Abstract
Introduction:
Cutaneous leishmaniasis is a vector borne disease caused by Leishmania major and Leishmania tropica. Bikaner is an endemic pocket for cutaneous leishmaniasis caused by Leishmania tropica.
Materials and Methods:
A prospective study was done to evaluate the efficacy of different concentrations of intralesional amphotericin B as a treatment modality for cutaneous leishmaniasis in Bikaner, Rajasthan, India from January 2016 to June 2017. Fifty patients were randomized into two groups, A and B. Twenty-five patients from group A, received intralesionl amphotericin B (2.5 mg/ml) 0.5 ml/cm2, weekly for 8 weeks. Another group of 25 patients were treated by intralesional amphotericin B (5.0 mg/ml) weekly for same period. The cases were followed-up for response, side effects, and recurrence of disease.
Results:
The results at the end of 8 weeks, showed complete response in 18 (72%) patients, partial response in 5 (20%) and 2 (8%) patients were non responders in group A. In group B, complete response was observed in 14 (56%), partial response in 7 (28%) patients and 4 (16%) patients did not show response. The difference was statistically insignificant (P > 0.05). No side effects were observed in both groups.
Conclusion:
The difference between the efficacy of 5 mg/ml and 2.5 mg/ml concentrations of Amphotericin B injections was found to be statistically insignificant. So, weekly injections of amphotericin B looks promising, however, larger sample size is required to assess the efficacy of both concentrations in the treatment of cutaneous leishmaniasis.
Keywords: Amphotericin B, cutaneous leishmanisis, intralesional
Introduction
Cutaneous leishmaniasis (CL) is one of the important protozoan zoonotic disease, second highest amongst parasitic disease after malaria.[1] Leishmaniasis threatens 350 million people in 88 countries where the disease is endemic i.e., 10% of world population suffers from it. With 12 million cases worldwide, over 1.5 million people report as new cases of cutaneous leishmaniasis annually, while many more cases go unreported.[2] It is also known by various local names as first diagnosed viz. Delhi boil, Oriental sore, Tropical sore, Baghdad sore, Lahore ulcer. In India, CL is reported primarily in some pockets in the Thar Desert of Rajasthan state, located in the Northwestern part of the country and bordering Pakistan.[3] Spreading in nonendemic areas, few cases of cutaneous leishmaniasis were detected by Sharma et al.[4] from Himachal Pradesh.
Old world cutaneous leishmaniasis is caused by L. major, L. tropica, and L. aethiopica. New world cutaneous leishmaniasis is caused by L. Mexicana, L. amazonesis, and L. brazilienisis.[5]
Clinical presentations of cutaneous leishmaniasis are extremely diverse and depend on parasite species, size of inoculum and host cellular immune response. Cutaneous leishmaniasis begins as a red papule, may transform into a nodule and plaque that later develop into skin ulcers in weeks to months after infection.[6]
Often the lesions of cutaneous leishmaniasis heal spontaneously with scar formation. However, treatment is recommended to accelerate cure and to reduce the scar formation, especially at cosmetically important sites of the body.[7] Antimoniate compounds have been used to treat cutaneous leishmaniasis since 1950 and are considered as the most appropriate drugs and the first line of treatment.[8] Nowadays, with an increase in the resistance to antimoniates, alternative therapies including amphotericin B (AmB) have been highly considered.[9]
Amphotericin B, even though a very effective option, carries the risk of severe systemic toxicity. The dose is l mg/kg per day with a maximum of 50 mg per dose. It is prepared by diluting it in 500 mL of 5% dextrose and delivered on alternate days up to a total dose of 1 to 1.5 g, while liposomal amphotericin is used at 2 to 3 mg/kg as a total dose over the course of 20 days. Intralesional injection of Amphotericin B offers a much better alternative in terms of adverse effect profile.
We undertook this study to evaluate the efficacy and safety of two different concentrations of intralesional amphotericin B in treatment of cutaneous leishmaniasis, and to find out the minimum effective dose with lesser side effects of AmB.
Materials and Methods
The prospective study was performed on the patients between January to June 2016-17, who had attended the outpatient department of our institute. The patients considered for this study had confirmed diagnosis of cutaneous leishmaniasis based on skin smear for Leishman Donovan (LD) bodies.
Fifty patients from both sexes, were randomly divided into two groups, group A and B. Twenty-five patients from group A, received intralesionl amphotericin B (2.5 mg/ml), 0.5 ml/cm2 of lesion, once a week, for 8 weeks. The other group of 25 patients (group B) were treated by intralesional amphotericin B (5.0 mg/ml) once a week for same duration. The cases were followed up for 6 months for response, possible side effects and recurrence of disease.
Multiple injections were applied at the margin of the lesions at 0.5 cm interval, with blanching as the end point of injection at each site. Maximum dose did not exceed the systemically permitted maximum dose limit (50 mg per dose).
Routine hematological investigations including complete blood cell count, liver function tests and renal function tests were done at baseline and again at the end of the study.
Epidemiological data, clinical features, investigations, treatment, and follow up were recorded. Patients less than 5 years of age, associated with systemic illness, previous history of anti-leishmanial therapy, defaulters, and pregnant or lactating females were excluded.
The final concentration of 2.5 mg/ml, was prepared by diluting 0.25 ml of stock solution (10 mg/ml) with 0.75 ml of distilled water and for concentration of 5.0 mg/ml, 0.5 ml of stock solution was diluted with 0.5 ml of distilled water and then injected intralesionally.
Size, induration, and ulceration of the lesion and smear positivity were measured before starting the treatment and then at the end of 8th week and 12th week. The obtained results were categorized as, Complete remission (more than 90% reduction in size, induration, and ulceration; skin smear negative), Partial remission (60–90% reduction in size, induration, and ulceration; smear negative), and no response (less than 60% reduction in size, induartion and ulceration; persistent smear positivity).
Written informed consent was taken from all the patients. Ethical clearance was taken from the institutional ethical clearance board.
Statistical analysis was carried out by using API -INFO software. Data were presented as number (%) or Mean ± SD. Categorical variables were compared between the groups using Chi square test. P value <0.05 was considered statistically significant.
Results
Fifty patients were included in this study for intralesional amphotericin B injection. In Group A, the mean age of the patients was 33.00 ± 19.17 years (range 6-64 years), 40% of them were males and 60% were females. In Group B, the mean age of the patients was 28.79 ± 17.08 years (range, 8–67 years), 52% of them were males and 48% were females. All the cases, in both groups, were positive for skin smear for LD bodies. Demographic characteristics of the patients in both groups have been shown in Table 1.
Table 1.
Demographic and cutaneous leishmaniasis characteristics of the studied population
| Group A (AmB 2.5 mg/ml) | Group B (AmB 5.0 mg/ml) | P | |
|---|---|---|---|
| No. of patients | 25 | 25 | |
| Sex | |||
| Male | 10 | 16 | |
| Female | 15 | 9 | |
| Age | |||
| (mean±SD) | 33.00±19.17 | 28.79±17.08 | |
| Habitat | |||
| Rural | 18 | 16 | |
| Urban | 7 | 9 | |
| Duration of lesions | |||
| 0-6 months | 15 | 18 | |
| >6 months | 10 | 7 | |
| Type of lesions | |||
| Ulcerated Plaque | 20 | 16 | |
| Erythematous Plaque | 12 | 09 | |
| Erythematous Nodule | 8 | 03 | |
| Site of lesions | |||
| Head and Neck | 11 | 09 | |
| Upper limb | 20 | 10 | |
| Lower limb and trunk | 9 | 9 |
As far as distribution of lesions was concerned, out of total 68 lesions, maximum number of the lesions were present over exposed parts of the body like, face (29.41%) followed by hands (25%) and forearm (19.12%), in both the groups.
Regarding the disease duration, 15 patients (60%) in Group A and 18 patients (72%) in Group B had disease for less than 6 months.
In our study, out of total 68 lesions, maximum number of lesions were ulcerated plaques (36) followed by erythematous plaque (21), and erythematous nodules (11) in both the groups.
Out of fifty, maximum number (34) of patients belonged to rural areas in both the groups and most of the patients (29) had single lesion.
The results were based on the change in size, induration and smear positivity at the end of 8th week and 12th week. Table 2 compares the therapeutic response after 8 weeks in the studied groups. Group A- Figure 1a and b. Group B- Figure 2a and b.
Table 2.
Therapeutic response in both studied groups at the end of 8 weeks
| Treatment response | Group A (AmB 2.5 mg/ml) (n=25)/% | Group B (AmB 5.0 mg/ml) (n=25)/% | P |
|---|---|---|---|
| Complete response | 18 (72) | 4 (56) | 0.472 |
| Partial response | 5 (20) | 7 (28) | |
| No response | 2 (8) | 4 (16) |
Figure 1.
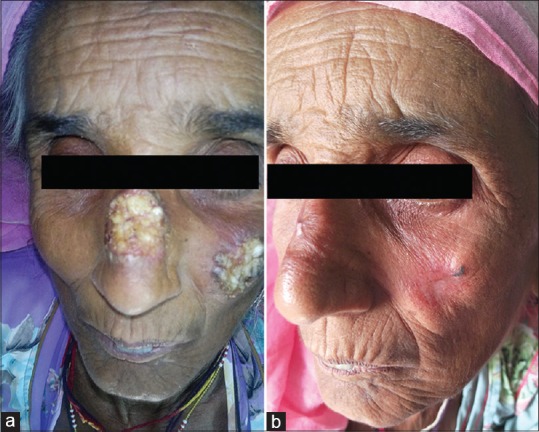
(a) Ulcerated indurated plaques, confirmed case of cutaneous leishmaniasis, treated with intra-lesional injections of 2.5mg/ml Amphotericin B, photograph taken at baseline. (b) Complete resolution of the lesion post treatment (with 8 weekly injections of 2.5 mg/ml Amphotericin B), with minimal residual scarring, photograph recorded 12 weeks after first session (Group A)
Figure 2.
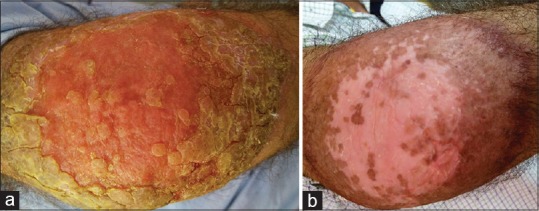
(a) Ulcerated plaque on the right elbow, confirmed case of cutaneous leishmaniasis, treated with intra-lesional injection of 5.0 mg/ml Amphotericin B, photograph taken at baseline. (b) Complete resolution of the lesion post treatment (with 8 weekly injections of 5 mg/ml Amphotericin B), with residual hypopigmentation and hyperpigmentation, photograph recorded 12 weeks after first session (Group B)
As seen in Table 2, at the end of 8 weeks, in group A, 18 (72%) patients showed complete response, 5 (20%) had partial remission and 2 (8%) were non responders while in group B 14 (56%) patients recovered completely, and 7 (28%) patients had partial response. The difference was not statistically significant (P = 0.47).
Table 3 summarizes the results after 12 weeks in the studied groups.
Table 3.
Therapeutic response in both studied groups at the end of 12 weeks
| Treatment response | Group A (AmB 2.5 mg/ml) (n=25)/% | Group B (AmB 5.0 mg/ml) (n=25)/% | P |
|---|---|---|---|
| Complete response | 22 (88) | 16 (64) | |
| Partial response | 2 (8) | 6 (24) | 0.139 |
| No response | 1 (4) | 3 (12) |
At the end of 12 weeks, in group A, 22 (88%) patients recovered completely, 2 (8%) showed partial remission and 1 was non responder. In group B 16 (64%) patients showed complete response, 6 (16%) had partial response and 3 (12%) patients did not show any response (P = 0.139).
Out of 50, 8 Patients had more than single lesion. The clinical response was similar in patients having more than single lesion.
The side effects of intralesional injection were only pain during injection, was perceived by all the patients but it did not last for more than 30 minutes and did not require discontinuation of treatment. Upon 6 months of follow-up, there was no recurrence of the disease in either group.
Discussion
On the basis of geographic distribution, cutaneous leishmaniasis is divided into Old World and New World leishmaniasis. While the Old World species mostly cause benign and often self-limiting disease, the New World species can cause severe manifestations, including mucosal involvement.[10]
Bikaner is an endemic pocket for cutaneous leishmaniasis, mainly caused by Leishmania tropica.[11] Pentavalent antimoniates have long been used for the treatment of cutaneous leishmaniasis, but acquired drug resistance has increased during recent years.[12,13] It requires a strong need for new alternative treatment modalities. Although cutaneous leishmaniasis is a self-limiting disease, the main goal in its treatment would be controlling the spread of the disease in endemic region along with minimizing incidence of scar formation.[14,15,16] Systemic liposomal amphotericin B has been used in the treatment of drug resistant cutaneous leishmaniasis.[14,15,16]
Amphotericin B is a polyene antibiotic, first isolated from streptomyces nodosus, is a broad antimycotic and antiparasitic agent.[17,18] It is a membrane active drug that forms channel-like structures (pores), spanning the lipid bilayer.[17,18,19] AmB deoxycholate is active against leishmania species and commonly administered intravenously in treatment of visceral and mucocutaneous leishmaniasis but has high incidence of adverse reactions like hyperpyrexia, hypotension, hypokalemia, renal toxicity, hepatitis, and anemia. However, their cost has limited their use in cutaneous leishmaniasis[11]
Several modifications of AmB molecule and changes in delivery systems, have been used to improve efficacy and reduce its toxicity.[20] Liposomal preparations of amphotericin B are superior to AmB emulsions or colloidal formulations in terms of bioavailability and side effects. AmB in plasma remain largely associated with liposomes for longer duration and is slowly released by liposomal delivery system.[21] However, toxic side effects of a drug greatly decrease with intralesional injection of amphotericin B for CL and can be considered as an alternative treatment in the areas of resistance to antimoniate.[22]
Previous studies have shown the usefulness of amphotericin B in the treatment of CL. Layegh et al.[13] compared the efficacy of topical liposomal amphotericin B lotion and intralesional antimoniate in Iran. 56.4% improvement in amphotericin B treated group versus 67.6% response in antimoniate treated group, was observed. Vardy et al.[23] reported the first successful topical use of amphotericin B in a 5% ethanol solution for the treatment of cutaneous leishmaniasis. Vahid et al.[24] studied the efficacy of intralesional amphotericin B for treatment of cutaneous leishmaniasis in Mashhad, Iran, AmB 2mg/ml was injected into lesions weekly for up to 12 weeks. At the end of 12th week, 61.4% of the patients showed complete recovery and 21.6% had partial remission.
Pentavalent antimonial drugs given parenterally or intralesionally, remains the first line therapy.[25] Intralesional pentavalent antimoniate reduces systemic toxic side effects and cost of therapy. But local treatment can only be applied in Old World cutaneous leishmaniasis and L. mexicana infection with small single lesions. In all the other New World species and Old World cutaneous leishmaniasis presenting with multiple or large lesions (>5 cm) and metastatic lesions, parenteral drugs should be used.[11]
Alternative regimens include parenteral pentamidine, topical paromomycin, miltefosin, oral rifampicin, and thermotherapy depending on the leishmania species.[25]
Oral imidazole (fluconazole, itraconazole) may be considered in complex lesions and those which have potential to land as mucosal leishmaniasis.[26]
A pilot study was done by Bumb et al.,[27] in 2002, role of oral rifampicin 600 mg bid or 20 mg/kg body weight in CL with multiple lesions, study showed 83.3% parasitological and clinical cure after four weeks of therapy. Again Bumb et al.,[28] in 2010, did a study on efficacy of intralesional sodium stibogluconate, 50 mg/cm2 of lesion in treatment of cutaneous leishmaniasis, found that short duration, twice weekly intralesional SSG treatment for CL accelerates cure, and highly effective. Bumb et al.[29] compared the long-term efficacy of radiofrequency induced heat therapy versus intralesional sodium stibogluconate in 100 patients of CL, found that single dose heat therapy was effective in 94% cases and SSG in 92% of cases.
Topical treatment of cutaneous leishmaniasis still poses a challenge. Nowadays, due to increased resistance and unavailability of antimoniates in the market, we tried weekly intralesional injections of amphotericin B as an alternative treatment modality.
This study compares the therapeutic efficacy of two different concentrations of intralesional amphotericin B for 50 cases of cutaneous leishmaniasis. There was no significant difference (P > 0.05) in both the groups, indicating that both concentrations of intralesional amphotericin B has a similar efficacy in the treatment of cutaneous leishmaniasis. But the subjective response to 2.5 mg/ml was more than to 5.0 mg/ml with faster healing of lesions. So, lower dose of amphotericin B can be considered as an alternative treatment modality for cutaneous leishmaniasis. However, larger sample size is required to support the evidence of our study.
The systemic toxic side effects of amphotericin B were not seen due to much lower doses of drug absorption and reached to kidney compared to systemic administration. Our study showed that intralesional AmB is safer with no recurrence during follow up and is given in outpatient setting. Local pain at injection sites did not cause discontinuation of treatment in any patient. There was no relapse during 6-months of follow up period. It should be kept in mind that multiple painful injections are required, so treatment would be quite difficult in children.
Our study had limitation that we did not determine the parasite species by molecular methods and culture. Also, histopathology was not done to assess the lesions pre- and post-treatment.
Conclusion
Intralesional amphotericin B is equally effective at a dose of 2.5 mg/ml and 5.0 mg/ml. It accelerates cure; hence, it can be considered as an alternative treatment modality for treatment of cutaneous leishmaniasis. As there is no significant difference in efficacy between the two concentrations, 2.5 mg/ml should be preferred due to lower systemic absorption as well as a lower risk of toxicity.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–9. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.Grevelink SA, Lerner EA. Leishmaniasis. J Am Acad Dermatol. 1996;34:257–72. doi: 10.1016/s0190-9622(96)80121-6. [DOI] [PubMed] [Google Scholar]
- 3.Lodha KR, Singh BB, Jatkar PR. Cutaneous leshmaniasis in Bikaner, Rajasthan. Indian Vet J. 1971;48:121–3. [PubMed] [Google Scholar]
- 4.Sharma RC, Mahajan VK, Sharma NL, Sharma A. A new focus of cutaneous leishmaniasis in Himachal Pradesh (India) Indian J Dermatol Venerol Leprol. 2003;69:170–72. [PubMed] [Google Scholar]
- 5.Bari Afran UL. Epidemiology of cutaneous leishmaniasis. J Pak Assoc Dermatol. 2006;16:156–62. [Google Scholar]
- 6.Vega-Lopez F, Hay RJ. 8th ed. Blackwell Publishing; 2010. Parasitic worms and Protozoa. Rook's Textbook of Dermatology; pp. 37.32–43. [Google Scholar]
- 7.Murray HW, Berman JD, Davis CR. Advances in leishmania. Lancet. 2005;366:1561–77. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 8.Layegh P, Rahsepar S, Rahsepar AA. Systemic meglumine antimoniate in acute cutaneous leishmaniasis: Children versus adult. Am J Trop Med Hyg. 2011;84:539–42. doi: 10.4269/ajtmh.2011.10-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Mohammed HI, Chance ML, Bates PA. Production and characterization of stable amphotericin resistant amastigote and promastigotes of Leishmania Mexicana. Antimicrob Agents Chemother. 2005;49:3274–80. doi: 10.1128/AAC.49.8.3274-3280.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum J, Desjeux P, Schwart E. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53:158–66. doi: 10.1093/jac/dkh058. [DOI] [PubMed] [Google Scholar]
- 11.Kumar R, Bumb RA, Ansari NA, Mehta RD, Salotra P. Cutaneous Leishmaniasis caused by L. tropica in Bikaner, India: Parsite identification and charactorization using molecular and immunological tools. Am J Trop Med Hyg. 2007;76:896–901. [PubMed] [Google Scholar]
- 12.Hadighi R, Mohebali M, Boucher P. Unresponsiveness to glucantime treatment in Iranian CL due to drug resistence Leishmania tropica parasites. PLoS Medicine. 2006;3:659–67. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Layegh P, Rahsepar S, Rahsepar AA. Systemic meglumine antimoniate in acute cutaneous leishmaniasis: Children versus adult. Am J Trop Med Hyg. 2011;84:539–42. doi: 10.4269/ajtmh.2011.10-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paradisi A, Capizzi R, Zampetti A, Proietti I, De Simone C, Feliciani C, et al. A typical multifocal cutaneous leishmaniasis in an immunocompetent patient treated by liposomal amphotericin B. J Infect. 2007;54:208. doi: 10.1016/j.jinf.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Amato VS, Rabello A, Rotondo-Silva A, Kono A, Maldonado TP, Alves IC, et al. Successful treatment of cutaneous leishmaniasis with lipid formulation of amphotericin B in two immunocompromised patients. Acta Tropica. 2004;92:127–132. doi: 10.1016/j.actatropica.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Brown M, Noursadeghi M, Boyle J, Davidson RN. Succeesful liposomal amphotericin B treatment of braziliensis cutaneous leishmaniasis. British J Dermatol. 2005;153:203–5. doi: 10.1111/j.1365-2133.2005.06670.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohen BE. Amphotericin B toxicity and lethality: A tale of two channels. Int J Pharm. 1998;162:95–106. [Google Scholar]
- 18.Lemke A, Kinderle AF, Kayser O. Amphotericin B. Appl Microbiol Biotechnol. 2005;68:151–62. doi: 10.1007/s00253-005-1955-9. [DOI] [PubMed] [Google Scholar]
- 19.Kleinberg ME, Finkelstein A. Single length and double length channels formed by nystatin in lipid bilayer membrane. J Membr Biol. 1984;80:257–69. doi: 10.1007/BF01868444. [DOI] [PubMed] [Google Scholar]
- 20.Brajtburg J, Bolard J. Carrier effect on biological activity of amphotericin B. Microbiol Rev. 1996;9:512–31. doi: 10.1128/cmr.9.4.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekersky I, Fielding, Dressler DE, Lee JW, Buell DN. Plasma protein binding of Amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B and amphotericin deoxycholate. Antimicrob Agents Chemother. 2002;46:834–40. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman JD. Human leishmanisis: Clinical, diagnostic and chemotherapeutic developments in the last 10 years. Clin Infect Dis. 1997;24:684–703. doi: 10.1093/clind/24.4.684. [DOI] [PubMed] [Google Scholar]
- 23.Vardy D, Barenholz Y, Cohen R, Zvulunov A, Biton A. Topical Amphotericin B for cutaneous leishmaniasis. Archi Dermatol. 1999;135:856–57. doi: 10.1001/archderm.135.7.856. [DOI] [PubMed] [Google Scholar]
- 24.Vahid MG, Elham V, Bita K, Yalda N. Efficacy of intralesional amphotericin B in cutaneous leishmaniasis. Indian J Dermatol. 2014;59:631. doi: 10.4103/0019-5154.143571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reithinger R, Dujardin JC, Louzir B. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7:581–96. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- 26.Baily MS, Lockwood DN. Cutaneous leishmaniasis. Clin Dermatol. 2007;25:203–11. doi: 10.1016/j.clindermatol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Bumb RA, Mehta RD. Oral rifampicin in cases of CL with multiple lesions (pilot study) Indian J Dermatol Venereol Leprol. 2002;68:272. [PubMed] [Google Scholar]
- 28.Bumb RA, Mehta RD, Ghiya BC, Jakhar R, Prasad N, Soni P. Efficacy of short duration intralesional sodium stibogluconate in treatment of cutaneous leishmaniasis in India. Br J Dermatol. 2010;163:854–8. doi: 10.1111/j.1365-2133.2010.09865.x. [DOI] [PubMed] [Google Scholar]
- 29.Bumb RA, Prasad N, Khandelwal K, Aara N, Mehta RD, Ghiya BC, et al. Long term efficacy of single dose radiofrequency induced heat therapy vs. intralesional antimonials for cutaneous leishmaniasis in India. Br J Dermatol. 2013;168:1114–9. doi: 10.1111/bjd.12205. [DOI] [PubMed] [Google Scholar]


