Plant aquaporins and hormones regulate root water uptake in mildly stressed poplar together with the participation of fungal aquaporins from the ectomycorrhizal fungus Laccaria bicolor.
Keywords: Abscisic acid, aquaporins, cytokinins, ectomycorrhiza, mild drought, poplar, root water transport
Abstract
The relatively better performance of mycorrhizal plants subjected to drought stress has commonly been linked to improved root water uptake through the fungal regulation of plant aquaporins and hormones. In this study, we examined the role of ectomycorrhizal fungi in plant water relations and plant hormonal balance under mild drought using split-root seedlings of Populus trichocarpa × deltoides either with or without inoculation with Laccaria bicolor. The root compartments where the drought treatment was applied had higher ABA and lower cytokinin tZR contents, and greater expression of the plant aquaporins PtPIP1;1, PtPIP1;2, PtPIP2;5, and PtPIP2;7. On the other hand, the presence of L. bicolor within the roots down-regulated PtPIP1;4, PtPIP2;3, and PtPIP2;10, and reduced the abundance of PIP2 proteins. In addition, expression of the fungal aquaporins JQ585595 and JQ585596 were positively correlated with root ABA content, while tZR content was positively correlated with PtPIP1;4 and negatively correlated with PtPIP2;7. The results demonstrate a coordinated plant–fungal system that regulates the different mechanisms involved in water uptake in ectomycorrhizal poplar plants.
Introduction
Drought stress is well known to greatly affect the development and yield of plants (Hogg et al., 2008; Allen et al., 2010), and it is expected to be one of the major issues for agriculture and forestry crops in the coming years due to unpredictable climate change (Lesk et al., 2016). Although the exact responses of plants to drying soil are species-dependent (Liu et al., 2010; Pinheiro and Chaves, 2011), a sudden drought event will typically cause stomatal closure to decrease transpiration (Cowan, 1977) and adjustments of root water uptake to avoid desiccation (Perrone et al., 2012).
Water uptake through the roots is tightly controlled by its radial transport, where the presence of apoplastic barriers and cell plasma membranes determine how water is transferred from the soil to the xylem. Following the ‘composite model’ for radial transport (Steudle and Peterson, 1998), water will be transferred from the soil to the root following two main paths: (1) through the cell-wall continuum (apoplastic path), which is driven by the hydrostatic gradient created by the transpiration stream and is controlled by the Casparian strip and suberin at the exodermis and endodermis; and (2) through the cell-to-cell path, which includes the transfer of water through plasmodesmata and plasma membranes using the specialized water-channel aquaporin proteins, and which always following the driving force of an osmotic gradient. Plant aquaporins belong to the major-intrinsic proteins family (MIPs) that are present in both plants and fungi (Aroca et al., 2009) and are divided into five subfamilies: plasma membrane (PIPs), tonoplast (TIPs), Nodulin26-like (NIPs), X (XIPs), and small basic (SIPs) . Aquaporins have a major role in the control of water transfer under drought stress (Zargar et al., 2017), and their function is closely related to post-translational regulation, in the form of phosphorylation, as well as their coordination with plant hormones (Sánchez-Romera et al. 2018).
Phytohormones respond to drought as signal molecules that regulate plant water relations (Wolters and Jürgens, 2009). Among them, abscisic acid (ABA), salicylic acid (SA) (Miura and Tada, 2014), and jasmonic acid (JA) (de Ollas et al. 2015) are induced in response to drought, while others such as cytokinins (CK) are typically reduced (reviewed in Cortleven et al., 2019). A complex regulatory network connects the different pathways and enables each hormone to assist or antagonize the others in responding to stress (Peleg and Blumwald, 2011; Munné-Bosch and Müller, 2013). ABA is the most studied and the most abundant under water-deficit conditions, where it is usually implicated in stomatal closure and leaf senescence (Wilkinson and Davies, 2010). ABA is also known to affect root hydraulic conductivity (Lpr), both by increasing it (Thompson et al., 2007; Aroca et al., 2008; Parent et al., 2009; Kudoyarova et al., 2011) and by inhibiting it (Beaudette et al., 2007). Other hormones, although less studied in water relations, have also been reported to enhance Lpr (JA; Sánchez-Romera et al., 2014) or to decrease it (SA, Quiroga et al., 2018). Information regarding how hormones and their multiple interactions affect water transport requires is scarce.
Plants form mutualistic associations with specialized fungi that affect how they obtain water and nutrients from the soil, including under extreme conditions. Among these different mycorrhizal associations, arbuscular mycorrhizal fungi are the most widespread in the plant kingdom, while ectomycorrhizal fungi are more specific to tree species from temperate forests. Ectomycorrhizal fungi generally form an extensive hyphal network in the soil in symbiosis with trees (Nehls et al., 2010) that is related with improvements in the overall water and nutritional status of the host plant (Rousseau and Reid, 1990; Lehto and Zwiazek, 2011). Increases in Lpr in the presence of some ectomycorrhizal fungi have commonly been reported (Marjanović et al., 2005; Yi et al., 2008; Lee et al., 2010, Xu et al. 2015), but other studies have found no changes or even reductions (Calvo-Polanco et al., 2008, 2009; Xu et al., 2016). The beneficial effects of ectomycorrhizal fungi on root water transport have been attributed to the resulting increase in the absorbing surface area and the exploration of larger soil volumes by the extramatrical mycelia, and to their effects on the expression of plant aquaporins (Lee et al., 2010; Navarro-Ródenas et al., 2013; Xu et al., 2015). As in arbuscular mycorrhizal fungi (Quiroga et al., 2018; Sánchez-Romera et al., 2018), it is presumed that ectomycorrhizal fungi also play a major role in regulating plant hormones, although our current knowledge is limited to a few studies that have shown reductions in ABA content under osmotic stress in ectomycorrhizal plants (Rincón et al., 2005) or have demonstrated a priming effect to drought of the ectomycorrhizal fungi on ABA and SA contents in poplar trees (Luo et al., 2009).
In this study, we examine how the interaction of Populus trichocarpa × deltoides with the ectomycorrhizal fungus Laccaria bicolor affects water uptake under drought through aquaporin and hormonal regulation. We used a split-root system in order to determine the responses of the plants to the presence of fungi and water stress. The objectives of the study were to evaluate whether plants can compensate the loss of water in different parts of the root system via the hormonal balance and expression of aquaporins, to evaluate whether ectomycorrhizal fungi can regulate plant hormones and aquaporins, and to test whether fungal aquaporins are involved in root water uptake.
Materials and methods
Plant material and growth conditions
Saplings of hybrid poplar (Populus trichocarpa × deltoides, clone H11-11, supplied by Dr Uwe Hacke, University of Alberta), were produced from cuttings (4 cm long) with one to two non-flushed buds. The cuttings were immersed in 0.8% indole-3-butyric acid (IBA) solution for 1 min, planted in a mixture of peat moss and vermiculate (2:1), and maintained for 4 weeks under relative high humidity. The roots of the cuttings were then divided into two equal parts and transferred into 1-l pots (17×10 cm) filled with perlite and configured in a split-root system. After transplanting, the different compartments of the split-root system were inoculated with the fungus Laccaria bicolor (Orton (R. Maire) and the plants were divided into three groups: (1) plants left as non-inoculated, to which plain Melin–Norkans liquid media was added instead of inoculum; (2) plants with both sides of the split-root system inoculated with L. bicolor; (3) plants with only one side of the root compartment inoculated with L. bicolor. At 12 weeks after transplanting, plants were subjected to the drought treatment, where half of the different inoculation groups of the split root-system (with or without inoculation) were left without any water for 10 d. The soil water potential was calculated at the time of harvesting in each of the compartments of the split-root system by using a HR-33T dew-point microvoltmeter together with a C-52 sample chamber (Wescor Inc.).
At the time of harvest, there was a total of 10 different treatment groups (Table 1), as follows. (1) Both compartments were non-inoculated and well-watered (NI-Ww/NI-Ww). (2) One compartment was non-inoculated and the other was inoculated with Laccaria, and both were well-watered (NI-Ww/Lacc-Ww). (3) Both compartments were inoculated and both were well-watered (Lacc-Ww/Lacc-Ww). (4) Both compartments were non-inoculated, one was well-watered and the other under drought (NI-Ww/NI-D). (5) One compartment was non-inoculated and under drought, and the other was inoculated and well-watered (NI-D/Lacc-Ww). (6) One compartment was non-inoculated and well-watered and the other was inoculated and under drought (NI-Ww/Lacc-D). (7) Both compartments were inoculated, one was well-watered and the other under drought (Lacc-Ww/Lacc-D). (8) Both compartments were non-inoculated and under drought (NI-D/NI-D). (9) One compartment was non-inoculated and the other inoculated and both were under drought (NI-D/Lacc-D). (10) Both compartments were inoculated and under drought (Lacc-D/Lacc-D).
Table 1.
Summary of drought and fungal treatment combinations applied to poplars in the split-root system.
| Treatments | Compartment 1 | Compartment 2 | |||
|---|---|---|---|---|---|
| L. bicolor | Drought | L. bicolor | Drought | ||
| 1 | NI-Ww / NI-Ww | – | – | – | – |
| 2 | NI-Ww / Lacc-Ww | – | – | Yes | – |
| 3 | LaccWw / LaccWw | Yes | – | Yes | – |
| 4 | NI-Ww / NI-D | – | – | – | Yes |
| 5 | NI-D / Lacc-Ww | – | Yes | Yes | – |
| 6 | NI-Ww / Lacc-D | – | – | Yes | Yes |
| 7 | Lacc-Ww / Lacc-D | Yes | – | Yes | Yes |
| 8 | NI-D / NI-D | – | Yes | – | Yes |
| 9 | NI-D / Lacc-D | – | Yes | Yes | Yes |
| 10 | Lacc-D / Lacc-D | Yes | Yes | Yes | Yes |
NI, Non-inoculated roots; Lacc, roots inoculated with L. bicolor; Ww, well-watered roots; D, roots under drought stress. ‘Yes’ indicates that the presence of L. bicolor or drought in the compartment.
Laccaria bicolor was obtained from the University of Alberta Microfungus Collection and Herbarium (UAMH8232). The inoculation of plant roots was done 1 d after transplanting and again after 4 weeks. The fungus was grown for 4 weeks in modified Melin–Norkans (MMN) liquid medium into 0.5-l Erlenmeyer flasks that were continuously shaken. The inoculum was blended to OD=260 and applied to the roots of the plants at a rate of 20 ml per root compartment. The plants were grown in a controlled-environment growth chamber under a 18/6 h day/night photoperiod with a photosynthetic photon flux density (PPFD) of 450–500 µmol m–2 s–1 at 22/18 °C, and 50% relative humidity for 3 months. During the growing period, 40% modified Hoagland’s solution was applied twice per week (Epstein, 1972): 2.5 mM KNO3, 0.5 mM KH2PO4, 2.5 mM Ca(NO3)2, 1 mM MgSO4, 23 µM H3BO3, 5 µM MnCl2, 0.3 µM ZnSO4, 0.2 µM CuSO4, 0.01 µM (NH4)6Mo7O24, and 90 µM EDTA-Fe.
Ectomycorrhizal colonization rates
Ectomycorrhizal root colonization rates were estimated by stereomicroscopic examination of the root tips in eight different root sections (3–4 cm) in each of the root compartments within each treatment, with a total of six seedlings per treatment combination being examined. The percentage of root tips with distinct ectomycorrhizal structures was calculated by determining the number of mycorrhizal versus non-mycorrhizal root tips in each of the root compartments per seedling (Brundrett et al., 1996).
Plant physiological measurements
Root and shoot fresh weights, stomatal conductance (gs), and leaf relative water content (RWC) were determined at the time of harvest for six plants per treatment combination (i.e. n=6). gs was measured 2 h into the photoperiod in the youngest fully developed mature leaf of each plant using a portable AP4 porometer (Delta-T Devices Ltd). RWC was determined in mature, fully developed leaves, which were excised from the main shoot, weighed (W0), and placed into 15-ml centrifuge tubes (BD Falcon, Fisher Scientific) with a piece of moist cotton for 24 h at 4 °C. The leaves were then weighed again (Wh) and dried at 65 °C for 2 d so that the dry weight could be obtained (Wd). The RWC was then calculated as [(W0–Wd)/(Wh–Wd)] × 100. Leaf water potential (Ψ w) was determined using a pressure chamber (SF-PRES-35, SolFranc Technologies SL, Tarragona, Spain) on six mature leaves per treatment after gs had been determined. Leaves were excised from the main shoot under water and then pressurized in the chamber until xylem sap was visible at the cut surface (Boyer, 1967).
Root hydraulic conductivity (Lpr) was determined in the whole roots of six plants per treatment combination between 4–5 h after the beginning of the photoperiod within the growth chamber. Whole roots were detached from the shoot and connected to a high-pressure flow meter (HPFM, Dynamax, Inc., Houston, TX). Water was pressurized into the roots from 0–0.5 MPa in the transient mode to calculate root hydraulic conductance (Kr). The root volume was determined using the volume displacement method (Kamaluddin and Zwiazek, 2002), and Lpr was calculated by dividing Kr by the root volume (Calvo-Polanco et al., 2016). Although measurements of whole-root hydraulics have been used successfully to determine the water transport capacity of plants under stress (Calvo-Polanco et al., 2016), it should be noted that the technique could be improved by determining the conductivity of each part of the root system (Dodd et al., 2008) as they have been shown to differ from the whole-system conductivity in split-root experiments (Hu et al., 2011).
Quantification of transcript abundance of poplar PIPs and L. bicolor aquaporins
Expression of root and fungal aquaporins was determined in each of the compartments of the split-root set-up. After 10 d of drought treatment, the roots of each compartment were harvested, carefully washed, cut in pieces, mixed, and divided into 0.5-g samples that were then stored at –80 °C prior to analysis.
For the detection of poplar aquaporins, total RNA was isolated using the phenol/chloroform protocol as described by Calvo-Polanco et al. (2014). Expression was determined for 11 Populus PIP genes, using the primers described by Almeida-Rodriguez et al. (2011). The expression of the different aquaporins was determined using an iCycler RT-qPCR system (Bio-Rad). Each 23-µl reaction mixture contained 1 µl of cDNA (80 ng), 10.5 µl of Master Mix (Bio-Rad), 8.6 µl of deionized water, and 0.45 µl of each primer pair at a final concentration of 0.2 mM. The PCR program consisted of 3 min incubation at 95 °C, followed by 32 cycles of: 30 s at 95 °C, 30 s of annealing temperature of 58 °C, and 72 °C for 30 s. Elongation Factor‐1 alpha was used as the reference gene, as it was the most stable one in all the treatments (Almeida-Rodriguez et al., 2011). Three different root RNA samples for each treatment were used for analysis (i.e. n=3), with each of them repeated three times. Negative controls without cDNA were used in all the PCR reactions.
For the detection of L. bicolor aquaporins, total RNA was isolated following the CTAB-PVP protocol described by Chang et al. (1993). The expression levels of the L. bicolor aquaporins JQ585592 to JQ585597(Xu et al., 2015) were determined using a QuantStudio3 RT-qPCR system (Applied Biosystems) with the same reaction mixture as described above except for the use of PowerUp SYBR Green Master Mix (Applied Biosystems). The PCR program consisted of 2 min incubation at 50 °C plus another 2 min at 95 °C, followed by 40 cycles of: 15 s at 95 °C, 30 s of annealing temperature of 58 °C, and 72 °C for 1 min. The translation elongation factor EF2 was used as the reference gene as it was the most stable across all the samples, as proposed by Xu et al., (2015). For each aquaporin, three different root RNA samples for each treatment were used for the analysis, with each of them repeated three times. Negative controls without cDNA were used in all the PCR reactions.
Root enzyme-linked immunosorbent assays (ELISAs)
Microsomes for the immunoassay determination were isolated and proteins extracted as described by Calvo-Polanco et al. (2014). As primary antibodies we used two antibodies (at a dilution of 1:1000) that recognize several PIP1s and PIP2s and three antibodies that recognize the phosphorylation of PIP2 proteins at their C-terminal region at serine 280 (PIP2280), serine 283 (PIP2283), and at both serine 280 and 283 (PIP2280/283). Goat anti-rat IgG coupled with horseradish peroxidase (Sigma-Aldrich) was used as the secondary antibody for PIP1 (at 1:10 000). Goat anti-rabbit Ig coupled to horseradish peroxidase (Sigma-Aldrich) was used as a secondary antibody for PIP2 and PIP2280, PIP2283, and PIP2280/283 (at 1:10000). Protein quantification was done in three different independent root samples per treatment, repeated three times each.
Root contents of ABA, SA, JA, and CKs
The following hormones were analysed in roots with four replicates per compartment and treatment combination: ABA, SA, JA, and CKs trans- and cis-zeatin riboside (t-ZR and c-ZR), N6-(∆2-isopentenyl) adenine (iP), and isopentenyl adenosine (iPR). The analyses were done using an HPLC-ESI-MS/MS with a 2795 Alliance HT HPLC device (Waters) coupled to a 3200 Q TRAP LC/MS/MS system (Applied Biosystems/MDS Sciex, Ontario, Canada), equipped with an electrospray interface.
The extraction and purification of ABA and CKs was carried out following the procedure described by Bacaicoa et al. (2009), for JA following the procedure described by Sánchez-Romera et al. (2014), and for SA following the procedure described by Sánchez-Romera et al. (2016).
Statistical analysis
All data were analysed using the MIXED procedure in SAS (v. 9.2, SAS Institute Inc.). MANOVA was used for all the parameters except for the analyses of fungal aquaporins where we used ANOVA directly plus Tukey’s test. MANOVA was used in order to detect the main contributions of the two treatments (drought and fungi) to the different parameters studied. Where MANOVA indicated a significant effect, we conducted the following tests. (1) ANOVA and post-hoc Tukey’s test for the leaf RWC, gs, Ψ w, and whole-root Lpr. (2) ANOVA in the split-root system to test if there was a local or long-distance response according to how the treatments (well-watered/drought or non-inoculated/L. bicolor) were applied to each side of the compartments (one side or two sides). (3) Pearson correlations analyses with the use of the command ‘proc corr’ in SAS. (4) Student’s t-test to compare partial root drying versus total root drying, and partial root inoculation versus complete root inoculation with L. bicolor.
Results
Soil conditions and mycorrhizal colonization rates
Drought stress was applied to the selected compartments of the split-root system by leaving them without water for 10 d. The final mean water soil potential of the well-watered compartments was –0.19±0.01 MPa (±SE), while in the drought compartments the mean potential was –0.40±0.01 MPa (Supplementary Table S2 at JXB online). The presence of L. bicolor within the compartments did not result in any significant difference in the water potential compared with the non-inoculated compartments, either under well-watered or drought conditions.
Examination of colonization rates indicated the presence of mycorrhizal tips in 30–35% of the roots inoculated with L. bicolor, while there were none present in the non-inoculated compartments.
Seedling growth and physiological parameters
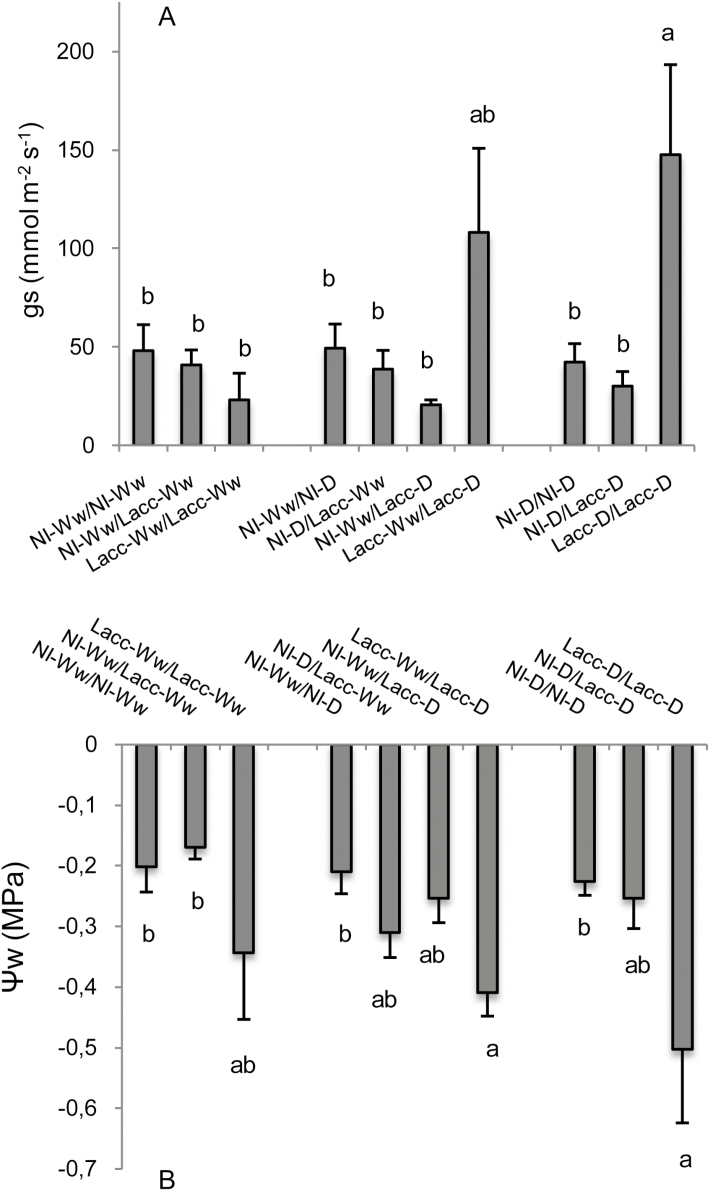
Although inoculation with L. bicolor to at least one compartment of the split-root system tended to increase biomass (Supplementary Table S1), the differences were not large enough to be detected as significant by the MANOVA analyses. Hence, there were no differences among the treatments in FW of roots (P=0.326), shoots (P=0.503), and leaf RWC (P=0.380). However, we were able to detect significant differences in leaf gs (P=0.003), leaf Ψ w (P=0.010), and in whole-root Lpr (P=0.019). The treatment Lacc-D/Lacc-D had the highest gs and Lpr, and the lowest Ψw (Figs 1, 2), while the NI-Ww/NI-D treatment had the lowest Lpr values and was among the treatments with the lowest gs.
Fig. 1.
Effects of drought and inoculation with L. bicolor on (A) leaf stomatal conductance (gs) and (B) leaf water potential (Ψ w) in poplar seedlings with roots divided between different treatments in a split-root system. The root compartments were either inoculated with L. bicolor (Lacc) or non-inoculated (NI) and either subjected to well-watered (Ww) or drought (D) conditions in different combinations. Data are means (±SE), n=8 Different letters indicate significant differences among the treatments as determined using ANOVA followed by Tukey’s test (α=0.05).
Fig. 2.
Effects of drought and inoculation with L. bicolor on whole-root hydraulic conductivity (Lpr) in poplar seedlings with roots divided between different treatments in a split-root system. The root compartments were either inoculated with L. bicolor (Lacc) or non-inoculated (NI) and either subjected to well-watered (Ww) or drought (D) conditions in different combinations. Data are means (±SE), n=6 Different letters indicate significant differences among the treatments as determined using ANOVA followed by Tukey’s test (α=0.05).
Plants with both compartments inoculated with L. bicolor (Lacc-Ww/Lacc-Ww, Lacc-Ww/Lacc-D, Lacc-D/Lacc-D) had a lower mean leaf Ψ w (–0.42±0.05 MPa) than plants with only one compartment inoculated (NI-Ww/Lacc-Ww, NI-D/Lacc-Ww, NI-Ww/Lacc-D) (–0.25±0.02 MPa; P=0.008), independently of water stress (Fig. 1B). There was a negative correlation between Ψ w and gs (r=–0.562, P<0.0001) that was unaffected by L. bicolor inoculation (Fig. 1), while Lpr was not correlated either with Ψ w (r=–0.141, P=0.28) or gs (r=0.145, P=0.27).
Expression of poplar PIPs in roots
The expression of root aquaporins was first tested using MANOVA in order to detect general effects related either to drought treatment, inoculation with L. bicolor, or the interaction between the two (Supplementary Table S3). When we found a significant effect using MANOVA, we tested for the individual effects of the presence of drought and L. bicolor in none, one, or both compartments on root aquaporin expression, abundance, and phosphorylation state. The following trends were observed.
Drought treatment resulted in up-regulation of the expression of PtPIP1;1 (P=0.0028), PtPIP1;2 (P=0.0001), PtPIP2;5 (P=0.0006), and PtPIP2;7 (P<0.0001) (Supplementary Table S3). This up-regulation occurred when either one or both of the compartments were subjected to drought (Table 2, Supplementary Fig. S1). The presence of L. bicolor in both compartments of the split-root system resulted in the down-regulation of PtPIP1;4 (P=0.0207), PtPIP2;3 (P=0.037), and PtPIP2;10 (P=0.017) (Supplementary Table S3). PtPIP2;10 was also down-regulated when L. bicolor was present in only one compartment (Table 3, Fig. S2).
Table 2.
Effects of drought treatments applied to the split-root system on aquaporin expression, abundance of PIP2, and phosphorylation state of PIP2 in poplar roots.
| Aquaporin | Drought treatment applied to split-roots | ||
|---|---|---|---|
| One side dry | Two sides dry | Two sides well-watered | |
| PtPIP1;1 | 0.85±0.08 A | 0.81±0.09 A | 0.46±0.10 B |
| PtPIP1;2 | 1.23±0.10 A | 1.46±0.12 A | 0.83±0.11 B |
| PtPIP1;4 | 0.07±0.02 A | 0.06±0.01 A | 0.04±0.01 A |
| PtPIP2;3 | 0.82±0.07 A | 0.89±0.08 A | 0.83±0.08 A |
| PtPIP2;4 | 0.02±0.01 A | 0.05±0.03 A | 0.02±0.01 A |
| PtPIP2;5 | 0.25±0.04 B | 0.47±0.04 A | 0.13±0.04 C |
| PtPIP2;7 | 0.21±0.03 A | 0.26±0.03 A | 0.09±0.03 B |
| PtPIP2;10 | 0.05±0.01 A | 0.06±0.02 A | 0.05±0.02 A |
| PIP2 | 0.004± 0.0003 AB | 0.005±0.0003 A | 0.004±0.0003 B |
| PIP2Ser-280 | 0.007±0.0002 A | 0.007±0.0003 A | 0.007±0.0002 A |
Expression of root aquaporins is in relative units using Elongation Factor‐1 alpha as the reference gene. Abundance of PIP2 and its phosphorylation state are expressed as µg µg–1 protein. Data are means (±SE), n=3 Different letters within the same line indicate significant differences as determined using MANOVA (α=0.05).
Table 3.
Effects of the presence of L. bicolor in the split-root system on aquaporin expression, abundance of PIP2, and phosphorylation state of PIP2 in poplar roots.
| Aquaporin | L. bicolor treatment applied to split-roots | ||
|---|---|---|---|
| No L. bicolor | One side L. bicolor | Two sides L. bicolor | |
| PtPIP1;1 | 0.68±0.06 A | 0.86±0.11 A | 0.61±0.07 A |
| PtPIP1;2 | 1.21±0.10 A | 1.29±0.11 A | 0.99±0.10 A |
| PtPIP1;4 | 0.07±0.01 A | 0.07±0.01 A | 0.02±0.01 B |
| PtPIP2;3 | 0.03±0.01 A | 0.02±0.01 AB | 0.01±0.01 B |
| PtPIP2;4 | 0.25±0.04 A | 0.22±0.03 A | 0.18±0.04 A |
| PtPIP2;5 | 0.26±0.04 A | 0.30±0.05 A | 0.26±0.04 A |
| PtPIP2;7 | 0.21±0.04 A | 0.25±0.03 A | 0.19±0.04 A |
| PtPIP2;10 | 0.08±0.02 A | 0.04±0.01 AB | 0.03±0.02 B |
| PIP2 | 0.005±0.0003 A | 0.004±0.0003 B | 0.004±0.0003 B |
| PIP2Ser-280 | 0.007±0.0002 A | 0.007±0.0003 A | 0.007±0.0003 A |
Expression of root aquaporins is in relative units using Elongation Factor‐1 alpha as the reference gene. Abundance of of PIP2 and its phosphorylation state are expressed as µg µg–1 protein. Data are means (±SE), n=3 Different letters within the same line indicate significant differences as determined using MANOVA (α=0.05).
None of the treatments had any effects on the expression of PtPIP1;5 (P=0.764), PtPIP2;1 (P=0.630), and PtPIP2;2 (P=0.733) (Supplementary Table S3).
The only treatment that induced an increase in Lpr, (Lacc-D/Lacc-D) was characterized by up-regulation of aquaporin expression in relation to drought (Table 2 , ‘two-sides dry’ compared with ‘two-sides well-watered’) and down-regulation of aquaporin expression and reduced PIP2 abundance in relation to L. bicolor (Table 3, ‘two-sides L. bicolor’ compared with ‘non-inoculated’).
Expression of L. bicolor aquaporin genes in poplar roots
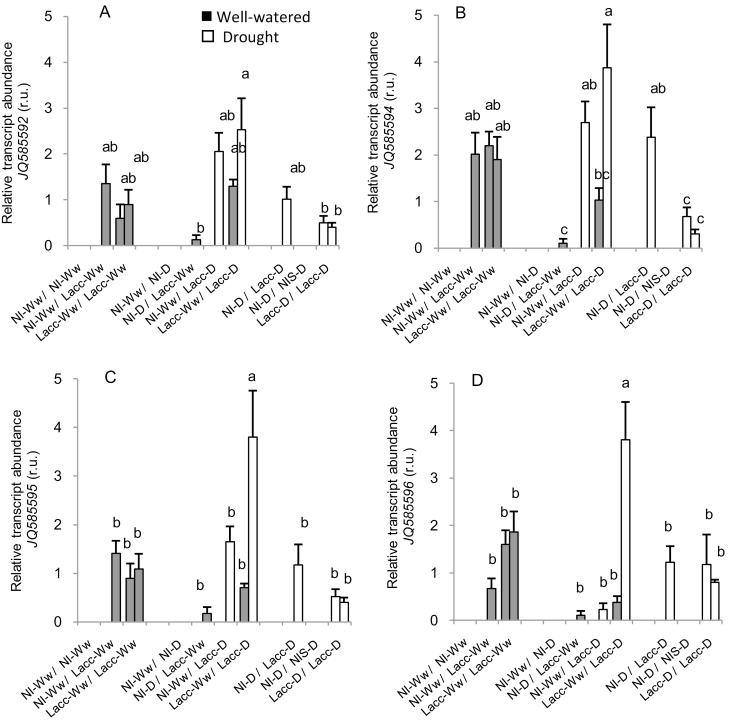
We examined the expression of six L. bicolor aquaporins in the roots of each compartment inoculated with the fungus in the split-root system. Expression was only detected for four of them, namely JQ585592, JQ585594, JQ585595, and JQ585596 (Fig. 3). Their expression did not follow a regular pattern and instead seemed to vary with the different water conditions applied to each of the compartments. The compartment under drought within the Lacc-Ww/Lacc-D treatment had the highest expression levels of JQ585595 and JQ585596 (Fig. 3C, D), and the treatment with the highest Lpr (Lacc-D/Lacc-D), had low expression.
Fig. 3.
Relative expression of fungal aquaporins in L. bicolor inoculated on roots of poplar seedlings divided between different treatments in a split-root system. The root compartments were either inoculated with L. bicolor (Lacc) or non-inoculated (NI) and either subjected to well-watered (Ww) or drought (D) conditions in different combinations. Data from well-watered and drought compartments are presented in grey and white, respectively. (A) JQ585592, (B) JQ585594, (C) JQ585595, and (D) JQ585596. Data are means (±SE), n=3 Different letters indicate significant differences among the treatments as determined using ANOVA followed by Tukey’s test (α=0.05).
Protein abundance and phosphorylation state of root aquaporins
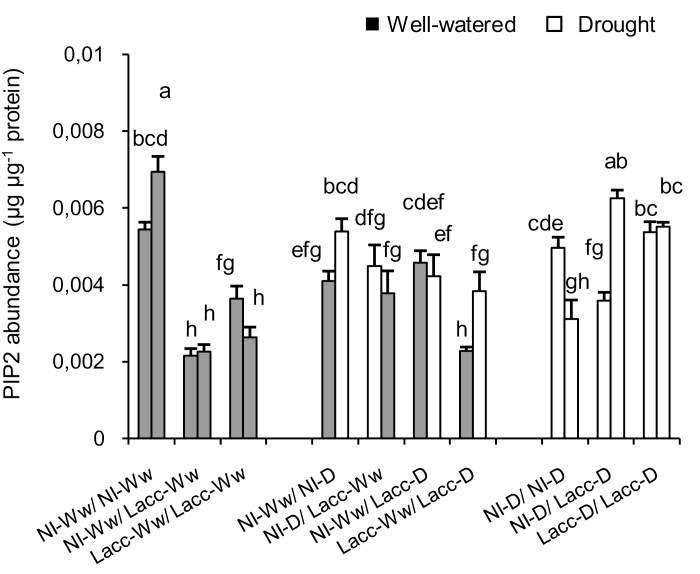
MANOVA showed that the abundance of PIP2 proteins was affected by the fungus × drought treatment interaction (P=0.0002; Supplementary Table S4, Fig. 4) and by the drought treatment (P=0.006), while the phosphorylation of PIP2280 was affected individually by the drought treatment (P=0.022) and the fungal treatment (P=0.022; Supplementary Fig. S3). PIP1, PIP2283, and PIP2280/283 were not affected by any of the treatments applied (Supplementary Table S4).
Fig. 4.
Effects of drought and inoculation with L. bicolor on the root abundance of PIP2 of poplar seedlings with roots divided between different treatments in a split-root system. The root compartments were either inoculated with L. bicolor (Lacc) or non-inoculated (NI) and either subjected to well-watered (Ww) or drought (D) conditions in different combinations. Data from well-watered and drought compartments are presented in grey and white, respectively. Data are means (±SE), n=3 Different letters indicate significant differences among the treatments as determined using ANOVA followed by Tukey’s test (α=0.05).
Following MANOVA, we examined whether the treatments applied to the different compartments followed the same general trends that were observed for aquaporin expression. We found only that the abundance of PIP2 increased when both of the compartments were under drought (Table 2, Fig. 4) and it decreased in the compartments inoculated with L. bicolor (Table 3, Fig. 4).
Root hormonal status
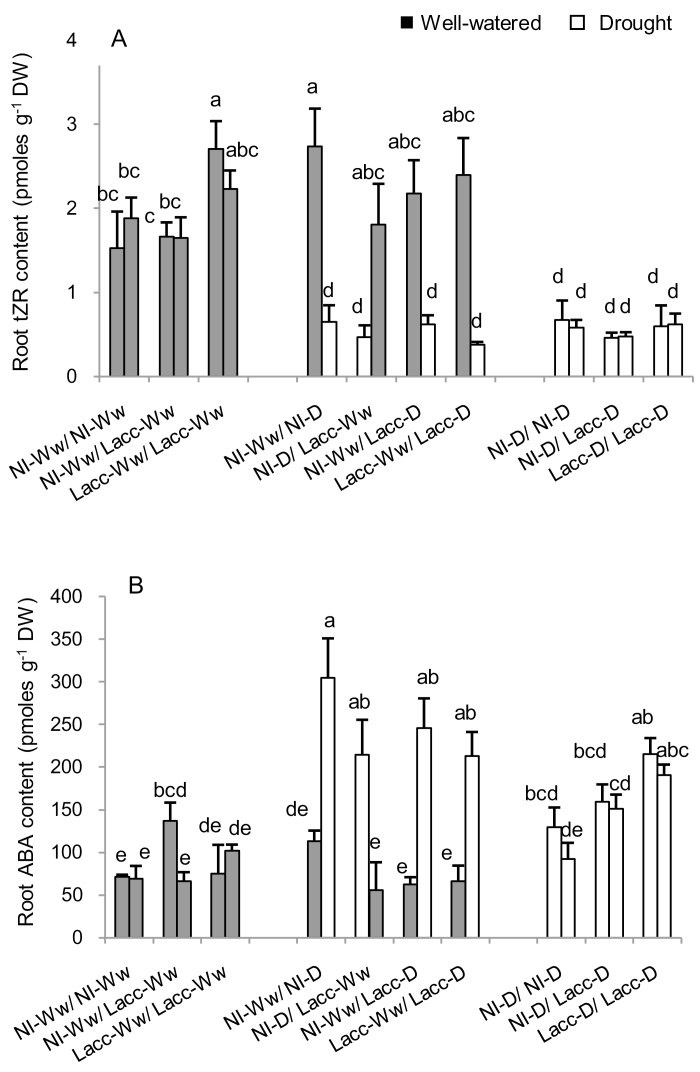
We analysed the contents of ABA, JA, SA, and various CKs in each of the compartments of the split-root system. MANOVA indicated that the drought treatment increased the root concentrations of ABA (P<0.0001), SA (P=0.014), and iPR (P=0.006), while it reduced tZR (P<0.0001; Supplementary Table S5). There was a significant fungus × drought interaction in the response of ABA (P=0.036), indicating a potential role of L. bicolor in the ABA concentrations of the roots.
We examine whether the presence of L. bicolor in the different compartments of the split-root system affected root hormone concentrations under different soil water conditions. Under drought stress, we could detect a general reduction of tZR and an increase of ABA concentrations that were independent of the presence of L. bicolor in the roots of the plants (Table 4, Fig. 5). Furthermore, ABA and tZR were negatively correlated (r=–0.37, P=0.001) (Supplementary Table S6).
Table 4.
Effects of drought treatments applied to the split-root system on hormone contents in poplar roots.
| Hormone | Drought treatment applied to split-roots | ||
|---|---|---|---|
| One side dry | Two sides dry | Two sides well-watered | |
| tZR | 0.27±0.03 B | 0.13±0.03 C | 0.36±0.03 A |
| iPR | 0.91±0.06 A | 0.84±0.07 A | 0.96±0.07 A |
| cZR | 0.37±0.04 A | 0.38±0.04 A | 0.37±0.04 A |
| ABA | 33.81±4.75 A | 40.26±5.20 A | 17.11±5.48 B |
| SA | 5648.6±681.3 A | 4875.4±746.3 A | 3834.1±786.7 A |
Data are means (±SE), n=4 (pmoles g–1 FW). Different letters within the same line indicate significant differences as determined using MANOVA (α=0.05).
Fig. 5.
Effects of drought and inoculation with L. bicolor on the root contents of (A) tZR (cytokinin) and (B) ABA of poplar seedlings with roots divided between different treatments in a split-root system. The root compartments were either inoculated with L. bicolor (Lacc) or non-inoculated (NI) and either subjected to well-watered (Ww) or drought (D) conditions in different combinations. Data from well-watered and drought compartments are presented in grey and white, respectively. Data are means (±SE), n=4. Different letters indicate significant differences among the treatments as determined using ANOVA followed by Tukey’s test (α=0.05).
We conducted Pearson correlation analyses with the root hormone contents, the hydraulic conductivity, and aquaporin expression, abundance, and phosphorylation state. We found that ABA was positively correlated with the fungal aquaporins JQ585595 (r=0.34, P=0.009) and JQ585596 (r=0.29, P=0.026), and that aquaporin JQ585596 was negatively correlated with PtPIP1;4 (r=–0.26, P=0.047) (Supplementary Table S7). In addition, tZR was positively correlated with PtPIP1;4 (r=0.41, P<0.001) and negatively correlated with PtPIP2;7 (r=–0.20, P=0.032) (Supplementary Table S6).
Discussion
We used a split-root system to study local and long-distance responses of ectomycorrhizal poplar trees to drought. The mild drought that we imposed allowed us to examine the role of L. bicolor in the regulation of root aquaporin expression and hormonal abundance in relation to rebalancing the root hydraulics and whole-plant water status. The results showed that homeostasis of root hydraulic conductivity, Lpr, was achieved without drastically affecting plant growth, stomatal conductance, leaf RWC and water potentials (Fig. 1, Supplementary Table S1).Drought commonly affects the stomatal opening and water balance in the aerial parts of plants, most likely driven by long-distance ABA signaling (Dodd, 2005), although the responses are species-dependent and will be affected by the intensity of the drought (Silim et al., 2009).
The mild drought that we applied to the different root compartments induced a local accumulation of ABA and a reduction of tZR content that were independent of L. bicolor inoculation if only half the root system was inoculated (Fig. 5, Table 4), together with an up-regulation of the plant aquaporins PtPIP1;1, PtPIP1;2, PtPIP2;5, and PtPIP2;7 (Table 2). PIP2;5, and PIP2;7 have been shown to have high water transport capacity in Xenopus laevis oocyte swelling assays (Marjanović et al., 2005; Secchi and Zwieniecki, 2010), and PIP2;5 has been shown to have a key role in water transport under water stress when overexpressed in poplar plants (Ranganathan et al., 2017). PIP1 aquaporins are channels with low water transport capacity (Marjanović et al., 2005; Almeida-Rodriguez et al., 2011), although it has been demonstrated that they are quite responsive to drought (Mahdieh et al., 2008). In addition, the functionality of PIP1 aquaporins in cell membranes is commonly related to their interaction with PIP2 isoforms (Fetter et al., 2004). The overexpression of aquaporins that we observed under mild drought (Table 2, Supplementary Fig. S1) would indicate a combined response of these aquaporins for the control of water uptake under drought that seems not to be directly regulated by hormone concentrations, as only PtPIP2;7 was negatively correlated with tZR abundance, and none of the aquaporins was correlated with ABA (Supplementary Table S6).
ABA is one of the main factors affecting the responses of plants to drought as it regulates protective responses by regulating water uptake (Aroca et al., 2003) and by interacting with several other hormones (Peleg and Blumwald, 2011; Zwack and Rashotte, 2015). The effects of ABA on Lpr have been reported as either being positive (Veselov et al., 2016) or negative (Beaudette et al., 2007), although it is well establish that ABA is a key regulator of aquaporin genes (Jang et al., 2004; Wan et al., 2004; Zhu et al., 2005; Ruiz-Lozano et al., 2009). In our experiments, ABA abundance was not correlated with any of the plant aquaporins (Supplementary Table S6), but it was correlated with the fungal aquaporins JQ585595 and JQ585596 (Supplementary Table S7). These are known to have high water-transport capacities (Xu et al., 2015) and JQ585595 is essential for the formation of mycorrhizas in poplar seedlings (Navarro-Ródenas et al., 2015). The levels of ABA were similar in all the drought-treated root compartments (Fig. 5B); however, plants under partial drought (NI-Ww/NI-D, NI-D/Lacc-Ww, NI-Ww/Lacc-D, LaccWw-Lacc-D) had a higher mean ABA content than those under total drought (NI-D/NI-D, NI-D/Lacc-D, Lacc-D/Lacc-D) (244.1±22.6 pmol g-1 DW versus 150.3±11.8 pmol g-1 DW, P=0.006). In addition, among the plants subjected to total drought, those with both compartments inoculated with L. bicolor (Lacc-D/Lacc-D) had a higher ABA content (203.8±9.8 pmol g-1 DW) than those with only one compartment inoculated (NI-D/Lacc-D) (155.1±11.7 pmol g-1 DW, P=0.011), or with both compartments non-inoculated (NI-D/NI-D) (106.2±15.1 pmol g-1 DW, P<0.001). This may indicate that plants have different ABA-related mechanisms to cope with drought when inoculated with mycorrhizal fungi and when partial versus total drying is present within the soil. The other hormone that was responsive to drought was the cytokinin tZR, which had lower abundance in the compartments where drought was applied (Fig. 5A). Cytokinins are known for their critical roles in many biological mechanisms in plants (Wybouw and De Rybel, 2019), although their response to water stress is limited to decreases in their abundance (Bano et al., 1993; Goicoechea et al., 1996). In our study, the reduction in abundance of tZR under drought was positively correlated with the plant aquaporin PtPIP1;4 and negatively correlated with PtPIP2;7 (Supplementary Table S6). As noted above, PtPIP2;7 is known for its high water-transport capacity, while PtPIP1:4 has been shown to have high expression in root tips and in the cells of the inner cortex, and is related to the water movement from the cortex to the stele (Almeida-Rodriguez et al., 2011). How tZR interacts with PtPIP1;4 and PtPIP2;7 under drought stress remains to be determined, as not only their expression but also their localization at the root level and their post-translational regulation will be key elements that determine their functions in water transport.
The presence of ectomycorrhizal fungi in the different compartments of the split-root system added another level of complexity to the system. We found negative effects of the presence of L. bicolor on the expression of PtPIP1;4, PtPIP2;3, and PtPIP2;10 both under well-watered and drought conditions (Table 3, Supplementary Fig. S2). Xu et al. (2016) found the same trend in the same poplar and L. bicolor system, where the presence of this specific fungi induced a reduction of the expression of all plant aquaporins under well-watered conditions. The down-regulation of aquaporins has been related to the formation of suberin at the endodermal apoplastic barriers in order to control water transport (Wang et al. 2019), although more studies will be needed to understand the role of L. bicolor in this respect. We found a negative correlation between the fungal aquaporin JQ585596 and the poplar aquaporin PtPIP1;4 (Supplementary Table S7). Negative correlations among fungal and plant aquaporins have been previously shown for other fungus–host systems (Aroca et al., 2009), and they could be integrated into the signaling networks between the two organisms to orchestrate water uptake and the different responses to environmental conditions. What seems to be clear is that different fungal and plant aquaporins form part of a complex system that needs to be coordinated at both the plant and the fungal levels and, at least in the case of poplar, does not seem to be totally controlled by the fungal partner (in this case L. bicolor).
Our experiments resulted in only one treatment where Lpr increased, which was when both compartments were under drought and inoculated with L. bicolor (Lacc-D/Lacc-D). In this case, the system presented high ABA and reduced tZR contents (Fig. 5), together with an up-regulation of aquaporins associated with the compartments subjected to drought (Table 2) and a down-regulation of aquaporins associated with L. bicolor (Table 3). These observations were quite puzzling and raise the question as to whether the presence of mycorrhizal fungi is necessary at the whole-root level in order to have a faster response to drought or, on the other hand, whether the resources that the fungi demand are so high that the plant is more reactive to external stresses.
In conclusion, our results demonstrate how poplar saplings regulate their water balance by different compensatory mechanisms at the root level in response to different combinations of water stress and inoculation with L. color. Under mild drought, ABA concentrations increase and tZR concentrations decrease, whilst the expression of the plant aquaporins PtPIP1;1, PtPIP2;2, and PtPIP2;7 is up-regulated. In the presence of L. bicolor, expression of PtPIP1;4, PtPIP2;3, and PtPIP2;10 is down-regulated. When the whole root system is colonized with L. bicolor and is under drought stress, Lpr is increased. How these mechanisms interact and are coordinated in order to maintain water uptake under drought seems to be related to ABA accumulation and coordinated with the overexpression of the fungal aquaporins QJ585595 and QJ585595, while the reduced accumulation of tZR interacts with the plant aquaporins PtPIP1;4 and PtPIP2;7.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Leaf and shoot fresh weights, leaf relative water contents, and root fresh weights in the various treatments.
Table S2. Soil water potentials in the different compartments of the split-root system.
Table S3. MANOVA main factor P-values for the expression of root aquaporins in poplar.
Table S4. MANOVA main factor P-values for root PIP1 and PIP2 abundance and PIP2 phosphorylation state in poplar.
Table S5. MANOVA main factor P-values for root hormone contents in poplar.
Table S6. Pearson correlation matrix among root hydraulic conductivity, aquaporin expression, abundance, and phosphorylation state, and ABA and tZR contents in poplar.
Table S7. Pearson correlations among fungal aquaporin and root aquaporin expression. Abundance, and phosphorylation state, and root ABA and tZR contents in ectomycorrhizal poplar.
Fig. S1. Root aquaporin mRNA expression of PtPIP1;1, PtPIP1;2, PtPIP2;5, and PtPIP2;7 in response to drought in poplar split-roots.
Fig. S2. Root aquaporin mRNA expression of PtPIP1;4, PtPIP2;3, PtPIP2;5, and PtPIP2;10 in response to the presence of L. bicolor in poplar split-roots.
Fig. S3. Root PIP2 phosphorylated state at serine 280 (PIP2280) in poplar in the different split-root treatments.
Acknowledgements
This work was supported by the Ministry of Economy and Competitiveness of Spain (Juan de la Cierva Program and AGL2011-25403 project) and Junta de Andalucía (P10-CVI-5920 project). We would like to thank Sonia Molina Arias for her help in extraction and analysis of the aquaporins of L. bicolor, and Dr Sabine Zimmerman for editing and correction of the manuscript.
References
- Allen CD, Macalady AK, Chenchouni H, et al. . 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management 259, 660–684. [Google Scholar]
- Almeida-Rodriguez AM, Hacke UG, Laur J. 2011. Influence of evaporative demand on aquaporin expression and root hydraulics of hybrid poplar. Plant, Cell & Environment 34, 1318–1331. [DOI] [PubMed] [Google Scholar]
- Aroca R, Bago A, Sutka M, Paz JA, Cano C, Amodeo G, Ruiz-Lozano JM. 2009. Expression analysis of the first arbuscular mycorrhizal fungi aquaporin described reveals concerted gene expression between salt-stressed and nonstressed mycelium. Molecular Plant-Microbe Interactions 22, 1169–1178. [DOI] [PubMed] [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, Pardossi A. 2003. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Science 165, 671–679. [Google Scholar]
- Aroca R, Vernieri P, Ruiz-Lozano JM. 2008. Mycorrhizal and non-mycorrhizal Lactuca sativa plants exhibit contrasting responses to exogenous ABA during drought stress and recovery. Journal of Experimental Botany 59, 2029–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacaicoa E, Zamarreño AM, Leménager D, Baigorri R, García-Mina JM. 2009. Relationship between the hormonal balance and the regulation of iron deficiency stress responses in cucumber (Cucumis sativus L.) plants. Journal of the American Society for Horticultural Science 134, 589–601. [Google Scholar]
- Bano A, Dörfling K, Bettin D, Hahn H. 1993. Abscisic acid and cytokinins as possible root-to-shoot signals in xylem sap of rice plants in drying soil. Australian Journal of Plant Physiology 20, 109–115. [Google Scholar]
- Beaudette PC, Chlup M, Yee J, Emery RJ. 2007. Relationships of root conductivity and aquaporin gene expression in Pisum sativum: diurnal patterns and the response to HgCl2 and ABA. Journal of Experimental Botany 58, 1291–1300. [DOI] [PubMed] [Google Scholar]
- Boyer JS. 1967. Leaf water potentials measured with a pressure chamber. Plant Physiology 42, 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N. 1996. Working with mycorrhizas in forestry and agriculture. Canberra, Australia: Australian Centre for International Agricultural Research. [Google Scholar]
- Calvo-Polanco M, Jones MD, Zwiazek JJ. 2009. Effects of pH on NaCl tolerance of American elm (Ulmus americana) seedlings inoculated with Hebeloma crustuliniforme and Laccaria bicolor. Acta Physiogia Plantarum 31, 515–522. [Google Scholar]
- Calvo-Polanco M, Molina S, Zamarreño AM, García-Mina JM, Aroca R. 2014. The symbiosis with the arbuscular mycorrhizal fungus Rhizophagus irregularis drives root water transport in flooded tomato plants. Plant & Cell Physiology 55, 1017–1029. [DOI] [PubMed] [Google Scholar]
- Calvo-Polanco M, Sánchez-Castro I, Cantos M, García JL, Azcón R, Ruiz-Lozano JM, Beuzón CR, Aroca R. 2016. Effects of different arbuscular mycorrhizal fungal backgrounds and soils on olive plants growth and water relation properties under well-watered and drought conditions. Plant, Cell & Environment 39, 2498–2514. [DOI] [PubMed] [Google Scholar]
- Calvo-Polanco M, Voicu MC, Zwiazek JJ. 2008. Responses of ectomycorrhizal American elm (Ulmus americana) seedlings to salinity and soil compaction. Plant and Soil 308, 189–200. [Google Scholar]
- Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T. 2019. Cytokinin action in response to abiotic and biotic stresses in plants. Plant, Cell & Environment 42, 998–1018. [DOI] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees. Plant Molecular Biology Reporter 11, 113–116. [Google Scholar]
- Cowan IR. 1977. Stomatal behaviour and environment. Advances in Botanical Research 4, 117–228. [Google Scholar]
- de Ollas C, Arbona V, Gómez-Cadenas A. 2015. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant, Cell & Environment 38, 2157–2170. [DOI] [PubMed] [Google Scholar]
- Dodd I. 2005. Root-to-shoot signalling: assessing the roles of ‘up’ in the up and down world of long-distance signalling in plant. In: Lambers H, Colmer TD. eds. Root physiology: from gene to function. Plant ecophysiology, vol. 4. Dordrecht: Springer, 251–270. [Google Scholar]
- Dodd IC, Egea G, Davies WJ. 2008. Accounting for sap flow from different parts of the root system improves the prediction of xylem ABA concentration in plants grown with heterogeneous soil moisture. Journal of Experimental Botany 59, 4083–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. 1972. Mineral nutrition of plants: principles and perspectives. New York: John Wiley and Sons, Inc. [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. 2004. Interactions between plasma membrane aquaporins modulate their water channel activity. The Plant Cell 16, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goicoechea N, Antolin MC, Strnad M, Sánchez-Díaz M. 1996. Root cytokinins, acid phosphatase and nodule activity in drought-stressed mycorrhizal or nitrogen-fixing alfalfa plants. Journal of Experimental Botany 47, 683–686. [Google Scholar]
- Hogg EH, Brandt JP, Michaelian M. 2008. Impacts of a regional drought on the productivity, dieback and biomass of western Canadian aspen forests. Canadian Journal of Forest Research 38, 1373–1384. [Google Scholar]
- Hu T, Kang S, Li F, Zhang J. 2011. Effects of partial root-zone irrigation on hydraulic conductivity in the soil–root system of maize plants. Journal of Experimental Botany 62, 4163–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JY, Kim DG, Kim YO, Kim JS, Kang H. 2004. An expression analysis of a gene family encoding plasma membrane aquaporins in response to abiotic stresses in Arabidopsis thaliana. Plant Molecular Biology 54, 713–725. [DOI] [PubMed] [Google Scholar]
- Kamaluddin M, Zwiazek JJ. 2002. Ethylene enhances water transport in hypoxic aspen. Plant Physiology 128, 962–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoyarova G, Veselova S, Hartung W, Farhutdinov R, Veselov D, Sharipova G. 2011. Involvement of root ABA and hydraulic conductivity in the control of water relations in wheat plants exposed to increased evaporative demand. Planta 233, 87–94. [DOI] [PubMed] [Google Scholar]
- Lee SH, Calvo-Polanco M, Chung GC, Zwiazek JJ. 2010. Role of aquaporins in root water transport of ectomycorrhizal jack pine (Pinus banksiana) seedlings exposed to NaCl and fluoride. Plant, Cell & Environment 33, 769–780. [DOI] [PubMed] [Google Scholar]
- Lehto T, Zwiazek JJ. 2011. Ectomycorrhizas and water relations of trees: a review. Mycorrhiza 21, 71–90. [DOI] [PubMed] [Google Scholar]
- Lesk C, Rowhani P, Ramankutty N. 2016. Influence of extreme weather disasters on global crop production. Nature 529, 84–87. [DOI] [PubMed] [Google Scholar]
- Liu CC, Liu YG, Guo K, Zheng YR, Li GQ, Yu LF, Yang R. 2010. Influence of drought intensity on the response of six woody karst species subjected to successive cycles of drought and rewatering. Physiologia Plantarum 139, 39–54. [DOI] [PubMed] [Google Scholar]
- Luo ZB, Janz D, Jiang X, Göbel C, Wildhagen H, Tan Y, Rennenberg H, Feussner I, Polle A. 2009. Upgrading root physiology for stress tolerance by ectomycorrhizas: insights from metabolite and transcriptional profiling into reprogramming for stress anticipation. Plant Physiology 151, 1902–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdieh M, Mostajeran A, Horie T, Katsuhara M. 2008. Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum plants. Plant & Cell Physiology 49, 801–813. [DOI] [PubMed] [Google Scholar]
- Marjanović Ž, Uehlein N, Kaldenhoff R, Zwiazek JJ, Weiss M, Hampp R, Nehls U. 2005. Aquaporins in poplar: what a difference a symbiont makes! Planta 222, 258–268. [DOI] [PubMed] [Google Scholar]
- Miura K, Tada Y. 2014. Regulation of water, salinity, and cold stress responses by salicylic acid. Frontiers in Plant Science 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S, Müller M. 2013. Hormonal cross-talk in plant development and stress responses. Frontiers in Plant Science 4, 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Ródenas A, Bárzana G, Nicolás E, Carra A, Schubert A, Morte A. 2013. Expression analysis of aquaporins from desert truffle mycorrhizal symbiosis reveals a fine-tuned regulation under drought. Molecular Plant-Microbe Interactions 26, 1068–1078. [DOI] [PubMed] [Google Scholar]
- Navarro‐Ródenas A, Xu H, Kemppainen M, Pardo AG, Zwiazek JJ. 2015. Laccaria bicolor aquaporin LbAQP1 is required for Hartig net development in trembling aspen (Populus tremuloides). Plant, Cell and Environment 38, 2475–2486. [DOI] [PubMed] [Google Scholar]
- Nehls U, Göhringer F, Wittulsky S, Dietz S. 2010. Fungal carbohydrate support in the ectomycorrhizal symbiosis: a review. Plant Biology 12, 292–301. [DOI] [PubMed] [Google Scholar]
- Parent B, Hachez C, Redondo E, Simonneau T, Chaumont F, Tardieu F. 2009. Drought and abscisic acid effects on aquaporin content translate into changes in hydraulic conductivity and leaf growth rate: a trans-scale approach. Plant Physiology 149, 2000–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z, Blumwald E. 2011. Hormone balance and abiotic stress tolerance in crop plants. Current Opinion in Plant Biology 14, 290–295. [DOI] [PubMed] [Google Scholar]
- Perrone I, Gambino G, Chitarra W, et al. . 2012. The grapevine root-specific aquaporin VvPIP2;4N controls root hydraulic conductance and leaf gas exchange under well-watered conditions but not under water stress. Plant Physiology 160, 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM. 2011. Photosynthesis and drought: can we make metabolic connections from available data? Journal of Experimental Botany 62, 869–882. [DOI] [PubMed] [Google Scholar]
- Quiroga G, Erice G, Aroca R, Zamarreño AM, García-Mina JM, Ruiz-Lozano JM. 2018. Arbuscular mycorrhizal symbiosis and salicylic acid regulate aquaporins and root hydraulic properties in maize plants subjected to drought. Agricultural Water Management 202, 271–284. [Google Scholar]
- Ranganathan K, Cooke JE, El Kayal W, Equiza MA, Vaziriyeganeh M, Zwiazek JJ. 2017. Over-expression of PIP2;5 aquaporin alleviates gas exchange and growth inhibition in poplars exposed to mild osmotic stress with polyethylene glycol. Acta Physiologiae Plantarum 39, 187. [Google Scholar]
- Rincón A, Priha O, Lelu-Walter MA, Bonnet M, Sotta B, Le Tacon F. 2005. Shoot water status and ABA responses of transgenic hybrid larch Larix kempferi × L. decidua to ectomycorrhizal fungi and osmotic stress. Tree Physiology 25, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Rousseau JVD, Reid CPP. 1990. Effects of phosphorus and ectomycorrhizas on the carbon balance of loblolly pine seedlings. Forest Science 36, 101–112. [Google Scholar]
- Ruiz-Lozano JM, Alguacil MM, Barzana G, Vernieri P, Aroca R. 2009. Exogenous ABA accentuates the differences in root hydraulic properties between mycorrhizal and nonmycorrhizal maize plants through regulation of PIP aquaporins. Plant Molecular Biology 70, 565–579. [DOI] [PubMed] [Google Scholar]
- Sánchez-Romera B, Calvo-Polanco M, Ruíz-Lozano JM, Zamarreño AM, Arbona V, García-Mina JM, Gómez-Cadenas A, Aroca R. 2018. Involvement of the def-1 mutation in the response of tomato plants to arbuscular mycorrhizal symbiosis under well-watered and drought conditions. Plant Cell Physiology 59, 248–261. [DOI] [PubMed] [Google Scholar]
- Sánchez-Romera B, Ruiz-Lozano JM, Li G, Luu DT, Martínez-Ballesta Mdel C, Carvajal M, Zamarreño AM, García-Mina JM, Maurel C, Aroca R. 2014. Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant, Cell & Environment 37, 995–1008. [DOI] [PubMed] [Google Scholar]
- Sánchez-Romera B, Ruiz-Lozano JM, Zamarreño AM, García-Mina JM, Aroca R. 2016. Arbuscular mycorrhizal symbiosis and methyl jasmonate avoid the inhibition of root hydraulic conductivity caused by drought. Mycorrhiza 26, 111–122. [DOI] [PubMed] [Google Scholar]
- Secchi F, Zwieniecki MA. 2010. Patterns of PIP gene expression in Populus trichocarpa during recovery from xylem embolism suggest a major role for the PIP1 aquaporin subfamily as moderators of refilling process. Plant, Cell and Environment 33, 1285–1297. [DOI] [PubMed] [Google Scholar]
- Silim S, Nash R, Reynard D, White B, Schroeder W. 2009. Leaf gas exchange and water potential responses to drought in nine poplar (Populus spp.) clones with contrasting drought tolerance. Trees 23, 959–969. [Google Scholar]
- Steudle E, Peterson CA. 1998. How does water get through roots? Journal of Experimental Botany 49, 775–788. [Google Scholar]
- Thompson AJ, Andrews J, Mulholland BJ, McKee JMT, Hilton HW, Horridge JS, Taylor IB. 2007. Overproduction of abscisic acid in tomato increases transpiration efficiency and root hydraulic conductivity and influences leaf expansion. Plant Physiology 143, 1905–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veselov DS, Sharipova GV, Veselov SY, Dodd IC, Ivanov I, Kudoyarova GR. 2016. Rapid changes in root HvPIP2;2 aquaporins abundance and ABA concentration are required to enhance root hydraulic conductivity and maintain leaf water potential in response to increased evaporative demand. Functional Plant Biology 45, 143–149. [DOI] [PubMed] [Google Scholar]
- Wan X, Steudle E, Hartung W. 2004. Gating of water channels (aquaporins) in cortical cells of young corn roots by mechanical stimuli (pressure pulses): effects of ABA and of HgCl2. Journal of Experimental Botany 55, 411–422. [DOI] [PubMed] [Google Scholar]
- Wang P, Calvo-Polanco M, Reyt G, et al. . 2019. Surveillance of cell wall diffusion barrier integrity modulates water and solute transport in plants. Scientific Reports 9, 4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson S, Davies WJ. 2010. Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell & Environment 33, 510–525. [DOI] [PubMed] [Google Scholar]
- Wolters H, Jürgens G. 2009. Survival of the flexible: hormonal growth control and adaptation in plant development. Nature Reviews Genetics 10, 305–317. [DOI] [PubMed] [Google Scholar]
- Wybouw B, De Rybel B. 2019. Cytokinin – a developing story. Trends in Plant Science 24, 177–185. [DOI] [PubMed] [Google Scholar]
- Xu H, Cooke JEK, Kemppainen M, Pardo AG, Zwiazek JJ. 2016. Hydraulic conductivity and aquaporin transcription in roots of trembling aspen (Populus tremuloides) seedlings colonized by Laccaria bicolor. Mycorrhiza 26, 441–451. [DOI] [PubMed] [Google Scholar]
- Xu H, Kemppainen M, El Kayal W, Lee SH, Pardo AG, Cooke JE, Zwiazek JJ. 2015. Overexpression of Laccaria bicolor aquaporin JQ585595 alters root water transport properties in ectomycorrhizal white spruce (Picea glauca) seedlings. New Phytologist 205, 757–770. [DOI] [PubMed] [Google Scholar]
- Yi H, Calvo-Polanco M, MacKinnon MD, Zwiazek JJ. 2008. Responses of ectomycorrhizal Populus tremuloides and Betula papyrifera seedlings to salinity. Environmental and Experimental Botany 62, 357–363. [Google Scholar]
- Zargar SM, Nagar P, Deshmukh R, Nazir M, Wani AA, Masoodi KZ, Agrawal GK, Rakwal R. 2017. Aquaporins as potential drought tolerance inducing proteins: towards instigating stress tolerance. Journal of Proteomics, 169, 233–238. [DOI] [PubMed] [Google Scholar]
- Zhu C, Schraut D, Hartung W, Schäffner AR. 2005. Differential responses of maize MIP genes to salt stress and ABA. Journal of Experimental Botany 56, 2971–2981. [DOI] [PubMed] [Google Scholar]
- Zwack PJ, Rashotte AM. 2015. Interactions between cytokinin signalling and abiotic stress responses. Journal of Experimental Botany 66, 4863–4871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.