Abstract
Seminal plasma is a rich source of proteins and serves as an ideal sample for proteomic analysis of male infertility. In varicocele-associated infertility, the contributory role of seminal plasma proteins specific to unilateral and bilateral varicocele is not clear. Furthermore, there is a lack of specific protein biomarker to differentiate bilateral from unilateral varicocele. The main objective is to identify the differentially regulated molecular and cellular pathways in bilateral varicocele. Furthermore, we intend to identify seminal plasma biomarkers to differentiate bilateral and unilateral varicocele patients in comparison with fertile healthy men. Global proteomic analysis of seminal plasma proteins has identified the functionality of differentially expressed proteins (DEPs) in varicocele patients. Bioinformatic analysis has revealed response to reactive oxygen species and oxidative stress, and tissue homeostasis as top process pathways that are affected in bilateral varicocele patients compared to fertile healthy men. In comparison with unilateral varicocele patients, inflammatory response pathways were dysregulated, especially interleukin 6 (IL-6) signaling and Janus kinase-signal transducer and activator of transcription (Jak-STAT) pathways, in bilateral varicocele patients, owing to the involvement of underexpressed DEPs. Key DEPs associated with oxidative stress (peroxiredoxin 2; PRDX2), DNA fragmentation (fatty acid synthase; FASN), and inflammatory response (fibronectin 1; FN1) validated by western blot analysis revealed differential expression of these proteins in unilateral and bilateral varicocele groups. Altered expression of DEPs and its association with key processes show that the seminal plasma homeostasis is compromised in bilateral varicocele patients. Furthermore, we propose PRDX2, FASN, and FN1 as potential noninvasive seminal plasma markers for the differentiation of unilateral and bilateral varicocele patients.
Keywords: biomarkers, male infertility, proteomics, seminal plasma, varicocele
INTRODUCTION
Varicocele is a major contributing factor for male infertility and prevalent in 15% of male population.1,2 It is also responsible for 40% and 80% of primary and secondary male factor infertility, respectively.1,3 Varicocele represents a major correctable cause of male infertility, whereby the occurrence of unilateral varicocele is more common than that of bilateral varicocele.4 Men with varicocele usually have altered semen parameters.5 Decades of research have revealed that seminal oxidative stress and sperm DNA damage are the major contributors of varicocele-mediated male infertility,5,6 wherein the damages are more severe in bilateral varicocele compared to unilateral varicocele.
Varicocele has been reported to trigger a series of inflammatory events with a negative impact on spermatogenesis.7 In fact, elevated levels of pro-inflammatory cytokines have been observed in the seminal plasma of varicocele patients.8 Inflammatory cytokines and reactive oxygen species (ROS) levels are known to increase during inflammation, thus impairing the process of sperm production. Altered expression of cytokines such as interleukin 6 (IL-6), IL-8, and IL-10 have been reported in the semen of subfertile men.9,10 Up till now, the molecular mechanisms by which varicocele affects sperm quality and male fertility are largely unknown. Studies on seminal plasma may help to find new information on this topic as it provides a protective environment to the spermatozoa, reflecting their functional state.11 The disturbance of seminal plasma proteome affects the fertilizing potential of the male germ cells.12
In the postgenomic era, a global proteomic approach is widely used to investigate the role of key proteins in the etiology of male infertility. Proteomic analysis of spermatozoa and seminal plasma provides new insights into the molecular pathways responsible for sperm function. Previous studies with varicocele patients have identified the altered expression of proteins in spermatozoa13,14,15 and seminal plasma16,17,18 related to sperm function. Studies on seminal plasma proteins in varicocele patients have demonstrated the proteins linked to inflammatory pathways.17,19 However, in varicocele patients, these processes are still poorly understood, and there are no specific protein biomarkers for the early diagnosis of unilateral or bilateral condition.
The current study is an extension of the report on sperm proteomics.15 Here, we have used proteomic approach to identify the differentially regulated molecular and cellular pathways affected in seminal plasma of bilateral varicocele patients. Furthermore, we intend to identify seminal plasma protein biomarkers to differentiate bilateral and unilateral varicocele patients.
PARTICIPANTS AND METHODS
Study participants
The current study was approved by the Institutional Review Board (IRB) of Cleveland Clinic (Cleveland, OH, USA). Signed written consent was obtained from all the participants (age of 20–40 years) enrolled in this study from March 2012 to 2014. Clinical varicocele was diagnosed by scrotal palpation and was graded and characterized into unilateral and bilateral varicocele as described in our previous publication.15 Semen samples were collected from 33 unilateral and 17 bilateral varicocele patients, and 10 healthy proven fertile men (without clinical varicocele) were included as a control group. The results of sperm proteomics in these patients have been analyzed and reported by us earlier.14 In the current study, Grade 1 to 3 varicocele patients were used for comparative seminal plasma proteomic experiment. The present study was conducted in compliance with the Minimum Information about a Proteomics Experiment (MIAPE) guidelines of the Human Proteome Organization's Proteomics Standards Initiative (HUPO-PSI) for reporting proteomics studies.20
All the participants included in the study were nonsmokers and had normal body mass index. These individuals were never exposed to any harmful radiation or environmental stress. All healthy fertile men without clinical varicocele included in the control group have initiated pregnancy or fathered a child in the last 2 years. Female partners of the infertile men were subjected to general gynecological evaluation and were reported to have normal reproductive health.
Men with leukocytospermia (Endtz positive), azoospermia, and oligozoospermia (<106 sperm per ml) were excluded from the study. Patients with recurring fever prior to 90 days of semen analysis were also excluded from the study. Infertile men with genetic defects and reproductive tract infection diagnosed by andrological examination were not included in the present study. Varicocele patients and fertile healthy men with a history of systemic illness, inflammation of reproductive tract (orchitis, epididymitis, urethritis, and testicular atrophy), or sexually transmitted disease were also excluded from the study.14,15
Semen analysis and preparation of samples for proteomic studies
Semen analysis was performed according to the WHO guidelines.21 Semen samples were collected by masturbation at the Andrology Laboratory (Cleveland Clinic, Cleveland, OH, USA) after sexual abstinence of at least 48 h. The samples were allowed to liquefy completely for 20 min at 37°C. After complete liquefaction, routine semen analysis was performed according to the WHO guidelines, and ROS levels and sperm DNA fragmentation (SDF) were measured by chemiluminescence assay using an AutoLumat LB 953 Multi-Tube luminometer (Berthold Technologies, Oak Ridge, TN, USA) and terminal deoxynucleotidyl transferase-mediated fluorescein end labeling (TUNEL) assay with an Apo-Direct™ kit (Pharmingen, San Diego, CA, USA), respectively.22,23 The semen samples were centrifuged for 7 min at 1000g (Eppendorf North America, Hauppauge, NY, USA) and checked for the presence of any sperm before the clear seminal plasma was aspirated and stored at −80°C for proteomic analysis.
Seminal plasma samples were thawed at room temperature and centrifuged at 3000g for 30 min at 4°C to completely remove contaminating spermatozoa and somatic cells completely.
The samples were mixed (1:1 ratio) with the protease inhibitor cocktail (Roche, Indianapolis, IN, USA) in phosphate-buffered saline to prevent proteolysis during sample handling. Protein concentration was determined using a bicinchoninic acid (BCA) kit (Thermo Fisher Scientific, Rockford, IL, USA). Pooled samples from the unilateral varicocele group (n = 5), bilateral varicocele group (n = 5), and fertile donor group (n = 5) were used for the proteomic analysis. Equal concentration of proteins from each individual sample was used to normalize the protein concentration in each group. In general, pooling of samples is accepted for seminal plasma proteomic analysis and it has been well documented in previous reports.14,24,25,26 The samples were run in triplicate in one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, each gel lane was cut into six pieces, digested using 5-μl trypsin (10 ng μl−1, Sigma-Aldrich, St. Louis, MO, USA) and 50 mmol l−1 ammonium bicarbonate (Sigma-Aldrich), and incubated overnight at room temperature. Prior to in-gel digestion, the samples (cut lanes) were alkylated with iodoacetamine (Sigma-Aldrich) and reduced with dithiothreitol (Sigma-Aldrich). The peptides from the digested gel were extracted in two aliquots of 30-μl acetonitrile (10%) with formic acid (5%) (Sigma-Aldrich). The two aliquots were pooled together and evaporated to <10 μl and then diluted with 1% acetic acid (Sigma-Aldrich) to make up a final volume of 30 μl.
Quantitative proteomics
Proteomic profiling of seminal plasma samples was carried out using a Finnigan LTQ linear ion trap mass spectrometer LC-MS/MS system (Thermo Fisher Scientific, Waltham, MA, USA). The peptides were fractionated by injecting 5 μl of above extracted mixture into a high-performance liquid chromatography (HPLC) column (Phenomenex Jupiter C18 reverse-phase capillary chromatography column). Fractions containing the peptides were eluted in acetonitrile/0.1% formic acid at a flow rate of 0.25 μl min−1 and were introduced into the source of the mass spectrometer online. The micro-electrospray ion source was operated at 2.5 kV. A full-spectral scan was performed by utilizing the data-dependent multitask ability of the instrument to determine peptide molecular weights and amino acid sequence of the peptides.22
A similar pipeline of analysis was followed to identify the proteins using Proteome Discoverer version 1.4.1.288. Mascot (version 2.3.02; Matrix Science, London, UK), Sequest (version 1.4.0.288; Thermo Fisher Scientific), and X!Tandem (version CYCLONE 2010.12.01.1; The Global Proteome Machine, thegpm.org). Protein identification criteria were established at >99% probability to achieve false detection rate (FDR) <1% as explained in our previous studies.14,15 The search was limited to the human protein reference database (http://www.hprd.org/) and the results were uploaded into the Scaffold (version 4.0.6.1; Proteome Software Inc., Portland, OR, USA) as previously described.27 Protein probabilities were assigned by the Protein Prophet (Systems Biology, Seattle, WA, USA) algorithm. Annotation of proteins was performed using Gene Ontology (GO) terms from the National Center for Biotechnology Information (NCBI).
Relative quantification of the proteins was performed by comparing the number of spectra and termed spectral counts in both varicocele and fertile donor groups. The abundance of the proteins was determined by matching the spectra (spectral counts) and classified as high (H), medium (M), low (L), or very low (VL). To overcome the sample-to-sample variation, normalization of spectral counts was done using the normalized spectral abundance factor (NSAF).15
Identification of differentially expressed proteins (DEPs) and bioinformatic analysis
As the focus of the study was to identify the DEPs to differentiate the molecular pathology associated with bilateral and unilateral varicocele, the following two different sets of comparison were performed using the proteomic data: (i) bilateral varicocele versus fertile men and (ii) unilateral varicocele versus bilateral varicocele to obtain two sets of DEPs (Figure 1). Furthermore, these two sets of DEPs were analyzed to identify the DEPs that were common in both the varicocele groups and differentially expressed in bilateral varicocele patients compared with fertile men. Such type of comparison was used to identify the molecular marker to distinguish bilateral and unilateral varicocele patients in comparison to fertile healthy men.
Figure 1.
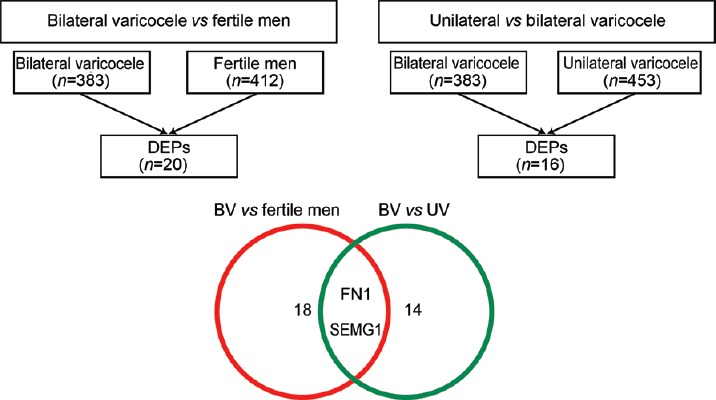
Comparison of protein profile of unilateral and bilateral varicocele and fertile men. Identification of DEPs specific to varicocele groups to distinguish between unilateral and bilateral varicocele. DEPs: differentially expressed proteins; UV: unilateral varicocele; BV: bilateral varicocele; FN1: fibronectin 1; SEMG1: semenogelin 1.
DEPs identified in two different comparisons were subjected to functional annotation and enrichment analysis using publicly available bioinformatic annotation tools; databases such as UniProt, Reactome, and Database for Annotation Visualization and Integrated Discovery (DAVID; http://david.niaid.nih.gov); and proprietary-curated databases such as Ingenuity pathway analysis (IPA) and Metacore™ (GeneGo Inc., St. Joseph, MI, USA) to analyze the involvement of DEPs in biological and cellular processes, pathways, cellular distribution, regulatory networks, and protein–protein interactions.
Protein selection and validation by western blot analysis
To distinguish the bilateral varicocele from unilateral varicocele condition, the proteins of fertility potential were selected for validation by western blot analysis. Three proteins (peroxiredoxin 2 [PRDX2], fatty acid synthase [FASN], and fibronectin 1 [FN1]) were selected for validation by western blot analysis in individual samples from the fertile healthy men group (n = 6), unilateral varicocele group (n = 6), and bilateral varicocele group (n = 6). Western blot analysis was carried out on the seminal plasma samples different from that used for proteomic analysis. All of these samples were selected using the same inclusion and exclusion criteria. A total of 50 μg of protein per sample was loaded into a 4%–15% SDS-PAGE for 2 h at 90 V. The resolved protein bands were then transferred onto polyvinylidene difluoride (PVDF) membranes and, for each protein analysis, specific primary antibodies were incubated at 4°C overnight (Supplementary Table 1). The membranes were incubated with the secondary antibody at room temperature for 1 h and finally reacted with enhanced chemiluminescence (ECL) reagent (GE Healthcare, Marlborough, MA, USA) for 5 min. The membranes were exposed to Chemi-Doc (ChemiDoc™ MP Imaging System, Bio-Rad, Hercules, CA, USA) to detect the chemiluminescence signals.
Supplementary Table 1.
List of primary and secondary antibodies
| Primary | ||||
|---|---|---|---|---|
| Protein | Antibody | Source | Manufacturer | Dilution |
| PRDX2 | Anti-human rabbit IgG | Rabbit polyclonal | Abcam ab 71533 | 1:1000 |
| FASN | Rabbit monoclonal | Abcam ab 128870 | 1:10000 | |
| FN1 | Rabbit monoclonal | Abcam ab 45688 | 1:5000 | |
| Secondary | ||||
| Anti-rabbit goat IgG | Goat polyclonal | Abcam ab97051 | 1:10000 | |
PRDX2: peroxiredoxin 2; FASN: fatty acid synthase; FN1: fibronectin 1; IgG immunoglobulin G
Initially, PRDX2 polyclonal antibody was used to probe the membranes and subsequently the same membrane was probed with FASN monoclonal antibody because the immunoreactive bands were absent from the area (molecular weight) corresponding to the expression of FASN protein. However, separate membranes were used to probe FN1 due to the heavy background produced by FN1 monoclonal antibody.
All the PVDF membranes used for protein identification were subjected to total protein staining. The membranes were briefly washed twice for 10 min in distilled water and stained with total colloidal gold protein stain (Bio-Rad) for 2 h at room temperature by gentle shaking. The stained membranes were washed twice with distilled water for 10 min, and the densitometry image was captured using the colorimetric mode on Chemi-Doc.
Statistical analyses
Data analysis was performed using MedCalc Statistical Software (version 17.8; MedCalc Software, Ostend, Belgium). After testing for normal distribution using the Kolmogorov–Smirnov test, Mann-Whitney U test was carried out to compare the semen parameters of the fertile donor group and the varicocele group, and P < 0.05 was considered statistically significant. The same test was used to compare the expression levels of the proteins validated using western blot technique in both the groups.
RESULTS
Semen parameters
Semen analysis revealed no statistically significant difference in sperm motility, sperm concentration, normal sperm morphology, and seminal ROS levels among unilateral and bilateral varicocele patients (all P > 0.05; Supplementary Table 2). The SDF detected using TUNEL assay was significantly higher in the bilateral varicocele group (P < 0.05, Supplementary Table 2).
Supplementary Table 2.
Semen parameters in healthy fertile men (control group) and in unilateral and bilateral varicocele patients
| Parameter | Healthy fertile men15 (n=10) | Varicocele patients14 | P | |
|---|---|---|---|---|
| Unilateral (n=17), n (%) | Bilateral (n=33), n (%) | |||
| Grade (1 or 2) | N/A | 26/31 (83.6) | 13/17 (76.4) | 0.62 |
| Grade (3 or 4) | N/A | 5/31 (16.1) | 4/17 (23.5) | |
| Sperm concentration (106/ml) | 69.90±37.65 | 26.35±25.57 | 35.51±44.86 | 0.76 |
| Sperm motility (%) | 57.1±16.0 | 40.23±17.09 | 41.76±19.78 | 0.86 |
| Normal sperm morphology (%) | 8.4±3.7 | 2.39±1.84 | 2.25±2.27 | 0.63 |
| ROS levels (RLU/s/106 sperm) | 142.7 (36.2, 337.7) | 766.2 (163.6, 3034.6) | 894.9 (149.2, 2842) | 0.75 |
| Sperm DNA fragmentation (%) | 14.4±3.9 | 12.0±8.8 | 16.4±10.1 | 0.02 |
These data were extracted from Agarwal et al., 2016,15 and Agarwal et al., 2015.14 Statistical analysis was performed between unilateral varicocele and bilateral varicocele patients. For all values, P<0.05 indicates a statistically significant difference based on the Mann–Whitney test. Sperm concentration, motility, morphology, and DNA fragmentation values are presented as mean±s.d. ROS levels are mentioned as median (25th and 75th percentiles), whereas sperm DNA fragmentation was represented as lower and upper limits. ROS: reactive oxygen species; RLU: relative light units; N/A: not available; s.d.: standard deviation
Identification of differentially expressed proteins
High-throughput proteomic analysis using Finnigan LTQ linear ion trap mass spectrometer detected a total of 453 and 383 proteins in unilateral and bilateral varicocele groups, respectively, whereas 412 proteins were identified in fertile healthy men group. Quantification of proteins was done based on the NSAF ratio. Comparison between bilateral varicocele and fertile healthy men revealed twenty DEPs, whereas another comparison between unilateral and bilateral varicocele groups yielded 16 DEPs.
From two different sets of DEPs, FN1 and semenogelin 1 (SEMG1) proteins were identified as suitable molecular markers to distinguish bilateral from unilateral varicocele patients. These two proteins were differentially expressed between bilateral and unilateral varicocele patients. Protein expression in the bilateral varicocele group was significantly different from the fertile donor group (P < 0.05, Figure 1).
Bilateral varicocele versus fertile donors: DEPs associated with top networks and pathways
Metacore™ analysis demonstrated response to ROS and oxidative stress as the top most differentially affected processes in bilateral varicocele versus fertile donors (Figure 2). DEPs in bilateral varicocele, including aldose reductase (AKR1B1), annexin 1 (ANXA1), FN1, PRDX1, and PRDX2 were involved in oxidative stress. The other pathways affected are involved in the negative regulation of apoptotic processes, programmed cell death, and tissue homeostasis (Figure 2). Lymphocyte and leukocyte activation pathways are also in the top ten most differentially affected processes in bilateral varicocele versus fertile group (Figure 2).
Figure 2.
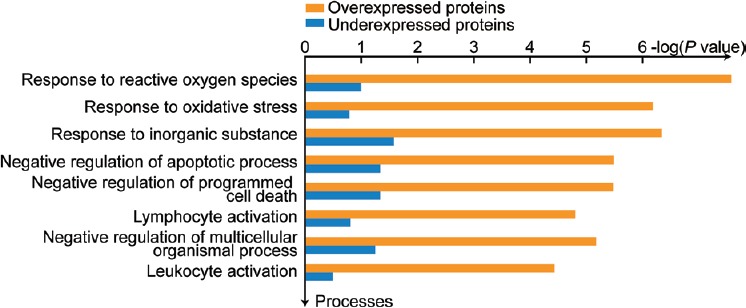
Top ten network processes affected in bilateral varicocele in comparison to fertile men.
The protein interaction network for DEPs in seminal plasma of bilateral varicocele revealed protein–protein interaction between DEPs involved in the key processes such as response to oxidative stress, negative regulation of apoptotic process, and tissue homeostasis (Figure 3). The DEPs in the network were found to be under the regulation of transcriptional regulators such as androgen receptor, interferon regulatory factor 1 (IRF1), c-Myc, nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB), activator protein 1 (AP-1), and nuclear factor erythroid 2 (NFE2)-related factor 2 (NRF2) (Figure 3).
Figure 3.
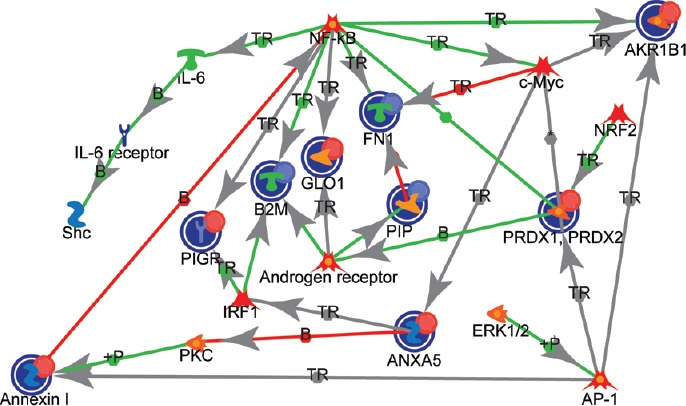
Interaction between the DEPs and their association with transcription regulators in bilateral varicocele in comparison to fertile men. IL-6: interleukin 6; Shc: Shc transforming protein; NF-κB: nuclear factor-kappa B, PIGR: polymeric immunoglobulin receptor; B2M: beta-2 microglobulin; GLO1: glyoxalase I; FN1: fibronectin1; IRF1: interferon regulatory factor 1; PKC: protein kinase C; ANXA5: annexin5; ERK1/2: extracellular signal-regulated kinases; AP-1: activator protein 1; PRDX2: peroxiredoxin 2; PRDX1: peroxiredoxin 1; NRF2: nuclear factor erythroid 2 (NFE2)-related factor 2; AKR1B1: aldose reductase; PIP: prolactin-induced protein; TR: transcription regulation; +P: phosphorylation; B: binding; AP-1: activating protein-1.
Bilateral varicocele versus unilateral varicocele: DEPs associated with top networks and pathways
Functional analysis projects the inflammatory response pathway as one of the top processes affected in the bilateral varicocele patients (Figure 4). Among the seven proteins involved in the inflammatory response, six proteins (alpha-1-acid glycoprotein 1 [ORM1], alpha-1-acid glycoprotein 2 [ORM2], alpha-1-antitrypsin [SERPINA1], glutathione hydrolase 1 proenzyme [GGT1], apolipoprotein D [APOD], and FN1) were underexpressed, whereas ECM1 was overexpressed in bilateral varicocele compared to unilateral varicocele group. The underexpressed proteins in bilateral varicocele are involved in the top network processes: inflammation IL-6 signaling and Jak-STAT pathways (Figure 5 and Supplementary Table 3).
Figure 4.
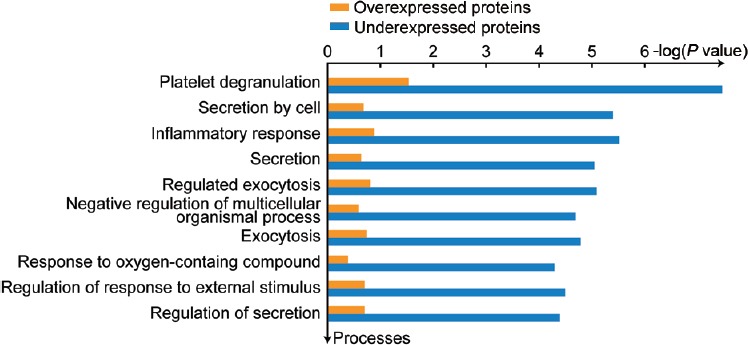
Top ten network processes affected in bilateral varicocele in comparison to unilateral varicocele.
Figure 5.
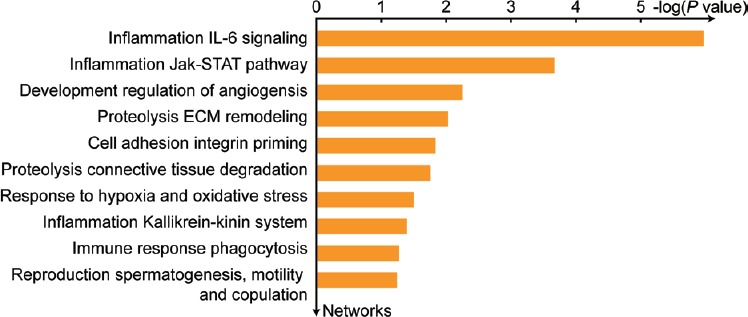
Top ten network processes affected due to the underexpression of proteins in bilateral varicocele in comparison to unilateral varicocele. IL-6: interleukin 6; Jak-STAT: Janus kinase-signal transducer and activator of transcription; ECM: extracellular matrix.
Supplementary Table 3.
Differentially expressed proteins associated with top processes affected in unilateral and bilateral varicocele patients
| Top processes | Comparison | DEPs |
|---|---|---|
| Response to ROS and oxidative stress |
BV versus fertile | AKR1B1, ANXA1, FN1, PRDX2, PRDX1 |
| Negative regulation of apoptotic process |
BV versus fertile | AKR1B1, ANXA1, ANXA5, FN1, GLO1, PIP, PRDX2 |
| Tissue homeostasis | BV versus fertile | AKR1B1, B2M, PIGR, PIP, PRDX2 |
| Inflammatory response | UV versus BV | ORM1, ORM2, SERPINA1, FN1, GGT1, APOD |
| sIL-6 signaling | UV versus BV | ORM1, ORM2, SERPINA1, FN1, APOD |
| Jak-STAT pathway | UV versus BV | AGT, CPE |
DEPs: differentially expressed proteins; ROS: reactive oxygen species; IL-6: interleukin 6; Jak-STAT: JAnus Kinase - signal transducer and activator of transcription; UV: unilateral varicocele; BV: bilateral varicocele; AKR1B1: aldose reductase; ANAX1: annexin 1; FN1: fibronectin; PRDX2: peroxiredoxin 2; PRDX1: peroxiredoxin 1; ANAX5: annexin 5; GLO1: glyoxalase I; PIP: prolactin-induced protein; ORM1: alpha-1-acid glycoprotein 1; ORM2: alpha-1-acid glycoprotein 2; SERPINA1: alpha-1-antitrypsin; APOD: apolipoprotein D; AGT: angiotensinogen; CPE: carboxypeptidase E; B2M: beta-2 microglobulin; PIGR: polymeric immunoglobulin receptor; ORM1: alpha-1-acid glycoprotein 1; GGT1: glutathione hydrolase 1 proenzyme
Selection and validation of expression profile of proteins by western blot analysis
The proteins for validation were selected based on their association with the vital pathways/processes dysregulated in varicocele patients. FN1 protein was involved in the top processes affected in bilateral varicocele versus fertile men and unilateral versus bilateral varicocele patients (Supplementary Table 3). PRDX2 was validated in unilateral and bilateral varicocele patients to demonstrate the difference in the effect of oxidative stress at molecular level. Similarly, FASN associated with DNA damage was selected as a biomarker to differentiate the varicocele patients based on the degree of SDF. Three selected proteins, namely PRDX2, FASN, and FN1, were validated using western blot analysis in unilateral and bilateral varicocele. Western blot analysis revealed that PRDX2 and FASN were significantly upregulated (1.62-fold variation to control, P < 0.05; and 7.68-fold variation to control, P < 0.05), whereas FN1 was significantly downregulated (0.38-fold variation to control, P < 0.05) (Figure 6).
Figure 6.
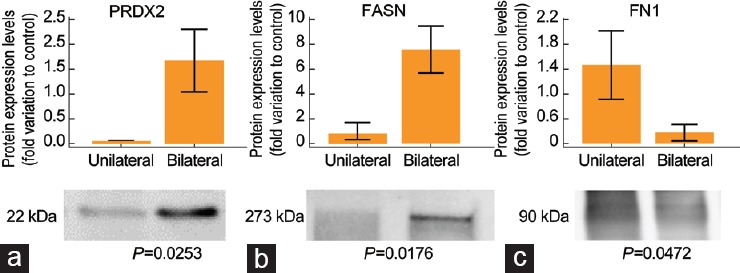
Protein expression levels of the DEPs selected for validation by western blot analysis in bilateral varicocele group (n = 6) relative to unilateral varicocele group (n = 6). (a) PRDX2. (b) FASN. (c) FN1. Results are expressed as mean ± standard error of the mean and in fold variation to control. DEPS: differentially expressed proteins; PRDX2: peroxiredoxin 2; FASN: fatty acid synthase; FN1: fibronectin 1.
DISCUSSION
Seminal plasma contains secretions from both primary and accessory male sex glands,12 and alterations in seminal plasma proteome have been reported in infertile men.19,22,28 Certain proteins serve as biomarkers to diagnose the condition associated with male infertility. Few studies have identified the differential expression of various seminal plasma proteins and dysregulation of the cellular pathways compromising the fertility potential of spermatozoa in varicocele pateints.16,17,29,30 Varicocele repair (varicocelectomy) results in regaining the homeostatic condition in seminal plasma. However, none of these proteomic studies have neither emphasized nor explained the differential processes or pathways affected in unilateral and bilateral varicocele. For the first time, using a high-throughput proteomic approach and bioinformatic analysis, we were able to identify DEPs in seminal plasma of unilateral and bilateral varicocele (Supplementary Table 3). We can therefore hypothesize that the homeostasis of seminal plasma proteins is affected in unilateral and bilateral varicocele.
Seminal plasma provides a nutritive and protective environment for spermatozoa,12,31 and maintenance of seminal plasma homeostasis is essential for sperm function. Any pathological state of male reproductive system affects seminal plasma composition and may elicit adverse effects on spermatozoa. In this study, we observed disturbance in the seminal plasma homeostasis of patients with bilateral varicocele (Figure 3) due to the overexpression of DEPs (polymeric immunoglobulin receptor [PIGR], aldose reductase [ALDR], and PRDX1), which are involved in tissue homeostasis pathway (Supplementary Table 3). PIGR expression is regulated by cytokines, which are elevated in the seminal plasma of varicocele patients due to inflammatory response.8,32 ALDR is responsible for the induction of the sperm capacitation process,33 and overexpression of ALDR in seminal plasma of bilateral varicocele patients indicates compromised sperm function due to the induction of early capacitation. Herwig et al.34 reported the differential expression of ALDR in infertile oligoasthenozoospermic men compared to fertile healthy men. PRDX1, involved in response to ROS and oxidative stress, was overexpressed in bilateral varicocele patients. Under the state of oxidative stress, PRDX1 expression is increased in seminal plasma.35 Overall, the upregulation of these DEPs ventures a state of disturbed homeostasis in seminal plasma and affects the normal physiological function of spermatozoa in infertile men with bilateral varicocele.
Varicocele patients are known to exhibit seminal oxidative stress.36 In the present study, irrespective of type (unilateral or bilateral), seminal ROS levels are higher than the cutoff value (93 RLU per second per 106 spermatozoa). Although this elevation was not significant among the (unilateral and bilateral) varicocele, the cellular and pathological damage incurred in bilateral varicocele was more, which suggests the compromise in semen quality.14 Functional analysis of the seminal plasma proteome indicated the involvement of DEPs in this process such as response to ROS and oxidative stress in the bilateral varicocele group (Figure 2). However, such differentially affected pathways were not observed in the unilateral varicocele condition (submitted elsewhere for publication). This shows that oxidative stress-associated mechanisms are pronounced in bilateral varicocele. Validation of the PRDX2 protein in seminal plasma suggests that PRDX2 may serve as a potential seminal plasma biomarker in the diagnosis of oxidative stress-mediated reproductive dysfunction in bilateral varicocele patients (Figure 6).
Infertile men with varicocele produce spermatozoa with poor fertilizing ability and increased SDF.5,6 Seminal oxidative stress has deleterious effect on the sperm DNA integrity and mainly occurs after spermiation.37,38 In the present study, we observed increased SDF in bilateral varicocele patients compared to unilateral varicocele patients (Supplementary Table 2). Proteomic analysis identified that the FASN was overexpressed in the seminal plasma of bilateral varicocele (Supplementary Table 4). FASN is associated with the molecular pathways related to DNA damage and vital for its regulation. Overexpression of FASN in the cytoplasm of the cell protects the nuclear DNA from damage.39,40 Therefore, absence of FASN in the spermatozoa indicates that the DNA repair mechanism is deactivated in the spermatozoa with high SDF. Intasqui et al.41 reported the absence of FASN protein in spermatozoa with high DNA fragmentation. However, the same authors in another study reported an increased expression of FASN in seminal plasma from samples with high DNA fragmentation.30,42 FASN is considered as a circulating biomarker as its expression increases in the extracellular fluid with decrease or depletion inside the cell.43 This makes FASN a reliable and accurate seminal plasma biomarker for differentiating the semen samples with high SDF in varicocele patients. Furthermore, our validation results of FASN in varicocele patients using western blot analysis warrant the use of FASN as a potential seminal plasma biomarker (Figure 6).
Supplementary Table 4.
Differentially expressed proteins and their abundance in bilateral varicocele group compared with fertile healthy men group
| Uniprot number | Gene name | Protein name | Fertile men group | Bilateral varicocele group | NSAF ratio | Expression | ||
|---|---|---|---|---|---|---|---|---|
| SC | Abund | SC | Abund | |||||
| P50991 | CCT4 | T-complex protein 1 subunit delta isoform a |
2.7 | VL | 0 | - | 0.00 | Unique to fertile group |
| Q9Y265 | RUVBL1 | RuvB-like 1 | 8.3 | L | 0 | - | 0.00 | Unique to fertile group |
| P26641 | EEF1G | Elongation factor 1-gamma | 10.3 | L | 1.3 | VL | 0.15 | UE |
| P07205 | PGK2 | Phosphoglycerate kinase 2 | 34.0 | M | 9.3 | L | 0.37 | UE |
| P61769 | B2M | Beta-2-microglobulin precursor | 31.3 | M | 19.3 | L | 0.49 | UE |
| P04279 | SEMG1 | Semenogelin-1 preproprotein | 698.3 | H | 486.7 | H | 0.65 | UE |
| P02751 | FN1 | Fibronectin isoform 3 preproprotein | 476.7 | H | 439.3 | H | 0.66 | UE |
| P12273 | PIP | Prolactin-inducible protein precursor | 258.3 | H | 185.7 | H | 0.67 | UE |
| P08758 | ANXA5 | Annexin A5 | 13.7 | L | 31.3 | M | 2.14 | OE |
| Q06830 | PRDX1 | Peroxiredoxin-1 | 13.3 | L | 35.3 | M | 2.18 | OE |
| P32119 | PRDX2 | Peroxiredoxin-2 | 11.3 | L | 31.3 | M | 2.49 | OE |
| P13796 | LCP1 | Plastin-2 isoform X2 | 10.0 | L | 33.3 | M | 3.13 | OE |
| P15121 | AKR1B1 | Aldose reductase | 6.0 | VL | 14.7 | L | 3.16 | OE |
| P04083 | ANXA1 | Annexin A1 | 7.7 | VL | 22.0 | M | 3.23 | OE |
| P49221 | TGM4 | Protein-glutamine gamma-glutamyltransferase 4 | 31.3 | M | 180.3 | H | 3.74 | OE |
| Q6UWW0 | LCN15 | Lipocalin-15 isoform X1 | 0.7 | VL | 6.0 | VL | 9.70 | OE |
| Q04760 | GLO1 | Lactoylglutathione lyase | 1.3 | VL | 11.3 | L | 10.65 | OE |
| P49327 | FASN | Fatty acid synthase | 0.7 | VL | 34.0 | M | 34.02 | OE |
| P01833 | PIGR | Polymeric immunoglobulin receptor isoform X1 |
0.3 | VL | 30.0 | VL | 62.38 | OE |
| O43278 | SPINT1 | Kunitz-type protease inhibitor 1 isoform 1 precursor |
0.0 | 2.7 | VL | 0.00 | Unique to bilateral varicocele group | |
VL: very low; L: low; M: medium; H: high; UE: underexpressed; OE: overexpressed; NSAF: normalized spectral abundance factor; Abund: abundance; SC: spectral count
Proteomic studies in seminal plasma of adolescent varicocele patients predicted biomarkers for spermatogenesis and homeostasis of testicular function. These studies, also, reported an increased immune response and state of chronic inflammatory profile.16,18 In our study, interacting DEPs in the seminal plasma of bilateral varicocele patients were under the influence of transcriptional regulators associated with sperm function. Differential expression of proteins linked to the transcriptional factor, androgen receptor, affects spermatogenesis, and the sperm maturation processes. Furthermore, NRF2 transcription factor responsible for oxidative stress response and NF-κB in the inflammatory response are linked to the interacting DEPs (Figure 3). Involvement of multiple transcription regulators may impair spermatogenesis by affecting testicular function in bilateral varicocele patients.
In varicocele patients, the IL-6-signaling and Jak-STAT pathways associated with inflammation are enriched with underexpressed DEPs (Figure 5 and Supplementary Table 3). In the current study, we demonstrated that proteins involved in the inflammatory response pathway were differentially expressed in varicocele patients (Figure 4). Among the inflammatory proteins, SERPINA1 is an acute-phase protein and responsible for the inhibition of proteases involved in stimulating the inflammatory response. During inflammation, their expression levels are high in plasma.44 Zylbersztejn et al.16 using conventional two-dimensional gel electrophoresis reported the absence of SERPINA1 in adolescents with varicocele. However, highly sensitive LC-MS/MS was able to detect the presence of the SERPINA1 in varicocele patients. Underexpression of SERPINA1 in seminal plasma of bilateral varicocele patients compared to unilateral varicocele patients (Supplementary Table 3) indicates an increased inflammatory response in bilateral varicocele patients. This is eventually due to the damage or injury to both left and right pampiniform plexus in bilateral varicocele condition.
FN1 is a multifunctional glycoprotein present in the seminal plasma. It is involved in the seminal gel formation after ejaculation45 and stimulates sperm capacitation.46 Previous studies reported the overexpression of FN1 in azoospermic patients47 and in patients with high sperm DNA fragmentation,41 whereas our study detected the presence of FN1 in the varicocele patients. Downregulation of FN1 in bilateral varicocele patients may lead to compromised sperm capacitation based on the severity of the condition. Our Western blot results substantiate the proteomic findings, suggesting FN1 as a potential biomarker to differentiate bilateral from unilateral varicocele patients. Validation of the DEPs (FN1, FASN, and PRDX2) in the seminal plasma of varicocele patients and fertile donors was in agreement with the high-throughput proteomic analysis (Supplementary Table 4 and 5).
Supplementary Table 5.
Differentially expressed proteins and their abundance in bilateral varicocele group compared with unilateral varicocele group
| Uniprot number | Gene name | Protein name | Unilateral varicocele group | Bilateral varicocele group | NSAF ratio | Expression | ||
|---|---|---|---|---|---|---|---|---|
| SC | Abund | SC | Abund | |||||
| P02763 | ORM1 | Alpha-1-acid glycoprotein 1 precursor |
86.0 | H | 6.0 | VL | 0.09 | UE |
| P19652 | ORM2 | Alpha-1-acid glycoprotein 2 precursor |
23.0 | M | 2.3 | VL | 0.17 | UE |
| P01019 | AGT | Angiotensinogen preproprotein | 16.7 | L | 2.0 | VL | 0.17 | UE |
| Q92896 | GLG1 | Golgi apparatus protein 1 isoform 2 precursor |
8.7 | L | 1.0 | VL | 0.19 | UE |
| P05090 | APOD | Apolipoprotein D precursor | 7.0 | VL | 1.3 | VL | 0.23 | UE |
| Q8WXA2 | PATE1 | Prostate and testis expressed protein 1 precursor | 12.3 | L | 3.0 | VL | 0.27 | UE |
| P19440 | GGT1 | Gamma-glutamyltranspeptidase 1 precursor |
36.3 | M | 13.7 | L | 0.39 | UE |
| P07195 | LDHB | L-lactate dehydrogenase B chain | 20.3 | M | 6.7 | VL | 0.41 | UE |
| P15144 | ANPEP | Aminopeptidase N isoform X1 | 284.3 | H | 131.3 | H | 0.48 | UE |
| P16870 | CPE | Carboxypeptidase E preproprotein | 110.7 | H | 50.7 | M | 0.59 | UE |
| P02751 | FN1 | Fibronectin isoform 3 preproprotein | 817.0 | H | 439.3 | H | 0.61 | UE |
| P01009 | SERPINA1 | Alpha-1-antitrypsin precursor | 190.7 | H | 96.7 | H | 0.64 | UE |
| Q16610 | ECM1 | Extracellular matrix protein 1 isoform 3 precursor |
76.3 | M | 86.0 | H | 1.53 | OE |
| P80303 | NUCB2 | Nucleobindin-2 isoform X1 | 17.3 | L | 26.7 | M | 2.02 | OE |
| P04279 | SEMG1 | Semenogelin-1 preproprotein | 304.3 | H | 487.0 | H | 2.39 | OE |
| Q02383 | SEMG2 | Semenogelin-2 precursor | 503.0 | H | 1026.7 | H | 2.75 | OE |
VL: very low; L: low; M: medium; H: high; UE: underexpressed; OE: overexpressed; NSAF: normalized spectral abundance factor; Abund: abundance; SC: spectral count
In conclusion, this is the first proteomic study comparing the seminal plasma proteome of unilateral and bilateral varicocele patients with normal fertile donors. Altered expression of DEPs and its association with key processes show that the seminal plasma homeostasis is compromised in bilateral varicocele patients. Furthermore, we propose PRDX2, FASN, and FN1 as potential noninvasive seminal plasma markers to differentiate the unilateral and bilateral varicocele patients.
AUTHOR CONTRIBUTIONS
AA conceived the idea and designed the study. MKPS carried out the experiment. Data analysis was carried out by MKPS and SB. The first draft was written by MKPS and revised by SB and AA. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors thank Ralf Henkel, PhD, and Kristian Leisegang, PhD, for their critical reading of the manuscript and helpful suggestions. Belinda Willard, PhD, Director of Proteomic Core Laboratory, Lerner Research Institute, assisted with proteomic analysis, while bioinformatics data analysis was conducted by Banu Gopalan, PhD. Research support was provided by the American Center for Reproductive Medicine at Cleveland Clinic.
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Alsaikhan B, Alrabeeah K, Delouya G, Zini A. Epidemiology of varicocele. Asian J Androl. 2016;18:179–81. doi: 10.4103/1008-682X.172640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmar JL. Varicocele and male infertility: part II: the pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update. 2001;7:461–72. doi: 10.1093/humupd/7.5.461. [DOI] [PubMed] [Google Scholar]
- 3.Silber S. The varicocele argument resurfaces. J Assist Reprod Genet. 2018;35:1079–82. doi: 10.1007/s10815-018-1160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, et al. Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol. 2011;60:796–808. doi: 10.1016/j.eururo.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Cho CL, Esteves SC, Agarwal A. Novel insights into the pathophysiology of varicocele and its association with reactive oxygen species and sperm DNA fragmentation. Asian J Androl. 2016;18:186–93. doi: 10.4103/1008-682X.170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A, Esteves SC. Varicocele and male infertility: current concepts and future perspectives. Asian J Androl. 2016;18:161–2. doi: 10.4103/1008-682X.172819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demirer Z, Uslu AU. More work needed in examining the relationship between mean platelet volume and inflammation in varicocele pathophysiology. Can Urol Assoc J. 2015;9:E639. doi: 10.5489/cuaj.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeinali M, Hadian Amree A, Khorramdelazad H, Karami H, Abedinzadeh M. Inflammatory and anti-inflammatory cytokines in the seminal plasma of infertile men suffering from varicocele. Andrologia. 2017;49:e12685. doi: 10.1111/and.12685. [DOI] [PubMed] [Google Scholar]
- 9.Seshadri S, Bates M, Vince G, Jones DI. The role of cytokine expression in different subgroups of subfertile men. Am J Reprod Immunol. 2009;62:275–82. doi: 10.1111/j.1600-0897.2009.00736.x. [DOI] [PubMed] [Google Scholar]
- 10.Bachir BG, Jarvi K. Infectious, inflammatory, and immunologic conditions resulting in male infertility. Urol Clin North Am. 2014;41:67–81. doi: 10.1016/j.ucl.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Jodar M, Soler-Ventura A, Oliva R. Semen proteomics and male infertility. J Proteomics. 2017;162:125–34. doi: 10.1016/j.jprot.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Samanta L, Parida R, Dias TR, Agarwal A. The enigmatic seminal plasma: a proteomics insight from ejaculation to fertilization. Reprod Biol Endocrinol. 2018;16:41. doi: 10.1186/s12958-018-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseinifar H, Sabbaghian M, Nasrabadi D, Modarresi T, Dizaj AV, et al. Study of the effect of varicocelectomy on sperm proteins expression in patients with varicocele and poor sperm quality by using two-dimensional gel electrophoresis. J Assist Reprod Genet. 2014;31:725–9. doi: 10.1007/s10815-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal A, Sharma R, Durairajanayagam D, Cui Z, Ayaz A, et al. Differential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicocele. Urology. 2015;85:580–8. doi: 10.1016/j.urology.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal A, Sharma R, Samanta L, Durairajanayagam D, Sabanegh E. Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J Androl. 2016;18:282–91. doi: 10.4103/1008-682X.170445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zylbersztejn DS, Andreoni C, Del Giudice PT, Spaine DM, Borsari L, et al. Proteomic analysis of seminal plasma in adolescents with and without varicocele. Fertil Steril. 2013;99:92–8. doi: 10.1016/j.fertnstert.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Camargo M, Lopes PI, Del Giudice P, Carvalho V, Cardozo K, et al. Unbiased label-free quantitative proteomic profiling and enriched proteomic pathways in seminal plasma of adult men before and after varicocelectomy. Hum Reprod. 2012;28:33–46. doi: 10.1093/humrep/des357. [DOI] [PubMed] [Google Scholar]
- 18.Del Giudice P, Belardin L, Camargo M, Zylbersztejn D, Carvalho V, et al. Determination of testicular function in adolescents with varicocoele – a proteomics approach. Andrology. 2016;4:447–55. doi: 10.1111/andr.12174. [DOI] [PubMed] [Google Scholar]
- 19.Camargo M, Intasqui P, Bertolla RP. Understanding the seminal plasma proteome and its role in male fertility. Basic Clin Androl. 2018;28:6. doi: 10.1186/s12610-018-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Bartolomé S, Deutsch EW, Binz PA, Jones AR, Eisenacher M, et al. Guidelines for reporting quantitative mass spectrometry based experiments in proteomics. J Proteomics. 2013;95:84–8. doi: 10.1016/j.jprot.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Switzerland: World Health Organization; 2010. [Google Scholar]
- 22.Sharma R, Agarwal A, Mohanty G, Du Plessis SS, Gopalan B, et al. Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013;11:85. doi: 10.1186/1477-7827-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma RK, Sabanegh E, Mahfouz R, Gupta S, Thiyagarajan A, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology. 2010;76:1380–6. doi: 10.1016/j.urology.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 24.Diz AP, Truebano M, Skibinski DO. The consequences of sample pooling in proteomics: an empirical study. Electrophoresis. 2009;30:2967–75. doi: 10.1002/elps.200900210. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal A, Ayaz A, Samanta L, Sharma R, Assidi M, et al. Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin Proteomics. 2015;12:23. doi: 10.1186/s12014-015-9094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogle O, Kumar K, Attardo-Parrinello C, Lewis S, Estanyol J, et al. Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology. 2017;5:10–22. doi: 10.1111/andr.12279. [DOI] [PubMed] [Google Scholar]
- 27.Hamada A, Esteves SC, Agarwal A. Definitions and epidemiology. In: Hamada A, Esteves SC, Agarwal A, editors. Varicocele and male infertility: current concepts, controversies and consensus. Cham: Springer International Publishing; 2016. pp. 1–3. [Google Scholar]
- 28.Agarwal A, Durairajanayagam D, Halabi J, Peng J, Vazquez-Levin M. Proteomics, oxidative stress and male infertility. Reprod Biomed Online. 2014;29:32–58. doi: 10.1016/j.rbmo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Fariello RM, Pariz JR, Spaine DM, Gozzo FC, Pilau EJ, et al. Effect of smoking on the functional aspects of sperm and seminal plasma protein profiles in patients with varicocele. Hum Reprod. 2012;27:3140–9. doi: 10.1093/humrep/des287. [DOI] [PubMed] [Google Scholar]
- 30.Camargo M, Intasqui P, Bertolla RP. Proteomic profile of seminal plasma in adolescents and adults with treated and untreated varicocele. Asian J Androl. 2016;18:194–201. doi: 10.4103/1008-682X.168788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juyena NS, Stelletta C. Seminal plasma: an essential attribute to spermatozoa. J Androl. 2012;33:536–51. doi: 10.2164/jandrol.110.012583. [DOI] [PubMed] [Google Scholar]
- 32.Nallella KP, Allamaneni SS, Pasqualotto FF, Sharma RK, Thomas AJ, et al. Relationship of interleukin-6 with semen characteristics and oxidative stress in patients with varicocele. Urology. 2004;64:1010–3. doi: 10.1016/j.urology.2004.05.045. [DOI] [PubMed] [Google Scholar]
- 33.Katoh Y, Takebayashi K, Kikuchi A, Iki A, Kikuchi K, et al. Porcine sperm capacitation involves tyrosine phosphorylation and activation of aldose reductase. J Androl. 2014;148:389–401. doi: 10.1530/REP-14-0199. [DOI] [PubMed] [Google Scholar]
- 34.Herwig R, Knoll C, Planyavsky M, Pourbiabany A, Greilberger J, et al. Proteomic analysis of seminal plasma from infertile patients with oligoasthenoteratozoospermia due to oxidative stress and comparison with fertile volunteers. Fertil Steril. 2013;100:355–66. doi: 10.1016/j.fertnstert.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 35.Intasqui P, Antoniassi MP, Camargo M, Nichi M, Carvalho VM, et al. Differences in the seminal plasma proteome are associated with oxidative stress levels in men with normal semen parameters. Fertil Steril. 2015;104:292–301. doi: 10.1016/j.fertnstert.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 36.Esteves SC, Agarwal A. Afterword to varicocele and male infertility: current concepts and future perspectives. Asian J Androl. 2016;18:319–22. doi: 10.4103/1008-682X.172820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aitken R, Smith T, Jobling M, Baker M, De Iuliis G. Oxidative stress and male reproductive health. Asian J Androl. 2014;16:31–8. doi: 10.4103/1008-682X.122203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John AR, De Iuliis GN, McLachlan R. Biological and clinical significance of DNA damage in the male germ line. Int J Androl. 2009;32:46–56. doi: 10.1111/j.1365-2605.2008.00943.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Dong Z, Wang CJ, Barlow LJ, Fako V, et al. FASN regulates cellular response to genotoxic treatments by increasing PARP-1 expression and DNA repair activity via NF-κB and SP1. Proc Natl Acad Sci U S A. 2016;113:E6965–73. doi: 10.1073/pnas.1609934113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J, Kim NH, Cui XS. Inhibition of fatty acid synthase reduces blastocyst hatching through regulation of the AKT pathway in pigs. PLoS One. 2017;12:e0170624. doi: 10.1371/journal.pone.0170624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Intasqui P, Camargo M, Del Giudice PT, Spaine DM, Carvalho VM, et al. Unraveling the sperm proteome and post-genomic pathways associated with sperm nuclear DNA fragmentation. J Assist Reprod Genet. 2013;30:1187–202. doi: 10.1007/s10815-013-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Intasqui P, Camargo M, Antoniassi MP, Cedenho AP, Carvalho VM, et al. Association between the seminal plasma proteome and sperm functional traits. Fertil Steril. 2016;105:617–28. doi: 10.1016/j.fertnstert.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez-Real JM, Menendez JA, Moreno-Navarrete JM, Blüher M, Vazquez-Martin A, et al. Extracellular fatty acid synthase: a possible surrogate biomarker of insulin resistance. Diabetes. 2010;59:1506–11. doi: 10.2337/db09-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carvalho MO, Souza AL, Carvalho MB, Pacheco AP, Rocha LC, et al. Evaluation of alpha-1 antitrypsin levels and SERPINA1 gene polymorphisms in sickle cell disease. Front Immunol. 2017;8:1491. doi: 10.3389/fimmu.2017.01491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katnik-Prastowska I, Przybysz M, Chełmońska-Soyta A. Fibronectin fragments in human seminal plasma. Acta Biochim Pol. 2005;52:557–60. [PubMed] [Google Scholar]
- 46.Martínez-León E, Osycka-Salut C, Signorelli J, Pozo P, Pérez B, et al. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Hum Reprod. 2015;30:2138–51. doi: 10.1093/humrep/dev154. [DOI] [PubMed] [Google Scholar]
- 47.Davalieva K, Kiprijanovska S, Noveski P, Plaseski T, Kocevska B, et al. Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia. 2012;44:256–64. doi: 10.1111/j.1439-0272.2012.01275.x. [DOI] [PubMed] [Google Scholar]


