Abstract
According to the World Health Organization (WHO), oxidative stress (OS) is a significant contributor to male infertility. Seminal OS can be measured by a number of assays, all of which are either costly or time sensitive and/or require large semen volume and complex instrumentation. One less expensive alternative is to quantify the oxidation-reduction potential (ORP) with the MiOXSYS. In this international multi-center study, we assessed whether ORP levels measured by the MiOXSYS could distinguish semen samples that fall within the 2010 WHO normal reference values from those that do not. Semen samples were collected from 2092 patients in 9 countries; ORP was normalized to sperm concentration (mV/106 sperm/ml). Only those samples with a concentration >1 × 106 sperm ml–1 were included. The results showed that 199 samples fell within the WHO normal reference range while the remaining 1893 samples did not meet one or more of the criteria. ORP was negatively correlated with all semen parameters (P < 0.01) except volume. The area under the curve for ORP was 0.765. The ORP cut-off value (1.34 mV/106 sperm/ml) was able to differentiate specimens with abnormal semen parameters with 98.1% sensitivity, 40.6% specificity, 94.7% positive predictive value (PPV) and 66.6% negative predictive value (NPV). When used as an adjunct to traditional semen analysis, ORP levels may help identify altered functional status of spermatozoa caused by OS in cases of idiopathic male infertility and in male partners of couples suffering recurrent pregnancy loss, and thereby directing these men to relevant medical therapies and lifestyle modifications.
Keywords: male infertility, oxidation-reduction potential, semen analysis, spermatozoa
INTRODUCTION
A physiological balance between oxidants and antioxidants (reductants) is required for important sperm functions such as testicular immunity, spermatogenesis, chromatin compaction, capacitation, hyperactivation, acrosome reaction, and sperm-oocyte fusion.1 However, excess production of reactive oxygen species (ROS) or a low concentration of seminal antioxidants drives the redox balance into a state of oxidative stress (OS). Clinically, OS in males reduces fertilization rates, impairs embryonic development, and leads to implantation failure, recurrent pregnancy loss, and poor assisted reproductive technology (ART) outcomes.1 The WHO considers OS a significant contributor to male infertility.2
A number of assays are available to measure OS including chemiluminescence for ROS, total antioxidant capacity (TAC) for antioxidants, and the malondialdehyde (MDA) assay for post hoc damage from lipid peroxidation.3,4 While useful, these tests are difficult to incorporate into routine use because they are expensive, complex, and time sensitive and may also require complex instrumentation, large and neat sample volumes, and extensive technical training.5 Additionally, these assay results do not correlate with one another and provide only a single marker of OS, either oxidant levels, antioxidant levels, or post hoc damage.6
The oxidation-reduction potential (ORP) is a measure of the overall balance between oxidants and antioxidants, which provides a comprehensive measure of the redox system and OS. Previous studies have used ORP to measure OS in the blood of patients with traumatic brain injury, stroke, metabolic syndrome, liver toxicity, sepsis, and extreme exercise habits.7 A novel technology based on a galvanic measure of electrons-the MiOXSYS (Aytu Bioscience, Englewood, CO, USA)–has been used to measure ORP in human semen and seminal plasma.8 The system overcomes many of the challenges presented by traditional OS measurement methods. It is inexpensive, requires only 30 μl of sample, and takes <4 min to complete the test.5 This method allows OS to be measured in real time; a high ORP level is indicative of OS. ORP levels have been shown to correlate negatively with semen parameters and positively with DNA damage. In one study, we used ORP to distinguish infertile men from fertile controls from two different centers.9
The current study aims to expand these previous findings from our group by evaluating ORP in a larger group of men with ethnic and regional differences from 9 institutions around the world. The objectives of this multicenter study were (1) to evaluate the predictability of the ORP measure using the MiOXSYS in comparison with standard semen analysis parameters (as defined by the WHO 5th edition2), (2) to evaluate the variability of all measures according to WHO criteria for the normal and abnormal semen analysis groups and (3) to verify the ORP cut-off value to distinguish men with normal semen parameters from those with abnormal semen parameters.
PARTICIPANTS AND METHODS
Study subjects
Following institutional approval by all 9 paricipating centers, we enrolled 2092 men who were seeking assistance from a fertility specialist. Written informed consent was obtained from all participants in the following 9 institutions: Cleveland Clinic, Cleveland, USA (n = 502); Hamad Medical Hospital, Doha, Qatar (n = 1078); Dokkyo University, Osaka, Japan (n = 152); The Doctor's Laboratory, London, UK (n = 106); VKF American Hospital, Istanbul, Turkey (n=100); Sohag University, Sohag, Egypt (n = 69); Bezmi Alem Vakif University, Istanbul, Turkey (n = 84); Assam University, Silchar, India (n = 48); and Tulane Medical Center, New Orleans, USA (n = 22).
The men were divided into two groups according to the WHO 5th edition criteria: those with normal semen parameters (normozoospermia; n = 199) and those with abnormal semen parameters (n = 1893) according to the WHO 5th edition reference values. Patients were excluded if they were taking medications for chronic diseases (irritable bowel disease, colitis, or a metabolic disorder) and/or had a sperm concentration <1 × 106 ml−1, leukocytospermia, genital tract infections, prior surgery or major trauma, or a history of infertility treatment or antioxidant supplement use. All enrolled patients were between the ages of 21 years and 45 years.
Semen analysis
Semen specimens were collected by masturbation after 2–3 days of sexual abstinence, and the sperm parameters were analyzed after complete liquefaction at 37°C for 20 min. Each sample was evaluated for both macroscopic parameters such as color, pH, ejaculate volume, the age of the sample and viscosity. An aliquot of the sample (5 μl) was examined for sperm concentration, total sperm count, sperm motility and round cell concentration. If the round cell concentration was >1 × 106 ml−1, the sample was examined for the presence of white blood cells either by the Endtz-test using a MicroCell counting chamber (Vitrolife, San Diego, CA, USA) with phase contrast optics set at ×20 or by using a CE-marked LeucoScreen™ kit (Microm, Bicester, UK) that differentiates round cells on the basis of their peroxidase content and confirmed on Papanicolaou stained slides with a cell differential count. Air-dried smears were prepared for morphological evaluation, and a minimum total of 200 sperm were scored with 4% normal morphology used as cut-off.
Measurement of oxidation-reduction potential
The ORP was measured with the MiOXSYS. A 30-μl sample was loaded into the sample port of the disposable MiOXSYS sensor within one hour of liquefaction and inserted into the MiOXSYS analyzer. The test starts immediately when the sample fills the reference cell, thereby completing the electrochemical circuit. After a short period, the ORP values are displayed on the screen in millivolts (mV). ORP provides a “snapshot” of the current balance of the redox system. A higher ORP level indicates an imbalance in the activity of all available oxidants relative to all available antioxidants in the seminal ejaculate - a state of OS. Both absolute ORP (mV) and normalized ORP values (mV/106 sperm/ml) were calculated.
Statistical analyses
Data were analyzed separately from each laboratory and subsequently combined and analyzed by IBM SPSS version 24 (IBM Corp., Armonk, NY, USA). Data are presented as mean ± standard deviation (s.d.). Data were tested for normal distribution with the Chi-squared test and subsequently analyzed with parametric or nonparametric tests. For group comparisons, only those samples with a concentration >1 × 106 sperm ml−1 were included. A logistic regression model was performed on the quantitative variables to determine the predictability of identifying abnormal and normal semen quality within the patient population. Because there was a 1:10 skewness in normal versus abnormal semen analysis, a random sampling technique was performed to compensate for any dominance by site with a large patient enrollment. A backward stepwise model was also applied to further verify the logistic regression model.10 Pearson correlations were used to measure associations between pairs of quantitative variables. A receiver operating characteristic (ROC) curve was used to identify the static ORP (sORP) (mV/106 sperm/ml) criterion that best differentiated normal and abnormal semen parameters in men. P < 0.05 was considered as statistically significant.
RESULTS
Of the 2092 patients examined in this study, 199 had normal semen parameters (normozoospermia) and 1893 had at least one abnormal parameter (Table 1). To examine the predictability of identifying abnormal semen quality, measures from the logistic regression model were categorized according to overall contribution and significance (Table 2). ORP ranked the highest (β = 2.494, P = 0.001) in terms of predicting abnormal and normal semen quality followed by progressive motility (β = 1.838, P = 0.001), and morphology (β = 0.242, P = 0.001).
Table 1.
Background information on the study population with a comparison of semen parameters between the normal and abnormal groups
| Semen parameters | Mean±s.d. | P | 95% CI | |
|---|---|---|---|---|
| Normal group (n=199) | Abnormal group (n=1893) | |||
| ORP (mV/106 sperm/ml) | 0.88±1.64 | 5.08±14.24 | 0.001 | −4.97–−3.43 |
| Sperm total (106) | 231.01±171.96 | 108.30±147.38 | 0.001 | 92.83–152.58 |
| Progressive motility (%) | 48.08±10.87 | 15.81±15.29 | 0.001 | 30.28–34.25 |
| Total motility (%) | 57.19±10.44 | 41.26±17.16 | 0.001 | 13.97–17.88 |
| Normal morphology (%) | 6.76±3.49 | 4.87±7.46 | 0.001 | 1.20–2.59 |
| Sperm concentration (106 ml−1) | 70.53±50.82 | 34.72±31.16 | 0.001 | 27.12–44.51 |
| Volume (ml) | 3.47±1.41 | 3.18±2.29 | 0.001 | −0.09–0.68 |
Sperm parameters and ORP values in patients with at least one abnormal semen parameter versus patients with normal semen parameters. ORP: oxidation-reduction potential; CI: confidence interval; s.d.: standard deviation
Table 2.
Logistic regression model of oxidation-reduction potential and semen analysis variables according to overall contribution and significance of predicting abnormal semen quality
| Semen parameters | β | Standard error of the mean | Difference | P |
|---|---|---|---|---|
| ORP (mV/106 sperm/ml) | 2.494 | 0.672 | 1 | 0.001 |
| Sperm total (106) | 0.290 | 0.135 | 1 | 0.032 |
| Progressive motility (%) | 1.838 | 0.152 | 1 | 0.001 |
| Total motility (%) | 0.154 | 0.135 | 1 | 0.254 |
| Normal morphology (%) | 0.242 | 0.075 | 1 | 0.001 |
| Sperm concentration (106 ml−1) | 0.027 | 0.112 | 1 | 0.811 |
| Volume (ml) | 0.101 | 0.125 | 1 | 0.420 |
ORP: oxidation-reduction potential
To account for the dominance of patients from the Hamad Medical Hospital (n = 1078) in the overall study, a random sample of 110 results were removed, and a revised logistic regression analysis was performed. By using a revised sample of 1124 patients for all sites, the results were categorized according to overall contribution and significance. ORP ranked the highest (β = 2.057, P = 0.001) followed by progressive motility (β = 1.851, P = 0.001) and total motility (β = 0.547, P = 0.001).
ORP was negatively correlated with sperm concentration, total sperm count, total motility, progressive motility, and normal morphological forms (all P < 0.0001; Table 3). Of the 2092 semen samples examined in the study, 199 were normal and 1893 had at least one abnormal parameter (Table 1). Comparisons of routine semen parameters (sperm concentration, total sperm count, total motility, progressive motility, volume and normal sperm forms) and ORP levels between patients with normal and abnormal semen parameters are shown in Table 1.
Table 3.
Correlation of oxidation-reduction potential with sperm parameters in all patients (n=1893)
| sORP | Sperm total (106) | Progressive motility (%) | Total motility (%) | Normal morphology (%) | Sperm concentration (106 ml−1) | Volume (ml) |
|---|---|---|---|---|---|---|
| Pearson correlation | −0.188** | −0.185** | −0.200** | −0.111** | −0.287** | −0.011 |
| P (two-tailed) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.678 |
sORP: static oxidation-reduction potential
ROC curves were generated from the test results of the six semen parameters and ORP (mV/106 sperm/ml), predicting normal versus abnormal semen quality values to calculate test sensitivity, specificity, and positive and negative predictive values (Figure 1). At a cut-off value of 1.34 mV/106 sperm/ml, ORP could be used to differentiate between normal and abnormal semen quality with 98.1% sensitivity, 40.6% specificity, 94.7% positive predictive value, and 66.6% negative predictive value (area under the curve [AUC] = 0.765). The distribution of patients in the normal and abnormal groups above or below the established cut-off value of 1.34 mV/106 sperm/ml is depicted in Figure 2. The median ORP (mV/106 sperm/ml) level was below the established cut-off value of 1.34 mV/106 sperm/ml in the normal group. In the abnormal group, the median ORP level was above the established cut-off value, and a greater range, as well as distribution of ORP values, can be observed in this group (P = 0.004).
Figure 1.
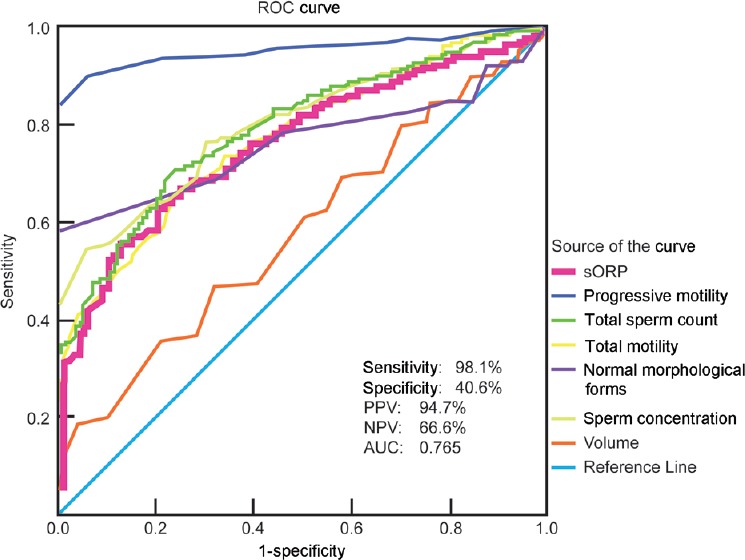
A ROC curve was used to identify the sORP (mV/106 sperm/ml) criterion, i.e., cut-off, sensitivity, and specificity, PPV, NPV, and AUC in conjunction with semen parameters that best predicted the normal and abnormal semen parameters. sORP: static oxidation-reduction potential; ROC: receiver operating characteristic; PPV: positive predictive value; NPV: negative predictive value; AUC: area under the curve.
Figure 2.
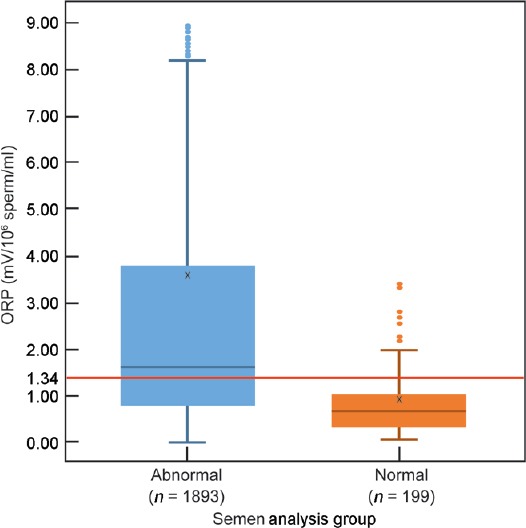
Distribution of ORP in patients with at least one abnormal sperm parameter versus patients with normal sperm parameters, showing the established cut-off value of 1.34 mV/106 sperm/ml. ORP: oxidation-reduction potential.
DISCUSSION
Although routine semen analysis is still used as the basis for male infertility evaluation, concerns about its technical and biological consistency and semen analysis guidelines raise questions about the reliability of this test.11 High intra-individual variability is common in semen specimens owing to both biological and technical reasons, and therefore, the results of a single semen specimen may not accurately reflect a man's fertility status and cannot be used as a specific surrogate of fertility potential.4 Additionally, semen analysis is unable to assess many of the key aspects of the fertilization process such capacitation, the state of the proteins required for zona pellucida binding and penetration, the ability of sperm to fertilize an egg, or any potential damage to the spermatozoa caused by OS or DNA fragmentation.12,13
Approximately 30% of infertile men have a normal semen parameters. In those cases, clinicians seek advanced tests (i.e., hypo-osmotic swelling test, computer-assisted sperm analysis, antisperm antibody test, sperm penetration assay, hemizona assay, and sperm chromatin integrity test) to identify other factors.13,14 However, many of these tests are costly and highly technical, making them inaccessible to small laboratories, and they do not provide complete information regarding a man's fertility status.13,15 As such, clinicians may be quick to categorize these men with idiopathic (unexplained) infertility. Considering the limited and often inconsistent results of semen analysis and the number of couples experiencing recurrent pregnancy loss, it is becoming more important to assess male fertility with advanced sperm function tests that are accurate, repeatable, and accessible.
It is well established that physiological levels of OS are required for sperm function. On the other hand, OS plays a key role in the pathogenesis of male infertility. Between 30% and 80% of infertile men present with OS caused by high levels of ROS or antioxidant depletion.16,17,18,19,20,21,22 Despite the prevalence of OS and the significance of its effects, clinicians do not routinely measure OS as part of a diagnostic workup due to the lack of a standardized protocol and differences between ROS reference values, given that many different tests can be used to assess OS.17,18,19,20,21,22 This makes it difficult to compare OS across different individuals or laboratories and to assess the significance of a sperm abnormality under a state of high OS and its impact on fertization or clinical pregnancy rates.
The MiOXSYS overcomes many of the challenges presented by previous OS single marker tests. The system is accurate, easy to use, and inexpensive, in which it does not require capital equipment or highly trained staff. In a study conducted by our group with 51 controls and 106 infertile patients, ROC curve analysis showed that a cut-off value of 1.36 mV/106 sperm/ml could distinguish infertile men from healthy men.12
In another previous study, we increased the sample size and applied findings to other laboratories by collaborating with a center in Doha, Qatar.13 In our two andology laboratories, there was a total of 101 fertile controls and 594 infertile patients.9 ORP was consistent across both centers, even when other semen parameters were not, and the ROC curve analysis generated an ORP cut-off value of 1.42 mV/106 sperm/ml to distinguish between healthy and infertile men.9
In the current study, we increased our sample size by enrolling men from 9 international centers and also measured ORP in conjunction with traditional semen analysis. We were able to detect abnormal semen quality with a 98.1% sensitivity, 40.6% specificity, 94.7% positive predictive value, and 66.6% negative predictive value. The cut-off value of the ORP measure was 1.34 mV/106 sperm/ml and is consistent with previous research.5
Using an ORP test in conjunction with the standard semen analysis resulted in slightly higher predictability (R2 = 0.538 versus R2 = 0.526) than performing standard semen analysis without ORP. This is a current limitation of the current study and can be addressed in the future by assessing clinical pregnancy as an endpoint. Despite this, ORP was able to distinguish abnormal semen samples from normal samples reliably.
In our multi-center study, the high positive and negative predictive values suggest that ORP levels measured by the MiOXSYS can identify abnormal and normal sperm quality especially well. This predictive ability is also reflected in the results of the logistic regression model in which ORP contributed greater than any other semen parameters in determining abnormal and normal semen quality.
ORP correlated with most of the semen parameters (sperm concentration, motility, total sperm count, progressive motility, and normal morphology) and body mass index (BMI). Although both measures (ORP and concentration) were significant in the logistic regression model, the contribution (β = 2.494) of ORP in the logistic regression model was greater than that of concentration (β = 0.027), suggesting that each measure is unique and influences the diagnosis differently. The negative correlation between ORP and concentration may be expected given that ORP is normalized to concentration, and as evidenced in previous studies, ORP can predict oligozoospermia with high sensitivity and specificity, suggesting that OS may play a key pathologic role in patients with reduced sperm concentration.9,21
One of the main advantages of the current study was that it included patients from all parts of the world, including those who were omitted from the development of the WHO 5th edition reference guidelines (Qatar, Japan, Turkey, Egypt, and India). This ensured that the ORP measurement cut-off values applied to multiple ethnic and geographical populations remained stable across laboratories in different countries where other semen parameters are not.9,11 Significant differences in semen parameters were found between the centers for samples that met the normal reference range of the WHO 5th edition guidelines and those that did not. Significant (P < 0.001) differences were found in progressive motility, total motility, and the morphology value for each center. Sperm concentration and semen volume were significantly different between centers that did not meet WHO criteria. Both ORP and total sperm count were nonsignificant. Although significant differences between the parameters were present, ORP levels for fertile and infertile patients did not differ significantly between the participating centers within the study (Table 4).
Table 4.
Sperm parameters and oxidation-reduction potential values (mV/106 sperm/ml) in infertile patients (n=2092) with at least one abnormal sperm parameter for combined dataset
| Criteria | Total sperm count (106) | Progressive motility (%) | Total motility (%) | Normal morphology (%) | Sperm concentration (106 ml−1) | Volume (ml) | ORP |
|---|---|---|---|---|---|---|---|
| WHO (5th) meets (n=199) | |||||||
| P | 0.191 | 0.001 | 0.001 | 0.001 | 0.245 | 0.393 | 0.955 |
| Difference of means values | 157.60–288.19 | 34.37–57.69 | 41.75–77.66 | 4.9–12.06 | 51.06–83.62 | 2.95–3.82 | 0.46–1.14 |
| WHO (5th) fails (n=1893) | |||||||
| P | 0.126 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 |
| Difference of means values | 98.71–139.75 | 12.18–51.54 | 37.53–58.80 | 1.38–4.83 | 31.60–51.05 | 2.75–3.97 | 2.73–4.64 |
Inter-rater reliability between participating centers was analyzed for the infertile patients. ORP: oxidation-reduction potential; WHO: World Health Organization
The limitations of our study include the following: (1) infertile patients were classified based on their clinical diagnosis of abnormal and normal semen parameters, but not by specific sperm abnormalities (i.e., oligiasthenoteratozoospermia versus isolated teratozoospermia); (2) the study did not balance the number of healthy controls with proven fertility and infertile patients, but to account for the dominance of patients in the overall study, a random sample of 110 results were removed and a revised logistic regression analysis was performed; and (3) while semen parameters are an important part of the assessment of the infertile male, the gold standard is the ability to determine the reproductive outcome. Finally, pregnancy outcomes were not prospectively measured in the infertile group.
CONCLUSION
ORP represents an advanced sperm function measure for OS that can reliably predict sperm abnormalities under a state of high OS. A reference value of 1.34 mV/106 sperm/ml for ORP provided the greatest predictability when identifying abnormal/normal semen quality among 2092 patients with male infertility. Abnormal ORP levels can be useful in identifying altered sperm functional status, especially in cases of idiopathic infertility and/or in male partners of couples who have suffered recurrent pregnancy loss. Ongoing studies are underway to further understand the clinical role of this new and reproducible measure of OS in ART. Similar to sperm DNA fragmentation studies, evaluation of the ORP measure will be closely assessed for fertility success in accordance with fertilization and embryo development rates.
AUTHOR CONTRIBUTIONS
Study design was planned by A Agarwal. A Agarwal, MA, HO, SH, AK, BB, RS, A Armagan, SR and SS were involved in analysis and interpretation of the data. Manuscript was written by A Agarwal and reviewed by MKPS, MA, HO, SH, AK, BB, A Armagan, SR and SS. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors thank Haitham Elbardisi, MD, Keisuke Suzuki, MD and Ahmet Ayaz, PhD, for their support in enrolling the subjects in the current study and Ramya Chandrakumar, BS, for the help with preparation of this manuscript. We also thank Christopher Nelson PhD, for providing assistance with statistical analysis. The authors are grateful to graphic specialist Sue Kido from the Cleveland Clinic Center for Medical Art and Photography for assistance with the figures. The authors acknowledge the efforts of Andrology technologists from all the participating centers and Aytu Bioscience, Englewood, CO, USA for supplying the MiOXSYS Analyzers for this clinical study. Aytu Bioscience did not participate in the design and implementation of this study as well as the manuscript submission. Research support was provided by the American Center for Reproductive Medicine at Cleveland Clinic.
REFERENCES
- 1.Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol. 2014;40:433–53. doi: 10.1590/S1677-5538.IBJU.2014.04.02. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Laboratory Manual for the Examination and Processing of Human Semen. 5th edi. Geneva: World Health Organization; 2010. [Google Scholar]
- 3.Grotto D, Santa Maria L, Boeira S, Valentini J, Charão M, et al. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography–visible detection. J Pharm Biomed Anal. 2007;43:619–24. doi: 10.1016/j.jpba.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Vessey W, Perez-Miranda A, Macfarquhar R, Agarwal A, Homa S. Reactive oxygen species in human semen: validation and qualification of a chemiluminescence assay. Fertil Steril. 2014;102:1576–83.e4. doi: 10.1016/j.fertnstert.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Roychoudhury S, Bjugstad KB, Cho CL. Oxidation-reduction potential of semen: what is its role in the treatment of male infertility? Ther Adv Urol. 2016;8:302–18. doi: 10.1177/1756287216652779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spanidis Y, Goutzourelas N, Stagos D, Kolyva AS, Gogos CA, et al. Assessment of oxidative stress in septic and obese patients using markers of oxidation-reduction potential. In Vivo. 2015;29:595–600. [PubMed] [Google Scholar]
- 8.Agarwal A, Roychoudhury S, Sharma R, Gupta S, Majzoub A, et al. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: clinical utility in male factor infertility. Reprod Biomed Online. 2017;34:48–57. doi: 10.1016/j.rbmo.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Arafa M, Chandrakumar R, Majzoub A, AlSaid S, et al. Multicenter study to evaluate oxidative stress by oxidation–reduction potential, a reliable and reproducible method. Andrology. 2017;5:939–45. doi: 10.1111/andr.12395. [DOI] [PubMed] [Google Scholar]
- 10.Venerables W, Ripley B. Modern applied statistics with S. Springer Science & Business Media. 2002. [Last accessed on 2002 Mar 15]. Available from: http://www.bagualu.net/wordpress/wpcontent/uploads/2015/10/Modern_Applied_Statistics_With_S.pdf .
- 11.Jarow JP, Fang X, Hammad TA. Variability of semen parameters with time in placebo treated men. J Urol. 2013;189:1825–9. doi: 10.1016/j.juro.2012.11.077. [DOI] [PubMed] [Google Scholar]
- 12.Esteves SC, Zini A, Aziz N, Alvarez JG, Sabanegh ES, et al. Critical appraisal of World Health Organization's new reference values for human semen characteristics and effect on diagnosis and treatment of subfertile men. Urology. 2012;79:16–22. doi: 10.1016/j.urology.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Björndahl L, Barratt CLR, Mortimer D, Jouannet P. 'How to count sperm properly': checklist for acceptability of studies based on human semen analysis. Hum Reprod. 2016;31:227–32. doi: 10.1093/humrep/dev305. [DOI] [PubMed] [Google Scholar]
- 14.van der Steeg JW, Steures P, Eijkemans MJ, Habbema JD, Hompes PG, et al. Role of semen analysis in subfertile couples. Fertil Steril. 2011;95:1013–9. doi: 10.1016/j.fertnstert.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102:1502–7. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovac JR, Pastuszak AW, Lamb DJ. The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril. 2013;99:998–1007. doi: 10.1016/j.fertnstert.2013.01.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahfouz R, Sharma R, Sharma D, Sabanegh E, Agarwal A. Diagnostic value of the total antioxidant capacity (TAC) in human seminal plasma. Fertil Steril. 2009;91:805–11. doi: 10.1016/j.fertnstert.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Sikka SC, Hellstrom WJ. Current updates on laboratory techniques for the diagnosis of male reproductive failure. Asian J Androl. 2016;18:392–401. doi: 10.4103/1008-682X.179161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–50. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- 20.Roychoudhury S, Sharma R, Sikka S, Agarwal A. Diagnostic application of total antioxidant capacity in seminal plasma to assess oxidative stress in male factor infertility. J Assist Reprod Genet. 2016;33:627–35. doi: 10.1007/s10815-016-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwal A, Wang SM, Tadros N, Sabanegh E. Involvement of oxidation reduction potential in the pathophysiology of male infertility in patients with varicocele: MP07-19. J Urol. 2017;197:e89. [Google Scholar]
- 22.Homa ST, Vessey W, Perez-Miranda A, Riyait T, Agarwal A. Reactive oxygen species (ROS) in human semen: determination of a reference range. J Assist Reprod Genet. 2015;32:757–64. doi: 10.1007/s10815-015-0454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


