Abstract
Background:
Focal dystonias are severe and disabling movement disorders of a still unclear origin. The structural brain networks associated with focal dystonia have not been well characterized. Here, we investigated structural brain network fingerprints in patients with blepharospasm (BSP) compared with those with hemifacial spasm (HFS), and healthy controls (HC). The patients were also examined following treatment with botulinum neurotoxin (BoNT).
Methods:
This study included matched groups of 13 BSP patients, 13 HFS patients, and 13 HC. We measured patients using structural-magnetic resonance imaging (MRI) at baseline and after one month BoNT treatment, at time points of maximal and minimal clinical symptom representation, and HC at baseline. Group regional cross-correlation matrices calculated based on grey matter volume were included in graph-based network analysis. We used these to quantify global network measures of segregation and integration, and also looked at local connectivity properties of different brain regions.
Results:
The networks in patients with BSP were more segregated than in patients with HFS and HC (p < 0.001). BSP patients had increased connectivity in frontal and temporal cortices, including sensorimotor cortex, and reduced connectivity in the cerebellum, relative to both HFS patients and HC (p < 0.05). Compared with HC, HFS patients showed increased connectivity in temporal and parietal cortices and a decreased connectivity in the frontal cortex (p < 0.05). In BSP patients, the connectivity of the frontal cortex diminished after BoNT treatment (p < 0.05). In contrast, HFS patients showed increased connectivity in the temporal cortex and reduced connectivity in cerebellum after BoNT treatment (p < 0.05).
Conclusions:
Our results show that BSP patients display alterations in both segregation and integration in the brain at the network level. The regional differences identified in the sensorimotor cortex and cerebellum of these patients may play a role in the pathophysiology of focal dystonia. Moreover, symptomatic reduction of hyperkinesia by BoNT treatment was associated with different brain network fingerprints in both BSP and HFS patients.
Keywords: Dystonia, blepharospasm, botulinum neurotoxin, MRI, structural brain networks, graph theory
Introduction
Dystonias are movement disorders characterized by sustained involuntary muscle contractions and abnormal postures. Blepharospasm (BSP) is a focal dystonia of the eyelids with bilateral involuntary periorbital spasms and hyperkinesia. It has a similar clinical manifestation as hemifacial spasm (HFS), a different type of movement disorder in which patients experience facial hyperkinesia with strictly unilateral paroxysmal contractions of the lower facial muscles.1,2 HFS is not regarded as a form of focal dystonia, but is due to neurovascular conflict leading to hyperexcitability and muscle contractions, and can be treated by solving this. The pathophysiology of BSP, however, still remains unclear.3 Local injections of botulinum neurotoxin (BoNT) type A are the treatment of choice for both conditions and beneficial in >85% of patients, leading to a considerable improvement of symptoms.4 The effects are apparent a few days after injection and last for 8–12 weeks, with maximal improvement after approximately 4 weeks.
Recent magnetic resonance imaging (MRI) studies of the brain have improved our pathophysiological understanding of focal dystonias by providing insight into the structural and functional connectivity of different brain regions in patients with this condition. Using functional MRI (fMRI), it has been shown that there is an altered connectivity within the sensorimotor and frontoparietal network components in patients with focal dystonia compared with healthy controls (HC).5 In a voxel-based morphometry (VBM) study, grey matter volume (GMV) was increased in the right middle frontal gyrus, and decreased in the left postcentral gyrus, and left superior temporal gyrus in BSP patients compared with HC.6 Another VBM study showed increased grey matter densities in the bilateral primary sensorimotor cortex in BSP patients compared with HC.7 Furthermore, a longitudinal study on patients presenting with cervical dystonia had shown an increased GMV in right precentral sulcus after BoNT treatment.8 In summary, these results suggest that focal dystonia is associated with structural and functional alterations across different brain regions, while predominantly in the motor circuitry.
However, studies addressing changes in overall structural brain networks in dystonia, rather than in specific brain regions, are still lacking. The coordinated alterations in brain morphology (e.g. volume) among the different brain regions across individuals have been used to infer large-scale structural brain networks.9 Moreover, so far studies have focused on comparisons between focal dystonia and HC.10,11 The next step towards elucidating the etiological origin of the dystonia is to compare patients with dystonia to those with other hyperkinetic disorders. This will make it possible to differentiate between causal, dystonia-specific abnormalities (primary) and secondary structural abnormalities that occur due to continuous hyperkinetic movements.12 Previous studies have demonstrated substantial brain circuit reorganization in prefronto-thalamic-cerebellar regions following extensive motor skill training.13 In focal dystonic patients, continuous involuntary eyelid movements might induce reorganization of functional and structural brain circuits and directly influence connectivity fingerprints of the sensory-motor, visual areas, and interconnected areas. A comparison merely to HC cannot distinguish these secondary alterations from pathophysiological specific alterations.12 The latter would be informative for the development of new therapies that target the cause, rather than just relieving the symptoms of the disease.
In a previous study, we analyzed regional grey matter microstructural integrity in patients with BSP and HFS.14 We showed that patients with BSP had lower cortical thickness in frontal-rostral, supramarginal, and temporal regions compared with patients with HFS. The current study follows up on this work by using graph theoretical analysis to quantify the relationship among different brain structures related to facial hyperkinesia, in order to elucidate the network origin of focal dystonia.15 Moreover, to delineate the causal and essential etiological components of focal dystonia from secondary effects due to hyperkinesia, we decided to include two control groups, namely HFS and HC. In addition, we studied the network reorganization associated with the decrease of hyperkinetic movements in both BSP and HFS patients as achieved through BoNT.
Subjects and methods
Study participants and design
A total of 13 patients with BSP and 13 patients with HFS were included in the study. The study participants were matched for age and sex. All patients had a structural MRI scan at baseline (pre). BSP and HFS patients were then injected with BoNT. These patients underwent a second structural MRI scan (post) 4 weeks after the injections. As a second control group, 13 HC were also studied. Participants demographic details are given in Table 1. All BoNT injections were performed by clinicians with expertise in BoNT application, who were blinded to the aim of this study. The study protocol was approved by the local ethics committee in the Medical Faculty of the University Kiel (Ethic approval number: A 152/01, A 123 7 05), and written informed consent was obtained from all participants before participating in the study.
Table 1.
Baseline characteristics and BoNT doses of BSP patients, HFS patients, and HC. Statistical differences between all three groups were analyzed using chi-squared test (sex), one-way ANOVA (age). The differences between BSP and HFS patient groups in BoNT dosage were tested by means of two-sample tests.
| BSP n = 13 | HFS n = 13 | HC n = 13 | χ 2/ F/ t | p | |
|---|---|---|---|---|---|
| Sex (M/F), n | 5/8 | 4/9 | 8/5 | χ2(2) = 2 | 0.2 |
| Age, years (mean ± SD) | 65 ± 6 | 60 ± 11 | 53 ± 7 | F(2,36) = 2 | 0.1 |
| BoNT_R, mU (mean ± SD) | 38 ± 26.7 | 31.5 ± 35.7 | – | t = 0.5 | 0.6 |
| BoNT_L, mU (mean± SD) | 37.3 ± 27.1 | 24.6 ± 34.6 | – | t = 1 | 0.3 |
1 mU, mouse units of Botox equivalent; BoNT, botulinum neurotoxin; BSP, blepharospasm, HC, healthy controls; HFS, hemifacial spasm; M/F, male/female; N, number of subjects; R/L, right/left side of the face.
Clinical assessment
The clinical symptoms of patients with BSP and HFS were assessed at baseline and 4 weeks after BoNT treatment, where the maximal and minimal manifestation of the symptoms was expected. Symptoms were assessed using the Blepharospasm Disability Scale (BDS), Blepharospasm Movement Scale (BMS), and Global Dystonia Severity rating scale (GDS), and calculated according to published methods.16,17 The BDS score is calculated based on the patient’s opinion of his own disability in seven daily living activities: speech, writing, feeding, eating, hygiene, dressing, and walking. The BMS comprises measures of dystonia severity and provoking factors in nine body areas, including eyes, mouth, speech and swallowing, neck, trunk, and both arms and legs. All these items are measured on a 5-point scale. The provoking factor rates the relationship between dystonia and activity, from 0 (no dystonia at rest or with activity) to 4 (dystonia at rest). The scores obtained for eyes, mouth, and neck were each multiplied by 0.5, before being entered into the calculation of the total score, in order to downweight them. The GDS is the sum of dystonia severity in 14 body areas that have been described elsewhere.16 On the GDS scale, severity ranges from 0 (no dystonia) to 10 (severe dystonia).
MRI data acquisition
Structural MRI was carried out using a 3T Achieva scanner (Philips Medical System, Best, The Netherlands) with an 8-channel head coil to acquire T1 weighted imaging sequences with a voxel resolution of 1 × 1 × 1 mm3, TR = 7.7 ms, TE = 3.6 ms, flip angle = 8° and 160 slices.
Voxel-based morphometry
The MR images were automatically preprocessed using the default procedure for longitudinal data in the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm) and the toolbox SPM8 (http://www.fil.ion.ucl.ac.uk/spm) running in Matlab 2015B (https://de.mathworks.com). Briefly, this included the following steps: after subject-specific realignment, the mean of the realigned images was calculated, and the mean image was used as a reference image. The realigned images were corrected for signal intensity inhomogeneity with regard to the reference mean image. Spatial normalization parameters were estimated using Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL) and applied to the segmented bias-corrected images.18 After normalization in each voxel, the determinant of the Jacobian matrix was multiplied by voxel volume, which corrected for volume error caused by the normalization procedure.19 Finally, the grey matter images were smoothed with an isotropic Gaussian kernel of 8 mm full-width at half-maximum (FWHM).
Structural correlation network reconstruction
Voxel-wise GMV values were obtained from the VBM8 toolbox as explained above. These were used to construct structural correlational networks. A total of 116 regions of interests (ROIs) were defined by the automated anatomical labeling (AAL) parcellation scheme.20 The average GMV of all voxels in the defined regions was extracted using the REX toolbox (http://web.mit.edu/swg/software.htm). An association matrix (116 × 116) was computed for each group (BSP patients, HFS patients, and HC), with each element ruv defined as the Pearson correlation coefficient between the average GMV of regions u and v across subjects.
Global network measures
Using the association matrix for each group (BSP patients, HFS patients, and HC), we calculated the following measures of network topology, which captures the segregation (modularity, clustering, and local efficiency) and integration (path length, and global efficiency) properties of structural networks. Modularity is calculated to detect the division of an entire network into modules, as well as the connectivity relationships among these modules. Clustering describes the connections between the neighbor nodes. The local efficiency explains the local integration and performance of the network. The average path length describes information flow across all regions in the network. Finally, global efficiency explains the global network integration and efficiency of information flow through the entire network. All network parameters were calculated by thresholding the connectivity matrices at a range of densities (0.2–0.4), where the lower boundary was determined as the minimum density in which the networks of the groups were not fragmented. The mathematical formulas and detailed descriptions of these network parameters are provided in the Supplementary Methods.
Regional network measures
In addition to studying global network topology, we also investigated regional structural networks to detect the differences between groups (BSP patients, HFS patients, and HC) in regional connectivity. We focused on the parameters regional degree and clustering. ‘Degree’ explains the number of connections to the region in the network,9 whereas regional ‘clustering’ explains the connectivity strength between neighboring regions. We calculated the degree and clustering for all 116 regions, followed by an evaluation of between-group differences.
Statistical analysis
Statistical analysis was performed using Statistica software (version 13.1, http://www.statistica.com). Post hoc tests were performed if the F values were significant (p < 0.05) with the Bonferroni correction method if not explicitly specified. To study the differences between patient groups, and also the effect of BoNT treatment, a repeated-measures analysis of variance (rmANOVA) was run including two factors, group (two levels: BSP, and HFS) and time (two levels: pre, and post), with the dependent measures of clinical scores. The significance of whole brain GMV differences was calculated by applying the general linear model using a voxel-wise height threshold of p < 0.001 combined with false discovery rate (FDR) correction. To examine the global network topology in patients with BSP relative to patients with HFS and HC, a one-way ANOVA was applied with the dependent measures of global network parameters. The longitudinal differences in global network topology due to BoNT treatment in patient groups were analyzed by means of a rmANOVA with two factors: group (two levels: BSP, and HFS) and time (two levels: pre, and post). The significance of the regional network differences (regarding degree and clustering) were tested by the nonparametric permutation test with 1000 repetitions (FDR corrected p < 0.05; two-tailed).
Results
Clinical description of the cohort
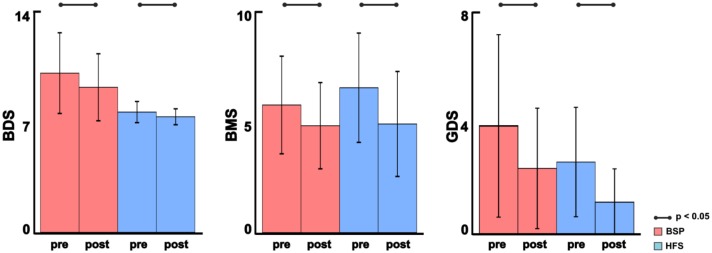
We found no significant differences in demographics between the studied groups and BoNT dosage between the patient groups (Table 1). BoNT treatment improved hyperkinesia in both patient groups after treatment as shown by BDS, BMS, and GDS scores (p < 0.05) (Figure 1).
Figure 1.
Clinical assessment of BSP patients and HFS patients at baseline (pre) and after (post) BoNT treatment: mean and standard deviations of clinical scores BDS, BMS, and GDS, respectively. BSP is represented in red, and HFS in blue. The significant comparisons between pre and post comparisons are shown using solid lines (p < 0.05, Bonferroni corrected).
BMS, blepharospasm movement scale; BoNT, botulinum neurotoxin; BSP, blepharospasm; GDS, global dystonia severity rating scale; HFS, hemifacial spasm.
Global brain network fingerprints
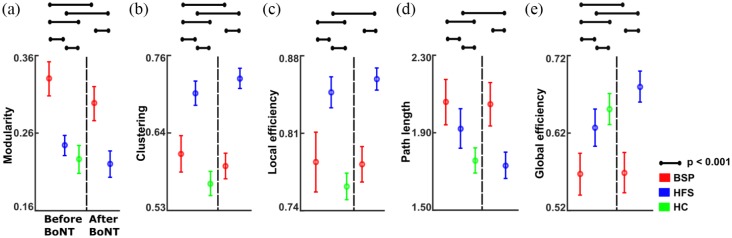
We initially studied the differences in global brain network fingerprints between patients with BSP, HFS, and HC at baseline, where BSP and HFS patients displayed the strongest symptoms. An increase in segregation is shown with higher modularity, clustering, and local efficiency, whereas increased integration is indicated by reduced path length, and increased global efficiency. We observed increased modularity in both patient groups relative to HC and in BSP relative to HFS (all at p < 0.001) (Figure 2a, Table 2). Both BSP and HFS patient groups showed increased clustering and local efficiency relative to HC, while both these parameters were higher in HFS patients compared with BSP patients (all at p < 0.001) (Figure 2b–c, Table 2). We further found increased path length in both patient groups relative to HC, and higher path length values in BSP relative to HFS patients (all at p < 0.001) (Figure 2d, Table 2). Global efficiency was decreased in both patient groups relative to HC, and was lower in patients with BSP compared with patients with HFS (all at p < 0.001) (Figure 2e, Table 2). Taken together, these results point towards an increased network segregation and decreased integration in both patient groups. These properties were more pronounced in patients with BSP than in patients with HFS.
Figure 2.
Comparison of global structural brain network parameters between BSP patients, HFS patients, and HC at baseline and after BoNT treatment: Mean values and standard deviations of global network parameters. (a) Modularity; (b) clustering; (c) local efficiency; (d) path length; and (e) global efficiency. BSP is represented in red, HFS in blue and HC in green. The significant post hoc comparisons for between and within group comparisons are shown using solid lines (denotes p < 0.001, Bonferroni corrected).
BoNT, botulinum neurotoxin; BSP, blepharospasm; HC, healthy controls; HFS, hemifacial spasm.
Table 2.
Differences between global brain network parameters of BSP patients, HFS patients and HC (group) at baseline. One-way ANOVA with dependent measures of global network parameters (modularity, clustering, local efficiency, path length, and global efficiency), and group (BSP, HFS, HC) as independent main effect.
| Parameter | Sum of squares | (df intercept, df error) | F | p | |
|---|---|---|---|---|---|
| Modularity | Group | 0.12 | (2, 63) | 191.1 | <0.001 |
| Clustering | Group | 0.19 | (2, 63) | 203.2 | <0.001 |
| Local efficiency | Group | 0.08 | (2, 63) | 109.3 | <0.001 |
| Path length | Group | 0.95 | (2, 63) | 50.05 | <0.001 |
| Global efficiency | Group | 0.08 | (2, 63) | 65.9 | <0.001 |
Regional brain network fingerprints
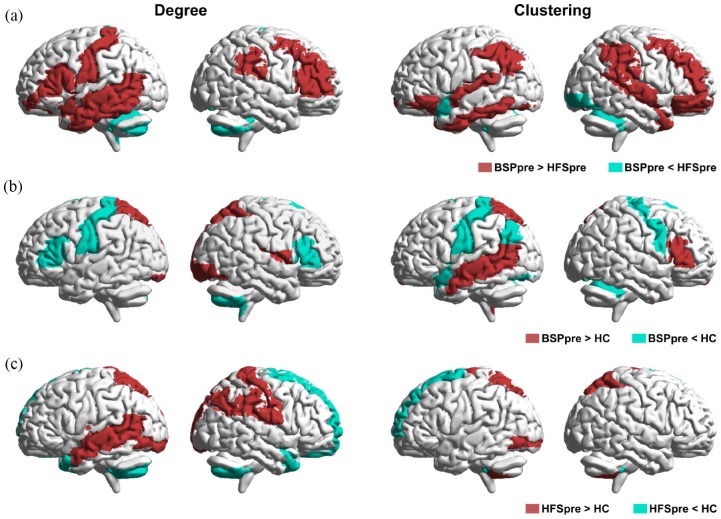
In regional network analysis, we assessed degree (number of connections to the region) and clustering (number of connections between neighbor regions) in different brain regions at baseline. Compared with HFS patients, BSP patients showed higher degree and clustering in the frontal [including bilateral primary sensorimotor cortex, right premotor cortex, and bilateral dorsolateral prefrontal cortex (DLPFC)] and temporal regions, and lower degree and clustering in the cerebellum (all at p < 0.05) (Figure 3a). Compared with HC, BSP patients showed higher degree and clustering in the temporal and parietal regions and lower degree and clustering in frontal and cerebellar regions (all at p < 0.05) (Figure 3b). A similar pattern was seen for patients with HFS at baseline, compared with HC (all at p < 0.05) (Figure 3c). In summary, patients with BSP showed impaired regional connectivity in the sensorimotor area and cerebellum relative to HFS patients and HC.
Figure 3.
Comparison of regional structural network changes between BSP patients, HFS patients, and HC at baseline: Representation of significant (FDR corrected, p < 0.05) regional between-group differences in degree (left panel) and clustering (right panel) before (pre) BoNT treatment for (a) BSPpre versus HFSpre; (b) BSPpre versus HC; (c) HFSpre versus HC.
BoNT, botulinum neurotoxin; BSP, blepharospasm; FDR, false discovery rate; HC, healthy controls; HFS, hemifacial spasm.
Modulation of global brain network fingerprints associated with symptom reduction
Global brain network fingerprints were analyzed after BoNT treatment to determine the effects of symptom and hyperkinesia reduction on global network fingerprints. Compared with baseline, following BoNT treatment patients with BSP had a reduction in modularity (Figure 2a, Table 3) and clustering (all at p < 0.001) (Figure 2b, Table 3), and increased global efficiency (p < 0.001) (Figure 2e, Table 3) that reflects the increased integration. In patients with HFS, we found decreased modularity (Figure 2a, Table 3) and an increase in both clustering (Figure 2b, Table 3) and local efficiency (Figure 2c, Table 3) (all at p < 0.001) after treatment. Moreover, patients with HFS showed increased global efficiency (Figure 2e, Table 3) and reduced path length (all at p < 0.001) (Figure 2d, Table 3) after treatment. These results show that global connectivity patterns related to BSP and HFS were modified by BoNT treatment.
Table 3.
Differences between global brain network parameters of BSP patients, HFS patients and HC (group) at baseline and after treatment with BoNT (time). The rmANOVA with dependent measures of global network parameters (modularity, clustering, local efficiency, path length, and global efficiency), and group (BSP, HFS) and time (pre, post) as independent main effects.
| Clinical score | Sum of squares | (df intercept, df error) | F | p | |
|---|---|---|---|---|---|
| Modularity | Group | 0.14 | (1, 40) | 192.9 | <0.001 |
| Time | 0.17 | (1, 40) | 712.1 | <0.001 | |
| Group × Time | 0.00 | (1, 40) | 12.9 | <0.001 | |
| Clustering | Group | 0.25 | (1, 40) | 311.7 | <0.001 |
| Time | 0.00 | (1, 40) | 1.97 | >0.1 | |
| Group × Time | 0.009 | (1, 40) | 222.1 | <0.001 | |
| Local efficiency | Group | 0.10 | (1, 40) | 168.0 | <0.001 |
| Time | 0.001 | (1, 40) | 10.3 | <0.01 | |
| Group × Time | 0.001 | (1, 40) | 18.1 | <0.001 | |
| Path length | Group | 1.06 | (1, 40) | 51.9 | <0.001 |
| Time | 0.21 | (1, 40) | 608.7 | <0.001 | |
| Group × Time | 0.16 | (1, 40) | 483.6 | <0.001 | |
| Global efficiency | Group | 0.15 | (1, 40) | 122 | <0.001 |
| Time | 0.16 | (1, 40) | 2894.6 | <0.001 | |
| Group × Time | 0.01 | (1, 40) | 2391.8 | <0.001 |
Modulation of regional brain network properties associated with symptom reduction
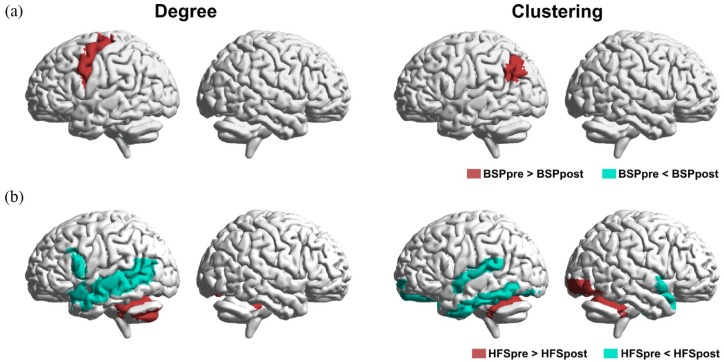
Our analysis revealed regional connectivity alterations in both BSP and HFS patient groups following BoNT treatment and subsequent symptom reduction. BoNT treatment induced a decrease in degree in the left sensorimotor cortex, and clustering in left angular gyrus in BSP patients, while we detected no increase in either parameter in any brain region (all at p < 0.05) (Figure 4a). In HFS patients, BoNT treatment was associated with a reduction of degree and clustering in the cerebellum and an elevation of both parameters in the temporal regions (all at p < 0.05) (Figure 4b). These results show that improvement of hyperkinesia following BoNT treatment is associated with modulation of the connectivity of cerebellum in HFS patients but not in patients with BSP.
Figure 4.
Comparison of regional structural network changes between BSP patients, HFS patients at baseline (pre) and after (post) BoNT treatment: Representation of significant (FDR corrected, p < 0.05) regional within group differences in degree (left panel) and clustering (right panel) for (a) BSP patients; (b) HFS patients.
BoNT, botulinum neurotoxin; BSP, blepharospasm; FDR, false discovery rate; HFS, hemifacial spasm.
VBM results
We also investigated differences in brain structure in BSP and HFS patients using voxel based comparison of GMV at baseline. The GMV abnormalities in BSP and HFS patients at baseline relative to HC survived the cluster level FDR correction for p < 0.05. In comparison with HC, BSP patients showed increased GMV in the bilateral precentral and left inferior occipital regions (Supplementary Figure S3a, Table S1). Compared with HC, HFS patients displayed increased GMV in the right precentral, bilateral paracentral, and left inferior occipital regions (Supplementary Figure S3b, Table S1), whereas GMV was reduced in the bilateral cerebellum and right parahippocampal regions (Supplementary Figure S3c, Table S1). The results of other comparisons between BSP and HFS before BoNT treatment are provided in the Supplementary Results.
Discussion
This study identified global and regional brain network fingerprints in BSP and HFS patients. We were able to disentangle dystonia-specific brain network fingerprints and contrast these to secondary alterations due to facial hyperkinesias. Moreover, we found hyperkinesia-related structural properties that are modulated with improvement of symptoms through BoNT treatment, as well as brain network features that remain unresponsive to this treatment and are thought of relevance for brain reorganization with dystonia or impaired compensation to malplasticity.
Dystonia-related network characteristics
On a global network level, we found that the structural brain networks were more segregated in patients with BSP than in HFS patients or HC. Higher modularity, longer path length, and a lack of global efficiency was characteristic for patients with BSP. Taken together, these results suggest that information has to travel longer distances in BSP patients, reflecting less efficient neural processing in focal dystonia compared with HFS patients and HC. Although such a lay interpretation oversimplifies the complex nature of the brain, it provides an intuitive model of a network architecture in dystonia as provided for other neurological or psychiatric disorders.21,22
Overall, local efficiency in BSP patients was lower than in HFS patients, but it was (mildly) elevated compared with HC. It therefore seems unlikely that a deficit in global efficiency or network integration is due simply to a lack of local efficiency in dystonia. Higher modularity, deficits in global efficiency, and greater network segregation in the brain have also been found in other neurological disorders and express a pathological state of the brain.23–25
Regional analyses provide conceptual links to previous studies in dystonia that revealed differences in local activity, connectivity, or metabolism in distinct brain regions, for example, the sensorimotor circuits.23,26,27 These regional alterations affect motor planning, motor execution or afferent sensorimotor processing.28–31 They partly explain the aberrant motor output and abnormal movement as seen in dystonia. Therapeutic modulation of these network areas (e.g. of the premotor cortex or cerebellum) by noninvasive transcranial magnetic stimulation (TMS) or direct current stimulation (TDCS) can improve dystonic symptoms.32–35
The current study demonstrated that BSP-specific changes occurred primarily in the cerebellum and in cortical sensorimotor areas. Compared with HC and patients with HFS, in BSP patients these areas showed reduced degree (local connectivity) and clustering, both of which affect information processing. The reduction in these regional parameters is consistent with higher segregation and reduced global efficiency in focal dystonia. These findings are in line with previous functional imaging reports that showed reduced functional activity and connectivity of the cerebellum and the sensorimotor cortex in dystonia,36–38 and particularly in blepharospasm.29,39,40 Furthermore, diffusion tensor imaging (DTI) studies could identify a lower density of fiber tracts projecting from or to these areas.26,41
In our study, we see modulation of cerebellar network parameters after BoNT treatment despite a reduction in symptoms, which supports a primordial role of the cerebellum in the underlying pathology of dystonia. Numerous previous studies suggesting an important role of the cerebellum in dystonia.27,37,42 However, we see reduced degree (local connectivity) in the cerebellum in HFS patients when related to HC. This observation challenges our pathophysiological model that differences in the cerebellum of patients with dystonia are causal for the disease. Our results instead suggest that abnormal cerebellar structure may be associated with facial hyperkinesias rather than being specific for dystonia as previously thought. Alternatively, there may be distinct cerebellar networks involved in dystonia and hyperkinesia due to other causes.
Unlike the cerebellum, the DLPFC displayed a more complex behavior on the regional network level in patients with BSP. The DLPFC is a high-level control area within the motor circuits, and has shown a broad range of abnormalities in previous studies of dystonia.42 BSP patients showed higher degree in DLPFC and left sensorimotor cortex compared with HFS, analoguous to increased functional connectivity of these areas.37 Increased communication in this frontoparietal motor executive function network could be a pathological correlate of dystonia in the presence of facial hyperkinesias. Although speculative, one could imagine that a gain of connectivity could facilitate disinhibited flow of information in the affected brain region (here: DLPFC and sensorimotor cortex). Such a model fits well with dystonia, which is clinically and neurophysiologically characterized by disinhibition in areas of motor planning and execution.43,44
While degree in DLPFC of BSP patients was increased relative to HFS patients, it was reduced relative to HC. Earlier studies identified loss of connectivity, activity, and metabolism in DLPFC in various forms of dystonia compared with HC,42 consistent with our findings. The multidimensional comparisons between BSP, HFS, and HC in the current study allow reconciling previously unresolved findings from other imaging and electrophysiological experiments. Earlier imaging studies had demonstrated altered functional connectivity and activity in the sensorimotor cortex,29,45 premotor cortex,28,46,47 DLPFC,42 and cerebellum37,42 in patients with dystonia. The dystonia-specific changes in the structural brain network found here converge with the previously detected functional changes in areas of motor planning and execution, thereby providing a structural framework for the observed functional abnormalities.
Common network fingerprints of facial hyperkinesia
In the current study, we were able to identify structural network characteristics that are associated with facial hyperkinesias by comparing both patients with BPS and HFS to HC. We postulated that facial hyperkinesias lead to brain network alterations, for example, by net increase of facial motor activity and secondary neuroplastic changes. However, our analysis showed similar global brain network fingerprints in BPS patients both before and after treatment, despite an improvement of symptoms.
On a regional level, patients with HFS and BSP both showed increased degree and clustering in the paramedian posterior parietal cortex. This region is a tertiary somatosensory area that is involved in sensorimotor processing. Previous investigations in dystonia have also found abnormal activity and connectivity in this area.48 The current findings suggest that these alterations might not be specific for dystonia, but could simply be due to hyperkinetic motor activity.
Hyperkinesia reduction related network dynamics
Injections of BoNT into the affected muscles are an effective treatment that reduce hyperkinesia in both BSP and HFS. However, symptom-improvement-related reorganization of brain circuits is not understood. A reduction in the motor drive or feedback information could lead to normalization of disinhibition,49 reorganization of brain circuits with abnormal cortical representations,50 or even restoration of aberrant (sub)cortical or subortico-cortical neuronal activity.29,39 Regionally modulated cerebellar motor outflow or whole brain network behavior, most likely mediated by a change of sensory afferent activity within sensorimotor loops, have also been discussed.30,51,52
Our analyses of BSP and HFS patient before and after BoNT treatment found changes in global and regional structural network fingerprints and grey matter characteristics, which were related to symptom improvement.
Segregation in BSP patients was reduced following BoNT treatment and symptom improvement, but not to the level of HC. By contrast, there was no change of path length or global efficiency in these patients after treatment despite symptom improvements, suggesting that these characteristics could be specific and causal for dystonia.
Relative to HC, path length and modularity were only mildly elevated in HFS patients before treatment. After BoNT treatment of HFS patients, these parameters were not significantly different to HC. The increase in these two parameters could be associated with, or be a consequence of, facial hyperkinesia irrespective of etiology, for example, due to a net increase of facial motor activity or secondary neuroplastic changes. Reduction of modularity after treatment could be related to relief of hyperkinetic burden in both disorders.
Prior to treatment, when symptoms were most pronounced, network clustering and local efficiency were increased in HFS patients, but only mildly elevated in BSP patients. BoNT therapy reduced clustering in BSP levels close to those of HC, while it increased clustering in HFS patients despite similar symptomatic relief in both conditions. We conclude from this that local clustering properties are not related to the severity of hyperkinetic movements or their improvement. Augmentation of local efficiency in HFS, and, to a lesser degree, in BSP, however, could reflect a mechanism of compensation that is deficient in dystonia. In BSP, local efficiency was unresponsive to BoNT therapy and could therefore indicate an endophenotypic trait.29,53
Taken together, network segregation in the form of longer path length and higher modularity correlates with facial hyperkinesia in patients with BSP and HFS. Increased local efficiency could reflect a mechanism of compensation for hyperkinetic movements that is effective in HFS but deficient in dystonia. Abnormal network integration in the form of reduced global efficiency and increased path length was unresponsive to BoNT therapy and seems to be a trait feature of dystonia. By contrast, path length and global efficiency were modulated by BoNT treatment in HFS; these parameters seem to be related to symptom severity rather than being an HFS-specific endophenotype.
Degree in the left sensorimotor cortex and clustering in the left intraparietal sulcus (IPS) were higher in BSP patients than in HC, but were reduced after treatment with BoNT. This correction following BoNT treatment was not found in the HFS group, and therefore seems to be specific for dystonia. While the sensorimotor cortex is directly related to the execution of movements, the left IPS is a multimodal sensory area and an important region for sensorimotor integration.
Structural correlates of network dynamics
In previous studies, GMV alterations in focal dystonia patients were assessed, on average, several years after onset of symptoms,6,54 while BoNT treatment-related changes were determined after 4–5 weeks of treatment.8 We supplemented our analysis of structural brain network fingerprints with a voxel-based analysis of GMV in BSP and HFS patients and HC. Higher degree in the left sensorimotor cortex was counterintuitively associated with regionally reduced GMV of these areas in BSP.
GMV was higher in a small area of the right superior temporal lobe in BSP patients relative to HFS patients. Regional network analysis revealed an increase of clustering and degree in larger parts of the right superior temporal lobe. While VBM and network analysis yielded consistent results, the network approach seems to be more sensitive for detecting the full magnitude of dystonia-specific etiology. In other words, the dystonia-specific features seem to be encoded in the brain’s network architecture rather than local grey matter volume. These findings are congruent with a reduced thickness of the fronto-rostral, supramarginal and temporal cortex in BSP relative to HFS patients.14
Both hyperkinetic disorders showed higher GMV in the primary motor cortex (and also in the primary somatosensory cortex) compared with HC. This finding indicates that facial hyperkinesias may lead to a GMV increase in areas intensely engaging in sensorimotor processing. A number of previous studies have shown abnormal activity, connectivity, and GMV in primary sensorimotor areas when patients with focal dystonia were compared with healthy subjects.7,40,55–58 The current results illustrate that some of the previously identified abnormalities in GMV might not be specific for dystonia, but could be related to facial hyperkinesias in general.
Limitations
A limitation of this study is the sample size (13 subjects per group). Nonetheless, our results provided the evidences for structural network abnormalities in focal dystonia. Second, the lateralization of the affected side in the HFS patients was not considered in the analysis, and this should be considered in future studies.
Conclusion
This work provides compelling support for structural network abnormalities in focal dystonia. By comparing BSP patients with HFS patients, we identified brain network fingerprints specific to focal dystonia, helping to distinguish between the etiology of the disease and secondary hyperkinesia. We identified loss of global connectivity and network efficiency, as well as as abnormal compensation properties, as causal and etiologic properties related to focal dystonic hyperkinesia. These findings are of clinical value as measurable parameters of disease manifestation or could be measured to trace treatment responses as hyperkinesia biomarkers.
Supplemental Material
Supplemental material, revised_supplementary_TAN-18-OR-0100 for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, sup1_inks_jpg for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supp1_jpg for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supplementary_Figure_2 for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental Material
Supplemental material, Supp_figure_3_inkscape_png_jpg for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Acknowledgments
Authors Muthuraman Muthuraman and Sergiu Groppa contributed equally. We thank Rosalind Gilchrist for proofreading the manuscript.
Footnotes
Funding: This study was supported by grants from German Research Council (DFG; CRC-TR-128, CRC-1193) and University of Mainz intramural funding.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Venkata C. Chirumamilla  https://orcid.org/0000-0002-0833-545X
https://orcid.org/0000-0002-0833-545X
Muthuraman Muthuraman  https://orcid.org/0000-0001-6158-2663
https://orcid.org/0000-0001-6158-2663
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Venkata C. Chirumamilla, Movement Disorders and Neurostimulation, Biomedical Statistics and Multimodal Signal Processing Unit, Department of Neurology, Focus Program Translational Neuroscience (FTN), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Christian Dresel, Movement Disorders and Neurostimulation, Biomedical Statistics and Multimodal Signal Processing Unit, Department of Neurology, Focus Program Translational Neuroscience (FTN), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Nabin Koirala, Movement Disorders and Neurostimulation, Biomedical Statistics and Multimodal Signal Processing Unit, Department of Neurology, Focus Program Translational Neuroscience (FTN), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Gabriel Gonzalez-Escamilla, Movement Disorders and Neurostimulation, Biomedical Statistics and Multimodal Signal Processing Unit, Department of Neurology, Focus Program Translational Neuroscience (FTN), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Günther Deuschl, Department of Neurology, University Hospital Schleswig-Holstein, University of Kiel, Kiel, Schleswig-Holstein, Germany.
Kirsten E. Zeuner, Department of Neurology, University Hospital Schleswig-Holstein, University of Kiel, Kiel, Schleswig-Holstein, Germany
Muthuraman Muthuraman, Movement Disorders and Neurostimulation, Biomedical Statistics and Multimodal Signal Processing Unit, Department of Neurology, Focus Program Translational Neuroscience (FTN), University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany.
Sergiu Groppa, Movement Disorders and Neurostimulation, Department of Neurology, Focus Program Translational Neuroscience (FTN), Rhine-Main Neuroscience network (rmn2), Johannes-Gutenberg-University Mainz, Langenbeckstr. 1, Mainz, 55131, Germany.
References
- 1. Jochim A, Li Y, Gora-Stahlberg G, et al. Altered functional connectivity in blepharospasm/orofacial dystonia. Brain Behav 2018; 8: e00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yuksel B, Genc F, Yaman A, et al. Evaluation of stigmatization in hemifacial spasm and quality of life before and after botulinum toxin treatment. Acta Neurol Belg. Epub ahead of print 3 September 2018. DOI: 10.1007/s13760-018-1018-5. [DOI] [PubMed] [Google Scholar]
- 3. Simonyan K, Cho H, Hamzehei Sichani A, et al. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain 2017; 140: 3179–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kopanidis P, Das C. Botulinum neurotoxin for the treatment of movement disorders. Aust J Gen Pract 2018; 47: 598–601. [DOI] [PubMed] [Google Scholar]
- 5. Battistella G, Termsarasab P, Ramdhani RA, et al. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 2017; 27: 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martino D, Di Giorgio A, D’Ambrosio E, et al. Cortical gray matter changes in primary blepharospasm: a voxel-based morphometry study. Mov Disord 2011; 26: 1907–1912. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki Y, Kiyosawa M, Wakakura M, et al. Gray matter density increase in the primary sensorimotor cortex in long-term essential blepharospasm. Neuroimage 2011; 56: 1–7. [DOI] [PubMed] [Google Scholar]
- 8. Delnooz CC, Pasman JW, van de Warrenburg BP. Dynamic cortical gray matter volume changes after botulinum toxin in cervical dystonia. Neurobiol Dis 2015; 73: 327–333. [DOI] [PubMed] [Google Scholar]
- 9. Hosseini SM, Black JM, Soriano T, et al. Topological properties of large-scale structural brain networks in children with familial risk for reading difficulties. Neuroimage 2013; 71: 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jochim A. Altered functional connectivity in blepharospasm/orofacial dystonia. Brain Behav. 2018; 8: e00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang W, Lan Y, Cen C, et al. Abnormal spontaneous neural activity of brain regions in patients with primary blepharospasm at rest. J Neurol Sci 2019; 403: 44–49. [DOI] [PubMed] [Google Scholar]
- 12. Lehericy S, Tijssen MA, Vidailhet M, et al. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord 2013; 28: 944–957. [DOI] [PubMed] [Google Scholar]
- 13. Neumann N, Domin M, Erhard K, et al. Voxel-based morphometry in creative writers: grey matter increase in a prefronto-thalamic-cerebellar network. Eur J Neurosci. Epub ahead of print 19 May 2018. DOI: 10.1111/ejn.13952. [DOI] [PubMed] [Google Scholar]
- 14. Alexandru H, Muthuraman M, Chirumamilla VC, et al. Grey Matter microstructural integrity alterations in blepharospasm are partially reversed by botulinum neurotoxin therapy. PLoS One 2016; 11: e0168652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fleischer V, Groger A, Koirala N, et al. Increased structural white and grey matter network connectivity compensates for functional decline in early multiple sclerosis. Mult Scler 2017; 23: 432–441. [DOI] [PubMed] [Google Scholar]
- 16. Comella CL, Leurgans S, Wuu J, et al. Rating scales for dystonia: a multicenter assessment. Mov Disord 2003; 18: 303–312. [DOI] [PubMed] [Google Scholar]
- 17. Albanese A, Sorbo FD, Comella C, et al. Dystonia rating scales: critique and recommendations. Mov Disord 2013; 28: 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007; 38: 95–113. [DOI] [PubMed] [Google Scholar]
- 19. Maier S, Perlov E, Graf E, et al. Discrete global but no focal gray matter volume reductions in unmedicated adult patients with attention-deficit/hyperactivity disorder. Biol Psychiatry 2016; 80(12): 905–915. [DOI] [PubMed] [Google Scholar]
- 20. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002; 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 21. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009; 10: 186–198. [DOI] [PubMed] [Google Scholar]
- 22. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010; 52: 1059–1069. [DOI] [PubMed] [Google Scholar]
- 23. Battistella G, Termsarasab P, Ramdhani RA, et al. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex 2017; 27: 1203–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crossley NA, Mechelli A, Scott J, et al. The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 2014; 137: 2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci 2010; 30: 9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Argyelan M, Carbon M, Niethammer M, et al. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci 2009; 29: 9740–9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shakkottai VG, Batla A, Bhatia K, et al. Current opinions and areas of consensus on the role of the cerebellum in dystonia. Cerebellum 2017; 16: 577–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castrop F, Dresel C, Hennenlotter A, et al. Basal ganglia-premotor dysfunction during movement imagination in writer’s cramp. Mov Disord 2012; 27: 1432–1439. [DOI] [PubMed] [Google Scholar]
- 29. Dresel C, Haslinger B, Castrop F, et al. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain 2006; 129: 36–46. [DOI] [PubMed] [Google Scholar]
- 30. Delnooz CC, Pasman JW, Beckmann CF, et al. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One 2013; 8: e62877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haslinger B, Altenmuller E, Castrop F, et al. Sensorimotor overactivity as a pathophysiologic trait of embouchure dystonia. Neurology 2010; 74: 1790–1797. [DOI] [PubMed] [Google Scholar]
- 32. Siebner HR, Tormos JM, Ceballos-Baumann AO, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology 1999; 52: 529–537. [DOI] [PubMed] [Google Scholar]
- 33. Brighina F, Romano M, Giglia G, et al. Effects of cerebellar TMS on motor cortex of patients with focal dystonia: a preliminary report. Exp Brain Res 2009; 192: 651–656. [DOI] [PubMed] [Google Scholar]
- 34. Furuya S, Nitsche MA, Paulus W, et al. Surmounting retraining limits in musicians’ dystonia by transcranial stimulation. Ann Neurol 2014; 75: 700–707. [DOI] [PubMed] [Google Scholar]
- 35. Bradnam LV, Graetz LJ, McDonnell MN, et al. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci 2015; 9: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Carbon M, Kingsley PB, Su S, et al. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol 2004; 56: 283–286. [DOI] [PubMed] [Google Scholar]
- 37. Dresel C, Li Y, Wilzeck V, et al. Multiple changes of functional connectivity between sensorimotor areas in focal hand dystonia. J Neurol Neurosurg Psychiatry 2014; 85: 1245–1252. [DOI] [PubMed] [Google Scholar]
- 38. Haslinger B, Noe J, Altenmuller E, et al. Changes in resting-state connectivity in musicians with embouchure dystonia. Mov Disord 2017; 32: 450–458. [DOI] [PubMed] [Google Scholar]
- 39. Dresel C, Bayer F, Castrop F, et al. Botulinum toxin modulates basal ganglia but not deficient somatosensory activation in orofacial dystonia. Mov Disord 2011; 26: 1496–1502. [DOI] [PubMed] [Google Scholar]
- 40. Jochim A, Li Y, Gora-Stahlberg G, et al. Altered functional connectivity in blepharospasm/orofacial dystonia. Brain Behav 2018; 8: e00894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vo A, Sako W, Niethammer M, et al. Thalamocortical connectivity correlates with phenotypic variability in dystonia. Cereb Cortex 2015; 25: 3086–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carbon M, Kingsley PB, Tang C, et al. Microstructural white matter changes in primary torsion dystonia. Mov Disord 2008; 23: 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ridding MC, Sheean G, Rothwell JC, et al. Changes in the balance between motor cortical excitation and inhibition in focal, task specific dystonia. J Neurol Neurosurg Psychiatry 1995; 59: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beck S, Richardson SP, Shamim EA, et al. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci 2008; 28: 10363–10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ceballos-Baumann AO, Sheean G, Passingham RE, et al. Botulinum toxin does not reverse the cortical dysfunction associated with writer’s cramp. A PET study. Brain 1997; 120: 571–582. [DOI] [PubMed] [Google Scholar]
- 46. Ibanez V, Sadato N, Karp B, et al. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology 1999; 53: 96–105. [DOI] [PubMed] [Google Scholar]
- 47. Gallea C, Horovitz SG, Najee-Ullah M, et al. Impairment of a parieto-premotor network specialized for handwriting in writer’s cramp. Hum Brain Mapp 2016; 37: 4363–4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Delnooz CC, Helmich RC, Toni I, et al. Reduced parietal connectivity with a premotor writing area in writer’s cramp. Mov Disord 2012; 27: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 49. Gilio F, Curra A, Lorenzano C, et al. Effects of botulinum toxin type A on intracortical inhibition in patients with dystonia. Ann Neurol 2000; 48: 20–26. [PubMed] [Google Scholar]
- 50. Byrnes ML, Thickbroom GW, Wilson SA, et al. The corticomotor representation of upper limb muscles in writer’s cramp and changes following botulinum toxin injection. Brain 1998; 121: 977–988. [DOI] [PubMed] [Google Scholar]
- 51. Hubsch C, Roze E, Popa T, et al. Defective cerebellar control of cortical plasticity in writer’s cramp. Brain 2013; 136: 2050–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Curra A, Trompetto C, Abbruzzese G, et al. Central effects of botulinum toxin type A: evidence and supposition. Mov Disord 2004; 19(Suppl. 8): S60–S64. [DOI] [PubMed] [Google Scholar]
- 53. Meunier S, Garnero L, Ducorps A, et al. Human brain mapping in dystonia reveals both endophenotypic traits and adaptive reorganization. Ann Neurol 2001; 50: 521–527. [DOI] [PubMed] [Google Scholar]
- 54. Horovitz SG, Ford A, Najee-Ullah MA, et al. Anatomical correlates of blepharospasm. Transl Neurodegener 2012; 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delmaire C, Vidailhet M, Elbaz A, et al. Structural abnormalities in the cerebellum and sensorimotor circuit in writer’s cramp. Neurology 2007; 69: 376–380. [DOI] [PubMed] [Google Scholar]
- 56. Obermann M, Yaldizli O, De Greiff A, et al. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord 2007; 22: 1117–1123. [DOI] [PubMed] [Google Scholar]
- 57. Granert O, Peller M, Gaser C, et al. Manual activity shapes structure and function in contralateral human motor hand area. Neuroimage 2011; 54: 32–41. [DOI] [PubMed] [Google Scholar]
- 58. Martino D, Di Giorgio A, D’Ambrosio E, et al. Cortical gray matter changes in primary blepharospasm: a voxel-based morphometry study. Mov Disord 2011; 26: 1907–1912. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, revised_supplementary_TAN-18-OR-0100 for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental material, sup1_inks_jpg for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental material, Supp1_jpg for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental material, Supplementary_Figure_2 for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders
Supplemental material, Supp_figure_3_inkscape_png_jpg for Structural brain network fingerprints of focal dystonia by Venkata C. Chirumamilla, Christian Dresel, Nabin Koirala, Gabriel Gonzalez-Escamilla, Günther Deuschl, Kirsten E. Zeuner, Muthuraman Muthuraman and Sergiu Groppa in Therapeutic Advances in Neurological Disorders