Abstract
Introduction:
Allergic contact dermatitis (ACD) is a growing concern due to increased use of cosmetics and topical medications routinely and exposure to a large number of allergens on day-to-day basis. Patch testing is a reliable method for detecting the causative antigens in suspected cases.
Aims and Objectives:
To assess the demographic profile, pattern of ACD, and patch test profile of suspected cases of ACD attending contact dermatitis clinic of our department.
Materials and Methods:
It was a retrospective study in which all the data enrolled in the contact dermatitis clinic of our department over a 7-year period were analyzed. Patch testing was done using the Indian Standard Series of 20 antigens primarily, and other batteries were used depending on patient requirement and availability.
Results:
A total of 582 patients were enrolled in the contact dermatitis clinic over a period of 7 years. Hand eczema was the most common pattern seen in 268 cases followed by feet eczema, hand and foot eczema, facial eczema, forearm and leg eczema and photoallergic contact eczema. A total of 177 patients (30.4%) gave positive patch test results, with nickel sulfate being the most common allergen identified followed by potassium dichromate, cobalt sulfate, paraphenylenediamine, neomycin sulfate, and fragrance mix.
Conclusion:
Common allergens identified in our study were more or less similar to studies from other parts of India. However, due to the unique climate of the valley, the profile of parthenium sensitivity was low in our study when compared to the rest of the country.
Keywords: Allergic contact dermatitis, patch testing, retrospective study
Introduction
Globally, the prevalence of allergic contact dermatitis (ACD) is increasing and the spectrum of its clinical patterns is expanding simultaneously. Contact dermatitis accounts for 4%–7% of all dermatological consultations.[1] Several predisposing factors increase the chances of sensitisation in certain individuals. Women are seen to suffer more from ACD than men (twice as common), possibly due to hormonal factors.[2] Ethnically, darker races are at a lower risk for ACD, due to the higher barrier function for certain substances.[3] Also, the genetic constitution of individuals and presence of atopic eczema are believed to be important risk factors for development of ACD as there are more chances of contact allergy due to impaired epidermal barrier in atopics.[4] No age is immune to development of ACD and its incidence is reported to be increasing in the pediatric population.[5,6,7] An acute response is often characterized by macular erythema, papules, vesicles, or bullae, depending on the intensity of the allergic response. Chronic ACD usually manifests as fissured, scaly, and lichenified dermatitis with or without accompanying papulovesicles.[8] A large number of allergens are present in our environment and are encountered daily in the form of cosmetics, skin care products, hair dyes, medications, accessories, jewellery, cement, plants, and so on. Nickel found in metal industry and household objects along with fragrances and preservatives are the most common allergens responsible for causing a significant number of cases of ACD globally. Allergens such as chromates (present in cement, paints, and coolants) and paraphenylenediamine (PPD) (in hair dyes) follow subsequently, but are more incriminated in occupational settings. Other commonly encountered antigens include rubber additives such as mercaptobenzothiazoles (in rubber gloves, shoes, etc.), cobalt, and plant-derived substances such as colophony, turpentine, and essential oils.[9] Accordingly, ACD is seen in a large number of occupational groups, with the frequency and pattern varying from one group to another. In many countries, occupational contact dermatitis ranks first among occupational diseases worldwide resulting in significant morbidity and work loss days.[10]
Patch testing is a reliable method for detecting the causative antigen(s) in suspected cases. The allergens that are included in standard series vary from country to country based on the local experience. Knowledge about the responsible allergen for ACD helps a long way in reducing morbidity in such cases by identifying the incriminating allergen and can thus help minimize the impact of ACD in the affected individuals.[11]
With this background, we attempted to assess the demographic profile, pattern of ACD, and patch test profile of suspected cases of ACD attending contact dermatitis clinic of our department over a 7-year period.
Materials and Methods
It was a retrospective study in which all the patient data enrolled in the contact dermatitis clinic of our department since its inception were analyzed. These data had been collected from those patients who attended the outpatient section of our dermatology department with suspected ACD and had been then referred to the contact dermatitis clinic where all the data were collected and maintained in proper files. A detailed history including the demographic data, occupational details, and exposure to different allergens was taken which was followed by clinical examination and relevant photographs for documentation. The various patterns of ACD observed were categorized into various groups like hand eczema involving primarily the dorsal and palmar aspects of fingers and hands upto the wrists; foot eczema involving primarily the dorsal and plantar aspects of feet upto the ankle joint; hand and foot eczema in which simultaneous involvement of both hands and feet was noticed; facial eczema in which the eczema was seen primarily affecting the convex surfaces of the face, eyelids, lips, and periorificial areas; forearm and leg eczema where primary involvement was of the forearms and legs with nil or minimal concurrent involvement of hands and feet; photoallergic contact eczema involving primarily the photoexposed areas such as face, V area of neck, and dorsal aspects of both hands and forearms with well-demarcated margins where the skin is covered with clothing with sparing of the Wilkinson's triangle, upper eyelids, and area under the chin and air-borne contact dermatitis (ABCD) affecting primarily the exposed areas of face, V area of neck, hands, and forearms, Wilkinson's triangle, both eyelids, nasolabial folds, and area under the chin. The involvement of both light-exposed and protected areas helps differentiate ABCD from photo-related dermatitis. Disseminated eczema was used for patients with extensive involvement of whole body, rarely proceeding to erythroderma. Nonspecific eczema was used for all such types of eczema which were not extensive but did not fit in any of the above-mentioned patterns of eczema and had a variable presentation.
All the patients (irrespective of age) were included in the study. However, patients on oral corticosteroids and other immunosupressants, pregnant, and lactating females were excluded. Those patients who had active dermatitis were patch-tested 2 weeks after their clinical symptoms subsided. Doubtful cases (requiring distinction from fungal infections, psoriasis, and other simulating dermatoses) were subjected to investigations like KOH mount and skin biopsy wherever necessary.
Patch testing was done by Finn chamber method using the Indian Standard Series (ISS) of 20 antigens recommended by Contact and Occupational Dermatoses Forum of India and other batteries such as ISS of 25 antigens, cosmetic and fragrance series, and footwear series depending on patient requirement and availability of the batteries. Cosmetic agents in the “as is” form were not used for patch testing. The patch tests were applied on nonhairy upper back of the patients. The results were read on day 2 (48 h) and day 4 (96 h) according to the guidelines laid down by the International Contact Dermatitis Research Group.[12] Day 4 reading was taken as final grade of positivity and was interpreted for clinical relevance. In doubtful cases, day 7 reading was also taken. All forms of topical and systemic medication were stopped 2 weeks prior to patch testing. Informed consent was taken from the patients prior to patch testing and before taking any photograph for record purpose. The relevance of positive patch test results was determined clinically using COADEX system[13,14] in which current and old relevance means that patient has been exposed to the allergen during the current and previous episodes of dermatitis, respectively, and there is improvement of the disease after cessation of exposure. When relevance is difficult to assess and no traceable relationship is found between the positive test and the disease, relevance is termed to be doubtful. The data of the entire 7-year period from January 2012 to December 2018 were tabulated, compiled, and subjected to statistical analysis.
Results
A total of 582 patients were enrolled in the contact dermatitis clinic over a period of 7 years. Of these, 371 were females (63.75%), while 211 were males (36.25%) giving a male: female ratio of 1:1.7. The mean age of the study population was 34.70 ± 11.27 years with age ranging from 9 to 68 years. In all, 310 (53.26%) patients had a rural background, while 272 (46.73%) were from urban areas. The mean disease duration was 5.11 ± 1.2 years with a range of 4 months to 12 years.
The pattern of clinical disease noticed in our study population was divided into various groups as mentioned in methodology. Table 1 shows the number of patients with different clinical patterns of eczema with hand eczema being the most common pattern seen in 268 cases (46.05%) [Figure 1] followed by feet eczema seen in 81 cases (13.92%) [Figure 2], hand and foot eczema in 70 cases (12.03%), facial eczema in 47 cases (8.08%), forearm and leg eczema in 41 cases (7.04%), photoallergic contact eczema in 29 cases (4.98%), ABCD in 17 cases (2.92%), nonspecific eczema in 17 cases (2.92%), and disseminated eczema in 12 cases (2.06%).
Table 1.
Number of patients with different clinical patterns of eczema in the study population
| Pattern of eczema | No. of patients with different clinical patterns of eczema (%) |
|---|---|
| Hand eczema | 268 (46.05%) |
| Feet eczema | 81 (13.92%) |
| Hand and foot eczema | 70 (12.03%) |
| Facial eczema | 47 (8.08%) |
| Forearm and leg eczema | 41 (7.04%) |
| Photoallergic contact eczema | 29 (4.98%) |
| Air-borne contact dermatitis | 17 (2.92%) |
| Nonspecific eczema | 17 (2.92%) |
| Disseminated eczema | 12 (2.06%) |
| Total (n) | 582 (100%) |
Figure 1.
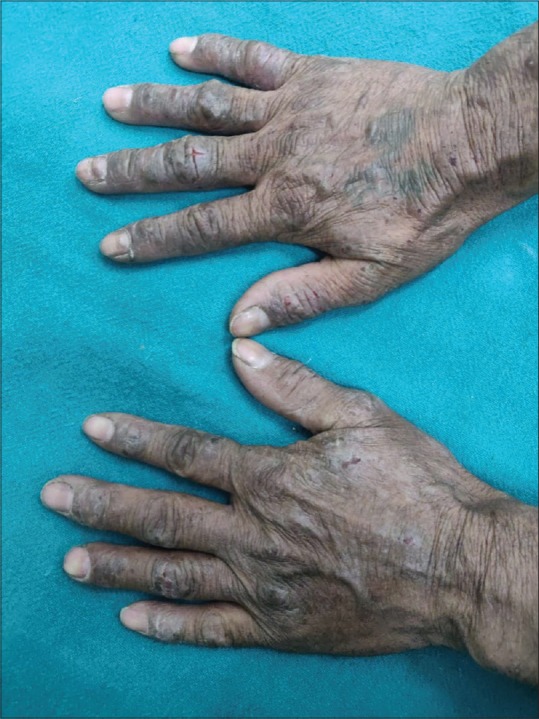
A patient with hand eczema involving primarily the dorsal surface of both hands with positive patch test reaction to potassium dichromate with current relevance
Figure 2.
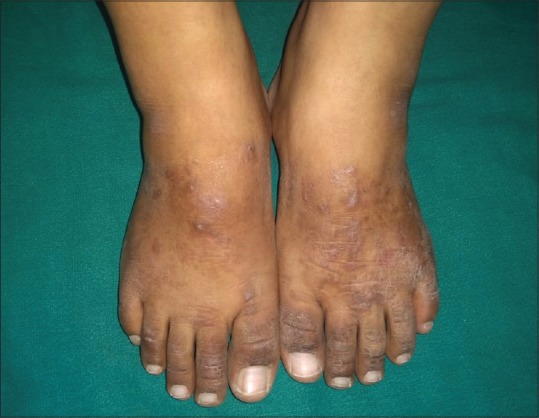
A patient with feet eczema with bilaterally symmetrical involvement of dorsal aspects of both feet and toes with positive patch test reaction to nickel sulfate with current relevance
Occupation-wise distribution of the study population included farmers, construction workers, housewives, students, office workers, food handlers, artisans, and medical/paramedical workers in that order as enumerated in Table 2.
Table 2.
Occupation-wise distribution of the study population
| Occupation of the patients | No. of patients in each occupation (%) |
|---|---|
| Farmers | 163 (28%) |
| Construction workers | 128 (21.99%) |
| Housewives | 99 (17%) |
| Students | 70 (12.07%) |
| Office workers | 52 (8.93%) |
| Food handlers | 29 (4.98%) |
| Artisans | 17 (2.91%) |
| Medical and paramedical workers | 12 (2.06%) |
| Others | 12 (2.06%) |
| Total (n) | 582 (100%) |
A total of 177 patients (30.4%) gave positive patch test results to various allergens used. A total of 242 positive reactions were seen in these 177 patients, among which 120 patients gave a single positive reaction while 38 patients gave positive reaction to two allergens and the rest 19 patients had more than two positive patch test reactions. Of the total 242 positive patch test reactions, 233 positive reactions were elicited from ISS of 20 and 25 antigens, while the rest 9 reactions were elicited from allergens in footwear and cosmetic series other than those included in ISS [Table 3].
Table 3.
Profile of patch test positivity in the study population with the relevance against individual allergens
| Name of antigen | No. of patients with positive reactions to individual antigens (%) | Relevance of positive patch test reactions |
|---|---|---|
| Nickel sulfate (nickel sulfate hexahydrate) | 49 | Current 45 Old 2 Unknown 2 |
| Potassium bichromate | 28 | Current 17 Old 8 Unknown 3 |
| Cobalt sulfate (Cobalt chloride hexahydrate) | 26 | Current 20 Old 2 Unknown 4 |
| Paraphenylenediamine | 23 | Current 15 Old 7 Unknown 1 |
| Neomycin sulfate | 16 | Current 8 Old 4 Unknown 4 |
| Fragrance mix | 15 | Current 12 Old 3 |
| Mercaptobenzothiazole | 10 | Current 6 Old 1 Unknown 3 |
| Parthenium | 10 | Current 2 Old 2 Unknown 6 |
| Thiuram mix | 10 | Current 3 Old 3 Unknown 4 |
| Formaldehyde | 9 | Current 4 Unknown5 |
| Colophony (colophonium) | 8 | Current 5 Unknown 3 |
| Perubalsam (myroxylon pereirae resin) | 6 | Current 5 Old 1 |
| Paraben mix | 4 | Current2 Old 2 |
| Nitrofurazon | 4 | Unknown 4 |
| Black rubber mix | 3 | Current 2 Unknown 1 |
| Wool alcohol (lanolin) | 3 | Unknown 3 |
| 4-Tert-butylphenolformaldehyde resin | 3 | Current 1 Unknown 2 |
| Epoxy resins | 2 | Current1 Unknown 1 |
| Benzocaine | 2 | Unknown 2 |
| Disperse blue | 2 | Current 1 Unknown 1 |
| Polyethylene glycol | 1 | Current 1 |
| Mercapto mix | 1 | Current 1 |
| Disperse orange | 1 | Unknown 1 |
| Jasmine absolute | 1 | Current 1 |
| Rose oil | 1 | Current 1 |
| Musk mix | 1 | Unknown 1 |
| Triclosan | 1 | Unknown 1 |
| Cetrimide | 1 | Current 1 |
| Sorbic acid | 1 | Unknown 1 |
| Total positive reactions seen with relevance of individual allergens | 242 | Current 154 Old 35 Unknown 53 |
Of the 233 positive reactions elicited from ISS of 20 and 25 antigens, nickel sulfate turned out to be the most common allergen identified in 49 cases followed by potassium dichromate in 28 cases, cobalt sulfate in 26 cases, PPD in 23 cases, neomycin sulfate in 16 cases, and fragrance mix in 15 cases. Figure 3 shows a patient with positive patch test reaction to nickel sulfate and cobalt sulfate, while Figure 4 shows a patient with positive patch test reaction to fragrance mix. Other allergens seen were mercaptobenzothiazole (10 cases), parthenium (10 cases), thiuram mix (10 cases), formaldehyde (9 cases), colophony (8 cases), peru balsam (6 cases), paraben mix (4 cases), nitrofurazon (4 cases), black rubber mix (3 cases), wool alcohol (3 cases), 4-tert-butylphenolformaldehyde resin (3 cases), epoxy resins (2 cases), benzocaine (2 cases), mercapto mix (1 case), and polyethylene glycol (1 case). Figure 5 shows a patient with positive patch test reaction to mercaptobenzothiazole and thiuram mix.
Figure 3.
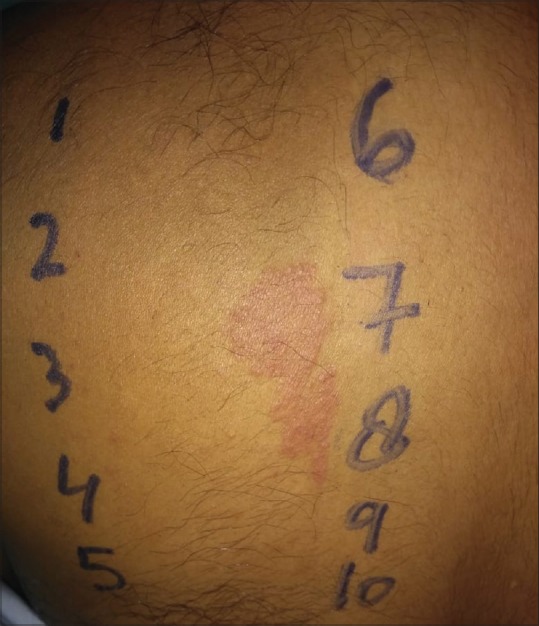
A farmer who presented with hand and foot eczema giving positive patch test reaction to nickel sulfate and cobalt sulfate with current relevance of positive patch test reactions
Figure 4.
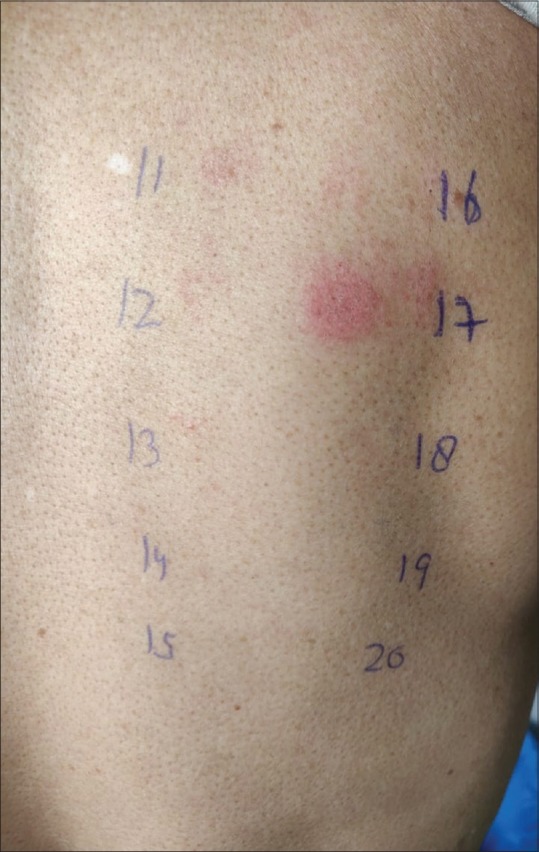
A housewive presenting with facial eczema giving positive patch test reaction to fragrance mix with current relevance of the positive reaction
Figure 5.
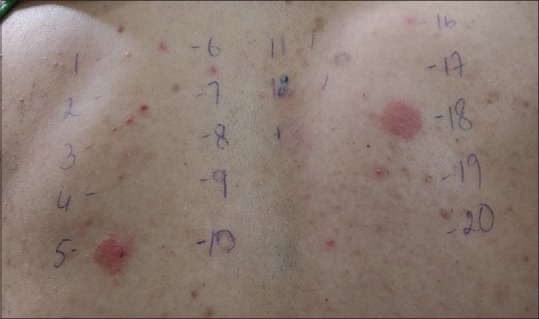
An artisan with hand eczema giving positive patch test reaction to mercaptobenzothiazole and thiuram mix with current relevance
Discussion
Clinical manifestations of ACD are highly varied, depending on the degree and frequency of contact with the allergen, the nature of the putative allergen, and host-related factors. The clinical presentation varies from patient to patient, often posing a diagnostic challenge to the treating dermatologist.
In our study, the most common allergen identified was nickel sulfate which accounted for 49 (20.24%) of the 242 positive patch test reactions seen in our study group followed by potassium dichromate accounting for 28 (11.57%) positive patch test reactions. Both these allergens have also been identified as the most common allergens in other studies done from Kashmir valley.[15,16] Nickel is present ubiquitously in the environment and was the most common allergen identified in females in our study. The reason for early development of nickel sensitivity in our population can be attributed to the common use of nickel-plated accessories and jewellery especially in females. As most of the population is Muslim, small girls are seen covering their heads with scarfs and using nickel-plated pins to hold the scarf in position. Also, ear piercing is done in almost all girls at a small age and they are found wearing artificial jewellery in the form of ear rings, necklaces, rings, and bracelets. These jewellery items and other accessories like eyeglass frames, belt buckles, pins, clips, zippers, coins, and keys may release nickel as there is poor quality control on the manufacture of these items in our country.[17] Most of the cases of nickel positivity had current relevance to the use of nickel-plated items and jewellery.
Potassium dichromate was the second most common allergen identified in our study. It was the most common allergen identified in males in our study population. Most of the patients giving positive patch test reactions to potassium dichromate were construction workers, while the rest were involved in other occupations but would occasionally do the small construction works at their houses or shops to save money. Other possible sources of exposure to chromates included use of paints, woods, glass, and cleaning products. Potassium dichromate has also been identified as a common allergen in other studies.[18,19,20,21]
Cobalt sulfate was the third most common allergen identified in our study population. It constituted for 26 (10.74%) positive patch test reactions. Cobalt is an invariable contaminant of nickel and is also found in cement.[22,23] Some patients with cobalt sensitivity in our study especially females had a concomitant allergy to nickel as well [Figure 3]. PPD was identified as the allergen in 23 (9.5%) cases with almost equal incidence in both sexes. The most common source of PPD in our study population was attributed to the use of hair dyes and dyes used occupationally by some artisans. Also, use of henna tattoos is very common in the local population of the valley (especially young girls) which also contains PPD.[24]
Other important sensitizers in our study population included neomycin sulfate and fragrance mix which constituted for 16 (6.6%) and 15 (6.19%) positive patch test results, respectively. Neomycin is available freely as an over-the-counter topical medication in the local markets of valley (especially in combination with other drugs). Neomycin and gentamicin have already been reported as an important allergen in many other studies.[11,16,25,26] Another important allergen identified in our study was fragrance mix similar to some other studies from North India.[16,21] The increased use of cosmetics, toiletries, and skin care products was thought to be responsible for more number of positive reactions to fragrance mix in our study.
Parthenium, being an important allergen in whole of India,[11,21] was only rarely encountered in our study. The reason for the lesser positivity to parthenium seen in our study and reported previously[15] has been attributed to the cold climate of the valley where the parthenium weed does not survive in the subzero temperature of winter. Four of the cases identified in our study had history of travel to areas outside the valley. However, the possibility of cross reaction to other members of the Compositae family could not be ruled out as other plants belonging to the same family like dahlias, sunflowers, and dandelion grow in abundance in the valley. More than 200 species of the Compositae family[27] containing a large number of allergens[28] have been reported to cause ACD. Other studies from the valley have identified contact dermatitis in saffron[29] and tulip workers[30] as these plants also grow in specific seasons in the valley. However, it was not possible for us to do patch testing with plant series in patients attending our contact dermatitis clinic on a routine basis due to nonavailability of these batteries which forms an important limitation of our study. Also, “as is” testing for certain cosmetics and food items was not done in our study.
Conclusion
Having an idea about the common allergens in a demographic area helps the clinician in pointing out the causative factors easily. Such retrospective studies are important to know the cumulative data from a particular geographical area as there can be variation in the allergen distribution which can affect the patch test profile. Common allergens identified in our study such as nickel sulfate, potassium dichromate, cobalt sulfate, and PPD are more or less similar to studies from other parts of India. However, due to the unique climate of the valley, the profile of parthenium sensitivity was low in our study when compared to the rest of the country.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mendenhall RG, Ramsay DL, Girard RA. A study of the practice of dermatology in the United States. Arch Dermatol. 1978;114:1456–62. [PubMed] [Google Scholar]
- 2.Thyssen JP, Linneberg A, Menné T, Johansen JD. The epidemiology of contact allergy in the general population – Prevalence and main findings. Contact Dermat. 2007;57:287–99. doi: 10.1111/j.1600-0536.2007.01220.x. [DOI] [PubMed] [Google Scholar]
- 3.Reed JT, Ghadially R, Elias PM. Skin type, but neither race nor gender, influence epidermal permeability barrier function. Arch Dermatol. 1995;131:1134–8. [PubMed] [Google Scholar]
- 4.Mortz CG, Lauritsen JM, Bindslev-Jensen C, Andersen KE. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents: The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523–32. doi: 10.1046/j.1365-2133.2001.04078.x. [DOI] [PubMed] [Google Scholar]
- 5.Stables GI, Forsyth A, Lever RS. Patch testing in children. Contact Dermatitis. 1996;34:341–4. doi: 10.1111/j.1600-0536.1996.tb02219.x. [DOI] [PubMed] [Google Scholar]
- 6.Katsarou A, Koufou V, Armenaka M, Kalogeromitros D, Papanayotou G, Vareltzidis A. Patch tests in children: A review of 14 years experience. Contact Dermatitis. 1996;34:70–1. doi: 10.1111/j.1600-0536.1996.tb02125.x. [DOI] [PubMed] [Google Scholar]
- 7.Sevila A, Romaguera C, Vilaplana J, Botella R. Contact dermatitis in children. Contact Dermatitis. 1994;30:292–4. doi: 10.1111/j.1600-0536.1994.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 8.Belsito DV. The diagnostic evaluation, treatment, and prevention of allergic contact dermatitis in the new millennium. J Allergy Clin Immunol. 2000;105:409–20. doi: 10.1067/mai.2000.104937. [DOI] [PubMed] [Google Scholar]
- 9.Sasseville D. Occupational contact dermatitis. Allergy Asthma Clin Immunonol. 2008;4:59–65. doi: 10.1186/1710-1492-4-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diepgen TL. Occupational skin-disease data in Europe. Int Arch Occup Environ Health. 2003;76:331–8. doi: 10.1007/s00420-002-0418-1. [DOI] [PubMed] [Google Scholar]
- 11.Bajaj AK, Saraswat A, Mukhija G, Rastogi S, Yadav S. Patch testing experience with 1000 patients. Indian J Dermatol Venereol Leprol. 2007;73:313–8. doi: 10.4103/0378-6323.34008. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson DS, Fregert S, Magnusson B, Bandmann HJ, Calnan CD, Cronin E, et al. Terminology of contact dermatitis. Acta Derm Venereol (Stockh) 1970;50:287–92. [PubMed] [Google Scholar]
- 13.Bruynzeel DP, Ferguson J, Andersen K, Goncalo M, English J, Goossens A, et al. Photopatch testing: A consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679–82. doi: 10.1111/j.1468-3083.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 14.Bourke J, Coulson I, English J. Guidelines for care of contact dermatitis. Br J Dermatol. 2001;145:877–85. doi: 10.1046/j.1365-2133.2001.04499.x. [DOI] [PubMed] [Google Scholar]
- 15.Hassan I, Rather PA, Jabeen Y, Wani ZA, Altaf H, Nisa N, et al. Preliminary experience of patch testing at Srinagar, Kashmir. Indian J Dermatol Venereol Leprol. 2013;79:813–6. doi: 10.4103/0378-6323.120737. [DOI] [PubMed] [Google Scholar]
- 16.Majid I. Contact allergens causing hand eczema in ethnic Kashmiri population: A study of 7-years. Indian J Dermatol. 2016;61:119. doi: 10.4103/0019-5154.174083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarma N, Ghosh S. Clinico-allergological pattern of allergic contact dermatitis among 70 Indian children. Indian J Dermatol Venereol Leprol. 2010;76:38–44. doi: 10.4103/0378-6323.58677. [DOI] [PubMed] [Google Scholar]
- 18.Sadagopan K, Kalappan D, Sivaprakasam N, Vinoth Patch test results from an occupational and contact dermatitis clinic in a tertiary care hospital of southern India: A retrospective study. J Clin Diagn Res. 2017;11:11–4. doi: 10.7860/JCDR/2017/26391.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iraji F, Asilian A, Enshaieh S, Shamoradi Z, Faghihi G. Contact dermatitis in cement workers in Isfahan. Indian J Dermatol. 2006;51:30–32. [Google Scholar]
- 20.Sharma V, Mahajan VK, Mehta KS, Chauhan PS. Occupational contact dermatitis among construction workers: Results of a pilot study. Indian J Dermatol Venereol Leprol. 2014;80:159–61. doi: 10.4103/0378-6323.129402. [DOI] [PubMed] [Google Scholar]
- 21.Handa S, Jindal R. Patch test results from a contact dermatitis clinic in North India. Indian J Dermatol Venereol Leprol. 2011;77:194–6. doi: 10.4103/0378-6323.77465. [DOI] [PubMed] [Google Scholar]
- 22.Beck MH, Wilkinson SM. Contact Dermatitis: Allergic. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. Vol. 2. West-Sussex, UK: Wiley-Blackwell; 2010. p. 1130. [Google Scholar]
- 23.Goh CL, Kowk SF, Gan SL. Cobalt and nickel content of Asian cements. Contact Dermatitis. 1986;15:169–72. doi: 10.1111/j.1600-0536.1986.tb01319.x. [DOI] [PubMed] [Google Scholar]
- 24.Sharma VK, Asati DP. Pediatric contact dermatitis. Indian J Dermatol Venereol Leprol. 2010;76:514–20. doi: 10.4103/0378-6323.69070. [DOI] [PubMed] [Google Scholar]
- 25.Shenoi DS, Srinivas CR, Balachandran C. Results of patch testing with a standard series of allergens at Manipal. Indian J Dermatol Venereol Leprol. 1994;60:133–5. [Google Scholar]
- 26.Morris SD, Rycroft RJ, White IR, Wakelin SH, McFadden JP. Comparative frequency of patch test reactions to topical antibiotics. Br J Dermatol. 2002;146:1047–51. doi: 10.1046/j.1365-2133.2002.04662.x. [DOI] [PubMed] [Google Scholar]
- 27.Andersen K, White I, Goossens A. Allergens from the standard series. In: Frosch PJ, Menné T, Lepoittevin J-P, editors. Contact Dermatitis. 4th ed. Berlin: Springer; 2006. p. 488. [Google Scholar]
- 28.Le Coz CJ, Ducombs G. Plants and plant products. In: Frosch PJ, Menné T, Lepoittevin J-P, editors. Contact Dermatitis. 4th ed. Berlin: Springer; 2006. pp. 751–800. [Google Scholar]
- 29.Hassan I, Kamili A, Rasool F, Nehvi F, Rather P, Yasmin S, et al. Contact dermatitis in saffron workers: Clinical profile and identification of contact sensitizers in a saffron-cultivating area of Kashmir Valley of North India. Dermatitis. 2015;26:136–41. doi: 10.1097/DER.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 30.Hassan I, Rasool F, Akhtar S, Kamili A, Rather P, Kanth R, et al. Contact dermatitis caused by tulips: Identification of contact sensitizers in tulip workers of Kashmir Valley in North India. Contact Dermatitis. 2018;78:64–9. doi: 10.1111/cod.12870. [DOI] [PubMed] [Google Scholar]


