Abstract
Background:
Skin problems are commonly encountered in the pediatric emergency department (PED). Although there are a few studies on the prevalence and spectrum of skin conditions in children attending the PED, only limited information is available on the outcome of the children with skin-related ailments requiring hospitalization.
Aim:
To study the clinical profile of skin manifestations in children presenting to the PED over a period of one year and assess the impact of skin lesions on the clinical outcome.
Materials and Methods:
All children <16 years of age attending the PED were screened and children with skin lesions were referred to the dermatologist for further evaluation, and those admitted were followed up until discharge. Children with skin lesions were categorized into seven subsets based on their diagnosis. Outcomes evaluated were duration of hospital stay, associated systemic inflammatory response syndrome (SIRS), and mortality.
Results:
Of the 24,324 patients screened, 203 (0.83%) had skin lesions, of whom 158 (77.83%) were discharged from the PED. Forty five (22.16%) patients required admission of whom 2 (0.99%) died. Inflammatory disorders were the most common, 102 (50.24%), followed by infections in 91 (44.82%) patients. Among the hospitalized patients, 25 (55.6%) had SIRS, which included infections in 14 (56%), vasculitis in 5 (20%), and urticaria in 3 (12%) patients. Two patients with SIRS died and the causes were purpura fulminans and febrile exanthem of probable viral etiology.
Conclusion:
Our study highlights the spectrum of pediatric cutaneous emergencies and their outcome. A subset of patients can present with severe skin ailments and SIRS in whom early diagnosis and prompt treatment can impact the outcome.
Keywords: Cutaneous emergencies, pediatric emergency department, skin manifestations, systemic inflammatory response syndrome
Introduction
Skin problems are commonly encountered in the pediatric emergency department (PED) and are assumed that they do not cause any critical illness or impact the overall outcome. Skin disorders in children presenting to emergency department (ED) are broadly classified into patients with primary skin conditions, patients with systemic diseases associated with skin manifestations, and those with skin manifestations as a complication of treatment given for other systemic conditions.[1] Although most are not true emergencies, some are severe and life-threatening, requiring appropriate diagnosis and management.[2]
Few studies have looked into the prevalence and clinical profile of dermatological conditions presenting to the PED,[2,3,4,5,6] of which two are from India. However, very limited information is available on the outcome of the children with skin-related ailments requiring hospitalization. Our study is unique because we looked at the prevalence and spectrum of skin manifestations in children presenting to the PED and evaluated the associated systemic manifestations, abnormal laboratory parameters, and clinical outcomes of the admitted patients, whereas other studies have focused only on the skin.
Materials and Methods
A prospective cohort study was conducted in a tertiary care Institute in south India over a period of 1 year (August 2015–July 2016) after Institutional Review Board approval. All children <16 years of age presenting to the PED were screened and those with skin manifestations at initial presentation, skin manifestations detected by PED physician after screening, or developed skin lesions while under observation in the PED were included in the study after obtaining informed consent/assent from parents/children, respectively. Patients with cutaneous manifestations secondary to trauma were excluded. Children with skin lesions were referred to the dermatology unit for further evaluation and those admitted were followed-up until discharge. The spectrum of skin lesions, related systemic manifestations, and impact of skin lesions on the clinical outcome in those admitted were assessed. Based on the diagnosis, patients were categorized into seven subsets, as shown in the results below.[7] Outcomes evaluated were duration of hospital stay, associated systemic inflammatory response syndrome (SIRS), and mortality. We used the criteria proposed by Goldstein et al. in 2005 at the International Consensus Conference on Pediatric Sepsis, to diagnose SIRS in children.[8] The data entry was done using Epidata 3.1 software and the data was analyzed using Microsoft Excel and SPSS 16.0 software.
Results
In a year, 24,324 children were seen in the PED, of whom 203 (0.83%) had cutaneous manifestations. The mean age of the children was 4.88 ± 4.04 years (range: 1 month to 15 years); majority of them (43.84%) were between 1 and 5 years, and male:female ratio was 125:78 (1.60:1). Among the 203 children, 158 (77.83%) were discharged from the PED, 45 (22.16%) required admission, of whom 2 (0.99%) died. Among the 45 admitted children, 43 (95.55%) improved at discharge.
The prevalence of skin diseases was higher in the months of January (10.83%), March (10.34%), and May (10.83%) in comparision with the other months. In summer, viral infections were more frequent (25/51, 49.01%) than other skin conditions (35/152, 23.02%) (P = 0.024).
Spectrum of skin disorders
The skin lesions were categorized into seven categories: (1) skin manifestations secondary to infections—91 (44.82%); (2) inflammatory disorders—102 (50.24%); (3) disorders of epidermal differentiation and keratinization—4 (2%); (4) connective tissue diseases—2 (1%); (5) coagulation disorders—1 (0.5%); (6) immunobullous disorders—1 (0.5%); (7) skin appendageal disorders—2 (1%).[7] Although inflammatory disorders were the most common, infections were more prevalent in the 1–5 years age group (P = 0.006).
Inflammatory skin disorders
In total, 102 (50.24%) (M:F 62:40) children had inflammatory skin disorders [Table 1] of which urticaria was the most common. Among 45 children (M:F 28:17) with urticaria, one had associated angioedema; infectious etiology was proven in 13 patients and suspected in six patients in view of elevated counts; abnormal urine microscopy with elevated C-reactive protein (CRP) and drug-induced etiology was considered in four patients. Twenty-eight patients (62.2%) had associated systemic symptoms, of whom 5 (11.11%) were hospitalized with a mean stay duration of 2.8 days (range 2–5 days). The total WBC count was elevated in the hospitalized children (P = 0.014).
Table 1.
Spectrum of the inflammatory skin conditions (n=102/203)
| Diagnosis | No. of patients (%) |
|---|---|
| Urticaria | 45 (22.17) |
| Papular urticaria | 20 (9.85) |
| Vasculitis | 9 (4.4) |
| Henoch-Schönlein purpura | 6 |
| Kawasaki disease | 2 |
| Cutaneous small vessel vasculitis | 1 |
| Erythema multiforme | 5 (2.46) |
| Drug rash with eosinophilia and systemic symptoms | 4 (1.97) |
| Acute generalized exanthematous pustulosis | 2 (0.99) |
| Stevens-Johnson syndrome | 1 (0.5) |
| Toxic epidermal necrolysis | 3 (1.48) |
| Drug-induced exanthem | 1 (0.5) |
| Pityriasis rosea | 3 (1.48) |
| Seborrheic dermatitis | 4 (1.97) |
| Eczema | 3 (1.48) |
| Lichen striatus | 1 (0.5) |
| Anaphylaxis | 1 (0.5) |
Among the 9 (4.5%) children with vasculitis (M:F 6:3), 8 (88.88%) were admitted, which included six children with Henoch-Schönlein purpura (HSP). The mean period of hospitalization was 2.44 days (range 3–5 days). The most common systemic symptoms in HSP children were fever (83.3%) and abdominal pain (66.7%).
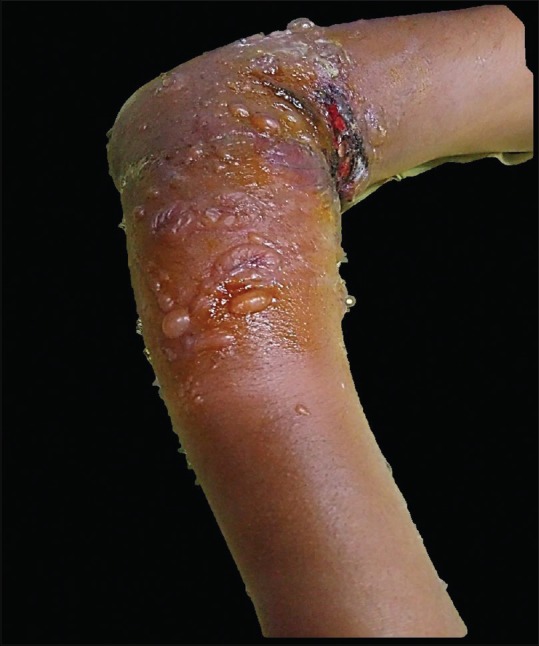
Twelve children (5.9%) presented with suspected cutaneous drug reactions, which included anticonvulsant-induced drug rash eosinophilia and systemic symptoms (DRESS) syndrome in four; toxic epidermal necrolysis (TEN) in three [Figure 1]; acute generalized exanthematous pustulosis (AGEP) in two; and Stevens-Johnson syndrome (SJS) in one [Figure 2]; co-trimoxazole-induced maculopapular exanthem and cefazolin-induced anaphylaxis were seen in one each. In two out of three children with TEN, a drug-induced etiology could not be established. Altered liver function was seen in 8/12 (66.7%) children.
Figure 1.
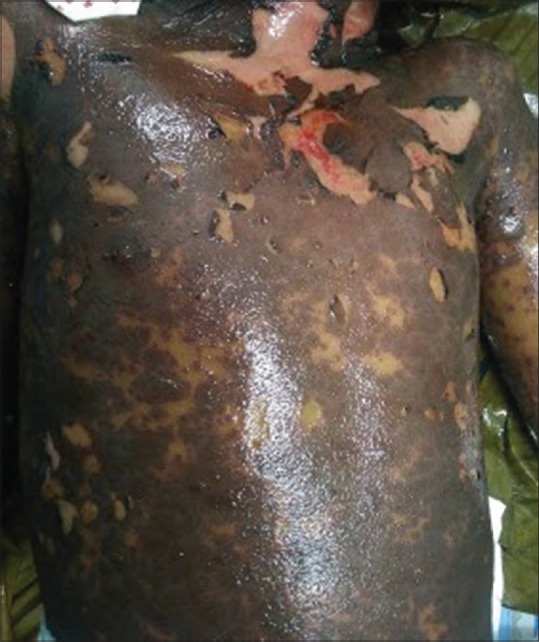
Sheets of skin detachment and erosions in toxic epidermal necrolysis
Figure 2.
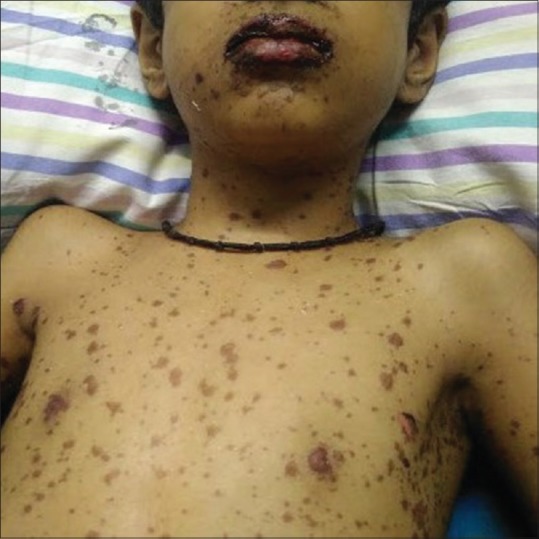
A child with Stevens-Johnson syndrome due to phenytoin
Infections
Table 2 shows that 91 (44.82%) patients had skin lesions secondary to infections and infestations: viral in 61 (67.03%), bacterial in 26 (28.57%), and parasitic infestations in 4 (4.39%) children.
Table 2.
Spectrum of the infectious skin conditions (n=91/203)
| Viral infections (n=61) | n (%) | Bacterial infections (n=26) | n (%) | Parasitic infections (n=4) | n (%) | |
|---|---|---|---|---|---|---|
| Hand, foot and mouth disease | 17 (18.68) | Primary cutaneous bacterial infections 19/26 | Impetigo | 10 (10.1) | Scabies (1/4 also had secondary bacterial infection) | 4 (4.4) |
| Varicella | 8 (8.79) | SSSS | 8 (8.8) | |||
| Dengue | 4 (4.4) | Cellulitis | 1 (1.1) | |||
| Gianotti Crosti syndrome | 3 (3.29) | Systemic bacterial infection with secondary cutaneous manifestations 7/26 | Rickettsial infection | 4 (4.4) | ||
| Measles | 2 (2.2) | Purpura fulminans | 2 (2.2) | |||
| Molluscum contagiosum | 1 (1.1) | Staphylococcal scarlatina | 1 (1.1) | |||
| Orofacial herpes | 1 (1.1) | |||||
| Suspected viral exanthem | 25 (27.47) | |||||
Viral exanthem was suspected in 25 children who presented with fever and maculopapular rash. Seven (11.47%) of 61 patients with viral infections were admitted with a mean period of hospitalization of 6.6 days (range 3–16 days). Fever was present in all admitted children (7/7) compared to those who were not admitted (19/54) (P = 0.004).
Eleven (42.30%) out of 26 patients with bacterial infections required hospitalization and the mean duration of hospitalization was 4.6 days (range 3–9 days). Fever was more prevalent among the hospitalized than those who were discharged (P = 0.011). Purpura fulminans [Figure 3] was seen in two children due to meningococcemia and rickettsial infection.
Figure 3.
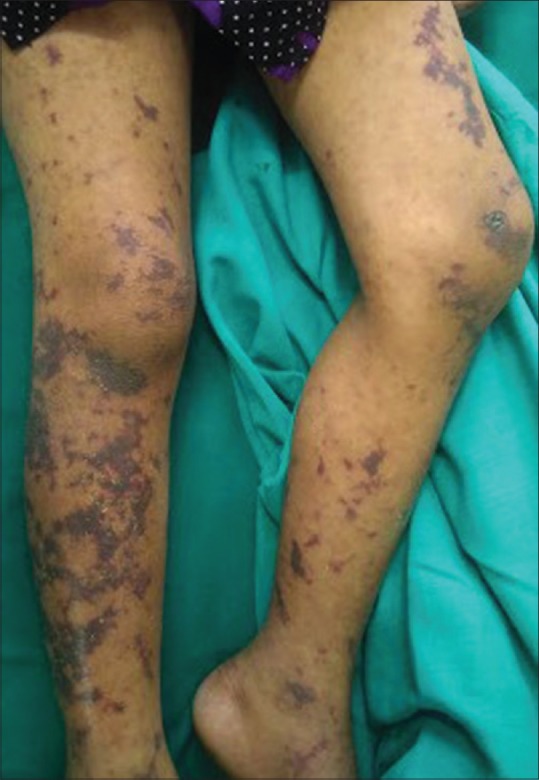
Sharply demarcated retiform purpura in a child with purpura fulminans
Connective tissue diseases
Two girls were diagnosed as systemic lupus erythematosus [Figure 4] of whom one had SIRS requiring admission.
Figure 4.
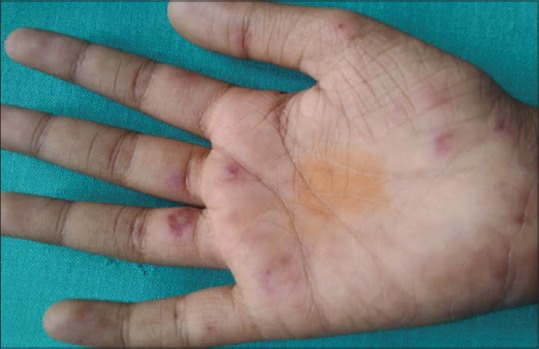
Multiple vasculitic lesions on the palm of a child with systemic lupus erythematosus
Immunobullous disorders
A 12-year-old girl presented to the PED with recalcitrant oral and genital erosions. She was admitted for evaluation and discharged a week later with a final diagnosis of paraneoplastic pemphigus (PNP), associated with anterior mediastinal Castleman's disease.
Others
Three patients had irritant contact dermatitis [Figure 5], two had miliaria, one patient had ichthyosis vulgaris and one patient with cortical venous thrombosis had ecchyomtic lesions.
Figure 5.
Irritant contact dermatitis
Systemic symptoms and SIRS
One hundred and sixteen (57.14%) patients with dermatological problems had associated systemic symptoms and signs, of whom 45/203 (22.16%) required admission and their diagnoses are shown in Table 3. The mean duration of hospital stay in patients with a primary dermatological diagnosis was higher (4.56 days) compared to other patients (3.83 days), but statistically insignificant (P = 0.503). Mean age of hospitalized patients was significantly higher (6.11 years) than the nonhospitalized (4.44 years) (P = 0.013). Among the hospitalized, 25 (55.55%) patients satisfied the criteria for SIRS [Figure 6]. The associated systemic symptoms and abnormal laboratory investigations in patients with SIRS are summarized in Table 4. The mean duration of hospital stay of children with SIRS vs. those without SIRS was 4.41 days (range 2–16 days) vs. 3.12 days (range 2–4 days) (P = 0.552).
Table 3.
Diagnoses of the admitted patients (n=45/203)
| Primary dermatological conditions (21/45) | Nonprimary dermatological conditions (24/45) | ||
|---|---|---|---|
| Diagnosis | No. of patients | Diagnosis | No. of patients |
| TEN | 3 | Dengue with exanthem | 3 |
| DRESS syndrome | 4 | Measles with exanthem | 2 |
| Erythema multiforme | 4 | Febrile exanthem | 2 |
| AGEP | 1 | Henoch-Schönlein purpura | 6 |
| SSSS | 7 | Urticaria | 5 |
| PNP | 1 | SLE | 1 |
| MRSA-induced scarlatina | 1 | Kawasaki disease | 2 |
| Purpura fulminans | 2 | ||
| Anaphylaxis | 1 | ||
TEN=Toxic epidermal necrolysis; DRESS=Drug rash, eosinophilia, systemic symptoms; AGEP=Acute generalized exanthematous pustulosis; SSSS=Staphylococcal scalded skin syndrome; PNP=Paraneoplastic pemphigus; MRSA=Methicillin-resistant Staphylococcus aureus; SLE=Systemic lupus erythematosus
Figure 6.
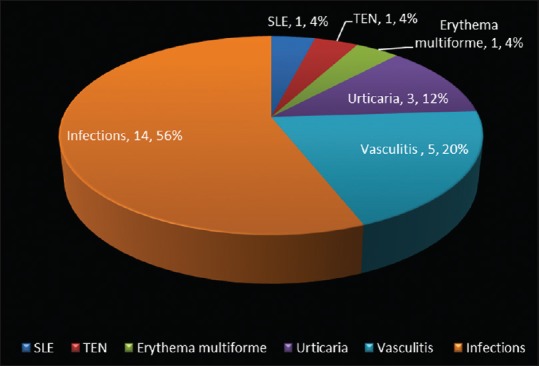
Spectrum of skin conditions associated with systemic inflammatory response syndrome in patients requiring hospitalization (n = 25)
Table 4.
Systemic symptoms and abnormal investigations in patients with SIRS (n=25)
| Systemic signs and symptoms | No. (%) |
|---|---|
| a) Fever | 25 (100) |
| b) Gastrointestinal | 10 (40) |
| c) Respiratory | 3 (12) |
| d) Joint | 2 (8) |
| Relevant abnormal laboratory results | Abnormal/total done |
| a) Total WBC | 22 (88) |
| b) Differential count | 16 (64) |
| c) CRP | 13 (81.25) |
| d) Liver function test | 5 (27.8) |
| e) Culture positivity: | |
| Blood Pus |
n=2 (meningococci, Gram-negative bacilli) n=3 (methicillin-resistant Staphylococcus aureus) |
SIRS=Systemic inflammatory response syndrome; WBC=White blood cells; CRP=C-reactive protein
Mortality
Two (0.9%) patients with SIRS died during their course of illness in the hospital. The diagnoses were purpura fulminans secondary to meningococcemia (died 3 days after admission) and febrile exanthem (probable viral) who died after 16 days.
Discussion
There are few studies describing the spectrum of skin conditions presenting to the PED, however we found that this is the first study looking at the presence of SIRS, and outcome of the children presenting to the PED.
Majority (61.08%) of our children were in the preschool age group (≤5 years) as similar to the literature from India and the West.[3,4,9] The prevalence of patients with skin lesions attending the PED in our study was lower than that reported in the world literature[2,3,6] but higher than the Indian studies.[4,5] As skin lesions secondary to trauma and burns were also included in some studies,[2,3] the reported prevalence was high. Additionally, the initial screening in our study was done by the PED pediatrician, raising the possibility of minor dermatological problems going unnoticed.
Urticaria secondary to infections, was the most common inflammatory condition in our study and in other similar studies,[3,10] however the prevalence (22.16%) was higher than other studies.[4,5] The percentage of patients with urticaria requiring admission in our study (11%) was slightly lower than that observed in France (17%).[2]
Though the prevalence of cutaneous adverse drug reactions was lower (5.9%) than other studies (13.33%),[4] we had patients with more severe forms such as TEN, DRESS, AGEP, and anaphylaxis. Mathias et al.[4] reported a higher prevalence of SJS/TEN than our study (10 vs. 1.9%). Anticonvulsants are the commonest triggers for DRESS,[11] which is reflected in our study as well. Although, mortality is reported to be 1.6%[12] and 5–10%[11] in TEN and DRESS syndrome respectively, none of our children died. In a study on anaphylaxis in children, 0.3% were due to cefazolin.[13] One of our patients had cefazolin-induced anaphylaxis emphasizing the need to be vigilant when prescribing drugs, especially parenteral administration. We had two children with AGEP, highlighting the necessity to have this condition in mind and differentiate from other causes of generalized pustular eruptions such as pustular psoriasis, folliculitis, or pustular bacterid eruption as the management is different. The most commonly observed systemic complication in our patients with drug reactions were altered liver function tests similar to published data.[14]
Cutaneous vasculitis among children attending the PED varies from 2.35 to 8.9%,[2,3,4] with HSP accounting for 2% of cases[15] similar to our observation. Among our patients with HSP and that of published data,[4,16] gastrointestinal symptoms were the most common but none of them died in contrast to the reported mortality rate of 1–3%.[15] Kawasaki disease was seen in 0.26% of children in the study done by Kramkimel et al.[2] vs. 0.99% in our study and in both studies all required admission.
Viral infections were most common in the West[8,17] similar to ours, but bacterial infections were common in India[4] and Argentina.[18] The pattern of viral infections seen in our study reflects the general Indian pattern. Hand foot and mouth disease was the most common viral infection in ours but unreported from the West,[2] where varicella was the commonest reported exanthem.[2,3] The increased prevalence of viral infections in summer was in concordance with another study.[2] In addition, nonspecific febrile exanthems were reported in a high proportion of our patients and most of them were self-resolving except for one child who died 16 days after hospitalization. Common viral etiologies including parvovirus, enterovirus, dengue, measles, and scrub typhus were ruled out in this child.
Impetigo was the most common bacterial infection similar to the published data[2,17] followed by Staphylococcal scalded skin syndrome (SSSS). Methicillin-resistant Staphylococcus aureus (MRSA) prevalence in children was 21.9% in a hospital-based Indian study,[19] which was seen in only two of our children (SSSS and Staphylococcal scarlatina). Fatality is reported in MRSA-induced SSSS,[20] stressing the importance to recognize and treat appropriately. The commonest presentation of rickettsial infection was fever with maculopapular exanthem except for one child who presented with purpura fulminans. Rickettsial infections are reported in children from South India,[21] hence it should be considered in the differentials of febrile exanthem. Patients with purpura fulminans accounted for 0.9% in our study as opposed to 12.2% in another Indian study.[4] The reported prevalence of meningococci induced purpura fulminans is 24.77% with 50% mortality.[22] In keeping with the high mortality reported in meningococcemia-induced purpura fulminans, our child also died.
In contrast to other studies, we had a child with PNP in our cohort. This shows that immunobullous disorders need to be considered when children present with intractable oral ulceration and systemic symptoms. The other common differentials of oral ulcerations-like herpetic gingivostomatitis and SJS must be ruled out in such scenarios.
Some of the nonemergency conditions such as scabies, papular urticaria, seborrheic dermatitis were also seen in our study which could be due to any of the following reasons: (1) all patients attending the PED were screened by the PED physician and these skin conditions might have been identified in those who presented for other concerns; (2) some might have directly presented to the PED when there was a delay in obtaining a routine outpatient clinic appointment, as most patients hailed from very far off places; or (3) due to an acute exacerbation of the existing skin condition.
Hospitalization rate was higher in our study (22.16%) than others (range: 5.2–8.2%)[2,3] and this might be influenced by the fact that our hospital is a tertiary referral center. Similar to other studies,[2,3] the most common reason for hospitalization was infections. Viral infections were the most common cause for admission in our study and from France.[2] In our study, hospitalization was significantly higher among older children (>6 years) (P = 0.013).
SIRS is a widely used measure to assess severity of illness and helps the clinician to identify patients who are at risk of progression to severe disease. Despite drug reactions and infections being the most common skin conditions causing SIRS, cutaneous vasculitis is also associated with SIRS.[23] In our study, 25 (55.55%) of the hospitalized patients satisfied the criteria for SIRS and the commonest underlying conditions were infections and vasculitis. The presence of SIRS did not influence the duration of hospitalization or outcome. Two (8%) out of 25 children with SIRS died during their course in the hospital. As patients with infections and associated SIRS are at risk of sepsis,[24] it is prudent to recognize and treat the infection at the earliest. The association of SIRS in children with pediatric dermatological conditions is not yet reported and our study is the first to report this association.
Our study is limited by the fact that it is a single-center study and done only over a one-year period.
Conclusion
Our study highlights the spectrum of pediatric cutaneous emergencies and their outcome. The wide spectrum of cutaneous manifestations in the PED with high hospitalization rate highlights that patients attending PED might have more severe skin ailments and careful skin examination will help to establish diagnosis earlier, especially in patients with multisystem diseases such as vasculitis, rickettsial infections, and collagen vascular diseases. Early referral of patients with primary skin conditions presenting to the ED to the dermatologists might result in better clinical outcome and decreased mortality. We would like to emphasize the role of the dermatologist in the PED and the need to train the residents in pediatric emergency about common skin conditions and dermatological emergencies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gupta S, Sandhu K, Kumar B. Evaluation of emergency dermatological consultations in a tertiary care centre in North India. J Eur Acad Dermatol Venereol. 2003;17:303–5. doi: 10.1046/j.1468-3083.2003.00690.x. [DOI] [PubMed] [Google Scholar]
- 2.Kramkimel N, Soussan V, Beauchet A, Duhamel A, Saiag P, Chevallier B, et al. High frequency, diversity and severity of skin diseases in a paediatric emergency department. J Eur Acad Dermatol Venereol. 2010;24:1468–75. doi: 10.1111/j.1468-3083.2010.03672.x. [DOI] [PubMed] [Google Scholar]
- 3.Landolt B, Staubli G, Lips U, Weibel L. Skin disorders encountered in a Swiss pediatric emergency department. Swiss Med Wkly. 2013;143:w13731. doi: 10.4414/smw.2013.13731. [DOI] [PubMed] [Google Scholar]
- 4.Mathias RC, Jayaseelan E, Augustine M. Spectrum of pediatric dermatological emergencies at a tertiary care hospital in India: A descriptive study. Int J Dermatol. 2013;52:27–31. doi: 10.1111/j.1365-4632.2011.05247.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar R, Basu S, Patwari AK, Sharma RC, Dutta AK, Sardana K. An appraisal of pediatric dermatological emergencies. Indian Pediatr. 2000;37:425–9. [PubMed] [Google Scholar]
- 6.AlKhater SA, Dibo R, Al-Awam B. Prevalence and pattern of dermatological disorders in the pediatric emergency service. J Dermatol Dermatol Surg. 2017;2:7–13. [Google Scholar]
- 7.McGraw-Hill Medical: Fitzpatrick's Dermatology - References [Internet] [Last cited on 2016 Sep 06]. Available from: http://www.fitzpatricksdermatology.com/references.php .
- 8.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 9.Moon AT, Castelo-Soccio L, Yan AC. Emergency department utilization of pediatric dermatology (PD) consultations. J Am Acad Dermatol. 2016;74:1173–7. doi: 10.1016/j.jaad.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Liu TH, Lin YR, Yang KC, Chou CC, Chang YJ, Wu HP. First attack of acute urticaria in pediatric emergency department. Pediatr Neonatol. 2008;49:58–64. doi: 10.1016/S1875-9572(08)60014-5. [DOI] [PubMed] [Google Scholar]
- 11.Alexander T, Iglesia E, Park Y, Duncan D, Peden D, Sheikh S, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:e945–9. doi: 10.1542/peds.2012-2117. [DOI] [PubMed] [Google Scholar]
- 12.Forman R, Koren G, Shear NH. Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: A review of 10 years’ experience. Drug Saf. 2002;25:965–72. doi: 10.2165/00002018-200225130-00006. [DOI] [PubMed] [Google Scholar]
- 13.Renaudin JM, Beaudouin E, Ponvert C, Demoly P, Moneret-Vautrin DA. Severe drug-induced anaphylaxis: Analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy. 2013;68:929–37. doi: 10.1111/all.12168. [DOI] [PubMed] [Google Scholar]
- 14.Patel TK, Thakkar SH, Sharma D. Cutaneous adverse drug reactions in Indian population: A systematic review. Indian Dermatol Online J. 2014;5(Suppl 2):S76–86. doi: 10.4103/2229-5178.146165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akbar DH. Fatal complication of Henoch-Schonlein purpura: Case report and literature review. Saudi J Gastroenterol. 2000;6:165–8. [PubMed] [Google Scholar]
- 16.Hung SP, Yang YH, Lin YT, Wang LC, Lee JH, Chiang BL. Clinical manifestations and outcomes of Henoch-Schönlein purpura: Comparison between adults and children. Pediatr Neonatol. 2009;50:162–8. doi: 10.1016/S1875-9572(09)60056-5. [DOI] [PubMed] [Google Scholar]
- 17.Auvin S, Imiela A, Catteau B, Hue V, Martinot A. Paediatric skin disorders encountered in an emergency hospital facility: A prospective study. Acta Derm Venereol. 2004;84:451–4. doi: 10.1080/00015550410021448. [DOI] [PubMed] [Google Scholar]
- 18.Dei-Cas I, Carrizo D, Giri M, Boyne G, Domínguez N, Novello V, et al. Infectious skin disorders encountered in a pediatric emergency department of a tertiary care hospital in Argentina: A descriptive study. Int J Dermatol. 2019;58:288–95. doi: 10.1111/ijd.14234. [DOI] [PubMed] [Google Scholar]
- 19.Senthilkumar K, Biswal N, Sistla S. Risk factors associated with methicillin-resistant Staphylococcus aureus infection in children. Indian Pediatr. 2015;52:31–3. doi: 10.1007/s13312-015-0562-9. [DOI] [PubMed] [Google Scholar]
- 20.Hörner A, Hörner R, Salla A, Nunes MS, Garzon LR, Rampelotto RF, et al. Staphylococcal scalded skin syndrome in a premature newborn caused by methicillin-resistant Staphylococcus aureus: Case report. Sao Paulo Med J. 2015;133:450–3. doi: 10.1590/1516-3180.2013.79400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamarasu K, Malathi M, Rajagopal V, Subramani K, Jagadeeshramasamy D, Mathai E. Serological evidence for wide distribution of spotted fevers & typhus fever in Tamil Nadu. Indian J Med Res. 2007;126:128–30. [PubMed] [Google Scholar]
- 22.Powars D, Larsen R, Johnson J, Hulbert T, Sun T, Patch MJ, et al. Epidemic meningococcemia and purpura fulminans with induced protein C deficiency. Clin Infect Dis. 1993;17:254–61. doi: 10.1093/clinids/17.2.254. [DOI] [PubMed] [Google Scholar]
- 23.Thomas M, Peter JV, Williams A, Job V, George R. Systemic inflammatory response syndrome in diseases of the skin. Postgrad Med J. 2010;86:83–8. doi: 10.1136/pgmj.2009.083824. [DOI] [PubMed] [Google Scholar]
- 24.Pavare J, Grope I, Gardovska D. Prevalence of systemic inflammatory response syndrome (SIRS) in hospitalised children: A point prevalence study. BMC Pediatr. 2009;3:9:25. doi: 10.1186/1471-2431-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]


