Abstract
Background:
Pityriasis versicolor (PV) is a superficial mycosis caused by Malassezia yeast; a lipophilic fungus. Dermoscopy may be a value addition in the diagnosis of PV in some cases, where results of KOH (potassium hydroxide) examination are ambiguous. There is paucity of Indian data on dermoscopy of PV.
Materials and Methods:
Thirty consecutive patients diagnosed clinically with pityriasis versicolor were recruited in this pilot cross sectional study. Patients were subjected to KOH mount of the skin scrapings from the lesions which were positive in all the patients. Dermoscopy was performed in all using Universal Serial Bus (USB) dermoscope [Dinolite AMZT 73915, Edge 3] and features were recorded for analysis.
Results:
Hypopigmented variant was the most common type (80%). Dermoscopic analysis revealed altered pigmentary network as most common finding (100%) followed by scaling seen in 25 cases (83.33%). Folliculocentric pattern was appreciated in 20 cases (66.67%). A characteristic contrast halo ring around the primary altered pigmentation was observed in 20 cases (66.67%). Invasion of hair follicles by yeast was evident in 6 patients (20%).
Conclusion:
Dermoscopy with characteristic features such as folliculocentricity, contrast halo sign, and yeast invasion of hair follicles can be a very useful aid in contributing towards diagnosing pityriasis versicolor.
Keywords: Dermoscopy, Malassezia, Pityriasisversicolor, superficial mycosis, tinea versicolor
Introduction
Pityriasis versicolor (PV) or tinea versicolor is a superficial mycosis caused by Malassezia genus that is a dimorphic lipophilic yeast.[1] It presents with multiple scaly macules of varying pigmentation that tend to coalesce to from larger lesions. The scaling may however be inconspicuous to the naked eye examination. The most commonly involved sites include upper trunk and proximal part of upper limbs. Lesions are either asymptomatic or mildly itchy. Triggering factors include hot climate, humidity, occlusion, and poor hygiene. Maximum numbers of cases are encountered in the hot and humid months of August to September.[2] Though the diagnosis can be made on clinical examination, PV can mimic various other skin conditions. Thus 10% potassium hydroxide (KOH) examination and culture may be required to confirm the diagnosis. KOH mounts lacks sensitivity and specificity and evaluation is subjective with variable interpretation. Culture is not routinely available and is time consuming. Studies have reported the utility of dermoscopy in dermatoses caused by Malassezia.[3,4] In the present study, we explored the potential of dermoscopy as an auxillary tool for the diagnosis of PV in Indian patients.
Materials and Methods
This cross sectional study was conducted at a tertiary care center, New Delhi. Thirty consecutive patients of PV, diagnosed on clinical grounds based on morphology and distribution of the lesions and confirmed on KOH examination were recruited in the study. Exclusion criteria included negative KOH examination and history of previous antifungal treatment in past one month. Demographic and clinical details were recorded on a predesigned proforma. Patients were classified into hypopigmented, hyperpigmented, and mixed variants of PV.
Dermoscopy was performed in all from the representative lesions as well as from the normal surrounding skin (control) using a Universal Serial Bus (USB) dermoscope [Dinolite AMZT 73915, Edge 3]. Images were taken with and without interface medium (immersion oil), stored and analyzed. Data was stored and assessed in MS Excel Sheet. SPSS version 20 was used for statistical analysis. Continuous variables were expressed as mean +/- SD and categorical variables wee expressed as frequencies in percentages.
Results
Of the 30 study patients, 20 were males and 10 were females (M;F = 2:1). The mean age of presentation was 18.33 years (range, 1–40 years) with more than half (17/30 = 56.66%) belonging to 11–20 years of age. Eight patients were of pediatric age (less than 14 years). Upper back was the most commonly affected sites in 25 (83.33%) followed by chest in 21 (70%) andface in 12 patients (40%). We observed that upper trunk was the site of predilection in adults (22/22) and face in pediatric cases (7/8).
Hypopigmented PV was seen in 24/30 (80%), hyperpigmented variant in 3 (10%) while a combination of lesions (mixed variant) was present in 3 (10%). Most of the patients were asymptomatic except 3 (10%) who complained of occasional mild pruritus. Microscopic examination of lesional skin scrapings in 10% KOH showed characteristic hyphae and spores (spaghetti and meat ball) in all patients.
The most common dermoscopic feature was alteration in the background pigmentation in all the patients regardless of the clinical variant (100%). There was a decreased reticulate pigmentary network in the hypopigmented variant [Figure 1a–d] while an increased reticulate pigmentary network was seen in the hyperpigmented lesions [Figure 2a–d]. The decrease or increase was in comparison to the normal surrounding skin. Scaling was observed in 83.33% of the cases (25/30) and the scales were predominantly along the dermatoglyphics [Figure 1b and c]. The lesions were found to be folliculocentric in 20 patients (66.67%) [Figure 1b and c].
Figure 1.
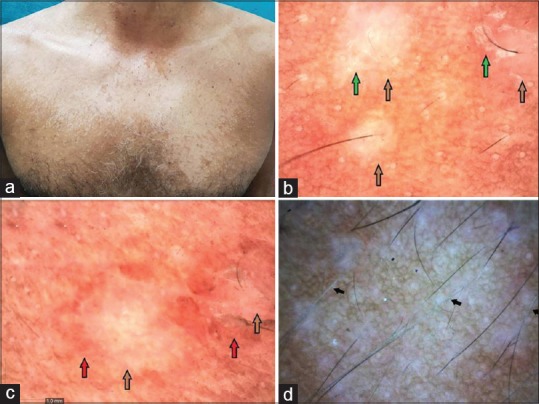
Hypopigmented pityriasis versicolor: (a) Adult patient with hypopigmented variant of pityriasis versicolor involving trunk. (b) Showing primary folliculocentric lesions with reduced pigmentary network (brown arrow) with scaling at the border of the primary lesion (green arrow) (Dinolite AMZT 73915, Edge 3; magnification ×50, polarizing mode). (c) Shows similar changes with a contrast halo (hyperpigmented) around the primary lesion (red arrow) (magnification ×50). (d) Hypopigmentation of the involved hair follicle as a result of follicular invasion of the yeast marked with black arrow (magnification 50×, polarizing mode)
Figure 2.
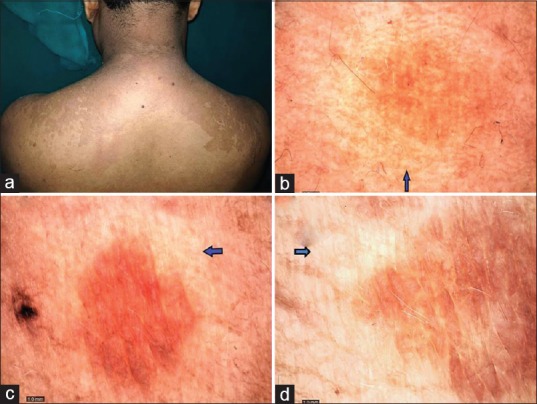
Hyperpigmented pityriasis versicolor: (a) adult patient with hyperpigmented variant of pityriasis versicolor involving upper trunk. (b-d) showing increased pigmentary network of the primary lesion surrounded by a contrast halo (hypopigmented) (Blue arrow) (magnification ×50, polarizing mode)
An interesting feature observed in 20 cases (66.67%) was a contrast halo ring around the primary lesion. In hypopigmented variant, this contrast halo ring was appreciated as a ring of increased pigmentation surrounding the primary lesion of decreased pigmentary network [Figure 1c]. In the hyperpigmented variant, the primary area of increased pigmentary network was surrounded by a halo of hypopigmentataion [Figure 2b–d]. We called it as “contrast halo sign”. Another characteristic feature appreciated was the invasion of the hair follicles by the yeast as evidenced by hypo pigmentation of the hair follicles which was noticed in 20% (6/30) as shown in Figure 1d. Hair follicles in the uninvolved surrounding skin were normal. Table 1 depicts all the dermoscopic features observed in the present study.
Table 1.
Dermoscopic features in Pityriasis Versicolor
| Dermoscopic Feature | Hypopigmented variant (24) | Hyperpigmented variant (3) | Mixed variant (3) | Total (30) |
|---|---|---|---|---|
| Altered pigmentary network | 24 (100%) | 3 (100%) | 3 (100%) | 30 (100%) |
| Scaling | 21 (87.5%) | 2 (66.66%) | 2 (66.66%) | 25 (83.33%) |
| Halo Sign | 22 (91.66%) | 2 (66.66%) | 1 (33.33%) | 20 (66.67%) |
| Folliculocentricity | 20 (83.33%) | 2 (66.66%) | 1 (33.33%) | 20 (66.67%) |
| Invasion of hair follicle | 6 (25%) | 0 | 0 | 6 (20%) |
Discussion
Pityriasis versicolor (PV) is a fairly common superficial mycosis in tropical countries.[1,2] Otherwise asymptomatic, patients usually are concerned about the pigmentary changes in the involved areas. It is essential to correctly diagnose the infection in order to ease the patient's anxiety and provide adequate mycological cure. The utility of dermoscopy as an auxillary tool to diagnose Malassezia infections is recently explored but still under-utilized.[3,4]
The epidemiological characteristics of our patients matched with the previous studies. The average patient's age was 18.33 years with majority (56.66%) falling in 11–20 years of age group; an observation in similar to the previous reports from India.[1,2,5] Likewise, we too noticed a slight male predominance (1.5:1) and distribution of lesions on upper back and chest followed by face and upper limb in adults.[1,2,5] In children, face was the most common site affected, as also reported by Jena et al.[6] Moreover, we observed that face was the only site involved in children less than 3 years of age (5 cases). The observation may be attributed to relatively increased facial sebaceous secretion in children as compared to the adults, probably under the influence of maternal androgens. In addition, we also observed hypopigmented variant to be far more common in children.
Dermoscopic analysis of the lesions revealed a consistent finding of altered pigmentary network (100%) that was found to be folliculocentric (66.67%) and associated with scaling (83.33%) in majority of the cases. Another common but interesting dermoscopic feature observed in our study was the characteristic contrast halo ring seen in in 20 cases (66.67%) around the primary lesion which has never been reported before. Follicular invasion resulting in hypopigmentation of the involved follicle was seen in six patients. To the best of our knowledge, there is only a single case of hyperpigmented pityriassis versicolor with dermoscopic analysis published so far in the English literature that showed a pigmentary network with fine scales.[4]
Various theories have been proposed for the pigmentary changes seen clinically in PV and its correlation with the altered pigmentary network observed on dermoscopy. It is believed that hypopigmentation in PV results from presence of fungus in the skin that initiates production of abnormal melanosome granules and possibly the faulty transfer of these granules to the keratinocytes.[2] Other have attributed to the release of dicarboxylic acid like azelaic acid by fungus which tends to inhibit enzyme tyrosinase and cause cytotoxic damage to the melanocyte. On the other hand, increased pigmentation reportedly is the result of thickened stratum corneum and perivascular lymphocytic inflammatory infiltrate in dermis that stimulates melanogenesis.[2] However, both hypotheses fail to explain the contrast halo sign seen around the primary lesion in our study. We propose that the contrast halo in hypopigmented variant could be a result of compensatory melanogenesis to the cytotoxic damage and abnormal melanosomes in the primary lesion. While in the hyperpigmented variant, this contrast halo could be due to consumption of melanocytes in the process of stimulated melanogenesis occurring as a result of perivascular inflammation in the primary lesion. Hypopigmentation of the hair follicle could be due to follicular invasion by Malassezia yeast which is known to show a similar tendency of invasion in pityrosporum folliculitis.[7]
The dermoscopic features seen in our study can help in ruling out close differentials of both hypo and hyperpigemented PV. The distinguishing dermoscopic features of these entities have been tabulated in Table 2.[8,9,10,11,12,13] There is paucity of literature on dermoscopy in infections caused by Malassezia species.[3,4] Our study shows that the characteristic dermoscopic features can be a useful aid in the diagnosisof PV.
Table 2.
| Diagnosis | Dermoscopic feature |
|---|---|
| Pityriasis versicolor | Altered pigmentary network, scaling, folliculocentricity and halo sign |
| Pityriasis rosea[8] | Peripheral scaling (so-called collarette scales) around a diffuse and structureless yellowish centerwith dotted vessels. |
| Pityriasis alba[9] | Pinkish areas with scaling |
| Pre vitiligo[10] | Reduced pigmentary network, perifollicularand perilesional pigmentation |
| Seborrhoeic dermatitis[11] | Orange-white areas, White structureless areas, dotted vessels and yellowish scaling. |
| Lichen planuspigmentosus[12] | Diffuse brown backgrounds, hem-like pigmentary pattern, perifollicular pigmentation, blue gray dots/globules |
| Confluent and reticulated papillomatosis[13] | Ridges and furrows, white structures, brown lines and -hairs |
Conclusion
Use of dermoscope in the evaluation of infections (entomodermoscopy) is still in evolving phase. The initial observations in dermoscopy of PV seem to be promising. As an adjunctive tool, dermoscopic evaluation of PV can give useful clues to the diagnosis. Further large scale studies correlating these findings with histopathology and electron microscopy are required to substantiate the findings.
Limitations
A smaller sample size was the limitation of our study. Culture and histopathology were not done in our study. A comparative group could have added more value to the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ghosh SK, Dey SK, Saha I, Barbhuiya JN, Ghosh A, Roy AK. Pityriasisversicolor: Aclinicomycological and epidemiological study from a tertiary care hospital. Indian J Dermatol. 2008;53:182–5. doi: 10.4103/0019-5154.44791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta D, Thappa DM. The enigma of color in tineaversicolor. Pigment Int. 2014;1:32–5. [Google Scholar]
- 3.Jakhar D, Kaur I, Chaudhary R. Dermoscopy of pityrosporumfolliculitis. J Am Acad Dermatol. 2018;80:e43–4. doi: 10.1016/j.jaad.2018.08.057. [DOI] [PubMed] [Google Scholar]
- 4.Zhou H, Tang XH, De Han J, Chen MK. Dermoscopy as an ancillary tool for the diagnosis of pityriasis versicolor. J Am Acad Dermatol. 2015;73:205–6. doi: 10.1016/j.jaad.2015.08.058. [DOI] [PubMed] [Google Scholar]
- 5.Rai MK, Wankhade S. Tineaversicolor - An epidemiology. J Microbial Biochem Technol. 2009;1:051–6. [Google Scholar]
- 6.Jena DK, Sengupta S, Dwari BC, Ram MK. Pityriasisversicolor in the pediatric age group. Indian J Dermatol Venereol Leprol. 2005;71:259–61. doi: 10.4103/0378-6323.16618. [DOI] [PubMed] [Google Scholar]
- 7.Akaza N, Akamatsu H, Sasaki Y, Kishi M, Mizutani H, Sano A, et al. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med Mycol. 2009;47:618–24. doi: 10.1080/13693780802398026. [DOI] [PubMed] [Google Scholar]
- 8.Lallas A, Kyrgidis A, Tzellos TG, Apalla Z, Karakyriou E, Karatolias A, et al. Accuracy of dermoscopic criteria for the diagnosis of psoriasis, dermatitis, lichen planus and pityriasisrosea. Br J Dermatol. 2012;166:1198–205. doi: 10.1111/j.1365-2133.2012.10868.x. [DOI] [PubMed] [Google Scholar]
- 9.Nayak SS, Mehta HH, Gajjar PC, Nimbark VN. Dermoscopy of general dermatological conditions in Indian population: A descriptive study. Clin Dermatol Rev. 2017;1:41–51. [Google Scholar]
- 10.Thatte SS, Khopkar US. The utility of dermoscopy in the diagnosis of evolving lesions of vitiligo. Indian J Dermatol Venereol Leprol. 2014;80:505–8. doi: 10.4103/0378-6323.144144. [DOI] [PubMed] [Google Scholar]
- 11.Errichetti E, Stinco G. Dermoscopy in general dermatology: Apractical overview. DermatolTher (Heidelb) 2016;6:471–507. doi: 10.1007/s13555-016-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neema S, Jha A. Lichen planuspigmentosus. Pigment Int. 2017;4:48–9. [Google Scholar]
- 13.BernardesFilho F, Quaresma MV, Rezende FC, Kac BK, Nery JA, Azulay-Abulafia L. Confluent and reticulate papillomatosis of Gougerot-Carteaud and obesity: Dermoscopic findings. An Bras Dermatol. 2014;89:507–9. doi: 10.1590/abd1806-4841.20142705. [DOI] [PMC free article] [PubMed] [Google Scholar]


