Abstract
This study compared the diagnostic efficacy of transrectal ultrasound (TRUS)-guided prostate biopsy (TRBx) and transperineal prostate biopsy (TPBx) in patients with suspected prostate cancer (PCa). We enrolled 2962 men who underwent transrectal (n = 1216) or transperineal (n = 1746) systematic 12-core prostate biopsy. Clinical data including age, prostate-specific antigen (PSA) level, and prostate volume (PV) were recorded. To minimize confounding, we performed propensity score-matching analysis. We measured and compared PCa detection rates between TRBx and TPBx, which were stratified by clinical characteristics and Gleason scores. The effects of clinical characteristics on PCa detection rate were assessed by logistic regression. For all patients, TPBx detected a higher proportion of clinically significant PCa (P < 0.001). Logistic regression analyses illustrated that PV had a smaller impact on PCa detection rate of TPBx compared with TRBx. Propensity score-matching analysis showed that the detection rates in TRBx were higher than those in TPBx for patients aged ≥ 80 years (80.4% vs 56.5%, P = 0.004) and with PSA level 20.1–100.0 ng ml−1 (80.8% vs 69.1%, P = 0.040). In conclusion, TPBx was associated with a higher detection rate of clinically significant PCa than TRBx was; however, because of the high detection rate at certain ages and PSA levels, biopsy approaches should be optimized according to patents' clinical characteristics.
Keywords: biopsy, demography, Gleason score, propensity score, prostatic neoplasms
INTRODUCTION
Transrectal ultrasound (TRUS)-guided prostate biopsy is the standard for the diagnosis of prostate cancer (PCa). To improve PCa diagnostic efficacy, different biopsy methods have been proposed, such as systematic biopsy, saturation biopsy, and magnetic resonance (MR)/ultrasound fusion targeted biopsy. Formerly, TRUS-guided sextant biopsy was considered the gold standard for PCa detection; however, it has been supplanted by 12-core biopsy because of the superior cancer detection rate of the latter.1,2 Whether saturation biopsy may maximize the cancer detection rate beyond that of systematic biopsy is controversial, besides it carries higher complication risks.3,4,5 Targeted biopsy has received attention from clinicians because recent studies have confirmed its higher diagnostic efficacy than that of systematic biopsy.6,7 The MR/ultrasound fusion technique still has limitations for cases with MR-negative signals.8 In addition, the need for more complex techniques and more expensive equipment in targeted biopsy could also be a burden for developing countries. As a result, the traditional systematic 12-core technique under TURS guidance still plays a vital role in PCa diagnosis.
There are two different approaches for systematic biopsy: transrectal prostate biopsy (TRBx) and transperineal prostate biopsy (TPBx). TRBx is considered to be the standard procedure whereas TPBx is also used in many medical institutes because of its comparable cancer detection efficacy9,10 and lower postprocedural infection rate.11 Some authors have proposed that the biopsy cores should be adjusted to different prostate volumes (PVs) or patients' clinical conditions.12,13 How to optimize biopsy approaches to adapt to different clinical characteristics, such as age, prostate-specific antigen (PSA) value, and PV, has rarely been reported. Furthermore, different biopsy approaches may detect PCa with different levels of clinical significance. Some researchers argue that TRUS-guided biopsy misses 20% of significant PCa.14 A good biopsy method should have a high detection rate and find a high proportion of clinically significant PCa. Which approaches have greater diagnostic efficacy for clinically significant PCa is important for evaluating the procedure and reducing unnecessary repeat biopsy and overtreatment. In addition, PCa prevalence and the disease management in China is different compared with Western countries.15 However, data from Chinese cohort are comparatively limited. In this study, we collected data retrospectively from two Chinese institutions to compare the diagnostic efficacy of TRBx and TPBx. To minimize confounding from age, PSA value, and PV, we introduced propensity score-matching analysis to adjust for selection bias. This study obtained distinct results compared with previous studies relied on data from Western countries, which would be more meaningful for treating Chinese patients. In all, we hope to provide useful recommendations for optimizing prostate biopsy.
PATIENTS AND METHODS
Patients
We retrospectively identified 2962 biopsy-naïve men who underwent prostate biopsy between January 2012 and December 2016. Among these patients, 1216 received TRBx in Shanghai General Hospital (Shanghai, China) and 1746 received TPBx in West China Hospital (Chengdu, China). Indications for prostate biopsy included PSA level >4 ng ml−1 and/or abnormal findings by digital rectal examination, TRUS, and prostatic MR imaging (MRI). This study was approved by the Ethic Committees of Shanghai General Hospital and West China Hospital. All patients enrolled were informed about the biopsy procedure, and written informed consent was obtained from all patients.
Clinical characteristics
Patients' age and baseline data including PSA level and PV were collected. PV was measured by TRUS and calculated using the ellipsoid volume formula: PV (ml) = (π/6) × (anterior − posterior diameter [cm]) × transverse diameter (cm) × (superior − inferior diameter [cm]).
Biopsy procedure
TRBx was performed with the patient in the left decubitus position under local infiltration anesthesia by lidocaine gel. TPBx was performed with the patient in the lithotomy position under subcutaneous infiltration plus periprostatic nerve block by 1% lidocaine. Biopsies were taken by two experienced urologists using BARD MAGNUM instrument needles with a penetration depth of 22 mm (Bard, Covington, GA, USA) under TRUS guidance. The schemes of biopsy cores in TRBx and TPBx are shown in Figure 1. An oral antibiotic, quinolone (Daiichi-Sankyo, Tokyo, Japan) or cephalosporin (Roche, Basel, Switzerland), was administered on the day of TPBx. Intravenous or oral antibiotic, cephalosporin, quinolone or metronidazole (Xinyi, Shanghai, China), and antihemorrhagic agent, tranexamic acid (Tiancheng, Changchun, China) or hemocoagulase (Aohong, Jinzhou, China), were administered immediately after the TRBx and last for 3 days.
Figure 1.
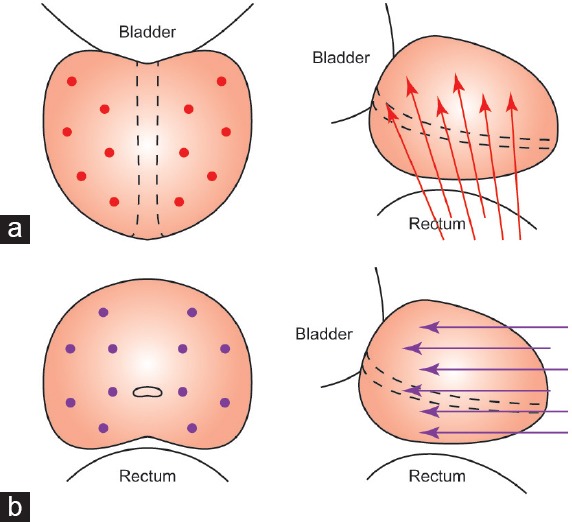
Schemes of cores in TRBx and TPBx. (a) Scheme of 12 cores in transrectal approach on prostatic coronal and sagittal plane. (b) Scheme of 12 cores in transperineal approach on prostatic cross-sectional and sagittal plane. TRBx: transrectal biopsy; TPBx: transperineal biopsy.
Pathology and PCa diagnosis
The biopsy specimens were fixed in formalin and embedded in paraffin. After slicing (4 μm thick) and histological staining, pathological sections were inspected and diagnosed. Gross description, site designation, pathological diagnosis, number of positive cores, and Gleason score were reported by experienced uropathologists. All of the PCa pathological diagnoses were confirmed by two independent pathologists.
Statistical analyses
Statistical analyses were carried out with SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (s.d.) or median (interquartile range [IQR]) and percentage for continuous and categorical variables, respectively. To minimize confounding from age, PV, and PSA level between TRBx group and TPBx group, a propensity score was generated for each patient from a multivariable logistic regression model based on patients' age, PSA value, and PV. TRBx patients were matched to TPBx patients at a 1:1 ratio using a nearest neighbor matching algorithm. A caliper width of 0.25 × s.d. of the logit of the propensity score was used. After matching, 376 patients in each group were selected. Subsequently, covariate balance was assessed between groups using the standardized difference:
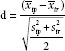
while x̄tp and x̄tr denote the sample mean of the covariate in TPBx and TRBx groups, respectively, while s2tp and s2tr denote the sample variance of the covariate in TPBx and TRBx groups, respectively. In addition, a jitter plot of individual cases, a line plot of individual differences, a histogram of individual differences, and a histogram of standardized differences were mapped (Figure 2). Student's t-test and Mann–Whitney U test were used to compare patients' clinical data between the groups. Pearson's Chi-square test or Fisher's exact test was used to compare the PCa detection rate between the groups. Predictors of a favorable outcome were assessed by stepwise multiple logistic regression. In both multivariable and univariable analyses, the magnitude of effects was expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Reported statistical significance levels were all two-sided and the threshold of statistical significance was set as P < 0.05.
Figure 2.
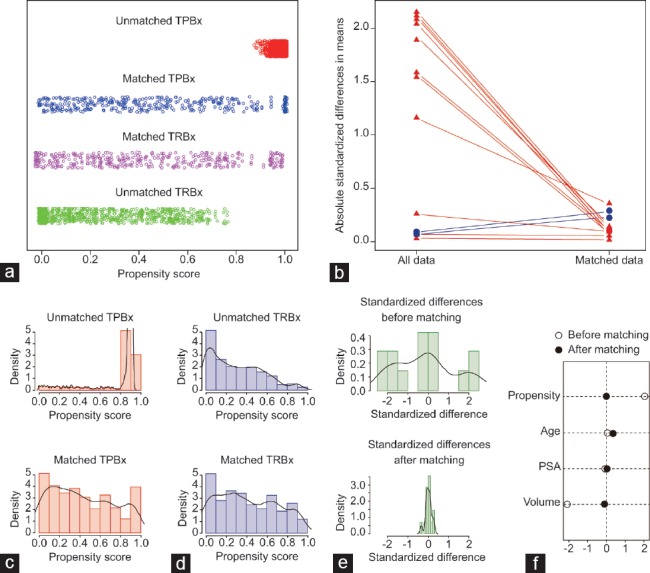
Balanced diagnostics for comparing the distribution of baseline covariates (age, PSA level and PV) between TRBx group and TPBx group. (a) Jitter plot of distribution of individual patients' propensity scores. (b) Line plot of standardized differences. (c) Histogram of distribution of patients' propensity scores in unmatched and matched TPBx groups. (d) Histogram of distribution of patients' propensity scores in unmatched and matched TRBx groups. (e) Histogram of standardized differences between the two groups before and after matching. (f) Differences of baseline covariates before and after matching. TRBx: transrectal biopsy; TPBx: transperineal biopsy; PSA: prostate-specific antigen; PV: prostate volume.
RESULTS
The overall PCa detection rate and patients' clinical data are listed in Table 1. In the TRBx group, 524 (43.1%) of 1216 cases were diagnosed with PCa. In the TPBx group, 785 (45.0%) of 1746 cases were diagnosed with PCa. The overall PCa detection rate between TRBx group and TPBx group was comparable (P = 0.314). Table 1 also shows the differences between the two groups in the patients' clinical characteristics, including age, PSA level, and PV. After propensity score matching, 376 patients in each group were matched in a 1:1 ratio. Figure 2 shows that the multivariate imbalance between the two groups was reduced remarkably after the matching.
Table 1.
Patients’ clinical data, and prostate cancer detection rate
| Characteristics | TRBx | TPBx | P |
|---|---|---|---|
| Cases (n) | 1216 | 1746 | |
| Overall detection rate, n (%) | 524 (43.1) | 785 (45.0) | 0.314 |
| Age (year) | |||
| Median (IQR) | 68 (63–75) | 71 (64–76) | 0.001 |
| Mean±s.d. | 69.20±8.03 | 69.72±8.93 | 0.106 |
| Range | 43–93 | 36–94 | |
| PSA (ng ml−1) | |||
| Median (IQR) | 11.19 (6.45–22.86) | 16.24 (8.93–48.15) | <0.001 |
| Mean±s.d. | 40.31±130.08 | 38.02±91.11 | 0.597 |
| Range | 0.29–1750.00 | 0.28–1848.00 | |
| PV (ml) | |||
| Median (IQR) | 50.57 (35.82–74.26) | 50.34 (32.14–62.59) | <0.001 |
| Mean±s.d. | 59.64±33.44 | 51.75±23.94 | <0.001 |
| Range | 8.89–245.42 | 8.71–233.83 | |
| Gleason score of PCa | |||
| Median (IQR) | 7 (6–7) | 7 (7–9) | <0.001 |
| Mean±s.d. | 6.8±1.4 | 7.6±1.0 | <0.001 |
| Range | 5–10 | 5–10 |
TRBx: transrectal biopsy; TPBx: transperineal biopsy; IQR: interquartile range; s.d.: standard deviation; PCa: prostate cancer; PSA: prostate-specific antigen; PV: prostate volume
The stratified detection rate is listed in Table 2. Before propensity score matching, the detection rate of TPBx was higher than that of TRBx for patients aged 60–69 years (38.6% vs 32.0%, P = 0.020). This difference was eliminated after matching (41.2% vs 36.5%, P = 0.455). For patients aged ≥80 years, TRBx had a higher detection rate than TPBx, both before (76.7% vs 56.8%, P < 0.001) and after (80.4% vs 56.5%, P = 0.004) propensity score matching. For patients with PSA level 10.1–20.0 ng ml−1, detection rate of TRBx was higher than that of TPBx before propensity score matching (41.6% vs 28.4%, P < 0.001). This difference was eliminated after matching (42.4% vs 34.2%, P = 0.233). A higher detection rate was obtained in patients who underwent TRBx when PSA level was 20.1–100.0 ng ml−1 before (69.2% vs 53.9%, P < 0.001) and after (80.9% vs 69.1%, P = 0.040) propensity score matching. With respect to PV, the detection rate between the groups was comparable in all stratification.
Table 2.
Prostate cancer detection rate stratified by age, prostate-specific antigen value, and prostate volume before and after propensity score matching
| Groups | Unmatched | Propensity score matched | ||||
|---|---|---|---|---|---|---|
| Detection rate in TRBx | Detection rate in TPBx | P | Detection rate in TRBx | Detection rate in TPBx | P | |
| Overall detection rate, n/total (%) | 524/1216 (43.1) | 785/1746 (45.0) | 0.314 | 184/376 (48.9) | 182/376 (48.4) | 0.884 |
| Age (year), n/total (%) | ||||||
| ≤59 | 24/122 (19.7) | 50/206 (24.3) | 0.335 | 5/33 (15.2) | 13/41 (31.7) | 0.099 |
| 60–69 | 176/550 (32.0) | 226/585 (38.6) | 0.020 | 57/156 (36.5) | 40/97 (41.2) | 0.455 |
| 70–79 | 212/398 (53.3) | 380/728 (52.2) | 0.731 | 81/136 (59.6) | 77/146 (52.7) | 0.249 |
| ≥80 | 112/146 (76.7) | 129/227 (56.8) | <0.001 | 41/51 (80.4) | 52/92 (56.5) | 0.004 |
| PSA (ng ml−1), n/total (%) | ||||||
| ≤4.0 | 14/103 (13.6) | 16/98 (16.3) | 0.587 | 7/30 (23.3) | 2/14 (14.3) | 0.695a |
| 4.1–10.0 | 103/430 (24.0) | 101/416 (24.3) | 0.912 | 40/138 (29.0) | 25/83 (30.1) | 0.858 |
| 10.1–20.0 | 136/327 (41.6) | 134/472 (28.4) | <0.001 | 39/92 (42.4) | 38/111 (34.2) | 0.233 |
| 20.1–100.0 | 191/276 (69.2) | 258/479 (53.9) | <0.001 | 76/94 (80.9) | 114/165 (69.1) | 0.040 |
| >100.0 | 80/80 (100) | 276/281 (98.2) | 0.591a | 22/22 (100) | 3/3 (100) | NA |
| PV (ml), n/total (%) | ||||||
| <25 | 65/105 (61.9) | 146/249 (58.6) | 0.567 | 64/106 (60.4) | 57/106 (53.8) | 0.331 |
| 25≤PV<50 | 243/487 (49.9) | 360/765 (47.1) | 0.327 | 72/139 (51.8) | 71/138 (51.4) | 0.954 |
| 50≤PV<75 | 122/325 (37.5) | 202/477 (42.3) | 0.173 | 33/83 (39.8) | 40/86 (46.5) | 0.376 |
| ≥75 | 94/299 (31.4) | 77/255 (30.2) | 0.752 | 15/48 (31.2) | 14/46 (30.4) | 0.932 |
aFisher’s exact test. TRBx: transrectal biopsy; TPBx: transperineal biopsy; PSA: prostate-specific antigen; PV: prostate volume; NA: not available.
Table 3 shows the bioptic Gleason score of PCa patients in each group after propensity score matching. PCa with Gleason score = 7 in total propensity score-matched patients was 48.9% for the TRBx group and 40.1% for the TPBx group. PCa with bioptic Gleason score ≥8 was 19.6% for the TRBx group and 42.3% for the TPBx group. For all the patients, TPBx detected more patients with Gleason score ≥7 PCa than TRBx detected (P < 0.001). In the PCa patients with PSA ≤20 ng ml−1 after propensity score matching, the proportion of patients with bioptic Gleason score = 7 was 51.2% for the TRBx group and 55.4% for the TPBx group. The proportion of patients with bioptic Gleason score ≥8 was 7.0% for the TRBx group and 13.8% for the TPBx group. Thus, for patients with PSA ≤20 ng ml−1, the detection rate for Gleason score ≥7 PCa between the two groups was comparable (P = 0.093), although TPBx had an advantage of about 4% and 7% for detection of PCa with Gleason score = 7 and ≥8, respectively.
Table 3.
Distribution of different Gleason score in prostate cancer patients between transrectal biopsy and transperineal biopsy after propensity score matching
| Gleason score, n (%) | Total | PSA ≤20 ng ml−1 | ||||
|---|---|---|---|---|---|---|
| TRBx (n=184) | TPBx (n=182) | P | TRBx (n=86) | TPBx (n=65) | P | |
| ≤6 | 58 (31.5) | 32 (17.6) | 36 (41.9) | 20 (30.8) | ||
| 7 | 90 (48.9) | 73 (40.1) | <0.001a | 44 (51.2) | 36 (55.4) | 0.093a |
| ≥8 | 36 (19.6) | 77 (42.3) | 6 (7.0) | 9 (13.8) | ||
aMann-Whitney U test to compare the distribution of Gleason scores between TRBx and TPBx. TRBx: transrectal biopsy; TPBx: transperineal biopsy; PSA: prostate-specific antigen
In the propensity score-adjusted multivariable and univariable logistic regression analysis (Table 4), patients' age and PSA level were discovered as independent predictors of obtaining a higher detection rate both in the TRBx and TPBx groups. Patients who underwent TRBx with lower PV were inclined to have a higher detection rate (multivariable, OR: 0.982, 95% CI: 0.973–0.991, P < 0.001; univariable, OR: 0.990, 95% CI: 0.983–0.997, P = 0.004). A similar result was not found in the TPBx group (multivariable, OR: 0.993, 95% CI: 0.985–1.000, P = 0.065; univariable, OR: 0.995, 95% CI: 0.988–1.001, P = 0.111), which indicated that PV did not have a noticeable impact on TPBx.
Table 4.
Multivariable and univariable logistic regression model for analyzing the effects of patients’ clinical characteristics on prostate cancer detection rate after propensity score matching
| Characteristics | Multivariable | Univariable | ||
|---|---|---|---|---|
| TRBx, OR (95% CI), P | TPBx, OR (95% CI), P | TRBx, OR (95% CI), P | TPBx, OR (95% CI), P | |
| Age | 1.101 (1.064–1.139), <0.001 | 1.038 (1.014–1.063), 0.002 | 1.118 (1.085–1.152), <0.001 | 1.045 (1.023–1.067), <0.001 |
| PSA | 1.059 (1.037–1.081), <0.001 | 1.035 (1.026–1.044), <0.001 | 1.064 (1.042–1.087), <0.001 | 1.035 (1.026–1.044), <0.001 |
| PV | 0.982 (0.973–0.991), <0.001 | 0.993 (0.985–1.000), 0.065 | 0.990 (0.983–0.997), 0.004 | 0.995, (0.988–1.001), 0.111 |
TRBx: transrectal biopsy; TPBx: transperineal biopsy; OR: odds ratio; CI: confidence interval; PSA: prostate-specific antigen; PV: prostate volume
DISCUSSION
TRUS-guided systematic prostate biopsy is an effective diagnostic strategy for PCa. TRBx and TPBx are two approaches for performing the operation, and previous studies documented the difference in overall PCa detection rate between the approaches is comparable.9,10 However, there are obvious differences between these two methods in the procedure and complication rate. Compared with the complicated steps of TPBx, TRBx is time-saving and easier to perform. Periprostatic nerve block before TPBx is complicated and may not relieve the pain from core sampling,16 whereas intrarectal local infiltration using lidocaine gel is simple and sufficient for TRBx.17 Nevertheless, TPBx is less likely to present serious postoperative complications such as infection and hemorrhage. Infectious complications including bacteriuria, acute bacterial prostatitis, epididymitis, and sepsis are major reasons for postoperative hospitalization and have a higher incidence in TRBx compared with TPBx.11 In addition, the two methods have distinct blind areas for detection, especially in enlarged prostate. The transrectal approach rarely detects tumors in the anterior prostate. Patients who receive TPBx may benefit from its high detection rate of anterior and apical tumors.18
Prior studies compared the overall detection rate, while we suggest that patients with different clinical characteristics should be treated separately. Patients' characteristics include age, PSA level, and PV is different; therefore, which approach can obtain a higher detection rate is confusing to clinicians. In addition, differences in the distribution of cores between TRBx and TPBx mean that their different efficacies to detect clinically significant PCa. Thus, prostate biopsy should be optimized for different patients according to their baseline characteristics. In the present study, to compare the detection rate of TRBx and TPBx for different stratification of patients' clinical characteristics, we performed propensity score-matching analysis. We hope that our study will provide practical advice to clinicians for optimizing prostate biopsy especially for Chinese patients.
Patients' age is the first factor that clinicians should consider before performing prostate biopsy. Here, through comparing the detection rate regarding stratified age after propensity score matching, we found that TRBx had a higher efficacy over TPBx for patients aged ≥80 years. It is known that incidence of PCa is positively correlated with age. Previous study reported that PCa was found in 30.5% of patients aged >75 years compared with 5.2% in those aged <50 years,19 although most of those may not have been clinically significant cancer. Moreover, elderly patients often have one or more chronic diseases, such as hypertension, diabetes mellitus, cardiovascular disease, or may have potential organ dysfunction. Although most procedures for prostate biopsy are safe, comorbidity is often a motivation of death because of serious postoperative complications, including sepsis and severe hemorrhage.20 In addition, PSA level and PV elevation along with age may lead to unnecessary prostate biopsy.21,22
PSA level is the most important value for PCa screening, as well as for tracking the effectiveness of treatment and possible disease progression. However, the specificity of PSA is imperfect. Increased PSA level may also result from benign prostatic hyperplasia or lower urinary tract infection. Finding an effective way to distinguish noncancerous diseases with elevated PSA from PCa could reduce unnecessary biopsy. Moreover, PSA level increases with age, while age-stratified PSA varies among regions.23 It is generally acknowledged that the gray zone for PSA level is 4–10 ng ml−1 in Western countries. However, some researchers argue that the upper limit of the PSA gray zone should be higher for Chinese men.24,25 This means that the appropriate screening strategies and therapies for PCa in China differ from those in Western countries. PCa detection rate by systematic biopsy in a Western cohort with PSA level of 4.0–10.0 ng ml−1 varied from 30% to 50%,1,26,27,28 whereas the detection rate in a Chinese cohort with the same PSA range was lower. For example, the detection rate was 25.3% among a Chinese cohort from Shanghai with a PSA level of 4.0–10.0 ng ml−1 and 36.5% among those with PSA level of 10.0–20.0 ng ml−1.22 Another study from Shanghai reported a detection rate of 26.2% in patients with PSA level of 2.0–20.0 ng ml−1.29 The detection rate in a Chinese cohort from Guangzhou with PSA level of 2.5–20.0 ng ml−1 was only 25.6%.30 Besides, clinically insignificant PCa accounts for a large proportion in positive biopsy result for Chinese patients with gray zone PSA level.31 In the present study, we found that the detection rate of two approaches showed no difference after propensity score matching in patients with PSA level of 4.1–20.0 ng ml−1. Thus, our data indicated that, for Chinese men with PSA level of 4.1–20.0 ng ml−1, there was no difference in diagnostic efficacy between TRBx and TPBx. Furthermore, when PSA ranged from 20.1 to 100.0 ng ml−1, TRBx showed a greater detection rate than TPBx. This may result from the advantage of TRBx for detecting transitional zone tumor which also contributes to the detection rates.
The inverse association between PV and cancer detection by prostate biopsy is acknowledged by many studies.24,27,28 Increased PSA caused by enlarged prostate creates a dilemma about whether and when patients should undergo biopsy. In general, the enlarged prostatic transitional zone has lower cancer incidence rate and its tumor often has insignificant Gleason score.32 Hence, some researchers consider that additional transitional zone sampling is unnecessary. Nevertheless, cores in the transitional zone may increase the detection rate for patients with gray-zone PSA levels or in repeat biopsy.10,33 In this study, the PV-stratified detection rate between TRBx and TPBx was similar. The data showed that PV increment could result in detection rate decrease in both approaches. Through univariate and multivariate logistic regression, we found that age and PSA level were positively associated with PCa detection rate in both methods. However, PV did not have an apparent impact on cancer detection rate of TPBx in the propensity score-adjusted cohort. This may result from distinct schemes for obtaining cores in the two methods. Both lateral and medial cores of TRBx may contain more transitional zone components in large prostates. Medial cores of TPBx may penetrate the enlarged transitional zone while all of lateral cores of TPBx are mainly located in the peripheral zone. Regardless of the PV, most TPBx cores collect apical tissues, which are usually the tumor foci.18
To distinguish clinically significant PCa from all biopsy-detected PCa is a vital criterion for evaluating a biopsy method. A procedure that can find a high proportion of clinically significant PCa will help reduce repeat biopsy rate and overtreatment.34 Systematic TRBx and TPBx detect a certain proportion of insignificant PCa, especially in patients with PSA ≤10 ng ml−1. However, Gleason score ≤6 in biopsy should not be designated simply as insignificant PCa because Gleason score may upgrade after radical prostatectomy.35 In the present study, TPBx in addition to TRBx resulted in 13% more PCa with Gleason score ≥7 after propensity score adjustment. For patients with PSA ≤20 ng ml−1, TPBx still had an advantage over TRBx to certain extent. Thus, TRBx may miss more clinically significant PCa as compared with TPBx. A further study, with a larger number of patients with PSA ≤20 ng ml−1, should be carried out to compare efficacy between the two approaches. According to our present data, we propose that TPBx has greater diagnostic efficacy than TRBx for detecting clinically significant PCa.
This study had some limitations. First, it is a retrospective study. Although confounding factors of age, PSA, and PV were minimized after propensity score matching, the selection bias was not eliminated entirely. Second, the different geographic location of the patients could have affected the detection rate and characteristics of PCa patients because our data were from two hospitals in different Chinese provinces. Besides, range of PSA levels or age was wide in this study. Furthermore, postoperative complications were not included in the present study, although we admit that distinct complications rate is critical for optimizing biopsy. We suggest that good preparation for biopsy and postoperative application of antibiotics, α-blockers, and antihemorrhagic agents36 are useful to reduce the complications no matter which approaches were performed.
CONCLUSIONS
Our results suggested that systematic 12-core TRBx and TPBx had comparable efficacy for patients aged <80 years and with PSA level ≤20 ng ml−1. However, PV had greater impact on PCa detection rate of TRBx compared with that of TPBx. In addition, TPBx was associated with a higher detection rate of clinically significant PCa. Although both TRBx and TPBx offer similar outcomes for all patients, patients' clinical characteristics should also be taken into consideration for optimizing prostate biopsy.
AUTHOR CONTRIBUTIONS
CYJ and PFS conceived of the study, collected data, performed the statistical analysis, and drafted the manuscript. CW and HJG helped to collect data and performed the statistical analysis. YR, HZ, and SJX participated in design and coordination of the study. FJZ and QW conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (No. 81570682, No. 81772746, and No. 81870516) and the grants from Shanghai Municipal Health Bureau (No. 2013ZYJB0102).
REFERENCES
- 1.Emiliozzi P, Scarpone P, DePaula F, Pizzo M, Federico G, et al. The incidence of prostate cancer in men with prostate specific antigen greater than 4.0 ng/ml: a randomized study of 6 versus 12 core transperineal prostate biopsy. J Urol. 2004;171:197–9. doi: 10.1097/01.ju.0000099824.73886.f3. [DOI] [PubMed] [Google Scholar]
- 2.Elabbady AA, Khedr MM. Extended 12-core prostate biopsy increases both the detection of prostate cancer and the accuracy of Gleason score. Eur Urol. 2006;49:49–53. doi: 10.1016/j.eururo.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Giannarini G, Autorino R, di Lorenzo G. Saturation biopsy of the prostate: why saturation does not saturate. Eur Urol. 2009;56:619–21. doi: 10.1016/j.eururo.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Zaytoun OM, Moussa AS, Gao T, Fareed K, Jones JS. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011;186:850–4. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 5.Irani J, Blanchet P, Salomon L, Coloby P, Hubert J, et al. Is an extended 20-core prostate biopsy protocol more efficient than the standard 12-core? A randomized multicenter trial. J Urol. 2013;190:77–83. doi: 10.1016/j.juro.2012.12.109. [DOI] [PubMed] [Google Scholar]
- 6.Borkowetz A, Platzek I, Toma M, Laniado M, Baretton G, et al. Comparison of systematic transrectal biopsy to transperineal magnetic resonance imaging/ultrasound-fusion biopsy for the diagnosis of prostate cancer. BJU Int. 2015;116:873–9. doi: 10.1111/bju.13023. [DOI] [PubMed] [Google Scholar]
- 7.Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, et al. A randomized controlled trial to assess and compare the outcomes of two-core prostate biopsy guided by fused magnetic resonance and transrectal ultrasound images and traditional 12-core systematic biopsy. Eur Urol. 2016;69:149–56. doi: 10.1016/j.eururo.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313:390–7. doi: 10.1001/jama.2014.17942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara R, Jo Y, Fujii T, Kondo N, Yokoyoma T, et al. Optimal approach for prostate cancer detection as initial biopsy: prospective randomized study comparing transperineal versus transrectal systematic 12-core biopsy. Urology. 2008;71:191–5. doi: 10.1016/j.urology.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka A, Hara R, Ishimura T, Fujii T, Jo Y, et al. A prospective randomized comparison of diagnostic efficacy between transperineal and transrectal 12-core prostate biopsy. Prostate Cancer Prostatic Dis. 2008;11:134–8. doi: 10.1038/sj.pcan.4500985. [DOI] [PubMed] [Google Scholar]
- 11.Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, et al. Sepsis and 'superbugs': should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014;114:384–8. doi: 10.1111/bju.12536. [DOI] [PubMed] [Google Scholar]
- 12.Cormio L, Scattoni V, Lorusso F, Perrone A, Di Fino G, et al. Prostate cancer detection rates in different biopsy schemes. Which cores for which patients? World J Urol. 2014;32:341–6. doi: 10.1007/s00345-012-0989-8. [DOI] [PubMed] [Google Scholar]
- 13.Ou Y, Shen D, Zeng J, Sun L, Moul J, et al. Sampling the spatial patterns of cancer: optimized biopsy procedures for estimating prostate cancer volume and Gleason score. Med Image Anal. 2009;13:609–20. doi: 10.1016/j.media.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Futterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, et al. Can clinically significant prostate cancer be detected with multiparametric magnetic resonance imaging? A systematic review of the literature. Eur Urol. 2015;68:1045–53. doi: 10.1016/j.eururo.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Xia SJ, Cui D, Jiang Q. An overview of prostate diseases and their characteristics specific to Asian men. Asian J Androl. 2012;14:458–64. doi: 10.1038/aja.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingber MS, Ibrahim I, Turzewski C, Hollander JB, Diokno AC. Does periprostatic block reduce pain during transrectal prostate biopsy? A randomized, placebo-controlled, double-blinded study. Int Urol Nephrol. 2010;42:23–7. doi: 10.1007/s11255-009-9621-2. [DOI] [PubMed] [Google Scholar]
- 17.Imani F, Moghaddam Y, Shariat Moharari R, Etezadi F, Khajavi MR, et al. Intrarectal lidocaine-diltiazem-meperidine gel for transrectal ultrasound guided prostate biopsy. Anesth Pain Med. 2015;5:e22568. doi: 10.5812/aapm.5(3)2015.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossack T, Patel MI, Huo A, Brenner P, Yuen C, et al. Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J Urol. 2012;188:781–5. doi: 10.1016/j.juro.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Pignot G, Salomon L, Lebacle C, Neuzillet Y, Lunardi P, et al. Prostate cancer incidence on cystoprostatectomy specimens is directly linked to age: results from a multicentre study. BJU Int. 2015;115:87–93. doi: 10.1111/bju.12803. [DOI] [PubMed] [Google Scholar]
- 20.Jha GG, Anand V, Soubra A, Konety BR. Challenges of managing elderly men with prostate cancer. Nat Rev Clin Oncol. 2014;11:354–64. doi: 10.1038/nrclinonc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia SJ, Xu XX, Teng JB, Xu CX, Tang XD. Characteristic pattern of human prostatic growth with age. Asian J Androl. 2002;4:269–71. [PubMed] [Google Scholar]
- 22.Chen R, Huang Y, Cai X, Xie L, He D, et al. Age-specific cutoff value for the application of percent free prostate-specific antigen (PSA) in Chinese men with serum PSA levels of 4.0-10.0 ng/ml. PLoS One. 2015;10:e0130308. doi: 10.1371/journal.pone.0130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11:197–212. doi: 10.1038/nrurol.2014.42. [DOI] [PubMed] [Google Scholar]
- 24.Tang P, Jin XL, Uhlman M, Lin YR, Deng XR, et al. Prostate volume as an independent predictor of prostate cancer in men with PSA of 10-50 ng ml−1. Asian J Androl. 2013;15:409–12. doi: 10.1038/aja.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Na R, Ye D, Liu F, Chen H, Qi J, et al. Performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and the prostate health index (PHI) in a Chinese hospital-based biopsy population. Prostate. 2014;74:1569–75. doi: 10.1002/pros.22876. [DOI] [PubMed] [Google Scholar]
- 26.Naughton CK, Miller DC, Mager DE, Ornstein DK, Catalona WJ. A prospective randomized trial comparing 6 versus 12 prostate biopsy cores: impact on cancer detection. J Urol. 2000;164:388–92. [PubMed] [Google Scholar]
- 27.Ficarra V, Novella G, Novara G, Galfano A, Pea M, et al. The potential impact of prostate volume in the planning of optimal number of cores in the systematic transperineal prostate biopsy. Eur Urol. 2005;48:932–7. doi: 10.1016/j.eururo.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Al-Azab R, Toi A, Lockwood G, Kulkarni GS, Fleshner N. Prostate volume is strongest predictor of cancer diagnosis at transrectal ultrasound-guided prostate biopsy with prostate-specific antigen values between 2.0 and 9.0 ng/mL. Urology. 2007;69:103–7. doi: 10.1016/j.urology.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 29.Wu YS, Wu XB, Zhang N, Jiang GL, Yu Y, et al. Evaluation of PSA-age volume score in predicting prostate cancer in Chinese population. Asian J Androl. 2018;20:324–9. doi: 10.4103/aja.aja_81_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin YR, Wei XH, Uhlman M, Lin XT, Wu SF, et al. PSA density improves the rate of prostate cancer detection in Chinese men with a PSA between 2.5-10.0 ng ml-1 and 10.1-20.0 ng ml-1: a multicenter study. Asian J Androl. 2015;17:503–7. doi: 10.4103/1008-682X.142129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Covarrubias F, Gonzalez-Ramirez A, Aguilar-Davidov B, Castillejos-Molina R, Sotomayor M, et al. Extended sampling at first biopsy improves cancer detection rate: results of a prospective, randomized trial comparing 12 versus 18-core prostate biopsy. J Urol. 2011;185:2132–6. doi: 10.1016/j.juro.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Wang CC, Carter HB, Epstein JI. Value of transition zone biopsy in active surveillance of prostate cancer. J Urol. 2014;191:1755–9. doi: 10.1016/j.juro.2013.11.058. [DOI] [PubMed] [Google Scholar]
- 33.de la Rosette JJ, Wink MH, Mamoulakis C, Wondergem N, ten Kate FJ, et al. Optimizing prostate cancer detection: 8 versus 12-core biopsy protocol. J Urol. 2009;182:1329–36. doi: 10.1016/j.juro.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 34.Chang DT, Challacombe B, Lawrentschuk N. Transperineal biopsy of the prostate– Is this the future? Nat Rev Urol. 2013;10:690–702. doi: 10.1038/nrurol.2013.195. [DOI] [PubMed] [Google Scholar]
- 35.Gao Y, Jiang CY, Mao SK, Cui D, Hao KY, et al. Low serum testosterone predicts upgrading and upstaging of prostate cancer after radical prostatectomy. Asian J Androl. 2016;18:639–43. doi: 10.4103/1008-682X.169984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71:353–65. doi: 10.1016/j.eururo.2016.08.004. [DOI] [PubMed] [Google Scholar]


