Short abstract
Introduction
Patients with multiple sclerosis may have a distinct gut microbiota profile. Delayed-release dimethyl fumarate is an orally administered drug for relapsing–remitting multiple sclerosis, which has been associated with gastrointestinal side-effects in some patients.
Objectives
The purpose of this study was to determine if dimethyl fumarate alters the abundance and diversity of commensal gut bacteria, and if these changes are associated with gastrointestinal side-effects.
Methods
Thirty-six patients with relapsing–remitting multiple sclerosis received either dimethyl fumarate (n = 27) or an injectable multiple sclerosis disease-modifying therapy (glatiramer acetate or interferons, n = 9) for 12 weeks. Stool samples were collected at baseline, two and 12 weeks. We included 165 healthy individuals as controls.
Results
At baseline, 16 microbial genera were altered in multiple sclerosis patients compared with healthy controls. In the dimethyl fumarate-treated patients (n = 21) we observed a trend of reduced Actinobacteria (p = 0.03, QFDR = 0.24) at two weeks, mainly driven by Bifidobacterium (p = 0.06, QFDR = 0.69). At 12 weeks, we observed an increased abundance of Firmicutes (p = 0.02, QFDR = 0.09), mostly driven by Faecalibacterium (p = 0.01, QFDR = 0.48).
Conclusions
This pilot study did not detect a major effect of dimethyl fumarate on the gut microbiota composition, but we observed a trend towards normalization of the low abundance of butyrate-producing Faecalibacterium after 12 weeks treatment. The study was underpowered to link microbiota to gastrointestinal symptoms.
Keywords: Gastrointestinal microbiome, dimethyl fumarate, multiple sclerosis, faecalibacterium, gastrointestinal symptoms, clinical trial
Introduction
Several recent studies suggest that patients with multiple sclerosis (MS) have a distinct gut microbiota profile.1–3 Whether this has an impact on the disease in terms of aetiology or disease progression is unknown. Development of experimental allergic encephalomyelitis (EAE) has been shown to depend on the presence of gut microbiota.4 In addition, transplantation of gut microbiota from untreated MS patients to mice worsened EAE compared to transplanted microbiota from healthy individuals,5,6 providing evidence of the gut microbiota as a disease modifier.
Dimethyl fumarate (DMF) is an oral disease-modifying drug used to treat adult patients with relapsing–remitting MS (RRMS). The mechanisms of action are not fully known but may include effects on different T cell subsets.7 The nrf2 antioxidant pathway8 and membrane-bound hydroxycarboxylic acid receptor 2 on leukocytes9 may also be implicated. Side-effects may give clues to the underlying effect mechanisms. While there was a low frequency of serious adverse events (AEs) in the phase 3 trials of DMF, gastrointestinal (GI)-related AEs like nausea, diarrhoea and upper abdominal pain were common.10,11 GI symptoms were reported by ∼30% of the patients and caused 4% treatment discontinuation, but for the majority of patients these issues occurred during the first 4–5 weeks of treatment and lasted for less than two weeks.12 The mechanism for GI symptoms is not known. It is therefore of importance to identify possible causes and approaches to alleviate this problem.
DMF is a derivative of fumaric acid, a metabolic intermediate in the citric acid cycle. In the dynamic microbiota community of the gut, the ability of some bacteria to metabolise fumaric acid is essential for maintaining competitive advantage.13,14 Conversely, it has been reported that fumaric acid possesses antimicrobial properties.15,16 These observations suggest that treatment with DMF may alter the microbiota composition, potentially favouring certain pathogenic bacteria that could contribute to the GI-related AEs. To test this hypothesis, we designed a study to analyse the microbiota abundance and diversity of patient stool samples before and during DMF treatment along with monitoring of GI side-effects.
Patients and methods
Participants
Patients ≥18 years old with confirmed diagnosis of RRMS who satisfied the therapeutic indication as described in the summary of product characteristics (SmPC) were eligible for inclusion in the study. Exclusion criteria were history of malignancy, medication that potentially could affect the gut microbiota during the last 30 days prior to study entry (including antibiotics, teriflunomide, fingolimod, natalizumab or alemtuzumab), pregnancy and GI conditions. No patients used immunomodulatory medications at the time of inclusion. For baseline comparison, a panel of previously collected samples from 165 healthy individuals with the same geographic distribution, recruited from the Norwegian Bone Marrow Donor Registry, served as controls.
Study design
At the baseline visit, patients were screened for eligibility and blood samples and food frequency questionnaires (FFQs) were collected. Treatment compliance, adverse events and blood samples were monitored after four and 12 weeks. In addition, study participants filled out GI scoring records and provided stool samples at baseline, at week two and week 12. The early timepoint at week 2 was chosen because GI side-effects from DMF occur primarily in the first month,17 while 12 weeks would capture the majority of long-term microbiota changes.
Endpoints
The primary endpoint was change in gut microbiota composition after treatment with DMF, described as change in alpha diversity, beta diversity or abundance of bacterial taxa on phylum, family and genus level in subjects pre- versus post-DMF. Secondary endpoints were relations between GI symptoms and baseline microbial composition, changes in microbial composition, changes in microbiota composition in the DMF group compared to the injectable group, and changes in microbial composition after resolution of GI symptoms. Exploratory endpoints were GI symptoms and changes in blood markers, baseline diet or baseline differences of gut microbiota composition between MS patients and healthy individuals.
Questionnaires
All subjects completed a validated self-administered Norwegian FFQ before study start.18 The contribution of each nutrient to total energy intake (EI) was calculated and denoted as E%.19 A 15-item self-reported validated questionnaire, the Gastrointestinal Symptoms Rating Scale (GSRS) was used to measure GI symptoms.20 Each item was scored in seven steps from ‘No discomfort’ to ‘Very severe discomfort’, corresponding to a score of 1–7, and a total GSRS score ranging from 15–105, with a higher score reflecting more symptoms. An increase of two or more points (≥2) in GSRS was regarded as GI symptoms.
Gut microbiota
Stool samples were collected by the patient at home in special tubes with preservatives (PSP tubes, Stratec), shipped to the central study laboratory and stored at –80°C. Microbial DNA were extracted from stool samples using PSP Spin Stool DNA Kit (Stratec Molecular GmbH) and sequenced on the Illumina MiSeq platform, targeting the V3–V4 region of the 16 s rRNA. The sequences were analysed using QIIME v1.9.1 (see Supplementary Material Methods). The same methods for stool collections and analysis where used for the healthy controls (HCs).
Circulating markers
Serum levels of markers associated with gut microbiota alterations in other conditions were analysed in duplicate using commercially available enzyme-linked immunosorbent assays (ELISAs): soluble (s) CD14, CD25, CD163 and LPS-binding protein (LBP), all from RnD (Minneapolis, Minnesota, USA) with intra- and inter-assay coefficients of variation <10%.
Statistical analysis
Power calculations are not well developed in microbiota research and were not formally performed. However, based on the assumption that 25% would acquire GI side-effects and allowing for some dropouts, the aim was to include 60 DMF patients and 10 non-DMF patients.
All continuous variables are presented as median and interquartile range (IQR). The statistical approach included: (a) case-control study of baseline MS samples compared with healthy individuals, using Mann-Whitney U test on all available taxa on genus level (n = 198), corrected with Benjamini Hochberg false discovery rate (FDR); (b) for changes in microbial abundance after intervention with DMF or an injectable drug, the Wilcoxon matched-pairs test was applied at phylum and genus level, and all non-adjusted p-values <0.05 are reported, given the exploratory nature of this pilot study. FDR is also reported for these tests. Statistical analyses were performed in SPSS Statistics v25.0 (IBM Corporation, Armonk, New York, USA) and R version 3.4.1 for comparisons of differential abundance at baseline between patients and HCs. Graphical presentations were made using Prism V7.0d software (GraphPad, San Diego, California, USA).
Ethics and approvals
The study was performed according to the Declaration of Helsinki. All participants gave written informed consent. The study was approved by the Norwegian Medicines Agency (EudraCT NOR-BGT-14-10665) and the Regional Committee for Health Research Ethics in South-Eastern Norway, and registered at clinicaltrials.gov (identifier NCT02471560).
Results
Baseline characteristics
Thirty-seven RRMS patients from seven different study sites (Supplementary Material Table 1) were recruited between November 2015–March 2017. Twenty-seven patients initiated treatment with DMF and nine patients with an injectable disease-modifying MS drug. One patient withdrew before the study start (Table 1). Patients in the injectable control group started on glatiramer acetate (n = 3), peginterferon beta-1a (n = 3), interferon beta-1b (n = 2) or interferon beta-1a (n = 1).
Table 1.
Baseline characteristics between treatment groups.
| DMF(n = 27) | Injectable(n = 9) | p-Value | |
|---|---|---|---|
| Age, years (IQR) | 45 (39–52) | 44 (33–59) | 0.91 |
| Female gender, n (%) | 19 (70.3%) | 7 (77.8%) | 0.75 |
| HLA-DR*15 carrier, n (%) | 11 (45.8%) | 5 (55.6%) | 0.62 |
| Smoking, n (%) | 2 (7.4%) | 1 (11.1%) | 0.87 |
| BMI, kg/m2 (IQR) | 25.2 (22.5–28) | 20.5 (20.3–23.7) | 0.09 |
| Medication with PPI, n (%) | 2 (7.4%) | 2 (22.2%) | 0.52 |
| Previous treatment with DMT, n (%)a | 6 (22.2%) | 1 (11.1%) | 0.64 |
| EDSS score (IQR) | 1.75 (1.38–3) | 1 (0.5–2.5) | 0.20 |
| GSRS score (IQR) | 19 (16–23) | 23 (21–29) | 0.11 |
| Nutritional values | |||
| EI (kJ/day) | 9939 (8259–12,514) | 10581 (7940–12,296) | 0.83 |
| Fat E% | 35.9 (33.7–39) | 37 (27.7–41.15) | 0.98 |
| Proteins E% | 15.1 (13.8–17.7) | 18.7 (17.45–19.6) | 0.01 |
| Carbohydrates E% | 43.8 (38.5–47.5) | 41.2 (37–47.6) | 0.51 |
| Fibre E% | 2.1 (1.9–2.7) | 2.9 (2–3.4) | 0.15 |
| Sugar E% | 5.7 (3.9–10.3) | 4.7 (2.8–6.15) | 0.19 |
| Ethanol E% | 0.5 (0–3.3) | 0.4 (0.05–2.45) | 0.86 |
BMI: body mass index; DMF: dimethyl fumarate; DMT: dimethylfumarate; EDSS: Expanded Disability Status Scale; EI: energy intake; GSRS: Gastrointestinal Symptoms Rating Scale; HLA-DR: human leukocyte antigen-DR; IQR: interquartile range; PPI: proton pump inhibitor.
DMF-group (glatiramer acetate = 2, interferons = 2, teriflunomide = 2). Injectable group (interferons = 1).
Microbiota profiles from 34 of 36 patients were available for baseline analysis between MS patients and HCs (Table 2), as two stool samples did not meet quality criteria. At two weeks, four individuals were excluded from analysis because of discontinuation of study drug due to an AE (n = 1), loss to follow-up (n = 1) or missing samples (n = 2). At 12 weeks, five individuals were excluded from analysis due to AEs leading to discontinuation (n = 2), loss of follow-up (n = 1) and missing samples (n = 2). Thus, 21 (DMF) and nine (injectable) patients were included in longitudinal analyses from baseline to week 2. Correspondingly, 20 (DMF) and nine (injectable) patients were analysed from baseline till week 12.
Table 2.
Baseline characteristics healthy controls and multiple sclerosis (MS) individuals.
| Healthy controls(n = 165) | MS individuals(n = 34) | p-Value | |
|---|---|---|---|
| Age, years (IQR) | 47 (41–53) | 46 (39–53) | 0.76 |
| Female gender, n (%) | 104 (63%) | 25 (74%) | 0.23 |
| Smoking, n (%) | 15 (9%) | 2 (6%) | 0.53 |
| BMI, kg/m2 (IQR) | 25.8 (23.5–29.3) | 24 (22.3–27.8) | 0.04 |
| Medication with PPI, n (%) | 13 (8%) | 4 (12%) | 0.48 |
BMI: body mass index.; IQR: interquartile range.
Reduced Faecalibacterium in MS patients at baseline
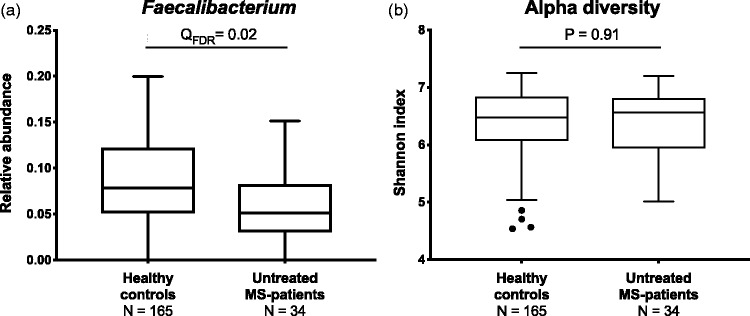
When comparing baseline global gut microbiota composition in MS patients and HCs, we found minor, but significant, differences as measured by beta diversity (weighted unifrac, adonis p-value = 0.01, R2 = 0.01, Supplementary Material Figure 1). At genus level, 16 taxa had significantly different abundance in MS patients compared to HCs (QFDR<0.05, Supplementary Material Table 2). Considering in total 40 different genera previously reported to be associated with MS (Supplementary Material Table 3), only one was confirmed in the present data; a lower abundance in MS patients of Faecalibacterium (QFDR = 0.02, Figure 1(a)). No differences in intra-individual microbial (alpha) diversity were observed between MS patients and HCs (p = 0.91, Figure 1(b)).
Figure 1.
Faecalibacterium and alpha diversity in multiple sclerosis (MS) patients and healthy controls (HCs). (a) At baseline, MS patients had reduced levels of butyrate-producing Faecalibacterium compared to HCs. (b) No differences in alpha diversity measures of the Shannon index were observed. Mann-Whitney U-test, corrected with Benjamini Hochberg FDR.
GI symptoms in patients treated with DMF
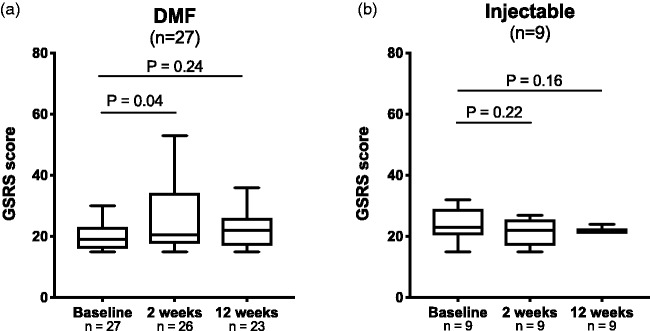
GSRS scores at baseline were 19 (16–23) for the DMF group and 23 (21–29) for the injectable group (p = 0.11, Table 1). After two weeks, 12 (46%) of the patients receiving DMF experienced a minimum of two (median 12, range 4–21) points increase of the GSRS score (p = 0.04, Figure 2(a)), predefined as on-treatment GI symptoms. After 12 weeks, the worsening of GSRS score in the DMF group was not significant compared to baseline (p = 0.24). No significant increases of GI symptoms were observed in patients receiving injectable drugs (Figure 2(b)).
Figure 2.
Changes in gastrointestinal (GI) symptoms after intervention with dimethyl fumarate (DMF). A significant increase in Gastrointestinal Symptoms Rating Scale (GSRS) score from baseline was seen in patients treated with DMF after two weeks of treatment, but not in patients treated with an injectable drug. Wilcoxon matched pair tests including individuals with available data at both timepoints.
AEs
Besides the questionnaire-based registration of GI-related symptoms, adverse events were formally registered (Table 4). Two patients discontinued treatment with DMF due to AEs (lip oedema and vomiting), one because of an MS attack and one was lost to follow-up. All patients starting on injectable drugs completed the study. The total incidence of adverse events was 56% in the DMF group and 89% in the injectable group (mostly injection site and flu-like reactions). Overall, 27 (71%) of the AEs were mild, 10 (26%) moderate and one severe. The most common AEs in the DMF group were gastrointestinal (n = 9) and flushing (n = 5).
Table 3.
Changes in selected microbiota profile parameters from baseline.
|
DMF |
Injectable |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks | p | QFDR | 12 weeks | p | QFDR | Baseline | 2 weeks | p | QFDR | 12 weeks | p | QFDR | |
| Actinobacteria | 0.016(0.01–0.03) | 0.011(0.005–0.02) | 0.03 | 0.24 | 0.016(0.01–0.04) | 0.78 | 0.86 | 0.027(0.01–0.05) | 0.024(0.02–0.04) | 1 | 0.95 | 0.02(0.008–0.03) | 0.30 | 0.62 |
| Bifidobacterium | 0.011(0.004–0.02) | 0.007(0.001–0.01) | 0.06 | 0.69 | 0.012(0.002–0.02) | 0.83 | 1 | 0.021 (0.005–0.04) | 0.015(0.004–0.03) | 0.82 | 0.96 | 0.011(0.004–0.03) | 0.16 | 0.58 |
| Firmicutes | 0.569(0.51–0.75) | 0.63(0.52–0.77) | 0.50 | 0.71 | 0.679(0.56–0.78) | 0.02 | 0.09 | 0.63(0.51–0.74) | 0.589(0.53–0.73) | 0.91 | 0.95 | 0.659(0.46–0.72) | 1 | 0.95 |
| Bacteroidetes | 0.272 (0.18–0.43) | 0.195(0.15–0.44) | 0.73 | 0.85 | 0.246(0.13–0.35) | 0.01 | 0.09 | 0.319(0.21–0.43) | 0.359 (0.21–0.45) | 0.50 | 0.79 | 0.292(0.23–0.49) | 1 | 0.95 |
| Faecalibacterium | 0.039(0.03–0.07) | 0.055(0.03–0.09) | 0.13 | 0.69 | 0.067(0.04–0.11) | 0.01 | 0.48 | 0.07(0.05–0.12) | 0.06(0.04–0.10) | 0.25 | 0.70 | 0.06(0.05–0.08) | 0.30 | 0.58 |
| Bacteroides | 0.15 (0.10–0.32) | 0.14 (0.09–0.30) | 0.81 | 0.92 | 0.14 (0.07–0.23) | 0.01 | 0.67 | 0.142(0.08–0.22) | 0.117(0.09–0.24) | 0.91 | 0.99 | 0.165(0.09–0.28) | 0.82 | 0.86 |
| Clostridium(sensu stricto) | 9 × 10–5(0–9 × 10–4) | 9 × 10–5(0–0.001) | 0.66 | 0.86 | 5 × 10–5(0–7 × 10–4) | 0.75 | 0.89 | 0(0–7 × 10–4) | 0(0–1 × 10–4) | 0.88 | 0.97 | 2 × 10–4(0–4 × 10–4) | 0.63 | 0.77 |
| Escherichia–Shigella | 2 × 10–4(0–0.001) | 9 × 10–5(0–9 × 10–4) | 0.63 | 0.86 | 0(0–0.001) | 0.30 | 0.69 | 0(0–4 × 10–4) | 0(0–4 × 10–4) | 0.34 | 0.73 | 2 × 10–4(0–0.002) | 0.03 | 0.58 |
DMF: dimethyl fumarate.
Wilcoxon matched pair tests including individuals with available data at both timepoints. Corrected with Benjamini Hochberg false discovery rate (FDR).
Gut microbiota alterations during disease-modifying therapy
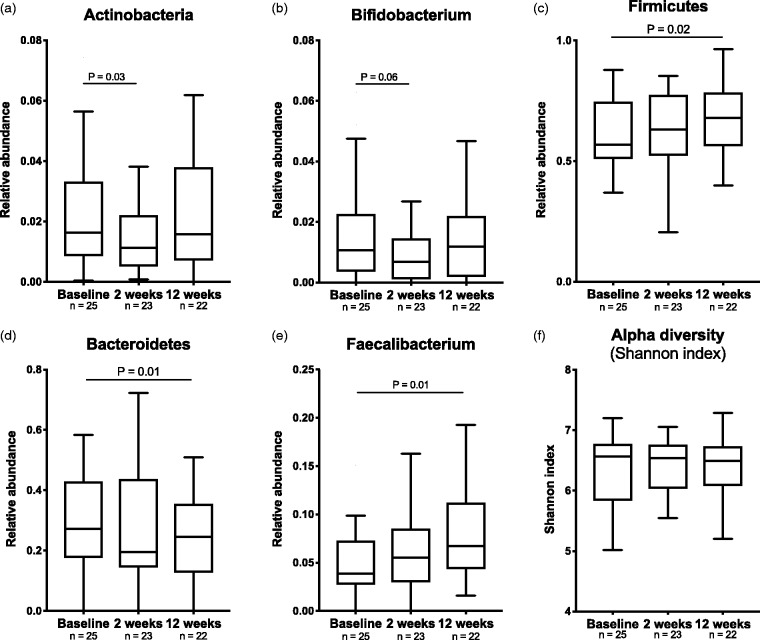
After two weeks on DMF, at phylum level we observed a reduction of Actinobacteria (median abundance reduced from 1.6% to 1.1%, p = 0.03, Figure 3(a)) mainly driven by a reduction of Bifidobacterium (1.1% to 0.7%, p = 0.06, Figure 3(b)). After 12 weeks of intervention, these changes were reversed. After 12 weeks of intervention there was an increase in the ratio between the two major phyla Firmicutes and Bacteroidetes (FB-ratio) from 1.97 to 2.67 (p = 0.02) in patients receiving DMF. The relative abundance of the Bacteroidetes phylum decreased from 27% to 25% (p = 0.01, Figure 3(c)) and Firmicutes increased from 57% to 68% (p = 0.02, Figure 3(d)). Within the Firmicutes phylum, changes were seen in the genus of Faecalibacterium (3.9% to 6.9%, p = 0.01, Figure 3(e)). In the injectable group, no changes were seen at phylum level at either two weeks or 12 weeks (Table 3). No changes were seen in measures of alpha diversity in either of the treatment groups after two weeks or 12 weeks (Figure 3(f)). None of the statistical tests had a QFDR<0.05 (Table 3).
Figure 3.
Changes in bacterial phyla after intervention with dimethyl fumarate (DMF). At phylum-level, Actinobacteria (a) were reduced from baseline to two weeks in patients treated with DMF, mainly explained by a reduction of Bifidobacterium (b). After three months of treatment with DMF, there was an increase in the ratio between the two major phylum Bacteroidetes (c) and Firmicutes (d). Changes in Firmicutes were mostly because of an increase of Faecalibacterium (e). No changes were seen in measures of alpha diversity with the Shannon index (f). Wilcoxon matched pair tests including individuals with available data at both timepoints.
Table 4.
Registered adverse events from the clinical trial during the 12-week study period.
| Adverse event (n (%)) | DMF(n = 27) | Injectable(n = 9) |
|---|---|---|
| Any adverse events | 15 (56) | 8 (89) |
| Serious adverse events | 1 (4) | 1 (11) |
| MS relapse | 1 (4) | 0 |
| Optic neuritis | 0 | 1 (11) |
| Common non-serious adverse events | ||
| Flushing | 5 (19) | 0 |
| GI adverse event | 9 (33) | 0 |
| Nausea | 4 (15) | 0 |
| Vomiting | 2 (7) | 0 |
| Abdominal pain | 2 (7) | 0 |
| Obstipation | 1 (4) | 0 |
| GI discomfort | 1 (4) | 0 |
| Gastroenteritis | 1 (4) | 0 |
| Sinusitis | 2 (7) | 0 |
| Muscle pain | 0 | 2 (22) |
DMF: dimethyl fumarate; GI: gastro-intestinal; MS: multiple sclerosis.
Microbial changes, GI symptoms and diet
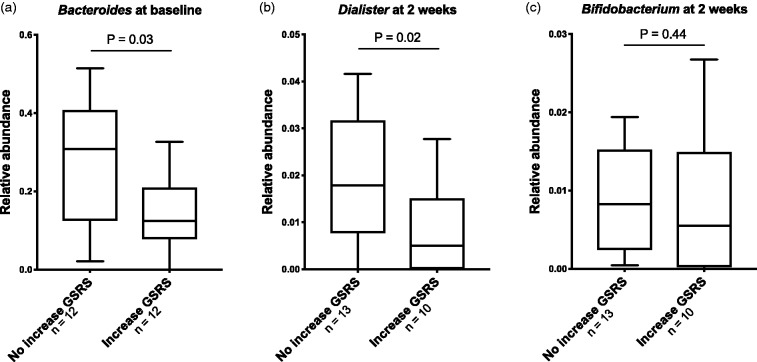
Twelve patients experienced GI symptoms at two weeks after initiation with DMF. Among these, we found no significant changes in gut microbiota composition between baseline and week 2 (Supplementary Material Table 4). Given the aim of understanding the GI side-effects of DMF, we performed an exploratory analysis (Supplementary Material Table 5). DMF patients with GI symptoms at two weeks had a lower abundance of Bacteroides (p = 0.03, Figure 4(a)) at baseline and a lower abundance of Dialister (p = 0.02, Figure 4(b)) after two weeks compared to those without GI symptoms, but none of these associations were robust to correction for multiple testing. There were no significant differences in Bifidobacterium abundance at two weeks (Figure 4(c)) or 12 weeks between patients in the DMF group with GI symptoms compared to those without such symptoms.
Figure 4.
Differences in bacterial phyla in patients with gastrointestinal (GI) symptoms after intervention with dimethyl fumarate (DMF). DMF patients with an increase of Gastrointestinal Symptoms Rating Scale (GSRS) score from baseline to two weeks had lower levels of Bacteroides (a) at baseline, lower levels of Dialister (b) at two weeks and a tendency of reduced abundance of Bifidobacterium (c) at two weeks, compared to those without an increase of GSRS score. Mann-Whitney U test.
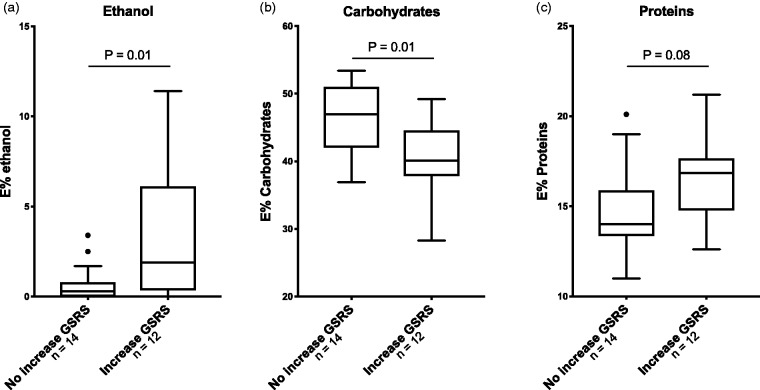
Patients in the DMF group with GI symptoms after two weeks of intervention had a higher relative intake of ethanol (1.9 E% vs 0.3 E%, p = 0.01), a lower intake of carbohydrates (40 E% vs 47 E%, p = 0.01) and a tendency to higher intake of proteins (16.9 E% vs 14 E%, p = 0.08, Figure 5, Supplementary Material Table 6) at baseline. Concentrating on microbial phyla and food intake, we observed a positive correlation between fibre intake and the abundance of Firmicutes (r = 0.47, p = 0.01) and Faecalibacterium (r = 0.44, p = 0.01) at baseline.
Figure 5.
Differences in energy intake at baseline in patients with gastrointestinal (GI) symptoms after intervention with dimethyl fumarate (DMF). DMF patients with an increase of Gastrointestinal Symptoms Rating Scale (GSRS) score from baseline to two weeks had (a) a higher intake of ethanol, (b) lower intake of carbohydrates and (c) higher intake of proteins at baseline, compared to those without increase of GSRS score. Mann-Whitney U test.
Changes in circulating biomarkers during intervention
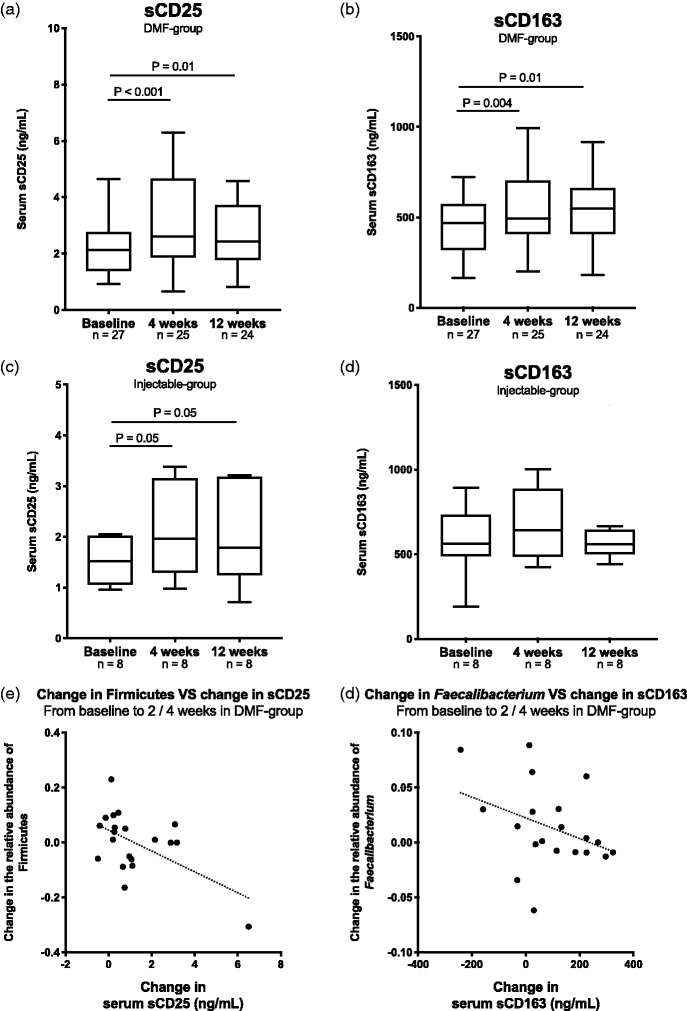
In the DMF group, we observed a significant increase from baseline to four and 12 weeks of the T cell activation marker sCD25 (Figure 6(a)) and the macrophage activation marker sCD163 (Figure 6(b)). No changes were seen in the microbial translocation markers sCD14 or LBP. In the injectable group, after four and 12 weeks there were increased levels of sCD25 (Figure 6(c)) but not sCD163 (Figure 6(d)), sCD14 or LBP.
Figure 6.
Changes in gut microbial serum biomarkers of inflammation after intervention with dimethyl fumarate (DMF). In the DMF group an increase of gut microbial serum biomarkers of inflammation (a) sCD25 and (b) sCD163 were observed. In the injectable group an increase of (c) sCD25 was observed, but no change in (d) sCD163. A negative correlation was observed between (e) the increase of Firmicutes after two weeks and the increase of sCD163 after four weeks and (f) between Faecalibacterium and sCD163, i.e. patients with higher increase of Firmicutes or Faecalibacterium after two weeks had lesser increase of the inflammatory biomarkers sCD25 or sCD163 after four weeks. Wilcoxon matched pair tests including individuals with available data at both timepoints and Spearman rank correlation tests.
In the DMF group, we found borderline significant correlations between change in Firmicutes from baseline to two weeks and change in sCD25 from baseline to four weeks (r = –0.46, p = 0.04, Figure 6(e)), and between change in Faecalibacterium from baseline to two weeks and change in sCD163 from baseline to four weeks (r = –0.42, p = 0.06, Figure 6(f)). There were no such correlations between changes in bacterial phyla and circulating biomarkers from baseline to 12 weeks.
Discussion
In this study, a three-month intervention with DMF was not associated with significant alterations in the gut microbiota profile after FDR correction. There was however a trend towards normalization of the low abundance of Faecalibacterium observed in MS patients compared to controls at baseline, and also an early transient depletion of Bifidobacterium, providing a rationale for further studies of a link between the gut microbiota and effects and side-effects of DMF in MS patients.
The most prominent trends in the DMF group seemed to follow different courses. There was a transient drop in Bifidobacterium at two weeks, while Faecalibacterium gradually increased from baseline to 12 weeks. No comparable interventional studies are available for other disease-modifying MS treatments. There is, however, an increasing interest in pharmamicrobiomics, i.e. how drugs act on the microbiota and vice versa.21 Many common drugs that are not considered typical antibiotics have anti-microbial effects,22 and some may primarily act on the gut microbiota to influence the host. The most prominent example, metformin in type 2 diabetes,23 is also associated with GI side-effects. Moreover, the observed antimicrobial effects of DMF against some intestinal pathogens suggest that a part of the changes observed at two weeks could be related to drug-microbe interactions.15,16
In a recent cross-sectional study of the gut microbiota in MS patients, individuals using DMF had a higher abundance of Bacteroidetes and lower abundance of Firmicutes compared to treatment-naïve MS patients.24 This differs from our findings, which revealed a reduction of Bacteroidetes and an increase of Firmicutes after initiation with DMF. These conflicting results might be explained by demographic differences, different study design or a treatment longer than 12 weeks which might change the microbiota differently. Taken together, this suggests that treatment with DMF has an impact on the microbiome of patients with MS, but further studies are needed to explore the changes.
Wang et al.15 demonstrated antibacterial activity of DMF against Escherichia coli and Rumah et al.16 showed that DMF inhibited growth of Clostridium perfringens. Since 16S rRNA-based microbiota profiling generally yields genus level resolution, these species are not well captured. In our data, the genera Escherichia-Shigella and Clostridium, which include the respective species, were captured in about half of the individuals. No changes were seen after intervention (Table 3).
The baseline microbiota profiles suggest that MS patients have a different microbiota composition compared to HCs. In line with previous studies, we found no difference in intra-individual (alpha) diversity, but modest significant differences for global composition (beta diversity).1–3 A low abundance of Faecalibacterium is observed in multiple studies of patients treated for MS (Supplementary Material Table 3). Faecalibacterium is a well-known butyrate-producing bacterium which is also reduced in inflammatory bowel disease.25 Butyrate is a short-chain fatty acid (SCFA) produced by gut bacteria from dietary fibre and is important as a nutrient to the intestinal epithelium and as a regulator of the mucosal immune system.26 In a recent study of the non-obese autoimmune diabetes model, specialised diets designed to maximise SCFA production in the gut protected against disease by reducing the number of autoreactive T cells and increasing T regulatory cells.27 Mice with EAE showed an improved clinical score both with a high fibre diet and supplementation with SCFA.28 Taken together with our findings of a close relationship between fibre intake and Faecalibacterium in MS patients, it could be speculated that a fibre-rich diet might be beneficial in MS.
The early and transient drop in Actinobacteria at two weeks, driven by Bifidobacterium was in line with the hypothesis of the present study. Unfortunately, our study was underpowered to address whether this observed change was associated with the reported GI side-effects of DMF. However, a low abundance of Bifidobacterium has been observed in many phenotypes of chronic inflammation or GI symptoms. A recent study suggested that probiotics per se could influence immunity in MS patients.29 Although faecal samples might have some limitations reflecting GI symptoms from the upper GI tract, our results provide a rationale for further studies addressing specifically whether probiotics could modify GI symptoms in DMF-treated MS patients
To investigate possible inflammatory effects of gut microbial changes, we evaluated serum markers of inflammation previously associated with gut microbiota composition.30–32 We found no relationship between DMF intervention or GI symptoms and sCD14 or LBP, suggesting that microbial translocation is not relevant. However, increases of sCD163 and sCD25, which also correlated with microbial alterations, but not GI symptoms, were observed after initiation of DMF. We also observed an increase of sCD25 in the injectable group. In a recent study, half of the studied MS patients had a non-significant increase in sCD25 after initiation of interferon beta.33 This suggests a short-term increased systemic immune activation after initiation of disease-modifying therapy, warranting further studies.
The main limitation of this study is the small sample size. This was largely due to a change in centrally regulated priorities for oral immunomodulatory drugs in the Norwegian market leading to a slow inclusion rate. None of the statistical tests of the longitudinal data met the significant threshold using Benjamini Hochberg QFDR. However, due to the exploratory approach of the study, we consequently have chosen to report the uncorrected p-values. All analyses performed were pre-specified, but given the large number of investigated variables in a small population, the results must be interpreted with caution.
Around 20% of the included patients had a history of previous treatment with disease-modifying therapy, which may have influenced the outcomes. Also, most of the included patients had early-stage RRMS. It was recently reported that patients with secondary progressive MS have reduced expression of memory T cells expressing the gut homing chemokine receptor.34 Further studies should seek to include late-stage MS. Dietary information was only obtained at baseline. Although patients were instructed not to change their diet, it is common to take DMF with a meal containing protein and fat, which could potentially have impacted on the microbial composition. Likewise, we have no information on the use of antibiotics except during and 30 days prior to the study and can, therefore, not exclude confounding from use of antibiotics before that. Moreover, FFQs were not administered to HCs, and gender matching between MS patients was not perfect. We can, therefore, not exclude the possibility that differences in diet or gender may have influenced the cross-sectional results. Lastly, our study was too short to link clinical outcome to alterations in the microbiome, an investigation which would require a different design.
In conclusion, 12 weeks of treatment with DMF was not associated with significant alterations in the gut microbiota profile after correction for multiple comparisons in this pilot study. There was however a trend towards a near-normalization of the low abundance of butyrate-producing Faecalibacterium seen in MS patients, and also a short-term depletion of Bifidobacterium after initiation of DMF treatment. Thus, a direct effect on the gut microbiota might be a part of the therapeutic action of DMF. The study was underpowered to firmly link microbiota changes to GI symptoms, and not designed to assess the impact of microbiota on treatment responses. Further clinical trials should evaluate if alterations in microbiota are associated with treatment effects, and whether microbial profiles can predict drug efficacy.
Conflict of interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/ or publication of this article: CSL: nothing to disclose. KMM: has received speakers honoraria, participated in clinical trials, received unrestricted research grants or attended advisory meetings for one or more of the following companies last three years; Almirall, Biogen, Sanofi Genzyme, Merck, Novartis, Roche or Teva. KMM is the president for the academic council for the Norwegian MS-association, Norwegian representative of MS International federation (MSIF) and EAN Scientific Panel Multiple Sclerosis. EF: has participated in advisory boards and received lecture honoraria from Biogen, Genzyme, Novartis, Roche and Merck. Unrestricted research grant from Novartis. RM: has served on scientific advisory boards for Novartis Norway and Merck Norway and has received travel funding and/or speaker honoraria from Biogen, Novartis Norway, Sanofi Genzyme. KN: nothing to disclose. LB: nothing to disclose. PBH: has received funding for travel or speaker's fees from Novartis, UCB and Teva. AB: nothing to disclose. KH: nothing to disclose. LEF: previous employee at Biogen. EBA: Biogen employee. JRH: reports research funding from Biogen in conjunction with the present study, and serving on advisory boards for Novartis and Orkla Health. TH: has received speakers honoraria, and/or served on advisory board, and/or received unrestricted research grants from Biogen, Roche, Merck, Novartis and Genzyme.
Supplemental Material
Supplemental material, MSO888767 Supplemetal Material1 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch A Buness L-E Fallang E Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental Material
Supplemental material, MSO888767 Supplemetal Material2 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch A Buness K Holm, T Ueland, L-E Fallang E Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental Material
Supplemental material, MSO888767 Supplemetal Material3 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch P Berg-Hansen, A Buness K Holm, T Ueland, L-E Fallang E Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental Material
Supplemental material, MSO888767 Supplemetal Material4 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch A Buness L-E FallangE Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Contributor Information
C Storm-Larsen, Institute of Clinical Medicine, University of Oslo, Norway; Norwegian PSC Research Center, Oslo University Hospital, Norway; Research Institute of Internal Medicine, Oslo University Hospital, Norway.
K-M Myhr, Department of Clinical Medicine, University of Bergen, Norway; Department of Neurology, Haukeland University Hospital, Norway.
E Farbu, Department of Clinical Medicine, University of Bergen, Norway; Neuroscience Research Group, Stavanger University Hospital, Norway.
R Midgard, Department of Neurology, Molde Hospital, Norway; Unit for Applied Clinical Research, NTNU, Norway.
K Nyquist, Department of Neurology, Innlandet Hospital Trust, Norway.
L Broch, Department of Neurology, Drammen Hospital, Norway.
P Berg-Hansen, Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology, Oslo University Hospital, Norway.
A Buness, Norwegian PSC Research Center, Oslo University Hospital, Norway.
K Holm, Institute of Clinical Medicine, University of Oslo, Norway; Norwegian PSC Research Center, Oslo University Hospital, Norway.
T Ueland, Institute of Clinical Medicine, University of Oslo, Norway; Research Institute of Internal Medicine, Oslo University Hospital, Norway; K.G. Jebsen TREC, University of Tromsø, Norway.
L-E Fallang, Previous Biogen, Norway.
E Burum-Auensen, Biogen, Norway.
JR Hov, Institute of Clinical Medicine, University of Oslo, Norway; Norwegian PSC Research Center, Oslo University Hospital, Norway; Research Institute of Internal Medicine, Oslo University Hospital, Norway; Section of Gastroenterology, Oslo University Hospital, Norway.
T Holmøy, Institute of Clinical Medicine, University of Oslo, Norway; Department of Neurology, Akershus University Hospital, Norway.
Funding
The author(s) disclosed receipt of the following financialsupport for the research, authorship, and/or publicationofthis article: This work was supported by Biogen. This clinical trial is registered at clinicaltrials.gov (identifier NCT02471560).
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016; 7: 12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016; 6: 28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyake S, Kim S, Suda W, et al. Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to Clostridia XIVa and IV clusters. PLoS One 2015; 10: e0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berer K, Mues M, Koutrolos M, et al. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011; 479: 538–541. [DOI] [PubMed] [Google Scholar]
- 5.Berer K, Gerdes LA, Cekanaviciute E, et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A 2017; 114: 10719–10724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A 2017; 114: 10713–10718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoglund RA, Polak J, Vartdal F, et al. B-cell composition in the blood and cerebrospinal fluid of multiple sclerosis patients treated with dimethyl fumarate. Mult Scler Relat Disord 2018; 26: 90–95. [DOI] [PubMed] [Google Scholar]
- 8.Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 2011; 134: 678–692. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Assmann JC, Krenz A, et al. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate's protective effect in EAE. J Clin Invest 2014; 124: 2188–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 11.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 12.Meltzer L, Selmaj K, Gold R, et al. Gastrointestinal tolerability events in relapsing–remitting multiple sclerosis (RRMS) patients treated with oral BG-12 (dimethyl fumarate) in DEFINE and CONFIRM (P01.164). Neurology 2013; 80: P01.164. [Google Scholar]
- 13.Jones SA, Gibson T, Maltby RC, et al. Anaerobic respiration of Escherichia coli in the mouse intestine. Infect Immun 2011; 79: 4218–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones SA, Chowdhury FZ, Fabich AJ, et al. Respiration of Escherichia coli in the mouse intestine. Infect Immun 2007; 75: 4891–4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HH, Sun DW, Kuang R. Inhibition of Escherichia coli by dimethyl fumarate. Int J Food Microbiol 2001; 65: 125–130. [DOI] [PubMed] [Google Scholar]
- 16.Rumah KR, Vartanian TK, Fischetti VA. Oral multiple sclerosis drugs inhibit the in vitro growth of epsilon toxin producing gut bacterium, Clostridium perfringens. Front Cell Infect Microbiol 2017; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips JT, Selmaj K, Gold R, et al. Clinical significance of gastrointestinal and flushing events in patients with multiple sclerosis treated with delayed-release dimethyl fumarate. Int J MS Care 2015; 17: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andersen LF, Solvoll K, Johansson LR, et al. Evaluation of a food frequency questionnaire with weighed records, fatty acids, and alpha-tocopherol in adipose tissue and serum. Am J Epidemiol 1999; 150: 75–87. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997; 65: 1220S–1228S; discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 20.Svedlund J, Sjodin I, Dotevall G. GSRS–a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Dig Dis Sci 1988; 33: 129–134. [DOI] [PubMed] [Google Scholar]
- 21.Haiser HJ, Turnbaugh PJ. Is it time for a metagenomic basis of therapeutics? Science 2012; 336: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 22.Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 2018; 555: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Esteve E, Tremaroli V, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017; 23: 850–858. [DOI] [PubMed] [Google Scholar]
- 24.Katz Sand I, Zhu Y, Ntranos A, et al. Disease-modifying therapies alter gut microbial composition in MS. Neurol Neuroimmunol Neuroinflamm 2019; 6: e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol Res Pract 2014; 2014: 872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gill PA, van Zelm MC, Muir JG, et al. Review article: Short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther 2018; 48:15–34. [DOI] [PubMed] [Google Scholar]
- 27.Marino E, Richards JL, McLeod KH, et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017; 18: 552–562. [DOI] [PubMed] [Google Scholar]
- 28.Mizuno M, Noto D, Kaga N, et al. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One 2017; 12: e0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tankou SK., Regev K., Healy BC., et al A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann Neurol. 2018; 83: 1147–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kummen M, Mayerhofer CCK, Vestad B, et al. Gut microbiota signature in heart failure defined from profiling of 2 independent cohorts. J Am Coll Cardiol 2018; 71: 1184–1186. [DOI] [PubMed] [Google Scholar]
- 31.Jorgensen SF, Troseid M, Kummen M, et al. Altered gut microbiota profile in common variable immunodeficiency associates with levels of lipopolysaccharide and markers of systemic immune activation. Mucosal Immunol 2016; 9: 1455–1465. [DOI] [PubMed] [Google Scholar]
- 32.Nowak P, Troseid M, Avershina E, et al. Gut microbiota diversity predicts immune status in HIV-1 infection. AIDS 2015; 29: 2409–2418. [DOI] [PubMed] [Google Scholar]
- 33.Ferrarini AM, Sivieri S, Bulian P, et al. Time-course of interleukin-2 receptor expression in interferon beta-treated multiple sclerosis patients. J Neuroimmunol 1998; 84: 213–217. [DOI] [PubMed] [Google Scholar]
- 34.Kadowaki A, Saga R, Lin Y, et al. Gut microbiota-dependent CCR9+CD4+ T cells are altered in secondary progressive multiple sclerosis. Brain 2019; 142: 916–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSO888767 Supplemetal Material1 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch A Buness L-E Fallang E Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, MSO888767 Supplemetal Material2 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch A Buness K Holm, T Ueland, L-E Fallang E Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, MSO888767 Supplemetal Material3 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch P Berg-Hansen, A Buness K Holm, T Ueland, L-E Fallang E Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, MSO888767 Supplemetal Material4 for Gut microbiota composition during a 12-week intervention with delayed-release dimethyl fumarate in multiple sclerosis – a pilot trial by C Storm-Larsen, K-M Myhr, E Farbu, R Midgard, K Nyquist L Broch A Buness L-E FallangE Burum-Auensen, JR Hov and T Holmøy in Multiple Sclerosis Journal – Experimental, Translational and Clinical