Exposure to Pb2+ increases NRT1.1-mediated uptake of nitrate in Arabidopsis and is associated with decreased uptake of Pb into the plant, which is a consequence of decreased acidification in the rhizosphere.
Keywords: Lead, nitrate, nitrate transporters, Pb resistance, pH, phytoremediation
Abstract
Identification of the mechanisms that control lead (Pb) concentration in plants is a prerequisite for minimizing dietary uptake of Pb from contaminated crops. This study examines how nitrate uptake by roots affects Pb uptake and reveals a new resistance strategy for plants to cope with Pb contamination. We investigated the interaction between nitrate transporter (NRT)-mediated NO3– uptake and exposure to Pb in Arabidopsis using NRT-related mutants. Exposure to Pb specifically stimulated NRT1.1-mediated nitrate uptake. Loss of function of NRT1.1 in nrt1.1-knockout mutants resulted in greater Pb toxicity and higher Pb accumulation in nitrate-sufficient growth medium, whereas no difference was seen between wild-type plants and null-mutants for NRT1.2, NRT2.1, NRT2.2, NRT2.4, and NRT2.5. These results indicate that only NRT1.1-mediated NO3− uptake alleviated Pb toxicity in the plants. Further examination indicated that rhizosphere acidification, which favors Pb entry to roots by increasing its availability, is prevented when NRT1.1 is functional and both NO3– and NH4+ are present in the medium.
Introduction
Consumption of lead (Pb) can result in a variety of health-related ailments ranging from learning disabilities to cardiovascular disease (Chiodo et al., 2007; Navas-Acien et al., 2008). Crops can take up Pb from the soil and, under certain conditions, high levels can accumulate in the edible parts of crops (Jin et al., 2005a, 2005b). The contamination of soil by Pb has continuously increased over recent decades as a result of various anthropogenic activities, such as waste disposal and the use of sewage water for irrigation (Clemens, 2006; Pourrut et al., 2011). Consequently, minimizing Pb in soil, particularly in an agricultural context, is a subject of increasing concern for researchers across a number of different scientific disciplines. Using plant-based technology to remove Pb from contaminated soils (phytoremediation) has gained a great deal of attention because of its low costs and environmental friendliness (Tangahu et al., 2011; Selamat et al., 2014). However, there are few natural hyperaccumulator plants for Pb and most of those that have been reported grow slowly and have small biomass, which limits their efficiency in cleaning contaminated soil (Ernst, 2000; van der Ent et al., 2013). Using a biological engineering approach to increase Pb accumulation in non-food crops with rapid growth and large biomass may be an alternative strategy to hyperaccumulating plants. A clear understanding of the factors that affect Pb uptake by plants is a prerequisite for ensuring that such a strategy is successful.
The amount of Pb taken up by plant roots is closely associated with its bioavailability in the growth medium (Kopittke et al., 2008; Uzu et al., 2009). The bioavailability of Pb in soil is affected by numerous factors including the cation exchange capacity (CEC), texture, redox potential, clay mineralogy, organic content, and pH of the soil, and also by the levels of other elements that are present (i.e. phosphorus and sulfur) (Jin et al., 2005b). Among these factors, soil acidity has been recognized as one of the most important (Fischer et al., 2014), because as pH decreases, the solubility of Pb increases in the form of free or solvated ions and ion pairs. Nutrient management, particularly nitrogen fertilization, significantly affects the pH of soil (Thomson et al., 1993). Nitrogen is taken up by plants mainly in the form of ammonium (NH4+) and nitrates (NO3−). Physiologically, when NO3− is taken up by plants, there is a simultaneous uptake of protons (H+) resulting in an increase in the pH of the rhizosphere. Conversely, when NH4+ is taken up, H+ is released into the rhizosphere, resulting in a decrease in the pH (Marschner, 1995). In this context, the uptake of NO3− by roots may decrease the amount of Pb2+ acquired by plants by consumption of rhizospheric H+, which thus lowers the level of soluble Pb2+, whereas the opposite may be true for uptake of NH4+.
Owing to the fact that Pb is a non-essential element for plants, its entry into root cells may rely on transporters/channels for various bivalent nutrient cations (Pourrut et al., 2011). In addition to H+, our previous studies have shown that decreased uptake of the anion NO3− by roots is accompanied by decreased uptake of several cations, including Ca2+, K+, Fe2+, and Cd2+ (Luo et al., 2012; Mao et al., 2014), indicating that the uptake of NO3− may be non-specifically coupled with the uptake of cations. Hence, the uptake of nitrate may favor the uptake of Pb2+. However, this is in contrast with the effect noted above that nitrate uptake may decrease Pb bioavailability in the rhizosphere. Thus, it is not clear how NO3− uptake by roots affects Pb2+ uptake. The pathways of NO3– uptake by plant roots are complex. It has been reported that six NO3– transporters (NRTs) are involved in uptake in Arabidopsis (Wang et al., 2012, 2018; Léran et al., 2014). NRT1.1 is one of the most common NRTs with dual-affinity, being involved in both high- and low-affinity uptake (Liu et al., 1999; Ye et al., 2019), NRT1.2 is a low-affinity transporter (Huang et al., 1999), whilst NRT2.1, NRT2.2, NRT2.4, and NRT2.5 only take part in high-affinity uptake (Cerezo et al., 2001; Li et al., 2007; Kiba et al., 2012; Lezhneva et al., 2014). These NRTs often show different responses to various stressors (Krapp et al., 2011; Mao et al., 2014; Fang et al., 2016). Given the presumed association between NO3− and Pb2+ uptake, it is clearly important to determine whether the activities of the different NRTs affect the uptake of Pb.
In the present study, we investigated the interaction between NRT-mediated NO3– uptake and Pb2+ exposure in Arabidopsis. Our results revealed a new mechanism by which the plants responded to Pb2+ stress: exposure to Pb specifically induced NRT1.1-mediated NO3– uptake, which consequently prevented acidification of the rhizosphere and thus decreased entry of Pb into the plants by decreasing its bioavailability. Our findings may provide a new strategy for manipulating Pb levels in plants, such as improving the efficiency of removal of Pb from contaminated soils and minimizing the accumulation of Pb in food crops.
Materials and methods
Plant material
The following Arabidopsis thaliana plants were used in this study: the mutants chl1-5 (Huang et al., 1996), nrt1.1-1 (salk_097431), nrt1.2 (cs859605), nrt2.1 (salk_141712), nrt2.2 (salk_043543), nrt2.5 (GK 213H10), nlp7-2 (cs868891), and chl1-9, the double-mutant nrt2.1 nrt2.2 (cs859604), the triple-mutants abi1 hab1 abi2 and abi1 hab1 pp2ca, together with pNRT1.1::NRT1.1-GFP transgenic plants with a Columbia (Col-0) background, and also the mutants chl1-6 (cs6154) and nrt2.4 (cs27332) with a Landsberg erecta (Ler) background. Seeds of chl1-5 and the pNRT1.1::NRT1.1-GFP transgenic plants were obtained from Dr Philippe Nacry (Biochimie et Physiologie Moléculaire des Plantes, Montpellier, France), seeds of chl1-9 were obtained from Dr Yi-Fang Tsay (Institute of Molecular Biology, Academia Sinica, Taiwan), and seeds of the abi1 hab1 abi2 and abi1 hab1 pp2ca triple-mutants were obtained from Dr Jian-Kang Zhu (Purdue University, USA). The insertions in these lines were verified using the primers listed in Supplementary Table S1 at JXB online.
Cultivation conditions
The experiments were performed on plants grown on agar medium in sterile square Petri dishes (10 × 10 cm). Briefly, the seeds were surface-sterilized and sown on basal agar medium containing 1% sucrose (w/v). The nutrient composition of the basal agar medium had a NO3−/NH4+ ratio of 3:1 and was as follows: 750 μM NaH2PO4, 500 μM MgSO4, 375 μM K2SO4, 2.25 mM KNO3, 375 μM (NH4)2SO4, 1 mM CaCl2, 10 μM H3BO3, 0.5 μM MnSO4, 0.5 μM ZnSO4, 0.1 μM CuSO4, 0.1 μM (NH4)6Mo7O24, and 25 μM Fe-EDTA. Because nrt1.1 mutants are hypersensitive to low pH (Fang et al., 2016), the agar medium was adjusted to pH 6.5. For treatment with Pb, 4-d-old seedlings were transferred to Petri dishes with fresh basal medium with or without 300 μM lead acetate, Pb(CH3COO)2. Each dish contained 15 seedlings. To examine the effects of N source on the response to Pb2+, we used basal medium adjusted to contain different NO3−:NH4+ ratios. The total N concentration fixed at 3.0 mM and the ratio was adjusted using KNO3 and (NH4)2SO4. The resulting differences in K concentrations were balanced by adjusting the K2SO4 concentration.
Measurement of Pb2+ concentrations
After 7 d of Pb2+ treatment, the seedlings were washed with pure water, divided into shoots and roots, and dried at 70 °C for 48 h. The dried samples were then wet-digested as previously described (Guan et al., 2018), and diluted with ultrapure water. The concentrations of lead were analysed using a Microwave Plasma-Atomic Emission Spectrometer (4210 MP-AES, Agilent Technologies). Four biological replicates per treatment were analysed, each of which consisted of root or shoot tissues from 90 seedlings.
GFP expression analysis
Seedlings of the pNRT1.1::NRT1.1-GFP (green fluorescent protein) transgenic plants were cultured on complete nutrient agar medium with or without 300 μM Pb(CH3COO)2 for 3 d. The distribution and intensity of the green fluorescence in their roots were then observed under a microscope (Eclipse NI; Nikon) and imaged using a camera attached to the microscope. Four replicate seedlings per treatment were analysed.
Measurement of NO3– uptake
The net fluxes of NO3– were measured along the root axis in the meristematic, elongation, and maturation zones using a non-invasive microelectrode ion flux measurement system (SIET IPA-2, Applicable Electronics, Inc., Forestdale, MA, USA) , which can directly record transmembrane ion influx and efflux in a non-contact manner by detecting the diffusion potentials outside of the membrane (Shabala and Newman, 1997). Seedlings were pre-cultured in complete nutrient medium with or without 300 μM Pb(CH3COO)2 for 3 d, and were then transferred to 5-cm diameter plates with a nutrient solution containing 750 μM NaH2PO4, 500 μM MgSO4, 375 μM K2SO4, 2.25 mM KNO3, 375 μM (NH4)2SO4, 1000 μM CaCl2, and 0.05% MES buffer (w/v), with a pH of 5.8. using NMT The NO3− fluxes of the roots were measured The flux of NO3– in each section of the roots was then measured for 30 min using an electrode as described by Hawkins et al. (2008). Six replicate seedlings per treatment were analysed.
Measurement of relative expression of NRT genes
Root samples of ~100 mg (FW) were collected and frozen in liquid nitrogen. Total RNA was extracted using RNAisoPlus (Takara) and was then used to synthesize first-strand cDNA using a PrimeScript RT reagent kit (Takara). The cDNA was mixed with an SYBR Green RT-PCR kit (Takara) and the corresponding primers in a 25-μl reaction system; the primers are listed in Supplementary Table S1. All quantitative reverse-transcription (qRT-)PCR analyses were performed in an Opticon 2 Real-Time PCR System (MJ Research, MA, USA). Relative transcript levels were determined and corrected with efficiency calculations as described in Fang et al. (2016), and normalizeding to the geometric mean of expression of UBQ10 and EF1α for each sample (Vandesompele et al., 2002). Four biological replicates per treatment were analysed, each of which consisted of 75–90 seedlings.
Determination of lipid peroxidation
We measured malondialdehyde (MDA), a product of lipid peroxidation, according to the method described by Zhang et al. (2016). Samples of ~0.1 g of root or shoot tissue were frozen in liquid nitrogen and homogenized in 1.5 ml of 10% trichloroacetic acid (TCA) at 4 °C. After centrifugation at 15 000 g for 10 min, the supernatant was collected and incubated with 1 ml of 0.6% thiobarbituric acid (TBA) (dissolved in 10% TCA) at 100 °C for 20 min. The absorbance was measured at 440, 532, and 600 nm using an ELISA (SpectraMax 13X, Melceemlar Devices, USA). A total of 4–5 biological replicates per treatment were analysed, each of which consisted of 45–90 seedlings.
Determination of pH in the agar rooting medium
The pH of the agar rooting medium was determined based on the method described by Hachiya et al. (2012). The rooting medium was collected into a 50-ml centrifuge tube, which was frozen at –20 °C overnight and thawed at room temperature to free the aqueous phase from the agar. The mixture was then filtered at room temperature and the pH of the supernatant was determined using a desktop pH electrode (Sartorius). Measurements were taken on agar from five replicate plates per treatment, each of which contained 15 seedlings.
Results
Pb2+ stress stimulates NO3– uptake by roots
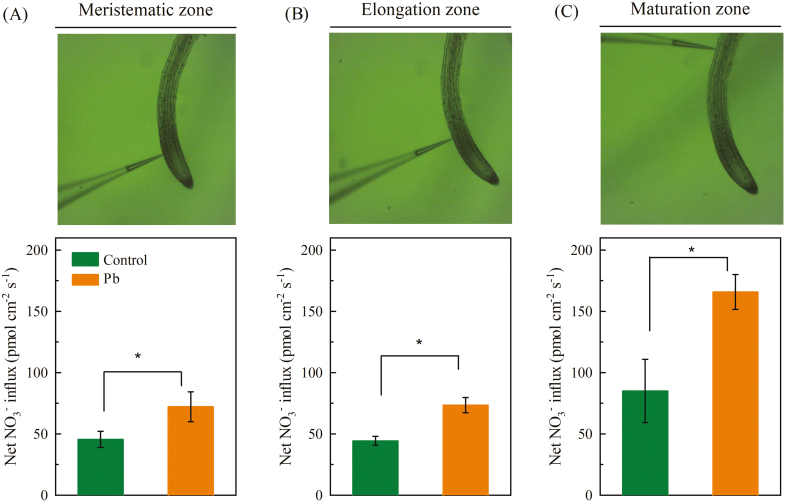
Abiotic stress often negatively affects NO3− uptake by the roots (Mao et al., 2014; Bai et al., 2017). In the present study, we first evaluated the effect of Pb2+ exposure on NO3− uptake in the roots of Arabidopsis Columbia-0 (Col-0) using a non-invasive technique. The transmembrane NO3− fluxes of the roots were measured along the root axis in the meristematic, elongation, and maturation zones. Interestingly, although Pb2+ is a toxic metal for plants, 3 d of exposure to 300 μM Pb2+ in the agar medium increased the rate of net NO3− influx in all zones (Fig. 1). The net NO3− influx in the mature root zone was greater than that in the other two zones, and exposure to Pb2+ resulted in about twice the uptake of NO3− compared to the controls.
Fig. 1.
Effects of Pb2+ stress on net NO3− influx in the roots of Arabidopsis Col-0 plants. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and net NO3− influx was measured after 3 d using a non-invasive microelectrode ion flux measurement system. Measurements were taken in the meristematic (A), elongation (B), and maturation zones (C) of the roots. Data are means (±SE) of six biological replicates. Significant differences were determined using two-tailed Student’s t-tests: *P<0.05. (This figure is available in colour at JXB online.)
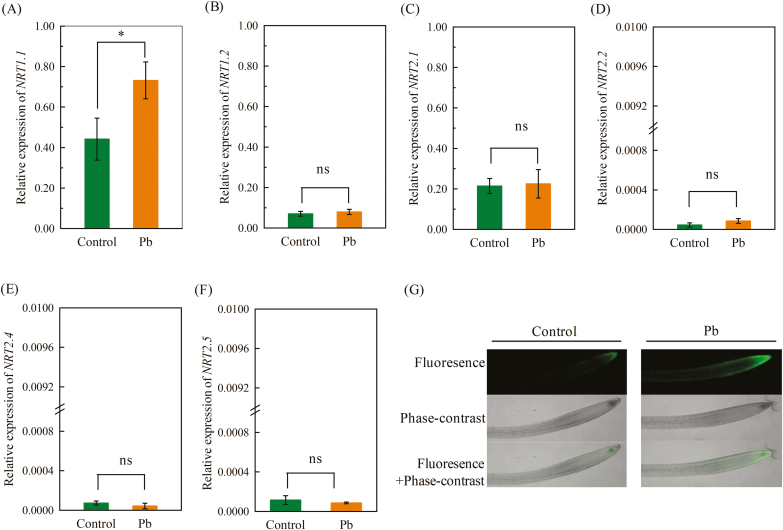
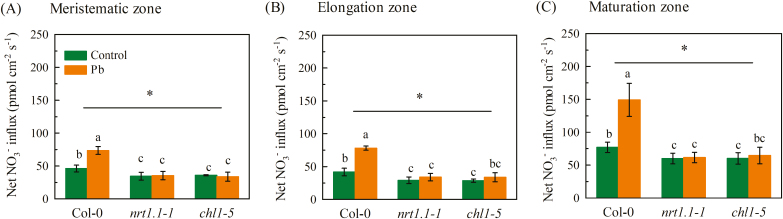
To investigate the molecular basis underlying the stimulation of root NO3− uptake in response to Pb2+ exposure, we examined the expression of six NRT genes that are involved in NO3− uptake by roots. We found that only the expression of NRT1.1 was significantly increased after 3 d of 300 μM Pb2+ exposure (Fig. 2A) whilst the expression of the other five genes, NRT1.2, NRT2.1, NRT2.2, NRT2.4, and NRT2.5 were not significantly affected (Fig. 2B–E). We then examined the time-course of NRT1.1 expression in response to exposure to Pb2+ and found that there was limited induction during the first 12 h of exposure, but after that it increased significantly (Supplementary Fig. S1). These results indicated that the stimulation of NO3− uptake by Pb2+ may have been mediated by NRT1.1. Consistent with this, GFP fluorescence was observed in the roots of pNRT1.1::NRT1.1-GFP transgenic plants (which are in the chl1-5 background, an NRT1.1-null mutant) and indicated that exposure to Pb2+ caused a clear increase in NRT1.1-GFP protein levels (Fig. 2F). We then compared the rate of net NO3− influx by the roots of wild-type Col-0 plants and two NRT1.1-null mutants, chl1-5 and nrt1.1-1. Exposure to Pb2+ had no effect on influx in the nrt1.1 mutant in any of the three root zones that we examined (Fig. 3), which was in contrast with the results for the Col-0 plants. These results demonstrated that induction of NRT1.1 activity was responsible for the increase in root NO3− uptake in the presence of exposure to Pb2+.
Fig. 2.
Effects of Pb2+ stress on the expression of (A) NRT1.1, (B) NRT1.2, (C) NRT2.1, (D) NRT2.2, (E) NRT2.4, and (F) NRT2.5 in the roots of Arabidopsis Col-0 plants, and (G) the localization of expression of NRT1.1-GFP in pNRT1.1::NRT1.1-GFP transgenic plants. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and measurements were taken after 3 d. Relative expression levels were normalized to the geometric mean expression of UBQ10 and EF1α. Data are means (±SE) of four biological replicates. Significant differences were determined using two-tailed Student’s t-tests: *P<0.05; ns, non-significant. (This figure is available in colour at JXB online.)
Fig. 3.
Effects of Pb2+ stress on net NO3− influx in different root sections of Arabidopsis wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and net NO3− flux was measured after 3 d using a non-invasive microelectrode ion flux measurement system (Fig. 1). Measurements were taken in the meristematic (A), elongation (B), and maturation zones. Data are means (±SE) of four biological replicates. Different letters indicate significant differences between means as determined using two-way ANOVA followed by Tukey’s multiple comparisons test (P<0.05). Significant interactions between Pb treatment and genotype are indicated by an asterisk (*P<0.05). (This figure is available in colour at JXB online.)
Pb2+ resistance in Arabidopsis is specifically associated with NRT1.1
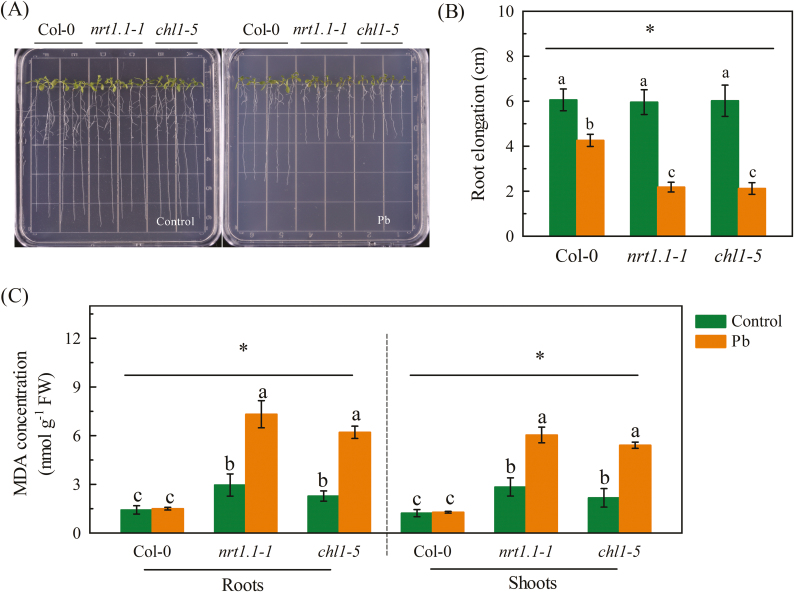
We investigated the association between NRTs and Pb resistance in Arabidopsis. Because NRT1.1 appeared likely to be responsible for the stimulation of NO3− uptake by Pb2+, we first examined the effects of its loss of function on resistance to Pb stress. After 7 d of exposure to 300 μM Pb2+, the inhibition of root growth in the NRT1.1-null mutants chl1-5, nrt1.1-1, and chl1-6, was greater than that in their corresponding wild-type plants (Fig. 4A, B, Supplementary Fig. S2). In addition, reductions in root elongation over time in response to exposure to Pb2+ were greater in the NRT1.1-null mutants compared to the wild-types (Supplementary Fig. S3) and there was a significant interaction between NRT1.1 function and exposure to Pb2+ (P<0.05). These results indicated that NRT1.1 is required for Pb2+ resistance, and further evidence for this was provided by Measurements of root growth in pNRT1.1::NRT1.1-GFP transgenic plants, which showed that the inhibition of root elongation in the NRT1.1-null mutants could be rectified by complementation with NRT1.1 (Supplementary Fig. S4). Lipid peroxidation is often used as an indicator of stress effects and therefore we measured the amounts of MDA (a product of this process; Hodges et al., 1999) in the roots and shoots. After 7 d of exposure to 300 μM Pb2+, the MDA levels in both the roots and shoots of the chl1-5 and nrt1.1-1 mutants were greatly increased, whereas there was no significant effect in the Col-0 plants (Fig. 4C). This provided further support for NRT1.1 being required for Pb2+ resistance in Arabidopsis.
Fig. 4.
Effects of Pb2+ stress on Arabidopsis wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and measurements were taken after 7 d. (A) Representative images of plants, (B) root elongation, and (C) concentrations of malondialdehyde (MDA, a product of lipid peroxidation) in the shoots and roots. Data are means (±SE) of 4–5 biological replicates. Different letters indicate significant differences between means as determined using two-way ANOVA followed by Tukey’s multiple comparisons test (P<0.05). Significant interactions between Pb treatment and genotype are indicated by an asterisk (*P<0.05). (This figure is available in colour at JXB online.)
NRT1.1 also functions as a NO3– sensor, and this is independent of its uptake activity (Ho et al., 2009; Bouguyon et al., 2015). We therefore examined Pb2+ resistance in the chl1-9 mutant, which is defective in terms of NO3– uptake but has a normal NO3– sensing function (Ho et al., 2009). We found that chl1-9 had a similar Pb2+ resistance phenotype in terms of root growth to the other nrt1.1-knockout mutants (Supplementary Fig. S5), indicating that NO3− uptake rather than sensing was the likely mechanism behind NRT1.1-conferred Pb2+ resistance. We also checked the Pb2+ resistance of the nlp7-knockout mutant, which shares common features with the nrt1.1-knockout mutant in terms of loss of many NO3– sensing functions, but it retains normal root NO3– uptake activity (Castaings et al., 2009; Marchive et al., 2013). As expected, the Pb2+ resistance of nlp7 was similar to that of the wild-type plants (Supplementary Fig. S5). These results provided further support for NO3−-uptake activity rather than NO3−-sensing activity being involved in NRT1.1-mediated Pb2+ resistance.
We then examined associations between Pb toxicity and other NRTs and found that the nrt1.2, nrt2.1, nrt2.2, nrt2.4, and nrt2.5 mutants all had similar root elongation to their corresponding wild-types (Supplementary Fig. S6). Owing to the fact that NRT2.1, NRT2.2, NRT2.4, and NRT2.5 are high-affinity NO3− uptake transporters, we also evaluated the effects of Pb2+ exposure on root growth in the corresponding NRT-null mutants under growth conditions with 0.2 mM NO3−. We found that root elongation in nrt2.1, nrt2.2, nrt2.4, and nrt2.5 was similar to that of the corresponding wild-type plants (Supplementary Fig. S7). We also examined Pb sensitivity in the nrt2.1 nrt2.2 double-mutant, which has only 20–40% of the high-affinity NO3− uptake of the wild-type (Li et al., 2007; Lezhneva et al., 2014). Again, we found that this double-mutant also had similar root elongation compared to the wild-type in growth medium containing 0.2 mM NO3− (Supplementary Fig. S8). These results suggested that NRT1.2, NRT2.1, NRT2.2, NRT2.4, and NRT2.5 are not involved in Pb2+ resistance in Arabidopsis, or at least they do not act like NRT1.1 in conferring resistance.
The level of Pb accumulated in Arabidopsis is specifically controlled by NRT1.1
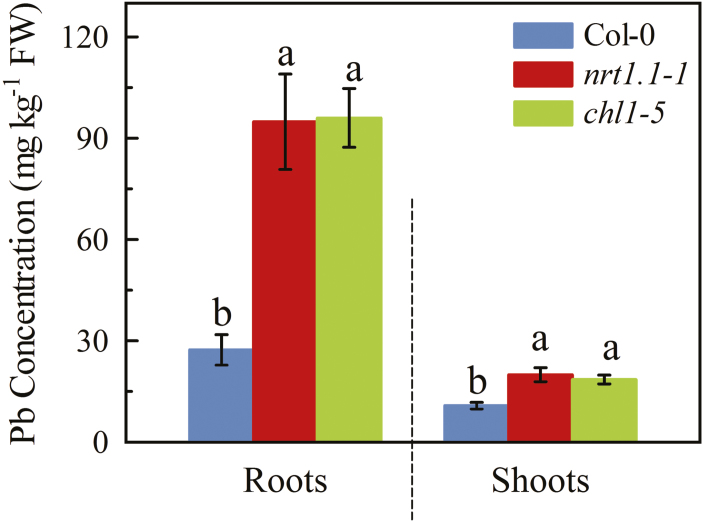
We investigated how NRT1.1 affected the levels of Pb accumulated in plants. After 7 d of exposure to 300 μM Pb2+, the concentrations in the roots of the chl1-5 and nrt1.1-1 mutants were ~3-fold higher than that in the Col-0 plants (Fig. 5). In addition, the mutants also had ~80% higher concentrations in their shoots. Similar differences was also found between the chl1-6 mutant and the Ler wild-type (Supplementary Fig. S9). The concentrations in the pNRT1.1::NRT1.1-GFP transgenic plants demonstrated that the increase in Pb levels in the NRT1.1-null mutants could be rectified by complementation with NRT1.1 (Supplementary Fig. S10). These results indicated that NRT1.1 negatively regulated the Pb concentrations in the plant tissues, providing further evidence that NRT1.1 is required for resistance to Pb2+ exposure in Arabidopsis. It is worth noting that in all the plant lines examined, the Pb concentrations in the shoots were much lower than that in the roots. This was probably because Pb has extremely low mobility in plants (Pourrut et al., 2011), and hence most will remain in the root tissues after being absorbed from the growth medium.
Fig. 5.
Pb concentrations in Arabidopsis wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants exposed to Pb2+ stress. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and measurements were taken after 7 d. Data are means (±SE) of four biological replicates. Different letters indicate significant differences between means as determined using two-way ANOVA followed by Tukey’s multiple comparisons test (P<0.05). (This figure is available in colour at JXB online.)
We also examined whether the other NRTs had a role in affecting the accumulation of Pb, and found that the nrt1.2, nrt2.1, nrt2.2, nrt2.4, and nrt2.5 mutants had similar Pb concentrations in their roots and shoots as their corresponding wild-types when grown in both sufficient (2.25 mM) or low-NO3− (0.2 mM) conditions (Supplementary Fig. S11). in addition, the nrt2.1 nrt2.2 double-mutant also had similar Pb concentrations to the Col-0 plants when grown with 0.2 mM NO3− (Supplementary Fig. S8). Thus, the NRT1.2, NRT2.1, NRT2.2, NRT2.4, and NRT2.5 transports do not play a role in affecting the Pb in Arabidopsis, leading us to conclude that NRT1.1 negatively regulates the Pb in a relatively specific manner.
NRT1.1-mediated reduction of Pb2+ uptake is associated with increased pH in the rhizosphere
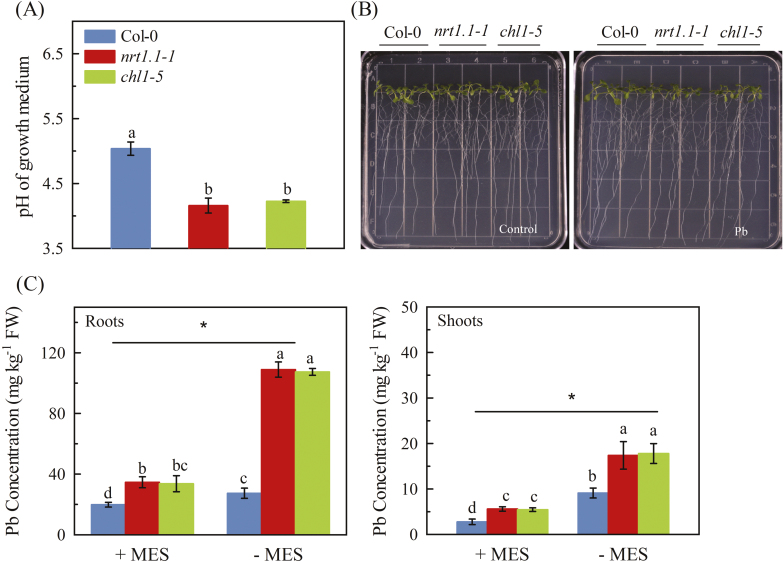
As noted in the Introduction, uptake of NO3− may non-specifically couple with the uptake of cations and thus favor uptake of Pb2+, whilst the simultaneous uptake of H+ will increase the pH of the rhizosphere and thus reduce the bioavailability of Pb2+. Given that we observed a decrease in uptake of Pb2+, we measured the pH in the agar growth medium, which was initially set to 6.5. After 7 d of treatment with 300 μM Pb2+, the pH in the media of the NRT1.1-knockout mutants decreased to ~4.0, whilst the pH in the Col-0 medium was ~5.0 (Fig. 6A).Computer modelling using GEOCHEM-PC (Parker et al., 1995) indicated that the activity of Pb2+ in our growth medium was sharply reduced when the pH increased (Supplementary Fig. S12). It is therefore likely that the higher concentrations of Pb accumulated in the nrt1.1-knockout mutants probably resulted from the increased Pb2+ availability that occurred because of the lower pH in the rhizosphere.
Fig. 6.
Effects of pH on Pb2+ toxicity in Arabidopsis wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and with or without the pH buffer MES, and measurements were taken after 7 d. The initial pH of the growth medium was 6.5. (A) The pH of the growth medium with Pb2+ and without pH-buffer. (B) Representative images of plants grown with pH buffer and with or without Pb2+. (C) Pb concentrations in roots and shoots of plants grown with Pb2+ and with or without pH buffer. Data are means (±SE) of 4–5 biological replicates. Different letters indicate significant differences between means as determined using either one- or two-way ANOVA (depending on whether one or two different variables were considered) followed by Tukey’s multiple comparisons test (P<0.05). Significant interactions between Pb treatment and genotype are indicated by an asterisk (*P<0.05). (This figure is available in colour at JXB online.)
We then used MES to buffer the pH in the growth media. After 7 d of Pb2+ treatment, the presence of 0.05% MES (w/v) substantially reduced the differences in pH between the Col-0 rooting medium and those of the two nrt1.1 mutants (Supplementary Fig. S13). This was associated with significantly reduced concentrations of Pb in both the roots and shoots of the mutants (Fig. 6C), suggesting that there was an interaction between NRT1.1 and the pH of the rhizosphere in the uptake of Pb. Thus, the results provided direct evidence that the negative effect of NRT1.1 on Pb uptake was associated with pH-determined Pb availability in the rhizosphere. Given that the toxicity of Pb is closely associated with the concentration that is accumulated in plant tissues (Pourrut et al., 2011), we evaluated the effect of NRT1.1 on plant resistance to exposure in the presence of MES. As expected, the MES treatment almost completely removed the Pb2+-related inhibition of root growth in the nrt1.1 mutants (Fig. 6B). Taken together, the results indicated that upon exposure to Pb2+, up-regulation of NRT1.1 in the roots reduced acidification of the rhizosphere and hence reduced Pb2+ uptake, thus creating a mechanism whereby the plants increased their resistance to Pb2+ toxicity.
An efficient contribution of NRT1.1 to Pb2+ resistance requires the co-supply of NO3– and NH4+
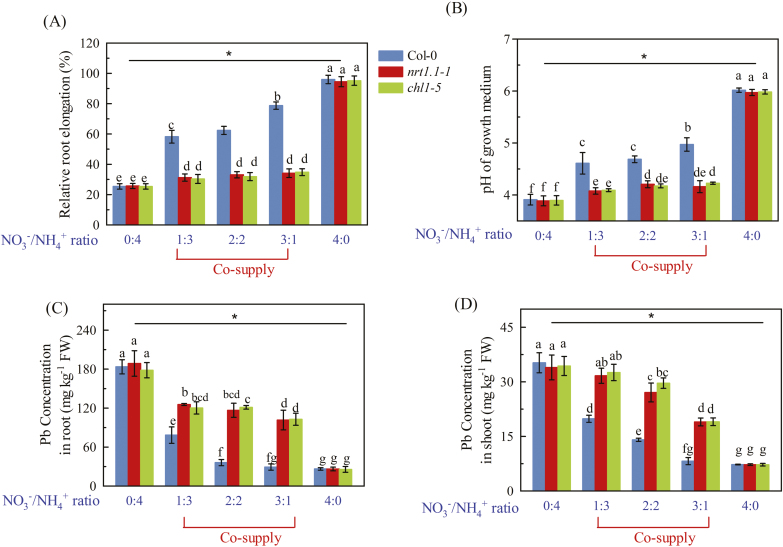
As the resistance to entry of Pb2+ into the plants associated with NRT1.1 depended on alterations in the pH of the rhizosphere, we wanted to assess how this was affected by the form in which nitrogen was supplied. We therefore fed Col-0, nrt1.1-1, and chl1-5 plants with different ratios of NO3– to NH4+ (N:A ratio). When NH4+ was supplied as the sole N source, the root growth of Col-0 plants was greatly inhibited by exposure to Pb2+, and they had similar root elongation to the nrt1.1 mutants (Fig. 7A). This was associated with equally strong acidification of the rhizosphere (Fig. 7B), which would have resulted in high Pb2+ availability (Supplementary Fig. S12). When NO3– was supplied together with NH4+, the root growth of the Col-0 plants improved progressively as the N:A ratio increased, whilst in the nrt1.1 mutants there was only an initial small increase in growth at an N:A ratio of 1:3. The variations in root growth were associated with corresponding changes in the pH of the growth media (Fig. 7B). When NO3– was supplied as the sole N source, exposure to Pb2+ had little effect on the root growth of any of any of the plants and this was associated with high pH in the growth media. These results indicated that an efficient contribution of NRT1.1 to Pb2+ resistance in Arabidopsis required the co-supply of NO3– and NH4+. We then measured the Pb concentrations in the plants and found that in growth media with either NH4+ or NO3− supplied as the sole N source there were no differences between Col-0 and the mutants in either the roots or shoots (Fig. 7C, D). However, when NH4+ and NO3− were co-supplied, both the mutants had higher Pb concentrations in their roots and shoots compared with the Col-0 plants, and this correlated with the differences in pH in the growth media and with the root growth phenotypes. These results further indicated that the presence of both NO3– and NH4+ in the growth medium enhances the contribution of NRT1.1 to Pb2+ resistance in Arabidopsis.
Fig. 7.
Interactions between the effects of Pb2+ stress and the ratio of NO3– to NH4+ supplied to Arabidopsis wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants. Seedlings at 4 d old were transferred to medium with or without 300 μM Pb(CH3COO)2 and containing different ratios of NO3– to NH4+, and measurements were taken after 7 d. (A) Root elongation, calculated as elongation with Pb relative to elongation of the same genotype without Pb under the same NO3−/NH4+ ratio. (B) pH of the growth medium, and Pb concentrations in (C) the roots and (D) the shoots. Data are means (±SE) of four biological replicates. Different letters indicate significant differences between means as determined using two-way ANOVA followed by Tukey’s multiple comparisons test (P<0.05). Significant interactions between NO3−/NH4+ ratio and genotype are indicated by an asterisk (*P<0.05). (This figure is available in colour at JXB online.)
We also examined the effects of Pb2+ exposure on the expression of NRT1.1 and on the influx of NO3− into roots in response to different N:A ratios. In Col-0, both the expression of NRT1.1 and the influx of NO3− were similar between control and Pb2+-treated plants when either NH4+ or NO3− was supplied as the sole N source, but both were up-regulated by exposure to Pb2+ when NH4+ and NO3− were co-supplied (Supplementary Figs S14, S15).Furthermore, two-way ANOVA of the root NO3− influx data confirmed that the NRT1.1 transporter was required for the up-regulation of NO3− influx that resulted from exposure to Pb2+ in the growth media containing both NH4+ and NO3−.These results suggested that co-supply of NH4+ and NO3− ensured the induction of NRT1.1-mediated NO3− uptake in response to exposure to Pb2+.
Discussion
The removal of heavy metals from contaminated soils and minimizing their accumulation in food crops are two important challenges that span scientific disciplines (Alexander et al., 2006; Tangahu et al., 2011). Identification of the mechanisms that control the entry of these pollutants into plant tissues is essential if we wish to address these challenges by using biotechnology to engineer modifications of relevant metabolic pathways. In this study, we have demonstrated the existence of a mechanism by which Arabidopsis prevents uptake of Pb2+ by its roots, namely that exposure to Pb2+ stimulates NRT1.1-mediated uptake of NO3− and this results in removal of H+ from the rhizosphere, which in turn lowers the bioavailability of Pb2+, thus reducing its uptake into the plant.
Pb2+ can cause damage to plants at both the physiological and molecular levels (Pourrut et al., 2011). The presence of high levels of Pb2+ can lead to inhibition in photosynthesis, an increase in the ATP/ADP ratio, detrimental changes in the cell cycle, breaks in single- and double-strands of DNA, and the accumulation of reactive oxygen species (ROS) (Verma and Dubey, 2003; Patra et al., 2004; Romanowska et al., 2005, 2006; Gichner et al., 2008). Thus, Pb2+ stress might be expected to have an adverse effect on NO3− uptake by roots; however, we found that exposure to Pb2+ stimulated NO3− uptake in Arabidopsis roots under our experimental growth conditions (Fig. 1). Although several nitrate transporters (NRTs) are involved in NO3− uptake in Arabidopsis, we found that Pb2+ only stimulated NRT1.1, as evidenced by its increased expression in seedlings and increased levels of transcripts in the roots (Fig. 2). Furthermore, the loss of function of NRT1.1 in nrt1.1 mutants abolished the effect of Pb2+ on root NO3− uptake (Fig. 3). These results raised the question of how NRT1.1-controlled NO3− uptake was induced by exposure to Pb2+. Time-course analysis showed that the induction of NRT1.1 was initially slow and significant increases were not detected until after12 h of exposure (Supplementary Fig. S1), suggesting that induction was probably the result of the progression of Pb toxicity. Several studies have shown that Pb2+ stress results in a significant increases in the level of endogenous abscisic acid (ABA) in plants (Parys et al., 1998; Atici et al., 2005; Cenkci et al., 2010), and ABA has been shown to significantly up-regulate the expression of NRT1.1 in roots (Kiba et al., 2011). This may provide a mechanism by which Pb2+ stimulates NRT1.1-controlled uptake ofNO3− but further research is needed.
Comparison of growth responses to Pb2+ exposure in various NRT-related mutants also indicated that only the NRT1.1 transporter was required for plant resistance to Pb2+ (Fig. 4, Supplementary Figs S2, S3). NRT1.1 was initially identified as a NO3– transporter responsible for root NO3– uptake (Tsay et al., 1993), but it has also been shown to function as a NO3– sensor and an auxin transporter, and these two functions are independent of its uptake activity (Ho et al., 2009; Krouk et al., 2010; Bouguyon et al., 2015). Consequently, NRT1.1 has been shown to be involved in many physiological processes, including the regulation of root growth (Guo et al., 2001; Remans et al., 2006), control of the expression of the gene for another NO3− transporter, NRT2.1 (Muños et al., 2004; Ho et al., 2009; Bouguyon et al., 2015), and regulation of resistance to NH4+, Cd2+, and H+ stress (Hachiya et al., 2011; Mao et al., 2014; Fang et al., 2016). Most of these NRT1.1-associated processes are dependent on the presence of NO3− in the growth medium, as they act through either NO3− uptake activity or the NO3− sensing function (Wang et al., 2012). In our current study, we found that the NRT1.1-conferred resistance to Pb2+ probably depends on the NO3−-uptake activity (Supplementary Fig. S14). Thus, the induction of NRT1.1-mediated NO3−-uptake as a result of exposure to Pb2+ (Fig. 1) may be part of the response mechanisms to enhance Pb resistance. As noted above, an increase in endogenous ABA in plants may be a factor leading to NRT1.1 induction under Pb2+ stress. In relation to this, found that the ABA-hypersensitive triple-mutants abi1 hab1 abi2 and abi1 hab1 pp2ca (Fujii et al., 2009) had a greater resistance to Pb2+ stress than the wild-type plants (Supplementary Fig. S16). Measurement of Pb concentrations showed that the effect of NRT1.1 resulted from a reduction in the entry of Pb2+ into the plants (Fig. 5). The amount of Pb2+ taken up by roots is highly associated with its availability in the rhizosphere, which is significantly affected by pH (Fischer et al., 2014). Here, we found that the loss function of NRT1.1 in nrt1.1-knockout mutants resulted in a lower pH in the growth medium (Fig. 6A), which favored an increase in Pb2+ activity, and thus increasing its uptake by the plants. This effect was greatly reduced when the pH-buffer MES was present in the growth medium (Fig. 6B, C), leading us to conclude that the reduction in uptake of Pb2+ associated with NRT1.1 occurred because of the consumption of H+ by root cells during NO3− uptake, which increased the pH of the rhizosphere and thus decreased Pb2+ solubility. This was supported by the observation that the H+ concentration of the growth medium was highly correlated with the Pb concentrations in the tissues of wild-type and mutant plants supplied with various N:A ratios (Supplementary Fig. S17).
NO3− uptake activity is a common function for NRTs (Wang et al., 2012; Léran et al., 2014), but we found that only NRT1.1-mediated uptake played a role in plant resistance to Pb2+, whilst the other NRTs that we examined had little effect. Our previous study had shown that efficient prevention of acidification in the rhizosphere requires a significant consumption of H+, which can be achieved through adequate NO3– uptake by the roots (Fang et al., 2016). Thus, insufficient uptake of NO3– may lead to insufficient consumption of H+ that is inadequate to decrease Pb2+ solubility in the rhizosphere. In the current study, we found that knockout of NRT1.1 resulted in a decrease in NO3− uptake rate of >50% in the presence of Pb2+ (Fig. 3), which suggested that the other five NRTs (i.e. NRT1.2, NRT2.1, NRT2.2, NRT2.4, and NRT2.5) are responsible for <50% of the total root NO3– uptake. The AtNRT2 transporters have functional redundancy for high-affinity nitrate uptake (Li et al., 2007; Lezhneva et al., 2014); however, the nrt2.1nrt2.2 double-mutant had similar root elongation and Pb concentrations as wild-type plants when grown in low-nitrate medium (Supplementary Fig. S8). Hence, relatively low levels of NO3– uptake by the other five NRTs may explain why they were not as effective as NRT1.1 in inhibiting Pb2+ uptake. It is worth noting that Pb2+ can be easily precipitated by phosphates, which may have affected the phosphate availability under our growth conditions. Lowering the pH would increase the solubility of Pb-phosphate precipitates in the medium (Sauvé et al., 1998) and hence the lower pH in the rhizosphere of the nrt1.1-knockout mutants compared with that of the wild-type would favor greater solubilization of phosphate precipitates. This suggests that it is more likely that the inhibition of root growth in the mutants was the result of greater Pb2+ solubility rather than insufficient phosphate availability.
Interestingly, co-supply of NH4+ and NO3− in the growth medium was required to ensure the induction of NRT1.1-mediated NO3− uptake in response to Pb2+ exposure (Supplementary Figs S14, S15). Although the NRT1.1-mediated process of resistance depended on NO3– uptake, the presence of NH4+ was also required, as evidenced by the fact that there were no differences in root growth and the pH in the growth medium between the wild-type and mutants when NO3– was the sole N source (Fig. 7A, B). As previously noted, NO3− uptake by roots consumes H+, whereas NH4+ uptake produces H+ (Marschner, 1995), and thus if a plant only takes up NO3– and not NH4+ then the pH in the rooting medium would increase. In addition, if a plant is fed with NO3– as its sole N source, feedback inhibition would limit the increase in pH in the rooting medium to a level that the plant could tolerate (Imsande, 1986; Helali et al., 2010), because beyond that the plant would be damaged. This may be the reason why the pH of the growth medium for both the wild-type and the nrt1.1 mutants increased and reached a maximum of ~6.0 as the N:A ratio increased and NO3– became the sole N source (Fig. 7B). This led to a similar root growth between these plants in the presence of Pb2+ (Fig. 7A). When NO3– and NH4+ were both present in the growth medium, the loss of function of NRT1.1 may have resulted in H+ consumption through NO3– uptake mediated by the other NRTs, but this was not sufficient to counteract H+ production by NH4+ uptake, hence leading to greater acidification in the rhizosphere. This may explain why only the co-supply of NO3– and NH4+ could result in higher Pb accumulation and lower Pb2+ resistance in the nrt1.1 mutants compared to the wild-type. It may be possible to use biotechnological modifications of NRT1.1 activity to effectively manipulate Pb levels in plants because NO3– and NH4+ are often both present in agricultural soils.
In summary, in this study we found that NRT1.1 negatively regulates Pb levels in Arabidopsis by preventing acidification of the rhizosphere through consumption of H+ in the process of NO3– uptake, which has the effect of reducing the bioavailability of Pb2+. Induction of NRT1.1 may therefore be considered as a regulatory mechanism used by Arabidopsis to cope with Pb2+ stress.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primers used in this study
Fig. S1. Time-course of induction of NRT1.1 as the result of exposure to Pb2+.
Fig. S2. Root growth responses of wild-type Ler and the chl1-6 mutant to exposure to Pb2+.
Fig. S3. Time-course of root growth responses of wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants to exposure to Pb2+.
Fig. S4. Root growth responses of wild-type Col-0, the chl1-5 mutant, and pNRT1.1::NRT1.1-GFP transgenic plants to exposure to Pb2+.
Fig. S5. Root growth responses of wild-type Col-0, and the nrt1.1-1, chl1-5, chl1-9, and nrt7-2 mutants to exposure to Pb2+.
Fig. S6. Root growth responses of wild-type Col-0, the nrt1.2, nrt2.1, and nrt2.2 mutants, and wild-type Ler and the nrt2.4 mutant to exposure to Pb2+ in standard growth medium.
Fig. S7. Root growth responses of wild-type Col-0, and the nrt2.1, nrt2.2, and nrt2.5 mutants, and wild-type Ler and the nrt2.4 mutant to exposure to Pb2+ low-nitrate growth medium.
Fig. S8. Root elongation and tissue Pb concentrations in wild-type Col-0 and the nrt2.1 nrt2.2 double-mutant in low-nitrate growth conditions.
Fig. S9. Pb concentrations in tissues of wild-type Ler and the chl1-6 mutant exposed to Pb2+.
Fig. S10. Pb concentrations in tissues of wild-type Col-0, the chl1-5 mutant, and pNRT1.1::NRT1.1-GFP transgenic plants exposed to Pb2+.
Fig. S11. Pb concentration in tissues of wild-type Col-0, the nrt1.2, nrt2.1, nrt2.2, and nrt2.5 mutants, wild-type Ler and the mutant nrt2.4 exposed to Pb2+ in sufficient (2.25 mM) or low (0.2 mM) nitrate growth conditions.
Fig. S12. Activity of free Pb in growth media at different values of pH as calculated using GEOCHEM-PC.
Fig. S13. pH values in growth media of wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants exposed to Pb2+ in the presence of the MES pH buffer.
Fig. S14. Expression of NRT1.1 in the roots of wild-type Col-0 plants exposed to Pb2+ and supplied with different ratios of NO3– to NH4+.
Fig. S15. Net NO3− flux in roots of wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants exposed to Pb2+ and supplied with different ratios of NO3– to NH4+.
Fig. S16. Root growth responses of wild-type Col-0, and the abi1 hab1 abi2 and abi1 hab1 pp2ca triple-mutants to exposure to Pb2+.
Fig. S17. The relationships between H+ concentration in the growth media and Pb concentrations in the roots and shoots of wild-type Col-0, and the nrt1.1-1 and chl1-5 mutants exposed to Pb2+ and supplied with different ratios of NO3– to NH4+.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31622051 and 31670258), and the Fundamental Research Funds for the Central Universities (2017XZZX002-06).
References
- Alexander PD, Alloway BJ, Dourado AM. 2006. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environmental Pollution 144, 736–745. [DOI] [PubMed] [Google Scholar]
- Atici Ö, Ağar G, Battal P. 2005. Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biologia Plantarum 49, 215–222. [Google Scholar]
- Bai JH, Jia J, Huang C, Wang QG, Wang W, Zhang GL, Cui BS, Liu XH. 2017. Selective uptake of nitrogen by Suaeda salsa under drought and salt stresses and nitrogen fertilization using 15N. Ecological Engineering 102, 542–545. [Google Scholar]
- Bouguyon E, Brun F, Meynard D, et al. 2015. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nature Plants 1, 15015. [DOI] [PubMed] [Google Scholar]
- Castaings L, Camargo A, Pocholle D, et al. 2009. The nodule inception-like protein 7 modulates nitrate sensing and metabolism in Arabidopsis. The Plant Journal 57, 426–435. [DOI] [PubMed] [Google Scholar]
- Cenkci S, Cigerci IH, Yildiz M, Özay C, Bozdag A, Terzi H. 2010. Lead contamination reduces chlorophyll biosynthesis and genomic template stability in Brassica rapa L. Environmental and Experimental Botany 67, 467–473. [Google Scholar]
- Cerezo M, Tillard P, Filleur S, Muños S, Daniel-Vedele F, Gojon A. 2001. Major alterations of the regulation of root NO3–uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiology 127, 262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, Ager J, Greenwald M, Delaney-Black V. 2007. Blood lead levels and specific attention effects in young children. Neurotoxicology and Teratology 29, 538–546. [DOI] [PubMed] [Google Scholar]
- Clemens S. 2006. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88, 1707–1719. [DOI] [PubMed] [Google Scholar]
- Ernst WHO. 2000. Evolution of metal hyperaccumulation and phytoremediation hype. New Phytologist 146, 357–358. [Google Scholar]
- Fang XZ, Tian WH, Liu XX, Lin XY, Jin CW, Zheng SJ. 2016. Alleviation of proton toxicity by nitrate uptake specifically depends on nitrate transporter 1.1 in Arabidopsis. New Phytologist 211, 149–158. [DOI] [PubMed] [Google Scholar]
- Fischer S, Kühnlenz T, Thieme M, Schmidt H, Clemens S. 2014. Analysis of plant Pb tolerance at realistic submicromolar concentrations demonstrates the role of phytochelatin synthesis for Pb detoxification. Environmental Science & Technology 48, 7552–7559. [DOI] [PubMed] [Google Scholar]
- Fujii H, Chinnusamy V, Rodrigues A, Rubio S, Antoni R, Park SY, Cutler SR, Sheen J, Rodriguez PL, Zhu JK. 2009. In vitro reconstitution of an abscisic acid signalling pathway. Nature 462, 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichner T, Znidar I, Száková J. 2008. Evaluation of DNA damage and mutagenicity induced by lead in tobacco plants. Mutation Research 652, 186–190. [DOI] [PubMed] [Google Scholar]
- Guan MY, Zhang HH, Pan W, Jin CW, Lin XY. 2018. Sulfide alleviates cadmium toxicity in Arabidopsis plants by altering the chemical form and the subcellular distribution of cadmium. The Science of the Total Environment 627, 663–670. [DOI] [PubMed] [Google Scholar]
- Guo FQ, Wang R, Chen M, Crawford NM. 2001. The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. The Plant Cell 13, 1761–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya T, Mizokami Y, Miyata K, Tholen D, Watanabe CK, Noguchi K. 2011. Evidence for a nitrate-independent function of the nitrate sensor NRT1.1 in Arabidopsis thaliana. Journal of Plant Research 124, 425–430. [DOI] [PubMed] [Google Scholar]
- Hachiya T, Watanabe CK, Fujimoto M, Ishikawa T, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, Terashima I, Noguchi K. 2012. Nitrate addition alleviates ammonium toxicity without lessening ammonium accumulation, organic acid depletion and inorganic cation depletion in Arabidopsis thaliana shoots. Plant & Cell Physiology 53, 577–591. [DOI] [PubMed] [Google Scholar]
- Hawkins BJ, Boukcim H, Plassard C. 2008. A comparison of ammonium, nitrate and proton net fluxes along seedling roots of Douglas-fir and lodgepole pine grown and measured with different inorganic nitrogen sources. Plant, Cell & Environment 31, 278–287. [DOI] [PubMed] [Google Scholar]
- Helali SM, Nebli H, Kaddour R, Mahmoudi H, Lachaâl M, Ouerghi Z. 2010. Influence of nitrate–ammonium ratio on growth and nutrition of Arabidopsis thaliana. Plant and Soil 336, 65–74. [Google Scholar]
- Ho CH, Lin SH, Hu HC, Tsay YF. 2009. CHL1 functions as a nitrate sensor in plants. Cell 138, 1184–1194. [DOI] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. [DOI] [PubMed] [Google Scholar]
- Huang NC, Chiang CS, Crawford NM, Tsay YF. 1996. CHL1 encodes a component of the low-affinity nitrate uptake system in Arabidopsis and shows cell type-specific expression in roots. The Plant Cell 8, 2183–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NC, Liu KH, Lo HJ, Tsay YF. 1999. Cloning and functional characterization of an Arabidopsis nitrate transporter gene that encodes a constitutive component of low-affinity uptake. The Plant Cell 11, 1381–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. 1986. Nitrate–ammonium ratio required for pH homeostasis in hydroponically grown soybean. Journal of Experimental Botany 37, 341–347. [Google Scholar]
- Jin CW, He YF, Zhang K, Zhou GD, Shi JL, Zheng SJ. 2005a. Lead contamination in tea leaves and non-edaphic factors affecting it. Chemosphere 61, 726–732. [DOI] [PubMed] [Google Scholar]
- Jin CW, Zheng SJ, He YF, Zhou GD, Zhou ZX. 2005b. Lead contamination in tea garden soils and factors affecting its bioavailability. Chemosphere 59, 1151–1159. [DOI] [PubMed] [Google Scholar]
- Kiba T, Feria-Bourrellier AB, Lafouge F, et al. 2012. The Arabidopsis nitrate transporter NRT2.4 plays a double role in roots and shoots of nitrogen-starved plants. The Plant Cell 24, 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiba T, Kudo T, Kojima M, Sakakibara H. 2011. Hormonal control of nitrogen acquisition: roles of auxin, abscisic acid, and cytokinin. Journal of Experimental Botany 62, 1399–1409. [DOI] [PubMed] [Google Scholar]
- Kopittke PM, Asher CJ, Kopittke RA, Menzies NW. 2008. Prediction of Pb speciation in concentrated and dilute nutrient solutions. Environmental Pollution 153, 548–554. [DOI] [PubMed] [Google Scholar]
- Krapp A, Berthomé R, Orsel M, Mercey-Boutet S, Yu A, Castaings L, Elftieh S, Major H, Renou JP, Daniel-Vedele F. 2011. Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiology 157, 1255–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 877–878. [DOI] [PubMed] [Google Scholar]
- Léran S, Varala K, Boyer JC, et al. 2014. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends in Plant Science 19, 5–9. [DOI] [PubMed] [Google Scholar]
- Lezhneva L, Kiba T, Feria-Bourrellier AB, Lafouge F, Boutet-Mercey S, Zoufan P, Sakakibara H, Daniel-Vedele F, Krapp A. 2014. The Arabidopsis nitrate transporter NRT 2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. The Plant Journal 80, 230–241. [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Okamoto M, Crawford NM, Siddiqi MY, Glass AD. 2007. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiology 143, 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KH, Huang CY, Tsay YF. 1999. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. The Plant Cell 11, 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo BF, Du ST, Lu KX, Liu WJ, Lin XY, Jin CW. 2012. Iron uptake system mediates nitrate-facilitated cadmium accumulation in tomato (Solanum lycopersicum) plants. Journal of Experimental Botany 63, 3127–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao QQ, Guan MY, Lu KX, Du ST, Fan SK, Ye YQ, Lin XY, Jin CW. 2014. Inhibition of nitrate transporter 1.1-controlled nitrate uptake reduces cadmium uptake in Arabidopsis. Plant Physiology 166, 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchive C, Roudier F, Castaings L, Bréhaut V, Blondet E, Colot V, Meyer C, Krapp A. 2013. Nuclear retention of the transcription factor NLP7 orchestrates the early response to nitrate in plants. Nature Communications 4, 1713. [DOI] [PubMed] [Google Scholar]
- Marschner H. 1995. Mineral nutrition of higher plants, 2nd edn London, UK: Academic Press. [Google Scholar]
- Muños S, Cazettes C, Fizames C, Gaymard F, Tillard P, Lepetit M, Lejay L, Gojon A. 2004. Transcript profiling in the chl1-5 mutant of Arabidopsis reveals a role of the nitrate transporter NRT1.1 in the regulation of another nitrate transporter, NRT2.1. The Plant Cell 16, 2433–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Schwartz BS, Rothenberg SJ, Hu H, Silbergeld EK, Guallar E. 2008. Bone lead levels and blood pressure endpoints. Epidemiology 19, 496–504. [DOI] [PubMed] [Google Scholar]
- Parker DR, Norvell WA, Chaney RL. 1995. GEOCHEM-PC-a chemical speciation program for IBM and compatible personal computers. In: Loeppert RH, Schwab AP, Goldberg S. Soil chemical equilibrium and reaction models. Madison, WI: Soil Science Society of America, 253–269. [Google Scholar]
- Parys E, Romanowska E, Siedlecka M, Poskuta JW. 1998. The effect of lead on photosynthesis and respiration in detached leaves and in mesophyll protoplasts of Pisum sativum. Acta Physiologiae Plantarum 20, 313–322. [Google Scholar]
- Patra M, Bhowmik N, Bandopadhyay B, Sharma A. 2004. Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environmental and Experimental Botany 52, 199–223. [Google Scholar]
- Pourrut B, Shahid M, Dumat C, Winterton P, Pinelli E. 2011. Lead uptake, toxicity, and detoxification in plants. Reviews of Environmental Contamination and Toxicology 213, 113–136. [DOI] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E,Tillard P, Forde BG, Gojon A. 2006. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proceedings of the National Academy of Sciences, USA 103, 19206–19211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowska E, Pokorska B, Siedlecka M. 2005. The effects of oligomycin on content of adenylates in mesophyll protoplasts, chloroplasts and mitochondria from Pb2+ treated pea and barley leaves. Acta Physiologiae Plantarum 27, 29–36. [Google Scholar]
- Romanowska E, Wróblewska B, Drozak A, Siedlecka M. 2006. High light intensity protects photosynthetic apparatus of pea plants against exposure to lead. Plant Physiology and Biochemistry 44, 387–394. [DOI] [PubMed] [Google Scholar]
- Sauvé S, McBride M, Hendershot W. 1998. Lead phosphate solubility in water and soil suspensions. Environmental Science & Technology 32, 388–393. [Google Scholar]
- Selamat SN, Abdullah SR, Idris M. 2014. Phytoremediation of lead (Pb) and arsenic (As) by Melastoma malabathricum L. from contaminated soil in separate exposure. International Journal of Phytoremediation 16, 694–703. [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA. 1997. H+ flux kinetics around plant roots after short-term exposure to low temperature: identifying critical temperatures for plant chilling tolerance. Plant, Cell and Environment 20, 1401–1410. [Google Scholar]
- Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, Mukhlisin M. 2011. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International Journal of Chemical Engineering 2011, 1–31. [Google Scholar]
- Thomson CJ, Marschner H, Römheld V. 1993. Effect of nitrogen fertilizer form on pH of the bulk soil and rhizosphere, and on the growth, phosphorus, and micronutrient uptake of bean. Journal of Plant Nutrition 16, 493–506. [Google Scholar]
- Tsay YF, Schroeder JI, Feldmann KA, Crawford NM. 1993. The herbicide sensitivity gene CHL1 of Arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 72, 705–713. [DOI] [PubMed] [Google Scholar]
- Uzu G, Sobanska S, Aliouane Y, Pradere P, Dumat C. 2009. Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environmental Pollution 157, 1178–1185. [DOI] [PubMed] [Google Scholar]
- van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H. 2013. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant and Soil 362, 319–334. [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology 3, research0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Dubey RS. 2003. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Science 164, 645–655. [Google Scholar]
- Wang YY, Cheng YH, Chen KE, Tsay YF. 2018. Nitrate transport, signaling, and use efficiency. Annual Review of Plant Biology 69, 85–122. [DOI] [PubMed] [Google Scholar]
- Wang YY, Hsu PK, Tsay YF. 2012. Uptake, allocation and signaling of nitrate. Trends in Plant Science 17, 458–467. [DOI] [PubMed] [Google Scholar]
- Ye JY, Tian WH, Jin CW. 2019. A reevaluation of the contribution of NRT 1.1 to nitrate uptake in Arabidopsis under low-nitrate supply. FEBS letters 593, 2051–2059. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang R, Fang X, Song T, Cai X, Liu H, Du S. 2016. Toxic effects of graphene on the growth and nutritional levels of wheat (Triticum aestivum L.): short- and long-term exposure studies. Journal of Hazardous Materials 317, 543–551. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.