Abstract
Ginsenosides, the key components isolated from ginseng, have been extensively studied in antitumor treatment. Numerous studies have shown that ginsenosides have direct function in tumor cells through the induction of cancer cell apoptosis and the inhibition of cancer cell growth and enhance the antitumor immunity through the activation of cytotoxic T lymphocytes and natural killer cells. However, little is known about the function of ginsenosides on myeloid immunosuppressive cells including dendritic cells in tumor, tumor-associated macrophages, and myeloid-derived suppressor cells in the tumor microenvironments. Those myeloid immunosuppressive cells play important roles in promoting tumor angiogenesis, invasion, and metastasis. In the review, we summarize the regulatory functions of ginsenosides on myeloid immunosuppressive cells in tumor microenvironment, providing the novel therapeutic methods for clinical cancer treatment.
Keywords: ginsenosides, cancer therapies, tumor microenvironment, myeloid immunosuppressive cells, tumor-associated macrophages, myeloid-derived suppressor cells
Introduction
Ginsenosides are the major active components extracted from ginseng. According to its chemical properties, ginsenosides have been divided into 3 types: ginsenoside diol type (type A), including Rb1, Rb2, RC, Rd, Rh2, and so on; ginsenoside trial type (type B), including Re, Rf, Rg1, Rg2, Rhr; and phenolic acid type (type C), such as Ro, Rh3, Ri, F4.1 Recently, rare saponins and glycosides in ginsenosides can be also provided by biotransformation.2,3 The results demonstrated that ginsenoside and its biotransformation products have remarkable pharmacological activities such as antitumor and improving immune system.4 Most data have shown that ginsenosides can improve the immune function of the body to carry out the antitumor role through increasing T-cell antitumor activity and enhancing the cytotoxicity of natural killer cells on tumor.5-8 Ginsenosides also inhibit the proliferation of tumor cells and induce their apoptosis directly.9-13 However, little is known about the function of ginsenosides on immunosuppressive cells in the tumor microenvironment. A majority of tumor immunosuppressive cells are myeloid-derived hematopoietic cells, including dendritic cells (DCs) in tumor, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs).14,15 Plenty of evidence indicates that the tumor microenvironment alters myeloid cells by converting them into potent immunosuppressive populations (tumor immunosuppressive cells) that facilitate tumor growth.16,17
In human body, myeloid cells arise from multipotent hematopoietic stem cells that develop into mature myeloid cells with multiple functions through sequential steps of differentiation physiologically. The 3 major groups of terminally differentiated myeloid cells—macrophages, DCs, and granulocytes—are essential for the physiological functions of the immune system. These cells protect organisms from pathogens, eliminate necrotic cells, and mediate tissue remodeling through the innate and/or adaptive immune response.18,19 However, in the tumor microenvironment, the roles of those myeloid cells were changed to promote tumor angiogenesis, invasion, and metastasis.14,20,21
In the review, we have summarized the regulatory function of ginsenosides in myeloid immunosuppressive cells in the tumor microenvironment, providing novel therapeutic methods for clinical use.
Components and Transformation of Ginsenosides
There are 3 key constituents of ginseng, namely, saponins, polysaccharides, and phenolic compounds.22 Ginseng’s saponins are generally called ginsenosides, the basic structure of which consists of a steroidal core with various sugar moieties and arabinose attached to the C3, C6, and C20 positions. Classically, ginsenosides are grouped into 2 major categories (panaxadiol saponin and panaxatriol saponin) based on different functional structures on the C6 position. Panaxadiol saponin, which contains 1 hydrogen, includes Rb1, Rb2, Rb3, Rd, Rg3, Rg5, Rh2, Rs11, Rk1, F2, and CK. Panaxatriol saponin, which contains a sugar side-chain, includes Re, Rg1, Rg18, Rh1, Rh4, Rp1, Rf, and F1.23,24 Most ginsenosides have been extensively investigated and emphasized in cancer chemoprevention and therapeutics (Table1).1,12,22 However, little was known about the functions of some rare ginsenosides due to the quantity and processing of those products.25
Table 1.
Major Ginsenosides With Anti-Tumor Activity.
| Number | Ginsenoside | Molecular Formula | Diagram | Reference |
|---|---|---|---|---|
| 1 | 20(S)-Ginsenoside-Rg2 | C42H72O13 |
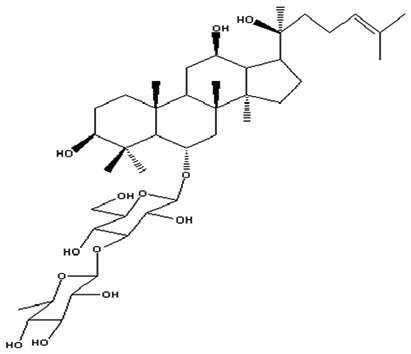
|
Ma et al54 |
| 2 | 20(R)-Ginsenoside-Rg2 | C42H72O13 |

|
Gui et al55 |
| 3 | Ginsenoside Rg1 | C42H72O14 |
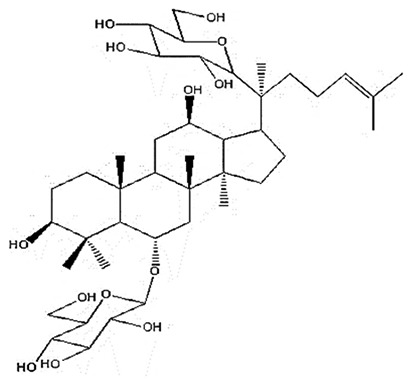
|
Shang et al34 |
| 4 | Ginsenoside Rf | C42H72O14 |
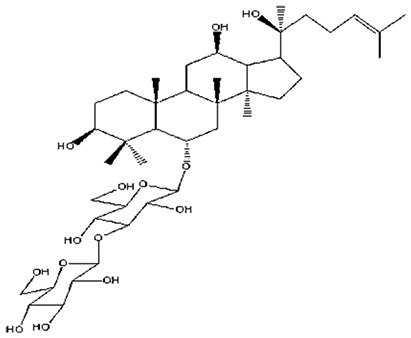
|
Song et al56 |
| 5 | Ginsenoside Rb1 | C54H92O23 |
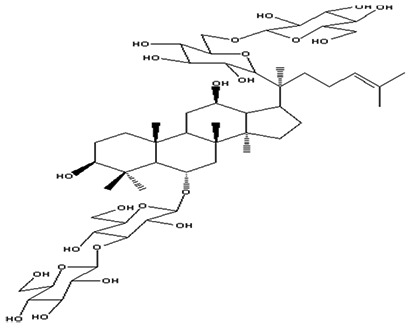
|
Lei et al57 |
| 6 | Ginsenoside Rb2 | C53H90O22 |
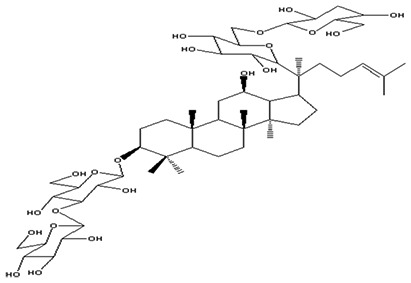
|
Gurung et al58 |
| 7 | β-D-glucopyranosyl 3-(β-D-glactopyranosyl (1→2)(β-D-glucopyranosyloxy))-oleanate | C42H72O18 |

|
Khan et al59 |
| 8 | Ginsenoside Rh1 | C36H62O9 |
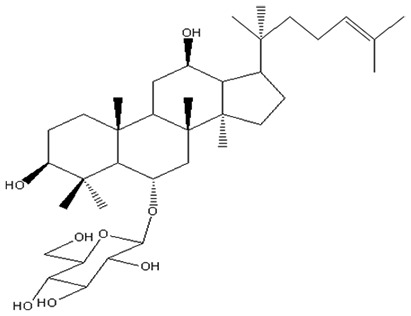
|
Odashima et al60 |
| 9 | 20(S)-Ginsenoside Rg3 | C42H72O13 |
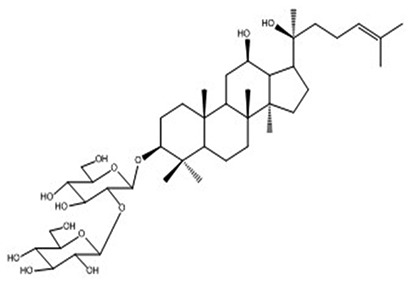
|
Anufriev et al61 |
| 10 | Gypenoside LXXV | C42H72O13 |
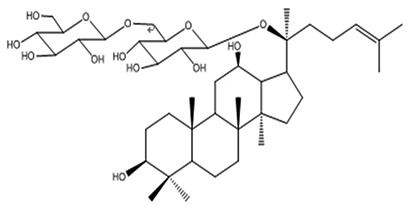
|
An et al62 |
| 11 | Ginsenoside Rk1 | C42H70O12 |
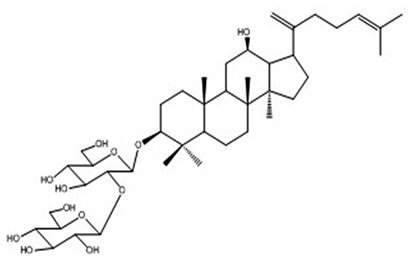
|
Park et al63 |
| 12 | Ginsenoside Re | C48H82O18 |
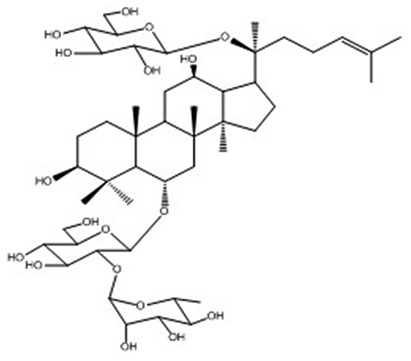
|
Sekiya et al64 |
| 13 | Notoginsenoside G | C48H80O19 |

|
Yoshikawa et al65 |
| 14 | Quinquefoloside-Le | C48H80O18 |
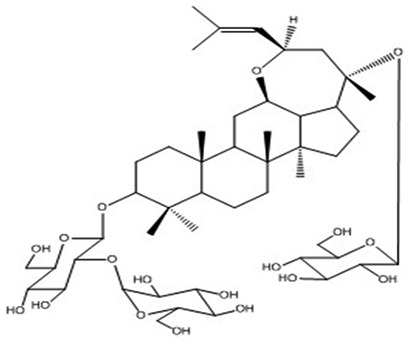
|
Yoshikawa et al66 |
| 15 | Vina-ginsenoside-R9 | C48H82O19 |
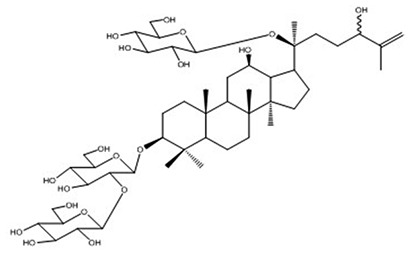
|
Nguyen et al67 |
| 16 | Vina-ginsenoside-R8 | C48H82O19 |
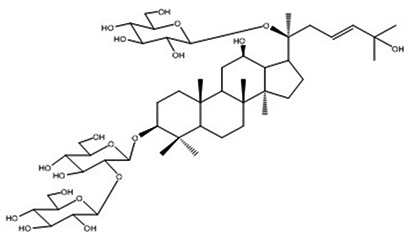
|
Ngyuen et al67 |
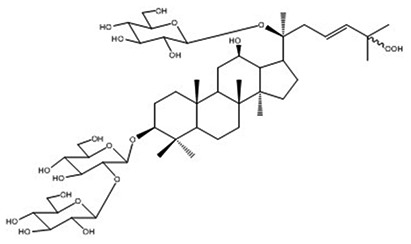
| 17 | Notoginsenoside E | C48H82O20 |
|
Yoshizaki et al68 |
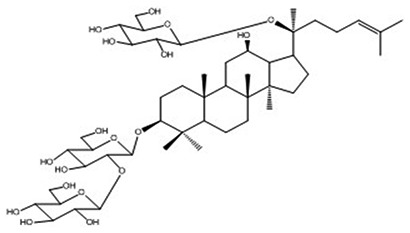
| 18 | Ginsenoside Rd | C48H82O18 |
|
Jung et al69 |
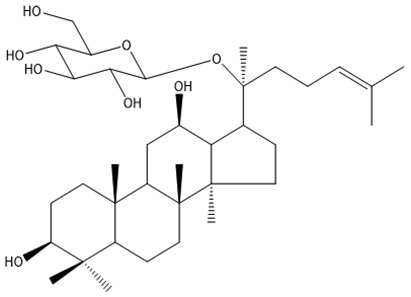
| 19 | Ginsenoside CK | C36H62O8 |
|
Wang et al51 and Huang et al70 |
| 20 | Ginsenoside F2 | C42H72O13 |

|
Ko et al71 |
| 21 | Notoginsenoside ST-8 | C42H72O14 |

|
Xu et al25,26 |
| 22 | Ginsenoside Re8 | C54H92O23 |
|
Xu et al25,27 |
| 23 | Ginsenoside Rb4 | C54H92O23 |
|
Xu et al and Wang et al26,28 |
| 24 | Ginsenoside Rb5 | C60H102O28 |

|
Xu et al26,27 |
Our research group spent several years on the biotransformation of Panax ginseng as a fermentation matrix and its active single saponin compounds as biocatalytic substrates to solve the problems of the quantity and production of rare ginsenosides. Fruitful proceedings have been accomplished through our research, including the establishment and optimization of biotransformation methods for the ample supply of some rare saponins, the discovery and elucidation on new saponin structures that provide potential anticancer agents, and others (Table1).26-28 We also found that several rare or new ginsenosides enhance antitumor immune response in the tumor microenvironment,25,26 and unpublished data, which will provide some novel medical products for clinical use of ginsenosides.
Regulatory Roles of Ginsenosides on Myeloid Immunosuppressive Cells
Ginsenosides Induce the Alteration of Tumor-Associated Macrophages
It is well-known that the tumor microenvironment is important for cancer development and metastasis. TAMs, one of the myeloid immunosuppressive cells in the tumor microenvironment, correlate with tumor progression and poor prognosis.29 The accumulating evidence supports that TAMs are typical pro-tumor macrophages (M2), which are responsible for releasing immunosuppressive cytokines, chemokines, and growth factors such as arginase, vascular endothelial growth factor (VEGF), platelet-derived growth factor, and interleukin-10 (IL-10), rendering tumor-specific cytotoxic T lymphocytes hyporesponsive and promoting tumor angiogenesis.30
Accumulating reports have shown that ginsenosides, the major active component of ginseng, had a potential to effectively convert TAM to the M1 subset of macrophages and enhance anti-tumor activity of M1 macrophages (Figure 1). Li et al31 demonstrated that ginsenoside Rh2 preferably decreases the expression levels of vascular endothelial growth factor, MMP2, and MMP9 (matrix metalloproteinase-2 and -9) on TAM to convert TAM from the M2 to M1 type, and further inhibiting/killing tumors. And ginsenosides Rg1 and Rg3 could facilitate macrophages with enhanced tumor cell killing ability by nitric oxide (NO) production (Figure1).32-34 20(S)-Protopanaxatriol (PPT) is one of the major metabolites of ginsenosides. Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) are important enzymes that mediate inflammatory processes. Improper upregulation of iNOS and/or COX-2 has been associated with the pathogenesis of inflammatory diseases and certain types of human cancers. PPT blocked the increase in lipopolysaccharides-induced iNOS and COX-2 expressions through inactivation of NFκB by preventing I-κBα phosphorylation and degradation. Thus, it may be possible to develop PPT as a useful agent for the prevention of cancer or inflammatory diseases (Figure1).10
Figure 1.
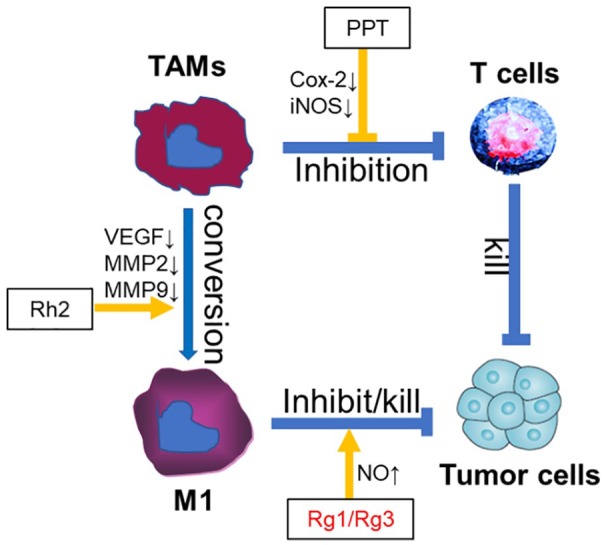
Roles of ginsenosides on function of tumor-associated macrpophages (TAMs) in tumor microenvironment.
In tumor microenvironment, TAMs inhibit the antitumor ability of T cells. Ginsenosides can block the inhibition function of TAM, promote TAMs conversion from M2 to M1 type, further inhibiting or killing tumor. PPT, 20(S)-Protopanaxatriol. Blue lines demonstrated the promotion ( ) or inhibition (
) or inhibition ( ) function among immune cells (TAM or T cells) and tumor cells. Yellow lines indicated the promotion (
) function among immune cells (TAM or T cells) and tumor cells. Yellow lines indicated the promotion ( ) or inhibition (
) or inhibition ( ) functions of ginsenosides.
) functions of ginsenosides.
Ginsenosides Enhance Antigen-Presenting Capability of Dendritic Cells
DCs, the most powerful professional antigen-presenting cells, which are involved in the interaction between innate and adaptive immune responses, play an important role in the suppression of tumorigenesis and tumor development.35,36 However, the suppression function of DCs in cancer patients contributes to tumor evasion of immune recognition and tumor progression.37 Both immature and mature DCs in tumor-bearing hosts may be coordinated by the tumor microenvironment to suppress T functions.14 MHC-II+CD11c+ tumor DCs have been shown to suppress the antitumor immune responses of CD8+ T cells through arginase 1 production, which is the same immunosuppressive mechanism as those of TAMs and MDSCs.38,39 Interestingly, plasmacytoid DC in prostate tumors also use arginase 1 and IDO to inhibit anti-tumor function of CD8+ T cells, suggesting that immunosuppressive mechanisms might be shared across different myeloid cells in the tumor microenvironment.40,41
The functional activation or conversion of DC in tumor patients is an essential mechanism of ginseng as an immunomodulator.42,43 There are emerging data that ginsenosides, as the functional contents of ginseng, are involved in enhancing the function of DCs in the tumor microenvironment. Ginsenosides activate the DCs and promote adaptive immune responses to exert anticancer effects in tumor-bearing mice.44,45 Huang et al46 found that ginsenoside Rg1 had adjuvant effects on DCs by promoting the production and secretion of cytokines (such as tumor necrosis factor-α) and chemokines (such as IL-8, IP-10), and further explored its antitumor activity combined with OVA in the lymphoma mouse model. Ginsenoside Rg1, as a cancer vaccine adjuvant, also activated antitumor immune responses directly. Ginsenoside Rg1 and Rh1 stimulate DCs to promote T cell proliferation and enhance the antitumor activity of LPAK by the treatment of PHA and IL-2, indicating that Rg1 and Rh1 may be the effective immunotherapeutic compounds.47 Recent studies have shown that ginsenoside Rg3 is a ginseng saponin that has its cytotoxic effect on tumor cells. In addition, Rg3-induced immunogenic cell death on tumor cells is characterized by the cell surface exposure of some chaperone proteins, which affect DC maturation and the uptake and presentation of tumor antigens. The data suggest that Rg3 has antitumor activity due to its cytotoxic effect and its ability to induce DC function.45,48
Ginsenosides Downregulate the Number and Function of MDSCs
MDSCs, which were dramatically increased in peripheral blood of patients or mouse models with cancer, are very important in tumor immune evasion in the tumor microenvironment.14,49 Some data have shown that intraperitoneal administration of Ginseng compromises MDSC function to induce T cell proliferation and the secretion of IL-2 and interferon-γ in tumor-bearing mice. The downregulation of iNOS and IL-10 suggest that Ginseng can restrain the suppressive function of MDSCs.50 Furthermore, study in colorectal cancer xenograft bearing mice model had shown that ginseng-derived compound K (C-K) had a significant effect on MDSCs. It was found that the percentages of apoptosis in early and late MDSCs was higher in the C-K treatment groups than those in the control groups. C-K also significantly decreased the expressions of immunosuppression-related genes COX-2 and Arg-1, and inhibited the secretion of IL-1β, IL-6, and IL-17 to inhibit CT26 cancer progression in mice.51 The effect of ginseng activity on macrophages extends to the immunosuppressive phenotype, such as Korean Red Ginseng, which blocks abnormal differentiation and suppressive function of MDSCs resulting in immune-activating events such as T-cell proliferation and the secretion of interferon-γ and IL-2, which were regulated by STAT3 (Figure 2).50,51
Figure 2.
Effect of ginsenosides on differentiation and function of myeloid-derived suppressor cells (MDSCs) in tumor microenvironment.
In tumor microenvironment, MDSCs from immature myeloid cells inhibit the antitumor ability of T cells. In each process, ginsenosides play a negative role. KRG, Korean Red Ginseng; C-K, ginseng-derived compound K; SQF, Sheng Qi formula. Blue lines demonstrated the promotion ( ) or inhibition (
) or inhibition ( ) roles among immature myeloid cells, MDSCs, and T cells. Yellow lines indicated the promotion (
) roles among immature myeloid cells, MDSCs, and T cells. Yellow lines indicated the promotion ( ) or inhibition (
) or inhibition ( ) roles of ginsenosides.
) roles of ginsenosides.
Lee et al52 found that one herbal extract, H9, enhanced antitumor roles of chemotherapeutic drugs doxorubicin/cyclophosphamide (AC). Ginsenosides Rg1 and Rb1, as 2 major marker compounds of H9, were involved in decreasing the number of MDSC population in 4T1 tumor-bearing mice (Figure 2).
Recently, the scientists from the National Cancer Institute in the United States found that Sheng Qi formula (SQF) delivered in the drinking water of mice bearing 4T1 inflammatory breast tumors resulted in reduction in all the following factors: tumor growth, numbers of tumor MDSC, functionality of the remaining MDSC, and circulating levels of IL-1 and granulocyte-colony stimulating factor. Combination therapy with SQF and paclitaxel or cyclophosphamide resulted in additive reduction in tumor growth and synergistic effects on MDSC. Ginsenosides Rg1, Re, Rb1, and Rd have been found as major functional components of SQF further (Figure 2).53
Conclusion
In the review, we summarized which ginsenosides can regulate the number and function of myeloid immunosuppressive cells (tumor DCs, TAMs, and MDSCs) to enhance the anticancer ability of the body and avoid the growth, metastasis, and recurrence of tumor cells in the tumor microenvironment. These data suggest that the roles of ginsenosides on tumor myeloid immunosuppressive cells may be regarded as one novel therapeutic method for clinical cancer treatment.
Future Prospect
Ginsenosides, as the immunomodulatory drugs, may assist with other clinical antitumor antibodies such as anti-PD-1 antibodies by reducing the number of myeloid immunosuppressive cells and inhibiting their functions to improve tumor microenvironment for enhancing antitumor activity of those antibodies. Recently, we and some other scientists improved the production process of several rare ginsenosides by the biotransformation of Panax ginseng. We found that those ginsenosides also had strong antitumor immune activity and inhibited the function of myeloid immunosuppressive cells in the tumor microenvironment, suggesting that more ginsenosides may be found to improve tumor microenvironment by inhibiting tumor myeloid immunosuppressive cells, and become strong immunoregulatory drugs for cancer treatment.
Acknowledgments
The authors thank the Pharmacy Department staff who technically supported us for the realization of this article.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (Grant Nos. 81572868, 81803680, 81803796, 81803649); Science Foundation of Shangdong (Grant No. ZR2018LC012); Jilin Province Science and Technology Development Project in China (20170307031YY, 20180520050JH); and Foundation of Jilin Educational Committee (JJKH20170726KJ).
ORCID iD: Peng Qu  https://orcid.org/0000-0002-1968-5455
https://orcid.org/0000-0002-1968-5455
References
- 1. Leung KW, Wong AS. Pharmacology of ginsenosides: a literature review. Chin Med. 2010;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng LQ, Na JR, Bang MH, Kim MK, Yang DC. Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry. 2008;69:218-224. [DOI] [PubMed] [Google Scholar]
- 3. Joh EH, Lee IA, Jung IH, Kim DH. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation—the key step of inflammation. Biochem Pharmacol. 2011;82:278-286. [DOI] [PubMed] [Google Scholar]
- 4. Liu KK, Wang QT, Yang SM, Chen JY, Wu HX, Wei W. Ginsenoside compound K suppresses the abnormal activation of T lymphocytes in mice with collagen-induced arthritis. Acta Pharmacol Sin. 2014;35:599-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mochizuki M, Yoo YC, Matsuzawa K, et al. Inhibitory effect of tumor metastasis in mice by saponins, ginsenoside-Rb2, 20(R)- and 20(S)-ginsenoside-Rg3, of red ginseng. Biol Pharm Bull. 1995;18:1197-1202. [DOI] [PubMed] [Google Scholar]
- 6. Keum YS, Park KK, Lee JM, et al. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000;150:41-48. [DOI] [PubMed] [Google Scholar]
- 7. Nakata H, Kikuchi Y, Tode T, et al. Inhibitory effects of ginsenoside Rh2 on tumor growth in nude mice bearing human ovarian cancer cells. Jpn J Cancer Res. 1998;89:733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I. Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid-conjugate of protopanaxatriol. Biol Pharm Bull. 2002;25:861-866. [DOI] [PubMed] [Google Scholar]
- 9. Liu WK, Xu SX, Che CT. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000;67:1297-1306. [DOI] [PubMed] [Google Scholar]
- 10. Oh GS, Pae HO, Choi BM, et al. 20(S)-Protopanaxatriol, one of ginsenoside metabolites, inhibits inducible nitric oxide synthase and cyclooxygenase-2 expressions through inactivation of nuclear factor-kappaB in RAW 264.7 macrophages stimulated with lipopolysaccharide. Cancer Lett. 2004;205:23-29. [DOI] [PubMed] [Google Scholar]
- 11. Oh M, Choi YH, Choi S, et al. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869-875. [DOI] [PubMed] [Google Scholar]
- 12. Mai TT, Moon J, Song Y, et al. Ginsenoside F2 induces apoptosis accompanied by protective autophagy in breast cancer stem cells. Cancer Lett. 2012;321:144-153. [DOI] [PubMed] [Google Scholar]
- 13. Qi Z, Chen L, Li Z, et al. Immunomodulatory effects of (24R)-pseudo-ginsenoside HQ and (24S)-pseudo-ginsenoside HQ on cyclophosphamide-induced immunosuppression and their anti-tumor effects study. Int J Mol Sci. 2019;20:E836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634-1642. [PubMed] [Google Scholar]
- 17. Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263-266. [DOI] [PubMed] [Google Scholar]
- 18. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [DOI] [PubMed] [Google Scholar]
- 19. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cubillos-Ruiz JR, Silberman PC, Rutkowski MR, et al. ER stress sensor XBP1 controls anti-tumor immunity by disrupting dendritic cell homeostasis. Cell. 2015;161:1527-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256-272. [DOI] [PubMed] [Google Scholar]
- 23. Chen T, Li B, Qiu Y, Qiu Z, Qu P. Functional mechanism of Ginsenosides on tumor growth and metastasis. Saudi J Biol Sci. 2018;25:917-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee DG, Lee AY, Kim KT, Cho EJ, Lee S. Novel dammarane-type triterpene saponins from Panax ginseng root. Chem Pharm Bull (Tokyo). 2015;63:927-934. [DOI] [PubMed] [Google Scholar]
- 25. Xu W, Yan QX, Liu YY, Chang BJ, Liu X, Qiu ZD. Three new triterpenoid saponins from notoginseng medicinal fungal substance. Chem Biodivers. 2017;14(12). doi: 10.1002/cbdv.201700195 [DOI] [PubMed] [Google Scholar]
- 26. Xu W, Liu X, Liu XL, Jia AL, Wang XW, Qiu ZD. Two new dammarane-type triterpenoid saponins from notoginseng medicinal fungal substance. J Asian Nat Prod Res. 2016;18:1138-1142. [DOI] [PubMed] [Google Scholar]
- 27. Xu W, Zhang JH, Wang XW, Liu XL, Liu X, Qiu ZD. Two new triterpenoid saponins from ginseng medicinal fungal substance. J Asian Nat Prod Res. 2016;18:865-870. [DOI] [PubMed] [Google Scholar]
- 28. Wang WN, Yan BX, Xu WD, Qiu Y, Guo YL, Qiu ZD. Highly selective bioconversion of ginsenoside Rb1 to compound K by the mycelium of Cordyceps sinensis under optimized conditions. Molecules. 2015;20:19291-19309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310-327. [DOI] [PubMed] [Google Scholar]
- 30. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [DOI] [PubMed] [Google Scholar]
- 31. Li H, Huang N, Zhu W, et al. Modulation the crosstalk between tumor-associated macrophages and non-small cell lung cancer to inhibit tumor migration and invasion by ginsenoside Rh2. BMC Cancer. 2018;18:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fan ZH, Isobe K, Kiuchi K, Nakashima I. Enhancement of nitric oxide production from activated macrophages by a purified form of ginsenoside (Rg1). Am J Chin Med. 1995;23:279-287. [DOI] [PubMed] [Google Scholar]
- 33. Park D, Bae DK, Jeon JH, et al. Immunopotentiation and antitumor effects of a ginsenoside Rg(3)-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011;31:397-405. [DOI] [PubMed] [Google Scholar]
- 34. Shang JH, Xu GW, Zhu HT, Wang D, Yang CR, Zhang YJ. Anti-inflammatory and cytotoxic triterpenes from the rot roots of Panax notoginseng. Nat Prod Bioprospect. 2019;9:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405-411. [DOI] [PubMed] [Google Scholar]
- 36. Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437-3446. [DOI] [PubMed] [Google Scholar]
- 37. Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562-567. [DOI] [PubMed] [Google Scholar]
- 38. Norian LA, Rodriguez PC, O’Mara LA, et al. Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res. 2009;69:3086-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qu P, Yan C, Du H. Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood. 2011;117:4476-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watkins SK, Zhu Z, Riboldi E, et al. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361-1372. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339-1346. [DOI] [PubMed] [Google Scholar]
- 42. Chen R, Deng X, Wu H, et al. Combined immunotherapy with dendritic cells and cytokine-induced killer cells for malignant tumors: a systematic review and meta-analysis. Int Immunopharmacol. 2014;22:451-464. [DOI] [PubMed] [Google Scholar]
- 43. Guo Q, Li J, Lin H. Effect and molecular mechanisms of traditional Chinese medicine on regulating tumor immunosuppressive microenvironment. Biomed Res Int. 2015;2015:261620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang W, Cho SY, Xiang G, Min KJ, Yu Q, Jin JO. Ginseng berry extract promotes maturation of mouse dendritic cells. PLoS One. 2015;10:e0130926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cho M, Choi G, Shim I, Chung Y. Enhanced Rg3 negatively regulates Th1 cell responses. J Ginseng Res. 2019;43:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang Y, Zou Y, Lin L, Zheng R. Ginsenoside Rg1 activates dendritic cells and acts as a vaccine adjuvant inducing protective cellular responses against lymphomas. DNA Cell Biol. 2017;36:1168-1177. [DOI] [PubMed] [Google Scholar]
- 47. Tam DNH, Truong DH, Nguyen TTH, et al. Ginsenoside Rh1: a systematic review of its pharmacological properties. Planta Med. 2018;84:139-152. [DOI] [PubMed] [Google Scholar]
- 48. Son KJ, Choi KR, Lee SJ, Lee H. Immunogenic cell death induced by ginsenoside Rg3: significance in dendritic cell-based anti-tumor immunotherapy. Immune Netw. 2016;16:75-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qu P, Wang LZ, Lin PC. Expansion and functions of myeloid-derived suppressor cells in the tumor microenvironment. Cancer Lett. 2016;380:253-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jeon C, Kang S, Park S, Lim K, Hwang KW, Min H. T Cell stimulatory effects of Korean red ginseng through modulation of myeloid-derived suppressor cells. J Ginseng Res. 2011;35:462-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang R, Li Y, Wang W, Zhou M, Cao Z. Compound K suppresses myeloid-derived suppressor cells in a mouse model bearing CT26 colorectal cancer xenograft [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:748-752. [PubMed] [Google Scholar]
- 52. Lee S, Han S, Jeong AL, et al. Combined treatment of herbal mixture extract H9 with trastuzumab enhances anti-tumor growth effect. J Microbiol Biotechnol. 2015;25:1036-1046. [DOI] [PubMed] [Google Scholar]
- 53. Jia L, Lin H, Oppenheim J, et al. US National Cancer Institute-China Collaborative Studies on Chinese medicine and cancer. J Natl Cancer Inst Monogr. 2017;2017(52). doi: 10.1093/jncimonographs/lgx007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma LY, Zhou QL, Yang XB, Wang HP, Yang XW. Metabolism of 20(S)-ginsenoside Rg(2) by rat liver microsomes: bioactivation to SIRT1-activating metabolites. Molecules. 2016;21(6):E757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gui FJ, Yang XW, Li LY, Tian JM. Simultaneous enantiomer determination of 20 (R)- and 20 (S)-ginsenoside-Rg2 in rat plasma after intravenous administration using HPLC method. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;850:1-6. [DOI] [PubMed] [Google Scholar]
- 56. Song HH, Moon JY, Ryu HW, et al. Discrimination of white ginseng origins using multivariate statistical analysis of data sets. J Ginseng Res. 2014;38:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lei J, Li X, Gong XJ, Zheng YN. Isolation, synthesis and structures of cytotoxic ginsenoside derivatives. Molecules. 2007;12:2140-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gurung B, Bhardwaj PK, Rai AK, Sahoo D. Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Nat Prod Res. 2018;32:234-238. [DOI] [PubMed] [Google Scholar]
- 59. Khan A, Muller S, Hecht S. Practical synthesis of an amphiphilic, non-ionic poly(para-phenyleneethynylene) derivative with a remarkable quantum yield in water. Chem Commun (Camb). 2005:584-586. [DOI] [PubMed] [Google Scholar]
- 60. Odashima S, Ohta T, Kohno H, et al. Control of phenotypic expression of cultured B16 melanoma cells by plant glycosides. Cancer Res. 1985;45:2781-2784. [PubMed] [Google Scholar]
- 61. Anufriev VP, Malinovskaya GV, Denisenko VA, et al. Synthesis of ginsenoside Rg3, a minor constituent of Ginseng radix. Carbohydr Res. 1997;304:179-182. [DOI] [PubMed] [Google Scholar]
- 62. An DS, Cui CH, Lee HG, et al. Identification and characterization of a novel Terrabacter ginsenosidimutans sp nov beta-glucosidase that transforms ginsenoside Rb1 into the rare gypenosides XVII and LXXV. Appl Environ Microbiol. 2010;76:5827-5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Park IH, Kim NY, Han SB, et al. Three new dammarane glycosides from heat processed ginseng. Arch Pharm Res. 2002;25:428-432. [DOI] [PubMed] [Google Scholar]
- 64. Sekiya K, Kadota S, Katayama K, Koizumi T, Namba T. Study on baths with crude drug. III. The effect of ligustici chuanxiong rhizoma extract on the percutaneous absorption of some natural compounds. Biol Pharm Bull. 1997;20:983-987. [DOI] [PubMed] [Google Scholar]
- 65. Yoshikawa M, Murakami T, Ikebata A, et al. Bioactive saponins and glycosides. X. On the constituents of zizyphi spinosi semen, the seeds of Zizyphus jujuba Mill var spinosa Hu (1): structures and histamine release-inhibitory effect of jujubosides A1 and C and acetyljujuboside B. Chem Pharm Bull (Tokyo). 1997;45:1186-1192. [DOI] [PubMed] [Google Scholar]
- 66. Yoshikawa M, Murakami T, Ueno T, et al. Bioactive saponins and glycosides. VIII. Notoginseng (1): new dammarane-type triterpene oligoglycosides, notoginsenosides-A, -B, -C, and -D, from the dried root of Panax notoginseng (Burk) F H Chen. Chem Pharm Bull (Tokyo). 1997;45:1039-1045. [DOI] [PubMed] [Google Scholar]
- 67. Nguyen MD, Kasai R, Ohtani K, et al. Saponins from Vietnamese ginseng, panax vietnamensis HA et Grushv. Collected in central Vietnam. II. Chem Pharm Bull (Tokyo). 1994;42:115-122. [DOI] [PubMed] [Google Scholar]
- 68. Yoshizaki K, Yahara S. New triterpenoid saponins from fruits specimens of Panax japonicus collected in Kumamoto and Miyazaki prefectures. Chem Pharm Bull (Tokyo). 2012;60:354-362. [DOI] [PubMed] [Google Scholar]
- 69. Jung J, Jang HJ, Eom SJ, Choi NS, Lee NK, Paik HD. Fermentation of red ginseng extract by the probiotic Lactobacillus plantarum KCCM 11613P: ginsenoside conversion and antioxidant effects. J Ginseng Res. 2019;43:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang Y, Liu H, Zhang Y, et al. Synthesis and biological evaluation of ginsenoside compound K derivatives as a novel class of LXRalpha activator. Molecules. 2017;22:E1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ko SR, Suzuki Y, Suzuki K, Choi KJ, Cho BG. Marked production of ginsenosides Rd, F2, Rg3, and compound K by enzymatic method. Chem Pharm Bull (Tokyo). 2007;55:1522-1527. [DOI] [PubMed] [Google Scholar]



