Abstract
The utilization of trastuzumab biosimilar medications is of particular interest in HER2-positive breast cancer as these drugs have the potential for cost savings and increased utilization/access to HER2 targeted therapy in both early stage and metastatic HER2-positive breast cancers. Five trastuzumab biosimilars: MYL-1401O (Ogivri), CT-P6 (Herzuma), SB3 (Ontruzant), PF-05280014 (Trazimera), and ABP980 (Kanjinti), have now been approved by the US Food and Drug Administration (FDA) for use in HER2-positive breast cancers. This review provides an overview of these agents with special consideration of the development and approval process, including available clinical data results for these trastuzumab biosimilars. Adoption in the clinic will depend on the degree of comfort with the overall evidence.
Keywords: ABP980, biosimilar, CT-P6, equivalence, HER2-positive breast cancer, interchangeability, MYL-1401O, PF-05280014, SB3, trastuzumab
Introduction
Over the past 10 years, biological medications have become increasingly common in the treatment of cancers and immunological diseases. With regard to breast cancer specifically, trastuzumab was approved by the US Food and Drug Administration (FDA) for use in HER2-overexpressing breast cancer, based on evidence of improved overall survival (OS) in both early stage and metastatic breast cancer.1,2 However, this revolutionary addition to breast cancer therapy comes at a high cost, with some estimates reaching at least US$70,000 for 1 year of therapy in the US.3,4 Often, financial factors, including reimbursement, out-of-pocket costs, and administrative factors are barriers to patient access for this recommended treatment.
In recent years, the development of biosimilar medications for trastuzumab, and other similar biological medications has provided an opportunity for expanded patient utilization of these treatments at a potentially lower cost than the original drug. With increased usage of biosimilar medications, some estimates predict potential cost savings of approximately US$54 billion over 10 years, largely based on data observed with biosimilar immunomodulatory medications.5 However, estimates of cost savings are largely dependent on regulatory and utilization factors that have yet to be fully predicted.
Biosimilar medications are engineered medications that provide similar pharmacokinetic and pharmacodynamic properties to the original biological medication. Specifically, a biosimilar is defined as a biological medication that is highly similar to a reference product, with only minor differences in clinically inactive components, and has no clinically meaningful differences between the two medications with regard to safety, purity, and potency.6 These medications typically undergo an abbreviated approval process that is based on the overall evidence supporting the proposed medication as a biosimilar product (Figure 1). Analytical assessment of the structural and functional characteristics of the potential biosimilar are required in extensive detail. Evaluations include the amino acid sequence of the molecule, structural conformation, and target binding factors and nonclinical evaluation of activity, clinical assessment of pharmacokinetic and pharmacodynamic properties, and evidence of comparable clinical efficacy and safety with the original product.6,7 Based on the combination of this evidence, supporting structural and functional similarity and equivalent pharmacologic properties and efficacy, biosimilar medications, including those for the reference trastuzumab, have been approved for use in multiple indications.
Figure 1.
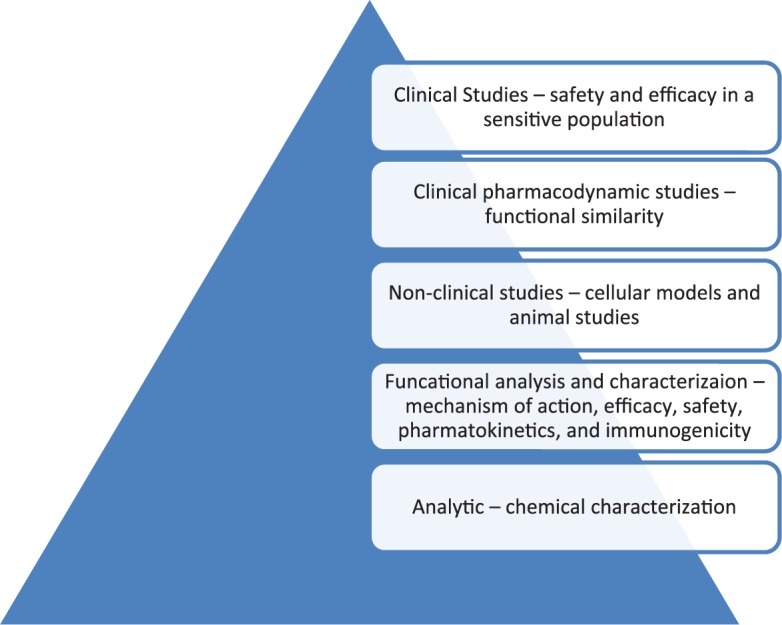
Biosimilar development and approval schematic leading to ’totality of evidence’.
Trastuzumab is a monoclonal antibody that targets HER2 on the surface of breast cancer cells and inhibits dimer dependent HER2 signaling and induction of antibody-dependent cellular toxicity, among other potential mechanisms of action. Since its initial approval in 1998 for the treatments of metastatic breast cancer, trastuzumab has been studied in multiple settings for the treatment of HER2-overexpressing breast cancers. In 2005 interim analysis of combined results for NSABP B31 and NCCTG N9831 were published. Both of the included studies compared the efficacy of typical doxorubicin/cyclophosphamide therapy followed by paclitaxel given with or without trastuzumab in early stage breast cancer. Results of this study noted median follow-up of 2 years with disease free survival (DFS) hazard ratio (HR) 0.48 (CI 95% 0.39–0.59) and OS HR 0.67 (CI 95% 0.48–0.93).8 Subsequent analysis noted a long-term DFS (HR 0.60) and OS (HR 0.63) advantage when adjuvant chemotherapy was given in combination with trastuzumab.9 These trials, combined with others, confirmed trastuzumab as standard of care in combination with chemotherapy followed by trastuzumab alone to complete 1 year of therapy. In the metastatic setting, trastuzumab was shown to produce a large increase in objective response rate, time to progression, and OS when given in combination with chemotherapy, either doxorubicin and cyclophosphamide or paclitaxel. The response rate in the group treated with chemotherapy plus trastuzumab was 50% versus a response rate of 32% in those treated with chemotherapy alone. The median time to progression was 7.4 months with chemotherapy plus trastuzumab compared with 4.6 months with chemotherapy alone. Similarly, OS was increased to 25.1 months with the addition of chemotherapy versus 20.3 months with chemotherapy alone.10
We review the five trastuzumab biosimilar medications currently approved for the US market with consideration of data leading to overall evidence supporting similarity to trastuzumab. Additional trastuzumab biosimilars are in development in both Europe and the US, however, these have not yet gained approval for utilization. Of note, the clinical comparison of biosimilar trastuzumab with the originator product was based on demonstrating statistical equivalence in well-established endpoints derived from meta-analyses of prior clinical trials. Such endpoints included overall response rate (ORR) and progression free survival (PFS) in advanced HER2-positive breast cancer and pathological complete response (pCR) rate in the neoadjuvant setting for primary HER2-positive breast cancer. Safety was assessed by measuring adverse events (AE) and immunogenicity was evaluated during and after the trial.
Methods
In December 2017 the US FDA approved trastuzumab-dkst (MYL-1401O), brand name Ogivri, for use in HER2-overexpressing breast cancer, in both the adjuvant and metastatic setting and HER2-overexpressing metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma making it the first trastuzumab biosimilar to gain approval in the US. Approval was, in part, based on the pharmacokinetic bioequivalence between MYL-1401O and trastuzumab, which was established based on similar peak serum concentration, max concentration, half-life, safety, and immunogenicity.11 In addition, HERITAGE was a double-blind, randomized clinical trial that evaluated equivalence of ORR (defined as within a range of 0.81–1.24) for MYL-1401O compared with trastuzumab when given once every 3 weeks in combination with either paclitaxel or docetaxel for at least 8 cycles as first-line therapy for metastatic breast cancer. The 24 week ORR was 69.6% (CI 90% 64.57–74.56) for MYL-1401O versus 64% (CI 90% 58.81–69.26) in the trastuzumab group (rate ratio 1.09), similarly PFS was at identical in the two groups at 11.1 months.12,13 Similar rates of serious AEs were seen in the biosimilar group and trastuzumab group, leading the authors to conclude that safety was equivalent.12,13 Serious AEs were seen in 38.1% of patients who received MYL-1401O compared with 36.2% of patients who received trastuzumab with the most frequent AE in both groups being neutropenia.13
Trastuzumab-pkrb (CT-P6), brand name Herzuma, was approved by the US FDA 1 year later, in December 2018, for the treatment of HER2-overexpressing breast cancers in the adjuvant and metastatic setting. Cellular data in both HER2-positive breast cancer and gastric cancer models indicated similar mechanism of action for CT-P6 and trastuzumab.14 A phase I, single dose, randomized, double-blind, parallel-group study compared CT-P6 with the reference trastuzumab (6 mg/kg over 90 min) with regards to area under the concentration time curve (AUC)and maximum serum concentration.15 This study demonstrated pharmacokinetic equivalence (predefined as a 90% CI of 80–125) of CT-P6 with reference trastuzumab and a similar safety profile in the healthy trial population.15 A randomized, double-blind, active controlled, phase III trial to evaluate equivalence (with a predefined equivalence margin of −0.15–0.15) of early stage HER2-positive breast cancers compared neoadjuvant CT-P6 with trastuzumab, each in 8 cycles lasting 3 weeks for 24 weeks (8 mg/kg on day 1 of cycle 1 and 6 mg/kg on day 1 of cycles 2–8) in conjunction with neoadjuvant docetaxel 75 mg/m2 on day 1 of cycles 1–4, and fluorouracil 500 mg/m2, epirubicin 74 mg/m2, and cyclophosphamide 500 mg/m2 (FEC) on day 1 of cycles 5–8. This was followed by surgery 3–6 weeks after the last neoadjuvant drug dose and then adjuvant therapy for up to 1 year. The results of this study indicated equivalence of CT-P6 with regards to pCR of 46.8% (CI 95% 40.4–53.2) compared with 50.4% (CI 95% 44.1–56.7) for reference trastuzumab. There was also equivalence of AEs, including febrile neutropenia (1% versus < 1%) and grade 3 or worse treatment related AEs (6% versus 8%).16,17 Secondary endpoints of this study included pharmacokinetic and pharmacodynamic comparison of CT-P6 and trastuzumab, that were similar in this patient population.16,17 Subsequently, the 2-year follow-up data was presented at the 2018 San Antonio Breast Symposium revealing the 2-year DFS in the CT-P6 group of 86% (CI 95% 80–90) compared with 90% (CI 95% 85–93) in the trastuzumab group.18 OS at 2 years was 97% (CI 95% 93–98) in the CT-P6 group and 98% (CI 95% 96–99) in the trastuzumab group further supporting the similarity of CT-P6 to the reference trastuzumab.18
Trastuzumab-dttb (SB3), brand name Ontruzant, became the third US FDA approved trastuzumab biosimilar in January of 2019 with indications for HER2-overexpressing breast cancer and metastatic gastric or GEJ adenocarcinoma. In 2016 a phase I pharmacokinetic study compared SB3 with trastuzumab in healthy subjects. This study established pharmacokinetic equivalence between SB3 and trastuzumab with regard to AUC, maximum concentration, immunogenicity, and adverse reactions.19 This led to a phase III double-blind randomized clinical trial comparing safety, immunogenicity, and efficacy in patients receiving SB3 versus trastuzumab after neoadjuvant and adjuvant therapy for early stage breast cancer.20,21 Patients were randomized to receive neoadjuvant SB3 or trastuzumab for 8 cycles every 3 weeks in combination with 4 cycles of docetaxel followed by 4 cycles of FEC with the primary endpoint of equivalence of pCR (using the definition of no invasive cancer in the breast). Post-surgery, patients continued either SB3 or trastuzumab for 10 additional cycles. The analysis of 800 randomized patients who completed the neoadjuvant therapy and went on to definitive surgery revealed pCR rates of 51.7% with SB3 and 42.0% with trastuzumab, adjusted risk ratio (RR) 1.259 (CI 95% 1.085–1.460) within the equivalence margins (defined as 0.785–1.546).20 The ORR were 96.3% and 91.2% for SB3 and trastuzumab, respectively. The safety profile was similar between arms, with infusion related reactions in 8.2% and 10.0%, left ventricular systolic dysfunction in 0.9% and 0.7% and congestive heart failure in 0.5% and 0% for SB3 and trastuzumab respectively.20 Subsequent phase III data added further support for similarity based on secondary endpoints of event free survival 30 days post adjuvant therapy of 92.2% for SB3 and 91.6% for trastuzumab with 1 year event free survival rates of 93.7% for SB3 and 93.4% for trastuzumab, indicating comparable survival rates between the groups.21 This study revealed similar incidence of treatment related AEs of 97.5% for SB3 and 96.1% for trastuzumab.21 With regards to immunogenicity, the incidence of antidrug antibodies (ADAs) was low, three patients in each group and there was, therefore, no significant difference noted.21
Soon after the approval of Ontruzant, a fourth trastuzumab biosimilar, trastuzumab-qyyp (PF-05280014), was approved by the US FDA in March 2019 under the brand name Trazimera. Approved indications include HER2-overexpressing breast cancer in both adjuvant and metastatic setting and metastatic gastric or GEJ adenocarcinoma. As with other trastuzumab biosimilars, preclinical trials established the biosimilarity of PF-05280014 with the reference trastuzumab with regards to maximum concentration, AUC, half-life, and immunogenicity in animal models.22 REFLECTIONS B327-01 was a phase I, double-blinded trial that compared the pharmacokinetics of PF-05280014 with trastuzumab in healthy volunteers in order to assess bioequivalence, defined as 80.00–125.00%. AUC and maximum concentration were similar between comparison groups. PF-05280014 had Cmax ratio 91.49% (CI 90% 85.32–98.09), EU-sourced trastuzumab Cmax ratio 106% (CI 90% 99.20–114.30), and US-sourced trastuzumab Cmax ratio 97.41% (CI 90% 90.71–104.62) respectively. Similar results were noted for both AUC(0,tlast) and AUC(0,∞) with 90% CI between the predefined equivalence margins, thus establishing the bioequivalence of PF-05280014.23 Similarly, the study noted similar rates of AEs among groups, 71.4% for PF-05280014 and 68.6%/65.7% for trastuzumab-EU/US-sourced.23 Following this, REFLECTIONS B327-02, a randomized, double-blinded, parallel-group study was conducted to compare safety, efficacy, and immunogenicity of PF-05280014 with that of trastuzumab. Patients with HER2-positive, metastatic breast cancer were randomized to receive weekly PF-05280014 or trastuzumab (first dose 4mg/kg over 90 minutes with subsequent doses of 2 mg/kg over 30–90 mg depending on tolerability) in combination with paclitaxel 80 mg/m2 on days 1, 8, and 15 of each 28 day cycle for at least 6 cycles after which time PF-05280014 or trastuzumab could be changed to 6 mg/kg every 3 weeks at the prescribers discretion. The ORR for PF-05280014 was 62.5% compared with 66.5% for trastuzumab with a RR of ORR of 0.94 (CI 95% 0.842–1.049), that was within the predefined equivalence margin of 0.8–1.25.24 In addition, there were no notable differences between PFS survival (median 12.16 months for PF-05280014 versus 12.06 months for trastuzumab) or 1-year OS (89.31% for PF-05280014 versus 87.36% for trastuzumab).24 Safety and immunogenicity outcomes assessed in this trial noted similarity between the groups with only one previously negative patient in each group developing ADAs and neutralizing antibodies following exposure.24
An additional trial, REFLECTIONS B327-04, was a randomized, double-blind, noninferiority trial to evaluate the efficacy, safety, immunogenicity, and pharmacokinetics of PF-05280014 compared with EU-sourced trastuzumab in the setting of neoadjuvant treatment for operable, HER2-positive, early stage breast cancer. Patients were randomized to receive PF-05280014 or trastuzumab-EU (8 mg/kg loading dose and 6 mg/kg thereafter) in combination with a standard regimen of docetaxel and carboplatin every 3 weeks for 6 cycles.25 The primary endpoint of Ctrough in cycle 5 was similar, 92.1% for PF-05280014 and 93.3% for trastuzumab-EU, that was above the noninferiority margin. The pCR was 47% for PF-05280014 and 50.0% for trastuzumab-EU. The objective response, which was assessed by central radiologist review, was also comparable at 88.1% for PF-05280014 and 82% for trastuzumab-EU. The AEs were similar, 38.1% grade 3–4 AEs for PF-05280014 and 45.5% for trastuzumab-EU. Overall, this trial found that PF-05280014 was noninferior to trastuzumab-EU when combined with standard chemotherapy in the neoadjuvant setting for early stage, operable, HER2-positive breast cancer.25
Another trastuzumab biosimilar, ABP980, is analytically similar to reference trastuzumab with regard to structure, function, and pharmacokinetic profile.26 It was the fifth trastuzumab biosimilar approved in June 2019 under the name trastuzumab-anns, brand name Kanjinti.27 A phase III global equivalence trial comparing ABP980 with trastuzumab in the neoadjuvant/adjuvant setting was conducted with the primary endpoints being risk difference and RR of pCR to neoadjuvant therapy.28 This study included a switching design with three arms: ABP980 in the neoadjuvant and adjuvant setting, trastuzumab in the neoadjuvant and adjuvant setting, or neoadjuvant trastuzumab followed by adjuvant ABP980. The neoadjuvant portion comprised 24 weeks, consisting of 12 weeks of run-in anthracycline cyclophosphamide chemotherapy followed by 4 cycles of trastuzumab or ABP980 with the drugs given every 3 weeks at 6 mg/kg after a loading dose of 8 mg/kg in combination with paclitaxel, every 3 weeks × 4, or weekly × 12. Of the 827 patients enrolled, 725 were randomized, and 696 were evaluated for pCR after surgery. pCR was achieved in 48% of patients receiving ABP980 and 41% of trastuzumab exposed patients had pCR for a risk difference of 7.3%.28 The RR was 1.188 (CI 90% 1.033–1.366), which did not technically meet the primary endpoint because the upper boundary of the equivalence margin was actually slightly higher than the predefined value of 13% (1.318) for ABP980 versus trastuzumab. While establishing equivalence was inconclusive based on the statistical possibility of superiority for ABP980, the lower boundary of the CI was within this margin indicating noninferiority of ABP980 versus trastuzumab. The AE rate was similar between arms. In the neoadjuvant portion, < 1% of patients had serious AEs attributed to study drugs. There was no difference in AEs in the adjuvant phase between patients who continued trastuzumab and patients who switched to ABP980. Grade 3 AEs occurred in 9% of continuing ABP980, 6% in continuing trastuzumab, and in 8% of patients who switched from trastuzumab to ABP980. Left ventricular ejection fraction decline occurred in 1.8–3.5% of patients across the treatment groups and were similar in the trastuzumab and switching groups. Clinical cardiac events, all grade 1–2, developed in 2% of the ABP980 patients and <1% of trastuzumab patients. All patients received all doses of the investigated products. Antibodies to trastuzumab developed in two patient and in two patients in the ABP980 group, no neutralizing antibodies were detected. Long-term follow-up of these patients, including late cardiac evaluation and invasive DFS, was not described.28
Discussion
Trastuzumab is a monoclonal antibody that binds to HER2 to inhibit cellular proliferation and activate antibody-dependent cell mediated toxicity thereby exerting antitumor activity. This mechanism of action is preserved across trastuzumab’s indications for HER2-overexpressing early stage breast cancer, HER2-overexpressing metastatic breast cancer, and HER2-overexpressing metastatic gastric or GEJ adenocarcinoma, in all cases, HER2 remains the target.
The approval of biosimilars is based on the totality of evidence demonstrating similarity to the reference medication, clinical data is only typically available for one indication of the reference production. Therefore, biosimilars are often approved for multiple indications, that are held by the reference product, without available clinical evidence for all indications. This is probably the most unfamiliar concept in the approval process for biosimilars. This unique aspect of approval does create a significant barrier to the utilization of biosimilars because of the misconceptions of the level of evidence. However, given the evidence required to establish a product as a biosimilar, the scientific evidence to support such extrapolation is typically available.
In order to establish a product as a biosimilar of trastuzumab, it must undergo analytical testing to establish structural and functional similarity. For each of the approved trastuzumab biosimilars, these analytical factors have been found to be the same or highly similar to the reference trastuzumab. Structural similarity requires that preclinical data demonstrates that the biosimilar structure is virtually identical to the original product with regard to amino acid sequence, final protein product including the three-dimensional structural confirmation, and Fc binding regions. Once established as being structurally similar to trastuzumab, biosimilars are then subjected to HER2-binding assays, inhibition of proliferation assays, and antibody-dependent cellular cytotoxicity assays in order to establish functional similarity to trastuzumab prior to any clinical testing.
The rigorous establishment of similarity allows for utilization of clinical trials designed with short-term primary endpoints, including OR as seen in the HERITAGE study and pCR as seen in studies for CT-P6, and secondary end points such as PFS and DFS that can be measured at long-term follow-up.12,16 The clinical trials discussed have all provided similar outcomes for the respective biosimilars tested against trastuzumab.
In combination, the analytical establishment of these biosimilars as structurally and functional similar to trastuzumab and the clinically proven equivalent efficacy allows for the extrapolation that these biosimilars will also perform in an equivalent manner in other settings when HER2 is the target. For this reason, MYL-1401O, SB3, PF-05280014, and ABP-980 gained approval for all of the indications originally proven for trastuzumab. Only CT-P6 (Herzuma) is not approved for use in metastatic gastric cancer because this indication was not sought in the amended FDA approval application.
The FDA has recently provided guidance on the extrapolation of biosimilars to indications not formerly studied. In the context of biosimilars, extrapolation is not based on the assumption that the data from one study or population is sufficient to support approval for multiple, nonstudied, indications.29 However, in keeping with the abbreviated approval process supported by the overall evidence, extrapolation is based on all of the available data on the biosimilar, previous findings of safety and efficacy for other approved indications of the reference product, and the knowledge of various scientific factors for each indication. The scientific factors that are studied during establishment of biosimilarity include mechanism of action, pharmacokinetics, pharmacodynamics, and immunogenicity and allow for the FDA to extrapolate safety and efficacy of a biosimilar to indications or populations not formerly studied. Safety and efficacy have been established for trastuzumab in neoadjuvant, adjuvant and metastatic breast cancer, and metastatic gastric and GEJ tumors. Based on the FDA’s guidelines for extrapolation, additional indications not based on clinical trials were approved for the discussed trastuzumab biosimilars.
Clinically, it can be a challenge to approve the use of an oncological medication without well-established efficacy that is based on clinical trials. The traditional model would have clinical trial evidence to support each individual indication. However, reviewing the experiences with previously marketed biosimilar medications, it is reassuring that extrapolated indications have later been supported by trial data.30 For example, INN-filgrastim (Zarzio, Kundl, Austria) was approved in Europe for all indications of the reference filgrastim based on analytic data in combination with a study of patients with breast cancer who had chemotherapy-induced neutropenia. Following release in the European market, additional studies have demonstrated safety and efficacy in extrapolated indications including stem cell mobilization.31,32 It is possible that similar comparison studies will be conducted, to confirm the efficacy of trastuzumab biosimilars for the extrapolated indications.
Interchangeability, switching from the reference trastuzumab to a biosimilar or vice versa, is a separate issue. In the US, establishing a product as a biosimilar to the reference product does not mean it is interchangeable. Following FDA guidelines, to establish interchangeability a biological product must be a biosimilar of the reference product, it is expected to have the same clinical results as the reference product in any given patient and, if the product is administered over time, the risk of safety or diminished efficacy of alternating or switching between the reference and biosimilar products must not be greater than the risk of using the reference product without switching.29 Of note, in the US, interchangeable products can be substituted for the reference product without prescriber involvement, although this is regulated at the state level. Normally, establishing a biological product as interchangeable requires scientific evidence based on a ‘switching study’ where the pharmacokinetic, pharmacodynamics, and immunogenicity are compared between the reference product and a group that switches between the reference product and the proposed biosimilar. Currently, to the best of our knowledge, none of the US-approved trastuzumab biosimilars are labeled as interchangeable.
Despite approval of five trastuzumab biosimilars by the US FDA, market launch and availability has been halted due to remaining patents on Herceptin, the trastuzumab originator drug marketed by Genentech, South San Francisco, CA, USA. The trastuzumab patent is set to expire mid-2019 making it very likely that trastuzumab biosimilars will be launched shortly after this in the US. Sales of trastuzumab biosimilars are expected to lead to cost savings overall.
Herceptin has previously been the only available trastuzumab on the market with a cost of approximately US$70,000 for 1 year of treatment, the standard treatment duration for early stage HER2 breast cancers. For many patients, high copays, or out-of-pocket expenses, make this standard of care treatment cost prohibitive. While biosimilars are expensive to develop and market, the addition of alternatives to branded trastuzumab will introduce market competition and a projected cost savings of 20–30%.33,34 This cost savings appears modest in comparison with the 80–90% cost savings of typical generic medications. The development costs of a biosimilar are markedly different than those involved in bringing a generic small molecule drug to market. Of note, in many markets, the utilization of branded trastuzumab has been historically limited due to cost. Therefore, a competitive market combined with modest cost savings is expected to allow for greater access to HER2 monoclonal antibodies in the US and globally. Ultimately, the economic impact and access expansion will depend on the market price of biosimilars, provider utilization, and insurance acceptance. We remain optimistic about overall cost effectiveness allowing for improved patient access and savings over time.
While the utilization of trastuzumab has revolutionized the treatment of HER2-positive breast cancers over the years, some limitations remain. For instance, HER2-targeted antibodies do not cross the blood brain barrier and tumor resistance to trastuzumab is well described. In addition, they traditionally lack efficacy as a monotherapy and are instead given in combination with chemotherapy which limits utilization in certain patient populations. Trastuzumab biosimilars will have similar limitations. Studies investigating both bispecific and trifunctional antibodies are ongoing in attempts to overcome resistance.35 In addition, future alterations in the Fc binding regions may allow for expanded utilization of trastuzumab. These investigations allow for future areas of investigation for trastuzumab biosimilars.
Conclusion
There are five currently approved trastuzumab biosimilar medications available in the US for use in both HER2-overexpressing adjuvant breast cancer and metastatic breast cancer treatment (Table 1). The development and testing of these medications included rigorous analytical testing of the structure and binding function in order to establish them as biosimilars of trastuzumab. In gaining approval this data, along with clinical data, demonstrating similar efficacy and safety compared with trastuzumab was reviewed. These characteristics of biosimilars allow for more rapid approval and the potential for significant cost savings and expanded patient access to targeted therapy for breast cancer.
Table 1.
Comparison of the five approved trastuzumab biosimilars.
| Agent | Brand name | Approval | Indication | Trial population | Trial results |
|---|---|---|---|---|---|
| MYL-1401O | Ogivri | December 2017 | Adjuvant early stage and metastatic breast cancer, metastatic gastric or GEJ adenocarcinoma | Metastatic breast cancer (n = 500) | MYL-1410 versus trastuzumab: Noninferior ORR: 69.6% versus 64% (HR 1.09; CI 95% 0.95–1.24)12,13 |
| CT-P6 | Herzuma | December 2018 | Adjuvant early stage and metastatic breast cancer | Early stage breast cancer (n = 549) | CT-P6 versus trastuzumab: noninferior pCR 46.8% versus 50.4% (risk ratio 0.92)17,18 |
| SB3 | Ontruzant | January 2019 | Adjuvant early stage and metastatic breast cancer, metastatic gastric or GEJ adenocarcinoma | Early stage breast cancer (n = 800) | SB3 versus trastuzumab: Equivalent in breast pCR 51.7% versus 42.0% (adjusted ratio 1.259)20 |
| PF-05280014 | Trazimera | March 2019 | Adjuvant early stage and metastatic breast cancer, metastatic gastric or GEJ adenocarcinoma | Metastatic breast cancer (n = 707) | PF-05280014 versus trastuzumab: Equivalent in breast ORR 62.5% versus 66.5% (RR for ORR 0.94)24 |
| ABP980 | Kanjinti | June 2019 | Adjuvant early stage and metastatic breast cancer, metastatic gastric or GEJ adenocarcinoma | Early stage breast cancer (n = 725) | ABP980 versus trastuzumab: pCR 48% versus 41% (risk ratio 1.188)28 |
GEJ, gastroesophageal junction; HR, hazard ratio; ORR, Overall Response Rate; pCR, pathological complete response; RR, risk ratio.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: Lee Schwartzberg has acted as a consultant for Amgen, Pfizer, AstraZeneca, and Genentech and received research funding from Amgen. Emily Miller has no conflicts of interest to declare.
ORCID iD: Lee S. Schwartzberg  https://orcid.org/0000-0002-7433-3428
https://orcid.org/0000-0002-7433-3428
Contributor Information
Emily M. Miller, Division of Hematology/Oncology, Department of Medicine, University of Tennessee Health Science Center, Germantown, TN, USA
Lee S. Schwartzberg, West Cancer Center, 7945 Wolf River Boulevard, Germantown, TN 38183, USA. Division of Hematology/Oncology, Department of Medicine, University of Tennessee Health Science Center, Germantown, TN, USA.
References
- 1. Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012; 4: CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 2007; 357: 39–51. [DOI] [PubMed] [Google Scholar]
- 3. Grady D. Good news on early breast cancer: herceptin treatment can be shortened. The New York Times, 2018. [Google Scholar]
- 4. Nordqvist C. One year on herceptin for breast cancer ideal, https://www.medicalnewstoday.com/articles/250912.php (2012, accessed 23 June 2019).
- 5. Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q 2018; 7: 3. [PMC free article] [PubMed] [Google Scholar]
- 6. Rugo HS, Rifkin RM, Declerck P, et al. Demystifying biosimilars: development, regulation and clinical use. Future Oncol 2019; 15: 777–790. [DOI] [PubMed] [Google Scholar]
- 7. Santos SB, Sousa Lobo JM, Silva AC. Biosimilar medicines used for cancer therapy in Europe: a review. Drug Discov Today 2019; 24: 293–299. [DOI] [PubMed] [Google Scholar]
- 8. Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 9. Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 2014; 32: 3744–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 11. Waller CF, Vutikullird A, Lawrence TE, et al. A pharmacokinetics phase 1 bioequivalence study of the trastuzumab biosimilar MYL-1401O vs. EU-trastuzumab and US-trastuzumab. Br J Clin Pharmacol 2018; 84: 2336–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rugo HS, Barve A, Waller CF, et al. Heritage: a phase III safety and efficacy trial of the proposed trastuzumab biosimilar Myl-1401O versus Herceptin. J Clin Oncol 2016; 34(18 Suppl.): LBA503–LBA503. [Google Scholar]
- 13. Rugo HS, Barve A, Waller CF, et al. Effect of a proposed trastuzumab biosimilar compared with trastuzumab on overall response rate in patients with ERBB2 (HER2)-positive metastatic breast cancer: a randomized clinical trial. JAMA 2017; 317: 37–47. [DOI] [PubMed] [Google Scholar]
- 14. Jeong SA, Choi JM, Park JM, et al. Mechanism of action of the trastuzumab biosimilar CT-P6. Expert Opin Biol Ther. Epub ahead of print 13 December 2018. DOI: 10.1080/14712598.2019.1554052 [DOI] [PubMed] [Google Scholar]
- 15. Esteva FJ, Stebbing J, Wood-Horrall RN, et al. A randomised trial comparing the pharmacokinetics and safety of the biosimilar CT-P6 with reference trastuzumab. Cancer Chemother Pharmacol 2018; 81: 505–514. [DOI] [PubMed] [Google Scholar]
- 16. Esteva FJ, Saeki T, Kim H, et al. Efficacy and safety of the trastuzumab biosimilar candidate CT-P6. Future Oncol 2018; 14: 1909–1919. [DOI] [PubMed] [Google Scholar]
- 17. Stebbing J, Baranau Y, Baryash V, et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol 2017; 18: 917–928. [DOI] [PubMed] [Google Scholar]
- 18. Esteva FJ, Yu S, Kim M, et al. 24 months results from a double-blind, randomized phase III trial comparing the efficacy and safety of neoadjuvant then adjuvant trastuzumab and its biosimilar candidate CT-P6 in HER2 positive early breast cancer (EBC). Paper presented at: an Antonio Breast Cancer Symposium; 5–8 December 2018, San Antonia, Texas. [Google Scholar]
- 19. Pivot X, Curtit E, Lee YJ, et al. A randomized phase I pharmacokinetic study comparing biosimilar candidate SB3 and trastuzumab in healthy male subjects. Clin Ther 2016; 38: 1665–1673.e1663. [DOI] [PubMed] [Google Scholar]
- 20. Pivot X, Bondarenko I, Nowecki Z, et al. Phase III, randomized, double-blind study comparing the efficacy, safety, and immunogenicity of SB3 (trastuzumab biosimilar) and reference trastuzumab in patients treated with neoadjuvant therapy for human epidermal growth factor receptor 2-positive early breast cancer. J Clin Oncol 2018; 36: 968–974. [DOI] [PubMed] [Google Scholar]
- 21. Pivot X, Bondarenko I, Nowecki Z, et al. A phase III study comparing SB3 (a proposed trastuzumab biosimilar) and trastuzumab reference product in HER2-positive early breast cancer treated with neoadjuvant-adjuvant treatment: final safety, immunogenicity and survival results. Eur J Cancer 2018; 93: 19–27. [DOI] [PubMed] [Google Scholar]
- 22. Hurst S, Ryan AM, Ng CK, et al. Comparative nonclinical assessments of the proposed biosimilar PF-05280014 and trastuzumab (Herceptin((R))). BioDrugs 2014; 28: 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yin D, Barker KB, Li R, et al. A randomized phase 1 pharmacokinetic trial comparing the potential biosimilar PF-05280014 with trastuzumab in healthy volunteers (REFLECTIONS B327-01). Br J Clin Pharmacol 2014; 78: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pegram MD, Bondarenko I, Zorzetto MMC, et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br J Cancer 2019; 120: 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lammers PE, Dank M, Masetti R, et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer 2018; 119: 266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanes V, Chow V, Zhang N, et al. A randomized, single-blind, single-dose study evaluating the pharmacokinetic equivalence of proposed biosimilar ABP 980 and trastuzumab in healthy male subjects. Cancer Chemother Pharmacol 2017; 79: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Food and Drug Administration. Trastuzumab-anns prescribing information. FDA; 2019. [Google Scholar]
- 28. von Minckwitz G, Colleoni M, Kolberg H-C, et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol 2018; 19: 987–998. [DOI] [PubMed] [Google Scholar]
- 29. Food and Drug Administration. Considerations in Demonstrating Interchangeability with Reference Product Guidance for Industry, https://www.fda.gov/media/124907/download.
- 30. Krendyukov A, Schiestl M. Extrapolation concept at work with biosimilar: a decade of experience in oncology. ESMO Open 2018; 3: e000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schmitt M, Hoffmann JM, Lorenz K, et al. Mobilization of autologous and allogeneic peripheral blood stem cells for transplantation in haematological malignancies using biosimilar G-CSF. Vox Sang 2016; 111: 178–186. [DOI] [PubMed] [Google Scholar]
- 32. Becker P, Schwebig A, Brauninger S, et al. Healthy donor hematopoietic stem cell mobilization with biosimilar granulocyte-colony-stimulating factor: safety, efficacy, and graft performance. Transfusion 2016; 56: 3055–3064. [DOI] [PubMed] [Google Scholar]
- 33. Henry D, Taylor C. Pharmacoeconomics of cancer therapies: considerations with the introduction of biosimilars. Semin Oncol 2014; 41(Suppl. 3): S13–S20. [DOI] [PubMed] [Google Scholar]
- 34. Blackwell K, Gligorov J, Jacobs I, et al. The global need for a trastuzumab biosimilar for patients with HER2-positive breast cancer. Clinical Breast Cancer 2018; 18: 95–113. [DOI] [PubMed] [Google Scholar]
- 35. Yu S, Liu Q, Han X, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol 2017; 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]


