 0.05, Table 7).
Table 6.
Sensitivity and specificity analyses for Gleason score (4+4) downgrade
| Characteristics | No downgrade, n (%) | Downgraded, n (%) | P | Sensitivity (%) | Specificity (%) | Correctly classify (%) | ROC area |
|---|---|---|---|---|---|---|---|
| Core+ ≤1 | 0.011* | 80.4 | 44.4 | 62.6 | 0.62 | ||
| No | 20 (69.0) | 9 (31.0) | |||||
| Yes | 25 (40.3) | 37 (59.7) | |||||
| Percentage of tumor in maximum core+ ≤16.7% | 0.008* | 84.8 | 40.0 | 62.6 | 0.62 | ||
| No | 18 (72.0) | 7 (28.0) | |||||
| Yes | 27 (40.9) | 39 (59.1) | |||||
| Maximum core+ length ≤5 mm | 0.145 | 78.3 | 35.6 | 57.1 | 0.57 | ||
| No | 16 (61.5) | 10 (38.5) | |||||
| Yes | 29 (44.6) | 36 (55.4) | |||||
| Percentage of PCa in total specimen ≤12% | 0.799 | 59.2 | 43.2 | 51.2 | 0.51 | ||
| No | 19 (52.8) | 17 (47.2) | |||||
| Yes | 25 (50.0) | 25 (50.0) | |||||
| Age ≤65 years | 0.731 | 41.3 | 62.2 | 51.7 | 0.52 | ||
| No | 28 (50.9) | 27 (49.1) | |||||
| Yes | 17 (47.2) | 19 (52.8) | |||||
| PSA ≤6.05 ng ml−1 | 0.342 | 52.2 | 57.8 | 55.0 | 0.55 | ||
| No | 26 (54.2) | 22 (45.8) | |||||
| Yes | 19 (44.2) | 24 (55.8) | |||||
| PSAD ≤0.20 ng ml−1 | 0.241 | 63.2 | 50.0 | 56.4 | 0.57 | ||
| No | 20 (58.8) | 14 (41.2) | |||||
| Yes | 20 (45.5) | 24 (54.5) | |||||
| Total number of biopsy cores ≤6 | 0.964 | 15.2 | 84.4 | 49.5 | 0.50 | ||
| No | 38 (49.4) | 39 (50.6) | |||||
| Yes | 7 (50.0) | 7 (50.0) |
*P<0.05 was considered statistically significant. PCa: prostate cancer; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; ROC: receiver operating characteristic
Table 7.
Multivariate logistic regression models for Gleason score (4+4) downgrade
| Characteristics | OR | 95% CI for OR | P |
|---|---|---|---|
| Age ≤65 years (yes vs no) | 2.18 | 0.68–6.98 | 0.188 |
| Race (Caucasian vs others) | 3.36 | 0.79–14.22 | 0.099 |
| PSA ≤6.05 ng ml−1 (yes vs no) | 1.95 | 0.52–7.25 | 0.321 |
| PSAD ≤0.20 ng ml−1 (yes vs no) | 1.38 | 0.36–5.29 | 0.642 |
| Clinical stage (cT2–cT3 vs cT1) | 1.09 | 0.37–3.19 | 0.878 |
| Number of biopsy cores £6 (yes vs no) | 1.06 | 0.24–4.77 | 0.937 |
| Number of Gleason score (4+4) positive cores ≤1 (yes vs no) | 2.36 | 0.68–8.15 | 0.174 |
| Number of positive cores/total biopsy cores ≤0.33% (yes vs no) | 2.66 | 0.49–14.27 | 0.254 |
| Biopsy that includes Gleason score 6–7 (yes vs no) | 3.15 | 0.64–15.55 | 0.159 |
| Gleason score (4+4) maximum positive core length ≤5 mm (yes vs no) | 2.31 | 0.61–8.75 | 0.218 |
| Total positive core length ≤10.5 mm (yes vs no) | 0.22 | 0.03–1.80 | 0.158 |
| Percentage of PCa (total positive core length/total cores length) ≤12% (yes vs no) | 1.58 | 0.30–8.32 | 0.587 |
*P<0.05 was considered statistically significant. ASAP: atypical small acinar proliferation; CI: confidence interval; HGPIN: high-grade prostatic intraepithelial neoplasia; OR: odds ratio; PCa: prostate cancer; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density
BCR occurred in 28 (30.8%) patients with 9 (19.6%) in the downgraded and 19 (42.2%) in nondowngraded group, in a mean (IQR) follow-up of 60 (41–75) months. There was no difference between the downgraded and nondowngraded patients in terms of follow-up time (72 vs 76 months, P = 0.577). According to the Kaplan–Meier survival analysis, the 5-year BCR-free survival rate was detected significantly higher in the downgraded group (71.1% vs 50.8%, P = 0.032, Figure 1b).
DISCUSSION
Multiple studies have addressed the frequent clinical observation that prostate biopsy GS and RP GS will be discordant10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27 in 12%–92% of cases. The published explanations could include repeat review of specimens17,18,19,21,22,24,26,27,28 by dedicated genitourinary pathologist,13,16,22,24 or the major revision of the Gleason grading system at the International Society of Urological Pathology (ISUP) consensus conference in 2005.8 Our study suggests that, beyond interpretation issues, the acquisition of the rest of the gland provides such a different sample size than biopsy that the high prevalence of mixed Gleason 3 and 4 pattern disease results in a regression to the mean GS7, even when biopsies indicate the more extreme GS 6 or GS 8. Our data suggest a difference between low-volume GS 6 and high-volume GS 6; although of interest, the high correlation of high-volume GS 6 to upgrading did not result in a large difference in recurrence rate. At the other extreme, nonupgraded GS 6 did not recur, in keeping with the modern concept of pathologic GS 6 not demonstrating the definition of a malignancy. Moving forward, our study explores two follow-up areas on the impact of GS changes: (1) would they change clinical management and (2) would they change biochemical recurrence rates?
Clinicians may recommend additional testing – with added expense – to refine clinical decision-making. In some cases, the biopsy GS may be accepted at face value for clinical decision-making. While MRI and targeted biopsy may be an improved method to obtain the correct pathologic GS,29,30,31 our study can provide some useful preliminary information and patient selection guidance.
At the low end of the scale, GS (3+3) is often selected for AS, although a surprising number of our patients who opted for surgery were upgraded to GS (3+4) or higher (70.9%) overall. This rate of upgrading has been described before27 and, in our case, includes a cohort of biopsy GS 6 cases selecting surgery where 48.9% were PRIAS candidates. Truong et al.23 evaluated all the AS protocols including PRIAS criteria and reported an upgrade rate of 30.9% (P < 0.0001) in patients eligible for PRIAS criteria. Fortunately, the majority of these upgrades were only to GS (3+4) (90.9%) with only 3.4% BCR. We would hypothesize, therefore, that, although giving the pathologist “the rest of the gland” is highly correlated with finding Gleason pattern 4 somewhere, the biology of the disease in the intermediate-term follow-up (5 years) is almost benign. In pure GS 6 confirmed at surgery, BCR was not observed.
Nevertheless, our statistical modeling shows various predictors for upgraded Gleason score at RP: higher age, clinical stage, PSAD, and presence of HGPIN and/or ASAP. In Table 4, we illustrate the spectrum of GS 6 biopsies and how number of cores+ and length of tumor foci can correlate with a >90% chance of upgrading. The ideal patient for lowest risk of upgrading would be age under 60 years, 1 core with <2 mm, PSA <4.3 ng ml-1, PSAD <0.13 ng ml-1, cT1c, and Hispanic. On the other hand, a very young patient with multiple risk factors for upgrading might be counseled as a GS (3+4). Then, the patient and clinician can focus upon whether or not that is enough information to make a decision versus add MRI and/or genomic testing to break the tie between surveillance and intervention.
It is noteworthy that, although many of these prognostic features and different ones have been reported,10,11,13,14,16,18,19,21,22,23,24,26,28,32,33,34 PSAD seems very consistent as a prognostic feature and does not add any cost.11,14,20,21,23 Sfoungaristos and Perimenis21 identified the best PSAD cutoff for upgrade as 0.15. The study by Seisen et al.27 found biopsy volume metrics as predictive of upgrading, and a recent review35 looked at studies from 2000 to 2016 – noteworthy nonpredictive features including clinical stage, race, smoking status, family history of PCa, HGPIN, and/or ASAP,18,21,22 and ethnicity22,23,24 were evaluated in very few studies and no correlation was found with GS upgrade.
These are “treated” statistics, and if the decision is for surveillance, then GS 7 still has to be discussed in terms of its success rates in the long term. Dall'Era and Klotz35 have shown that some intermediate-risk patients are at higher risk of metastases on surveillance, and the ProTect trial36 also showed differences in metastases compared to treated cohorts. Perhaps, the recently published PRECISION30 trial will lead to greater numbers of MRI before primary biopsy such that more patients are allocated to the correct GS at first biopsy. Indeed, the study by Radtke et al.31 showed >90% accuracy in identifying index lesions. Therefore, in the circumstance of available highly expert MRI image acquisition, interpretation, and fusion (possibly cognitive) biopsy techniques, the final grade issues should shift in a favorable direction: (1) MR-fusion biopsied patients with GS 7 will more likely be identified that way at biopsy and (2) MR-fusion biopsied patients with GS 6 should have lower risk of upgrading.
On the high end of the scale, most guidelines suggest that high-risk PCa consider active intervention as well as clinical trials. High-risk patients choosing radiation would have different androgen deprivation therapy (ADT) recommendations compared to intermediate-risk patients – 24 months versus 6 months.37,38,39 It certainly makes sense that biopsy pattern 5 will likely correlate with RP pattern 5 – usually a final score of 4+5 or 5+4. However, with biopsy GS (4+4), all you need is additional areas of pattern 3 in the predominant foci to downgrade it to 4+3 or 3+4. In addition, biopsy GS (4+4) patients may have that finding in isolation, or with other positive cores showing lower grade. We considered both possibilities, and the overall downgrading rate was 50.5% with multiple prognostic factors shown in Table 6 and 7. Moussa et al.12 and Epstein et al.26 also reported a similar rate of downgrade from GS 8 as 50.0% and 51.3%, respectively. Moussa et al.12 evaluated the downgrade factors generally for all GS and found a significant relation with higher prostate volume, more obtained biopsy cores, and low maximum percentage cancer in any core. Epstein et al.26 detected lower PSA level and lower maximum percentage of cancer per core as significant predictors for downgrade for any biopsy GS. Although having a single core of GS (4+4) was prognostic for downgrading, none of our features held up in multivariate analysis – perhaps sample size limiting. Five-year BCR shown in Figure 1b was improved by 20.3% in the downgraded group. These might be more subtle aspects of clinical utility, as patients seem to merit treatment either way, but perhaps useful statistics for further study and refinement for defining more truly high-risk patients for clinical trials and/or radiation candidates for reduced ADT exposure. On the other hand, it is a reasonable argument that the 28.9% 5-year BCR of downgraded high-risk cases merits consideration for combination therapy and/or trials.
Our study is limited by being retrospective, single institution/tertiary referral location, and specific to the biases of patients selecting RP (i.e., age, comorbidity, etc.). Our follow-up metrics are limited to BCR given the more recent era of study. BCR may be associated with metastatic progression and further survival endpoints, but with heterogeneous timing and significance – these would be areas for future study.
CONCLUSION
In counseling men with clinically localized prostate cancer, the odds of GS change should be presented, and certain men with high volume GS 6 or low volume GS 8 managed as if they are GS 7.
AUTHOR CONTRIBUTIONS
MA participated in the design, collection, and analyses of the data, drafted the manuscript, and participated in the revision. JWD designed the study concept, interpreted the data, performed the critical revision for important intellectual content, and supervised the drafting of the manuscript. MFA carried out the data collection. PT reviewed the pathological slides and the manuscript. GNG performed the statistical analyses. SFM provided patient data and reviewed the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We sincerely thank the patients for their participation in this study.
REFERENCES
- 1.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 2.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–8. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 3.Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohler JL, Armstrong AJ, Bahnson RR, D'Amico AV, Davis BJ, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 5.Klotz L. Active surveillance for intermediate risk prostate cancer. Curr Urol Rep. 2017;18:80. doi: 10.1007/s11934-017-0726-3. [DOI] [PubMed] [Google Scholar]
- 6.Ristau BT, Cahn D, Uzzo RG, Chapin BF, Smaldone MC. The role of radical prostatectomy in high-risk localized, node-positive and metastatic prostate cancer. Future Oncol. 2016;12:687–99. doi: 10.2217/fon.15.355. [DOI] [PubMed] [Google Scholar]
- 7.Boström PJ, Bjartell AS, Catto JW, Eggener SE, Lilja H, et al. Genomic predictors of outcome in prostate cancer. Eur Urol. 2015;68:1033–44. doi: 10.1016/j.eururo.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL Committee IG. The 2005 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 9.Bokhorst LP, Valdagni R, Rannikko A, Kakehi Y, Pickles T, et al. A decade of active surveillance in the PRIAS study: an update and evaluation of the criteria used to recommend a switch to active treatment. Eur Urol. 2016;70:954–60. doi: 10.1016/j.eururo.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Pinthus JH, Witkos M, Fleshner NE, Sweet J, Evans A, et al. Prostate cancers scored as Gleason 6 on prostate biopsy are frequently Gleason 7 tumors at radical prostatectomy: implication on outcome. J Urol. 2006;176:979–84. doi: 10.1016/j.juro.2006.04.102. [DOI] [PubMed] [Google Scholar]
- 11.Serkin FB, Soderdahl DW, Cullen J, Chen Y, Hernandez J. Patient risk stratification using Gleason score concordance and upgrading among men with prostate biopsy Gleason score 6 or 7. Urol Oncol. 2010;28:302–7. doi: 10.1016/j.urolonc.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Moussa AS, Li J, Soriano M, Klein EA, Dong F, et al. Prostate biopsy clinical and pathological variables that predict significant grading changes in patients with intermediate and high grade prostate cancer. BJU Int. 2009;103:43–8. doi: 10.1111/j.1464-410X.2008.08059.x. [DOI] [PubMed] [Google Scholar]
- 13.Dong F, Jones JS, Stephenson AJ, Magi-Galluzzi C, Reuther AM, et al. Prostate cancer volume at biopsy predicts clinically significant upgrading. J Urol. 2008;179:896–900. doi: 10.1016/j.juro.2007.10.060. [DOI] [PubMed] [Google Scholar]
- 14.Corcoran NM, Casey RG, Hong MK, Pedersen J, Connolly S, et al. The ability of prostate-specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumour grade due to reduced PSA secretion per unit tumour volume. BJU Int. 2012;110:36–42. doi: 10.1111/j.1464-410X.2011.10681.x. [DOI] [PubMed] [Google Scholar]
- 15.Corcoran NM, Hong MK, Casey RG, Hurtado-Coll A, Peters J, et al. Upgrade in Gleason score between prostate biopsies and pathology following radical prostatectomy significantly impacts upon the risk of biochemical recurrence. BJU Int. 2011;108:202–10. doi: 10.1111/j.1464-410X.2011.10119.x. [DOI] [PubMed] [Google Scholar]
- 16.Suer E, Gokce MI, Gulpinar O, Guclu AG, Haciyev P, et al. How significant is upgrade in Gleason score between prostate biopsy and radical prostatectomy pathology while discussing less invasive treatment options? Scand J Urol. 2014;48:177–82. doi: 10.3109/21681805.2013.829519. [DOI] [PubMed] [Google Scholar]
- 17.Chun FK, Steuber T, Erbersdobler A, Currlin E, Walz J, et al. Development and internal validation of a nomogram predicting the probability of prostate cancer Gleason sum upgrading between biopsy and radical prostatectomy pathology. Eur Urol. 2006;49:820–6. doi: 10.1016/j.eururo.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Kulkarni GS, Lockwood G, Evans A, Toi A, Trachtenberg J, et al. Clinical predictors of Gleason score upgrading: implications for patients considering watchful waiting, active surveillance, or brachytherapy. Cancer. 2007;109:2432–8. doi: 10.1002/cncr.22712. [DOI] [PubMed] [Google Scholar]
- 19.Capitanio U, Karakiewicz PI, Valiquette L, Perrotte P, Jeldres C, et al. Biopsy core number represents one of foremost predictors of clinically significant gleason sum upgrading in patients with low-risk prostate cancer. Urology. 2009;73:1087–91. doi: 10.1016/j.urology.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Magheli A, Hinz S, Hege C, Stephan C, Jung K, et al. Prostate specific antigen density to predict prostate cancer upgrading in a contemporary radical prostatectomy series: a single center experience. J Urol. 2010;183:126–31. doi: 10.1016/j.juro.2009.08.139. [DOI] [PubMed] [Google Scholar]
- 21.Sfoungaristos S, Perimenis P. Clinical and pathological variables that predict changes in tumour grade after radical prostatectomy in patients with prostate cancer. Can Urol Assoc J. 2013;7:93–7. doi: 10.5489/cuaj.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moussa AS, Kattan MW, Berglund R, Yu C, Fareed K, et al. A nomogram for predicting upgrading in patients with low- and intermediate-grade prostate cancer in the era of extended prostate sampling. BJU Int. 2010;105:352–8. doi: 10.1111/j.1464-410X.2009.08778.x. [DOI] [PubMed] [Google Scholar]
- 23.Truong M, Slezak JA, Lin CP, Iremashvili V, Sado M, et al. Development and multi-institutional validation of an upgrading risk tool for Gleason 6 prostate cancer. Cancer. 2013;119:3992–4002. doi: 10.1002/cncr.28303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedland SJ, Kane CJ, Amling CL, Aronson WJ, Terris MK, et al. Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology. 2007;69:495–9. doi: 10.1016/j.urology.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boorjian SA, Thompson RH, Siddiqui S, Bagniewski S, Bergstralh EJ, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007;178:864–70. doi: 10.1016/j.juro.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61:1019–24. doi: 10.1016/j.eururo.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seisen T, Roudot-Thoraval F, Bosset PO, Beaugerie A, Allory Y, et al. Predicting the risk of harboring high-grade disease for patients diagnosed with prostate cancer scored as Gleason ≤ 6 on biopsy cores. World J Urol. 2015;33:787–92. doi: 10.1007/s00345-014-1348-8. [DOI] [PubMed] [Google Scholar]
- 28.Boorjian SA, Karnes RJ, Crispen PL, Rangel LJ, Bergstralh EJ, et al. The impact of discordance between biopsy and pathological Gleason scores on survival after radical prostatectomy. J Urol. 2009;181:95–104. doi: 10.1016/j.juro.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 29.El-Shater Bosaily A, Parker C, Brown LC, Gabe R, Hindley RG, et al. PROMIS--Prostate MR imaging study: a paired validating cohort study evaluating the role of multi-parametric MRI in men with clinical suspicion of prostate cancer. Contemp Clin Trials. 2015;42:26–40. doi: 10.1016/j.cct.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–77. doi: 10.1056/NEJMoa1801993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radtke JP, Schwab C, Wolf MB, Freitag MT, Alt CD, et al. Multiparametric magnetic resonance imaging (MRI) and MRI-transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol. 2016;70:846–53. doi: 10.1016/j.eururo.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 32.King CR, McNeal JE, Gill H, Presti JC. Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys. 2004;59:386–91. doi: 10.1016/j.ijrobp.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 33.San Francisco IF, DeWolf WC, Rosen S, Upton M, Olumi AF. Extended prostate needle biopsy improves concordance of Gleason grading between prostate needle biopsy and radical prostatectomy. J Urol. 2003;169:136–40. doi: 10.1016/S0022-5347(05)64053-0. [DOI] [PubMed] [Google Scholar]
- 34.Mian BM, Lehr DJ, Moore CK, Fisher HA, Kaufman RP, et al. Role of prostate biopsy schemes in accurate prediction of Gleason scores. Urology. 2006;67:379–83. doi: 10.1016/j.urology.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Dall'Era MA, Klotz L. Active surveillance for intermediate-risk prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:1–6. doi: 10.1038/pcan.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane JA, Donovan JL, Davis M, Walsh E, Dedman D, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014;15:1109–18. doi: 10.1016/S1470-2045(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 37.Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–27. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 38.Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12:451–9. doi: 10.1016/S1470-2045(11)70063-8. [DOI] [PubMed] [Google Scholar]
- 39.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]


